2. Summary
B cell-based cellular vaccines represent a promising approach to active immunotherapy of cancer complementing the use of dendritic cells, especially in pediatric patients and patients with low bone marrow reserves. B cells can be easily prepared in large numbers and readily home to secondary lymphoid organs, the primary site of induction of cytotoxic T lymphocyte (CTL) responses. However, most B cell-based vaccines tested so far failed to induce functional and protective CTLs in in vivo models. Here we demonstrate that B cells activated via the Toll like receptor-9 (TLR-9) and CD40 up-regulate surface expression of MHC and costimulatory molecules, produce IL-12, and exhibit potent antigen-presenting properties in vitro. Importantly, while administration of peptide-coated or transiently transfected B cells fails to induce immune responses, therapeutic immunization with low numbers of genetically modified B cells stably expressing antigen results in an induction of functional CTLs and protection against the growth of tumor in an animal model. Following activation, B cells partially loose their ability to home to organized lymphoid tissue due to the shedding of CD62L; however, this property can be restored by expression of protease-resistant mutant of CD62L. In summary, the data presented in this report suggest that genetically modified activated B cells represent a promising candidate for a cancer vaccine eliciting functional systemic CTLs.
Keywords: Cancer immunotherapy, Cellular vaccine, B cell, Gene delivery
3. Introduction
Characterization of dendritic cells (DCs) as highly efficient antigen-presenting cells (APCs) led to a number of clinical studies demonstrating the benefit of induction of tumor-specific immune responses in cancer patients 1. However, in sharp contrast to the results obtained in animal models, complete and long-lasting remissions were observed only in a fraction of patients 2-5. Large-scale application of DC-based vaccines is significantly complicated by the fact that clinical grade DCs are difficult to produce in large quantities, cannot be efficiently expanded ex vivo, and are highly sensitive to cryopreservation 5. Suboptimal quantities of DCs are frequently employed in clinical trials. The lack of adequate cell numbers is most profound in pediatric patients and in patients with low bone marrow reserves 6-9. In addition, only a fraction (< 1 %) of exogenously transferred DCs appears to migrate to the draining lymph nodes, while most remain at the site of immunization 10, 11. This precludes effective use of DCs as vehicles for the delivery of costimulatory factors such as cytokines and chemokines to the organized secondary lymphoid tissue, the primary site of induction of T cell responses. Identification of alternative sources of antigen-presenting cells for active immunization complementing the use DCs is a high priority in the field.
B cells represent a promising alternative to DCs as cell-based vaccines for immunotherapy 5,12-14. Large numbers of autologous B cells can be easily prepared from the blood of patients and further expanded ex vivo up to 1,000 fold in the presence of CD40L 14. While only about 106 DCs can be prepared from 10 ml of blood, 109-1010 B cells can be generated from the same volume. Importantly, in contrast to DCs, intravenously administered B cells readily migrate to immune inductive sites in secondary lymphoid tissues. Activated B cells can be loaded with antigen by pulsing with peptides, proteins, tumor lysates, transfection with DNA or RNA, or transduction with viral vectors 6, 13, 15, 16. Multiple studies have demonstrated that antigen-presenting activated B cells induce potent expansion of antigen-specific CD4+ and CD8+T cells in vitro 5, 6, 12-15, 17-22. However, with the exception of studies utilizing re-arranged immunoglobulin molecule encoding antigenic epitopes in the complementarity determining regions 17, 20, most reports suggest that immunization with B cells pulsed with antigenic peptides or transfected to express whole antigenic proteins fails to induce significant cytotoxic T cell responses 23-25. Thus, optimal B cell vaccination strategy eliciting cytotoxic T cell responses remains to be established.
In this study, we demonstrate that B cells activated via the TLR-9 and CD40 receptors exhibit powerful APC capacity in vitro. We show that immunization with as few as 5×104 genetically modified live B cells stably expressing antigen results in an induction of functional cytotoxic T cell responses in vivo. Importantly, we show that immunization with B cells stably expressing model antigen but not with peptide-coated B cells protects hosts against tumor growth in a therapeutic setting. Thus, it is likely that prolonged antigen presentation and delivery by proliferating B cells is required for an efficient immunization. Since lentivirally-transduced cellular vaccines are increasingly used in gene therapy and cancer immunotherapy clinical trials, the data presented here provide evidence supporting the use of genetically modified B cells as a promising candidate for a cellular vaccine for cancer immunotherapy and an alternative complementing the use of DCs, especially in pediatric patients and patients with low bone marrow reserves.
4. Results
B cells activated via TLR-9 and CD40 receptors efficiently present antigen to CD8+ and CD4+ T cells in vitro
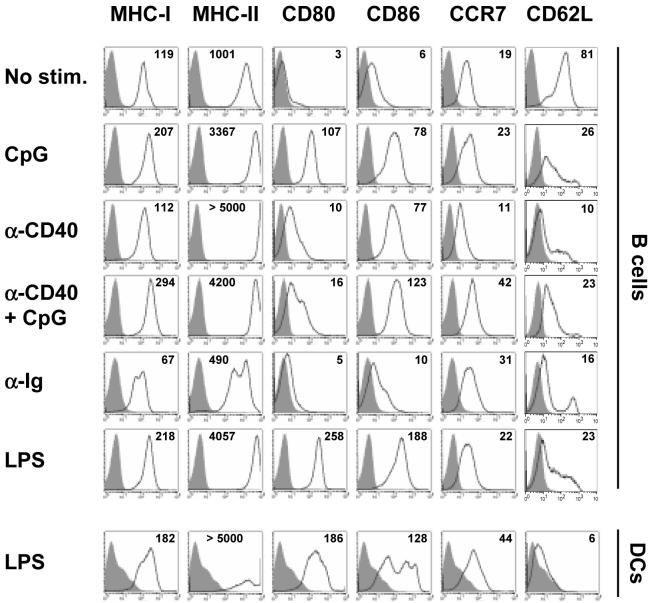
We addressed whether the antigen-presenting capacity of B cells can be enhanced by a combination of signals send through the surface immunoglobulin receptor, CD40 receptor, and a “danger” signal transmitted by the TLRs. As shown on Fig. 1, stimulation of B cells with TLR-9 receptor ligand CpG motif-containing oligonucleotide (CpG), activating anti-CD40 antibodies (α-CD40), or TLR-4 ligand bacterial lipopolysaccharide (LPS) significantly increased the expression of MHC-I, MHC-II and costimulatory molecules CD80 (B7.1) and CD86 (B7.2). In contrast, stimulation with antibodies engaging surface immunoglobulin (α-Ig) alone or in combination with other stimuli did not increase the expression of molecules involved in antigen presentation. Surface CD62L (L-selectin), a homing molecule involved in the targeting of lymphocytes to secondary lymphoid tissues, was down-modulated by all activating stimuli. Levels of CCR7 chemokine receptor and α4β7 integrin were not significantly affected by any treatment (Fig. 1 and data not shown). Importantly, the levels of antigen-presenting and costimulatory molecules on B cells activated with CpG, α-CD40, or their combination resembled those detected on LPS-matured DCs (Fig. 1).
Figure 1. Expression of MHC, costimulatory, and homing molecules on the surface of activated B cells and DCs.
The expression of surface molecules was determined by flow cytometry on freshly isolated unstimulated B cells (No stim.), B cells activated with various agents for 20 hrs, or bone marrow-derived DCs stimulated with LPS for 20 hrs. Numbers represent mean fluorescent intensities. Representative results of four similar experiments are shown.
