Abstract
Higher coffee consumption has been associated inversely with the incidence of chronic liver disease in population studies. We examined the relationship of coffee consumption with liver disease progression in individuals with advanced hepatitis C related liver disease. Baseline coffee and tea intake was assessed in 766 participants of the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis (HALT-C) trial who had hepatitis C-related bridging fibrosis or cirrhosis on liver biopsy and failed to achieve a sustained virological response to peginterferon plus ribavirin treatment. Participants were followed for 3.8 years for clinical outcomes and for those without cirrhosis, a 2 point increase in Ishak fibrosis score on protocol biopsies. At baseline, higher coffee consumption was associated with less severe steatosis on biopsy, lower serum AST/ALT ratio, alpha-fetoprotein, insulin, and HOMA2 score, and higher albumin (p<0.05 for all). 230 patients had outcomes. Outcome rates declined with increasing coffee intake: 11.1/100 person-years for none, 12.1 for <1 cup/day, 8.2 for 1 to <3 cups/day, and 6.3 for ≥ 3 cups/day (p-trend=0.0011). Relative risks (95% confidence intervals) were 1.11 (0.76–1.61) for < 1 cup/day; 0.70 (0.48–1.02) for 1 to <3 cups/day; and 0.47 (0.27–0.85) for ≥3 cups/day (p-trend = 0.0003), versus not drinking. Risk estimates did not vary by treatment assignment or cirrhosis status at baseline. Tea intake was not associated with outcomes.
Conclusion
In a large prospective study of participants with advanced hepatitis-C related liver disease, regular coffee consumption was associated with lower rates of disease progression.
Keywords: hepatitis C, cirrhosis, disease progression
Hepatitis C virus (HCV) infects approximately 2.2% of the World’s population (including more than three million individuals in the United States).(1) Treatment with peginterferon and ribavirin for 24–48 weeks clears virus and resolves chronic hepatitis in approximately half of patients.(2) The remaining patients and patients ineligible for or unable to tolerate treatment have few additional options, such that identification of modifiable risk factors for disease progression is a clinically important issue.
Coffee intake may have beneficial effects on the liver. Increasing coffee consumption has been inversely associated with liver enzyme concentrations, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), and γ-glutamyltransferase.(3–8) In population studies, among persons with unknown diagnosis of liver disease, greater coffee intake has been associated with lower risk of cirrhosis,(6, 9–12) chronic liver disease,(13) and hepatocellular carcinoma.(14, 15) Laboratory studies suggest that several components of coffee, including caffeine(16–19), diterpenes,(20) and polyphenols,(21) may have beneficial effects on the liver. But, heretofore, no studies have examined the relationship between coffee consumption and progression of liver disease among persons known to have advanced hepatic fibrosis.
We prospectively examined the association of coffee intake and liver disease progression in participants of the HALT-C trial, a large randomized controlled trial evaluating the role of long-term peginterferon alfa-2a for the prevention of disease progression in patients with hepatitis C-related bridging fibrosis and cirrhosis who had failed to respond to peginterferon plus ribavirin therapy.
MATERIALS AND METHODS
Study population
The design and results of the HALT-C trial has been published.(22) Randomized subjects had detectable HCV RNA and met the following criteria: failure to achieve a sustained virological response with previous peginterferon/ribavirin therapy; advanced hepatic fibrosis on liver biopsy (Ishak stage ≥ 3); no history of hepatic decompensation or HCC; and absence of defined exclusion criteria (such as liver disease other than hepatitis C, uncontrolled medical or psychiatric conditions). All participants were required to have an ultrasound, computed tomography or magnetic resonance imaging with no evidence of hepatic mass lesion suspicious of HCC. Participants were randomized to low dose maintenance therapy (peginterferon alfa-2a 90 mcg weekly) or no treatment. Liver biopsies were repeated 1.5 and 3.5 years after randomization. All biopsies were reviewed in conference by a panel of twelve hepatic pathologists. The Ishak scoring system was used to assess inflammation (0–18) and fibrosis (0–6).(23) The homeostatic model assessment (HOMA2) score of insulin resistance was calculated as previously described.(24) Treated and untreated participant data from the randomized phase of the trial were combined, because maintenance low dose peginterferon therapy did not affect clinical outcome or histologic progression.(22)
Of 1,050 randomized participants, we excluded 241 participants who did not complete the food frequency questionnaire (FFQ), 1 participant with extreme caloric intake (total energy intake more than two interquartile ranges from the median), and 42 participants lacking follow-up biopsies. After exclusions, our dataset included 766 participants.
Exposure assessment
At study baseline, participants completed the Block 98.2 FFQ (Block data systems, Berkeley, CA), which has been extensively validated.(25, 26) Participants were asked to report their typical frequency of intake and portion size over the past year, using 9 frequency categories ranging from ‘never’ to ‘every day’ and 4 categories of portion size (1 cup, 2 cups, 3–4 cups, and 5+ cups). One question assessed coffee consumption and a second question assessed black or green tea intake. Participants also completed a second Block 98.2 FFQ approximately 13 months (median= 12.9 months, interquartile range= 12.3–15.4 months) after randomization. We calculated typical intake (cups per day) from the questionnaire. For analysis, we created categorical variables of coffee (never, > 0 to <1, ≥1 to <3, and ≥ 3 cups/day) and tea intake (never, > 0 to <1, ≥1 to <2, and ≥ 2 cups/day). Participants also completed a short form 36 item quality of life questionnaire (SF-36)(27) at baseline.
Assessment of outcomes
Participants were seen every three months during the study period. Complete blood counts, liver chemistry panel and alpha-fetoprotein (AFP) were tested at each clinical site. Participants had at least one ultrasound examination every 12 months. Predefined clinical outcomes included ascites, Child-Turcotte-Pugh score of ≥7 on two consecutive study visits, liver disease related death, hepatic encephalopathy, hepatocellular carcinoma, spontaneous bacterial peritonitis, or variceal hemorrhage; and for participants with bridging fibrosis at baseline, a ≥ 2 point increase in Ishak fibrosis score on either of the follow-up biopsies. All outcome reports were reviewed by an Outcomes Review Panel consisting of three investigators from the participating clinical centers. Excluding hepatocellular carcinoma (23 events) did not alter results (data not shown).
