Abstract
We use unique experimental data on daily reproduction and survival of individual fruit flies from eight cohorts eclosed at different dates in 2004 and 2005 who were treated with varying proportions of sugar and yeast and subject to different caloric restrictions (CR). We investigate the relationship between eclosion date and longevity across diets and reproduction in Anastrepha ludens. We show that eclosion date can be associated with uncontrolled external or internal factor(s) which can modulate longevity of males and females independently of diet and reproduction to the extent similar to the effect of diet on longevity. The effect of diet manipulation on longevity is sensitive to date of eclosion with the role of CR in life extension ranging from beneficial to harmful. Interaction of date of eclosion with compositional changes of sugar and yeast but not with CR is responsible for life extension. Highly protein-enriched diets reliably maximize reproduction but not life span. Decreased longevity of flies treated with high-protein diets may be associated with harmful consequences of protein ingestion but is unlikely a result of high reproduction rates. We present evidence for the presence of two frailty-sensitive weakly interacting mechanisms of longevity in female flies associated with differences in predisposed fitness.
Keywords: Longevity extension, diet, caloric restriction, protein, reproduction, sex differentials
INTRODUCTION
The majority of studies on effects of dietary restriction (DR) on longevity show life span (LS) extension under DR (Partridge et al., 2005), including model organisms ranging from yeasts (e.g., Saccharomyces cerevisaie (Lin et al., 2002)) to invertebrates (e.g., the nematode worm C. elegans (Walker et al., 2005)) and mammals (e.g., the rhesus monkey, Macaca mulatta (Lane et al., 2004)). Common belief that similar mechanisms can be inherent to humans stimulates intense scrutiny of the longevity benefits in model organisms (see recent review in (Kennedy et al., 2007)). However, recent studies that better represent the integrative nature of diet and resource allocation (using dietary restriction gradients, analyses of body composition, or measurement or control of food intake) show that these manipulations merely scratch the surface of the biological changes that are induced by diet (Carey et al., 2008; Lee et al., 2008; Maklakov et al., 2008; Skorupa et al., 2008).
The objective of feeding is to meet the nutritional requirements for maximal reproductive success, which can be decomposed into longevity and rate of reproduction. These requirements are sources of energy and nutrients needed for growth and repair most notably amino acids (in particular essential amino acids), sterols, vitamins, co-factors, salts and micro-elements. While it is obvious that any shortage will prevent an organism from reaching its potential, over ingestion of nutrients also comes at a cost. Because food sources will rarely, if ever, exactly match the nutritional requirements of an organism, individuals employ strategies to balance the cost of ingesting fewer of some nutrients and the harm of ingesting others (Douglas, 2009; Raubenheimer and Simpson, 1996; 2003; Raubenheimer et al., 2009; Simpson and Raubenheimer, 2001; Simpson et al., 2004). In particular in exothermic invertebrates such as insects, sources of amino acids (protein, nitrogen) are often limiting factors and dietary restriction studies therefore focus on caloric and protein content of diets. Then dietary composition refers to compositional proportion of carbohydrates (sugars) and proteins (yeasts), denoted as SY, whereas caloric restriction (CR) refers to the number of consumed calories. Some authors may use the acronym DC for ‘dietary congruence’ to indicate to what extent a resource is congruent with the nutritional requirements (Boggs, 2009) which is in practice often simplified to our definition of SY.
The phenotypic response to dietary restriction is often interpreted as part of an organisms adaptation to available diets in the wild. In particular, organisms are thought to invest in longevity when they perceive or expect that food is scarce, so that individuals will have the opportunity to reproduce when food becomes available later in life (Carey et al., 2008; Morris et al., 2008). While there is little information on nutrient intake of important model organisms in the wild, studies on some insect species indicate that restricted diets can be common in nature (Molleman et al., 2008; Molleman et al., 2009; Murphy, 1983). Therefore, it has been suggested that the normal nutritional regime in the laboratory is harmfully nutrient rich.
Physiological mechanisms which underlie DR-related life extension are not yet clear (Mair et al., 2004). It is increasingly recognized that calories do not explain the life-extending effects at least in Drosophila (Mair et al., 2005). Instead, recent studies suggest that diet composition could be more relevant to life extension than CR (Carey et al., 2008; Lee et al., 2008; Magwere et al., 2004; Skorupa et al., 2008). These findings are supported by the metabolic role of proteins which can be in a causative pathway linked with intrinsic senescence (Raubenheimer and Simpson, 2003; Tavernarakis, 2008).
A possible trade off between reproduction and longevity is an important topic in life history theory. Flies that lay eggs experience higher mortality rates than those in which reproduction is restricted, regardless of the nature of the eggs arresting manipulation (Carey et al., 2001; Mair et al., 2004; Piper et al., 2005; Rogina et al., 2007). Underlying biological rationale is that reproduction can require reallocation of the energetic resources to egg laying at the cost of maintenance that eventually limits life span (Novoseltsev et al., 2004). Nevertheless, a clear and dominant reproduction/life span trade off is not often apparent. For example, recent experiments with Drosophila do not support the leading role of the reproductive system in longevity (Mair et al., 2004).
While early life experience plays an important role in the life history trajectories of many types of organisms, this is especially important for species with a distinct larval stage during which particular nutrients can be gathered to compensate for deficiencies in the adult diet (Boggs, 1997; Mevi-Schutz and Erhardt, 2005; O'Brien et al., 2004; 2005), important decisions about adult phenotype are made (Brakefield et al., 2005; Brakefield et al., 1998) and symbionts may be acquired (Behar et al., 2008a; Behar et al., 2008b; Ben-Yosef et al., 2008; Douglas, 2009). In rats the offspring of mothers that experienced protein restriction during pregnancy or lactation have dramatically delayed sexual maturation and premature ageing of reproductive function (Guzman et al., 2006). Sensitivity of LS to birth date is also known for humans (Lowell and Davis, 2008). Accumulating evidence suggests that mean LS in various experimental species including Drosophila varies from experiment to experiment even if they are performed under the same controllable conditions for the same genetic lines of animals (Izmaylov et al., 2005). Studies where the effect of different larval diets on adult life span and reproduction is measured generally find important effects (i. e. Bauerfeind and Fischer, 2009; Boggs and Freeman, 2005; Krainacker et al., 1987).
In this study we use unique experimental data gathered by J. Carey and colleagues on daily reproduction and survival of individual flies raised using the same protocols who were under different combinations of SY and CR treatments and eclosed at different dates in 2004 and 2005 (Carey et al., 2008). We investigated the relationship between eclosion date and LS across dietary-restriction gradients and female reproduction in Anastrepha ludens (A. ludens). The large sample sizes used in this experiment (4800 flies in total) allowed us to use a novel approach to the study of DR using the sensitivity of effects of diet treatment to date of eclosion. Our results provided unique insights into the role of date of eclosion in life extension in A. ludens, the lack of generality of diet effect on life span, and individual differences in responses to diet and reproduction.
DATA AND METHODS
Design and methods of the experiments with A. ludens (known as the Mexican fruit flies, mexflies) are detailed in (Carey et al., 2008). Briefly, 100 mexflies of each sex were treated with 24 different diets arranged in a grid of four SY levels, i.e., sugar-to-yeast (SY) ratios of 1:0 (100% sugar), 24:1 (96% sugar), 9:1 (90% sugar), and 3:1 (75% sugar), and six levels of CR for each SY level, i.e., 100% (i.e., full diet), and 75%, 50%, 25%, 10%, and 0% (water only) of full diet. This yielded samples of 2400 mexflies of each sex, totaling to 4800 individuals. Each treatment group (i.e., 100 mexflies) was composed of 12 flies randomly selected from seven cohorts eclosed on July 23 (Jul23); July 26 (Jul26); and November 15 (Nov15) of 2004 and Jan14 (Jan14), February 22 (Feb22), April 8 (Apr8), and May 4 (May4) of 2005 as well as of 16 flies born in August 5 (Aug5), 2005.
