Abstract
We show that phenoxy-auxin herbicides and lipid-lowering fibrates inhibit human but not rodent T1R3. T1R3 as a co-receptor in taste cells responds to sweet compounds and amino-acids; in endocrine cells of gut and pancreas T1R3 contributes to glucose sensing. Thus, certain effects of fibrates in treating hyperlipidemia and type II diabetes may be via actions on T1R3. Likewise, phenoxy-herbicides may have adverse metabolic effects in humans that would have gone undetected in studies on rodents.
Keywords: T1R3 receptor, GPCR, fibrates, phenoxy herbicides, PPAR
INTRODUCTION
The type I taste receptors (T1Rs) are G-protein-coupled receptors that underlie sweet and umami (amino acid) taste 1. The T1R2+T1R3 heterodimer responds to sugars and sweeteners; T1R1+T1R3 responds to glutamate and other amino-acids 2, 3. T1R3 alone, possibly as a homodimer, may serve as a low-affinity sweet receptor for carbohydrates 3. T1R receptors, the taste G protein gustducin and other taste transduction proteins are expressed in taste cells of the tongue and in a number of non-taste tissues including enteroendocrine cells of the gastrointestinal tract and pancreatic islets 4, 5 (Kokrashvili et al., unpublished).
Sugars and artificial sweeteners are powerful agonists of the sweet taste receptors of both tongue and gut 6–8. Activated sweet receptors in taste cells signal the presence of carbohydrate-rich foods to the brain; the same receptors in intestinal enteroendocrine cells regulate secretion of glucagon-like peptide-1 (GLP-1) and induce expression of sodium-glucose co-transporter-1 (SGLT1) leading to enhanced absorption of carbohydrates 9, 10. Knockout mice lacking gustducin are deficient in detecting sweet and umami compounds and have dysregulated glucose homeostasis 7, 9. Yet, little attention has been paid to the physiological effects of artificial sweeteners beyond their sweet taste. Studies now indicate that artificial sweeteners activate intestinal T1R receptors 7, 8, 10. Of potential relevance is the observation that ingestion of diet soda is associated with an increased risk for metabolic syndrome thereby increasing the risk for heart disease, stroke, and diabetes 11. These studies indicate that taste receptors and other taste signaling proteins expressed in gut and other endocrine organs may have an important role in glucose homeostasis and energy metabolism and that their altered activity may contribute to pathologies such as type II diabetes and obesity.
A number of naturally occurring anti-sweet or sweet-modifying substances are suspected to be ligands of the sweet receptor, but to date the site(s) of action of only a few of these compounds have been identified 12–14. The best described inhibitor of sweet taste is lactisole (methoxy-phenoxy-propionic acid), originally isolated from coffee beans 15. Lactisole’s site of action has been mapped to the transmembrane domain of human T1R3 6, 14, 16. This region of T1R3 receptors is well conserved in humans, old world monkeys and primates, but differs in other species. Indeed, it was shown that lactisole specifically inhibits human T1R3 but not the rodent form of the receptor 17, 18 . Lactisole is a broad acting inhibitor of all or most sweeteners, and a suspected inverse agonist of the sweet receptor that on wash-out produces a sweet after-taste in humans 18–20. Compared to activities of agonists of T1R receptors very little is known of physiological and medicinal roles for sweet and umami receptor antagonists. However, there is a longstanding tradition in folk medicine of using extracts of plants naturally producing sweet-inhibiting substances to treat various diseases, particularly diabetes. For example, Gymnema sylvestre, a tropical plant containing anti-sweet gymnemic acids, has been used for more than 2,000 years in India to treat diabetes and obesity 12. In western countries, these plants and the anti-sweet substances are now becoming recognized as alternative therapies for lowering high blood sugar levels and treating obesity 21.
