Abstract
This study assessed 9 kinematic characteristics of infants’ reach and grasp to test the hypothesis that iron deficiency anemia (IDA) delays upper extremity motor development. Reach and grasp movements, recorded with a 3D-motion capture system, were compared in 9- to 10-month-old infants (4 IDA vs. 5 iron-sufficient [IS]). Based on normative motor development data available for 6 characteristics, the results indicated poorer upper extremity control in IDA infants: 2 characteristics showed statistically significant group differences despite small n, and the other 4 had strong indications for such results (effect sizes [Cohen's d] > 1.2). The remaining 3 measures, for which normative studies do not show developmental changes in this age period, showed significant or moderate-to- large effect differences. Poorer upper-extremity control in IDA infants in the short-term in this study and in the long-term despite iron therapy in other studies suggests that a motor intervention may be warranted when IDA is detected in infancy.
Keywords: Iron deficiency, anemia, infants, fine-motor, motor development, reach and grasp
Introduction
According to the World Health Organization (WHO, 2001), “iron deficiency, and specifically iron deficiency anemia, remains one of the most severe and important nutritional deficiencies in the world today” (p. xii). In developing countries, 23–33% of children under four years are anemic due to iron deficiency (ID);(Stoltzfus, 2001). Both of these publications consider impaired cognitive development as one of the negative effects of early IDA but do not mention delayed motor development. Yet there is considerable evidence that IDA adversely affects motor development. Nine of 12 previous studies that assessed infant motor development reported lower motor scores in infants with iron deficiency anemia (IDA), compared to non-anemic infants(Lozoff & Georgieff, 2006). Moreover, two recent longitudinal studies showed that iron deficiency in infancy appears to have long-term effects on motor development(Shafir, Angulo-Barroso, Calatroni, Jimenez, & Lozoff, 2006; Gunnarsson, Thorsdottir, Palsson, & Gretarsson, 2007). In addition, among randomized controlled trials that looked at iron supplementation (in contrast to iron treatment for infants with IDA/ID), 7 of 9 that assessed motor function reported motor benefits of iron supplementation(Lozoff & Georgieff, 2006).
These studies used global motor developmental tests or parental report of developmental milestones. For young infants, such measures generally emphasize whole body postures and movements such as independent standing and walking. Neither approach is very sensitive in uncovering differences in motor control of the upper extremity. Yet good manual control is an important consideration. It has been necessary for many manual vocations across cultures and history, including today’s work environment with its emphasis on computers and other manually controlled machines. Furthermore, children’s level of fine-motor control may affect their social interaction, especially since video games, which can require high levels of fine-motor control, are often replacing gross-motor physical activities as the preferred mode of children’s play. Thus, a more accurate assessment of the effects of IDA on upper extremity motor development is warranted.
In this initial in-depth exploration of the effects of IDA in infancy on upper extremity motor development, we used an optoelectric three-dimensional (3D) motion capture system (Optotrak). These sensitive systems are the current gold standard for upper extremity research. We used Optotrak to study reach and grasp, an upper-extremity skill that develops over the first two years of life. The reach and grasp skill, which starts to develop around 4–5 months of age(Berthier & Keen, 2006; Thelen, Corbetta, & Spencer, 1996), is usually divided into two main components: the reach movement (hand transport) and the grasp movement (object pick-up). Each component is typified by several kinematic characteristics with magnitudes that change with development. Accurate measurement of these kinematic variables with systems such as Optotrak can quantify more precisely the infant’s developmental stage of upper extremity control. Developmental studies using 3D motion capture systems have typically included 4–12 infants. Such small samples have been adequate to detect significant developmental changes with these sensitive techniques.
Our overarching hypothesis was that arm and hand motor development would be delayed in IDA infants due to iron’s role in myelination. ID has been shown to impede brain myelination in animal models(Connor & Menzies, 1996; Ortiz et al., 2004; Beard & Connor, 2003; Beard, Wiesinger, & Connor, 2003)(see review(Lozoff et al., 2006)). Impaired myelination could affect all parts of the brain and thus all aspects of motor development. We predicted that the skill level of IDA infants in the performance of reach and grasp, as expressed quantitatively in specific kinematic characteristics, would be developmentally behind that of same-age infants with good iron status. To illustrate this general prediction with a specific example, we expected that IDA infants would show hand trajectories during the reach movement that were less straight than those of iron-sufficient (IS) infants, since the reach trajectory becomes substantially straighter during the first two years of life(Berthier & Keen, 2006; Konczak & Dichgans, 1997; Thelen, Corbetta, & Spencer, 1996).
Methods
Subjects
This initial in-depth exploration of upper extremity control in early ID involved a subset of the infants in a study of the neurodevelopmental effects of early iron deficiency(Shafir, Angulo-Barroso, Jing, Jacobson, & Lozoff, 2007). This convenience sample was similar to the larger sample in background factors and iron status variables by group (data available upon request). Optotrak recordings of reach and grasp were obtained for 9 infants whose mothers were willing to come to the lab a second time for this additional motor testing: 4 with iron deficiency anemia (IDA; 3 males, 1 female), and 5 who were iron-sufficient (IS; 2 males, 3 females). Participants were otherwise healthy, full-term singleton African-American infants, identified at the 9-month-old health maintenance visit, with birth weight > 5th percentile, born to mothers ≥ 18 years, no perinatal complications, no maternal diabetes or emergency C-section, not in foster care, and no chronic health problem or hospitalization more than once or for > 5 days. All infants attending a health maintenance visit were approached for screening. For infants who met inclusion criteria and had mothers who agreed to screening, a venous blood sample was obtained for determination of iron status. Details of subject enrollment in the larger sample have been published previously(Shafir, Angulo-Barroso, Jing, Jacobson, & Lozoff, 2007). Table 1 describes the background characteristics of IDA and IS infants with Optotrak data. There were no statistically significant background differences between the iron status groups.
Table 1.
