Abstract
Background
Study of endocrine pathology in animal models is critical to understanding endocrine pathology in humans.
Methods
We evaluated 434 endocrine-related diagnoses from 4,619 baboon necropsies, established the incidence of spontaneous endocrine pathology, and analyzed the clinical and biochemical data associated with the individual cases.
Results
The most common diagnoses in descending order, were pancreatic islet cell amyloidosis (n=259), ovarian cysts (n=50), pituitary adenoma (n=37), pancreatic islet cell adenoma (n=20), granulosa cell tumor (n=15), thyroid adenoma (n=11), adrenal hyperplasia (n=10), thyroid carcinoma (n=8), and pheochromocytoma (n=6). The incidence of pancreatic islet cell amyloidosis progressively increased with age. Pheochromocytomas were associated with renal and heart failure. The incidence of pancreatic islet cell amyloidosis and adrenal pathology was similar to humans; the incidence of pituitary adenoma and thyroid pathology was lower than in humans.
Conclusions
Endocrine disease in baboons is common and shares clinical and biochemical characteristics with endocrine disease in humans.
Keywords: Papio, nonhuman primate, thyroid, pancreas, endocrine, disease, cancer
INTRODUCTION
Baboons have greater than 97% genetic similarity to humans, and therefore, have great potential to serve as models of human disease [9]. They have been proposed as a model for the study of obesity and its related co-morbidities and spontaneously develop a variety of endocrine diseases that occur in humans such as insulin resistance, pancreatic amyloid deposits, type 2 diabetes [7,11,12,22], and cancer [8]. Previous reports have described baboons with spontaneous proliferative and neoplastic endocrine disease such as ovarian tumors, including granulosa cell tumors [16], and adrenal lesions including hyperplasia, and neoplasia [19]. Primary hyperparathyroidism has also been described in the baboon [13]. These observations confirm that the baboon may present spontaneously with many of the endocrine diseases that are seen in humans.
In this work, we describe endocrine diseases diagnosed in baboons over a 27-year period and compare the baboon disease with that seen in humans.
MATERIAL AND METHODS
We searched the Southwest National Primate Research Center at the Southwest Foundation for Biomedical Research (SFBR) computerized pathology database (apath) for endocrine-related diagnoses, which included all 4,619 baboon necropsies done over 27 years (1980 to 2007). All endocrine-related diagnoses were based on histological analysis of the tissues obtained during the necropsy of each baboon.
Over the 27 year period, the baboon colony population averaged approximately 4,000 animals, 63% female and 37% male. Baboons were housed in two 6-acre corrals and metal and concrete gang cages; individual metal cages were used for special handling. Commercial monkey chows fed over the years were supplemented with an enrichment fare of grains, fruits, and vegetables; water was provided ad libitum. If there was any question that the endocrine diagnosis could be related to experimental use of the baboon, the baboon was excluded from this analysis. All animal care and procedures were approved by the Southwest Foundation for Biomedical Research Institutional Animal Care and the Use Committee in this AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care, International) accredited facility.
Baboons that died or were euthanized were necropsied and a complete gross examination was performed for all tissues. Tissues collected for histologic evaluation were fixed in 10% neutral buffered formalin, processed conventionally, embedded in paraffin, cut at 5 microns and stained with hematoxylin and eosin or other stains as required diagnostically. All of the tissues and the vast majority of the necropsies were evaluated by two board certified veterinary pathologists (GBH and EJD, Jr). All the pathological findings were classified according to conventional nomenclature. If deemed necessary, cases were referred to the Armed Forces Institute of Pathology (AFIP) or other individual pathologists with expertise in the specific organ system involved.
We evaluated clinical and biochemical data from the last 3 to 6 months of life for each baboon using a computerized data base (EZPro Micromedical Interface Inc., Ft Worth, TX) retrieval system.
Statistics
Data are presented as mean±SEM, proportions, and/or percentages. ANOVA and chi square or exact fisher test were used for comparisons between the different groups. Groups with 2 or less cases were excluded from the statistical analysis
RESULTS
Table 1 provides summary statistics for baboons with endocrine disease. Table 2 summarizes the occurrence of disease in the endocrine system by organ. Table 3 lists the total number of cases for each specific diagnosis.