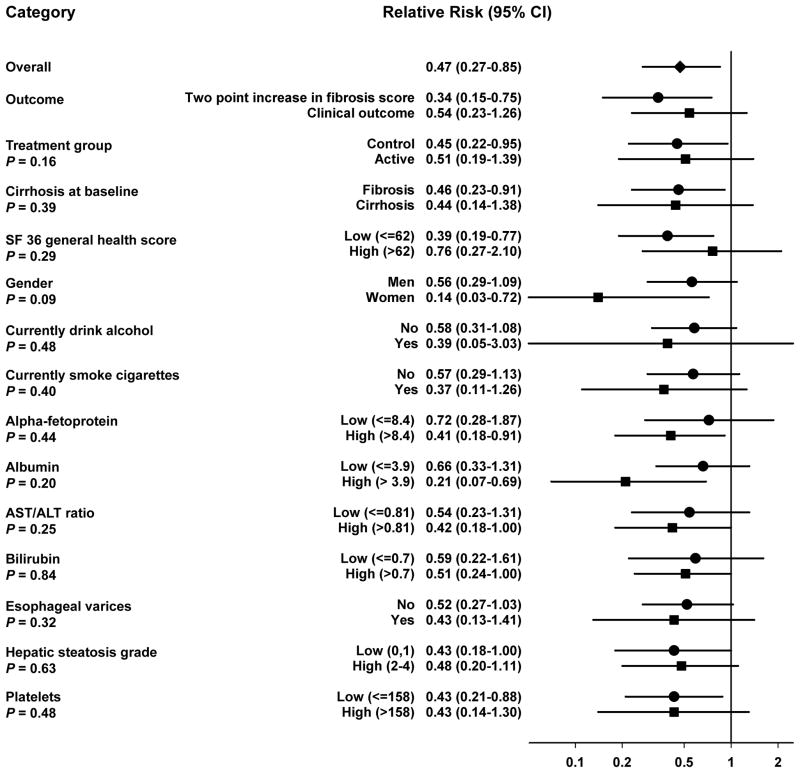
We present results for a combined endpoint, which included both clinical outcomes and, for those with fibrosis at randomization, a two point increase in Ishak fibrosis score. Results for individual endpoints were similar (Figure 1).
Figure 1.
Subgroup analysis of the association of baseline coffee intake with liver disease progression in the HALT-C trial. Relative Risk estimates shown are for drinking ≥ 3 cups of coffee per day relative to non-drinking and are adjusted for age, body mass index, education, ethnicity, gender, baseline ishak fibrosis score, total energy intake, lifetime alcohol intake, pack-years of cigarette use, and tea intake. Black diamond indicates the overall point estimate. Black circles and squares represent the point estimate for each indicated subgroup. Horizontal lines represent 95% confidence intervals (CI). The solid vertical line indicates a relative risk of 1. P-values are for the interaction between coffee intake and each stratifying variable and are taken from the Wald-test for the cross-product term of each stratifying variable and continuous coffee intake. Abbreviations: CI: confidence interval.
All details of this study were approved by the local Institutional Review Board at each participating institution and all participants gave written informed consent.
Statistical Analyses
Analyses were performed with SAS release 9.1 (SAS Institute, Cary, NC). An alpha level of <0.05 was considered statistically significant and all tests were two sided.
We tabulated baseline demographic, behavioral, and clinical factors by categories of coffee (Table 1) or tea intake (data not shown). Statistically significant variation across categories of increasing coffee or tea intake was assessed with the Mantel-Haenszel test for trend for categorical variables and the Jonckheere-Terpstra test for trend for continuous variables.
Table 1.
Baseline demographic, clinical, and lab features of 766 participants of the HALT-C trial by category of coffee intake
| Variables | Coffee consumption | P for trend* | |||
|---|---|---|---|---|---|
| Non-drinkers | > 0 to <1 cups/day | ≥ 1 to <3 cups/day | ≥ 3 cups/day | ||
| Number in cohort | 131 | 222 | 324 | 89 | |
| Treatment group, No. (%) | 63 (48.1%) | 113 (50.9%) | 166 (51.2%) | 42 (47.2%) | 0.925 |
| Coffee intake (cups/day), Median (IQR) | 0 | 0.29 (0.03–0.5) | 2 (1–2) | 3.5 (3.5–3.5) | |
| Age, years, Median (IQR) | 48 (45–53) | 50 (46–54) | 50 (46–54) | 49 (46–53) | 0.30 |
| Gender, female, No. (%) | 47 (35.9%) | 66 (29.7%) | 88 (27.2%) | 16 (18.0%) | 0.007 |
| General health score from SF-36, Median (IQR) | 62 (37–77) | 64 (47–77) | 62 (42–77) | 57 (40–67) | 0.29 |
| Physical functioning score from SF-36, Median (IQR) | 85 (50–95) | 90 (61–100) | 85 (60–95) | 87.5 (67.5–95.0) | 0.78 |
| Vitality score from SF-36, Median (IQR) | 55 (35–80) | 60 (45–75) | 55 (40–70) | 50 (35–65) | 0.018 |
| Race/ethnicity | |||||
| Caucasian, No. (%) | 90 (68.7%) | 141(63.5%) | 262 (80.9%) | 82 (92.1%) | <0.0001 |
| African American, No. (%) | 30 (22.9%) | 53 (23.9%) | 35 (10.8%) | 1 (1.1%) | |
| Hispanic, No. (%) | 6 (4.6%) | 23 (10.4%) | 23 (7.1%) | 3 (3.4%) | |
| Other, No. (%) | 5 (3.8%) | 5 (2.3%) | 4 (1.2%) | 3 (3.4%) | |
| Education† | |||||
| High school or less, No. (%) | 41 (31.3%) | 69 (31.1%) | 101 (31.3%) | 36 (40.5%) | 0.21 |
| Some post high school, No. (%) | 49 (37.4%) | 95 (42.8%) | 133 (41.2%) | 33 (37.1%) | |
| Completed college, No. (%) | 41 (31.3%) | 58 (26.1%) | 89 (27.6%) | 20 (22.5%) | |
| Lifetime alcohol consumption, # of drinks, Median (IQR) | 4,117 (438–16,445) | 5,050 (768–16,318) | 8,875 (1,463–22,877) | 18,083 (3,854–37,944) | <0.0001 |
| Pack-years of cigarettes, Median (IQR) | 4.0 (0–15.0) | 3.0 (0–14.5) | 13.5 (1.4–26.0) | 23.5 (10.0–37.0) | <0.0001 |
| Green and black tea intake, cups per day, Median (IQR) | 0.08 (0–0.57) | 0.14 (0.01–0.5) | 0.14 (0.01–0.79) | 0.11 (0.01–0.57) | 0.029 |
| Total energy, Kcal, Median (IQR) | 1,959 (1,289–2,612) | 1,811 (1,292–2,349) | 1,907 (1,399–2,540) | 2,302 (1,554–2,809) | 0.019 |
| Body Mass Index, Median (IQR) | 29.2 (25.9–32.4) | 29.1 (26.4–32.3) | 29.0 (26.2–32.8) | 28.7 (25.7–31.8) | 0.54 |
| Diabetes, Glucose ≥126, No. (%) | 42 (32.1%) | 47 (21.2%) | 61 (18.8%) | 20 (22.5%) | 0.107 |
| Baseline HOMA2,† Median (IQR) | 5.7 (3.7–7.1) | 4.3 (2.9–6.7) | 4.1 (3.0–6.5) | 4.0 (2.5–6.5) | 0.001 |
| Insulin,† Median (IQR) | 43.9 (26.7–79.6) | 34.1 (22.3–55.0) | 33.4 (23.2–51.2) | 31.4 (19.2–53.0) | 0.002 |
| AFP, ng/mL, Median (IQR) | 9.7 (5.2–18.5) | 11.1 (5.9–23.0) | 7.5 (5.0–15.3) | 6.2 (4.0–10.4) | <0.0001 |
| AST, Median (IQR) | 71 (46–108) | 77 (55–128) | 70 (50.5–107) | 60 (43–96) | 0.021 |
| ALT, Median (IQR) | 82 (53–116) | 94 (59–141) | 88 (60–133) | 72 (58–122) | 0.77 |
| AST/ALT Ratio, Median (IQR) | 0.86 (0.71–1.07) | 0.88 (0.71–1.07) | 0.79 (0.66–1.00) | 0.75 (0.63–0.86) | <0.0001 |
