Abstract
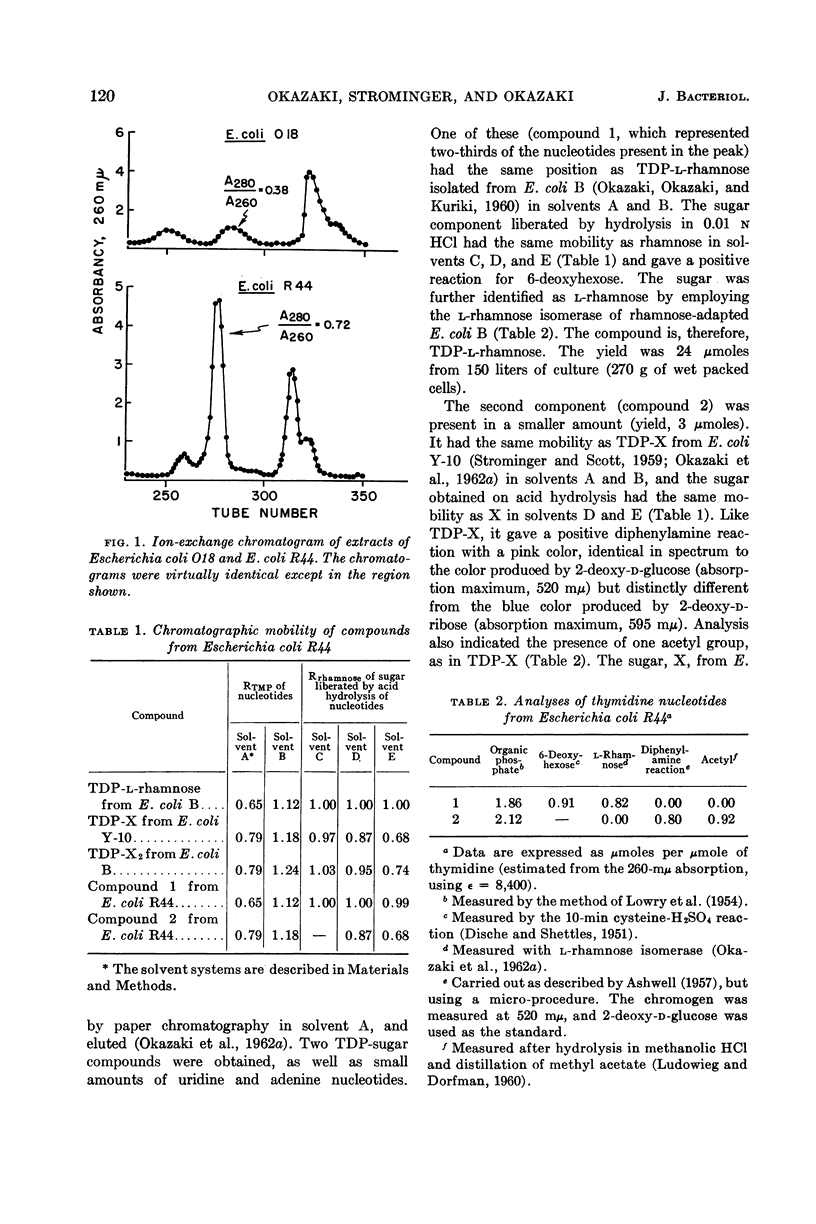
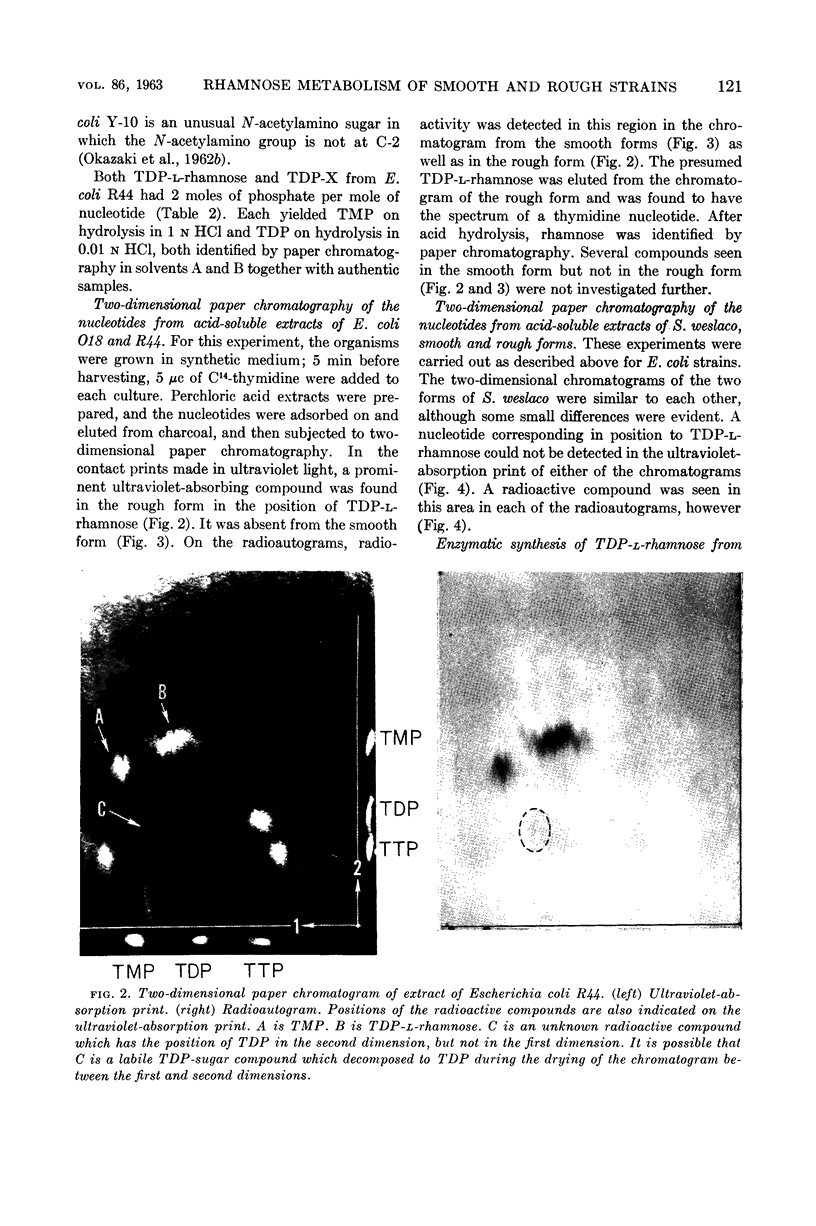
Okazaki, Tuneko (Washington University School of Medicine, St. Louis, Mo.), Jack L. Stominger, and Reiji Okazaki. Thymidine diphosphate-l-rhamnose metabolism in smooth and rough strains of Escherichia coli and Salmonella weslaco. J. Bacteriol. 86:118–124. 1963.—Logarithmic-phase cells of Escherichia coli O18, which have rhamnose in their lipopolysaccharide, contained only traces of thymidine diphosphate (TDP)-l-rhamnose. Extracts of this organism, however, catalyzed the synthesis of TDP-l-rhamnose from TDP-d-glucose. On the other hand, cells of E. coli R44, a rough variant of this strain which contains no rhamnose in its lipopolysaccharide, contained a large amount of TDP-l-rhamnose. Like the smooth form, this organism was able to synthesize TDP-l-rhamnose. The rough variant is apparently a mutant blocked in some manner in utilization of TDP-l-rhamnose for lipopolysaccharide synthesis. Similar studies of smooth (rhamnose-containing) and rough (rhamnose-lacking) forms of Salmonella weslaco showed that both organisms can synthesize TDP-l-rhamnose from TDP-d-glucose. In contrast to the smooth and rough forms of E. coli O18, only traces of TDP-l-rhamnose were detected in extracts of both forms. A second thymidine diphosphosugar compound isolated from E. coli R44 is similar or identical to TDP-X, previously isolated from E. coli Y-10.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DISCHE Z., SHETTLES L. B. A new spectrophotometric test for the detection of methylpentose. J Biol Chem. 1951 Oct;192(2):579–582. [PubMed] [Google Scholar]
- GLASER L., KORNFELD S. The enzymatic synthesis of thymidine-linked sugars. II. Thymidine diphosphate L-rhamnose. J Biol Chem. 1961 Jun;236:1795–1799. [PubMed] [Google Scholar]