Ad libitum (full diet) requirement for the minimum volume of mother stock was estimated in pilot studies similarly as in earlier studies (see Figure 1 in Carey et al., 2002). The stock was then diluted while the volume of the droplet of food offered to each fly each day was held constant. This method is equivalent to a rodent-like system where animals have access to a fixed amount of food and fundamentally different from simply diluting a diet with different yeast content while the flies can eat as much as they want. Each treatment group was fed using food prepared in the same way. Throughout this study we ignored CR=0% as trivial.
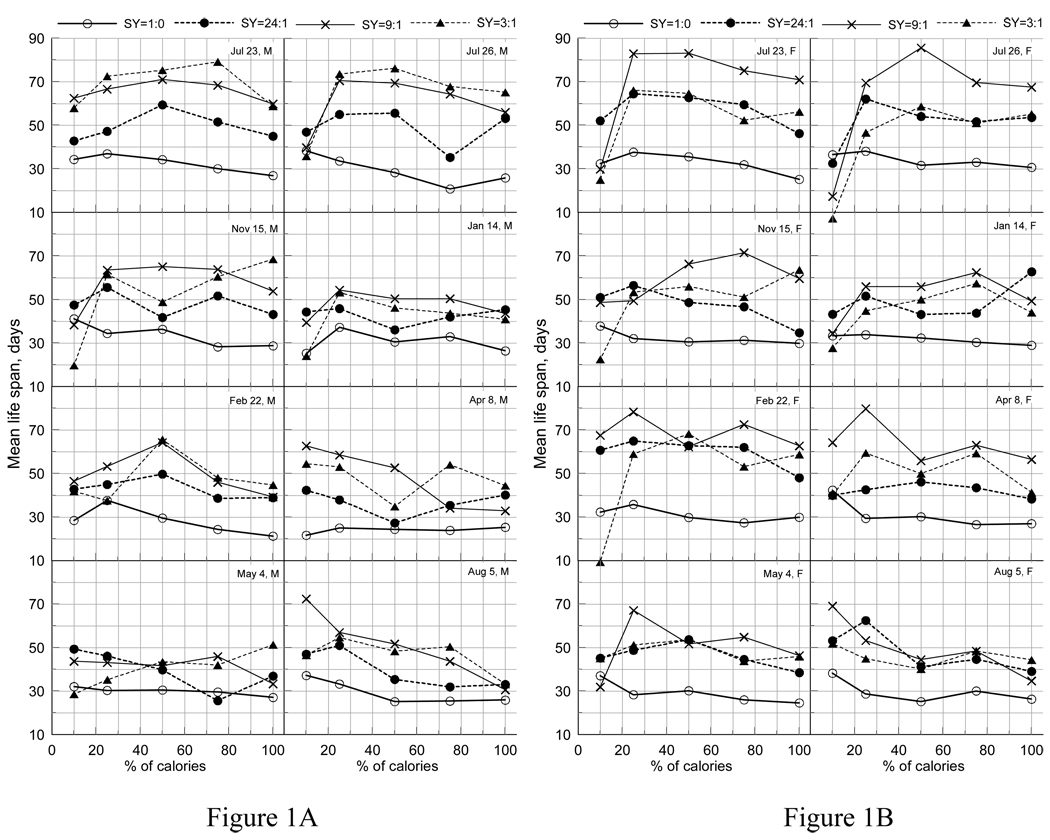
Figure 1.
CR-patterns of mean life span for different sugar and yeast (SY) composition treatments for (A) males (M) and (B) females (F) from cohorts eclosed on dates shown in the inset. July 23, July 26, and November 15 cohorts eclosed in 2004. The remaining cohorts eclosed in 2005.
RESULTS
Empirical evidences
From previous analyses of the data it was concluded that life-extending effects of different DR regimes (i.e., combinations of SY and CR treatments) in the considered sample of mexflies are better attributable to SY than to CR with the longest life spans for SY=9:1 (i.e., 90% sugar and 10% yeast) diet for all CR levels combined (Carey et al., 2008). Given this result, we connected mean LS for each of four SY treatments (SY=1:0, 24:1, 9:1, and 3:1) as a function of caloric content (i.e., CR-pattern) for each of the eight cohorts of males and females (Figure 1).
Despite small sample size that results in relatively large standard errors (not shown), four important observations follow from Figure 1. First, there is no consistently beneficial effect of CR on LS, i.e., there is no consistently increasing CR-patterns for different SY treatments and cohorts as CR changes from 100% to 10%. For instance, CR-patterns of LS can be flat (Figure 1A; May4), increasing (Figure 1A; Aug5), or declining (Figure 1A; Nov15) depending on eclosion cohort and SY. Second, mexflies from distinct cohorts responded markedly differently to SY composition. The two most SY-sensitive cohorts were those eclosed on Jul23 and Jul26. The least sensitive were Jan14, May4, and Aug5 cohorts of males and females. Third, this different responsiveness is weakly sex-sensitive, i.e., CR-patterns of mean LS are much more similar across the sexes than across cohorts (Figures 1A,B). This observation (along with the other; see next paragraph) partly offsets possible statistical uncertainty associated with small sample size. Fourth, pure sugar diet (i.e., SY=1:0) is consistently unfavorable for longevity across cohorts.
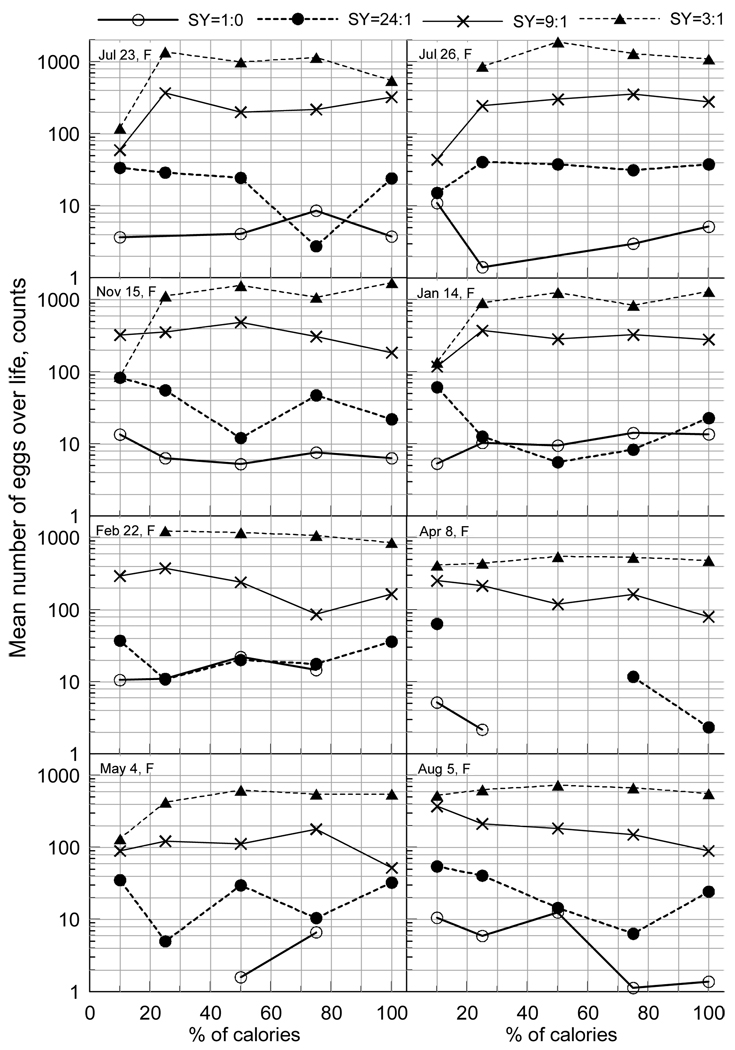
Figure 2 shows cohort- and DR-specific mean life time reproduction. The first observation is that unlike the results for LS in Figure 1, these results are remarkably consistent across cohorts. For instance, rich protein diet (SY=3:1) is practically always associated with maximal egg production. Decreased proportions of yeasts resulted in smaller numbers of eggs. This observation further offset possible statistical uncertainty in Figure 1. Second, females with the same mean LS at a given CR level lay markedly different number of eggs, e.g., mean LS of Feb22 cohort for CR=50% is the same for three protein-enriched diets (Figure 1B) but the egg production for these three diets is tremendously different (see Figure 2 and note logarithmic scale).