| Albumin, g/dL, Median (IQR) | 3.8 (3.6–4.1) | 3.9 (3.5–4.1) | 4.0 (3.7–4.2) | 4.0 (3.8–4.2) | <0.0001 |
| Bilirubin, mg/dL, Median (IQR) | 0.7 (0.5–0.9) | 0.8 (0.6–1.0) | 0.7 (0.5–1.0) | 0.6 (0.5–0.8) | 0.053 |
| Platelets, 1000/mm3, Median (IQR) | 159 (106–220) | 146 (112–196) | 163 (119–203) | 161 (127–215) | 0.25 |
| Prothrombin time, INR, Median (IQR) | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) | 0.188 |
| Log HCV RNA level, Median (IQR) | 6.4 (6.1–6.7) | 6.4 (6.1–6.8) | 6.5 (6.2–6.8) | 6.7 (6.3–6.9) | 0.034 |
| HCV genotype 1, No. (%) | 123 (93.9%) | 202 (91.0%) | 312 (96.3%) | 79 (88.8%) | 0.80 |
| Cirrhosis on biopsy, No. (%) | 57 (43.5%) | 103 (46.4%) | 143 (44.1%) | 28 (31.5%) | 0.073 |
| Hepatic steatosis | |||||
| Grade 0, No. (%) | 16 (12.2%) | 34 (15.3%) | 65 (20.1%) | 17 (19.1%) | 0.047 |
| Grade 1, No. (%) | 59 (45.0%) | 89 (40.1%) | 130 (40.1%) | 37 (41.6%) | |
| Grade 2, No. (%) | 39 (29.8%) | 69 (31.1%) | 105 (32.4%) | 25 (28.1%) | |
| Grade 3 or 4, No. (%) | 17 (13.0%) | 30 (13.5%) | 24 (7.4%) | 10 (11.2%) | |
| Ishak inflammation score, Median (IQR) | 7 (6–9) | 8 (6–9) | 8 (6–9) | 7 (6–9) | 0.51 |
| Esophageal varices†, No. (%) | 35 (27.1%) | 67 (30.5%) | 84 (26.6%) | 16 (18.2%) | 0.068 |
Abbreviations: No: Number; IQR: Interquartile range
Mantel-Haenszel test for trend for categorical variables. Jonckheere-Terpstra test for trend for continuous variables and race/ethnicity.
Data not available for all participants: Education available for 765 participants; Esophageal varices for 753 participants; Insulin and Homa2 score for 593 participants; Pack-years of cigarettes for 757 participants.
Relative risks and 95% confidence intervals for the association of coffee and tea intake and liver disease progression were calculated by use of Cox proportional hazards regression.(28) We calculated follow-up time in person-years from baseline to first outcome, end of study, or date of patient withdrawal. Linear trend tests across increasing categories of coffee and tea intake were performed by assigning participants the median intake for their categories and entering that term as a continuous variable in the regression model. We tested the proportional hazards assumption by modeling interaction terms of time and the trend variable for coffee or tea intake and found no significant deviations.
We examined risk estimates from crude and multivariate models that were adjusted for known and suspected confounders, including age, baseline Ishak fibrosis score, body mass index, education, gender, race and ethnicity, lifetime alcohol intake, pack-years of cigarette use, and intake of total energy, and coffee or tea with progression of liver disease.
Possible effect modification by randomization group, cirrhosis at baseline, self-reported health, sex, use of alcohol or cigarettes at baseline, hepatic steatosis grade, esophageal varices, serum AFP levels, AST/ALT ratio, bilirubin, and albumin were assessed by stratification and formally tested by including an interaction term between each stratifying variable and continuous coffee intake in the model.
The concordance of reported coffee intake between the two food frequency questionnaires was assessed by the weighted kappa statistic. We also examined whether the consistency of reported coffee intake across questionnaires affected the association coffee intake with liver disease progression. These analyses were restricted to those who completed both questionnaires (n=633) and did not have an outcome prior to completion of their second questionnaire (n=586); follow-up time began at the date of the second questionnaire. For analysis, a single variable with mutually exclusive categories from intake reported on each questionnaire was used.
RESULTS
Coffee intake varied from zero to five or more cups per day, with a median intake of one cup per day. At baseline, higher coffee consumption was associated with male gender, Caucasian race, higher lifetime alcohol and cigarette use, increased tea consumption and total energy intake, lower baseline HOMA2 score, lower fasting insulin levels, higher log HCV RNA level, and less severe liver disease (lower AFP levels, serum AST/ALT ratio, hepatic steatosis grade, and higher albumin), p <0.05 for all (Table 1). We observed no association between coffee intake and age, body mass index, cirrhosis status, diabetes, educational attainment, HCV genotype, Ishak inflammation score, platelets, prothrombin time, or treatment group (Table 1). We also examined the association of SF-36 quality of life domains with coffee intake. The physical functioning score had no association with coffee intake (p=0.78), though coffee drinkers tended to have poorer general health (p=0.29) and vitality scores (p=0.018) than non-drinkers. Among baseline variables examined, higher tea consumption was associated only with total energy intake and coffee intake (data not shown).