- HEATH E. C., ELBEIN A. D. The enzymatic synthesis of guanosine diphosphate colitose by a mutant strain of Escherichia coli. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1209–1216. doi: 10.1073/pnas.48.7.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HURLBERT R. B., SCHMITZ H., BRUMM A. F., POTTER V. R. Nucleotide metabolism. II. Chromatographic separation of acid-soluble nucleotides. J Biol Chem. 1954 Jul;209(1):23–39. [PubMed] [Google Scholar]
- KAUFFMANN F., BRAUN O. H., LUEDERITZ O., STIERLIN H., WESTPHAL O. [Immunochemistry of O-antigens of Enterobacteriaceae. IV. Analysis of the sugar constituents of Escherichia O-antigens]. Zentralbl Bakteriol. 1960 Oct;180:180–188. [PubMed] [Google Scholar]
- KAUFFMANN F., KRUEGER L., LUEDERITZ O., WESTPHAL O. [On the immunochemistry of the O-antigen of Enterobacteriaceae. VI. Comparison of the sugar components of polysaccharides from S and R forms of Salmonella]. Zentralbl Bakteriol. 1961 May;182:57–66. [PubMed] [Google Scholar]
- KAUFFMANN F., LUEDERITZ O., STIERLIN H., WESTPHAL O. [On the immunochemistry of O antigens of Enterobacteriaceae. I. Analysis of the sugar component of Salmonella O antigens]. Zentralbl Bakteriol. 1960 May;178:442–458. [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- LUDOWIEG J., DORFMAN A. A micromethod for the colorimetric determination of N-acetyl groups in acid mucopolysaccharides. Biochim Biophys Acta. 1960 Feb 26;38:212–218. doi: 10.1016/0006-3002(60)91233-6. [DOI] [PubMed] [Google Scholar]
- NIKAIDO H., JOKURA K. Isolation of cytidine diphosphate 3,6-dideoxyhexoses from Salmonella. Biochem Biophys Res Commun. 1961 Nov 29;6:304–309. doi: 10.1016/0006-291x(61)90384-9. [DOI] [PubMed] [Google Scholar]