Interestingly, lactisole shares structural similarity with two well-known classes of compounds: fibrates and phenoxy-herbicides. Their use in medicine and/or agriculture, respectively, is determined by their predominant activities. The fibrates are a class of amphipathic carboxylic acids with a phenoxy acid motif. Fibrates are used to treat hyperlipidemias: they bind and activate peroxisome proliferator-activated receptor alpha (PPAR-alpha) which affects lipid metabolism to lower triglycerides predominantly, along with a modest lowering of LDL and increase of HDL 22. Some fibrates also have an effect on glycemia and insulin resistance 22, 23. Phenoxy herbicides are a class of organo-auxins used extensively in agriculture to control broad-leafed weeds 24. Approximately 55 million pounds of phenoxy herbicides are used annually in the United States, with 2,4-D comprising 86% of total use or about 47 million pounds of acid equivalent 25. Fibrates and phenoxy herbicides are structurally, and to some extent functionally, similar. For example one of the first widely used fibrates, clofibric acid, actually has demonstrated herbicidal activity 26. Conversely, it has been shown that some phenoxy herbicides such as 2,4D (2,4-dichlorophenoxyacetic acid) and MCPA (4-chloro-2-methylphenoxyacetic acid) have fibrate-like effects to lower lipids in rats 27.
In the present work we set out to determine if phenoxy-herbicides and fibrates, because of their chemical similarity to lactisole, act as antagonists of the T1R3 chemosensory receptor. Our results indicate that the T1R3 receptor is indeed blocked by phenoxy-herbicides and fibrates at micromolar concentrations, and that this action is specific to T1R receptors found in humans and old-world monkeys. These findings may have important health implications.
RESULTS
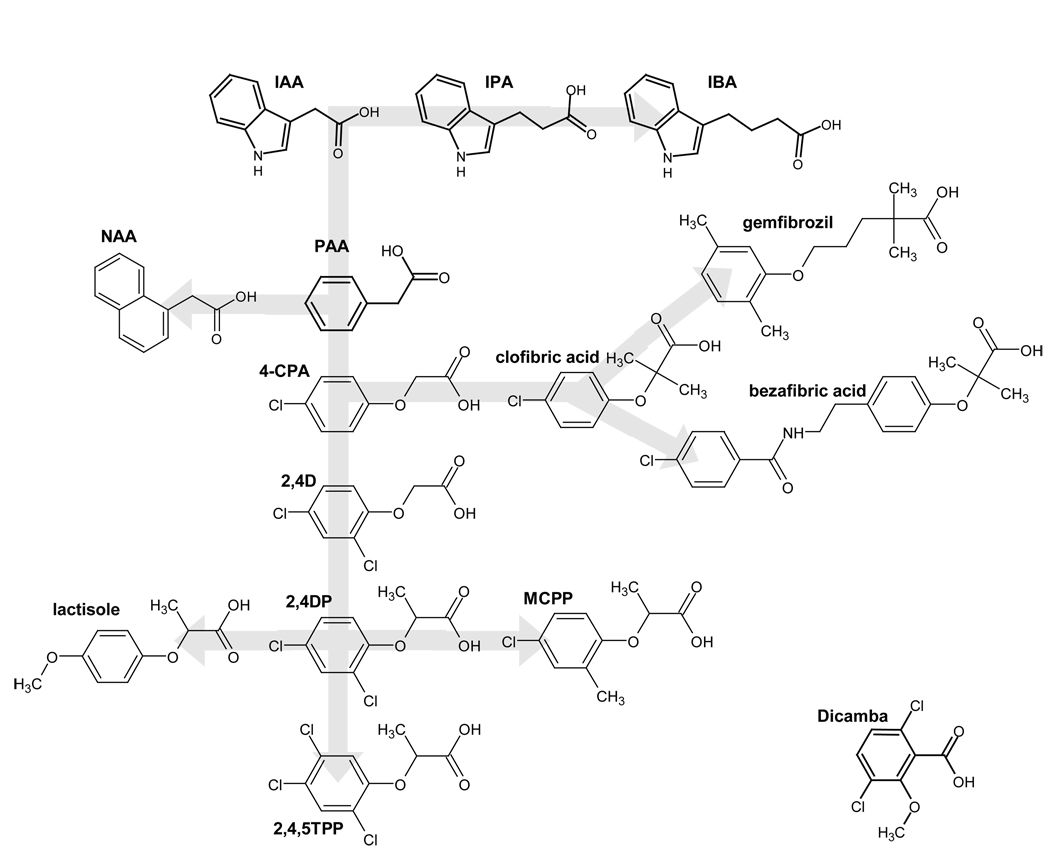
In searching for novel antagonists of the sweet taste receptor we noted the marked structural similarity of lactisole with phenoxy- herbicides and clofibric acid and other anti-lipidemia drugs (Fig. 1).
Figure 1. Structural similarity between lactisole, phenoxy-auxin–herbicides and fibrates.