Table 1.
Summary statistics for baboons with endocrine disease.
| Weight (kg) |
|||||
|---|---|---|---|---|---|
| Disease | N | Age (yr) | Male/Female | Male | Female |
| Adrenal adenoma | 3 | 22±1.5 | 1/2 | 28.8±0 | 14.5±1.5 |
| Adrenal hyperplasia | 10 | 15.7±2.1 | 2/8 | 28.9±10.1 | 18.0±1.9 |
| Pheochromocytoma | 6 | 25.5±2.3a | 1/5 | 21.0±0 | 15.0±1.2 |
| Ovarian cyst | 50 | 16.6±0.7b | 0/50a | -- | 16.4±0.5 |
| Granulosa cell tumor | 15 | 21.5±2.0a,d | 0/15 | -- | 15.5±0.8 |
| Pancreatic islet adenoma | 20 | 21.2±1.3 | 1/19 | -- | 19.6±2.8 |
| Pituitary adenoma | 37 | 20.6±0.8a,d | 13/24d | 30.5±1.7 | 16.6±0.8 |
| Thyroid carcinoma | 8 | 22.8±1.6a,d | 1/7 | 25.4±0 | 19.4±2.6 |
| Thyroid adenoma | 11 | 18.2±1.6b | 7/4d,c,e | 36.1±5.4 | 15.3±1.6 |
| Thyroiditis | 5 | 16.0±3.9b,e | 1/4 | 6.3±0 | 14.5±1.1 |
p<0.05 vs Adrenal hyperplasia
p<0.05 vs Pheochromocytoma
p<0.05 vs Granulosa cell tumor
p<0.05 vs Ovarian cyst
p<0.05 vs Thyroid carcinoma
Table 2.
Number of cases of disease by the organ involved.
| Organ | Cases |
|
|---|---|---|
| No. | % | |
| Pancreatic islets | 281 | 64.8 |
| Ovary | 67 | 15.4 |
| Pituitary | 37 | 8.5 |
| Thyroid | 26 | 6.0 |
| Adrenal | 20 | 4.6 |
| Parathyroid | 3 | 0.7 |
| Total | 434 | 100 |
Table 3.
Number of cases by endocrine disease.
| Disease | Cases |
|
|---|---|---|
| No. | % | |
| Pancreatic islet cell amyloidosis | 259 | 59.6 |
| Ovarian cyst | 50 | 11.6 |
| Pituitary adenoma | 37 | 8.6 |
| Pancreatic islet cell adenoma | 20 | 4.6 |
| Granulosa cell tumor | 15 | 3.4 |
| Thyroid adenoma | 11 | 2.5 |
| Adrenal hyperplasia | 10 | 2.3 |
| Thyroid carcinoma | 8 | 1.8 |
| Pheochromocytoma | 6 | 1.4 |
| Thyroiditis | 5 | 1.2 |
| Adrenal adenoma | 3 | 0.7 |
| Parathyroid hyperplasia | 2 | 0.46 |
| Goiter | 2 | 0.46 |
| Adrenal carcinoma | 1 | 0.23 |
| Gonadal stromal tumor | 1 | 0.23 |
| Parathyroid adenoma | 1 | 0.23 |
| Pancreatic islet cell carcinoma | 1 | 0.23 |
| Pancreatic islet cell hyperplasia | 1 | 0.23 |
| Sex cord stromal tumor | 1 | 0.23 |
| Total | 434 | 100 |
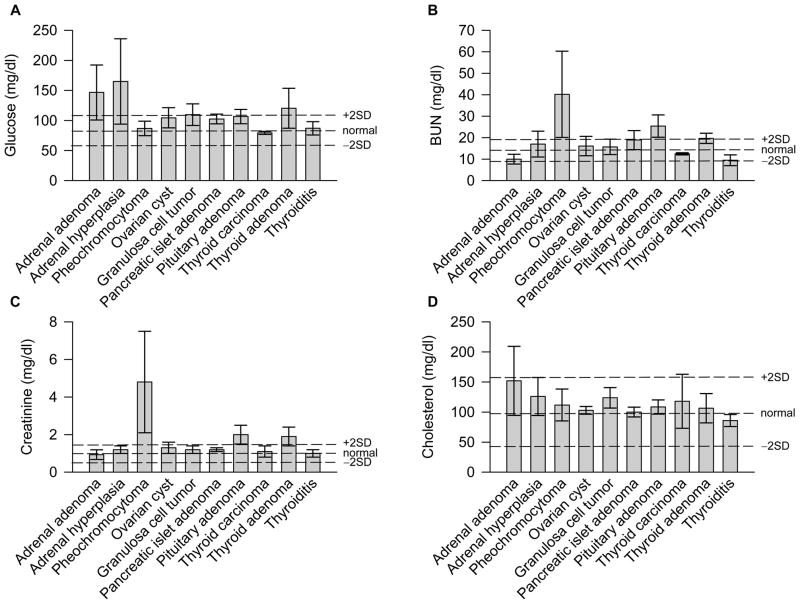
Figure 1 presents the clinical and biochemical data grouped by disease diagnosis occurring in at least three baboons, but excluding the baboons with pancreatic islet cell amyloidosis as these are presented elsewhere [12,15].