Figure 2.
Mean number of eggs laid by females during their entire life for each diet. Cohorts eclosed on dates shown in the inset. Missing symbols indicate less than one mean egg laid for life.
Further analyses are designed to rigorously quantify the observations suggested by Figure 1 and Figure 2. For these analyses we used unadjusted proportional hazard Cox regression model because LS was known for each individual fly. We evaluated relative risks (RR) of death and survival patterns in: (i) groups with aggregated DR treatments, (ii) groups with individual DR treatments, and (iii) more and less homogenous groups eclosed at different dates.
Survival in SY- and CR-aggregated groups
In this Section we show the results of the analyses for two types of DR aggregations. First, we aggregated four SY treatments and considered CR as an explanatory variable. Second, we aggregated five CR treatments and considered SY as an explanatory variable.
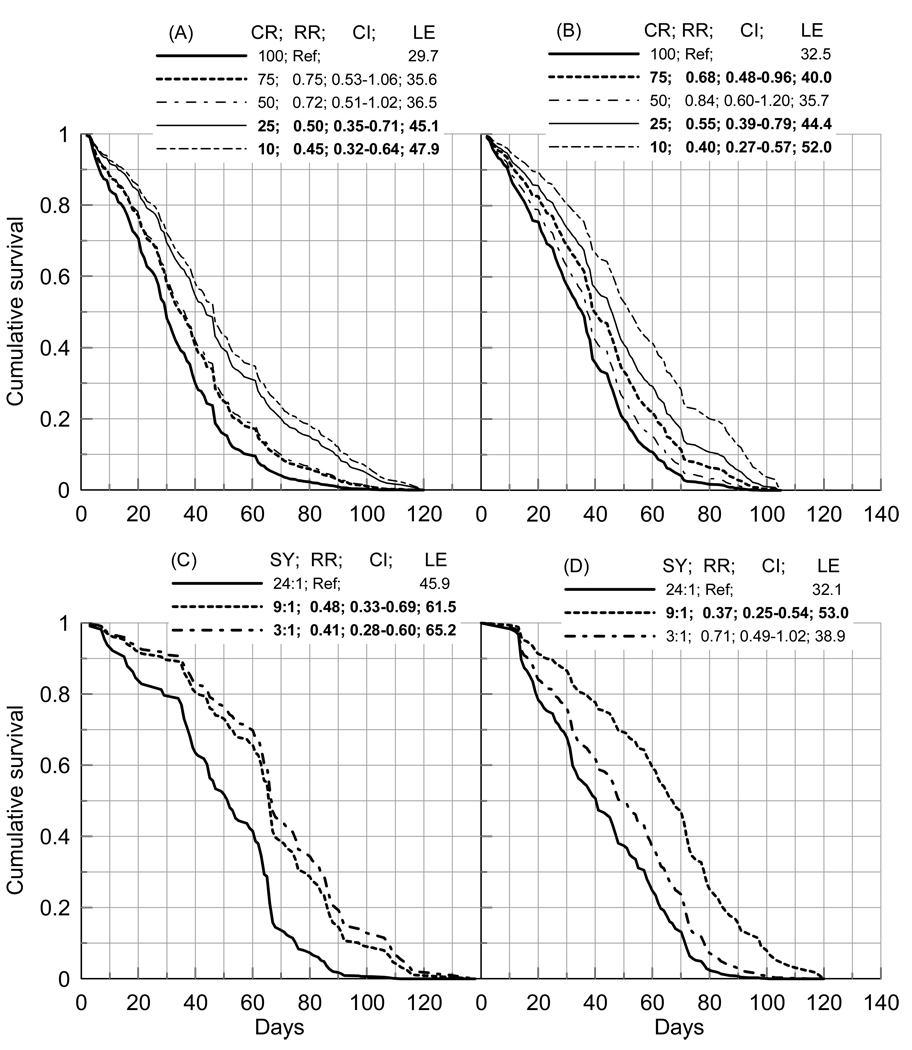
For aggregated SY levels the analyses showed no significant differences in RR of death between CR levels for five of eight male cohorts, i.e., Jul23, Jul26, Nov15, Jan14, and May4. Two male cohorts (Feb22 and Apr8) show modest variation in RR when only one CR level had significantly smaller RR, e.g., for Feb22 cohort RR=0.50 (95% Confidence Interval [CI]=0.33–0.76) for CR=50% compared to the least favorable for LS CR=100% treatment. The largest effect of CR is found for Aug5 cohort (N=320; Figure 3A). The shortest life expectancy for aggregated SY levels, i.e., 34.2 days (it was calculated as an integral over the survival curve), is found for May4 cohort and the greatest life expectancy was for Jul23 cohort (50.4 days). Because the greatest life expectancy (50.4 days) is observed for the CR-insensitive cohort (i.e., it is greater than the life expectancy which can be obtained by applying CR to the most CR-sensitive Aug5 cohort, Figure 3A), we conclude that CR does not really extend the LS in males.
Figure 3.
Cumulative survival of (A,C) male and (B,D) female mexflies from (A,B) Aug5, (C) Jul23, and (D) Apr8 cohorts showing maximal sensitivity of the relative risks (RR) of death to changes in: (A,B) caloric restriction (CR, %, shown in the insets) for aggregated levels of diet composition and (C,D) diet composition (SY, shown in the insets) for aggregated levels of CR. Insets also show 95% Confidence intervals (CI) of the RR, and life expectancy (LE). Cumulative survival is evaluated using the proportional hazard Cox regression model with (A,B) caloric restriction or (C,D) diet composition as an explanatory variable. LE is evaluated as an integral over the survival curve. Bold font denotes significant RRs.