Structural relationships between lactisole, phenoxy-herbicides and fibrates used in the study, generated by the ChemSketch software 2.0. Compounds are arrayed (note underlying gray arrow) according to structural similarity.
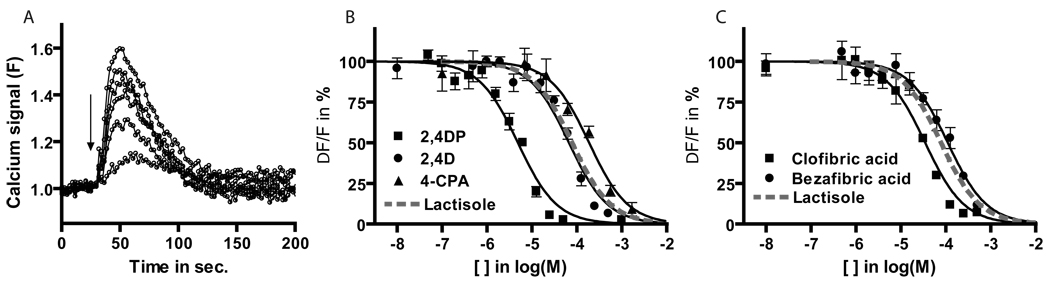
To determine if these compounds had inhibitory properties we used a cell-based assay with HEK 293 cells expressing the human sweet receptor composed of hT1R3 and hT1R2. Activation of the receptor by sugars or sweeteners was detected by intracellular calcium mobilization (Fig. 2a). Heterologously-expressed human sweet receptors were inhibited by herbicides with chlorinated and/or methylated phenoxy-propionic acid motifs (2,4DP, MCPP and 2,4,5TPP) (IC50s from 5–12 µM) even more potently than by lactisole (IC50 70 µm) (Fig. 2bc, Table 1). Bi-chlorinated phenoxy-acetic acid 2,4D also strongly inhibited the human receptor (IC50 70 µM), while the mono-chlorinated 4-CPA and non-phenoxy-herbicides PAA and NAA inhibited only at higher concentrations (IC50s from 142 to >500 µM) (Fig. 2ab). Indole type auxins (IPA, IAA, IBA) at up to 500 µM did not inhibit the human sweet receptor (Table 1). Dicamba (3,6 dichloromethoxybenzoic acid), and trichloro (2,3/4,5/6) benzoic acids at up to 1mM did not inhibit T1R3 (data not shown).
Figure 2. Phenoxy-herbicides and fibrates potently inhibit the human sweet-sensing receptor T1R2+T1R3.
T1R2+T1R3 subunits were transfected into HEK cells along with the promiscuous Gq-type protein G16-gust44. Activation of receptors by agonists was followed using the calcium-sensitive fluorescent dye fluo4-AM in a 96-well format with the fluorescent microplate reader Flexstation II (Molecular Devices). Inhibitors were added concomitantly with the sweetener sucralose at 2.5mM (saturating concentration).
A. Example of time-resolved raw traces of sucralose-activated T1R2+T1R3 receptor calcium response in the presence of increasing concentrations of the lactisole (10 to 100µM). Fluorescence signal (F) is expressed in arbitrary units (AU). The down arrow indicates the injection of the sweetener-inhibitors mixture on the cells. B,C. Dose-response curves showing inhibition of sucralose-activated human sweet receptor T1R2+T1R3 by phenoxy-herbicides and fibrates. Peak calcium signal is expressed as ΔF/F in %, normalized to control (sucralose alone). Data are from a representative experiment, means ± SD, done in quadruplicate.
Table 1. IC50 inhibition values of several phenoxyauxins and fibrates on the sweet-sensing receptor.
IC50 values of the tested compounds were determined from the calcium mobilization assays with heterologously expressed human T1R2+T1R3 receptor activated by 2.5mM sucralose in the presence of increasing concentrations of inhibitors. IC50s are means ± SD from at least 3 independent experiments.
| compound | IC50 | µM(SD) |
|---|---|---|
| Lactisole | 70 | (13) |
| IPA | >1000 | |
| IAA | >1000 | |
| IBA | >1000 | |
| PAA | >500 | |
| NAA | 233 | (82) |
| 4-CPA | 142 | (19) |
| 2,4D | 50 | (10) |
| 2,4DP | 5.3 | (1.3) |
| 2,4,5TPP | 5.7 | (1.2) |
| MCPP | 12 | (3) |
| Clofibric acid | 28 | (8) |
| Gemfibrozil | 69 | (20) |
| Bezafibric acid | 100 | (11) |
We then tested several fibrates with anti-hyperlipidemia properties that are structurally similar to phenoxy-herbicides and lactisole (Fig. 1). Clofibric acid, bezafibric acid and gemfibrozil potently inhibited the human sweet receptor (IC50s from 30 to 100 µM) (Fig. 2ab, Table 1).