Figure 1.
Clinical chemistry values for baboons with endocrine disease (mean ± SEM). Dashed lines indicate normal values ± 2 SD (based on data from Hainsey et al. [11]).
Pancreatic Disease
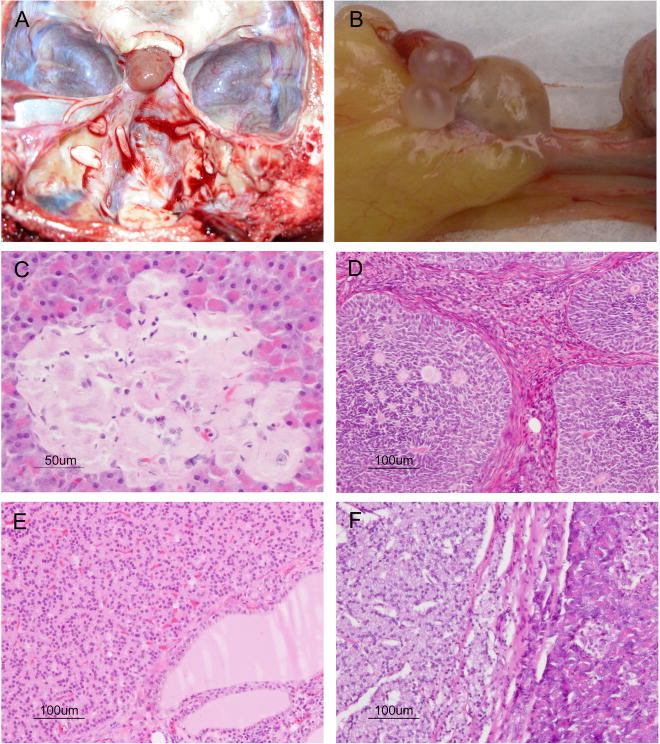
There were 259 baboons with amyloid deposits in the pancreatic islets (Figure 2C), with a prevalence of 6.98% in the whole population, which increased to 11.0% in adult baboons (> 8 years of age). Other findings included pancreatic islet cell adenoma (n=20, 4.6%), pancreatic islet cell carcinoma (n=1, 0.23%; Figure 2F), and pancreatic islet cell hyperplasia (n=1, 0.23%).
Figure 2.
Selected endocrine pathologies of the baboon. A) Pituitary adenoma. B) Ovarian cysts. C) Pancreatic islet cell amyloidosis. D) Granulosa cell tumor. E) Thyroid neuroendocrine carcinoma (carcinoid). F) Pancreatic islet cell carcinoma. H&E (C–F).
Ovarian Disease
The most common ovarian pathology was cystic change (n=50, 74.6% of all ovarian lesions; Figure 2A); 15 baboons (22.4%) had granulosa cell tumors (Figure 2D). One baboon had a gonadal stromal tumor with sertoli cell differentiation and one had a luteinized sex cord stromal tumor; these two baboons were older than the baboons in all the groups (32 and 28.5 years, respectively), but were not included for the statistical analysis because an n of 1 lacked sufficient power.
