Abstract
Inflammation is linked clinically and epidemiologically to cancer, and NF-κB appears to play a causative role, but the mechanisms are poorly understood. We show that transient activation of Src oncoprotein can mediate an epigenetic switch from immortalized breast cells to a stably transformed line that forms self-renewing mammospheres that contain cancer stem cells. Src activation triggers an inflammatory response mediated by NF-κB that directly activates Lin28 transcription and rapidly reduces let-7 microRNA levels. Let-7 directly inhibits IL6 expression, resulting in higher levels of IL6 than achieved by NF-κB activation. IL6-mediated activation of the STAT3 transcription factor is necessary for transformation, and IL6 activates NF-κB, thereby completing a positive feedback loop. This regulatory circuit operates in other cancer cells lines, and its transcriptional signature is found in human cancer tissues. Thus, inflammation activates a positive feedback loop that that maintains the epigenetic transformed state for many generations in the absence of the inducing signal.
Keywords: NF-κB, let-7, Lin 28, IL6, inflammation, cancer, cellular transformation, epigenetics, positive feedback loop
INTRODUCTION
Tumorigenesis is a multistep process that requires constitutive cell division, growth, and survival. The molecular events governing the onset and progression of malignant transformation involve the inactivation of tumor suppressor genes and the acquisition of oncogenic mutations (Hahn and Weinberg, 2002; Vogelstein and Kinzler, 2004). These genetic perturbations help cancer cells override the normal mechanisms controlling cellular proliferation. In addition to genetic changes, tumor suppressor genes can be inactivated by epigenetic silencing through DNA methylation (Baylin, 2005), and this can occur through an elaborate pathway triggered by an oncoprotein (Gazin et al., 2007). Lastly, the progression from normal cells to cancer is strongly influenced by environmental conditions and extracellular signaling pathways that affect the activity of tumor suppressors and oncoproteins.
Clinical and epidemiological studies have suggested a strong association between inflammation and different types of cancer, and inflammatory molecules can provide growth signals that promote the proliferation of malignant cells (Balkwill and Mantovani, 2001; Naugler and Karin, 2008; Pierce et al., 2009). For example, interleukin-6 (IL6) is up-regulated in epithelial cancers such as breast and prostate (Sasser et al., 2007; Wegiel et al., 2008). NF-κB, a transcription factor that regulates the expression of anti-apoptotic genes and activates different pro-inflammatory cytokines and chemokines, seems to be a key molecular link between inflammation and oncogenesis initiation and progression (Naugler and Karin, 2008). Constitutively active NF-κB occurs in many types of cancer, and mouse models provide genetic and biochemical evidence for a causative role of NF-κB in malignant conversion and progression (Luedde et al., 2007; Sakurai et al., 2008). However, the mechanistic linkage between inflammation and cancer remain to be elucidated.
microRNAs play critical roles in many biological processes including cancer by directly interacting with specific mRNAs through base pairing and then inhibiting expression of the target genes through a variety of molecular mechanisms (Bartel, 2009; Ventura and Jacks, 2009). MicroRNAs can undergo aberrant regulation during carcinogenesis (Lu et al., 2005), and they can act as oncogenes or tumor suppressor genes. For example, Let-7 is an important microRNA family consisting of 12 members located in genomic locations frequently deleted in human cancers (Calin et al., 2004). In addition, expression of let-7 RNAs is reduced in non small cell lung cancer patients and associated with poor prognosis (Johnson et al., 2005), and let-7 overexpression substantially reduces tumor burden in a K-Ras murine lung cancer model (Kumar et al., 2008). Although microRNAs are important for cancer development and progression, there is limited understanding of their roles in the molecular pathways and regulatory circuits that are involved in the process of cellular transformation.
Epigenetic inheritance is a phenomenon in which cellular phenotypes and gene expression patterns are faithfully transmitted through multiple generations via a mechanism that involves something beyond DNA sequence. The term was invented to explain how the same genome can give rise to phenotypically different cell types. An epigenetic switch occurs when a stable cell type changes to another stable cell type without any change in DNA sequence. Epigenetic switches require an initiating event (which could be a specific molecular process or stochastic fluctuation), but the phenotypes of the new cell type are inherited in the absence of the initiating signal. Epigenetic switches occur in prokaryotes, and hence in the absence of chromatin, although chromatin often plays an important role in eukaryotes. As illuminated by classic studies of an epigenetic switch in bacteriophage λ (Ptashne, 2009), positive feedback loops involving transcriptional regulatory proteins are the fundamental principle for epigenetic inheritance of gene expression patterns.
Here, we show that transient activation of Src causes an epigenetic switch in which non-transformed MCF10A cells are converted into mammospheres that can be propagated for many generations in the absence of the initiating signal. This epigenetic switch is activated by an inflammatory signal, and epigenetic inheritance is mediated by a positive feedback loop involving the NF-κB transcription factor, the microRNA processing factor Lin28, Let-7 microRNA, and interleukin 6. This regulatory circuit links inflammation to cellular transformation, and it appears to be important for other forms of cancer.
RESULTS
Transient activation of Src causes an epigenetic switch from normal to transformed cells
We described an experimental model of oncogenesis involving a derivative of MCF10A, a spontaneously immortalized cell line derived from normal mammary epithelial cells (Soule et al., 1990), that contains ER-Src, a fusion of the Src kinase oncoprotein (v-Src) and the ligand binding domain of the estrogen receptor (Hirsch et al., 2009)(Figure S1). Treatment of these cells with tamoxifen (TAM) for 36h results in phenotypic transformation, formation of multiple foci, the ability to form colonies in soft agar, increased motility and invasive ability, and tumor formation upon injection in nude mice. This model permits the opportunity to kinetically follow the pathway of cellular transformation in a manner similar to that used to study viral infection and other temporally ordered processes
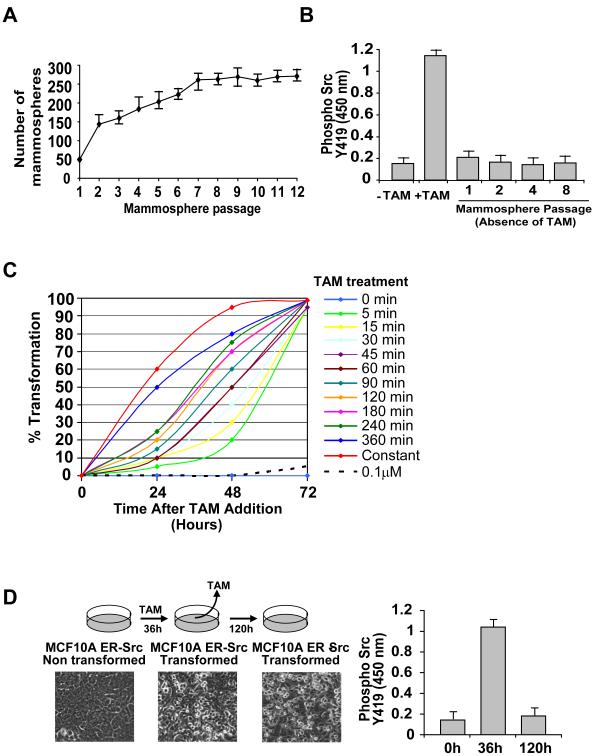
Mammospheres are multicellular structures enriched in “cancer stem cells” (also called tumor initiating cells) that form when transformed cells are placed in non-adherent and non-differentiating conditions (Liao et al., 2007; Grimshaw et al., 2008). Transformed ER-Src cells have mammosphere formation ability, whereas the untransformed cells do not. Strikingly, mammospheres derived from ER-Src transformed cells can be passaged in vitro for twelve generations in the absence of TAM, with the number of mammospheres increasing upon passage (Figure 1A). As expected from the absence of TAM, the passaged mammospheres do not contain activated Src (assayed by phosphorylation of Y419), unlike the initially transformed cells in the presence of TAM (Figure 1B).