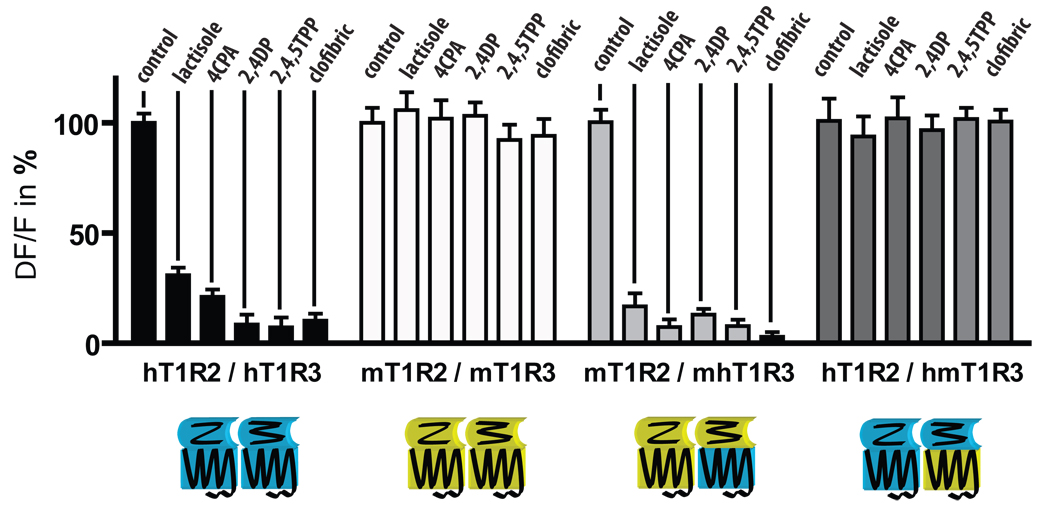
We next set out to determine if the inhibitory effect of the tested compounds was specific for human receptors. Lactisole inhibits the human sweet taste receptor, however, it has no effect on the mouse receptor. The phenoxy-herbicides and fibrates displayed the same species specificity in inhibiting the human but not the mouse sweet receptor (Fig. 3). Assays with human/mouse chimeric sweet receptors showed that the ability of phenoxy-auxins and fibrates to inhibit the sweet receptor depended on the presence of the human form of the seven-transmembrane domain of T1R3 (Fig. 3) as has been shown for lactisole 14.
Figure 3. The human T1R3 transmembrane domain determines sensitivity to lactisole, phenoxy-herbicides and fibrates.
Human (hT1R2+hT1R3), mouse (mT1R2+mT1R3) and chimeric mouse/human sweet receptors (as indicated by m/h prefix) were activated by 2.5mM sucralose and assayed for inhibition by indicated phenoxy-herbicides, fibrates and lactisole at sub maximal concentrations (~5-times IC50). Chimaeric receptors of T1R3 contained the extracellular portion (VFTM and CRD) of human or mouse receptor, and the transmembrane domain and C-terminal from mouse or human receptor (h.1–567.mT1R3 and m.1–568.hT1R3, respectively). Inhibition of the sucralose response occurred in the presence of the transmembrane domain of human T1R3. Data in the figure are from a representative experiment, means ± SD, done in quadruplicate.
DISCUSSION
We have determined that widely used herbicides 2,4DP, 2,4D and MCPP potently inhibit the human T1R2+T1R3 receptor. We also found that clofibrate and related anti-lipid drugs such as bezafibrate and gemfibrosil also strongly inhibit the human T1R2+T1R3 receptor. We determined that these compounds act specifically on the human and not the rodent form of the sweet receptor. Furthermore, it is the transmembrane portion of human T1R3 that is targeted by these compounds. Because only old world monkeys and primates (including humans) have similar T1R3 receptors that would respond to lactisole and related compounds 14, 28, 29 most animal models would not have shown any effects through their T1R3 receptors, either in taste cells or gut endocrine cells.