During 2,407 person-years of follow-up (median: 3.8 years per patient, interquartile range: 2.6–3.8 years), 230 individuals had a two point increase in fibrosis score from baseline or a clinical outcome for liver disease. Combining these endpoints, we observed an inverse association between coffee intake and liver disease progression (Table 2). In crude models, the relative risk (RR) associated with a one cup per day increase in coffee consumption was 0.88 (95% confidence interval (CI): 0.79–0.98). In analyses involving categorical variables, we found that individuals drinking three or more cups of coffee per day had a RR of 0.56 (95%CI: 0.33–0.97) relative to non-coffee drinkers. Across increasing categories of coffee consumption, the p for trend was 0.0013. In multivariate models adjusted for age, baseline Ishak fibrosis score, body mass index, education, gender, race and ethnicity, lifetime alcohol intake, pack-years of cigarette use, tea intake, and total energy intake, we observed similar risk estimates to those from crude models. Relative to non-drinkers, drinkers of three or more cups of coffee per day had a RR of 0.47 (95%CI: 0.27–0.85) for reaching an endpoint indicative of disease progression.
Table 2.
Association of baseline coffee intake with liver disease progression in 766 participants of the HALT-C trial.
| RR per cup/day | Non-drinkers | > 0 to <1 cups/day | ≥ 1 to <3 cups/day | ≥ 3 cups/day | p-for trend | |
|---|---|---|---|---|---|---|
| Cohort, person-years (%) | 404 (16.8) | 678 (28.2) | 1,039 (43.2) | 286 (11.9) | ||
| Cases, No. (%) | 45 (19.6) | 82 (35.7) | 85 (37.0) | 18 (7.8) | ||
| Crude RR (95% CI) | 0.88 (0.79–0.98) | 1.00 (ref) | 1.09 (0.76–1.57) | 0.73 (0.51–1.05) | 0.56 (0.33–0.97) | 0.0013 |
| Multivariate adjusted RR* | 0.85 (0.76–0.96) | 1.00 (ref) | 1.11 (0.76–1.61) | 0.70 (0.48–1.02) | 0.47 (0.27–0.85) | 0.0003 |
| Multivariate RR additionally adjusted for general health score† | 0.85 (0.76–0.96) | 1.00 (ref) | 1.12 (0.77–1.63) | 0.70 (0.48–1.02) | 0.47 (0.27–0.84) | 0.0003 |
| RR additionally adjusted for markers of liver function‡ | 0.91 (0.81–1.02) | 1.00 (ref) | 1.15 (0.78–1.70) | 0.87 (0.58–1.29) | 0.66 (0.36–1.19) | 0.041 |
Abbreviations: No: Number; RR: relative risk; CI: confidence interval
Adjusted for age, body mass index, education, ethnicity, gender, baseline Ishak fibrosis score, total energy intake, lifetime alcohol intake, pack-years of cigarette use, and tea intake.
Adjusted for age, body mass index, education, ethnicity, gender, baseline Ishak fibrosis score, total energy intake, lifetime alcohol intake, pack-years of cigarette use, tea intake, and SF-36 general health score.
Adjusted for age, body mass index, education, ethnicity, gender, baseline Ishak fibrosis score, total energy intake, lifetime alcohol intake, pack-years of cigarette use, tea intake, SF-36 general health score, albumin, AST/ALT ratio, bilirubin, esophageal varices, hepatic steatosis grade, and platelets.
In contrast to coffee intake, no association between black and green tea intake with liver disease progression was observed. In crude models, the RR associated with a one cup per day increase in tea intake was 1.03 (95%CI: 0.91–1.18); the RR from multivariate adjusted models was 1.02 (95%CI: 0.90–1.17) (Table 3).
Table 3.
Association of baseline tea intake with liver disease progression in 766 participants of the HALT-C trial.
| RR per cup/day | Non-drinkers | > 0 to <1 cups/day | ≥ 1 to <2 cups/day | ≥ 2 cups/day | p-for trend | |
|---|---|---|---|---|---|---|
| Cohort, person-years (%) | 456 (18.9) | 1,441 (59.9) | 335 (13.9) | 175 (7.3) | ||
| Cases, No. (%) | 39 (17.0) | 146 (63.5) | 27 (11.7) | 18 (7.8) | ||
| Crude RR (95% CI) | 1.03 (0.91–1.18) | 1.00 (ref) | 1.19 (0.84–1.70) | 0.94 (0.58–1.54) | 1.21 (0.69–2.11) | 0.672 |
| Multivariate adjusted RR* | 1.02 (0.90–1.17) | 1.00 (ref) | 1.24 (0.87–1.79) | 0.96 (0.59–1.58) | 1.19 (0.67–2.10) | 0.576 |
| Multivariate RR additionally adjusted for general health score† | 1.02 (0.89–1.16) | 1.00 (ref) | 1.24 (0.87–1.78) | 0.94 (0.57–1.55) | 1.16 (0.65–2.05) | 0.498 |
| RR additionally adjusted for markers of liver function‡ | 1.08 (0.94–1.24) | 1.00 (ref) | 1.09 (0.75–1.58) | 0.82 (0.49–1.37) | 1.54 (0.85–2.78) | 0.982 |
Abbreviations: No: Number; RR: relative risk; CI: confidence interval
Adjusted for age, body mass index, education, ethnicity, gender, baseline Ishak fibrosis score, total energy intake, lifetime alcohol intake, pack-years of cigarette use, and coffee intake.
Adjusted for age, body mass index, education, ethnicity, gender, baseline Ishak fibrosis score, total energy intake, lifetime alcohol intake, pack-years of cigarette use, coffee intake, and SF-36 general health score.
Adjusted for age, body mass index, education, ethnicity, gender, baseline Ishak fibrosis score, total energy intake, lifetime alcohol intake, pack-years of cigarette use, coffee intake, SF-36 general health score, albumin, AST/ALT ratio, bilirubin, esophageal varices, hepatic steatosis grade, and platelets.