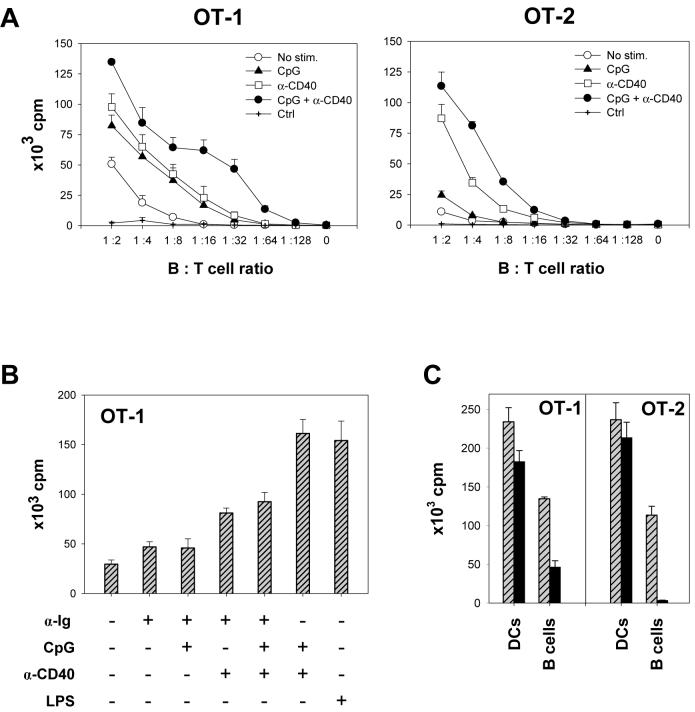
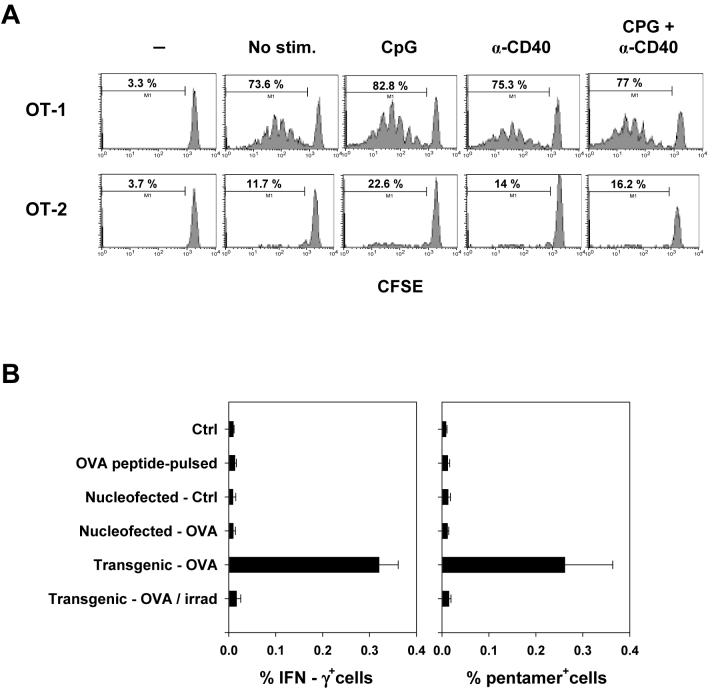
Next, we assessed the in vitro antigen-presenting capacity of activated B cells. B cells pulsed with ovalbumin-derived MHC-I H-2Kb-restricted OVA-1 peptide and MHC-II I-Ab-restricted OVA-2 peptide were incubated at various B:T cell ratios with CD8+ T cells and CD4+ T cells isolated from transgenic mice expressing respective cognate T-cell receptors (OT-1 and OT-2). While B cells stimulated either with CpG or α-CD40 antibody alone displayed increased ability to induce specific T cell proliferation, the effect was markedly enhanced by combined stimulation (Fig. 2A). Surprisingly, B cells stimulated with anti-Ig antibody alone or in combination with CpG and α-CD40 displayed lower APC capacity than CpG/CD40-stimulated B cells (Fig. 2B). Similar results were obtained with stimulator B cells derived from transgenic mice stably expressing OVA under a control of a ubiquitous promoter.
Figure 2. Activated B cells efficiently induce antigen-specific CD8+ and CD4+ T cell proliferation in vitro.
(A) OVA-specific CD8+ OT-1 or CD4+ OT-2 T cells were incubated for 72 hrs at indicated ratios with cognate peptide-pulsed irradiated freshly isolated B cells or B cells stimulated with indicated agents for 20 hrs. The extent of T cell proliferation was determined by thymidine incorporation assay. α-CD40, anti-CD40 antibodies; Ctrl, CpG/α-CD40 activated B cells not pulsed with peptide. Representative results of one of four independent experiments using peptide-pulsed or OVA-transgenic B cells are shown. (B), Proliferation of OT-1 cells following incubation with peptide-pulsed irradiated B cells stimulated with indicated agents for 20 hrs (B:T cell ratio 1:2). (C), Proliferation of OT-1 or OT-2 cells following incubation with peptide-pulsed DCs or CpG/CD40-activated B cells at APC:T cell ratio of 1:2 (lined bars) or 1:32 (solid bars). Error bars represent standard errors (SE).
Compared to peptide-pulsed or transgenic DCs, CPG/CD40-activated B cells were about 50 % as effective in inducing T cell proliferation at high APC:T cell ratios; however, DCs were significantly more effective at lower ratios (Fig. 2C). To exclude a possible confounding effect of DCs contaminating B cell preparations, B cells isolated by a negative selection for CD43- cells combined with a subsequent depletion of CD11c+ cells or by a positive selection of CD19+ cells were tested. Although these procedures eliminated almost all contaminating DCs (< 0.1 % CD11c+ cells present in the CD19+ cell preparation), the method of B cell purification did not have any significant effect on the rate of proliferation of OT-1 or OT-2 cells (Supplemental Fig. 1). Thus, the observed APC activity is mediated by B cells. In conclusion, B cells activated with CpG and α-CD40 efficiently expand antigen-specific T cells in vitro and can be utilized as APCs for ex vivo expansion of tumor-specific T cells for adoptive T cell therapy.
The interaction between antigen-presenting cell and specific T cell is regulated by a complex interplay of co-stimulatory and inhibitory signals mediated by an array of cell surface molecules and soluble factors. In our system, the efficacy of stimulation of proliferation of antigen-specific CD8+ T and CD4+ T cells appeared to best correlate with the expression of CD86 (B7.2), rather than the expression of MHC-I or II (Fig. 1). However, an effect of other factors cannot be excluded.
IL-12 produced by activated B cells does not affect T cell proliferation in vitro
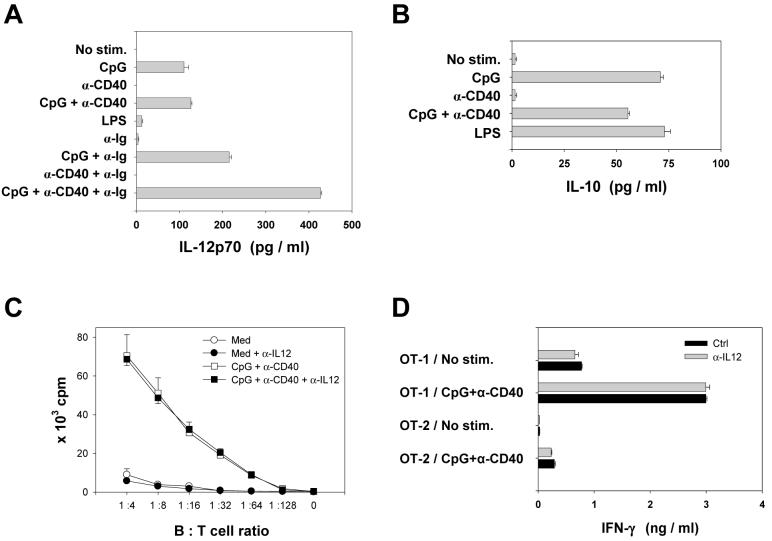
Under certain conditions, activated B cells can produce IL-12 26-29, a cytokine known to direct the differentiation of CD4+ T cells toward the Th1 phenotype and stimulate the proliferation of CD8+ T cells 30, 31. We assessed whether IL-12 might mediate some of the effect of CpG/CD40-activated B cells on antigen-specific T cell proliferation in vitro. B cells stimulated with CpG expressed the limiting p70 component of IL-12 as well as IL-10 (Fig. 3A, B). In contrast, stimulation with LPS resulted in the production of IL-10 without significant production of IL-12. Costimulation via surface immunoglobulin in a combination with α-CD40 and CpG significantly increased the levels of IL-12p70 produced. Importantly, antibody-mediated neutralization of IL-12 did not affect the rate of proliferation of OT-1 or OT-2 cells or production of IFN-γ or IL-4 (Fig. 3C, D, and data not shown). Thus, the ability of activated B cells to induce antigen-specific T cell proliferation in vitro is not significantly affected by the production of costimulatory IL-12. However, IL-12 produced by B cells might play a role in vivo, e.g. by modulating NK cell activity, modifying cytokine expression by T cells, exerting anti-angiogenic effect, and inducing chemokines and other regulatory factors modulating T cell trafficking and duration of conjugation events between CD8+ T cells and APCs 32.
Figure 3. IL-12 produced by activated B cells does not affect T cell proliferation in vitro.
(A, B) B cells were either not stimulated or stimulated with indicated agents for 20 hrs and the levels of released IL-12p70 (A) or IL-10 (B) were determined. One of three similar experiments is shown. (C) Proliferation of OT-1 cells incubated with fresh (Med) or activated (CpG + α-CD40) irradiated OVA-transgenic B cells in the absence or presence of IL-12-specific neutralizing antibodies (5 μg/ml). (D) IFN-γ production by OT-1 or OT-2 T cells incubated for 20 hrs with fresh or activated irradiated OVA-transgenic B cells at 1:2 B:T cell ratio. One of two experiments is shown; error bars represent SE.