Background characteristics by iron group
| Iron-deficient | Iron-sufficient | |
|---|---|---|
| Iron group | anemic | |
| (IDA) | (IS) | |
| N | 4 | 5 |
| Infant | ||
| Gender, % male (n) | 75 (3) | 40 (2) |
| Birth weight, kilograms | 3.4 ± 0.4 | 3.2 ± 0.4 |
| Gestational age, weeks | 40.0 ± 0.8 | 39.4 ± 1.1 |
| Age at testing, months* | 9.7 ± 0.4 | 10.3 ± 0.3 |
| Hemoglobin, g/L* | 104.3 ± 3.4 | 124.6 ± 4.2 |
| Mean corpuscular volume, fl * | 71.0 ± 6.1 | 78.8 ± 2.8 |
| Red cell distribution width, % | 14.2 ± 0.9 | 13.6 ± 0.4 |
| Zinc protoporphyrin/heme ratio, µmol/mol heme | 78.8 ± 16.6 | 64.6 ± 3.4 |
| Transferrin saturation, % | 19.4 ± 10.7 | 25.4 ± 2.1 |
| Ferritin, µg/L | 5.9 ± 6.1 | 21.8 ± 12.1 |
| Lead, µg/dL | 1.3 ± 0.5 | 2.4 ± 0.9 |
| Peabody fine motor standard score | 19.8 ± 2.9 | 22.0 ± 3.3 |
| Peabody gross motor standard score | 28.5 ± 3.8 | 29.8 ± 1.9 |
| Peabody total standard score | 48.3 ± 6.5 | 51.8 ± 4.3 |
| Number of Optotrak trials | 5.8 ± 3.4 | 5.6 ± 1.1 |
| Mother Age at delivery, years | 21.7 ± 2.9 | 21.4 ± 4.0 |
| Education, years | 12.5 ± 0.6 | 12.1 ± 0.2 |
| Medicaid, % (n) | 75 (3) | 60 (3) |
Note. Values are means ± SD or % (n) for categorical variables. N varies slightly due to occasional missing data for some measures. Significant group differences (p < .05) are indicated by an asterisk.
Iron status was determined based on venous blood samples, which were analyzed for hemoglobin (Hb), mean corpuscular volume (MCV), red cell distribution width (RDW), and zinc protoporphyrin/heme ratio (ZPP/H). Anemia was defined as Hb < 110 g/L(Life Sciences Research Office, 1984; Centers for Disease Control and Prevention, 1998). ID was suggested by 1 or more abnormal iron measure among the following: MCV < 74 fl(Centers for Disease Control, 2001), RDW > 14.0%(Centers for Disease Control and Prevention, 1998), and ZPP/H > 69 µmol/mol heme (corresponding to free erythrocyte protoporphyrin > 80 µg/dl(Looker, Dallman, Carroll, Gunter, & Johnson, 1997)). IDA was defined as anemia plus ID. Iron sufficiency was considered clear absence of anemia (Hb ≥ 115 g/L) and normal MCV, RDW, and ZPP/H. By definition, iron status measures differed across groups (Table 1), although small n resulted in limited power to reach statistical significance for some measures.
The study was approved by the Institutional Review Boards at Wayne State University and the University of Michigan. Signed informed consent was obtained from the infants’ mothers. Infants were recruited at Children’s Hospital of Michigan and evaluated in the Child Development Research Laboratory at Wayne State University School of Medicine.
Task and apparatus
The infant sat on his/her mother’s lap as the experimenter presented a small pellet (1 cm diameter colorful wooden ball) on top of a 20-cm diameter white flat plate. Infant reaching towards the pellet was recorded over 10–20 trials, depending on the infant’s attention span, interest in the pellet, and ability to keep on task. To prevent the infant from putting the pellet in his/her mouth, the pellet was tied to the plate by a 20-cm transparent fishing line. The pellet was presented at arm length in front of the infant, at the infant’s waist height and 45 degrees to the right of his/her torso, so that s/he had to reach with the right hand with a diagonally forward motion. This reach angle was chosen to minimize Optotrak missing data. To ensure reaching with the right hand, the mother was asked to gently hold the infant’s left hand. For each trial, Optotrak data collection started immediately prior to pellet presentation and continued for 10 seconds.
The 3D motion capture system (Optotrak) (Northern Digital, Inc) in this study consisted of one row of three cameras. Infra-red light emitting diodes (IREDs) were placed on the infant’s right shoulder (acromion process), right elbow (lateral epicondyle of the humerus), dorsal left side of the right wrist (distal end of the radius), dorsal aspect of the right hand above the fifth metacarpophalangeal joint, middle segment (phalanx) of the index finger, and distal segment (phalanx) of the thumb. The diameter of the IREDs on the finger and the thumb was 3 mm and that of the other IREDs 8 mm. IRED signals were collected at 120 Hz using Optotrak. In addition, the session was videotaped at 30 Hz with a standard video-8 camera. Initiation of each trial activated a time code generator that lasted for the duration of that trial (i.e., 10 sec). This time signal was embedded into the videotape. This procedure enabled the synchronization of video frames with Optotrak data. Optotrak examiners, video coders, and the infants’ mothers were blind to the iron status of the infants tested.
Data reduction and analysis
Videotapes were first examined to identify trials where the infant grasped the pellet. Reaching movements that did not end with an immediate grasp were not analyzed. For qualifying trials, times of reach onset, maximum aperture (i.e., the maximum distance between the tip of the thumb and the tip of the index finger), first contact with the pellet, and pellet pick-up were initially determined based on video coding (visual inspection). Time of reach onset was defined as the point in time when the infant intentionally (based on the infant’s visual attention) started to move his/her hand towards the pellet. This time was ascertained by frame-by-frame observation of the videotape, tracing the movement backwards from first contact(Corbetta & Thelen, 1995).
Optotrak data were analyzed with LabVIEW (National Instruments), using custom designed and commercial algorithms. Similar to some other infant Optotrak studies, many trials had to be discarded from analysis for a variety of reasons: the infant was not interested and did not reach for the pellet, the infant started reaching late and the first contact happened after the end of the 10-sec data collection of the specific trial, the infant played with the pellet or plate before grasping the pellet, or there were too many missing data points in otherwise adequate reaching trials (i.e., IRED signal was out of camera range because the infant turned arm or hand away from the camera). Thus, only 50 trials in total (5–6 per infant) qualified for analysis: 23 performed by IDA infants and 27 by IS infants.