Pituitary Disease
The only pituitary disease found was pituitary adenoma (n=37, 100%; Figure 2B). It was more common in females. Lesions were not observed in the pineal gland.
Thyroid Disease
Cases of thyroid disease included 11 (42.3%) adenomas, 8 (30.8%) carcinomas (Figure 2E), 5 (19.2%) cases of thyroiditis, and 2 (7.7%) cases of goiter. Included in the carcinomas was a neuroendocrine carcinoma (carcinoid) of the thyroid in a 27 year old female baboon. The neoplastic cells were positive for kermix and chromogranin, and negative for TTF1, thyroglobulin, calcitonin, and synaptophysin.
Adrenal Disease
Adrenal pathology included adrenal hyperplasia (n=10, 50%), pheochromocytoma (n=6, 30%), adenoma (n=3, 15%), and adrenal carcinoma (n=1, 5%). Of note, most of the baboons with pheochromocytomas had clinical signs of hypertension; four presented with clinical signs of pulmonary congestion (increased heart rate and difficulty breathing), and one had a seizure as a cause of death; all this was accompanied by higher levels of BUN, creatinine and potassium (n=3). The female baboons with adrenal hyperplasia were younger and heavier than the rest of the baboons with adrenal disease, but this was not statistically significant.
Parathyroid Disease
Parathyroid disease included parathyroid hyperplasia (n=2, 66.7%) and parathyroid adenoma (n=1, 33.3%); none were included for the statistical analysis because an n of 2 lacked sufficient power.
Incidence of Endocrine Disease in Baboons
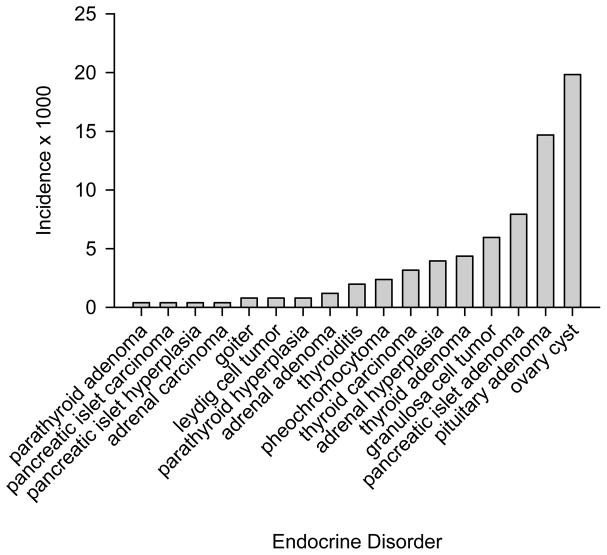
The most common endocrine diagnosis was pancreatic islet cell amyloidosis, with a global incidence of 11.0%. As shown in Figure 3, the next most common were cystic ovaries and pituitary adenomas, with an incidence of 19.8 and 14.6 in 1000, respectively; the least common endocrine diagnoses were parathyroid adenoma, adrenal carcinoma, pancreatic islet cell carcinoma, and pancreatic islet cell hyperplasia, with an incidence of less than 1 in 1,000.
Figure 3.
Incidence (× 1000) of endocrine diagnoses in baboons.
Multiple Endocrine Diseases in the Baboon
Several baboons had multiple endocrine diseases. The presence of adrenal hyperplasia was associated with ovarian cysts (n=2), sex cord stromal tumor and pituitary adenoma (n=1), and pituitary and thyroid adenoma (n=1). Adrenal adenomas were associated with ovarian cysts (n=1), pituitary adenoma (n=1), and granulosa cell tumor (n=1). Pheochromocytomas were associated with pituitary adenoma (n=1), adrenal carcinoma (n=1), and pituitary adenoma and parathyroid adenoma (n=1). Thyroid carcinoma was associated with thyroiditis and ovarian cysts (n=1), and thyroid adenoma was associated with pituitary adenoma (n=2).