Figure 1.
Epigenetic switch from untransformed to a transformed phenotype
(A) Number of mammosphere formed/1000 seeded transformed (TAM-treated for 36h; mean ± SD) ER-Src cells during 12 serial passages.
(B) Levels of Src-Y419 phosphorylation (ELISA assay; mean ± SD) in untreated, and TAM-treated (36h), and 1st, 2nd, 4th, and 8th generation mammospheres derived from TAM-treated ER-Src cells.
(C) Kinetics of cellular transformation as a function of TAM exposure. ER-Src cells were treated with 1 μM TAM for the indicated times, and then analyzed for cellular transformation (defined by morphological changes) 24, 48 and 72h post TAM treatment.
(D) Phase-contrast images and Src-Y419 phosphorylation levels (ELISA assay; mean ± SD) in ER-Src cells 36 and 120h post TAM treatment. Cells were treated with TAM for 36h and then TAM was removed from the medium.
To further characterize the switch between non-transformed and transformed cells, we varied the time of TAM treatment. Remarkably, TAM treatment for only 5 minutes results in transformation, although the process is slower (72h as opposed to 36h; Figures 1C, S2A). Furthermore, increasing the time of TAM treatment progressively reduces the time necessary for the transformed phenotype. The transformation that occurs upon very short TAM treatment is not due to residual TAM, because 10-fold lower TAM does are unable to induce transformation (Figure S2B), and transformed cells maintained in the absence of TAM lack activated Src yet retain the transformed phenotype (Figure 1D).
Collectively, these results demonstrate that transient activation of Src causes an epigenetic switch from a stable non-transformed cell line to a transformed state capable of forming self-renewing mammospheres. In accord with the classical definition of an epigenetic switch, both the initial and the final states are stably inherited, and the inducing signal necessary for the switch is no longer present in the transformed cells.
Src activation triggers a rapid inflammatory response that is mediated by NF-κB and required for transformation
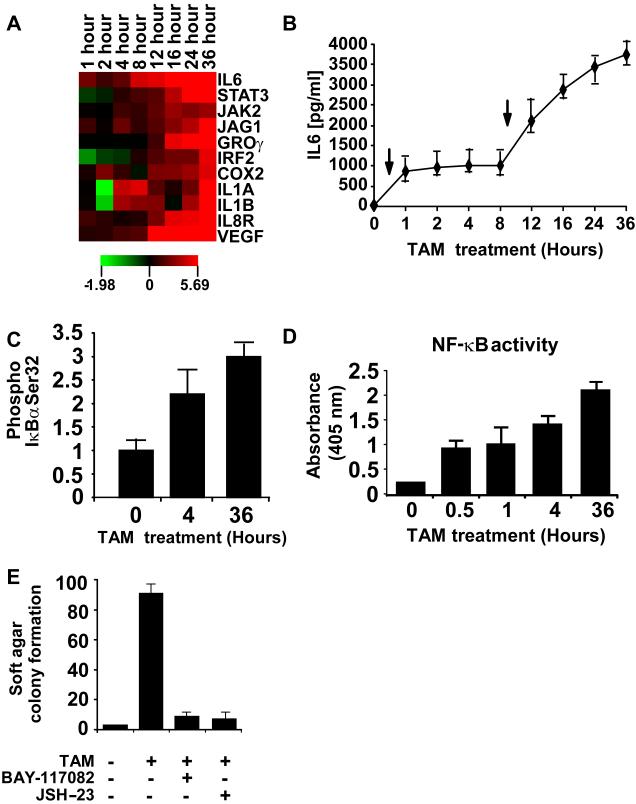
As will be described elsewhere, mRNA profiling during cellular transformation revealed activation of an inflammatory gene signature. The initial phase of this inflammatory response occurs very rapidly upon Src activation, and it expands qualitatively (more genes affected) and quantitatively (higher induction levels) during transformation (Figure 2A). In particular, IL6 is up-regulated very early (1h post TAM treatment) and highly in comparison to other inflammation-related genes. There is a second boost of IL6 expression (8h post TAM treatment), suggesting a biphasic regulation of IL6 expression (Figure 2B). Thus, activation of IL6 is an early step in the transformation process, whereas other steps (e.g. activation of STAT3) occur later.
Figure 2.
Src induces an inflammatory response mediated by NF-κB
(A) Heatmap representation of RNA levels for the indicted inflammatory genes at the indicated time points after TAM induction.
(B) IL6 levels (ELISA assay; mean ± SD)) at the indicated times after TAM treatment. The black arrows show the biphasic induction of IL6.
(C) Phosphorylation status of IκBα-serine 32 (ELISA assay) in cells treated with TAM for the indicated times.
(D) NF-κB/p65 ActivELISA assay (NF-κB nuclear localization) cells treated with TAM for the indicated times.
(E) Soft agar colony assay (mean ± SD) in untreated and TAM-treated cells in the presence or absence of NF-κB inhibitors (5 μM BAY-117082 and 6 μM JSH-23).
NF-κB is the critical transcription factor that mediates the inflammatory response, and it stimulates IL6 expression in response to inflammatory signals. NF-κB is activated through I-κBα phosphorylation (Figure 2C), translocates to the nucleus within 30 minutes of treatment with TAM and remains highly active until 36h post TAM treatment (Figure 2D). Furthermore, treatment of cells with inhibitors of NF-κB (5 μM BAY-117082 or 6 μM JSH-23) strongly inhibits Src-dependent transformation (Figure 2E) without affecting the growth of the non-transformed cells (Figure S3). Thus, Src activation triggers a rapid inflammatory response mediated by NF-κB that is critical for cellular transformation.
Let-7 is down-regulated at an early stage of cellular transformation by NF-κB activation of Lin28B, an inhibitor of microRNA processing
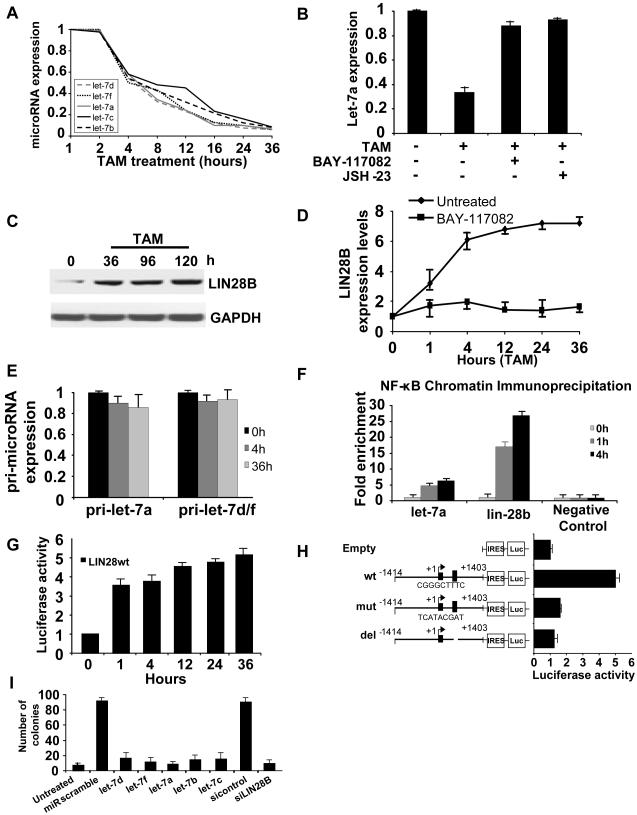
In work to be presented elsewhere, we analyzed the expression profile of 365 microRNAs at 8 different time points after TAM treatment. This analysis revealed 5 members of the let-7 family (let-7d, let-7f, let-7a, let-7b, let-7c) to be rapidly and strongly down-regulated, with a 2-fold reduction at the 4h time point followed by a continuous decrease such that these RNAs are reduced 20-fold 36h post-treatment (Figures 3A). The relatively short half-life (~4h) of let-7 RNAs suggests a regulated mechanism of RNA degradation.