Missing data up to 8 consecutive data points were interpolated using 3rd order general polynomial fit. The duration of 8 data points (0.67 sec) was equal to 5% of average reach duration and 11% of the shortest reach duration. Following the interpolation, data on the positions (X, Y and Z coordinates) of the IREDs were filtered using a low-pass, 2nd order Butterworth filter with 7 Hz cut-off frequency. Cut-off frequency was determined based on residual analysis(Jackson, 1979). The filtered 3D position data were then used to calculate continuous wrist resultant displacement, wrist resultant velocity, and aperture size, starting at one second before reach onset and ending one second after pellet pick-up. When wrist data were not available due to too many consecutive missing data points, elbow data were used to describe several reach characteristics (3 out of 50 reaching movements analyzed). This approach was considered reasonable, since elbow and wrist markers were placed on the same arm segment and analysis of a partial number of trials from several subjects yielded parallel velocity trajectories for the elbow and wrist.
Optotrak data were used for measuring 6 kinematic variables related to the reach movement: movement duration, wrist path length, wrist peak velocity, duration from reach onset to peak velocity, number of movement units (MU), and straightness of the hand trajectory. Three kinematic variables related to the grasp were also generated: amplitude of peak aperture, duration from reach onset to peak aperture, and duration from peak aperture to first contact. Kinematic variables (underlined) are further described below.
After interpolation and filtering of the Optotrak data, reach onset time was re-determined as the time of the wrist (or elbow) velocity minima (valley) that was closest to the onset time as previously determined based on visual inspection of the video (Figure 1;(Corbetta & Thelen, 1995). The reach segment was defined as starting at this re-determined reach onset and ending at first contact. Reach duration was calculated based on this definition. Peaks and valleys of wrist velocity trajectory during the reach segment were determined, and the highest peak was used to define peak velocity of the reach. The time interval between reach onset and peak velocity of the reach was calculated. Valley-to-peak and peak-to-valley differences in wrist velocity trajectory that were greater than 20% of the amplitude of the highest peak velocity were determined and used to establish the number of MU(Konczak, Borutta, Topka, & Dichgans, 1995; Newman, Atkinson, & Braddick, 2001). 1 MU = 1 acceleration (valley-to-peak velocity) + 1 deceleration (peak-to-valley velocity)(Konczak, Borutta, Topka, & Dichgans, 1995) (Figure 1). Minor changes in wrist velocity trajectory (i.e., less than 20% of maximum peak velocity) were not considered to constitute movement units. We also determined the straightness of hand trajectory by calculating the ratio between the total length of wrist displacement during the reach (wrist path length) and the straight line distance from the position of the wrist marker in space at reach onset and its position at the time of first contact (reach distance). Thus, the lower the value of this ratio and the closer to one, the straighter and more mature is the reach movement.
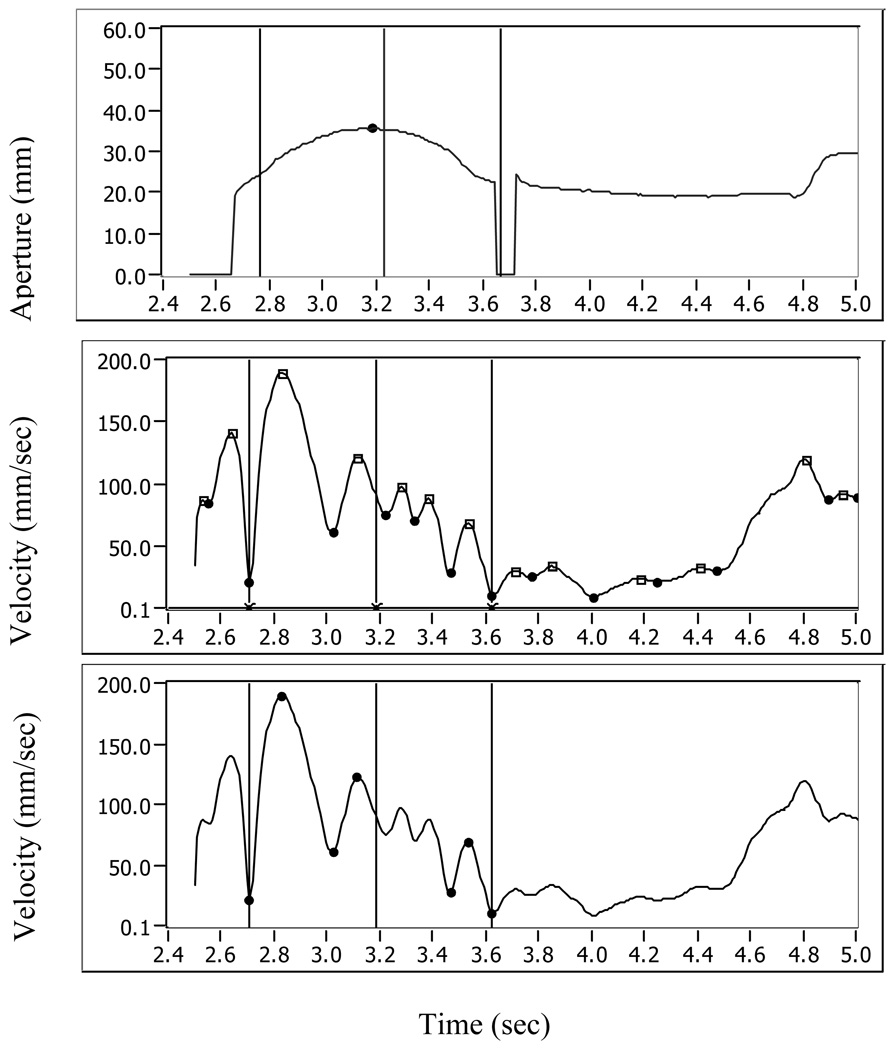
Figure 1.
Aperture (upper panel) and wrist velocity (middle and lower panels) data of one representative trial. Upper panel shows aperture data (in mm) during part of a trial. X axis represents time (in sec). Reach onset time, as determined by visual inspection (video coding), is marked by the first vertical line. The middle vertical line represents the time of peak aperture as determined by visual inspection, while peak aperture as determined based on the Optotrak data is marked by a small circle. The last (right) vertical line represents the time of first contact based on visual inspection. Times in which the aperture line drops to zero are times of missing data for either the thumb or pointer markers. The middle panel shows wrist velocity (in mm/sec) and its peaks (depicted by small squares) and valleys (depicted by small circles) plotted against time (X axis). Reach onset (left vertical line) was re-determined as occurring at minimum wrist velocity closest to the reach onset as determined by visual inspection. Peak aperture time (middle vertical line) was adjusted to represent peak aperture time based on Optotrak data.