B cells stably expressing antigen induce functional CTL responses in vivo
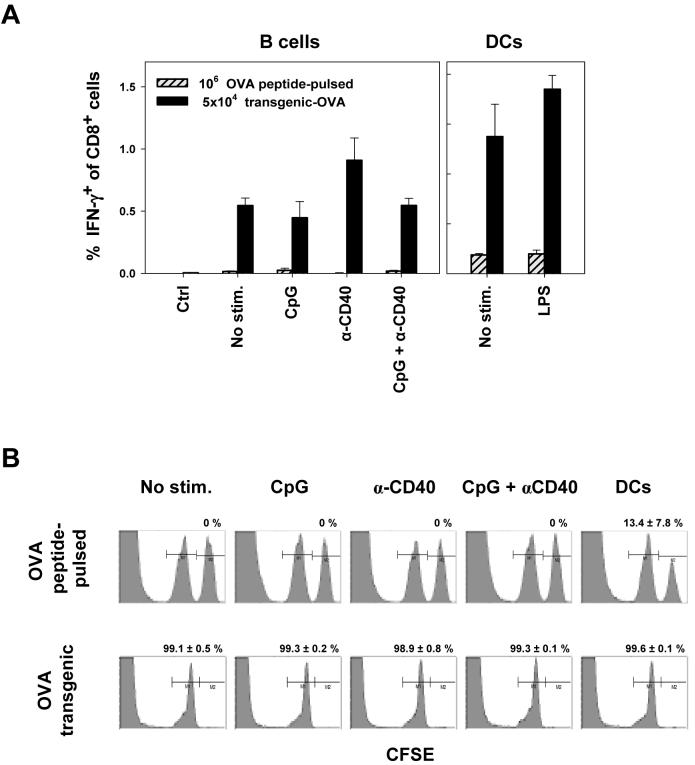
Next, we tested whether immunization with peptide-pulsed fresh or activated B cells induces endogenous T cell responses in naïve recipients. Intravenous administration of 5×104 to 2×106 OVA-1 and OVA-2 peptide-pulsed fresh or activated B cells resulted in low CD8+ T cell responses as determined by intracellular cytokine staining and pentamer binding assays (data with 106 B cells presented in Fig. 4A). In sharp contrast, immunization with as few as 5×104 resting or activated OVA-transgenic B cells stably expressing antigen induced OVA-1-specific CD8+ T cells at frequencies ranging from 0.5 to 1.5 % of total CD8+ T cells in the spleen and lymph nodes (Fig. 4A and data not shown). OVA-2-specific CD4+ T cells were induced at lower frequencies (0.1 to 0.2 % of total CD4+ T cells). Foot pad immunization with 2×104 to 5×104 CpG/CD40-activated OVA-transgenic B cells resulted in an induction of 0.5 - 3 % of OVA-1-specific T cells of all CD8+ splenocytes (data not shown). Surprisingly, activation status of B cells prior to administration did not have a significant effect on the magnitude of the ensuing T cell responses. Similarly, intravenously administered OVA-transgenic DCs were more effective at inducing CD8+ T cell response than peptide-pulsed DCs (Fig. 4A), consistently with previous reports 29, 33. Compared to immunization with activated OVA-transgenic DCs, immunization with activated B cells induced lower frequencies of antigen-specific CD8+ T cells (LPS-DCs versus CpG/CD40-B cells, p = 0.04). However, this difference could be compensated by administering higher doses of antigen-expressing B cells. We intentionally used low numbers of B cells for immunization since this better represents the cell number / kg body weight ratio employed in clinical protocols.
Figure 4. Immunization with B cells stably expressing antigen induces endogenous T cell responses irrespectively of their activation status.
(A) Naïve B6 mice were immunized intravenously with 5×104 OVA-transgenic B cells or 106 OVA peptide-pulsed B cells that were either unstimulated or stimulated with indicated agents (left panel). In a control experiment, mice were immunized with 5×104 OVA-transgenic or 106 OVA peptide-pulsed DCs (right panel). The frequency of OVA-1-specific CD8+ T cells in spleen 10 days after immunization was detected by ICS assay. 5 animals per group; error bars represent SE. Results of one of five similar experiments are shown. (B) Immunization with B cells induces functional cytotoxic T cell responses. Naïve B6 mice were immunized with 106 unstimulated or stimulated autologous B cells pulsed with OVA-1 peptide (upper panel) or 5×104 B cells purified from OVA-transgenic mice (lower panel). 9 days later, mice were injected with a mix of 107 splenocytes labeled with 0.35 μM CFSE (not pulsed) and 107 splenocytes labeled with 3.5 μM CFSE (pulsed with 1 μM OVA-1 peptide). Splenocytes were harvested 20 hrs later and CFSE+ cells were detected by flow cytometry. Numbers represent the percentages ± SE of killed pulsed target cells normalized to internal control population and external non-immunized mice control (0% killing). Four mice per group; representative results of one of three similar experiments are presented.
To address the functionality of T cell responses induced by immunization with fresh or activated B cells, in vivo cytotoxicity assay was performed. While immunization with 106 unstimulated or stimulated autologous B cells pulsed with OVA-1 peptide failed to induce detectable CTL responses, immunization with as few as 5×104 OVA-transgenic B cells resulted in efficient killing of target cells in vivo (Fig. 4B). Similarly, immunization with transgenic DCs was more efficient in CTL induction than immunization with peptide-pulsed DCs. These results were confirmed by ex vivo killing assays using splenocytes and lymph node cells as effector cells (data not shown).
To test whether antigenic peptide-pulsed CpG/CD40-ativated B cells can sustain proliferation of existing antigen-specific T cells in vivo, B6 mice were injected with OT-1 transgenic T cells labeled with CFSE and, 24 hrs later, with fresh or activated B cells pulsed with OVA-1 and OVA-2 peptides. Adoptively transferred CD8+ OT-1 T cells efficiently proliferated following in vivo stimulation; CD4+ OT-2 cells proliferated to a lower degree (Fig. 5A). Interestingly, there was little difference in the rate of proliferation of antigen-specific T cells stimulated by non-activated versus activated B cells.
Figure 5. Peptide-pulsed, transfected, or transgenic/irradiated B cells fail to induce endogenous T cells responses.
(A), Fresh and activated peptide-pulsed B cells sustain comparable proliferation of adoptively transferred antigen-specific T cells in vivo. B6.SJL (CD45.1+) mice were injected I.V. with 106 CFSE-labeled OT-1 and OT-2 cells (CD45.2+). Next day, 106 B6 B cells either unstimulated or stimulated with indicated agents for 20 hrs and pulsed with cognate OVA-1 and OVA-2 peptides were injected. Three days later, CFSE dilution in CD45.2+CD8+ (OT-1) or CD45.2+CD4+ (OT-2) cells isolated from spleen was analyzed by flow cytometry. (B) Naïve C57Bl/6 mice were immunized intravenously with 5×104 autologous CpG/CD-40-activated B cells that were not pulsed, pulsed with OVA-1 peptide, nucleofected with EGFP and empty control plasmids, nucleofected with EGFP and OVA-expressing plasmids, or with CpG/CD-40-activated B cells from OVA-transgenic mice that were either not irradiated or exposed to a lethal dose of radiation. OVA-specific immune response was determined in splenocytes 10 days later by ICS and pentamer binding assays.
To further address the mechanisms underlying B cell-elicited induction of CTLs, B cells were co-transfected by nucleofection with plasmids expressing EGFP and OVA and transfected cells were enriched by sorting of EGFP-expressing cells. 24 hrs post nucleofection, transfected B cells expressed equal or higher amount of OVA protein compared to OVA-transgenic cells, as determined by Western blot analysis. However, administration of nucleofected B cells induced only low frequencies of specific CD8+ T cells (Fig. 5B). Importantly, irradiation of OVA-transgenic B cells abolished their ability to induce CTLs. In contrast, immunization with relatively low numbers of live B cells stably expressing antigen efficiently induced antigen-specific T cell responses. Collectively, this data suggest that, although peptide-pulsed B cells present antigenic peptide to T cells in vitro and in vivo 23, stable antigen expression by proliferating B cells is required for an efficient induction of high frequencies of specific T cells by a B cell-based vaccine.
Activated B cells are less efficient in homing to secondary lymphoid tissues
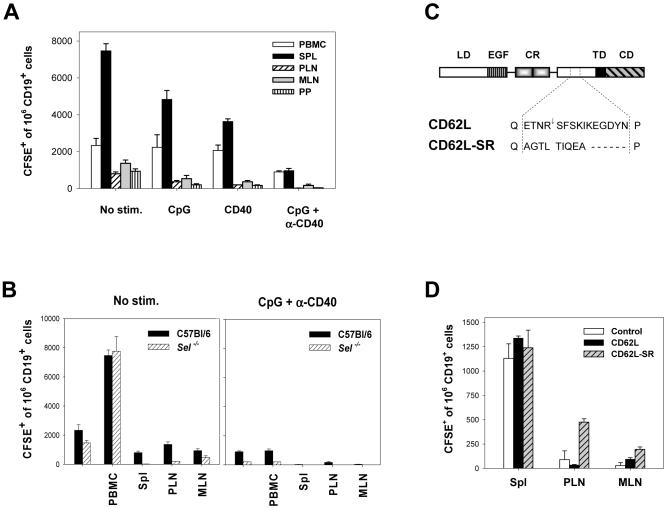
Observed difference in the antigen-presenting capacity of fresh versus activated B cells in vitro is in a sharp contrast with their comparable immunogenicity in vivo. Possible factors contributing to this discrepancy include decreased capacity of activated B cells to home to secondary lymphoid organs. To determine their homing properties, CFSE-labeled fresh and activated B cells were injected I.V. into recipient mice and the frequencies of CD19+ CFSE+ cells in various lymphoid organs were determined 4 and 24 hrs later (Fig. 6A and data not shown). Compared to unstimulated B cells, activation of B cells with CpG and α-CD40 significantly decreased their ability to home to the spleen, peripheral (PLN) and mesenteric (MLN) lymph nodes, and Peyer’s patches (PP). This data suggest that the inability of CpG/CD40-activated B cells to induce stronger immune responses in vivo despite their superior APC capacity in vitro may be caused in part by their inefficient homing to secondary lymphoid organs.