Pancreatic islet amyloidosis was associated with pituitary adenoma (n=11), ovarian cyst (n=10), adrenal hyperplasia (n=5), thyroid adenoma (n=5), adrenal adenoma (n=3), granulosa cell tumor (n=3), thyroid carcinoma (n=2), sex cord stromal tumor (n=1), gonadal stromal tumor (n=1), and goiter (n=1). Of the baboons with pancreatic islet amyloidosis, 4.2 and 3.8% had pituitary adenomas and ovarian cyst, respectively. Of the baboons with pituitary adenoma and ovarian cyst, 27 and 22%, respectively, had pancreatic islet amyloidosis.
DISCUSSION
The incidence of adrenal hyperplasia that we found was 3.9/1,000, a little bit higher than the most common form of adrenal hyperplasia in humans (nonclassic form, 1/1,000) [24]. This difference could be explained because this work was a necropsy study, and since we don’t have the biochemical and molecular confirmation of the cases, they could be less than what we are reporting.
Pheochromocytomas are catecholamine-producing tumors that arise from chromaffin cells. Most of them are situated within the adrenal medulla, although in between 9 and 23% of cases, tumors develop from extraadrenal chromaffin tissue. In clinical human studies the incidence of pheochromocytoma has been reported to be around 0.01–0.02/1,000 per year and as high as 12/1,000. However, in an autopsy study, also in humans, the reported incidence was 1 pheochromocytoma per 2,031 autopsies (0.5/1000) [14]. Here we found an incidence of 2.38/1,000 over the 27-year period studied, lower than the clinical and biochemical studies [3,14,20,21], and higher than the autopsy studies in humans [14]. Also, the clinical presentation in the baboons coincides with the clinical presentation frequently observed in humans [4]. On the other hand, the reported incidence of adrenal carcinoma in humans has been around 0.0016/1,000, here we found an incidence of 0.39/1,000, which is higher than the one reported in human autopsy studies [18]. Pheochromocytoma was the only condition that exhibited changes in the biochemical parameters. This is not unexpected as the majority of endocrine diseases encountered in this study do not present with altered biochemistry values in humans.
Regarding ovarian disease, the most common finding in baboons was ovarian cysts, but without any evident clinical or biochemical data of polycystic ovary syndrome (increased weight or hyperglycemia), these cysts may be part of a preclinical or asymptomatic stage of this condition [5]. Also, it is possible that some of these cysts are actually parovarian. We found several cases with testicular atrophy, 1 seminoma and 1 giant cell tumor in the testes of male baboons, however as these did not involve endocrine tissues, they were not included in this report.
In baboons, we found an incidence of pituitary adenomas of 14.68/1,000, lower than the reported in autopsy studies in humans, which has been between 50 – 200/1,000. We don’t have any information about the clinical presentation in the baboons, perhaps because most of them were asymptomatic, as in humans [2].
The reported incidence of thyroid nodules in humans is about 50/1,000, from which 95% are adenomas and only 5% carcinomas, [23], here we found a lower incidence in baboons (7.53/1,000 including both pathologies), with the thyroid adenomas being the most common (Fig. 3). In humans, both pathologies are more common in females. However, in these baboons only thyroid carcinomas were more common in females [23]. The male baboons with thyroid adenoma weighed more than the rest of the groups, which could mean, indirectly, the presence of a certain degree of hypothyroidism, since we know that one of the clinical manifestations of hypothyroidism is the increase in weight and decreased physical activity.
The pancreatic islet cell amyloidosis and its association with hyperglycemia has been reported previously in baboons [12] and in humans [10]. Now, we are reporting an estimated prevalence in the pancreatic islet cell amyloidosis according to age. Of note, pancreatic amyloid deposits increase directly proportional to age, with a global incidence of 11%. The association that we found between pancreatic islet cell amyloidosis and ovary cyst is not surprising, since in humans an association between type 2 diabetes and polycystic ovary syndrome has been shown [1,17].
The co-existence of different endocrine pathologies in some baboons opens the possibility of exploring multiple endocrine neoplasia in this species, particularly involving the pituitary, thyroid and adrenal glands [6]. Although we do not have more data to confirm these findings, there is an opportunity for the research of molecular biology and tumorogenesis of different endocrine tissues in this nonhuman primate model.