In the third panel wrist velocity is plotted again with only the peaks and valleys that were used for movement-units (MV) calculation. The trial shown in this panel has 3 movement units
The time of maximum aperture was also redefined, based on calculation of peak aperture using Optotrak position data for the finger and thumb (Figure 1). Establishing initial maximum aperture based on visual inspection enabled us to retain Optotrak data in trials for which aperture data was not available along the entire reach duration but was available around the time initially determined as time of maximum aperture based on visual video coding. The time interval (duration) between reach onset and peak aperture was determined, as well as the time interval between peak aperture and first contact.
Statistical analysis
All statistical analyses were performed using SAS 9.1(, 2003). Mixed models, which account for having repeated trials within a subject, were used to test for significant differences in motor performance in IDA vs. IS groups. On theoretical grounds, initial analyses controlled for infant age (in days) and gender, with additional control for hand size (calculated as hand width X hand length) for variables concerned with aperture size. A further reason for including infant age as a covariate was to control for the somewhat younger age in the IDA group compared to the IS group. Because the absence of statistically significant group differences in birth or family background characteristics could be due to small n, we also performed all analyses with and without control for background factors that correlated with a given outcome. Inclusion of covariates improved the fit of the models and therefore those models are reported here. In light of the small number of subjects, we also calculated Cohen’s effect size for all dependent variablesi
Results
For all variables, results (mean, standard error (SE), F statistic, p value and effect size (Cohen’s d)) from analyses performed with and without adjusting for covariates are reported in table 2. The following sections pertain to results from analyses that adjusted for covariates.
Table 2.
Results of the mixed models analyses performed with and without adjusting for covariates
| IDA | IS | Modeled Covariates | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariate Adjustment | Variable | mean | SE | mean | SE | F | p | effect size | significant | non-significant | |
| Unadjusted | Duration from peak aperture to first contact | 0.57 | 0.08 | 1.09 | 0.26 | (1,7)=3.60 | 0.1 | 1.45 | |||
| Adjusted | Duration from peak aperture to first contact | 0.56 | 0.13 | 1.1 | 0.24 | (1,5)=2.74 | 0.16 | 1.26 | gender, testage | ||
| Unadjusted | Straight path | 1.87 | 0.1 | 1.64 | 0.08 | (1,7)=3.28 | 0.11 | 1.38 | |||
| Adjusted | Straight path | 1.96 | 0.08 | 1.56 | 0.04 | (1,4)=15.50 | 0.02 | 3 | testage, bweight_g | gender | |
| Unadjusted | Duration from onset of movement to peak aperture | 0.55 | 0.05 | 0.52 | 0.05 | (1,7)=0.29 | 0.61 | 0.41 | |||
| Adjusted | Duration from onset of movement to peak aperture | 0.32 | 0.05 | 0.77 | 0.05 | (1,3)=21.51 | 0.02 | 3.53 | gender, testage, handsize9, iron ses | ||
| Unadjusted | Peak velocity | 471.34 | 64.83 | 322.95 | 37.53 | (1,7)=3.92 | 0.09 | 1.51 | |||
| Adjusted | Peak velocity | 468.86 | 15 | 325.06 | 12.31 | (1,4)=51.33 | 0.002 | 5.45 | gender, moage_t0 | testage | |
| Unadjusted | Peak aperture | 34.25 | 1.33 | 32.87 | 1.36 | (1,7)=0.53 | 0.49 | 0.56 | |||
| Adjusted | Peak aperture | 40 | 2.43 | 26.8 | 2.89 | (1,3)=6.19 | 0.09 | 1.89 | gender, moage_t0 | testage, handsize | |
| Unadjusted | Number of movement units | 2.26 | 0.29 | 1.96 | 0.22 | (1,7)=0.66 | 0.44 | 0.62 | |||
| Adjusted | Number of movement units | 2.38 | 0.17 | 1.86 | 0.23 | (1,5)=2.56 | 0.17 | 1.22 | gender, testage | ||
| Unadjusted | Duration from onset of movement to peak velocity | 0.36 | 0.03 | 0.56 | 0.14 | (1,7)=2.03 | 0.2 | 1.09 | |||
| Adjusted | Duration from onset of movement to peak velocity | 0.31 | 0.08 | 0.6 | 0.13 | (1,4)=2.36 | 0.2 | 1.17 | gender, testage, moage_t0 | ||
| Unadjusted | Reach duration | 1.33 | 0.15 | 1.81 | 0.31 | (1,7)=1.90 | 0.21 | 1.05 | |||
| Adjusted | Reach duration | 1.22 | 0.14 | 1.9 | 0.32 | (1,5)=3.12 | 0.14 | 1.35 | gender, testage | ||
| Unadjusted | Path length | 237.94 | 20.3 | 189.2 | 13.89 | (1,7)=3.93 | 0.09 | 1.51 | |||
| Adjusted | Path length | 221.36 | 15.21 | 203.32 | 7.39 | (1,4)=0.94 | 0.39 | 0.74 | testage | gender, moage_ t0 | |
Note: For each variable, the covariates used in the adjusted analysis and their significance are reported.
Measures of the reach movement
Reach duration, measured from reach onset to first contact, was shorter for IDA infants (1.22±0.44 sec) compared to the IS infants (1.90±1.01 sec). The difference between the groups did not reach statistical significance (F(1, 5) = 3.12, p =0.14) but the effect size was very large (d=1.35). Wrist path length, from reach onset to first contact, was not affected by iron status (221.4±82.8 mm and 203.3±142.3 mm for IDA and IS, respectively) but had a medium effect size (0.74). Consequently, IDA infants had significantly higher peak velocity than IS infants. Mean peak velocity for the IDA group was 468.9±233.8 mm/sec, compared to 325.1±283.7 mm/sec for IS infants (F(1, 4) = 51.33, p =.002). Although IDA infants had higher peak velocity, the time to reach that peak, i.e., their duration from reach onset to peak velocity (0.31±0.20 sec) was not statistically different from the IS group (0.60±0.69 sec) (F(1, 4) = 2.36, p =0.2) but the effect size was very large (d=1.17). There was also no significant difference between the two groups in the number of movement units along the reach (2.38±1.0 for IDA and 1.86±1.21 for IS F(1, 5) = 2.56, p =0.17) but again, the effect size was very large (d=1.22).