From our measured IC-50s of fibrates and phenoxy herbicides on T1R3 we can draw some preliminary conclusions about structure-activity relationships (SARs). It appears that the longer and more branched aliphatic chains in fibrates weaken the inhibitory activity toward the T1R3. However, a certain length or branching of the chain may be required for optimal inhibition of T1R3: for example 2,4DP is 10-fold more potent than is the 2,4D. The only difference between the two structures is that propionic acid is replaced by acetic acid (Fig.1). The inhibitory activity toward T1R3 is also affected by the modifications of the aromatic portion of the compounds. Naphthalene acetic acid, NAA, shows modest activity while its phenyl counterpart, PAA, is very weak, and the structures with indole rings (IAA, IPA) have no activity. Di-chloro substitutions (ortho or para) on the phenoxy group appear to improve T1R3 inhibitory potency in comparison to one single para-methoxy, single para-chloro substitutions, or double ortho-methyl/para-chloro substitutions (e.g. 2,4DP is 10X more potent than lactisole; and 2,4DP, and 2,4D gains 2-3-fold potency over MCPP and 4,CPA respectively). Tri-chloro substitutions do not have any further effect on T1R3 inhibitory activity (e.g. 2,4DP and 2,4,5TPP have about the same potency). The phenoxy-motif seems obligatory as its absence could be the reason that dicamba (and trichlorinated benzoic acid) do not inhibit T1R3 whereas 2,4D and 2,4,5TPP do.
T1R3’s role as a critical component of sweet and umami receptors in taste cells is well established 2, 3. T1R3, like gustducin, has also been implicated in glucose-sensing functions of gut endocrine cells and thereby in glucose homeostasis 7. From psychophysical self-experimentation conducted by one of the authors (BM) we found that clofibric acid potently inhibited both sweet and umami (MSG) taste in vivo (data not shown). We predict that fibrates and phenoxy herbicides would also inhibit T1R3 receptors within and outside of the taste system (e.g. enteroendocrine cells).
Fibrates are used in the treatment of many forms of hyperlipidemia: these compounds primarily lower triglyceride levels, modestly improve HDL and seem to improve insulin resistance when the dyslipidemia is associated with other features of the metabolic syndrome (hypertension and diabetes mellitus type 2) 30. The therapeutic target of fibrates is thought to be nuclear peroxisome proliferator activated receptor-alpha (PPAR-α), whose activation leads to increased transcription of several genes involved in lipid metabolism 22. Studies with PPAR agonists also reported lower plasma glucose, improved glucose tolerance, and enhanced insulin sensitivity 22, although the mechanism of action of fibrates on glucose homeostasis remains unclear. Our results show that IC50s (30 to 100 µM) of clofibric and bezafibric acids for inhibiting the sweet receptor are comparable to their EC50s (50–55 µM) for activating PPAR-alpha 23. Based on this calculation alone, the plasma level of clofibric acid attained in the course of the fibrate treatment would be sufficient to systemically block the T1R3 receptor. Thus, T1R3-containing receptors may be an important biological target of fibrates and could mediate certain of their effects on lipid metabolism and glucose homeostasis.
Phenoxy-auxin herbicides are synthetic herbicides that mimic the action of auxin plant hormones. These herbicides are very effective in killing broadleaf plants while leaving grasses largely unaffected, and are thus extensively used in crop agriculture and in landscape turf management 25. Several of the phenoxy-herbicides tested here are among the most widely used, e.g. 2,4D. They have low soil sorption, high leachability, and are prone to enter the human food chain. Long-term biological effects of these compounds in humans are largely unknown and based on our studies their actions on T1R3-containing receptors would not have manifested in rodent models. We think it prudent to evaluate effects of acute and chronic exposure to these compounds specifically on human metabolism and development 31.