The results remain qualitatively the same when the least favorable diet (pure sugar) is excluded (not shown). Similar weak sensitivity of maximal life expectancy to CR is seen for females. Specifically, for all DR levels combined, three cohorts (Nov15, Feb22, and Apr8) are CR-insensitive. For three other cohorts (Jul23, Jul26, and Jan14) the CR-sensitivity is attributed solely to the detrimental effect of the CR=10% treatment. May4 cohort shows modestly-significant sensitivity to CR=50% (RR=0.62, CI=0.41–0.93) and CR=25% (RR=0.59, CI=0.39–0.88) compared to the least favorable for LS CR=100% treatment. Again, as for males, the largest sensitivity was found for Aug5 cohort (N=320; Figure 3B). Consistently with males, CR does not extend longevity beyond maximal life expectancy (54.4 days for four aggregated CR levels, excluding the CR=10%) for the Jul23 female cohort.
Sensitivity of LS to SY treatment for aggregated CR levels is more pronounced compared to sensitivity to CR even if the most detrimental pure sugar diet is excluded from the analyses as trivial. Specifically, male Jan14, Feb22, and May4 cohorts are not responsive to changes in SY, i.e., risks of death for all remaining three SY levels (i.e., SY=24:1, 9:1, and 3:1) do not differ significantly. For two cohorts (Nov15 and Aug5) sensitivity to the SY composition is modest. For Jul23, Jul26, and Apr8 male cohorts the risks of death for SY=9:1 and 3:1 treatments are highly significant and smaller than the risks for the SY=24:1. The most sensitive cohort of males is Jul23 one (N=180; Figure 3C). Males from this cohort treated with SY=9:1 and 3:1 diets live the longest lives. Therefore, unlike the effect of CR, manipulation of SY can be associated with real life extension. For all five SY-sensitive male cohorts the RR for the 9:1 and 3:1 diets do not differ significantly.
Similarly, there are three SY-insensitive cohorts of females (ignoring pure sugar diet), i.e., Jan14, May4, and Aug5. The RR of death are the smallest for SY=9:1 diet and do not differ significantly between SY=24:1 and SY=3:1 diets for five remaining cohorts (i.e., Jul23, Jul26, Nov15, Feb22, and Apr8). The most responsive to SY treatment is Apr8 female cohort (N=180; Figure 3D). Similar to males, manipulation with SY can lead to real extension of LS, i.e., the most long-lived is the Jul23 cohort under SY=9:1 treatment (with 65.7 days of life expectancy). May4 female cohort is the shortest living for aggregated CR levels with life expectancy of 45.5 days.
Relative risks of death across DR treatments and cohorts
The effect of individual DR treatments was analyzed using Cox regression model with one explanatory variable (that excludes multiple comparisons) which characterized each of all 20 DR treatments for each of eight cohorts. Samples with the largest mean LS (i.e., SY=3:1 and CR=75% for Jul23 cohort of males and SY=9:1 and CR=50% for Jul26 cohort of females; see Figure 1) were chosen as referent. The results for SY=1:0 treatment show consistently high RR for each cohort, CR level, and sex (not shown) confirming the observations in Figure 1.
Table 1 and Table 2 quantitatively characterize three important observations. First, the RRs explicitly show that date of eclosion is an important factor which can affect LS of mexflies independently of diet and female reproduction. Indeed, Table 1 and Table 2 show that RRs significantly vary between cohorts for the same diet. This variation is seen for each sex. For instance, for SY=3:1 and CR=50% diet the risks of death for males can experience a 6.6-fold increase for Apr8 cohort compared to Jul23 cohort for which one of the smallest risks among all males (RR=1.00, CI=0.45– 2.24) is observed (Figure 1).
Table 1.
Relative risks of death for males from selected cohorts according to the dietary restriction treatment.
| DR | Cohorts | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SY | CR, % | Jul 23 | Jul 26 | Nov 15 | Jan 14 | Feb 22 | Apr 8 | May 4 | Aug 5 |
| 24:1 | 100 | 3.54** | 2.27* | 4.18*** | 3.31** | 4.94*** | 4.18*** | 5.53*** | 5.57*** |
| 75 | 2.94** | 5.81*** | 2.87** | 4.55*** | 4.51*** | 6.22*** | 15.2*** | 6.35*** | |
| 50 | 1.91 | 2.19 | 4.91*** | 5.85*** | 3.18** | 10.2*** | 4.97*** | 4.91*** | |
| 25 | 2.80* | 2.22 | 2.45* | 4.10** | 3.31** | 4.98*** | 3.73** | 2.61* | |
| 10 | 2.82* | 2.60* | 2.26* | 3.91** | 3.51** | 4.04** | 3.18** | 2.36* | |
| 9:1 | 100 | 1.58 | 1.79 | 2.60* | 4.64*** | 4.51*** | 5.19*** | 7.67*** | 5.71*** |
| 75 | 1.38 | 1.39 | 1.52 | 2.69* | 3.29** | 6.11*** | 3.58** | 3.00** | |
| 50 | 1.20 | 1.24 | 1.52 | 2.89** | 1.42 | 2.45* | 3.25** | 2.96** | |
| 25 | 1.20 | 1.20 | 1.60 | 2.49* | 2.62* | 2.02 | 4.70*** | 1.47 | |
| 10 | 1.47 | 2.60* | 3.69** | 2.40* | 3.09** | 1.37 | 3.22** | 1.06 | |
| 3:1 | 100 | 1.46 | 1.45 | 1.46 | 5.35*** | 3.55** | 2.98** | 2.94** | 3.45** |
| 75 | Reference | 1.24 | 1.82 | 3.70** | 3.04** | 2.57* | 3.78** | 2.42* | |
| 50 | 1.00 | 1.11 | 3.31** | 2.70* | 1.46 | 6.60*** | 3.14** | 2.91** | |
| 25 | 1.14 | 1.14 | 1.82 | 2.26* | 5.02*** | 2.33* | 6.49*** | 2.14* | |
| 10 | 1.26 | 2.33* | 7.92*** | 9.36*** | 2.98** | 2.04 | 7.79*** | 1.87 | |
0.01<p<0.05
0.0005<=p<=0.01
p<0.0005
SY=sugar and yeast composition; CR=caloric restriction; DR=SY and CR.
Bold font denotes risks which are significantly larger compared to the risks for the CR=100% diet as evaluated for samples stratified by cohort and SY (i.e., within SY levels) treatment in separate Cox regressions.
Table 2.
Relative risks of death for females from selected cohorts according to the dietary restriction treatment.
| DR | Cohorts | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SY | CR, % | Jul 23 | Jul 26 | Nov 15 | Jan 14 | Feb 22 | Apr 8 | May 4 | Aug 5 |
| 24:1 | 100 | 4.29*** | 3.75** | 9.79*** | 1.63 | 4.09** | 8.90*** | 6.22*** | 7.81*** |
| 75 | 1.99 | 3.70** | 4.40*** | 4.91*** | 2.49* | 5.38*** | 5.57*** | 4.42*** | |
| 50 | 1.59 | 3.59** | 4.62*** | 6.07*** | 2.07 | 4.28*** | 2.86* | 4.63*** | |
| 25 | 1.87 | 2.48* | 3.32** | 3.40** | 1.74 | 4.90*** | 4.42*** | 2.56* | |
| 10 | 2.14 | 5.98*** | 3.62** | 6.59*** | 2.33* | 6.46*** | 6.13*** | 3.19** | |
| 9:1 | 100 | 1.28 | 2.07 | 2.76* | 3.96** | 1.71 | 2.81* | 5.20*** | 9.93*** |
| 75 | 1.28 | 2.08 | 1.87 | 2.21 | 1.40 | 1.77 | 3.83** | 4.03*** | |
| 50 | 1.20 | Reference | 1.99 | 3.19** | 1.87 | 2.66* | 3.34** | 5.24*** | |
| 25 | 0.93 | 1.70 | 4.61*** | 2.96** | 1.23 | 1.42 | 2.04 | 2.94** | |
| 10 | 4.77*** | 19.7*** | 3.43** | 6.13*** | 1.43 | 2.02 | 9.25*** | 1.74 | |
| 3:1 | 100 | 3.03** | 2.82* | 2.88* | 6.23*** | 2.94** | 5.82*** | 5.18*** | 5.70*** |
| 75 | 3.41** | 4.14** | 4.48*** | 2.90** | 3.44** | 3.32** | 5.00*** | 4.60*** | |
| 50 | 2.00 | 2.97** | 3.53** | 4.30*** | 1.85 | 3.54** | 3.50** | 7.85*** | |
| 25 | 1.95 | 4.22*** | 4.24*** | 5.77*** | 2.35* | 2.70* | 3.89** | 4.67*** | |
| 10 | 10.9*** | 181.1*** | 9.29*** | 11.5*** | 113.6*** | 5.72*** | 4.28*** | 2.71** | |
0.01<p<0.05
0.0005<=p<=0.01
p<0.0005
SY=sugar and yeast composition; CR=caloric restriction; DR=SY and CR.