There was, however, a significant difference between the straightness of hand trajectory of IDA infants compared to the IS infants (F(1, 4) = 15.50, p =.02). While IS infants had a straightness ratio of 1.56 (±0.42), the IDA infants had straightness ratio of 1.96 (±0.60), i.e., their hand path was almost twice longer than the straight-line distance between the wrist location at the beginning and end of the reach movement.
Measures related to the grasp
There was a significant effect for IDA vs. IS infants for the duration from reach onset to peak aperture (F(1, 3) =25.21, p =.02). On average, it took IDA infants 0.32±0.23 sec after reach onset to get to their maximum aperture, compared to 0.77±0.24 sec for the IS infants. The duration from peak aperture to first contact was also much shorter for IDA infants (0.56±0.74 sec); about half the duration for IS infants (1.1±0.92 sec). Although this difference did not reach statistical significance, the effect size was very large: 1.26. As for the amplitude of peak aperture, IDA infants had almost a 2 x larger peak aperture (40±5.2 mm) as the IS infants (26.8±5.5 mm), p = 0.09, indicating a trend towards significance, and the effect size was huge (d=1.89). As mentioned above, the analysis of peak aperture controlled for hand size.
Overall, there were statistically significant differences for 3 of the 9 kinematic variables measured (peak velocity, straightness of hand trajectory, duration from movement onset to peak aperture), a trend towards significance with a huge effect size in an additional variable (peak aperture), a large effect size for another four variables (movement duration, duration from peak aperture to first contact, number of MU, duration from reach onset to peak velocity), and a medium effect size for the remaining variable (wrist path length). Table 3 summarizes the results, showing the direction of effects between study groups and in relation to expected changes during normal development as reviewed in the discussion section.
Table 3.
Performance of reach and grasp of IDA infants compared to non-anemic infants in light of normal development of reach and grasp around 9–10 months
| Optotrak kinematic variable | IDA compared to IS | Normal developmental change around 9–10 mo |
|---|---|---|
| Peak aperture | No difference Huge effect size and a trend towards: ↑ IDA>IS |
↓ |
| Reach duration | No difference Very large effect size: ↓ IDA<IS |
↑ |
| Duration from peak aperture to first contact | No difference Very large effect size: ↓ IDA<IS |
↑ |
| Peak velocity | ↑ IDA>IS | ↓ (3); General developmental ↑ with possible ↓ specifically at 9 mo (2) |
| Number of MU | No difference Very large effect size: ↑ IDA>IS |
↓ (3); no change (2) |
| Straightness ratio of hand trajectory | ↑ IDA<IS | ↓ (4); no change (1) |
| Path length | No difference Medium effect size: ↑ IDA>IS |
No change |
| Duration from reach onset to peak velocity | No difference Very large effect size: ↓ IDA<IS |
No change |
| Duration from reach onset to peak aperture | ↓ IDA>IS | No normative data |
Note. The direction of statistically significant differences is shown for each kinematic variable. Arrows show the direction of the difference to facilitate comparisons to expected changes during normal development. In the last column arrows show the direction of normal developmental changes expected around 9–10 months compared to earlier ages. When there is conflicting evidence in the normative longitudinal studies, the number of papers supporting each result is given in parenthesis.
Discussion
This study assessed various kinematic characteristics of infants’ reach and grasp to test the hypothesis that IDA is associated with delayed upper extremity motor development. Although the number of subjects and analyzable trials per subject was small, this limitation is shared by most other infant studies using 3D optoelectric camera. There are only 30 healthy infants in all previous longitudinal studies combined that used optoelectric camera techniques to follow the development of reach and grasp(Berthier & Keen, 2006; Konczak, Borutta, Topka, & Dichgans, 1995; Konczak & Dichgans, 1997; Newman, Atkinson, & Braddick, 2001; Thelen, Corbetta, & Spencer, 1996), and the biggest study had only 12 infants. Thus, our sample size of 9 infants is in keeping with the state of the field.
Differences in skill performance between IDA and IS infants will be considered for each variable in relation to available data on the development expected in healthy infants (Table 3). It should be noted, however, that not all earlier studies report data specific for the age of infants in our study (9–10 months). Our findings indicated developmental delay based on most, but not all previous studies. Existing normative data for 3 kinematic variables in IDA infants (peak aperture, reach duration, and duration from peak aperture to first contact) indicated the differences we observed support the hypothesis that IDA infants are delayed in upper extremity motor development. For three variables (peak velocity, number of MU, and straightness of hand trajectory), previous studies generally support our hypothesis, but there are some contradictory findings regarding changes during healthy development. We could not assess our hypothesis for the remaining three variables (path length, duration from reach onset to peak velocity and duration from reach onset to peak aperture), either because healthy infants have not shown developmental changes with age (for path length and duration from reach onset to peak velocity) or because there were not enough normative data from previous studies concerning changes during normal motor development in the age range of our study.
One of the variables that was clearly and significantly affected by iron status was the straightness of hand trajectory. Adults’ movements are characterized by straight-line hand trajectories, which represent the most efficient way to reach from one place to another. As infants gain better motor control, their movements become straighter. Although Newman et al.(Newman, Atkinson, & Braddick, 2001) did not observe changes in the straightness of hand trajectory from 5 to 15 months, all four other studies that used 3D optoelectric systems to collect accurate kinematic data reported a significant increase in trajectory straightness with development(Berthier & Keen, 2006; Konczak, Borutta, Topka, & Dichgans, 1995; Konczak & Dichgans, 1997; Thelen, Corbetta, & Spencer, 1996). Thus, our results, which showed less straight hand trajectory (i.e., higher straightness ratio) in IDA infants compared to IS infants, indicate delayed development of this motor pattern with IDA in infancy.
We also found that IDA infants had significantly higher wrist peak velocity than IS infants. These results are consistent with the hypothesized developmental delay, based on three previous studies in normally developing infants, which found a reduction in wrist peak tangential velocity around the age of 8–9 months(Berthier & Keen, 2006; Newman, Atkinson, & Braddick, 2001; Thelen, Corbetta, & Spencer, 1996). Two other previous studies reported a different pattern: Konczak & Dichgans(Konczak & Dichgans, 1997) found an increase in peak tangential hand velocity from 5 months to 3 years, and Konczak et al.(Konczak, Borutta, Topka, & Dichgans, 1995) found a non-significant increase in peak velocity from 4 to 15 months. However, a close examination of the data from the latter more age-restricted study showed a decrease in peak velocity in the transition from 8 to 9 months (Figure 4 of that paper). Thus, an overall increase in peak velocity over 2.5 years, as was found by Konczak and Dichgans (1997), does not preclude the possibility of a short-term period of decrease around 9 months.