Figure 3.
Let-7a is down-regulated during transformation by NF-κB.
(A) RNA levels of individual let-7 family members in cells treated with TAM for the indicated times.
(B) Levels of let-7a RNA in untreated and TAM-treated (36h) cells in the presence or absence of NF-κB inhibitor (5 μM BAY-117082 and 6 μM JSH-23).
(C) Lin28B protein levels (western blot) in ER-Src cells at the indicated times after TAM treatment.
(D) Lin28B mRNA levels (mean ± SD) during transformation in the presence or absence of an NF-κB inhibitor (5 μM BAY-117082).
(E) Levels of precursor RNAs (pri-let-7a and pri-let-7d/f) in untreated and TAM-treated cells.
(F) NF-κB occupancy (fold-enrichment) at the Lin-28B and let-7a loci as determined by chromatin immunoprecipitation of crosslinked cells that were or were not treated with TAM.
(G) Luciferase activity (mean ± SD)) of Lin28B vector containing the NF-κB binding site during transformation.
(H) Luciferase activity of Lin28B vector (wt, mutated or deleted in NF-κB site) in TAM-treated (36h) cells.
(I) Anchorage-independent growth assays (microscopic counting of 50 μm colonies) of untreated and TAM-treated cells transfected with the indicated let-7 family members or siRNA against Lin28B.
As TAM treatment results in rapid activation NF-κB and inhibition of let-7 expression, we considered the possibility that NF-κB inhibits let-7. In accord with this idea, inhibition of NF-κB (BAY-117082 or JSH-23) results in up-regulation of let-7a (Figure 3B). However, as NF-κB typically behaves as an activator protein, it seemed unlikely that it would directly inhibit let-7 expression. Instead, the hypothesized regulated mechanism of RNA degradation suggests the possibility that NF-κB activates an inhibitor of let-7 expression, and in this regard, let-7 is strongly inhibited by Lin28B at the transcriptional and post-transcriptional level (Viswanathan et al., 2008). Interestingly, Lin28B expression is rapidly induced upon TAM treatment (Figure 3C) in a manner dependent on NF-κB activity (Figure 3D). In addition, although mature let-7 RNA levels decrease rapidly and strongly during the cellular transformation process, levels of the primary let7a, c, and e RNAs are unaffected by (Figure 3E), suggesting that NF-κB has little if any direct effect on the rate of let-7 transcription.
Sequence analysis reveals a highly conserved NF-κB motif in the first intron of the lin-28B gene and moderately conserved NF-κB motifs ~4 kb upstream of the let-7a gene (Figures S4). As assayed by chromatin immunoprecipitation, NF-κB binds both the let-7 and Lin28B regions identified above, with binding to the highly conserved Lin28B site being stronger (Figure 3F). In accord with the kinetics of NF-κB activation, strong binding is observed 1h after TAM addition, but not in the absence of TAM. Moreover, a Lin28B genomic fragment including the highly conserved NF-κB site is sufficient to activate transcription of a luciferase reporter construct during the transformation process (Figure 3G) Similar constructs in which the NF-κB site was mutated or deleted do not support transcriptional activity (Figure 3H). These observations suggest that NF-κB directly activates Lin28B expression through a binding site in the first intron, and the increased levels of Lin28B inhibit let-7 expression through a post-transcriptional mechanism.
Inhibition of let-7 through activation of Lin28B is important for cellular transformation
To address whether the observed inhibition of let-7 microRNAs by Lin28B is important for cellular transformation, we examined the phenotypic consequences of modulating the expression of Lin28B and let-7 family members. First, inhibition of Lin28B expression by siRNA (Figure S5A) strongly reduces cellular transformation (Figure 3I). Second, in MCF-10A cells lacking the ER-Src construct, overexpression of Lin28B results in increased cell growth and motility, the ability to form colonies in soft agar and tumors in nude mice, reduced levels of let-7, and increased levels of IL6 (Figure S5B-G). Third, overexpression of individual let-7 microRNAs (let-7a, let-7b, let-7c, let-7d, and let-7f) strongly inhibits anchorage-independent growth in soft agar (Figure 3I), and it blocks the transformed morphology and formation of foci as well as the migratory and invasion activity of ER-Src transformed cells (Figure S6). Thus, Lin28B and its ability to rapidly inhibit let-7 microRNAs upon Src activation is a key early step that is important for cellular transformation. Moreover, as each let-7 family member acts as a suppressor of transformation, Lin28B is important to coordinately inhibit all the let-7 family members. Due to the redundancy between let-7 family members and because the slightly stronger effect of let-7a for inhibiting colony formation, migration and invasion activity, subsequent experiments have been performed with let-7a.
Let-7 microRNA directly inhibits expression of IL6
MicroRNAs exert their biological functions through suppression of target genes via RNA-RNA complementarity. Using three different selection criteria (Figure S7), we identified interleukin-6 (IL6) as a potential gene target of let-7. Moreover, the conservation between the microRNA and a putative target site in the 3′ UTR of the IL6 gene suggests the importance of this interaction during evolution (Figure 4A).
Figure 4.
Let-7a regulates IL6 expression during transformation
(A) Let-7a binding site in 3′UTR of IL6 with sequence complementarity and phylogenic conservation (yellow) of IL6 target sequence indicated.
(B) Luciferase assay using a reporter containing the 3′UTR of IL6 (wt or mutant in let-7a binding site) 24h after transfection with let-7a or a scrambled miR control.
(C) Physical interaction between let-7a and IL6 mRNA in the context of the RISC complex. IL6 RNA levels in HA-Ago1 immunoprecipitates in HEK-293 cells co-transfected with a plasmid expressing HA-Ago1 and let-7a or scrambled microRNA control.
(D) IL6 RNA levels after treatment for 24h with antisense-let-7a (100 nM) or Lin28B.
(E) Phosphorylation of STAT3-Y705 ELISA assay; mean ± SD) in untreated and TAM-treated (36h) cells in addition to treatment with increasing concentrations (50, 80, 100 nM) of let-7a and siRNA against Lin28B (80 nM).
(F) VEGF and IL6 production (ELISA assay; mean ± SD) in TAM-treated cells before and after transfection (100 nM) of the indicated let-7 microRNAs.
(G) Western blot analysis of RAS protein expression in 36h TAM-treated cells after let-7a (100 nM) overexpression; GAPDH levels were used as a loading control.
(H) IL6 production (ELISA assay during transformation after transfection with negative control siRNA or siRNA against Ras or let-7a (100 nM).
(I) Soft agar colony assay in TAM-treated cells after treatment with 2 μg/ml of monoclonal antibody against IL6 (Ab-IL6) and IgG isotype antibody (Ab-IgG) or siRNA negative control and siRNA against Ras (100 nM).
Several lines of evidence indicate that let-7a directly targets IL6 mRNA through binding its 3′UTR. First, let-7a overexpression inhibits the activity of a luciferase reporter construct containing the IL6 3′ UTR (Figure 4B). Second, immunoprecipitates of HA-Ago1, a protein that mediates microRNA functions, show markedly higher levels of IL6 mRNA binding upon let-7a overexpression (Figure 4C). Third, inhibition of let-7a via introduction of an antisense RNA or by Lin28B over-expression significantly induces IL6 mRNA levels (Figure 4D). Fourth, phosphorylation of STAT3, a downstream target of IL6, is inhibited by the addition of let-7a or by inhibition of Lin28B (Figure 4E). Fifth, individual expression of all let-7 family members tested results in reduced levels of both IL6 protein and the angiogenic cytokine VEGF, a direct transcriptional target of STAT3 (Figure 4F), indicating that let-7 inhibits the IL6-dependent signaling pathway.