Bold font denotes risks which are significantly larger compared to the risks for the CR=100% diet as evaluated for samples stratified by cohort and SY (i.e., within SY levels) treatment in separate Cox regressions.
Second, the effect of date of eclosion on LS can be of similar magnitude as that of DR treatment. This is evident from comparison of the ranges of variation of the RRs between cohorts for the same DR and between DR treatments for the same cohort (Table 1 and Table 2). For instance, given the above example with a 6.6-fold increase in RR of death for Apr8 male cohort (Table 1), change of diet for the same (e.g., Jul23) cohort leads to a modest 3.5-fold increase in RR (for SY=24:1 and CR=100% diet) compared to the same referent (SY=3:1, CR=50%, Jul23) level.
Third, although CR can be associated with extended LS for certain cohorts (e.g., RR for Apr8 cohort of males for SY=9:1 diet decline as CR changes from 100% to 10%; Table 1), it shows no beneficial effects for the others (see, e.g., Jul26 cohort for SY=9:1 diet; Table 1). Moreover, certain CR treatments can be harmful (see bold font in Table 1 and Table 2 showing significantly elevated risks of death for treatments with reduced caloric content).
Life span: insights across diet, reproduction, and date of eclosion
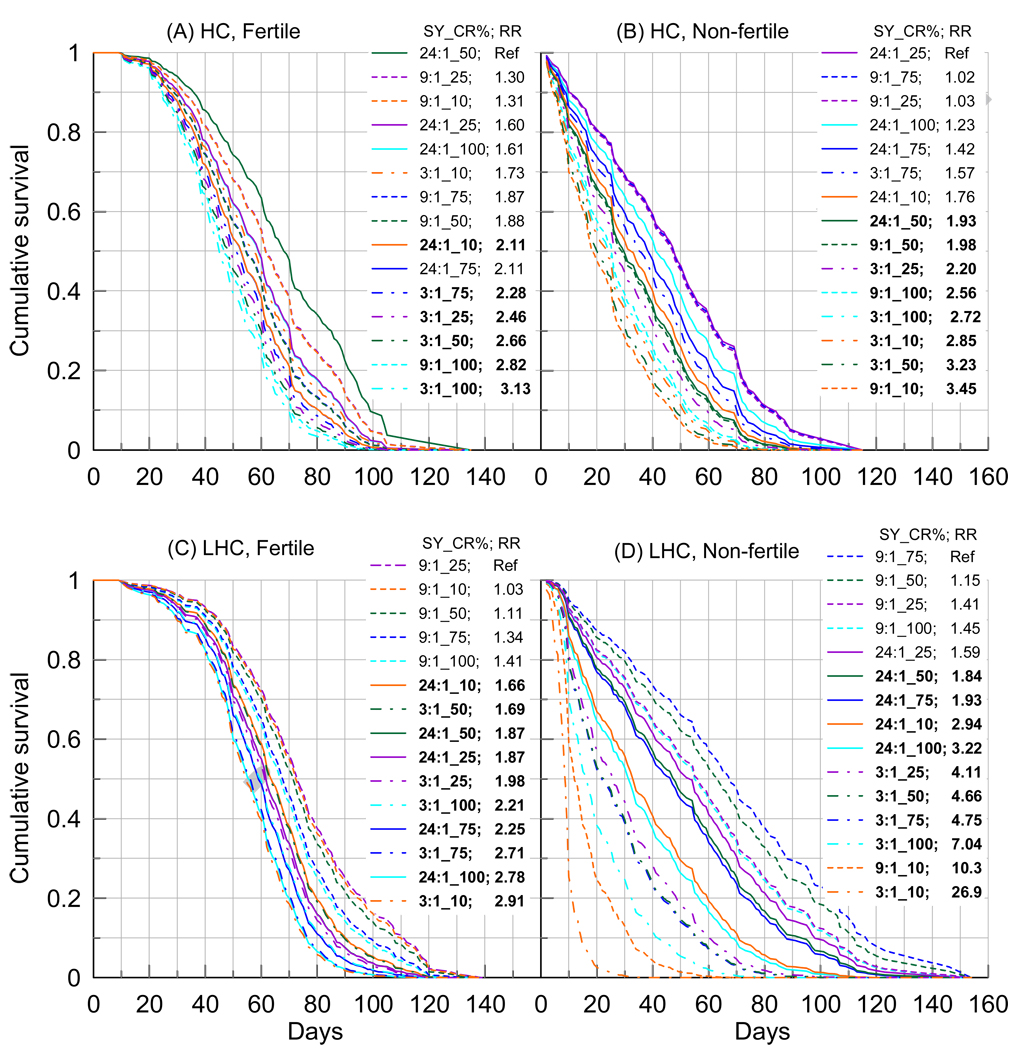
To evaluate connections among diet, reproduction, date of eclosion and LS, we divided the female sample into two more and less homogenous groups according to sensitivity of cohorts to SY treatments following the results presented in the “Survival in SY- and CR-aggregated groups” section. Specifically, we pooled three SY-insensitive (up to statistical uncertainty for a given sample size) cohorts of females (i.e., Jan14, May4, and Aug5) into the Homogenous Cohort (HC) and the remaining five SY-sensitive cohorts (i.e., Jul23, Jul26, Nov15, Feb22, and Apr8) into the Less Homogenous Cohort (LHC). Then, we used Cox regression to evaluate RR of death and cumulative survival of fertile and non-fertile females in HC and LHC groups for each of the DR level, excluding the pure sugar diet (Figure 4).
Figure 4.
Cumulative survival of samples of (A) fertile and (B) non-fertile females from homogenous Jan14, May4, and Aug5 cohorts (denoted as HC) and samples of (C) fertile and (D) non-fertile females from less homogenous Jul23, Jul26, Nov15, Feb22, and Apr8 cohorts (denoted as LHC) treated according to diets shown in the insets. Insets also show relative risks (RR) of death. Bold font denotes significant RRs. Continuous, dashed, and dashed-dotted lines denote SY=24:1, 9:1, and 3:1, respectively. Survival curves are ordered according to increasing magnitude of RRs to reliably distinguish between different treatments.