Our findings further indicated that the duration from reach onset to peak aperture was significantly shorter for IDA compared to IS infants. All other measured durations (duration from reach onset to peak velocity, duration from peak aperture to first contact, duration of the entire reach movement) were also shorter for the IDA compared to the IS infants, with a very large effect size. It is difficult to determine the normal developmental profile of reach durations due to conflicting results in the literature(Berthier & Keen, 2006; Newman, Atkinson, & Braddick, 2001; Konczak, Borutta, Topka, & Dichgans, 1995). However, thorough examination of previous studies seems to indicate a modest increase in movement duration shortly before or around 9 months of age. The only study of normal infants to report duration from reach onset to peak velocity(Berthier & Keen, 2006) did not find a significant age effect, and this can explain the lack of significant difference for this variable between the IDA and IS groups in our study.
We found a significant difference between the two groups in the duration from reach onset to peak aperture, but we could not find any normative developmental data concerning changes in this duration around that age. As for the duration from peak aperture to first contact, none of the longitudinal studies reported this variable. However, von Hofsten and Ronnqvist’s(Von Hofsten & Ronnqvist, 1988) observations in a cross-sectional study indicated a developmental increase in this duration between 5 and 13 months, predominantly between 9 and 13 months. Thus, our findings of a very large effect for shorter duration from peak aperture to first contact and for shorter duration of the entire reach movement in IDA infants, seem consistent with delayed maturation of their reaching skill.
Another kinematic variable that showed a huge effects size and a trend towards a significant effect for iron status was peak aperture. When individuals reach towards an object, in preparation for grasping it, they first open their hand, increasing the aperture until it reaches a certain peak, and then they start closing the hand as it further approaches the object to be grasped. The size of peak aperture is dependent on the size of the object to be grasped and the size of the safety margins the individual uses to ensure that the fingers are opened wide enough to bring them around the object. The more control individuals have over their movements and the higher their ability to accurately perceive the object size and plan the correct movement, the smaller the safety margins used. Since young children have less control over their movements than older children or adults, they use larger safety margins and their peak aperture for grasping the same object size is therefore larger(Kuhtz-Buschbeck, Stolze, Johnk, Boczek-Funcke, & Illert, 1998). The ability to adjust aperture magnitude to object size starts to develop at 7–9 months(Fagard, 2000; Von Hofsten & Ronnqvist, 1988). Thus, by about 9 months, better ability to accurately plan and control reach and grasp movements will result in smaller safety margins and consequently, smaller peak aperture. IDA infants displayed a trend towards a larger peak aperture, compared to IS infants, suggesting developmental delay expressed in poorer ability to plan and control reach and grasp movements.
For the number of movement units (MU) although we did not find a significant difference between the two groups, the mean number of MU for IDA infants was higher than that of the IS infants, with a very large effect size. Adult reaches are normally characterized by having only a single MU (one acceleration and one deceleration). Therefore, one might expect that the more “adult-like” the reach, the fewer MU involved. The higher number of MU in the IDA infants could thus be consistent with a developmental delay. Studies with normally developing infants, however, show conflicting evidence regarding developmental changes in the number of MU. For instance, Newman et al.(Newman, Atkinson, & Braddick, 2001) and Berthier & Keen(Berthier & Keen, 2006) found no significant change in the number of MU between 5 and 15–20 months, whereas other studies showed a decrease in the number of movement units during infancy(Thelen, Corbetta, & Spencer, 1996; Konczak, Borutta, Topka, & Dichgans, 1995; Konczak & Dichgans, 1997). Berthier and Keen(Berthier & Keen, 2006) suggested that such conflicting results are explained by subtle differences among the different studies in MU definition and experimental methodology, such as using different filtering of the data. It is not clear whether we failed to find a significant difference between the two iron groups because of methodological reasons or because the groups indeed did not differ enough from one another. However, the fact that the IDA infants had more MU, on average during their reach, is in line with the idea of delayed motor development.
Lastly, iron deficiency anemia did not have a significant effect on the path length of wrist displacement. Only two other studies reported path length in the normal development of reach and grasp(Thelen, Corbetta, & Spencer, 1996; Berthier & Keen, 2006). Neither found a significant change with age. Since age does not appear to affect path length around 9–10 months, our failure to bserve a significant difference between the two iron groups does not contradict the possibility of developmental delay in IDA.
Overall, our results indicate that sensitive measures focused specifically on the reach and grasp skill, instead of overall fine motor development, detect IDA effects on several upper extremity motor control characteristics. The mechanism by which ID might slow the development of the reach and grasp skill might be related to delayed maturation of the corticospinal tract. The corticospinal tract, which connects motor and sensory cortex with the spinal cord and constitutes the main path for motor commands to travel from the brain to the limbs, has been shown to have a major role in the control of reach and grasp(Lemon, Johansson, & Westling, 1995). The development and maturation of the corticospinal tract, which begins prenatally and continues until adulthood(Fietzek et al., 2000), is affected both by myelination and use, i.e., motor activity performed during infancy and childhood(Martin, 2005). Myelination of the corticospinal tract usually starts in the second trimester or beginning of the third and is not completed until the age of 2–3 years(ten Donkelaar et al., 2004). Iron deficiency has been shown to impair myelination in several animal studies(Beard, Wiesinger, & Connor, 2003; Connor & Menzies, 1996; Ortiz et al., 2004). Possible effects of ID on brain myelination have also been found in humans(Roncagliolo, Garrido, Walter, Peirano, & Lozoff, 1998; Algarin, Peirano, Garrido, Pizarro, & Lozoff, 2003; Burden et al., 2007). Thus, impaired myelination of the corticospinal tract due to ID could contribute to the observed developmental delays in the performance of reach and grasp in IDA infants.