In addition to the baseline FFQ, 633 participants also completed a second FFQ approximately 13 months later. Participants reported similar coffee intakes on both FFQs with a weighted kappa = 0.59, which indicates good agreement. The test of symmetry was not rejected (p=0.92) indicating that there was no systematic increase or decrease in coffee consumption between the two time points. In analyses restricted to events that occurred after administration of the second FFQ (n=599), we observed similar associations between coffee intake and progression of liver disease, whether we used coffee intake reported on the baseline or the subsequent FFQ. From multivariate adjusted models, an increase of one cup of coffee per day on the first FFQ was associated with a RR of 0.84 (95%CI: 0.72–0.97). Similarly, an increase of one cup of coffee per day on the second FFQ was associated with a RR of 0.78 (95%CI: 0.67–0.92). Relative to non-drinkers on both FFQs, coffee drinkers reporting up to three cups of coffee per day on either FFQ had a RR of 0.79 (95%CI: 0.50–1.24), drinkers of three or more cups of coffee per day on either FFQ had a RR of 0.60 (95%CI: 0.30–1.19), and drinkers of three or more cups of coffee per day on both FFQs had a RR of 0.38 (95%CI: 0.14–1.03).
We examined whether risk estimates for coffee intake and liver disease progression varied by treatment group, cirrhosis status at baseline, type of outcome, sex, current alcohol and cigarette use, and markers of liver disease severity, presenting results for drinking three or more cups per day relative to non-drinking (Figure 1). Risk estimates did not vary by treatment group (p for interaction= 0.16). We observed a RR of 0.51 (95%CI: 0.19–1.39) in the active treatment group (112 events) and 0.45 (95%CI: 0.22–0.95) in the control group (118 events) relative to non-drinkers. Estimates also did not vary by cirrhosis status. For those starting with bridging fibrosis (146 events), the RR was 0.46 (95%CI: 0.23–0.91), while for those starting with cirrhosis (84 events), it was 0.44 (95%CI: 0.14–1.38); p for interaction=0.39. Risk estimates were similar for the development of clinical outcomes (0.54, 95%CI: 0.23–1.26) and for a two point increase on Ishak score (0.34, 0.15–0.75). Risk estimates were similar in men and women (0.56, 95%CI: 0.29–1.09; in women: 0.14, 95%CI: 0.03–0.72) and did not vary by use of alcohol (p for interaction= 0.48) or cigarettes (p for interaction=0.40) at baseline.
We also observed similar risk estimates for those with above median values and below median values of AFP, albumin, AST/ALT ratio, bilirubin, hepatic steatosis grade, platelets, and those with and without esophageal varices at baseline (p for interaction > 0.2 for all) (Figure 1). Additionally adjusting for these markers of liver disease severity attenuated the association between coffee intake and liver disease progression (0.66, 95%CI: 0.36–1.19; p-trend across categories=0.041; without adjustment for these markers, RR: 0.47, 95%CI: 0.27–0.85; p-trend=0.0003; Table 2).
Adjustment for SF-36 general health score did not meaningfully alter risk estimates (0.47, 95%CI: 0.27–0.84; Table 2). Risk estimates were similar in those with a less than the median SF-36 general health score (RR: 0.39, 95%CI: 0.19–0.77, 126 events) and those with a greater than median general health score (RR: 0.76, 95%CI: 0.27–2.10, 96 events); p for interaction= 0.29 (Figure 1). Results for other SF-36 quality of life domains were similar (data not shown).
Finally, we examined whether risk estimates for coffee intake varied after adjustment for biochemical markers of insulin resistance and inflammation. Adjustment for diabetes or glucose as a continuous variable did not affect risk estimates (data not shown). In contrast, in models restricted to 593 participants with measured insulin, addition of insulin or HOMA2 score attenuated risk estimates slightly (three cups per day or more relative to non-drinking without insulin or HOMA2 score in the model: 0.48, 95%CI: 0.25–0.93; with insulin in the model: 0.56, 95%CI: 0.28–1.10; with HOMA2 in the model: 0.55 95%CI: 0.28–1.08). With regards to inflammation, results did not change after adjustment for Ishak inflammation score at baseline (HR for three or more cups per day relative to non-drinking: 0.46, 95%CI: 0.26–0.83).
DISCUSSION
In this prospective study of patients with chronic hepatitis C and advanced liver disease who had failed to achieve a sustained virological response with peginterferon plus ribavirin treatment, we observed an inverse association between coffee intake and liver disease progression. Drinkers of three or more cups of coffee per day had 53% lower risk of liver disease progression than non-coffee drinkers. Results were consistent for coffee intake assessed on the baseline questionnaire and coffee intake assessed on a second questionnaire 13 months later. Results were also similar for those with both bridging fibrosis and cirrhosis at baseline. In contrast to coffee, we observed no association with consumption of black or green tea.
This is the first study to address the association between coffee intake and liver disease progression. Previous studies in persons with unknown liver disease, however, have observed inverse associations between coffee intake and the risk of cirrhosis,(6, 9–12) chronic liver disease,(13) and hepatocellular carcinoma.(14, 15) A limitation of these and other previous studies has been the inclusion of persons without liver disease, who were not at risk of its complications. Inverse associations in these studies could simply reflect a lack of coffee drinking in persons with advanced liver disease. In contrast to these studies, all patients in the HALT-C trial had advanced liver disease documented by liver biopsy. Furthermore, all patients in HALT-C had chronic hepatitis C; patients with other causes of liver disease were excluded. Hence, the trial participants were homogeneous for the cause and stage of liver disease. This design and the ability to assess a large number of potentially confounding factors rendered this trial an excellent vehicle for studying risk factors for liver disease progression.
In support of our prospective findings regarding liver disease progression, we found that coffee drinking at baseline was associated with better status for many markers of liver disease and portal hypertension including lower AFP levels, serum AST/ALT ratio, hepatic steatosis grade, and higher albumin. Previous studies from the United States, Japan, and Italy(3–8) reported similar results in cross-sectional comparisons of coffee intake and liver disease markers (γ-glutamyltranspeptidase, ALT, AST, and the AST/ALT ratio).
Symptoms of poor health may cause individuals to decrease their coffee intake, perhaps resulting in the observed association between coffee intake and reduced disease progression. In our study, however, individuals with lower general health and vitality scores actually reported drinking more coffee than individuals with higher scores. Also, including SF-36 health scores in risk models did not alter results. We observed no evidence for an association between reduced coffee consumption on the second FFQ, relative to the first, and subsequent disease outcomes. Risk estimates were also similar for those with fibrosis and cirrhosis at baseline. Although coffee intake was associated inversely with several markers of severe liver disease at baseline, risk estimates appeared similar in those with higher and lower values for these markers. Including these markers in the models attenuated risk estimates, but evidence for an inverse association with coffee intake persisted even after adjustment.