Figure 6. Activated B cells do not efficiently home to secondary lymphoid organs.
(A) B cells were either unstimulated or stimulated with indicated agents for 20 hrs, labeled with CFSE, and 3×106 cells were injected I.V. into B6 mice. Four hours later, blood, spleen, peripheral (PLN) and mesenteric (MLN) lymph node cells, and Peyer’s patches (PP) were harvested and the percentage of CFSE+ CD19+ cells of total CD19+ cell population in each tissue was determined. Three mice per group; error bars represent SE. One of three similar experiments is presented. (B) Homing properties of unstimulated (left panel) or CpG/CD40-stimulated (right panel) B cells isolated from C57Bl/6 or selectin-knockout (Sel-l,p,e-/-) mice were analyzed as described in (A). (C) The structure of CD62L and CD62L-SR mutant. LD - lectin domain, EGF - epidermal growth factor-like domain, CR - short consensus repeats, TD - transmembrane domain, CD -cytoplasmic domain; arrow denotes cleavage site. (D) Activated B cells expressing sheddase-resistant mutant of CD62L (CD62L-SR) exhibit greater capacity to home to PLNs. CpG/CD40-activated B cells were co-transfected with plasmid expressing EGFP and either control empty plasmid, CD62L, or CD62L-SR-expressing plasmids and 5×105 transfected cells were injected intravenously. The frequency of CFSE+ B cells was determined in various organs 4 hrs post injection.
The altered homing capacity of activated B cells could be caused by a downregulation of CD62L molecule from the cell surface occurring as early as 30 min following activation (Fig. 1B). To address the role of CD62L in B cell trafficking, homing experiments were performed using B cells from selectin-deficient mice (Sel-l,p,e-/-). While freshly isolated B cells from selectin-knockout mice readily migrated to the spleen of recipient animals, homing to peripheral and mesenteric lymph nodes was severely reduced (P = 0.01 and 0.003 for PLN and MLN compared to control B6 B cells) (Fig. 6B). Following stimulation with CpG+CD40 antibody, selectin-knockout B cells exhibited diminished capacity to home to the spleen and virtually no activated cells were found in PLNs, MLNs, or Peyer’s patches (Fig. 6B, right panel). Thus, the loss of the ability of activated B cells to home to LNs and PPs can be partially attributed to decreased surface levels of selectin.
Next, we investigated whether decreased lymphoid tissue homing of activated B cells can be reversed by expression of proteolysis-resistant mutant of CD62L. The sheddase-mediated proteolysis occurs within the membrane proximal region between R283 and S284 of CD62L 34. We have constructed a CD62L mutant in which the proteolysis is inhibited by a substitution of the membrane-proximal region of CD62L with that of P-selectin lacking the cleavage site (Fig. 6C) 35. CpG/CD40-stimulated B cells nucleofected with plasmid encoding sheddase-resistant mutant of CD62L (CD62L-SR) expressed about 2.5 fold higher mean levels of total surface CD62L ectodomain compared to cells nucleofected with wild-type CD62L as determined by flow cytometry. Expression of CD62L-SR resulted in an increased migration of activated B cells to peripheral and mesenteric lymph nodes (Fig. 6D). These results confirm the role of CD62L in targeting B cells to secondary lymphoid organs and provide possible means for the enhancement of the immunogenicity of genetically-modified B cell-based vaccines. However, simple co-transfection of plasmids encoding antigen and CD62L-SR did not significantly increase in vivo immunogenicity of transfected B cells (data not shown). Stable expression of both proteins by proliferating cells is likely needed for enhanced immunogenicity.
Immunization with antigen-expressing B cells confers protection against tumor growth in a therapeutic setting
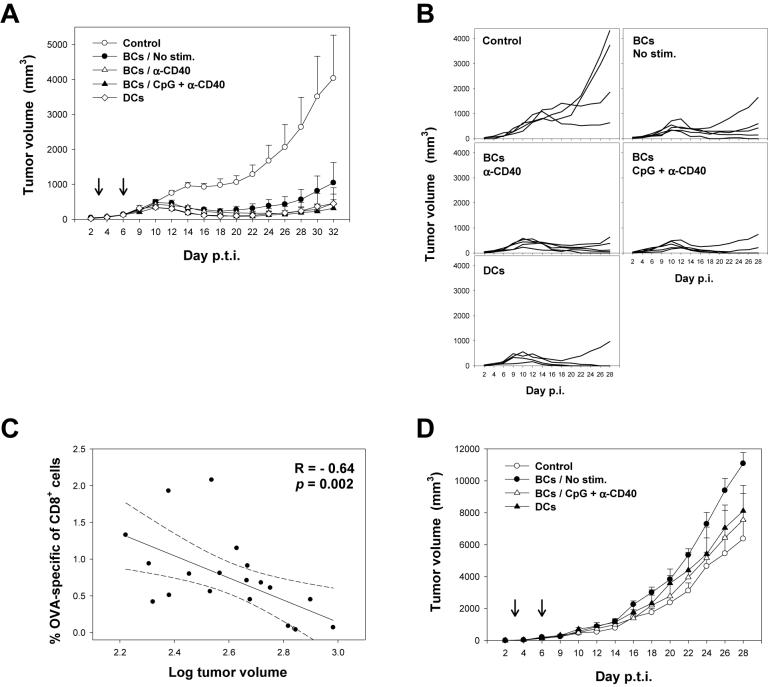
To investigate the ability of antigen-presenting B cells to protect against tumor, mice were inoculated subcutaneously with 106 EG-7 thymoma cells expressing OVA protein. 3 and 6 days post tumor inoculation, mice were immunized with 105 resting or activated OVA-transgenic B cells. As shown in Fig. 7A and B, mice immunized with fresh, CD40- or CpG/CD40-activated OVA-transgenic B cells or OVA-transgenic DCs displayed significant protection against tumor growth compared to controls (p = 0.027, 0.01, 0.009, and 0.023, respectively; analyzed by RM-ANOVA). At day 32 post inoculation, 3 of 5 mice in CpG/CD40-B cells-immunized group and 3 of 4 in DC-immunized group were tumor free (Fig. 7B). In contrast, only 1 of 5 mice immunized with fresh B cells was free of palpable tumor. The ability to control tumor growth correlated with the frequency of OVA-specific CD8+ T cells following the two rounds of immunization, suggesting a direct role of CTLs in tumor protection (p = 0.002; Fig. 7C). In contrast, therapeutic immunization with peptide-pulsed B cells did not significantly increase the frequency of OVA-specific CD8+ T cells or confer protection against tumor (Fig. 7D). This data demonstrate that stable expression of antigen by B cells is required for the protection against tumor growth.
Figure 7. Immunization with genetically modified B cells protects against tumor growth in a therapeutic setting.
(A) Mice were inoculated subcutaneously with 106 EG-7 tumor cells and either not immunized (Control), or immunized at days 3 and 6 post tumor inoculation (p.t.i.) with 105 OVA-transgenic unstimulated or stimulated B cells or bone marrow-derived DCs. Arrows represent times of immunization. Tumor volumes ± SE are presented as averages of 4 to 5 mice per group. (B) Tumor growth curves in individual mice. (C) Correlation between the frequency of OVA-1-specific CD8+ T cells in blood of mice at day 10 post tumor inoculation determined by specific pentamer staining and tumor volume at day 12. R, correlation coefficient. (D) As in (A) except that mice were immunized with OVA peptide-pulsed B cells.
5. Discussion
Since large number of B cells can be easily prepared from relatively small volume of blood, B cell-based cellular vaccines represent a highly promising approach to the immunotherapy of cancer complementing the use of dendritic cells, especially in select groups of patients such as pediatric patients and patients with low bone marrow reserves. In this report, we demonstrate that: 1) Activated B cells produce IL-12 and display potent antigen-presenting properties in vitro and in vivo; 2) Therapeutic immunization with low numbers of genetically modified B cells stably expressing antigen results in a protection against tumor growth; and 3) B cells can be genetically modified to deliver the antigen to organized lymphoid tissue. Although these results need to be further confirmed by studies using relevant tumor antigens in animal models and in humans, this report presents an initial set of conditions for the utilization of B cell-based cellular vaccines for the immunotherapy of cancer and other chronic diseases.
In order to break immune evasion, strong antigenic stimuli comprising multiple antigenic epitopes presented at immune induction sites distant from the immunosuppressive environment of the tumor is needed. In tumor-bearing host, functional TAA-specific effector T cells are continuously depleted by processes of functional exhaustion and activation-induced cell death [8]. Therefore, in contrast to vaccination against infectious agents where limited number of immunizations is sufficient to build up immune memory, successful immunotherapy of cancer will likely require multiple immunizations over prolonged period of time not only to induce, but also maintain functional effector T cell population [8-10]. Suboptimal induction of immune responses, due to the limited quantity of APCs and short time scale of immunizations, may be some of the major factors responsible for the limited efficacy of most cancer immunotherapy vaccines. In this respect, B cells represent a promising novel platform for cancer immunotherapy, as they can be easily prepared in large numbers 5, 12-14. Furthermore, B cells can be easily cryopreserved and retain their antigen-presenting capacity upon recovery, thus simplifying clinical procedures for repeated immunizations 14. Immunization with B cells will likely have to be performed in combination with other therapies that offset the suppressive environment of the tumor, such as therapies blocking the suppressive effect of cytokines and regulatory T cells or drugs inducing immunogenic death of cancer cells 1, 36, 37.