We would also like to note that the observed differences in motor performance should not be interpreted as a non-specific effect of fatigue, which is common in anemia. First, the Optotrak testing was performed on a separate day, and the infants did not go through any other testing that day. Thus, they did not have a reason to be fatigued. Second, the infants performed only 10–20 movements of reaching to a toy. This amount of movement cannot be considered as a fatiguing task for an infant. Lastly, if the IDA infants were fatigued, we would have expected them to move slower than the IS infants. The IDA group, however, had significantly higher peak velocity compared to the IS. Thus, we believe that the observed differences are truly due to developmental delay.
The infants whose reach and grasp Optotrak measurements are reported here were a subset of a sample whose motor performance was assessed by several other motor tests(Shafir, Angulo-Barroso, Jing, Jacobson, & Lozoff, 2007). Analysis of the fine-motor component (which relates to upper extremity motor control) of the Peabody Developmental Motor Scale test (PDMS-2)(Folio & Fewell, 2000) for this larger sample did not yield a significant IDA effect on fine-motor development, a result consistent with most previous studies (reviewed in(Lozoff & Georgieff, 2006)). This observation raises two questions: 1) Why are effects of IDA on upper extremity motor development not detected with motor-behavioral tests such as the Peabody? 2) What is the clinical significance of IDA effects that are detected by special equipment or targeted complex tests but not noticed behaviorally in a simple reach? A possible answer to the first question relates to the natural variability in motor development during infancy. The age range for achieving each upper extremity developmental milestone is quite wide. Therefore, in order to detect a statistically significant developmental delay, the effects have to be pronounced, the sample size large, or the measures very sensitive. We think that the sensitivity of Optotrak measures is a likely explanation for the discrepancy with behavioral test results.
In relation to the second question, two longitudinal studies that examined the effects of IDA and ID without anemia in infancy on motor development over time found poorer fine-motor performance at 5–6 years of age based on direct testing(Shafir, Angulo-Barroso, Calatroni, Jimenez, & Lozoff, 2006) or maternal report(Gunnarsson, Thorsdottir, Palsson, & Gretarsson, 2007). It is possible that, in infancy, when upper extremity skills are just starting to be refined, IDA effects on upper extremity motor development are not yet pronounced enough to be detected by an overall test of motor development like the Peabody. The recent findings that IDA/ID in infancy is associated with poorer fine-motor development later on suggest that subtle effects on upper extremity motor performance during infancy may become more apparent with time, despite iron therapy. The observation by Gunnarsson et al. that ID in infancy even without anemia seemed to affect fine-motor development years later needs to be confirmed in future studies. Nonetheless, findings of long-term effects of IDA/ID on fine-motor control despite iron therapy suggest that a motor intervention may be warranted as soon as ID is detected in infancy, even if no upper extremity motor deficits are perceived at the time. Enhanced practice of upper extremity motor skills might help prevent the long-term effects of iron deficiency in infancy, since motor activity itself can improve corticospinal tract maturation(Martin, 2005) and the reach and grasp skill continues to develop until late childhood(Kuhtz-Buschbeck, Stolze, Johnk, Boczek-Funcke, & Illert, 1998).
In sum, using a sensitive assessment of reach and grasp movements at 9–10 months, this study found poorer upper extremity motor function in IDA compared to IS infants on several variables tested. The observed deficits in upper extremity control, although undetected by common behavioral measures during infancy, may increase over time and become more obvious at later ages(Shafir, Angulo-Barroso, Calatroni, Jimenez, & Lozoff, 2006).
Acknowledgments
This research was supported by Grant Number P01 HD039386 from the National Institute of Child Health and Human Development, B. Lozoff, Principal Investigator. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health and Human Development or the National Institutes of Health. We are grateful to the study families and to student coders for their help in video coding. The entire group of investigators participating in the Brain and Behavior in Early Iron Deficiency Program Project contributed to our thinking and understanding of effects of ID on early motor development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- negligible effect (≥ −0.15 and <.15)
- small effect (≥ .15 and <.40)
- medium effect (≥ .40 and <.75)
- large effect (≥ .75 and <1.10)
- very large effect (≥ 1.10 and <1.45)
- huge effect (>1.45)
Contributor Information
Tal Shafir, The Molecular & Behavioral Neuroscience Institute (MBNI), University of Michigan, Ann Arbor, Michigan
Rosa Angulo-Barroso, Center for Human Growth and Development and Division of Kinesiology, University of Michigan, Ann Arbor, Michigan
Jing Su, Center for Human Growth and Development, University of Michigan, Ann Arbor, Michigan
Sandra W. Jacobson, Department of Psychiatry and Behavioral Neuroscience, Wayne State University School of Medicine, Detroit, Michigan
Betsy Lozoff, Center for Human Growth and Development and Department of Pediatrics and Communicable Diseases, University of Michigan, Ann Arbor, Michigan
Reference List
- SAS 9.1. Cary, NC: SAS Institute Inc.; 2003. [Google Scholar]
- Algarin C, Peirano P, Garrido M, Pizarro F, Lozoff B. Iron deficiency anemia in infancy: Long-lasting effects on auditory and visual systems functioning. Pediatric Research. 2003;53:217–223. doi: 10.1203/01.PDR.0000047657.23156.55. [DOI] [PubMed] [Google Scholar]
- Beard JL, Connor JR. Iron status and neural functioning. Ann Rev Nutr. 2003;23:41–58. doi: 10.1146/annurev.nutr.23.020102.075739. [DOI] [PubMed] [Google Scholar]
- Beard JL, Wiesinger JA, Connor JR. Pre- and postweaning iron deficiency alters myelination in Sprague-Dawley rats. Developmetal Neuroscience. 2003;25:308–315. doi: 10.1159/000073507. [DOI] [PubMed] [Google Scholar]