Let-7 inhibits IL6 expression indirectly through Ras and NF-κB
As mentioned previously, HMGA2 and Ras are let-7a gene targets (Johnson et al., 2005; Mayr et al., 2007). Although we did not detect any difference in HMGA2 expression during transformation of ER-Src cells (data not shown), transformed cells have high levels of Ras that are inhibited by expression of let-7a (Figure 4G). As Ras-induced secretion of IL6 is required for tumorigenesis (Ancrile et al., 2007), and IL6 transcription is induced directly by NF-κB, we hypothesized that let-7a might inhibit IL6 expression indirectly through the Ras-NF-κB pathway. In accord with this hypothesis, antisense inhibition of Ras expression (Fig. S8A) during cellular transformation reduces IL6 protein levels, albeit less effectively than achieved by let-7a overexpression (Figure 4H). In addition, NF-κB activity is strongly induced upon transformation, and this induction is partially blocked by inhibition of Ras (Figure S8B). Lastly, inhibition of Ras expression reduces the level of transformation, although much less effectively than inhibition of IL6 (Figure 4I). Thus, let-7a microRNA inhibits IL6 expression both directly through its 3′UTR and indirectly by an interaction with Ras that leads to a reduction in NF-κB activity. However it seems that let-7 regulates IL6 expression more effectively through direct inhibition rather than indirect inhibition through Ras.
IL6 inhibition of let-7 expression occurs through NF-κB and is important for transformation
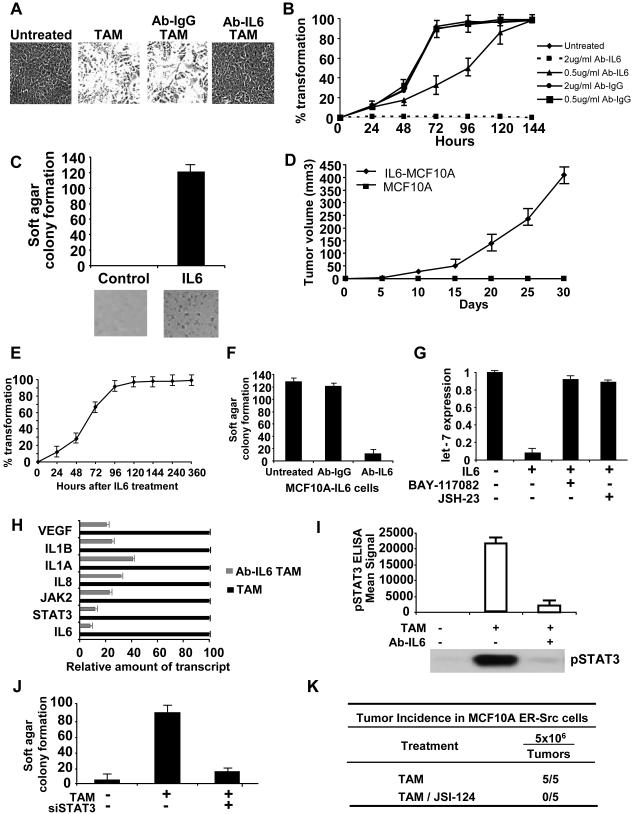
Depletion of IL6 by a monoclonal antibody blocks the morphological changes associated with transformed cells (Figure 5A), reduces colony formation and inhibits cell motility (Figure S9). At lower concentrations of the IL6 antibody that only partially reduce IL6 levels (Figure S9), transformation is significantly delayed (Figure 5B). Thus, IL6 is important for transformation, and the level of IL6 affects the rate at which cells become transformed.
Figure 5.
IL6 signaling pathway regulates MCF10A ER-Src transformation
(A) Representative phase contrast images of ER-Src cells that were or were not treated with TAM and an antibody against IL6 (2 μg/ml).
(B) Kinetics of cellular transformation (mean ± SD) in untreated and TAM-treated (5 minutes) cells together with 0.5 and 2 ug/ml of a monoclonal antibody against IL6 (Ab-IL6) or an isotype (Ab-IgG).
(C) Soft agar colony assay in untreated and IL6-treated (1h) MCF10A cells.
(D) Tumor growth of MCF10A and IL6-treated (1h) MCF10A cells in nude mice (5 mice/group). All the mice in the IL6-treated group developed tumors.
(E) Kinetics of cellular transformation in IL6-treated (1h) MCF10A cells.
(F) Soft agar colony assay in IL6-transformed MCF10A cells after treatment with 2ug/ml Ab-IL6 or Ab-IgG (control).
(G) Let-7a expression in untreated and IL6-transformed MCF10A cells in the presence or absence of NF-κB inhibitors (5 μM BAY-117082 and 6 μM JSH-23).
(H) RNA levels for the indicated genes in cells treated with TAM or an IL6 antibody.
(I) Levels of phosphorylated STAT3 (pStat3; ELISA assay and western blot) in cells that were or were not treated with TAM and an antibody against IL6 2 (νg/ml Ab-IL6).
(J) Colony formation assay of untreated and TAM-treated cells in the presence of absence of an siRNA against Stat3 (80nM).
(K) Tumor incidence of subcutaneously injected MCF10A ER-Src cells treated for 24h with Stat3 inhibitor (7uM JSI-124). The number of palpable tumors at 4 weeks is shown.
Conversely, IL6 treatment for only 1h is sufficient to induce transformation of MCF10A cells (soft agar assay) in the absence of Src activation (Figure 5C), and the resulting transformed cells were able to form tumors in xenografts (Figure 5D). The transformed phenotype of these IL6-induced cells was fully acquired 96–120 h after treatment, and it remained stable for at least 10 more days (Figure 5E). Furthermore, when these stable (15 day) IL6-transformed cells were treated with the IL6 antibody, the resulting cells were severely defective in forming colonies in soft agar (Figure 5F), indicating that transformation per se and maintenance of the transformed state depends on IL6 production.
Strikingly, IL6 treatment inhibits let-7a microRNA expression in a manner that depends upon NF-κB (Figure 5G), and it leads to increased cell motility (Figure S10A). This IL6-induced cell transformation of MCF10A cells is inhibited by overexpression of let-7a (Figure S10B). The finding that IL6 inhibits let-7 expression and that let-7 inhibits IL6 expression through a direct microRNA targeting interaction indicates that there is a negative feedback loop between let-7a and IL6. This negative feedback loop is controlled by NF-κB, and indeed is actually a sub-loop of a positive feedback loop controlled by NF-κB (see Discussion and Figure 7E).
Figure 7.
Positive inflammatory feedback loop in cancer cells, xenografts and cancer patients.
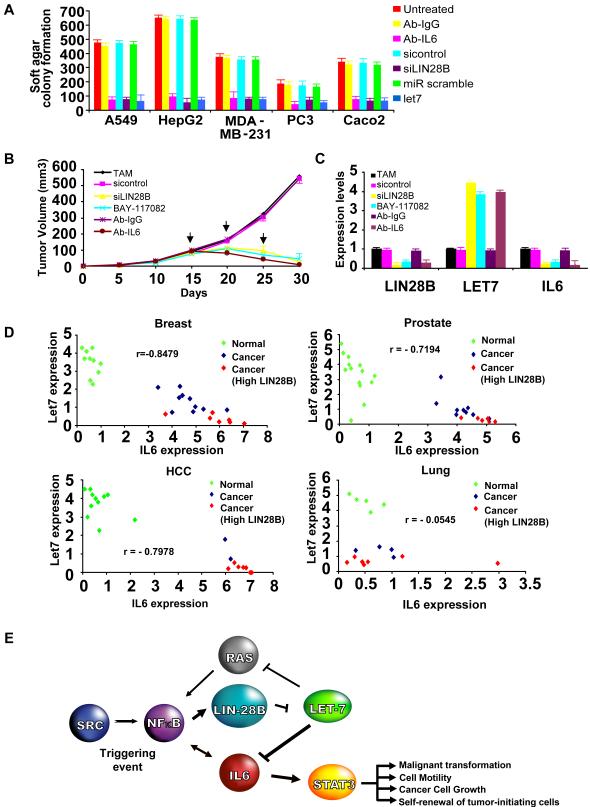
(A) Colony formation assay in A549 (lung), HepG2 (hepatocellular), MDA-MB-231 (breast), PC3 (prostate) and Caco2 (colon) cancer cell lines treated with 2 μg/ml Ab-IgG (control), 2 μg/ml Ab-IL6, 80 nM siRNA control, 80 nM siRNA against Lin28B, 100 nM microRNA scrambled control and 100nM let-7 microRNA for 24h.