Transduction with lentiviral vectors represents an efficient approach for generating B cells stably producing specific tumor antigens. Recently developed third generation of lentiviral vectors transduces primary cells with high efficiency, does not express any viral protein, and contains a multitude of safety features preventing the generation of replication-competent recombinants or oncogenic transformation 38. Several reports have demonstrated that DCs transduced with antigen-encoding lentiviral vectors induce strong and long-lasting T cell responses 29, 33 and several clinical trials currently evaluate the safety and efficacy of lentiviral vectors in cancer immunotherapy 38. Interestingly, Russo et al. have demonstrated that immunization of mice with 4-10×106 lymphocytes transduced with a retroviral vector encoding tumor antigen resulted in and induction of anti-tumor immunity via cross-presenting host DCs 39. Here we show that restricting antigen expression to activated B cells allows efficient immunization with as few as 5×104 cells, i.e. 100x lower cell dose. We are currently testing the efficacy of immunization with B cells transduced with lentiviral vectors co-expressing antigen and CD62L-SR. In addition, non-viral modes of genetic modification of somatic cells such as Sleeping beauty transposon are currently under development 40.
Since activated B cells pulsed with antigenic peptides or transfected with antigen-expressing plasmid or RNA efficiently present antigen and induce specific T cell proliferation in vitro and in vivo, it was believed that this simple manipulation would result in an effective cell-based vaccine 6, 13, 15, 16, 21. However, the data presented here and elsewhere 23, 24 suggest that peptide-pulsed or transfected B cells fail to induce protective CTLs in vivo. Importantly, we demonstrate that stable antigen expression is required for an effective vaccine. Multiple studies suggest that the presentation of MHC-I/peptide complexes by peptide or protein-loaded APCs is relatively short lived 41, 42 and epitopes derived from stabilized proteins are more efficiently cross-presented than epitopes derived from rapidly degrading proteins or short peptides 43. Sustained source of antigen provided by a cellular vaccine is advantageous for T cell induction due to the prolonged direct presentation or cross-presentation by host DCs 29, 33. A number of reports have suggested that persistent antigen expression is required for optimal expansion of CD8+ T cells and their differentiation into the memory phenotype 29, 44-47. Despite these observations, majority of currently undergoing clinical trials evaluating the efficacy of DC-based immunotherapy use DCs loaded with antigenic peptides, proteins, or tumor lysates. The requirement of sustained antigen expression is likely more critical for B cell-based vaccines due to the shorter presentation half-time of MHC-peptide complexes on the surface of B cells compared to mature DCs 42. The difference between DCs and B cells could explain current lack of reports on the efficacy of B cell immunization in vivo, despite an abundance of data on their ability to sustain T cell proliferation in vitro.
There is limited amount of data available regarding the capacity of B cells to induce protective CTL responses in vivo. Ritchie et al. demonstrated that mice immunized intravenously with peptide-loaded CD40L-activated B cells displayed a short delay in the growth of tumor expressing the corresponding epitope; however, no effect was observed in mice administered with freshly isolated B cells or B cells activated with lipopolysaccharide (LPS) 23. Importantly, the adhesion-based procedure for B cell purification used in this report could not preclude DC contamination. Lee et al. has demonstrated that immunization with B cells transfected with mRNAs encoding antigen and co-stimulatory molecules failed to induce CTLs and tumor protection, despite their potent APC activity in vitro 24.
Controversy exists in the field as to whether or not resting B cells can serve as APCs. Several groups have shown that these cells induce T cell tolerance resulting in decreased immune responses in vivo 48-50. Although the reason for this seems to be low expression of accessory and costimulatory molecules, Evans et al. suggested that unstimulated B cells are deficient in later functions necessary for a productive T cell response 51. Our results suggest that the immunization with low numbers of untouched B cells isolated using a negative selection procedure induces CTLs in vivo; however, the anti-tumor responses appear to be diminished compared to that induced by activated B cells. It is well established that B cells can internalize antigen via their surface Ig receptors and drive priming of naïve CD4+ T cells in vivo 52, 53. During a T helper cell-dependent B cell response, activated antigen-specific CD4+ T cells express CD40L and in turn activate B cells through the CD40 receptor. Following activation, B cells migrate to the B zone / T zone boundary in organized lymphoid tissue and greatly improve their ability to present antigen by upregulating surface MHC and costimulatory molecules 28, 52, 54. Activation via CD40 receptor dramatically increases the antigen-presenting ability of normal and malignant B cells and has therefore been studied as an approach to generate autologous APCs for immunotherapy 14. CD40-activated B cells not only expand antigen-specific CD4+ and CD8+ T cells, but also prime naïve T cells in vitro 13, 15,16. In addition to CD40L, B cells can be activated with a number of TLR ligands resulting in enhanced expression of MHC, costimulatory molecules, IL-12, and increased cytoskeletal activity26, 27, 55. The B cell receptor -induced expression of TLR-9 in naïve B cells prevents the polyclonal activation in primary response since it restricts stimulation to antigen-specific cells 56. Here we show that co-stimulation of B cells with CD40L and TLR-9 ligand results in an increase of expression of MHC and costimulatory molecules and enhancement of their ability to sustain specific T cell proliferation in vitro. This observation may be valuable for protocols employing B cells as APCs for the ex vivo expansion of T cells for passive adoptive T cell immunotherapy. It remains to be established whether B cells activated with CpG alone would be sufficient for induction of tumor protection in humans. Since CpG-stimulated B cells readily expand in vitro, immunotherapy with CpG-activated B cells represents an affordable alternative to more complex technologies involving amplification of B cells on CD40L-expressing cell lines 57.
Recently, it has been reported that IgD+ CD27- naïve mature B cells can uptake DNA by a process of spontaneous transgenesis turning B cells into functional APCs by upregulation of MHC class I and II, CD40, and CD86 17, 20. Using a re-arranged immunoglobulin molecule encoding well-defined CD4+ and CD8+ epitopes in the complementarity determining regions, Gerloni et al. have shown that single intravenous injection of as few as 70-300 transfected B cells induced protection against a lethal dose of influenza virus 17, 20. It is unclear whether spontaneous transgenesis might be associated with chromosomal integration events.
Multiple lines of evidence suggest that the antigen delivered by cellular vaccines is presented by the recipient’s secondary lymphoid organ-resident DCs by the process of cross-presentation 43, 58-60. B cells can present antigen both by direct presentation and cross-presentation 17, 23. Gerloni et al. showed that immunization with B lymphocytes transfected with antigen-encoding plasmid elicited similar CD4+ and CD8+ T cells responses in relB-/- and control mice suggesting that transgenic B cells can directly activate T cells in the absence of functional dendritic cells 17. Furthermore, while the CD4+ T cell response following a single injection of B cells from MHC-II-/- mice was virtually abolished compared to controls, immunization with MHC-I-/- B cells induced CD8+ T cell response that was only partially reduced compared to that induced by normal B cells 17. These results, further confirmed by Ritchie et al. 23, suggest that while the CD4+ T cells are primed directly by transgenic B cells used for immunization, priming of CD8+ T cells occurs by a combination of cross-presentation and direct presentation mechanisms. As B cells activated via various pathways differ in their APC function and homing properties, the importance of cross-priming may vary depending on their activation phenotype. In the experiments presented here, B cells activated by various stimuli displayed large differences in their capacity to stimulate specific T cell proliferation in vitro; however, their in vivo immunogenicity was comparable. This might be explained by a fact that B cells stably expressing antigen serve to a large degree as a source of antigen for cross-presentation by endogenous APCs; therefore, the type of in vitro stimulation preceding administration is less important for the resulting immunogenicity.