- NIKAIDO H. Studies on the biosynthesis of cell wall polysaccharide in mutant strains of Salmonella. II. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1542–1548. doi: 10.1073/pnas.48.9.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKAZAKI R., OKAZAKI T., KURIKI Y. Isolation of thymidine diphosphate rhamnose and a novel thymidine diphosphate sugar compound from Escherichia coli strain B. Biochim Biophys Acta. 1960 Feb 26;38:384–386. doi: 10.1016/0006-3002(60)91271-3. [DOI] [PubMed] [Google Scholar]
- OKAZAKI R., OKAZAKIT, STROMINGER J. L., MICHELSON A. M. Thymidine diphosphate 4-keto-6-deoxy-d-glucose, an intermediate in thymidine diphosphate L-rhamnose synthesis in Escherichia coli strains. J Biol Chem. 1962 Oct;237:3014–3026. [PubMed] [Google Scholar]
- OKAZAKI R. Studies of deoxyribonucleic acid synthesis and cell growth in the deoxyriboside-requiring bacteria, Lactobacillus acidophilus. III. Identification of thymidine diphosphate rhamnose. Biochim Biophys Acta. 1960 Nov 18;44:478–490. doi: 10.1016/0006-3002(60)91602-4. [DOI] [PubMed] [Google Scholar]
- OKAZAKI T., OKAZAKI R., STROMINGER J. L., SUZUKI S. Thymidine diphosphate N-acetylamino sugar compounds from Escherichia coli strains. Biochem Biophys Res Commun. 1962 May 4;7:300–305. doi: 10.1016/0006-291x(62)90195-x. [DOI] [PubMed] [Google Scholar]
- PAZUR J. H., SHUEY E. W. The enzymatic synthesis of thymidine diphosphate glucose and its conversion to thymidine diphosphate rhamnose. J Biol Chem. 1961 Jun;236:1780–1785. [PubMed] [Google Scholar]
- STROMINGER J. L., SCOTT S. S. Isolation of thymidine diphosphosugar compounds from Escherichia coli. Biochim Biophys Acta. 1959 Oct;35:552–553. doi: 10.1016/0006-3002(59)90412-3. [DOI] [PubMed] [Google Scholar]