Individuals who drank coffee had higher lifetime alcohol consumption and cigarette use and were also more likely to be current alcohol drinkers and cigarette smokers than non-coffee drinkers (data not shown). Adjustment for lifetime or current alcohol consumption or cigarette use did not affect risk estimates for the association of coffee and liver disease progression. Risk estimates for coffee and liver disease progression were similar in both individuals who smoked cigarettes or drank alcohol at baseline, and those who did not. Finally, lifetime or current use of alcohol or cigarettes was not associated with liver disease progression (data not shown). Together, these results suggest that the association between coffee and liver disease progression observed in this study was independent of alcohol intake and cigarette smoking.
Coffee intake could be a marker of socioeconomic status or another unmeasured or poorly measured exposure. With regards to socio-economic status, coffee intake did not vary by educational attainment. In addition, participants were part of a NIH-funded clinical trial and as such received excellent medical care during the study, regardless of their education or socioeconomic status. Inverse associations between coffee intake and liver disease have been observed in diverse geographic regions, each with different risk factors for liver disease, arguing against coffee as a surrogate for another protective factor. Coffee intake is also not generally considered to be part of a healthy lifestyle. Nevertheless, as in all observational analyses, observed associations in this study could be due to unmeasured or poorly measured confounders.
Coffee intake might protect against liver disease via several mechanisms. Coffee intake has been associated with a decreased risk of type 2 diabetes, (29) and diabetes has been associated with liver disease,(30) suggesting that coffee intake could affect liver disease by modulating insulin sensitivity. Supporting this mechanism, the coffee constituent chlorogenic acid has been shown to inhibit activity of glucose-6-phosphatase,(31) an important regulator of blood glucose levels.(32) In our study, we observed an inverse association between coffee intake and serum insulin levels and baseline HOMA2 score, but no association with serum glucose. Addition of insulin or HOMA2 score to the models slightly attenuated the results for coffee, suggesting that the effect of coffee may act, at least in part, through insulin signaling. Coffee intake could also act by reducing inflammation, as the inflammatory response to liver injury is thought to cause fibrosis and cirrhosis.(33) Several studies have found evidence for an inverse association between coffee intake and mortality from other inflammation related diseases(34, 35) as well as surrogate markers of inflammation such as C-reactive protein;(36) though not all studies are consistent.(37) In our study, coffee intake was not associated with hepatic inflammation at baseline, and adjusting for inflammation score did not affect risk estimates. Finally, coffee could act by reducing oxidative stress, which may play a role in hepatic damage and disease progression.(38)
Coffee contains more than 1000 chemical compounds. The most studied constituent is caffeine, which has potent pharmacologic effects and is present in coffee at high amounts. In a previous study, the association between caffeine and ALT levels was stronger than that between coffee and ALT levels.(7) In addition, caffeine has been shown to inhibit hepatic carcinogenesis in rats(39) and TGF-beta signaling in rat hepatocytes.(17) However, the association of other caffeinated beverages (including tea and soft drinks) with liver disease has been inconsistent, even in populations who consume large quantities of tea, such as in Japan.(6, 8, 10, 40–42) We observed no association with tea intake, although tea, which contains lower concentrations of caffeine than coffee, was consumed less frequently. In addition to caffeine, coffee provides a rich source of bioactive compounds including diterpenes(20) and polyphenols.(21) Future work is needed to identify bioactive components in coffee and determine their effect in vivo.
Our study benefited from a prospective design, assessment of diet at two time points, comprehensive assessment of clinical and histologic features, and careful assessment of clinical and histologic endpoints. Limitations include lack of information on decaffeinated coffee, soft drinks, and coffee brewing methods. As an observational study, the association observed with coffee intake could reflect another exposure or could have been the result of, rather than the cause of, liver disease progression. Also, the SF-36 questionnaire assessing self-reported health may not accurately assess gastro-intestinal symptoms that may affect coffee intake. Our data on coffee consumption was also self reported and may not accurately reflect consumption over the entire time period of liver disease progression. Finally, individuals in this study were hepatitis C positive, had advanced chronic liver disease, and did not respond to standard of care therapy. As such, our findings may not be generalizable to healthier populations. Nevertheless, as individuals who do not respond to standard of care therapy represent a sizable proportion of hepatitis C positive individuals, these findings are of potential importance to a large number of individuals worldwide.
In summary, in a prospective study of individuals with hepatitis C and bridging fibrosis or cirrhosis at baseline, we found a significant inverse association between regular coffee intake and liver disease progression.