A significant advantage of B cells as APCs in vivo is that they spontaneously home to organized lymphoid tissue, the optimal site of induction of immune responses. The lymph node microenvironment enhances the likelihood that antigen-loaded APCs will encounter naïve and memory CD4+ and CD8+ T cells while APCs in peripheral tissues are more likely to encounter effector memory T cells. Migration from the bloodstream into lymph nodes occurs through a multistep adhesion cascade regulated by tissue-specific chemokines and adhesion receptors facilitating the capture and arrest of lymphocytes from flowing blood 61. B lymphocytes express CD62L, LFA-1, and CCR7 molecules that play a crucial role in the entering of lymph nodes from blood by extravasation through high endothelial venules (HEVs) expressing peripheral node addressin, CCL21, and inter-cellular adhesion molecule-1 (ICAM-1) 62, 63. CD40-activated B cells express surface CCR7 and CXCR4 and decreased levels of CCR5 and CCR6 64. This pattern of expression suggests that CD-40 activated B cells should enter the T cell rich areas of secondary lymphoid organs rather than the germinal centers 62. The first step in entering the lymph node is the tethering and rolling of naïve lymphocytes on the surface of HEVs of peripheral lymph nodes mediated by CD62L 63, 65, 66. Here we show that the capacity of B cells to home to secondary lymphoid tissue is diminished following activation through TLR-9 and CD40 likely due to the shedding of CD62L from the cell surface. Decreased homing to secondary lymphoid organs, the primary site of induction of T cell responses, may be in part responsible for the lack of any advantage of CpG/CD40-activated B cells compared to B cells stimulated with any agent alone in CTL induction, despite their superior antigen-presenting capacity in vitro. The possibility of enhancing the antigen-presenting ability of B cells by various genetic modifications has been addressed by several investigators. Palena et al. has demonstrated that infection of CD40-activated B cells with fowlpox recombinant vector expressing a triad of co-stimulatory molecules, B7-1, ICAM-1, and LFA-3, significantly improves their function as APCs 19. Similarly, Biagi et al. have shown that molecular transfer of CD40 and OX40 ligands to leukemic B cells enhances their ability to expand tumor-reactive cytotoxic T cells 67. Transduction of a chimeric E/L selectin expressing the ectodomain of CD62L into mature DCs has been shown to enhance homing of DCs to peripheral lymph nodes 68. Here we show that the expression of sheddase-resistant mutant of CD62L results in an increased migration of activated B cells to the peripheral and mesenteric lymph nodes. Such modifications, possibly in combination with over-expression of CCR7 receptor targeting B cells to the B / T zone boundary in organized lymphoid tissue 62, should further enhance the immunogenicity of B cell-based vaccines.
In summary, immunization with activated autologous B cells presenting defined tumor antigens in combination with other therapies that offset the immunosuppressive environment created by the tumor represent a promising alternative strategy for the immunotherapy and prevention of cancer. Thanks to their availability, activated B cells can be used to test whether repetitive immunization over extended periods of time can overcome tumor tolerance and sustain long-term maintenance of functional anti-tumor T cell responses.
6. Materials and methods
Materials
OVA-1 (OVA257-264, SIINFEKL) and OVA-2 (OVA323-339, ISQAVHAAHAEINEAGR) peptides were synthesized by Genemed Synthesis (South Francisco, CA), CpG oligonucleotide (ODN 1668; TCCATGACGTTCCTGATGCT) by Invitrogen (Carlsbad, CA). Bacterial lipopolysaccharide (LPS) was purchased from Sigma (St Louis, Missouri). Anti-CCR7 antibody was purchased from Biolegend (San Diego, CA); all other antibodies from BD Pharmingen (San Diego, CA),
Mice
C57BL/6 (B6), B6/SJL, OVA-specific TCR transgenic OT1 and OT2, OVA-transgenic mice expressing chicken OVA under the control of chicken β-actin promoter/CMV immediate-early enhancer (# 005145), and selectin-knockout mice (Sel-l,p,e-/-, # 003817) were obtained from the Jackson Laboratory (Bar Harbor, Maine; all mice on B6 background). All experimental procedures were approved by the Institutional Animal Care and Use Committee.
B cell and DC purification and stimulation
B cells were purified from the spleen and lymph node cells by negative selection for CD43- cells on MACS column (Milteneyi Biotec, Auburn, CA) according to manufacturer’s directions resulting in 96-98 % purity of untouched CD19+ cells. In some experiments, B cells were further purified by negative selection of CD11c+ cells or by positive selection for CD19+ cells using MACS columns. B cells were stimulated as indicated by co-incubation with CpG (10 μM), activating anti-CD40 (HM40-3) or anti-Ig (187.1) antibodies (5 μg/ml), or LPS (10 μg/ml) at 2 × 106 cells/ml in complete RPMI medium (RPMI-1640, 10 % FBS, 100 U penicillin, 100 U streptomycin, 50 μM 2-mercaptoethanol; Invitrogen, Carlsbad, CA). Bone marrow-derived dendritic cells were prepared from harvested bone marrow cells by 8 day incubation in the presence of 20 ng/ml GM-CSF (R&D systems, Minneapolis, MN) as described 69 and either unstimulated or stimulated with LPS (10 μg/ml) for 20 hrs prior use. Flow cytometry was performed according to manufacturer’s directions (BD).
T cell proliferation assay in vitro
OVA-specific TCR transgenic T lymphocytes were purified from spleens and lymph nodes of OT-1 or OT-2 mice using Pan-T cell kit (Miltenyi). Fresh unstimulated B cells or B cells stimulated for 20 hrs with indicated agents were coated for 2 hrs with 10 μM of OVA-1 and OVA-2 peptides in complete RPMI medium and irradiated at 2500 rads. 5×104 OT-1 or OT-2 T cells were co-incubated with sequential dilutions of B cells for 72 h. T cell proliferation was determined by [3H]-thymidine incorporation 69, 70.
Cytokine production in vitro
B cells were incubated for 24 hrs at 2×106/ml in complete RPMI medium in the presence or absence of stimulators. In mixed cultures, 2.5×104 irradiated B cells were co-incubated with 5×104 OT-1 or OT-2 T cells in 200 μl complete medium for 72 hrs. Concentration of cytokines was determined using mouse IL-10, 12(p70), IL-4, and IFN-γ ELISA sets (BD).
T cell proliferation in vivo
OT-1 and OT-2 T cells were labeled with 5 μM 5-(6)-carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR). 106 OT-1 and OT-2 cells were injected IV into B6.SJL (CD45.1+) mice. 24 hrs later, 106 B6-derived B cells unstimulated or stimulated with indicated agents for 20 hrs were pulsed for 2 hrs with OVA-1 and OVA-2 peptides (10 μM) and injected into recipients. Three days later, CFSE dilution in CD45.2+CD8+ (OT-1) or CD45.2+CD4+ (OT-2) cells isolated from spleen and LNs of recipient mice was analyzed by flow cytometry.
Immunization and intracellular cytokine staining (ICS)
Mice were immunized by intravenous (tail vein) injection of indicated numbers of unstimulated or stimulated OVA-transgenic B cells or B6-derived B cells pulsed for 2 hrs with cognate OVA-1 and OVA-2 peptides (10 μM) in 200 μl of RPMI. To detect OVA-specific T cells, splenocytes or PBMCs were incubated with OVA-1-specific pentamer (ProImmune, Springfiled, VA) and CD8 antibodies 70. For ICS, 106 splenocytes or LN cells were incubated with OVA-1 and OVA-2 peptides (10 μM) and stained intracellularly with IFN-γ-FITC antibody as described 69, 70.
For immunization with transfected B cells, CpG/CD40-stimulated B cells were co-nucleofected with pCMV-1 plasmids (Gene Therapy Systems, San Diego, CA) encoding EGFP and OVA under the control of a CMV promoter (2 μg of each plasmid / 2×106 cells). Control sample was nucleofected with phCMV1-EGFP and empty phCMV1 plasmid. Nucleofection was done using a Nucleofector II device (Amaxa, Cologne, Germany) according to a protocol optimized for primary mouse B cells (mouse macrophage buffer, Y-001 program). Transfection efficacy in activated cells ranged at 60-80%, viability ranged from 50-60%. 24 hrs post nucleofection, transfected B cells expressed equal or higher amount of OVA protein compared to OVA-transgenic cells, as determined by Western blot analysis. For immunization, viable EGFP+ cells were sorted 24 hrs post nucleofection and 5×104 cells were injected intravenously.
In vivo cytotoxicity assay
9 days following immunization, mice were injected with 107 autologous splenocytes labeled with 0.35 μM CFSE (control) and 107 splenocytes labeled with 3.5 μM CFSE and pulsed with 10 μM specific peptide for 2 hrs (target population). The spleens and LNs were harvested 20 hrs later and the relative killing of target cells was determined as the ratio between the target and control populations standardized against non-immunized controls.
B cell homing assays
3×106 B cells labeled with 5μM CFSE were injected intravenously into recipients and 4 hours later spleens, peripheral blood, peripheral lymph nodes, mesenteric lymph nodes and Peyer’s Patches were harvested. The frequency of CFSE+ CD19+ positive cells was determined by flow cytometry. To investigate the role of CD62L in B cell homing, a CD62L-SR (sheddase-resistant) mutant was designed in which the cleavage is inhibited by a substitution of the membrane-proximal region of CD62L with that of P-selectin by PCR-based mutagenesis (Figure 6C) 35. CpG/CD40-stimulated B cells were co-nucleofected with plasmids encoding EGFP and CD62L or CD62L-SR under the control of CMV promoter. Nucleofection was performed on a Nucleofector II. Transfection efficacy in activated cells ranged at 60-80%, viability was about 50-60%. For homing assay, EGFP+ cells were sorted, co-labeled with 5 μM CFSE, and 5×105 cells were injected intravenously.
Tumor protection assay
Mice were inoculated subcutaneously in left flank with 106 thymoma-derived EG-7 cells expressing OVA and 3 and 6 days later immunized intravenously with 105 fresh or activated OVA-transgenic B cells, OVA-transgenic bone marrow-derived DCs, or OVA-1 and OVA-2 peptide pulsed B cells. Tumor size was monitored every two days by measuring two perpendicular diameters and the tumor volume was w: (width)2 x length.