The disadvantage of the present study is that it is a retrospective necropsy study, and hence we don’t have much of the clinical and biochemical data that usually are used in humans for the diagnosis of these diseases. A second disadvantage is that not all tissues from all animals were evaluated histologically. It is probable that some lesions that were only visible upon histologic exam were not identified. As a result, the incidence rates described above likely undercount the true incidence of these various lesions in the baboon. This however does not detract from the value of this species as a model of endocrine diseases, as even with this limitation, many pathologies in the baboon were identified at rates comparable to those reported in the human population. We believe this incidence data, even with this limitation, is important and could open a new window in the research area for the study of endocrine pathology in nonhuman primates.
Acknowledgments
This work was supported by the National Institutes of Health/National Center for Research Resources (NIH/NCRR) grant P51 RR013986 to the Southwest National Primate Center. Nonhuman primates were housed in facilities constructed with support from Research Facilities Improvement Program Grant C06 RR016228 from the NIH/NCRR.
The authors thank Marie Silva, Michaelle Hohmann and Denise Trejo for pathology support and the expert assistance of the Technical Publications personnel. We thank the personnel of the SFBR clinical pathology section and the Armed Forces Institute of Pathology for their individual contributions.
References
- 1.Amini M, Horri N, Farmani M, Haghighi S, Sattari G, Pornaghshband Z, Aminorroaya A. Prevalence of polycystic ovary syndrome in reproductive-aged women with type 2 diabetes. Gynecol Endocrinol. 2008;24:423–427. doi: 10.1080/09513590802306143. [DOI] [PubMed] [Google Scholar]
- 2.Arafah BM, Nasrallah MP. Pituitary tumors: pathophysiology, clinical manifestations and management. Endocr Relat Cancer. 2001;8:287–305. doi: 10.1677/erc.0.0080287. [DOI] [PubMed] [Google Scholar]
- 3.Beard CM, Sheps SG, Kurland LT, Carney JA, Lie JT. Occurrence of pheochromocytoma in Rochester, Minnesota, 1950 through 1979. Mayo Clin Proc. 1983;58:802–804. [PubMed] [Google Scholar]
- 4.Bravo EL, Tagle R. Pheochromocytoma: state-of-the-art and future prospects. Endocr Rev. 2003;24:539–553. doi: 10.1210/er.2002-0013. [DOI] [PubMed] [Google Scholar]
- 5.Buggs C, Rosenfield RL. Polycystic ovary syndrome in adolescence. Endocrinol Metab Clin North Am. 2005;34:677–705. x. doi: 10.1016/j.ecl.2005.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carney JA. Familial multiple endocrine neoplasia: the first 100 years. Am J Surg Pathol. 2005;29:254–274. doi: 10.1097/01.pas.0000147402.95391.41. [DOI] [PubMed] [Google Scholar]
- 7.Chavez AO, Lopez-Alvarenga JC, Tejero ME, Triplitt C, Bastarrachea RA, Sriwijitkamol A, Tantiwong P, Voruganti VS, Musi N, Comuzzie AG, DeFronzo RA, Folli F. Physiological and molecular determinants of insulin action in the baboon. Diabetes. 2008;57:899–908. doi: 10.2337/db07-0790. [DOI] [PubMed] [Google Scholar]
- 8.Cianciolo RE, Butler SD, Eggers JS, Dick EJ, Jr, Leland MM, de la Garza M, Brasky KM, Cummins LB, Hubbard GB. Spontaneous neoplasia in the baboon (Papio spp. J Med Primatol. 2007;36:61–79. doi: 10.1111/j.1600-0684.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- 9.Comuzzie AG, Cole SA, Martin L, Carey KD, Mahaney MC, Blangero J, VandeBerg JL. The baboon as a nonhuman primate model for the study of the genetics of obesity. Obes Res. 2003;11:75–80. doi: 10.1038/oby.2003.12. [DOI] [PubMed] [Google Scholar]