Acknowledgments
In addition to the authors of this manuscript, the following individuals were instrumental in the planning, conduct and/or care of patients enrolled in this study at each of the participating institutions as follows: University of Massachusetts Medical Center, Worcester, MA: (Contract N01-DK-9-2326) Gyongyi Szabo, MD, Barbara F. Banner, MD, Maureen Cormier, RN, Donna Giansiracusa, RN
University of Connecticut Health Center, Farmington, CT: (Grant M01RR-06192) Michelle Kelley, RN, ANP
Saint Louis University School of Medicine, St Louis, MO: (Contract N01-DK-9-2324) Bruce Bacon, MD, Brent Neuschwander-Tetri, MD, Elizabeth M. Brunt, MD, Debra King, RN
Massachusetts General Hospital, Boston, MA: (Contract N01-DK-9-2319, Grant M01RR-01066) Raymond T. Chung, MD, Andrea E. Reid, MD, Atul K. Bhan, MD, Wallis A. Molchen
University of Colorado School of Medicine, Denver, CO: (Contract N01-DK-9-2327, Grant M01RR-00051) Gregory T. Everson, MD, S. Russell Nash, MD, Jennifer DeSanto, RN, Carol McKinley, RN
University of California - Irvine, Irvine, CA: (Contract N01-DK-9-2320, Grant M01RR-00827) Timothy R. Morgan, MD, John R. Craig, MD, M. Mazen Jamal, MD, MPH, Muhammad Sheikh, MD, Choon Park, RN
University of Texas Southwestern Medical Center, Dallas, TX: (Contract N01-DK-9-2321, Grant M01RR-00633) Thomas E. Rogers, MD, Janel Shelton, Nicole Crowder, LVN, Rivka Elbein, RN, BSN, Nancy Liston, MPH
University of Southern California, Los Angeles, CA: (Contract N01-DK-9-2325, Grant M01RR-00043) Sugantha Govindarajan, MD, Carol B. Jones, RN, Susan L. Milstein, RN
University of Michigan Medical Center, Ann Arbor, MI: (Contract N01-DK-9-2323, Grant M01RR-00042) Robert J. Fontana, MD, Joel K. Greenson, MD, Pamela A. Richtmyer, LPN, CCRC, R. Tess Bonham, BS
Virginia Commonwealth University Health System, Richmond, VA: (Contract N01-DK-9-2322, Grant M01RR-00065) Richard K. Sterling, MD, Melissa J. Contos, MD, A. Scott Mills, MD, Charlotte Hofmann, RN, Paula Smith, RN
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD: T. Jake Liang, MD, David Kleiner, MD, PhD, Yoon Park, RN, Elenita Rivera, RN, Vanessa Haynes-Williams, RN
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: Leonard B. Seeff, MD, Patricia R. Robuck, PhD, Jay H. Hoofnagle, MD, Elizabeth C. Wright, PhD
University of Washington, Seattle, WA: (Contract N01-DK-9-2318) David R. Gretch, MD, PhD, Minjun Chung Apodaca, BS, ASCP, Rohit Shankar, BC, ASCP
New England Research Institutes, Watertown, MA: (Contract N01-DK-9-2328) Kristin K. Snow, MSc, ScD, Anne M. Stoddard, ScD, Margaret C. Bell, MS, MPH
Armed Forces Institute of Pathology, Washington, DC: Zachary D. Goodman, MD
Data and Safety Monitoring Board Members: (Chair) Gary L. Davis, MD, Guadalupe Garcia-Tsao, MD, Michael Kutner, PhD, Stanley M. Lemon, MD, Robert P. Perrillo, MD
Financial support: This study was supported by the National Institute of Diabetes & Digestive & Kidney Diseases. Additional support was provided by the National Institute of Allergy and Infectious Diseases, the National Cancer Institute, the National Center for Minority Health and Health Disparities and by General Clinical Research Center grants from the National Center for Research Resources, National Institutes of Health. This research was also supported in part by the Intramural Research Program of the National Cancer Institute. Additional funding to conduct this study was supplied by Hoffmann-La Roche, Inc., through a Cooperative Research and Development Agreement with the National Institutes of Health. The funding organizations had no role in the design and conduct of the study; the collection, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Financial disclosures
Financial relationships of the authors with Hoffmann-La Roche, Inc., are as follows: K.L. Lindsay is a consultant and receives research support; M.L. Shiffman is a consultant, on the speaker’s bureau, and receives research support; W.M. Lee receives research support; A.S. Lok is a consultant; A.M. Di Bisceglie is a consultant, on the speaker’s bureau, and receives research support; H.L. Bonkovsky receives research support; J.C. Hoefs is on the speaker’s bureau. Authors with no financial relationships related to this project are: N.D. Freedman, J.E. Everhart, M.G. Ghany, T.M. Curto, C.C. Abnet, R. Sinha, J.L. Dienstag, and C. Morishima.
List of abbreviations
- HALT-C
Hepatitis C Antiviral Long-Term Treatment against Cirrhosis
- HOMA2
homeostatic model assessment score
- FFQ
food frequency questionnaire
- AFP
alpha-fetoprotein
- RR
relative risk
- CI
confidence interval
Footnotes
This is publication #38 from the HALT-C Trial Group.
The HALT-C Trial was registered with clinicaltrials.gov (#NCT00006164).
References
- 1.Williams R. Global challenges in liver disease. Hepatology. 2006;44:521–526. doi: 10.1002/hep.21347. [DOI] [PubMed] [Google Scholar]
- 2.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 3.Arnesen E, Huseby NE, Brenn T, Try K. The Tromso Heart Study: distribution of, and determinants for, gamma-glutamyltransferase in a free-living population. Scand J Clin Lab Invest. 1986;46:63–70. doi: 10.3109/00365518609086483. [DOI] [PubMed] [Google Scholar]
- 4.Casiglia E, Spolaore P, Ginocchio G, Ambrosio GB. Unexpected effects of coffee consumption on liver enzymes. Eur J Epidemiol. 1993;9:293–297. doi: 10.1007/BF00146266. [DOI] [PubMed] [Google Scholar]
- 5.Honjo S, Kono S, Coleman MP, Shinchi K, Sakurai Y, Todoroki I, et al. Coffee consumption and serum aminotransferases in middle-aged Japanese men. J Clin Epidemiol. 2001;54:823–829. doi: 10.1016/s0895-4356(01)00344-4. [DOI] [PubMed] [Google Scholar]
- 6.Klatsky AL, Morton C, Udaltsova N, Friedman GD. Coffee, cirrhosis, and transaminase enzymes. Arch Intern Med. 2006;166:1190–1195. doi: 10.1001/archinte.166.11.1190. [DOI] [PubMed] [Google Scholar]
- 7.Ruhl CE, Everhart JE. Coffee and caffeine consumption reduce the risk of elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2005;128:24–32. doi: 10.1053/j.gastro.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka K, Tokunaga S, Kono S, Tokudome S, Akamatsu T, Moriyama T, et al. Coffee consumption and decreased serum gamma-glutamyltransferase and aminotransferase activities among male alcohol drinkers. Int J Epidemiol. 1998;27:438–443. doi: 10.1093/ije/27.3.438. [DOI] [PubMed] [Google Scholar]
- 9.Corrao G, Zambon A, Bagnardi V, D’Amicis A, Klatsky A. Coffee, caffeine, and the risk of liver cirrhosis. Ann Epidemiol. 2001;11:458–465. doi: 10.1016/s1047-2797(01)00223-x. [DOI] [PubMed] [Google Scholar]