Statistical analysis
All reported P values are two-sided. Group comparisons were performed using the Mann-Whitney rank sum test. Correlations were performed using Spearman rank sum test. The SigmaStat (SPSS, Chicago, Illinois) and NCSS (NCSS, Kaysville, Utah) statistical software packages were used.
7. Acknowledgements
This work was supported by National Institutes of Health grant AI063967 and by internal funds from Department of Pathology at UAB.
Footnotes
This work was supported by National Institutes of Health grant AI063967.
- CTL
- cytotoxic T lymphocyte
- TLR
- toll like receptor
- CpG/CD40-B cells
- B cells activated by CpG and anti-CD40 antibody
- TAA
- tumor-associated antigen
- LPS
- bacterial lipopolysaccharide
- CFSE
- 5-(6)-Carboxyfluorescein diacetate succinimidyl ester
- ICS
- intracellular cytokine staining
- PLN and MLN
- peripheral and mesenteric lymph node
- PP
- Peyer’s patch
- CD62L-SR
- sheddase-resistant mutant of CD62L
- ICAM-1
- inter-cellular adhesion molecule-1
- HEV
- high endothelial venule
8. Conflict-of-interest disclosure: The authors declare no competing financial interests.
9. References
- 1.Palucka AK, Ueno H, Fay JW, Banchereau J. Taming cancer by inducing immunity via dendritic cells. Immunol Rev. 2007;220:129–150. doi: 10.1111/j.1600-065X.2007.00575.x. [DOI] [PubMed] [Google Scholar]
- 2.Ridgway D. The first 1000 dendritic cell vaccinees. Cancer Invest. 2003;21:873–886. doi: 10.1081/cnv-120025091. [DOI] [PubMed] [Google Scholar]
- 3.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 4.Vulink A, Radford KJ, Melief C, Hart DN. Dendritic cells in cancer immunotherapy. Adv Cancer Res. 2008;99:363–407. doi: 10.1016/S0065-230X(07)99006-5. [DOI] [PubMed] [Google Scholar]
- 5.Schultze JL, Grabbe S, Bergwelt-Baildon MS. DCs and CD40-activated B cells: current and future avenues to cellular cancer immunotherapy. Trends Immunol. 2004;25:659–664. doi: 10.1016/j.it.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Coughlin CM, Vance BA, Grupp SA, Vonderheide RH. RNA-transfected CD40-activated B cells induce functional T-cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood. 2004;103:2046–2054. doi: 10.1182/blood-2003-07-2379. [DOI] [PubMed] [Google Scholar]
- 7.Geiger JD, Hutchinson RJ, Hohenkirk LF, McKenna EA, Yanik GA, Levine JE, et al. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res. 2001;61:8513–8519. [PubMed] [Google Scholar]
- 8.Dagher R, Long LM, Read EJ, Leitman SF, Carter CS, Tsokos M, et al. Pilot trial of tumor-specific peptide vaccination and continuous infusion interleukin-2 in patients with recurrent Ewing sarcoma and alveolar rhabdomyosarcoma: an inter-institute NIH study. Med Pediatr Oncol. 2002;38:158–164. doi: 10.1002/mpo.1303. [DOI] [PubMed] [Google Scholar]
- 9.Shilyansky J, Jacobs P, Doffek K, Sugg SL. Induction of cytolytic T lymphocytes against pediatric solid tumors in vitro using autologous dendritic cells pulsed with necrotic primary tumor. J Pediatr Surg. 2007;42:54–61. doi: 10.1016/j.jpedsurg.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Morse MA, Coleman RE, Akabani G, Niehaus N, Coleman D, Lyerly HK. Migration of human dendritic cells after injection in patients with metastatic malignancies. Cancer Res. 1999;59:56–58. [PubMed] [Google Scholar]
- 11.de Vries IJ, Lesterhuis WJ, Barentsz JO, Verdijk P, van Krieken JH, Boerman OC, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol. 2005;23:1407–1413. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- 12.Hel Z. Cancer immunotherapy with activated B cells. In: Hemorhat PL, editor. Cancer and gene therapy. 1st ed. Transworld Research Network; 2007. pp. 139–153. [Google Scholar]
- 13.Lapointe R, Bellemare-Pelletier A, Housseau F, Thibodeau J, Hwu P. CD40-stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Cancer Res. 2003;63:2836–2843. [PubMed] [Google Scholar]
- 14.Schultze JL, Michalak S, Seamon MJ, Dranoff G, Jung K, Daley J, et al. CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest. 1997;100:2757–2765. doi: 10.1172/JCI119822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergwelt-Baildon MS, Vonderheide RH, Maecker B, Hirano N, Anderson KS, Butler MO, et al. Human primary and memory cytotoxic T lymphocyte responses are efficiently induced by means of CD40-activated B cells as antigen-presenting cells: potential for clinical application. Blood. 2002;99:3319–3325. doi: 10.1182/blood.v99.9.3319. [DOI] [PubMed] [Google Scholar]
- 16.Kondo E, Topp MS, Kiem HP, Obata Y, Morishima Y, Kuzushima K, et al. Efficient generation of antigen-specific cytotoxic T cells using retrovirally transduced CD40-activated B cells. J Immunol. 2002;169:2164–2171. doi: 10.4049/jimmunol.169.4.2164. [DOI] [PubMed] [Google Scholar]
- 17.Gerloni M, Rizzi M, Castiglioni P, Zanetti M. T cell immunity using transgenic B lymphocytes. Proc Natl Acad Sci U S A. 2004;101:3892–3897. doi: 10.1073/pnas.0400138101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heit A, Huster KM, Schmitz F, Schiemann M, Busch DH, Wagner H. CpG-DNA aided cross-priming by cross-presenting B cells. J Immunol. 2004;172:1501–1507. doi: 10.4049/jimmunol.172.3.1501. [DOI] [PubMed] [Google Scholar]
- 19.Palena C, Zhu M, Schlom J, Tsang KY. Human B cells that hyperexpress a triad of costimulatory molecules via avipox-vector infection: an alternative source of efficient antigen-presenting cells. Blood. 2004;104:192–199. doi: 10.1182/blood-2003-09-3211. [DOI] [PubMed] [Google Scholar]
- 20.Zanetti M, Castiglioni P, Rizzi M, Wheeler M, Gerloni M. B lymphocytes as antigen-presenting cell-based genetic vaccines. Immunol Rev. 2004;199:264–278. doi: 10.1111/j.0105-2896.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- 21.Zentz C, Wiesner M, Man S, Frankenberger B, Wollenberg B, Hillemanns P, et al. Activated B cells mediate efficient expansion of rare antigen-specific T cells. Hum Immunol. 2007;68:75–85. doi: 10.1016/j.humimm.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Ahmadi T, Flies A, Efebera Y, Sherr DH. CD40 Ligand-activated, antigen-specific B cells are comparable to mature dendritic cells in presenting protein antigens and major histocompatibility complex class I- and class II-binding peptides. Immunology. 2008;124:129–140. doi: 10.1111/j.1365-2567.2007.02749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritchie DS, Yang J, Hermans IF, Ronchese F. B-Lymphocytes activated by CD40 ligand induce an antigen-specific anti-tumour immune response by direct and indirect activation of CD8(+) T-cells. Scand J Immunol. 2004;60:543–551. doi: 10.1111/j.0300-9475.2004.01517.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee J, Dollins CM, Boczkowski D, Sullenger BA, Nair S. Activated B cells modified by electroporation of multiple mRNAs encoding immune stimulatory molecules are comparable to mature dendritic cells in inducing in vitro antigen-specific T-cell responses. Immunology. 2008 doi: 10.1111/j.1365-2567.2008.02833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watt V, Ronchese F, Ritchie D. Resting B cells suppress tumor immunity via an MHC class-II dependent mechanism. J Immunother. 2007;30:323–332. doi: 10.1097/CJI.0b013e31802bd9c8. [DOI] [PubMed] [Google Scholar]
- 26.Wagner M, Poeck H, Jahrsdoerfer B, Rothenfusser S, Prell D, Bohle B, et al. IL-12p70-dependent Th1 induction by human B cells requires combined activation with CD40 ligand and CpG DNA. J Immunol. 2004;172:954–963. doi: 10.4049/jimmunol.172.2.954. [DOI] [PubMed] [Google Scholar]
- 27.Shirota H, Sano K, Hirasawa N, Terui T, Ohuchi K, Hattori T, et al. B cells capturing antigen conjugated with CpG oligodeoxynucleotides induce Th1 cells by elaborating IL-12. J Immunol. 2002;169:787–794. doi: 10.4049/jimmunol.169.2.787. [DOI] [PubMed] [Google Scholar]
- 28.Schultze JL, Michalak S, Lowne J, Wong A, Gilleece MH, Gribben JG, et al. Human non-germinal center B cell interleukin (IL)-12 production is primarily regulated by T cell signals CD40 ligand, interferon gamma, and IL-10: role of B cells in the maintenance of T cell responses. J Exp Med. 1999;189:1–12. doi: 10.1084/jem.189.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dullaers M, Breckpot K, Van Meirvenne S, Bonehill A, Tuyaerts S, Michiels A, et al. Side-by-side comparison of lentivirally transduced and mRNA-electroporated dendritic cells: implications for cancer immunotherapy protocols. Mol Ther. 2004;10:768–779. doi: 10.1016/j.ymthe.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 30.Valenzuela J, Schmidt C, Mescher M. The roles of IL-12 in providing a third signal for clonal expansion of naive CD8 T cells. J Immunol. 2002;169:6842–6849. doi: 10.4049/jimmunol.169.12.6842. [DOI] [PubMed] [Google Scholar]