- Berthier NE, Keen R. Development of reaching in infancy. Experimental Brain Research. 2006;169:507–518. doi: 10.1007/s00221-005-0169-9. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Westerlund AJ, Armony-Sivan R, Nelson CA, Jacobson SW, Lozoff B, Angelilli ML, Jacobson JL. An event-related potential study of attention and recognition memory in infants with iron-deficiency anemia. Pediatrics. 2007;120:e336–e345. doi: 10.1542/peds.2006-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control. Healthy People - 2000 National Health Promotion and Disease Prevention Objectives Final Review. Hyattsville, MD: Department of Health and Human Services; 2001. [Google Scholar]
- Centers for Disease Control Prevention. Morbidity and Mortality Weekly Report. Vol. 47. 1998. Recommendations to prevent and control iron deficiency in the United States; pp. 1–29. [PubMed] [Google Scholar]
- Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. GLIA. 1996;17:83–93. doi: 10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Corbetta D, Thelen E. A method for identifying the initiation of reaching mvoement in natural prehension. J Mot Behav. 1995;27:285–293. [PubMed] [Google Scholar]
- Fagard J. Linked proximal and distal changes in reaching behavior of 5-to 12-month-old human infants grasping objects of different sizes. Infant Behavior and Development. 2000;23:317–329. [Google Scholar]
- Fietzek UM, Heinen F, Berweck S, Maute S, Hufschmidt A, Schulte-Monting J, Lucking CH, Korinthenberg R. Development of the corticospinal system and hand motor function: central conduction times and motor performance tests. Dev Med Child Neurol. 2000;42:220–227. doi: 10.1017/s0012162200000384. [DOI] [PubMed] [Google Scholar]
- Folio MR, Fewell RR. Peabody Developmental Motor Scale. Second ed. Austin, TX: Pro-Ed; 2000. [Google Scholar]
- Gunnarsson BS, Thorsdottir I, Palsson G, Gretarsson SJ. Iron status at 1 and 6 years versus developmental scores at 6 years in a well-nourished affluent population. Acta Paediatr. 2007;96:391–395. doi: 10.1111/j.1651-2227.2007.00086.x. [DOI] [PubMed] [Google Scholar]
- Jackson KM. Fitting of mathematical functions to biomechanical data. IEEE Trans Biomed Eng. 1979;26:122–124. doi: 10.1109/tbme.1979.326551. [DOI] [PubMed] [Google Scholar]
- Konczak J, Borutta M, Topka H, Dichgans J. The development of goal-directed reaching in infants: hand trajectory formation and joint torque control. Experimental Brain Research. 1995;106:156–168. doi: 10.1007/BF00241365. [DOI] [PubMed] [Google Scholar]
- Konczak J, Dichgans J. The development toward stereotypic arm kinematics during reaching in the first 3 years of life. Experimental Brain Research. 1997;117:346–354. doi: 10.1007/s002210050228. [DOI] [PubMed] [Google Scholar]
- Kuhtz-Buschbeck JP, Stolze H, Johnk K, Boczek-Funcke A, Illert M. Development of prehension movements in children: a kinematic study. Experimental Brain Research. 1998;122:424–432. doi: 10.1007/s002210050530. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Johansson RS, Westling G. Corticospinal control during reach, grasp, and precision lift in man. Journal of Neuroscience. 1995;15:6145–6156. doi: 10.1523/JNEUROSCI.15-09-06145.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assessment of the Iron Nutrition Status of the U.S. Population Based on Data Collected in the Second National Health and Nutrition Survey, 1976–-1980. Bethesda: Federation of American Societies for Experimental Biology; 1984. Life Sciences Research Office. [Google Scholar]
- Looker AC, Dallman P, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. Journal of the American Medical Association. 1997;277:973–976. doi: 10.1001/jama.1997.03540360041028. [DOI] [PubMed] [Google Scholar]
- Lozoff B, Beard J, Connor J, Felt B, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutrition Reviews. 2006;64:S34–S43. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13:158–165. doi: 10.1016/j.spen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Martin JH. The corticospinal system: from development to motor control. Neuroscientist. 2005;11:161–173. doi: 10.1177/1073858404270843. [DOI] [PubMed] [Google Scholar]
- Newman C, Atkinson J, Braddick O. The development of reaching and looking preferences in infants to objects of different sizes. Developmental Psychology. 2001;37:561–572. doi: 10.1037//0012-1649.37.4.561. [DOI] [PubMed] [Google Scholar]
- Ortiz E, Pasquini JM, Thompson K, Felt B, Butkus G, Beard J, Connor JR. Effect of manipulation of iron storage, transport, or availability on myelin composition and brain iron content in three different animal models. J Neurosci Res. 2004;77:681–689. doi: 10.1002/jnr.20207. [DOI] [PubMed] [Google Scholar]
- Roncagliolo M, Garrido M, Walter T, Peirano P, Lozoff B. Evidence of altered central nervous system development in infants with iron deficiency anemia at 6 mo: Delayed maturation of auditory brain stem responses. American Journal of Clinical Nutrition. 1998;68:683–690. doi: 10.1093/ajcn/68.3.683. [DOI] [PubMed] [Google Scholar]
- Shafir T, Angulo-Barroso R, Calatroni A, Jimenez E, Lozoff B. Effects of iron deficiency on patterns of motor development over time. Hum Mov Sci. 2006;25:821–838. doi: 10.1016/j.humov.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafir T, Angulo-Barroso R, Jing Y, Jacobson S, Lozoff B. Iron deficiency and infant motor development. Early Human Development. 2007;84:479–485. doi: 10.1016/j.earlhumdev.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus RJ. Defining iron-deficiency anemia in public health terms: a time for reflection. Journal of Nutrition. 2001;131:565S–567S. doi: 10.1093/jn/131.2.565S. [DOI] [PubMed] [Google Scholar]
- ten Donkelaar HJ, Lammens M, Wesseling P, Hori A, Keyser A, Rotteveel J. Development and malformations of the human pyramidal tract. J Neurol. 2004;251:1429–1442. doi: 10.1007/s00415-004-0653-3. [DOI] [PubMed] [Google Scholar]
- Thalheimer W, Cook S. How to calculate effect sizes from published research articles: A simplified methodology. Retrieved March 3 2009 from http://work-learning.com/effect_sizes.htm. [Google Scholar]
- Thelen E, Corbetta D, Spencer JP. Development of reaching during the first year: Role of movement speed. Journal of Experimental Psychology : Human Perception and Performance. 1996;22:1059–1076. doi: 10.1037//0096-1523.22.5.1059. [DOI] [PubMed] [Google Scholar]
- Von Hofsten C, Ronnqvist L. Preparation for grasping an object: A developmental study. J Exp Psychol. 1988;14:610–621. doi: 10.1037//0096-1523.14.4.610. [DOI] [PubMed] [Google Scholar]
- WHO. Geneva: World Health Organization; Iron deficiency anaemia: assessment, prevention and control. A guide for programme managers. 2001