Both the survival of fertile (N=222; Figure 4A) and non-fertile (N=378; Figures 4B) females from the HC sample are responsive to DR treatment. Variability in RR is slightly higher for non-fertile females. Baseline mortality risks are higher for non-fertile females than for fertile females for all ages (not shown). In general, there is no consistent SY treatment which could be the most favorable for LS either for fertile or non-fertile flies (each of three protein-enriched SY levels is associated with the longest lived females for whom RR of death are not significant). Nevertheless, SY=3:1 diet is the least favorable for LS both for fertile and for non-fertile females because four of five CR treatments for SY=3:1 diet are associated with the shortest-lived females. SY=9:1 diet largely favors LS of fertile females. CR=50% treatment is unfavorable for LS for non-fertile flies for all SY treatments whereas it can be highly favorable for fertile females.
The situation is dramatically different for the LHC sample which includes N=366 non-fertile and N=534 fertile females. Figure 4D shows remarkable variability in LS with about 27-fold increase of the mortality risks for non-fertile females treated with SY=3:1 and CR=10% diet compared to the longest lived females treated with SY=9:1 and CR=75% diet. Sensitivity to DR treatment of fertile females is considerably lower (Figure 4C) and, in fact, it is the lowest for all four samples in Figure 4. Non-fertile females (Figure 4D) show remarkable consistency for the effect of SY on LS with SY=9:1 treatment (except CR=10% treatment) as the most and SY=3:1 treatment as the least favorable diet for LS. SY=9:1 treatment is also the most favorable for fertile females including the effect of CR=10% treatment. No consistent patterns are seen for changes in CR. Although the results for SY=24:1 and SY=3:1 are mixed, the SY=3:1 diet tends to be less favorable than the SY=24:1 diet for fertile females (Figure 4C). The SY=9:1 and CR=10% diet is favorable for fertile females and is highly unfavorable for non-fertile females (both from the HC and LHC samples). Fertile females have smaller mortality risks at younger ages (the survival patterns are more convex at early periods of life for fertile than for non-fertile females) whereas at older ages non-fertile females have smaller mortality risks and, thus, they live longer. Unlike the HC sample, baseline mortality risks for the LHC sample are higher for non-fertile females only at younger ages. After age of 80 days baseline mortality becomes larger for fertile females. Maximal fecundity for both the HC and LHC samples is found for SY=3:1 diet (not shown) which is unfavorable for both fertile (Figures 4A,C) and non-fertile (Figures 4B,D) females.
Figures 4A,C show that fertile females are weakly sensitive to date of eclosion, and this is in line with analyses of Figure 2. Specifically, Figures 4A,C show that both HC and LHC samples of fertile flies exhibit virtually the same-shape survival patterns and sensitivity to DR (maximal RR=2.9 for HC and RR=3.13 for LHC) as well as similar life expectancy (e.g., 68.2 days for the longest lived females from the HC sample and 74.5 days for those from the LHC sample). In contrast, non-fertile females are tremendously sensitive to date of eclosion (Figures 4B,D).
DISCUSSION AND CONCLUSIONS
Recent studies have addressed the complexity of diet effects on lifespan and other traits using dietary gradients with controlled or measured food intake (Carey et al., 2008; Lee et al., 2008; Maklakov et al., 2008; Skorupa et al., 2008). Our analyses of data collected by Carey and colleagues (2008) provide further insights by taking into account effects of cohort quality (as characterized by the eclosion date), without directly manipulating larval or pupal conditions. Given the complexity of the topic and the experiment, we had many findings, summarized in Table 3. These findings lead to the following major insights.
Table 3.
Summary of findings: Sensitivity of life span (LS) and reproduction responses to eclosion date across dietary-restriction gradients in the mexfly Anastrepha ludens
|
A first result is that there are uncontrolled external or internal factors which can significantly modulate LS in experimental animals independently of diet and reproduction and to the extent comparable to the effect of diet manipulation (see first and second observations for Table 1 and Table 2). These factors are assessed through date of eclosion which can be considered as an integrative characteristic of the unobserved life-limiting factors. The origin of these unobserved factors is unknown yet. It might well be that these forces originate from external factors, e.g., sun activity, geophysical factors (Davis and Lowell, 2004; Izmaylov et al., 2005). Different cohorts may have experienced different larval diets and thus had different opportunities to store reserves (Tu and Tatar, 2003), they may have acquired different gut faunas that modulated the effect of adult diet on demographic rates (Behar et al., 2008c), or the conditions may have caused different decisions of phenotypic plasticity (Westeberhard, 1989). An example for the latter would be that flies may have anticipated adult starvation or not, based on the temperature they experienced as pupae.
A second result is that the effect of diet manipulation on longevity is sensitive to date of eclosion. Given this sensitivity, the role of CR in life extension can be inconsistent ranging from beneficial for some samples to harmful for the others. Such inconsistency is evident from Figure 1 and Table 1 and Table 2 when we consider variability of mean LS and RR of death within the same SY treatment. Further analyses of different cohorts for aggregated SY treatments (Figures 3A,B) show that only few of them are significantly sensitive to CR (e.g., three of eight male cohorts). For such aggregated analyses, however, the CR-attributable life-extending effect is smaller than that of date of eclosion, i.e., maximal life expectancy is observed for CR-insensitive cohorts. In the SY-aggregated analyses CR=100% diet seems to be unfavorable for all CR-sensitive cohorts. Because the SY-aggregated analyses used a mixed sample of flies subject to different SY treatments, they could mimic a situation in a society of humans where individuals have different diets. Then, on a population-wide level (when the effect of diet is largely averaged) the effect of CR might be seen as beneficial. More detailed analyses of the effect of a given SY on LS, shows that the same CR treatment can benefit some flies whereas it can considerably increase the risks of death for others (see bold font in Table 1 and Table 2 and Figure 4B). The second result shows that a beneficial effect of CR in a heterogeneous sample can be due to the net effect of beneficial and detrimental CR contributions but is not due to a beneficial effect of CR for all members of this sample.
Manipulation with SY is more sensitive to date of eclosion than manipulation with CR. This is evident from Figure 1 and the fact that only three of eight cohorts of males and females are not responsive to changes in SY whereas only three male and two female cohorts are responsive to CR treatments (see the “Survival in SY- and CR-aggregated groups” section). Furthermore, unlike manipulation with CR, interaction of date of eclosion with SY can result in a real life extension (see Figures 3C,D and the relevant discussion in the “Survival in SY- and CR-aggregated groups” section). Both too little protein (sugar only diet for both sexes) and too much protein (for females) in the diet negatively affects survival. Too little protein may reduce lifespan as too few resources are available for maintenance, while too much protein may present a cost of excretion, or result in toxic products when amino acids are used as an energy source (Webb et al., 1998).
A third result is that our analyses support the view that reproduction plays a minor role in determining longevity for experimental flies. In particular, the deleterious effect of diets with high protein content can be explained by harmful consequences of protein ingestion but not with excessive reproduction as it is evident from the fact that SY=3:1 diet is typically harmful for both fertile and non-fertile flies (Figure 4). Nevertheless, cost of reproduction can be a life limiting factor under certain conditions because: (i) the longest lived females in our samples are non-fertile (Figure 4D) and (ii) baseline hazards for fertile females can be larger than that of non-fertile females although in selected samples (e.g., only in the LHC sample in our study; see discussion of Figure 4 in the Results section).