T1R3 receptors are now known to be expressed in a number of tissues: taste cells, endocrine cells in the gastrointestinal tract, and cells in pancreas and testes 5, 32–34. In rodents, T1R3, gustducin and other signaling molecules previously found in taste cells have been shown to be present in gut endocrine cells and implicated in nutrient sensing and regulation of glucose metabolism through release of intestinal hormones 7, 9, 10. To date, comparable studies in humans or old-world monkeys have not been conducted. In view of the number of compounds used in agriculture, medicine and food industry that may affect activity of T1R receptors much more research needs to be done on the health-related effects of these compounds. The results presented here exemplify the need of testing chemicals intended for human use and/or consumption on tissues, cells and organisms with pharmacological targets similar or identical to humans. Based on our present work compounds that selectively act on T1R3 but not PPAR might be identified and prove useful in the treatment of metabolic syndrome, obesity and type II diabetes.
EXPERIMENTAL PROCEDURES
Chemicals were obtained from Sigma-Aldrich
Purity of all chemicals used was at least >98%. Fibrates and herbicides were used as acids, their active forms, as shown in the Fig.1. 10mM solutions were prepared in DMSO or water according to the compounds’ solubility. Chemical structures were generated using the ChemSketch2.0 software.
DNA constructs
Human and mouse T1R2 and T1R3 chimeras, and the G 16-gust44 were prepared pCDNA3 vector as described in 6, 14. The integrity of all DNA constructs was confirmed by sequencing.
Heterologous calcium assay for sweet-sensing receptors
HEK293E cells were cultured at 37 °C in Optimem GlutaMAX culture medium (Invitrogen) supplemented with 4% dialyzed fetal bovine serum. Cells were transfected with the DNA constructs using lipofectamine2000 according to the manufacturer’s protocol (Invitrogen). Briefly, cells were seeded onto 96-well poly-D-lysine plates (Corning) at about 12,500 cells/well 18 h prior to transfection; and co-transfected with plasmid DNAs encoding T1Rs and G 16-gust44 (0.1 µg total DNA/well; 0.2 µl lipofectamine/well). After 24 h, the transfected cells were washed once with the culture medium and incubated for another 24 h. The cells were washed with HBSS supplemented with 20mM Hepes (HBSS-H), loaded with 3 µM Fluo-4AM (Molecular Probe) in HBSS-H buffer, incubated for 1.5 h at room temperature, and then washed with HBSS-H and maintained in HBSS-H at 25°C. The plates of dye-loaded transfected cells were placed into a FlexStation II apparatus (Molecular Devices) to monitor fluorescence (excitation, 488 nm; emission, 525 nm; cutoff, 515 nm). Sweeteners were added 30 s after the start of the scan at 2x concentration in 50 µl of HBSS-H while monitoring fluorescence for an additional 200 s at 2 s intervals. Inhibitors were added concomitantly to the sweeteners on the cells.
Data Analysis of Calcium responses
After obtaining a calcium mobilization trace for each sample, calcium responses to sweeteners were quantified as the percentage of change (peak fluorescence - baseline fluorescence level, denoted as F) from baseline fluorescence level (denoted as F); ΔF/F. Peak fluorescence intensity occurred about 20–30 s after the addition of agonists. Typically, wild type sweet receptors (T1R2+T1R3) along with G 16-gust44 show calcium signal increases (ΔF/F) from 40 to 100 % of the basal signal F. Buffer alone evokes no significant response from transfected cells and sweeteners and inhibitors evoke no significant responses from parent cells (3 % ≤ F/F ≤ 3 %, S.E). The data were expressed as the mean ± S.E. of quadruplicate or sextuplicate of the F/F values, or were normalized as described. The bar graph and curving-fitting routines were carried out using Graph-Pad Prism 3.0 (GraphPad Software, Inc.).
ACKNOWLEDGMENT
Funding
Supported in part by National Institutes of Health grants DC007399 and DK073248 to BM, DC008301 to RFM and DC007384 to ELM.