(B) Tumor growth of ER-Src cells after i.p treatment (days 15, 20, 25) with siRNA negative control, siRNA against Lin28B, BAY-117082, Ab-IgG and Ab-IL6.
(C) Expression levels of Lin28B, let-7 and IL6 from tumors derived from the experiment described above.
(D) Lin28B, let-7a and IL6 expression in breast, prostate, hepatocellular and lung cancer and normal tissues. Each data point represents an individual sample, and a correlation coefficient (r) between let-7a and IL6 expression is shown.
(E) Schematic overview of inflammatory positive feedback loop during cellular transformation.
IL6-STAT3 signaling pathway is important for transformation
As expected, depletion of IL6 results in reduced expression of several targets of the IL6 signaling pathway such as JAK2, STAT3, VEGF, IL8, IL1A, and IL1B (Figure 5H). IL6 acts primarily through its receptor to activate the JAK/STAT pathway, and inhibition of the IL6 receptor reduces transformation and tumorigenicity (Figure S11). STAT3, a DNA-binding transcriptional activator that is phosphorylated in response to IL6 and other inflammatory cytokines, is an important mediator of cellular transformation (Frank, 2007). Levels of STAT3 RNA (Figure 2A) and protein (Figure S12A) are induced during ER-Src transformation, but only at late time points; i.e. after NF-κB activation, let-7 inhibition, and IL6 super-activation. IL6 inhibition strongly reduces STAT3 expression (Figure 5H) and phosphorylation (Figure 5I), indicating that STAT3 activation is IL6-dependent. Inhibition of STAT3 blocks the morphological changes associated with ER-Src transformation (Figure S12B) and reduces colony formation (Figure 5J). In addition, pharmacological inhibition of the JAK/STAT3 pathway inhibited tumor formation in nude mice (Figure 5K). Lastly, Socs3, a negative regulator of the IL6 pathway, is down-regulated during the process of cellular transformation, and inhibition of Socs3 expression via siRNA causes increased tumorigenicity (Figure S13). Overall, these results suggest that activation of the IL6 pathway through IL6 receptor, STAT3 activation, and down-regulation of Soc3 are crucial late steps in cellular transformation.
The positive feedback loop involving NF-κB, Lin28B, let-7, and IL6 is required for maintenance of the transformed phenotype and stem cell population
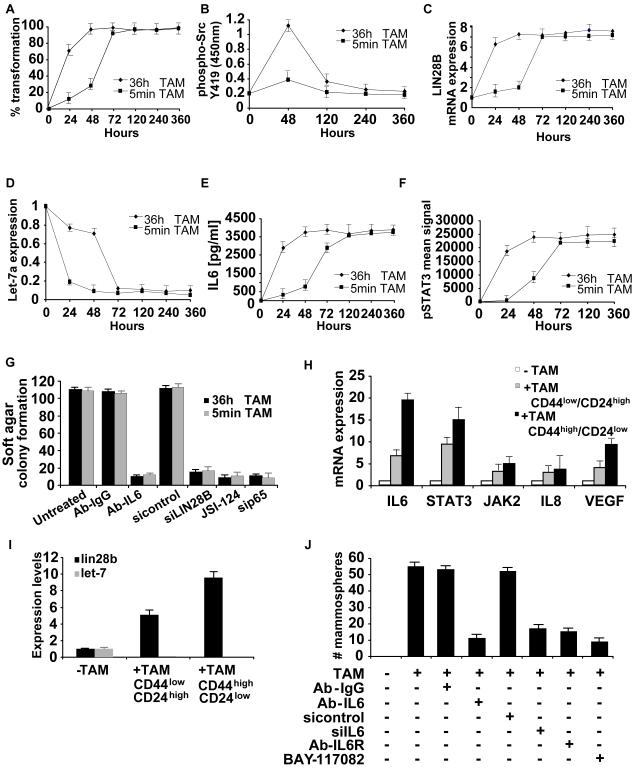
The above experiments suggest that a positive feedback loop involving NF-κB, Lin28B, let-7, and IL6 is required for transformation of MCF10A cells (See Figure 7E). To test whether this positive feedback loop is required for the maintenance and stability of the transformed phenotype, we examined transformed cells (generated by 5 min or 36 h TAM treatment) for up to 15 days after removal of TAM. Under these conditions, the transformed phenotype is maintained in the absence of Src activity, with high levels of Lin28B, IL6, and phosphorylated STAT3, low levels of let-7 (Figure 6A-F). Furthermore, breaking the regulatory circuit in these stably transformed cells by inhibition of IL6, Lin28B, STAT3, or NF-κB leads to loss of tumorigenicity and cell motility (Figures 6G, S14). Thus, the positive feedback loop is required to maintain the stability of the transformed phenotype.
Figure 6.
The positive feedback regulatory circuit is required for maintenance of the transformed phenotype and cancer stem cell population.
(A) Kinetics of cellular transformation in TAM-treated (5 minutes and 36h) cells.
(B) Levels of Src phosphorylation (ELISA assay), (C) Lin28B mRNA (real-time PCR), (D) let-7a (real-time PCR), (E) IL6 protein (ELISA assay) and (F) Stat3 phosphorylation (ELISA assay) in TAM-treated (5 minutes and 36h) cells
(G) Colony formation assay in ER-Src cells (15 days post TAM treatment) treated with 2 μg/ml monoclonal antibody against IL6 (Ab-IL6), 80 nM siRNA against Lin28B, STAT3 inhibitor (7 μM JSI-124) and 80 nM siRNA against NF-κB (p65). Treatments with 2 μg/ml isotype antibody (Ab-IgG) and 80 nM negative control siRNA were used as controls.
(H) Expression levels of the indicated RNAs (mean ± SD) in untreated cells and sorted CD44high/CD24low and CD44low/CD24high TAM-treated cells.
(I) Let-7a and Lin28B RNA levels (mean ± SD) in untreated and sorted CD44high/CD24low and CD44low/CD24high TAM-treated cells.
(J) Number of mammospheres/1000 seeded cells formed by TAM-treated cells in the presence or absence of 2 μg/ml Ab-IgG or Ab-IL6; 100 nM negative control siRNA or siRNA against IL6; 4 μg/ml Ab-IL6R; 5 μM BAY-117082.
ER-Src cells treated with TAM can form mammospheres with self-renewal properties (Hirsch et al., 2009)(Figure 1A), suggesting that they have attributes of “cancer stem cells” (also known as tumor initiating cells). Cancer stem cells express high levels of CD44 and low levels of CD24 antigen markers (Mani et al., 2008), and ~10% of the TAM-treated ER-Src cell population are stem cells (CD44high/CD24low) whereas 90% are non-stem transformed cells (CD44low/CD24high). This ratio of stem cells and cancer cells is typical for other cancer cell lines (Ponti et al., 2005; Chu et al., 2009). In accord with the defining features of cancer stem cells, the CD44high/CD24low cells can form mammospheres and tumors in nude mice, while CD44low/CD24high cannot.