Finally, the results of our work lead to an important conclusion on presence of at least two weakly interacting fertile-specific and non-fertile-specific mechanisms of longevity at least in female flies. This conclusion is broadly in line with differences in the effects of diet on demographic and behavioral aging which also support hypothesis on distinct mechanisms influencing aging-associated processes in flies (Bhandari et al., 2007). Our conclusion is supported by two observations. First, the same factor (date of eclosion) has small (Figures 4A,C) and large (Figures 4B,D) effects on survival of fertile (Figures 4A,C) and non-fertile (Figures 4B,D) flies. Second, in cohorts that do not individually respond to DR (i.e., those which compose HC sample) survival characteristics of fertile and non-fertile flies are similar and, thus, fecundity is likely not the cause of huge differences in survival of females from the cohorts that do respond to DR (Figures 4C,D). Consequently, date of eclosion can be selectively linked with one of the two potential longevity mechanisms in female mexflies. These mechanisms likely have some common pathways because DR can contribute to both of them (all samples in Figure 4 are DR-sensitive) and date of eclosion can modulate contribution of DR to longevity (this is evident from differential sensitivity of DR treatments and from qualitative changes in the effect of different proportions of sugar and yeast on LS between HC and LHC samples; Figure 4). The strongly asynchronous response of non-fertile and fertile flies to date of eclosion shows, however, that interaction between these two mechanisms is weak. Given that fecundity can be a proxy measure of fitness (Schnebel and Grossfield, 1988) and that reproduction itself is not always a risk factor for mortality (see our third result above), the proposed two potential longevity mechanisms in female flies can be associated with differences in predisposed fitness, i.e., be sensitive to frailty. Then, our results suggest an optimistic scenario for flies which could be deemed as being frailer: specifically such flies are the most responsive to modifiable factors that could make them more robust and, thus, longer lived. Conversely, individuals that are already predisposed for a short life span have to watch their diet even more than more robust individuals. This may contribute to the vast heterogeneity among individuals seen in nature. This opens an avenue for searching similar mechanisms of aging in humans which could be responsive to modifiable factors. Importance of our results warrants further exploration in specifically designed experiments with larger samples.
ACKNOWLEDGEMENTS
The research reported in this paper was supported by the National Institute on Aging grants 1R01 AG028259, 1R01-AG-027019, 5R01-AG-030612, and 5P01-AG-008761. We thank A. Oropeza, R. Bustamente, E. de Leon, S. Salgado, S. Rodriguez, R. Rincon and G. Rodas for technical assistance and the Moscamed-Moscafrut program in Mexico for their laboratory facilities at Metapa, Chiapas.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bauerfeind SS, Fischer K. Effects of larval starvation and adult diet-derived amino acids on reproduction in a fruit-feeding butterfly. Entomologia Experimentalis Et Applicata. 2009;130:229–237. [Google Scholar]
- Behar A, Jurkevitch E, Yuval B. Bringing back the fruit into fruit fly-bacteria interactions. Molecular Ecology. 2008a;17:1375–1386. doi: 10.1111/j.1365-294X.2008.03674.x. [DOI] [PubMed] [Google Scholar]
- Behar A, Yuval B, Jurkevitch E. Gut bacterial communities in the Mediterranean fruit fly (Ceratitis capitata) and their impact on host longevity. Journal of Insect Physiology. 2008b;54:1377–1383. doi: 10.1016/j.jinsphys.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Behar A, Yuval B, Jurkevitch E. Gut bacterial communities in the Mediterranean fruit fly (Ceratitis capitata) and their impact on host longevity. J Insect Physiol. 2008c;54:1377–1383. doi: 10.1016/j.jinsphys.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Ben-Yosef M, Behar A, Jurkevitch E, Yuval B. Bacteria-diet interactions affect longevity in the medfly Ceratitis capitata. Journal of Applied Entomology. 2008;132:690–694. [Google Scholar]
- Bhandari P, Jones MA, Martin I, Grotewiel MS. Dietary restriction alters demographic but not behavioral aging in Drosophila. Aging Cell. 2007;6:631–637. doi: 10.1111/j.1474-9726.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- Boggs CL. Reproductive allocation from reserves and income in butterfly species with differing adult diets. Ecology. 1997;11:181–191. [Google Scholar]
- Boggs CL. Understanding insect life histories and senescence through a resource allocation lens. Functional Ecology. 2009;23:27–37. [Google Scholar]
- Boggs CL, Freeman KD. Larval food limitation in butterflies: effects on adult resource allocation and fitness. Oecologia. 2005;144:353–361. doi: 10.1007/s00442-005-0076-6. [DOI] [PubMed] [Google Scholar]
- Brakefield PM, Gems D, Cowen T, Christensen K, Grubeck-Loebenstein B, Keller L, Oeppen J, Rodriguez-Pena A, Stazi MA, Tatar M, Westendorp RGJ. What are the effects of maternal and pre-adult environments on ageing in humans, and are there lessons from animal models? Mechanisms of Ageing and Development. 2005;126:431–438. doi: 10.1016/j.mad.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Brakefield PM, Kesbeke F, Koch PB. The regulation of phenotypic plasticity of eyespots in the butterfly Bicyclus anynana. American Naturalist. 1998;152:853–860. doi: 10.1086/286213. [DOI] [PubMed] [Google Scholar]
- Carey JR, Harshman LG, Liedo P, Muller HG, Wang JL, Zhang Z. Longevity-fertility trade-offs in the tephritid fruit fly, Anastrepha ludens, across dietary-restriction gradients. Aging Cell. 2008;7:470–477. doi: 10.1111/j.1474-9726.2008.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR, Liedo P, Harshman L, Zhang Y, Muller HG, Partridge L, Wang JL. Life history response of Mediterranean fruit flies to dietary restriction. Aging Cell. 2002;1:140–148. doi: 10.1046/j.1474-9728.2002.00019.x. [DOI] [PubMed] [Google Scholar]
- Carey JR, Liedo P, Muller HG, Wang JL, Love B, Harshman L, Partridge L. Female sensitivity to diet and irradiation treatments underlies sex-mortality differentials in the Mediterranean fruit fly. J Gerontol A Biol Sci Med Sci. 2001;56:B89–B93. doi: 10.1093/gerona/56.2.b89. [DOI] [PubMed] [Google Scholar]
- Davis GE, Jr, Lowell WE. The Sun determines human longevity: teratogenic effects of chaotic solar radiation. Med Hypotheses. 2004;63:574–581. doi: 10.1016/j.mehy.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Douglas AE. The microbial dimension in insect nutritional ecology. Functional Ecology. 2009;23:38–47. [Google Scholar]
- Guzman C, Cabrera R, Cardenas M, Larrea F, Nathanielsz PW, Zambrano E. Protein restriction during fetal and neonatal development in the rat alters reproductive function and accelerates reproductive ageing in female progeny. J Physiol. 2006;572:97–108. doi: 10.1113/jphysiol.2005.103903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izmaylov DM, Obukhova LK, Konradov AA. Correlations of life-span variation parameters in 128 successive generations of Drosophila melanogaster with changes in atmospheric pressure and geomagnetic activity. Int J Biometeorol. 2005;49:337–344. doi: 10.1007/s00484-004-0243-1. [DOI] [PubMed] [Google Scholar]