Abbreviations
- T1R3
type I taste receptor subunit 3
- PPAR
Peroxisome proliferator-activated receptor
- IPA
3-(1H-indol-3-yl)propanoic acid
- IAA
1H-indol-3-ylacetic acid
- IBA
4-(1H-indol-3-yl)butanoic acid
- PAA
phenylacetic acid
- NAA
naphthalen-1-ylacetic acid
- 4-CPA
(4-chlorophenoxy)acetic acid
- 2,4,5TPP
2-(2,4,5-trichlorophenoxy) propanoic acid
- 2,4D
(2,4-dichlorophenoxy)acetic acid
- 2,4DP
2-(2,4-dichlorophenoxy) propanoic acid
- MCPP
2-(4-chloro-2-methylphenoxy) propanoic acid
- Lactisole
2-(4-methoxyphenoxy) propanoic acid
- Clofibric acid
2-(4-chlorophenoxy)-2-methylpropanoic acid
- Gemfibrozil
5-(2,5-dimethylphenoxy)-2,2-dimethylpentanoic acid
- Bezafibric acid
2-[4-(2-{[(4-chlorophenyl)carbonyl]amino}ethyl)phenoxy]-2-methylpropanoic acid
Footnotes
Conflicts of interest
Dr. Margolskee has a personal financial interest in the form of stock ownership in the Redpoint Bio company, received consulting fees from the Redpoint Bio company, and is an inventor on patents and patent applications that have been licensed to the Redpoint Bio company. The remaining authors disclose no conflicts.
REFERENCES
- 1.Brauner-Osborne H, Wellendorph P, Jensen AA. Structure, pharmacology and therapeutic prospects of family C G-protein coupled receptors. Curr Drug Targets. 2007;8:169–184. doi: 10.2174/138945007779315614. [DOI] [PubMed] [Google Scholar]
- 2.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 3.Max M, Meyerhof W. Taste Receptors. In: Allan I, Basbaum AK, Shepherd Gordon M, Westheimer Gerald, Firestein Stuart, Beauchamp Gary K., editors. The Senses: A Comprehensive Reference. Vol. 4. San Diego: Academic Press; 2008. pp. 197–218. Olfaction & Taste. [Google Scholar]
- 4.Sternini C, Anselmi L, Rozengurt E. Enteroendocrine cells: a site of 'taste' in gastrointestinal chemosensing. Curr Opin Endocrinol Diabetes Obes. 2008;15:73–78. doi: 10.1097/MED.0b013e3282f43a73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toyono T, Seta Y, Kataoka S, Toyoshima K. CCAAT/Enhancer-binding protein beta regulates expression of human T1R3 taste receptor gene in the bile duct carcinoma cell line, HuCCT1. Biochim Biophys Acta. 2007;1769:641–648. doi: 10.1016/j.bbaexp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Xu H, Staszewski L, Tang H, Adler E, Zoller M, Li X. Different functional roles of T1R subunits in the heteromeric taste receptors. Proc Natl Acad Sci U S A. 2004;101:14258–14263. doi: 10.1073/pnas.0404384101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007;104:15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 2007;582:379–392. doi: 10.1113/jphysiol.2007.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egan JM, Margolskee RF. Taste cells of the gut and gastrointestinal chemosensation. Mol Interv. 2008;8:78–81. doi: 10.1124/mi.8.2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008;117:754–761. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- 12.Kanetkar P, Singhal R, Kamat M. Gymnema sylvestre: A Memoir. J Clin Biochem Nutr. 2007;41:77–81. doi: 10.3164/jcbn.2007010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurihara Y. Characteristics of antisweet substances, sweet proteins, and sweetness-inducing proteins. Crit Rev Food Sci Nutr. 1992;32:231–252. doi: 10.1080/10408399209527598. [DOI] [PubMed] [Google Scholar]
- 14.Jiang P, Cui M, Zhao B, Liu Z, Snyder LA, Benard LM, Osman R, Margolskee RF, Max M. Lactisole interacts with the transmembrane domains of human T1R3 to inhibit sweet taste. J Biol Chem. 2005;280:15238–15246. doi: 10.1074/jbc.M414287200. [DOI] [PubMed] [Google Scholar]
- 15.Flament I, Bessière-Thomas Y. Chap: The individual constituents. In: JW, aS, editors. Coffee flavor chemistry. p. 207. [Google Scholar]
- 16.Winnig M, Bufe B, Meyerhof W. Valine 738 and lysine 735 in the fifth transmembrane domain of rTas1r3 mediate insensitivity towards lactisole of the rat sweet taste receptor. BMC Neurosci. 2005;6:22. doi: 10.1186/1471-2202-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sclafani A, Perez C. Cypha [propionic acid, 2-(4-methoxyphenol) salt] inhibits sweet taste in humans, but not in rats. Physiol Behav. 1997;61:25–29. doi: 10.1016/s0031-9384(96)00316-2. [DOI] [PubMed] [Google Scholar]