Interestingly, the cancer stem cells display a stronger inflammatory gene signature (Figure 6H), more increased levels of nuclear NF-κB (Figure S15), higher levels of Lin28B, and more decreased let-7a expression (Figure 6I) than observed in the non-stem cancer cells. Thus, mammosphere formation involves the selection of a sub-population of cancer stem cells in which the inflammatory feedback loop is even more active than in the majority population of cancer cells. In mammospheres derived from ER-Src transformed cells, inhibition of IL6, IL6 receptor or NF-κB activity blocks mammosphere propagation (Figure 6J), indicating that the inflammatory feedback loop is required for growth of cancer stem cells. Conversely untransformed MCF10A cells can form mammospheres after IL6 treatment. This IL6-mediated mammosphere formation is dramatically reduced after let-7a overexpression or NF-κB inhibition, and the few mammospheres formed are smaller (Figure S16A). Similarly, inhibition of let-7a or Lin28B overexpression permits MCF10A cells to form mammospheres in a manner that depends on IL6 (Figure S16B). These data suggest that activation of NF-κB and the IL6 pathway through inhibition of let-7 by Lin28B is required for the self-renewal capacity of cancer stem cells.
The inflammatory regulatory circuit is important for cancer cells from diverse developmental lineages
Several lines of evidence indicate that the NF-κLin28B, let-7a, and IL6 is more generally involved in oncogenic transformation. First, in MCF-10A cells, RASV12 behaves similarly to Src in activating NF-κB and mediating transformation through let-7 and IL6 (Figures S17, S18). Second, 8 out of 15 different kinds of cancer cell lines show the characteristics of the inflammatory regulatory circuit, namely Lin28B overexpression, let-7 down-regulation, and high levels of IL6 (Figure S19). Third, perturbation of any component of the regulatory circuit (inhibition of Lin28B or IL6 or overexpression of let-7) significantly reduced the tumorigenicity and motility of lung (A549), hepatocellular (HepG2), breast (MDA-MB-231), prostate (PC3), and colon (Caco2) cancer cells (Figures 7A, S20A). In all cases, these perturbations resulted in reduced expression of IL6 (Figure S20B), suggesting the importance of IL6 in maintaining the transformed phenotype. Thus, the inflammatory feedback loop is important for cancer cells from diverse developmental lineages.
The inflammatory regulatory circuit is important for cancer cell growth in vivo
To address whether the inflammatory regulatory circuit was important for cancer growth in vivo, we injected subcutaneously injected TAM-treated cells into 30 nu/nu mice and obtained tumors with a size of 100 mm3 in all cases. The mice were randomly separated into 6 groups and treated intraperitoneally with siRNAs against Lin28B, the NF-κB inhibitor BAY-117082, or a monoclonal antibody against IL6; treatments were repeated for 3 cycles. After the second cycle of treatment, there was significant suppression of tumor growth in all treated mice (Figure 7B). After 30 days, the tumors were extremely small in size, and cells taken from these tumors had low expression of Lin28B and IL6 and high levels of let-7 (Figure 7C). Thus, perturbation of any component of the regulatory circuit strongly suppresses tumor growth, and restores gene expression patterns typical of non-transformed cells.
Let-7a and IL6 are negatively correlated in cancer and normal tissues
To address whether the inflammatory feedback loop is relevant to human cancer, we examined the expression of Lin28B, let-7a and IL6 in cancer and normal breast, prostate, hepatocellular and lung tissues (Figures 7D, S21). As expected, cancer tissues had lower levels of let-7a and higher levels of IL6 relative to normal tissues. More importantly, among both normal and diseased individuals, there is a striking inverse relationship between let-7a and IL6 expression levels in breast, prostate and hepatocellular tissues. This striking inverse relationship strongly argues for a mechanistic relationship between let-7 and IL6 in these tissues that is amplified in cancer. In contrast, while let-7a expression is reduced in lung cancer tissues, only a subset of these show high IL6 levels. Furthermore, Lin28B is overexpressed in a subset of breast (7/17), prostate (6/15), and hepatocellular (7/9) cancer tissues, suggesting a mechanistic relationship between Lin28B, let-7, and IL6 in different forms of cancer. These results suggest that the regulatory pathway identified in the ER-Src model (Fig. 7E) is relevant to human disease and specifically important for certain cancer types.
DISCUSSION
A molecular pathway that links inflammation to cellular transformation
In 1863, Rudolf Virchow proposed that chronic inflammation may lead to development of cancer, and there are now clinical, epidemiological, and molecular links between inflammation and oncogenic transformation (Balkwill and Mantovani, 2001; Naugler and Karin, 2008; Pierce et al., 2009). However, molecular pathways linking inflammation to cellular transformation are unknown, in large part due to the lack of experimental systems that can follow the process by which a non-transformed cell becomes transformed. Here, using an experimental model in which activation of an ER-Src oncoprotein by treatment with tamoxifen converts a non-transformed epithelial cell line (MCF-10A) to the transformed state in 24–36 hours, we describe such a molecular pathway that involves NF-κB, Lin28B, let-7 microRNA, and IL6 (Figure 7E).
The first step in the pathway is the activation of NF-κB, which occurs within 30 minutes after treatment with tamoxifen. By activating NF-κB, Src effectively provides the inflammatory signal that is critical for cellular transformation. Src is required for NF-κB activation in other biological contexts (Abu-Amer et al., 1998; Lee et al., 2007), so genetic changes or environmental conditions that activate Src might contribute to cancer via an inflammatory pathway. However, in our model, Src does not play a significant role in the transformation process other than providing the initial inflammatory signal. In this regard, activation of NF-κB by Ras or by IL6 also induces the oncogenic transition in MCF-10A cells lacking the ER-Src protein.
Down-regulation of the let-7 microRNA family is a critical early step in the oncogenic transition of the ER-Src cells, because expression on any individual let-7 family member blocks cellular transformation. Although NF-κB is required for down-regulating the level of let-7 RNAs, this inhibition occurs primarily at a post-transcriptional level, because the amounts of the let-7 precursor RNA are essentially unchanged during the transformation process. Lin28B plays a critical role in processing let-7RNA (Heo et al., 2008; Viswanathan et al., 2008), and our results indicate that NF-κB inhibits let-7 RNA levels primarily by activating transcription of lin-28B via direct binding to a highly conserved NF-κB site in the first intron. By using lin-28B as an intermediary, NF-κB rapidly transmits an inflammatory signal into a mechanism for coordinate inhibition of all let-7 microRNAs, which is essential for transformation. NF-κB also binds in vivo to a site upstream of the let-7 RNA coding region, but it is unclear whether this binding affects the regulation of let-7 RNA.
Expression of the cytokine IL6, a major mediator of the inflammatory response, is directly repressed by let-7 through a standard interaction of the microRNA with the 3′ UTR of the target mRNA. NF-κB activation and subsequent repression of let-7 therefore results in a dramatic and biphasic increase in IL6 levels, which is necessary for cellular transformation. These high levels of IL6 depend on both transcriptional activation by NF-κB and inhibition of Let7, and are required for sufficient binding to the IL6 receptor to cause phosphorylation and nuclear entry of the STAT3 transcription factor, which then activates multiple growth and survival genes such as VEGF (Niu et al., 2002). In the ER-Src cells, STAT3 is a key IL6 target that is required for cellular transformation. Consistent with these results, STAT3 is important for colitis-associated tumorigenesis (Bollrath et al., 2009; Grivennikov et al., 2009).
An epigenetic switch from non-transformed to transformed cells mediated by an inflammatory positive feedback loop
An epigenetic switch occurs when a stable cell type changes to another stable cell type without any change in DNA sequence. Epigenetic switches require an initiating event (which could be a specific molecular process or stochastic fluctuation), but the phenotypes of the new cell type are inherited in the absence of the initiating signal. Here, we describe an epigenetic switch in which a stable non-transformed cell type is converted to a stable transformed cell type via transient activation of the Src oncoprotein. Remarkably, this switch in cell type occurs even when cells are exposed to tamoxifen for only 5 minutes. Furthermore, mammospheres generated from the transformed cells can be propagated for at least 12 generations over 2.5 months in the absence of tamoxifen. As the transition between non-transformed to transformed cells occurs within 24–36 hours, it is extremely unlikely to involve changes in DNA sequence. Hence, the phenomenon we describe here fits all the definitions of an epigenetic switch.