- 10.Haataja L, Gurlo T, Huang CJ, Butler PC. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev. 2008;29:303–316. doi: 10.1210/er.2007-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hainsey BM, Hubbard GB, Leland MM, Brasky KM. Clinical parameters of the normal baboons (Papio species) and chimpanzees (Pan troglodytes) Lab Anim Sci. 1993;43:236–243. [PubMed] [Google Scholar]
- 12.Hubbard GB, Steele KE, Davis KJ, III, Leland MM. Spontaneous pancreatic islet amyloidosis in 40 baboons. J Med Primatol. 2002;31:84–90. doi: 10.1034/j.1600-0684.2002.01020.x. [DOI] [PubMed] [Google Scholar]
- 13.Leszczynski JK, Danahey DG, Ferrer KT, Hewett TA, Fortman JD. Primary hyperparathyroidism in an adult female olive baboon (Papio anubis) Comp Med. 2002;52:563–567. [PubMed] [Google Scholar]
- 14.McNeil AR, Blok BH, Koelmeyer TD, Burke MP, Hilton JM. Phaeochromocytomas discovered during coronial autopsies in Sydney, Melbourne and Auckland. Aust N Z J Med. 2000;30:648–652. doi: 10.1111/j.1445-5994.2000.tb04358.x. [DOI] [PubMed] [Google Scholar]
- 15.Mendoza RG, Davalli A, Chavez-Velazquez AO, Comuzzie A, Tejero E, Alvarenga JC, Bastarrachea R, Zuo P, Change Z, Dick EJ, Jr, Hubbard GB, Cruz AM, Perez CT, Halff G, Defronzo RA, Folli F. Effect of Obesity and Lipotoxicity on Pancreatic Islet of Langerhans Amyloidosis in the Baboon, a New Model of Type 2 Diabetes Mellitus (T2DM). American Diabetes Association 68th Scientific Sessions; June 6 – 10, 2008; San Francisco, California. Abstract number 1711-P. [Google Scholar]
- 16.Moore CM, Hubbard GB, Leland MM, Dunn BG, Best RG. Spontaneous ovarian tumors in twelve baboons: a review of ovarian neoplasms in non-human primates. J Med Primatol. 2003;32:48–56. doi: 10.1034/j.1600-0684.2003.00002.x. [DOI] [PubMed] [Google Scholar]
- 17.Pelusi B, Gambineri A, Pasquali R. Type 2 diabetes and the polycystic ovary syndrome. Minerva Ginecol. 2004;56:41–51. [PubMed] [Google Scholar]
- 18.Shin SJ, Hoda RS, Ying L, DeLellis RA. Diagnostic utility of the monoclonal antibody A103 in fine-needle aspiration biopsies of the adrenal. Am J Clin Pathol. 2000;113:295–302. doi: 10.1309/QFFN-6808-M4G5-UA1K. [DOI] [PubMed] [Google Scholar]
- 19.Skelton-Stroud PN, Ishmael J. Adrenal lesions in the baboon (Papio spp) Vet Pathol. 1985;22:141–146. doi: 10.1177/030098588502200208. [DOI] [PubMed] [Google Scholar]
- 20.Smythe GA, Edwards G, Graham P, Lazarus L. Biochemical diagnosis of pheochromocytoma by simultaneous measurement of urinary excretion of epinephrine and norepinephrine. Clin Chem. 1992;38:486–492. [PubMed] [Google Scholar]
- 21.Stenstrom G, Svardsudd K. Pheochromocytoma in Sweden 1958–1981. An analysis of the National Cancer Registry Data. Acta Med Scand. 1986;220:225–232. [PubMed] [Google Scholar]
- 22.Stokes WS. Spontaneous diabetes mellitus in a baboon (Papio cynocephalus anubis) Lab Anim Sci. 1986;36:529–533. [PubMed] [Google Scholar]
- 23.Utiger RD. The multiplicity of thyroid nodules and carcinomas. N Engl J Med. 2005;352:2376–2378. doi: 10.1056/NEJMp058061. [DOI] [PubMed] [Google Scholar]
- 24.White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev. 2000;21:245–291. doi: 10.1210/edrv.21.3.0398. [DOI] [PubMed] [Google Scholar]