- Kennedy BK, Steffen KK, Kaeberlein M. Ruminations on dietary restriction and aging. Cell Mol Life Sci. 2007;64:1323–1328. doi: 10.1007/s00018-007-6470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krainacker DA, Carey JR, Vargas R. Effect of larval host on the life history parameters of the Mediterranean fruit fly, Ceratitis capitata. Oecologia. 1987;73:583–590. doi: 10.1007/BF00379420. [DOI] [PubMed] [Google Scholar]
- Lane MA, Mattison JA, Roth GS, Brant LJ, Ingram DK. Effects of long-term diet restriction on aging and longevity in primates remain uncertain. J Gerontol A Biol Sci Med Sci. 2004;59:405–407. doi: 10.1093/gerona/59.5.b405. [DOI] [PubMed] [Google Scholar]
- Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JW, Taylor PW, Soran N, Raubenheimer D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc Natl Acad Sci U S A. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- Lowell WE, Davis GE., Jr The light of life: evidence that the sun modulates human lifespan. Med Hypotheses. 2008;70:501–507. doi: 10.1016/j.mehy.2007.05.053. [DOI] [PubMed] [Google Scholar]
- Magwere T, Chapman T, Partridge L. Sex differences in the effect of dietary restriction on life span and mortality rates in female and male Drosophila melanogaster. J Gerontol A Biol Sci Med Sci. 2004;59:3–9. doi: 10.1093/gerona/59.1.b3. [DOI] [PubMed] [Google Scholar]
- Mair W, Piper MDW, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. Plos Biology. 2005;3:1305–1311. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Sgro CM, Johnson AP, Chapman T, Partridge L. Lifespan extension by dietary restriction in female Drosophila melanogaster is not caused by a reduction in vitellogenesis or ovarian activity. Exp Gerontol. 2004;39:1011–1019. doi: 10.1016/j.exger.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Maklakov AA, Simpson SJ, Zajitschek F, Hall MD, Dessmann J, Clissold F, Raubenheimer D, Bonduriansky R, Brooks RC. Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Current Biology. 2008;18:1062–1066. doi: 10.1016/j.cub.2008.06.059. [DOI] [PubMed] [Google Scholar]
- Mevi-Schutz J, Erhardt A. Amino acids in nectar enhance butterfly fecundity: a long-awaited link. American Naturalist. 2005;165:411–419. doi: 10.1086/429150. [DOI] [PubMed] [Google Scholar]
- Molleman F, Ding J, Wang J-L, Brakefield PM, Carey JR, Zwaan BJ. Adult diet affects life span and reproduction of the fruit-feeding butterfly Charaxes fulvescens Entomologia Experimentalis et applicata. 2008 doi: 10.1111/j.1570-7458.2008.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molleman F, Ding J, Wang J-L, Carey JR. Nutrients in fruit increase fertility in wild-caught females of long-lived Euphaedra species (Lepidoptera, Nymphalidae) The Journal of Insect Physiology. 2009;55:375–383. doi: 10.1016/j.jinsphys.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris WF, Pfister CA, Tuljapurkar S, Haridas CV, Boggs CL, Boyce MS, Bruna EM, Church DR, Coulson T, Doak DF, Forsyth S, Gaillard JM, Horvitz CC, Kalisz S, Kendall BE, Knight TM, Lee CT, Menges ES. Longevity can buffer plant and animal populations against changing climatic variability. Ecology. 2008;89:19–25. doi: 10.1890/07-0774.1. [DOI] [PubMed] [Google Scholar]
- Murphy DD. The role of adult feeding in egg-production and population-dynamics of the checkerspot butterfly Euphydryas editha. Oecologia. 1983;56:257–263. doi: 10.1007/BF00379699. [DOI] [PubMed] [Google Scholar]
- Novoseltsev VN, Arking R, Carey JR, Novoseltseva JA, Yashin AI. How an individual fecundity pattern looks in Drosophila and medflies. Ann N Y Acad Sci. 2004;1019:577–580. doi: 10.1196/annals.1297.108. [DOI] [PubMed] [Google Scholar]
- O'Brien DM, Boggs CL, Fogel ML. Making eggs from nectar: the role of life history and dietary carbon turnover in butterfly reproductive resource allocation. Oikos. 2004;105:279–291. [Google Scholar]
- O'Brien DM, Boggs CL, Fogel ML. The amino acids used in reproduction by butterflies: A comparative study of dietary sources using compound-specific stable isotope analysis. Physiological and Biochemical Zoology. 2005;78:819–827. doi: 10.1086/431191. [DOI] [PubMed] [Google Scholar]
- Partridge L, Piper MDW, Mair W. Dietary restriction in Drosophila. Mechanisms of Ageing and Development. 2005;126:938–950. doi: 10.1016/j.mad.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Piper MD, Mair W, Partridge L. Counting the calories: the role of specific nutrients in extension of life span by food restriction. J Gerontol A Biol Sci Med Sci. 2005;60:549–555. doi: 10.1093/gerona/60.5.549. [DOI] [PubMed] [Google Scholar]
- Raubenheimer D, Simpson SJ. Meeting nutrient requirements: The roles of power and efficiency. Entomologia Experimentalis Et Applicata. 1996 Jul;:65–68. [Google Scholar]
- Raubenheimer D, Simpson SJ. Nutrient balancing in grasshoppers: behavioural and physiological correlates of dietary breadth. Journal of Experimental Biology. 2003;206:1669–1681. doi: 10.1242/jeb.00336. [DOI] [PubMed] [Google Scholar]
- Raubenheimer D, Simpson SJ, Mayntz D. Nutrition, ecology and nutritional ecology: toward an integrated framework. Functional Ecology. 2009;23:4–16. [Google Scholar]
- Rogina B, Wolverton T, Bross TG, Chen K, Muller HG, Carey JR. Distinct biological epochs in the reproductive life of female Drosophila melanogaster. Mech Ageing Dev. 2007;128:477–485. doi: 10.1016/j.mad.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnebel EM, Grossfield J. Antagonistic Pleiotropy - an Interspecific Drosophila-Comparison. Evolution. 1988;42:306–311. doi: 10.1111/j.1558-5646.1988.tb04134.x. [DOI] [PubMed] [Google Scholar]
- Simpson SJ, Raubenheimer D. The geometric analysis of nutrient-allelochemical interactions: A case study using locusts. Ecology. 2001;82:422–439. [Google Scholar]
- Simpson SJ, Sibly RM, Lee KP, Behmer ST, Raubenheimer D. Optimal foraging when regulating intake of multiple nutrients. Animal Behaviour. 2004;68:1299–1311. [Google Scholar]
- Skorupa DA, Dervisefendic A, Zwiener J, Pletcher SD. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell. 2008;7:478–490. doi: 10.1111/j.1474-9726.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavernarakis N. Ageing and the regulation of protein synthesis: a balancing act? Trends Cell Biol. 2008;18:228–235. doi: 10.1016/j.tcb.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Tu MP, Tatar M. Juvenile diet restriction and the aging and reproduction of adult Drosophila melanogaster. Aging Cell. 2003;2:327–333. doi: 10.1046/j.1474-9728.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- Walker G, Houthoofd K, Vanfleteren JR, Gems D. Dietary restriction in C. elegans: from rate-of-living effects to nutrient sensing pathways. Mech Ageing Dev. 2005;126:929–937. doi: 10.1016/j.mad.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Webb SC, Hedges REM, Simpson SJ. Diet quality influences the delta C-13 and delta N-15 of locusts and their biochemical components. Journal of Experimental Biology. 1998;201:2903–2911. doi: 10.1242/jeb.201.20.2903. [DOI] [PubMed] [Google Scholar]
- Westeberhard MJ. Phenotypic Plasticity and the Origins of Diversity. Annual Review of Ecology and Systematics. 1989;20:249–278. [Google Scholar]