As illuminated by classic studies of an epigenetic switch in bacteriophage λ, positive feedback loops involving transcriptional regulatory proteins are the fundamental principle for epigenetic inheritance of gene expression patterns (Ptashne, 2009). NF-κB, Lin28B, let-7 microRNA, and IL6 are the key components of the positive feedback loop underlying the epigenetic switch from a non-transformed cell type to a transformed cell type capable of forming self-renewing stem cells (Figure 7E). The switch is triggered by an initial inflammatory signal (activated Src here) that activates NF-κB. NF-κB generates high levels of IL6 by direct activation of IL6 transcription and indirect (via Lin28B) inhibition of let-7 microRNA. The resulting high levels of IL6 activate NF-κB, thereby completing the positive feedback loop that maintains the transformed phenotype, self-renewal of mammospheres, and tumor formation in nude mice in the absence of the triggering event (Src activation). High levels of IL6 are crucial for activating NF-κB, and this is why Let-7 is required for the regulatory circuit.
A related positive feedback loop involving Ras is likely to contribute to the epigenetic switch. As is the case for IL6, Ras is both a direct target of Let-7 and an activator of NF-κB. However, as the oncogenic form of Ras (Ras-V12) is not present in MCF-10A cells, we suspect that this regulatory circuit is not as important as the feedback loop involving IL6 in our experimental system. Our results do not exclude, and we would not be surprised by, the existence of analogous feedback loops involving NF-κB or other transcriptional regulatory proteins or microRNAs.
Three classes of experiments validate the positive feedback loop. First, overexpression of any positive factor (Lin28B, IL6) or inhibition of a negative factor (let-7) induces cellular transformation, indicating that the loop can be started at any step. Second, under conditions where the transformed state is stable in the absence of the inducing factor, perturbation at any step breaks the established positive feedback loop, thereby causing loss of the transformed state. Third, the strength of the initial signal (time of TAM treatment) or the amount of product generated by that signal (IL6) is inversely correlated with the rate of observing cellular transformation.
Ultimately, the epigenetic switch is an inflammatory positive feedback loop in which a transient inflammatory signal is converted into a chronic inflammatory state that is maintained by activated NF-κB. Once NF-κB is activated, the regulatory circuit is sufficient to generate and maintain the chronic inflammatory loop (i.e. the transformed state) without the original (or new) environmental signal. Importantly, this inflammatory feedback loop is a common feature of cellular transformation, because it occurs and is functionally relevant in cancer cells of diverse developmental origin.
Relevance to human cancer
Our experimental model has the advantage of studying the process of cellular transformation and cancer stem cell formation in a dynamic and well-defined manner, analogous to studies of viral infection. Although this model utilizes an immortalized cell line with an artificial oncogene, the key components of the switch between nontransformed and transformed cells, NF-κB, let-7, and IL6, have all been linked to human disease. Activation of the NF-κB pathway is correlated with carcinogenesis (Luo et al., 2005), Let-7 is down-regulated in several cancers (Johnson et al., 2005; Mayr et al., 2007; Sampson et al., 2007), Lin28 is overexpressed in primary human tumors (Viswanathan et al., 2009)(Figure 7D), and IL6 has been implicated as a growth factor for multiple myeloma, Hodgkin’s lymphoma, and epithelial cancers (Kawano et al., 1988; Nagel et al., 2005)(Sasser et al., 2007). Our finding of a striking inverse relationship between let-7 and IL6 expression in breast and prostate epithelial cancer tissues suggests the importance of inflammatory activation and the IL6-let-7 regulatory circuit in solid cancers. Lastly, the epigenetic switch can be triggered by v-Src or Ras-V12, which are oncogenes associated with many human cancers.
In our cellular transformation model, the epigenetic switch between non-transformed and transformed cells occurs very quickly in response to a transient inflammatory signal. Although a transient inflammatory signal is clearly insufficient to trigger such an epigenetic switch in normal cells, we believe that the epigenetic switch described here is relevant to human cancer. Specifically, we suggest that the epigenetic switch requires cells that are genetically altered to be at an intermediate stage in the transition between a primary cell and a cancer cell. The epigenetic switch requires cells that can inhibit let-7 and generate high levels of IL6 upon NF-κB stimulation, and it is likely that this requires multiple factors in addition to NF-κB itself. In addition, although IL6 is a key effector molecule, generation of the transformed state undoubtedly requires other factors. These additional factors are likely to depend on developmental state, extracellular stimuli, and mutational status, thus accounting for cell-type specificity of the epigenetic switch. Nevertheless, the results presented here provide a paradigm in which a key step in cancer progression involves an epigenetic switch in response to an inflammatory (or other environmental) signal as opposed to a mutational change in a tumor suppressor or oncogene.
EXPERIMENTAL PROCEDURES
Cell Culture, Cellular Transformation Assays, and Isolation of Cancer Stem Cells
MCF10A cells containing ER-Src, an integrated fusion of the v-Src oncoprotein and the ligand-binding domain of estrogen receptor were grown and induced to transform with 1 μM 4OH-tamoxifen (TAM)as described previously (Hirsch et al., 2009). In some experiments, cells were treated with human recombinant IL6, anti-IL6 antibody, and the NF-κB inhibitors BAY-117082 and JSH-23. Except where otherwise indicated, morphological changes, phenotypic transformation and foci formation occurred 24–36 h after TAM addition, and were monitored by phase-contrast microscopy. Cells were assayed for their ability to grow as anchorage-independent colonies in soft agar and to form mammospheres as described previously (Hirsch et al., 2009). For long-term propagation, mammospheres were collected by gentle centrifugation, dissociated to single cells as described (Dontu et al., 2003) and then cultured to obtain the next generation. Cancer stem cells (CD44high/CD24low) and non-stem transformed cells (CD44low/CD24high) from isolated from transformed cell populations by flow cytometric sorting on single cell suspensions stained with CD44 (FITC-conjugated) and CD24 (PE-conjugated) antibodies. Other cell lines were grown in DMEM, 10% fetal bovine serum, and penicillin/streptomycin.
Protein analysis
Western blotting was performed by standard procedures using antibodies against Ras, STAT3, the p65 subunit of NF-κB, Lin28B, Socs3, GAPDH, and β-actin with detection performed using HRP-conjugated antisera and chemiluminescence. ELISA assays for IL6, VEGF, NF-κB, phospho (Ser32)-IκBα, phospho (Tyr705)-STAT3 were performed in accord with manufacturers’ instructions. To examine the association of the RISC complex with IL6 mRNA, HEK-293 cells were co-transfected with a plasmid that expressed HA-Ago1 together with 100 nM microRNAs, followed by HA-Ago1 immunoprecipitation and analysis of IL6 RNA levels.
RNA analysis
For analyzing mRNAs, total RNA was reverse-transcribed to form cDNA, and the resulting material analyzed by quantitative PCR in real-time. Levels of Let-7 microRNAs were determined with the mirVana qRT-PCR miRNA Detection Kit and qRT-PCR Primer Sets, according to the manufacturer’s instructions, using RNU48 as a control. For analysis of patient samples, we only used samples lacking the absence of infiltrating macrophages, which was determined by expression of the macrophage marker CD11b, which is not expressed in epithelial cells.
Chromatin Immunoprecipitation
Potential NF-κB binding sites in the vicinity of the Let-7a3 and Lin28B mRNA initiation site were identified by DNA sequence motif, evolutionary conservation, and nucleosome occupancy. NF-κB(p65) binding in vivo to these putative sites was analyzed by chromatin immunoprecipitation and quantitative PCR analysis as described previously (Yang et al., 2006).
Transfection Experiments
Transcriptional activation by NF-κB and inhibition of IL6 mRNA levels by Let-7 microRNA were performed standard luciferase reporter gene assays upon transient transfection of the relevant DNAs. For other genetic experiments, siRNAs or plasmids capable of overexpressing the desired microRNAs or protein were transiently transfected into cells. After 24 h, the resulting cells were phenotypically analyzed for transformation and for levels of RNAs and proteins of interest.