- 10.Gallus S, Tavani A, Negri E, La Vecchia C. Does coffee protect against liver cirrhosis? Ann Epidemiol. 2002;12:202–205. doi: 10.1016/s1047-2797(01)00304-0. [DOI] [PubMed] [Google Scholar]
- 11.Klatsky AL, Armstrong MA, Friedman GD. Coffee, tea, and mortality. Ann Epidemiol. 1993;3:375–381. doi: 10.1016/1047-2797(93)90064-b. [DOI] [PubMed] [Google Scholar]
- 12.Tverdal A, Skurtveit S. Coffee intake and mortality from liver cirrhosis. Ann Epidemiol. 2003;13:419–423. doi: 10.1016/s1047-2797(02)00462-3. [DOI] [PubMed] [Google Scholar]
- 13.Ruhl CE, Everhart JE. Coffee and tea consumption are associated with a lower incidence of chronic liver disease in the United States. Gastroenterology. 2005;129:1928–1936. doi: 10.1053/j.gastro.2005.08.056. [DOI] [PubMed] [Google Scholar]
- 14.Larsson SC, Wolk A. Coffee consumption and risk of liver cancer: a meta-analysis. Gastroenterology. 2007;132:1740–1745. doi: 10.1053/j.gastro.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 15.Bravi F, Bosetti C, Tavani A, Bagnardi V, Gallus S, Negri E, et al. Coffee drinking and hepatocellular carcinoma risk: a meta-analysis. Hepatology. 2007;46:430–435. doi: 10.1002/hep.21708. [DOI] [PubMed] [Google Scholar]
- 16.Devasagayam TP, Kamat JP, Mohan H, Kesavan PC. Caffeine as an antioxidant: inhibition of lipid peroxidation induced by reactive oxygen species. Biochim Biophys Acta. 1996;1282:63–70. doi: 10.1016/0005-2736(96)00040-5. [DOI] [PubMed] [Google Scholar]
- 17.Gressner OA, Lahme B, Rehbein K, Siluschek M, Weiskirchen R, Gressner AM. Pharmacological application of caffeine inhibits TGF-beta-stimulated connective tissue growth factor expression in hepatocytes via PPARgamma and SMAD2/3-dependent pathways. J Hepatol. 2008;49:758–767. doi: 10.1016/j.jhep.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Ruiz JA, Leake DS, Ames JM. In vitro antioxidant activity of coffee compounds and their metabolites. J Agric Food Chem. 2007;55:6962–6969. doi: 10.1021/jf0710985. [DOI] [PubMed] [Google Scholar]
- 19.Lee C. Antioxidant ability of caffeine and its metabolites based on the study of oxygen radical absorbing capacity and inhibition of LDL peroxidation. Clin Chim Acta. 2000;295:141–154. doi: 10.1016/s0009-8981(00)00201-1. [DOI] [PubMed] [Google Scholar]
- 20.Cavin C, Holzhaeuser D, Scharf G, Constable A, Huber WW, Schilter B. Cafestol and kahweol, two coffee specific diterpenes with anticarcinogenic activity. Food Chem Toxicol. 2002;40:1155–1163. doi: 10.1016/s0278-6915(02)00029-7. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro H, Bruck R. Coffee and tea consumption and chronic liver disease. Gastroenterology. 2006;130:1931–1932. doi: 10.1053/j.gastro.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 22.Di Bisceglie AM, Shiffman ML, Everson GT, Lindsay KL, Everhart JE, Wright EC, et al. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med. 2008;359:2429–2441. doi: 10.1056/NEJMoa0707615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishak K, Baptista A, Bianchi L, Callea F, De GJ, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 24.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 25.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–1335. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 26.Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc. 1992;92:686–693. [PubMed] [Google Scholar]
- 27.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 28.Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society Series B-Statistical Methodology. 1972;34:187. [Google Scholar]
- 29.van Dam RM, Hu FB. Coffee consumption and risk of type 2 diabetes: a systematic review. JAMA. 2005;294:97–104. doi: 10.1001/jama.294.1.97. [DOI] [PubMed] [Google Scholar]
- 30.Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2003;124:71–79. doi: 10.1053/gast.2003.50004. [DOI] [PubMed] [Google Scholar]
- 31.Arion WJ, Canfield WK, Ramos FC, Schindler PW, Burger HJ, Hemmerle H, et al. Chlorogenic acid and hydroxynitrobenzaldehyde: new inhibitors of hepatic glucose 6-phosphatase. Arch Biochem Biophys. 1997;339:315–322. doi: 10.1006/abbi.1996.9874. [DOI] [PubMed] [Google Scholar]
- 32.Chou JY, Matern D, Mansfield BC, Chen YT. Type I glycogen storage diseases: disorders of the glucose-6-phosphatase complex. Curr Mol Med. 2002;2:121–143. doi: 10.2174/1566524024605798. [DOI] [PubMed] [Google Scholar]
- 33.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersen LF, Jacobs DR, Jr, Carlsen MH, Blomhoff R. Consumption of coffee is associated with reduced risk of death attributed to inflammatory and cardiovascular diseases in the Iowa Women’s Health Study. Am J Clin Nutr. 2006;83:1039–1046. doi: 10.1093/ajcn/83.5.1039. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Garcia E, van Dam RM, Li TY, Rodriguez-Artalejo F, Hu FB. The relationship of coffee consumption with mortality. Ann Intern Med. 2008;148:904–914. doi: 10.7326/0003-4819-148-12-200806170-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez-Garcia E, van Dam RM, Qi L, Hu FB. Coffee consumption and markers of inflammation and endothelial dysfunction in healthy and diabetic women. Am J Clin Nutr. 2006;84:888–893. doi: 10.1093/ajcn/84.4.888. [DOI] [PubMed] [Google Scholar]
- 37.Zampelas A, Panagiotakos DB, Pitsavos C, Chrysohoou C, Stefanadis C. Associations between coffee consumption and inflammatory markers in healthy persons: the ATTICA study. Am J Clin Nutr. 2004;80:862–867. doi: 10.1093/ajcn/80.4.862. [DOI] [PubMed] [Google Scholar]
- 38.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 39.Hosaka S, Kawa S, Aoki Y, Tanaka E, Yoshizawa K, Karasawa Y, et al. Hepatocarcinogenesis inhibition by caffeine in ACI rats treated with 2-acetylaminofluorene. Food Chem Toxicol. 2001;39:557–561. doi: 10.1016/s0278-6915(00)00175-7. [DOI] [PubMed] [Google Scholar]
- 40.Heilbrun LK, Nomura A, Stemmermann GN. Black tea consumption and cancer risk: a prospective study. Br J Cancer. 1986;54:677–683. doi: 10.1038/bjc.1986.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inoue M, Yoshimi I, Sobue T, Tsugane S. Influence of coffee drinking on subsequent risk of hepatocellular carcinoma: a prospective study in Japan. J Natl Cancer Inst. 2005;97:293–300. doi: 10.1093/jnci/dji040. [DOI] [PubMed] [Google Scholar]
- 42.Montella M, Polesel J, La Vecchia C, Dal ML, Crispo A, Crovatto M, et al. Coffee and tea consumption and risk of hepatocellular carcinoma in Italy. Int J Cancer. 2007;120:1555–1559. doi: 10.1002/ijc.22509. [DOI] [PubMed] [Google Scholar]