- 18.Schiffman SS, Booth BJ, Sattely-Miller EA, Graham BG, Gibes KM. Selective inhibition of sweetness by the sodium salt of +/−2-(4-methoxyphenoxy)propanoic acid. Chem Senses. 1999;24:439–447. doi: 10.1093/chemse/24.4.439. [DOI] [PubMed] [Google Scholar]
- 19.Bond RA, Ijzerman AP. Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery. Trends Pharmacol Sci. 2006;27:92–96. doi: 10.1016/j.tips.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Galindo-Cuspinera V, Winnig M, Bufe B, Meyerhof W, Breslin PA. A TAS1R receptor-based explanation of sweet 'water-taste'. Nature. 2006;441:354–357. doi: 10.1038/nature04765. [DOI] [PubMed] [Google Scholar]
- 21.Leach MJ. Gymnema sylvestre for diabetes mellitus: a systematic review. J Altern Complement Med. 2007;13:977–983. doi: 10.1089/acm.2006.6387. [DOI] [PubMed] [Google Scholar]
- 22.Staels B, Fruchart JC. Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes. 2005;54:2460–2470. doi: 10.2337/diabetes.54.8.2460. [DOI] [PubMed] [Google Scholar]
- 23.Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery. J Med Chem. 2000;43:527–550. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- 24.Troyer JR. In the beginning: the multiple discovery of the first hormone herbicides. Weed Science. 2001;49:290–297. [Google Scholar]
- 25.Szmedra P. Banning 2,4-D and the Phenoxy Herbicides: Potential Economic Impact. Weed Science. 1997;45:592–598. [Google Scholar]
- 26.Lahey KA, Yuan R, Burns JK, Ueng PP, Timmer LW, Kuang-Ren C. Induction of phytohormones and differential gene expression in citrus flowers infected by the fungus Colletotrichum acutatum. Mol Plant Microbe Interact. 2004;17:1394–1401. doi: 10.1094/MPMI.2004.17.12.1394. [DOI] [PubMed] [Google Scholar]
- 27.Vainio H, Linnainmaa K, Kahonen M, Nickels J, Hietanen E, Marniemi J, Peltonen P. Hypolipidemia and peroxisome proliferation induced by phenoxyacetic acid herbicides in rats. Biochem Pharmacol. 1983;32:2775–2779. doi: 10.1016/0006-2952(83)90091-6. [DOI] [PubMed] [Google Scholar]
- 28.Nofre C, Tinti JM, Glaser D. Evolution of the sweetness receptor in primates. II. Gustatory responses of non-human primates to nine compounds known to be sweet in man. Chem Senses. 1996;21:747–762. doi: 10.1093/chemse/21.6.747. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Danilova V, Cragin T, Roberts TW, Koposov A, Hellekant G. The sweet taste quality is linked to a cluster of taste fibers in primates: lactisole diminishes preference and responses to sweet in S fibers (sweet best) chorda tympani fibers of M. fascicularis monkey. BMC Physiol. 2009;9:1. doi: 10.1186/1472-6793-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steiner G. Atherosclerosis in type 2 diabetes: a role for fibrate therapy? Diab Vasc Dis Res. 2007;4:368–374. doi: 10.3132/dvdr.2007.067. [DOI] [PubMed] [Google Scholar]
- 31.Schreinemachers DM. Mortality from ischemic heart disease and diabetes mellitus (type 2) in four U.S. wheat-producing states: a hypothesis-generating study. Environ Health Perspect. 2006;114:186–193. doi: 10.1289/ehp.8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taniguchi K. Expression of the sweet receptor protein, T1R3, in the human liver and pancreas. J Vet Med Sci. 2004;66:1311–1314. doi: 10.1292/jvms.66.1311. [DOI] [PubMed] [Google Scholar]
- 33.Kiuchi S, Yamada T, Kiyokawa N, Saito T, Fujimoto J, Yasue H. Genomic structure of swine taste receptor family 1 member 3, TAS1R3, and its expression in tissues. Cytogenet Genome Res. 2006;115:51–61. doi: 10.1159/000094801. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa Y, Nagasawa M, Yamada S, Hara A, Mogami H, Nikolaev VO, Lohse MJ, Shigemura N, Ninomiya Y, Kojima I. Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS ONE. 2009;4:e5106. doi: 10.1371/journal.pone.0005106. [DOI] [PMC free article] [PubMed] [Google Scholar]