Xenograft experiments
Injections of non-transformed and transformed MCF-10A cells into nude mice, treatments of tumors by i.p injections, and measurements of tumor volume were performed as described previously (Hirsch et al., 2009).
For all quantitative experiments, data are presented as mean values ± SD from three independent experiments. Detailed experimental procedures are provided in the Supplemental Data.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Richard Gregory for suggesting the idea that lin-28 might be involved in the process, Philip N. Tsichlis for providing laboratory access and materials needed for the xenograft experiments, Fabio Petrocca for help in designing the mammosphere and cell sorting experiments, Savina A. Jaeger for bioinformatic analysis and identification of NF-KB binding sites in let-7a and lin-28B, Joan Brugge for providing the MCF10A ER-Src and control cell lines, William Farrar for providing the pGL3-IL6 luciferace vector, George Daley for providing the pBabe.Puro-Lin28B vector, Joshua T. Mendell for providing the Lin28B-P1 luciferase vector, and Koon Ho Wong for construction of the mutant luciferase vectors. This work was supported by a postdoctoral fellowship from the American Cancer Society to H.A.H. and a research grant to K.S. from the National Institutes of Health (CA 107486).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abu-Amer Y, Ross F, McHugh K, Livolsi A, Peyron JF, Teitelbaum S. Tumor necrosis factor-alpha activation on nuclear factor-kB in marrow macrophages is mediated by c-Src tryrosine phosphorylation of IKB. J Biol Chem. 1998;273:29417–29423. doi: 10.1074/jbc.273.45.29417. [DOI] [PubMed] [Google Scholar]
- Ancrile B, Lim KH, Counter CM. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev. 2007;21:1714–1719. doi: 10.1101/gad.1549407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylin SB. DNA methylation and gene silencing in cancer. Nat Clin Pract Oncol. 2005;2:S4–11. doi: 10.1038/ncponc0354. [DOI] [PubMed] [Google Scholar]
- Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitalla S, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu P, Clanton DJ, Snipas TS, Lee J, Mitchell E, Nguyen ML, Hare E, Peach RJ. Characterization of a subpopulation of colon cancer cells with stem cell-like properties. International journal of cancer. 2009;124:1312–1321. doi: 10.1002/ijc.24061. [DOI] [PubMed] [Google Scholar]
- Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DA. STAT3 as a central mediator of neoplastic cellular transformation. Cancer Lett. 2007;251:199–210. doi: 10.1016/j.canlet.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Gazin C, Wajapeyee N, Gobeil S, Virbasius CM, Green MR. An elaborate pathway required for Ras-mediated epigenetic silencing. Nature. 2007;449:1073–1077. doi: 10.1038/nature06251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimshaw MJ, Cooper L, Papazisis K, Coleman JA, Bohnenkamp HR, Chiapero-Stanke L, Taylor-Papadimitriou J, Burchell JM. Mammosphere culture of metastatic breast cancer cells enriches for tumorigenic breast cancer cells. Breast Cancer Res. 2008;10:R52. doi: 10.1186/bcr2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn WC, Weinberg RA. Rules for making human tumor cells. N Engl J Med. 2002;347:1593–1603. doi: 10.1056/NEJMra021902. [DOI] [PubMed] [Google Scholar]
- Heo I, Joo C, Cho J, Ha M, Han J, Kim VN. Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Mol Cell. 2008;32:276–284. doi: 10.1016/j.molcel.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells and acts together with chemotherapy to blocks tumor growth and prolong remission. Cancer research. 2009;69 doi: 10.1158/0008-5472.CAN-09-2994. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Kawano M, Hirano T, Matsuda T, Taga T, Horii Y, Iwato K, Asaoku H, Tang B, Tanabe O, Tanaka H, et al. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988;332:83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- Kumar MS, Erkeland SJ, Pester RE, Chen CY, Ebert MS, Sharp PA, Jacks T. Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proc Natl Acad Sci USA. 2008;105:3903–3908. doi: 10.1073/pnas.0712321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Moon C, Lee HW, Park EM, Cho MS, Kang JL. Src tyrosine kinases mediate activations of NF-kappaB and integrin signal during lipopolysaccharide-induced acute lung injury. J Immunol. 2007;179:7001–7011. doi: 10.4049/jimmunol.179.10.7001. [DOI] [PubMed] [Google Scholar]
- Liao MJ, Zhang CC, Zhou B, Zimonjic DB, Mani SA, Kaba M, Gifford A, Reinhardt F, Popescu NC, Guo W, et al. Enrichment of a population of mammary gland cells that form mammospheres and have in vivo repopulating activity. Cancer research. 2007;67:8131–8138. doi: 10.1158/0008-5472.CAN-06-4493. [DOI] [PubMed] [Google Scholar]
- Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R, Roskams T, Trautwein C, Pasparakis M. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119–132. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Luo J-L, Kamata H, Karin M. IKK/NF-kB signaling: balancing life and death-a new approach to cancer therapy. J Clin Invest. 2005;115:2625–2632. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr C, Hemann MT, Bartel DP. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science. 2007;315:1576–1579. doi: 10.1126/science.1137999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel S, Scherr M, Quentmeier H, Kaufmann M, Zaborski M, Drexler HG, MacLeod RA. HLXB9 activates IL6 in Hodgkin lymphoma cell lines and is regulated by PI3K signalling involving E2F3. Leukemia. 2005;19:841–846. doi: 10.1038/sj.leu.2403716. [DOI] [PubMed] [Google Scholar]
- Naugler WE, Karin M. NF-kappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18:19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, Baumgartner KB, Gilliland FD, Sorensen BE, McTiernan A, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27:3437–3444. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer research. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- Ptashne M. Binding reactions: epigenetic switches, signal transduction and cancer. Curr Biol. 2009;19:R234–241. doi: 10.1016/j.cub.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Sakurai T, He G, Matsuzawa A, Yu GY, Maeda S, Hardiman G, Karin M. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson VB, Rong NH, Han J, Yang Q, Aris V, Soteropoulos P, Petrelli NJ, Dunn SP, Krueger LJ. MicroRNA let-7a down-regulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer research. 2007;67:9762–9770. doi: 10.1158/0008-5472.CAN-07-2462. [DOI] [PubMed] [Google Scholar]
- Sasser AK, Sullivan NJ, Studebaker AW, Hendey LF, Axel AE, Hall BM. Interleukin-6 is a potent growth factor for ER-alpha-positive human breast cancer. FASEB J. 2007;21:3763–3770. doi: 10.1096/fj.07-8832com. [DOI] [PubMed] [Google Scholar]
- Soule HD, Maloney TM, Wolman SR, Peterson WD, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC. Isolation and characterization of a spontaneously immortallized human breast epithelial cell line, MCF10. Cancer research. 1990;50:6075–6086. [PubMed] [Google Scholar]
- Ventura A, Jacks T. MicroRNAs and cancer: short RNAs go a long way. Cell. 2009;136:586–591. doi: 10.1016/j.cell.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan SR, Powers JT, Einhorn W, Hoshida Y, Ng TL, Toffanin S, O’Sullivan M, Lu J, Phillips LA, Lockhart VL, et al. Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet. 2009;41:843–848. doi: 10.1038/ng.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- Wegiel B, Bjartell A, Culig Z, Persson JL. Interleukin-6 activates PI3K/Akt pathway and regulates cyclin A1 to promote prostate cancer cell survival. Int J Cancer. 2008;122:1521–1529. doi: 10.1002/ijc.23261. [DOI] [PubMed] [Google Scholar]
- Yang A, Zhu Z, Kapranov P, McKeon F, Church GM, Gingeras TR, Struhl K. Relationships between p63 binding, DNA sequence, transcription activity, and biological function in human cells. Mol Cell. 2006;24:593–602. doi: 10.1016/j.molcel.2006.10.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.