- 31.Chang J, Cho JH, Lee SW, Choi SY, Ha SJ, Sung YC. IL-12 priming during in vitro antigenic stimulation changes properties of CD8 T cells and increases generation of effector and memory cells. J Immunol. 2004;172:2818–2826. doi: 10.4049/jimmunol.172.5.2818. [DOI] [PubMed] [Google Scholar]
- 32.Henry CJ, Ornelles DA, Mitchell LM, Brzoza-Lewis KL, Hiltbold EM. IL-12 produced by dendritic cells augments CD8+ T cell activation through the production of the chemokines CCL1 and CCL17. J Immunol. 2008;181:8576–8584. doi: 10.4049/jimmunol.181.12.8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He Y, Zhang J, Mi Z, Robbins P, Falo LD., Jr. Immunization with lentiviral vector-transduced dendritic cells induces strong and long-lasting T cell responses and therapeutic immunity. J Immunol. 2005;174:3808–3817. doi: 10.4049/jimmunol.174.6.3808. [DOI] [PubMed] [Google Scholar]
- 34.Zhao LC, Edgar JB, Dailey MO. Characterization of the rapid proteolytic shedding of murine L-selectin. Dev Immunol. 2001;8:267–277. doi: 10.1155/2001/91831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galkina E, Tanousis K, Preece G, Tolaini M, Kioussis D, Florey O, et al. L-selectin shedding does not regulate constitutive T cell trafficking but controls the migration pathways of antigen-activated T lymphocytes. J Exp Med. 2003;198:1323–1335. doi: 10.1084/jem.20030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 37.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 38.Breckpot K, Aerts JL, Thielemans K. Lentiviral vectors for cancer immunotherapy: transforming infectious particles into therapeutics. Gene Ther. 2007;14:847–862. doi: 10.1038/sj.gt.3302947. [DOI] [PubMed] [Google Scholar]
- 39.Russo V, Cipponi A, Raccosta L, Rainelli C, Fontana R, Maggioni D, et al. Lymphocytes genetically modified to express tumor antigens target DCs in vivo and induce antitumor immunity. J Clin Invest. 2007;117:3087–3096. doi: 10.1172/JCI30605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hackett PB, Ekker SC, Largaespada DA, McIvor RS. Sleeping beauty transposon-mediated gene therapy for prolonged expression. Adv Genet. 2005;54:189–232. doi: 10.1016/S0065-2660(05)54009-4. [DOI] [PubMed] [Google Scholar]
- 41.Amoscato AA, Prenovitz DA, Lotze MT. Rapid extracellular degradation of synthetic class I peptides by human dendritic cells. J Immunol. 1998;161:4023–4032. [PubMed] [Google Scholar]
- 42.Zehn D, Cohen CJ, Reiter Y, Walden P. Extended presentation of specific MHC-peptide complexes by mature dendritic cells compared to other types of antigen-presenting cells. Eur J Immunol. 2004;34:1551–1560. doi: 10.1002/eji.200324355. [DOI] [PubMed] [Google Scholar]
- 43.Shen L, Rock KL. Cellular protein is the source of cross-priming antigen in vivo. Proc Natl Acad Sci U S A. 2004;101:3035–3040. doi: 10.1073/pnas.0308345101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stock AT, Mueller SN, van Lint AL, Heath WR, Carbone FR. Cutting edge: prolonged antigen presentation after herpes simplex virus-1 skin infection. J Immunol. 2004;173:2241–2244. doi: 10.4049/jimmunol.173.4.2241. [DOI] [PubMed] [Google Scholar]
- 45.Curtsinger JM, Johnson CM, Mescher MF. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J Immunol. 2003;171:5165–5171. doi: 10.4049/jimmunol.171.10.5165. [DOI] [PubMed] [Google Scholar]
- 46.Prlic M, Hernandez-Hoyos G, Bevan MJ. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J Exp Med. 2006;203:2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams MA, Bevan MJ. Shortening the infectious period does not alter expansion of CD8 T cells but diminishes their capacity to differentiate into memory cells. J Immunol. 2004;173:6694–6702. doi: 10.4049/jimmunol.173.11.6694. [DOI] [PubMed] [Google Scholar]
- 48.Bennett SR, Carbone FR, Toy T, Miller JF, Heath WR. B cells directly tolerize CD8(+) T cells. J Exp Med. 1998;188:1977–1983. doi: 10.1084/jem.188.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qin Z, Richter G, Schuler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat Med. 1998;4:627–630. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- 50.Reichardt P, Dornbach B, Rong S, Beissert S, Gueler F, Loser K, et al. Naive B cells generate regulatory T cells in the presence of a mature immunologic synapse. Blood. 2007;110:1519–1529. doi: 10.1182/blood-2006-10-053793. [DOI] [PubMed] [Google Scholar]
- 51.Evans DE, Munks MW, Purkerson JM, Parker DC. Resting B lymphocytes as APC for naive T lymphocytes: dependence on CD40 ligand/CD40. J Immunol. 2000;164:688–697. doi: 10.4049/jimmunol.164.2.688. [DOI] [PubMed] [Google Scholar]
- 52.Constant S, Schweitzer N, West J, Ranney P, Bottomly K. B lymphocytes can be competent antigen-presenting cells for priming CD4+ T cells to protein antigens in vivo. J Immunol. 1995;155:3734–3741. [PubMed] [Google Scholar]
- 53.Constant SL. B lymphocytes as antigen-presenting cells for CD4+ T cell priming in vivo. J Immunol. 1999;162:5695–5703. [PubMed] [Google Scholar]
- 54.Jaiswal AI, Croft M. CD40 ligand induction on T cell subsets by peptide-presenting B cells: implications for development of the primary T and B cell response. J Immunol. 1997;159:2282–2291. [PubMed] [Google Scholar]
- 55.Peng SL. Signaling in B cells via Toll-like receptors. Curr Opin Immunol. 2005;17:230–236. doi: 10.1016/j.coi.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 56.Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4504. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 57.Jiang W, Lederman MM, Harding CV, Rodriguez B, Mohner RJ, Sieg SF. TLR9 stimulation drives naive B cells to proliferate and to attain enhanced antigen presenting function. Eur J Immunol. 2007;37:2205–2213. doi: 10.1002/eji.200636984. [DOI] [PubMed] [Google Scholar]
- 58.Harshyne LA, Watkins SC, Gambotto A, Barratt-Boyes SM. Dendritic cells acquire antigens from live cells for cross-presentation to CTL. J Immunol. 2001;166:3717–3723. doi: 10.4049/jimmunol.166.6.3717. [DOI] [PubMed] [Google Scholar]
- 59.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 60.Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 61.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 62.Reif K, Ekland EH, Ohl L, Nakano H, Lipp M, Forster R, et al. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 2002;416:94–99. doi: 10.1038/416094a. [DOI] [PubMed] [Google Scholar]
- 63.Weninger W, Crowley MA, Manjunath N, von Andrian UH. Migratory properties of naive, effector, and memory CD8(+) T cells. J Exp Med. 2001;194:953–966. doi: 10.1084/jem.194.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bergwelt-Baildon M, Shimabukuro-Vornhagen A, Popov A, Klein-Gonzalez N, Fiore F, Debey S, et al. CD40-activated B cells express full lymph node homing triad and induce T-cell chemotaxis: potential as cellular adjuvants. Blood. 2006;107:2786–2789. doi: 10.1182/blood-2004-01-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- 66.Tang ML, Steeber DA, Zhang XQ, Tedder TF. Intrinsic differences in L-selectin expression levels affect T and B lymphocyte subset-specific recirculation pathways. J Immunol. 1998;160:5113–5121. [PubMed] [Google Scholar]
- 67.Biagi E, Dotti G, Yvon E, Lee E, Pule M, Vigouroux S, et al. Molecular transfer of CD40 and OX40 ligands to leukemic human B cells induces expansion of autologous tumor-reactive cytotoxic T lymphocytes. Blood. 2005;105:2436–2442. doi: 10.1182/blood-2004-07-2556. [DOI] [PubMed] [Google Scholar]
- 68.Robert C, Klein C, Cheng G, Kogan A, Mulligan RC, von Andrian UH, et al. Gene therapy to target dendritic cells from blood to lymph nodes. Gene Ther. 2003;10:1479–1486. doi: 10.1038/sj.gt.3302008. [DOI] [PubMed] [Google Scholar]
- 69.Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W. Current protocols in immunology. John Wiley & Sons, Inc.; 1991-2008. [Google Scholar]
- 70.Hel Z, Nacsa J, Tryniszewska E, Tsai WP, Parks RW, Montefiori DC, et al. Containment of simian immunodeficiency virus infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and postchallenge CD4+ and CD8+ T cell responses. J Immunol. 2002;169:4778–4787. doi: 10.4049/jimmunol.169.9.4778. [DOI] [PubMed] [Google Scholar]