Abstract
Whereas hepatocytes secrete the major human plasma high density lipoproteins (HDL)-protein, apo A-I, as lipid-free and lipidated species, the biogenic itineraries of apo A-II and apo E are unknown. Human plasma and HepG2 cell-derived apo A-II and apo E occur as monomers, homodimers and heterodimers. Dimerization of apo A-II, which is more lipophilic than apo A-I, is catalyzed by lipid surfaces. Thus, we hypothesized that lipidation of intracellular and secreted apo A-II exceeds that of apo A-I, and once lipidated, apo A-II dimerizes. Fractionation of HepG2 cell lysate and media by size exclusion chromatography showed that intracellular apo A-II and apo E are fully lipidated and occur on nascent HDL and VLDL respectively, while only 45% of intracellular apo A-I is lipidated. Secreted apo A-II and apo E occur on small HDL and on LDL and large HDL respectively. HDL particles containing both apo A-II and apo A-I form only after secretion from both HepG2 and Huh7 hepatoma cells. Apo A-II dimerizes intracellularly while intracellular apo E is monomeric but after secretion associates with HDL and subsequently dimerizes. Thus, HDL apolipoproteins A-I, A-II and E have distinct intracellular and post-secretory pathways of hepatic lipidation and dimerization in the process of HDL formation. These early forms of HDL are expected to follow different apolipoprotein-specific pathways through plasma remodeling and reverse cholesterol transport.
Keywords: apo A-I, apo A-II, apo E, HDL formation, HepG2, Huh7
1. Introduction
Human plasma high-density lipoproteins-cholesterol is a negative risk factor for cardiovascular disease for which current therapies are inadequate [1]. HDL promote reverse cholesterol transport (RCT) by carrying excess cellular cholesterol through the plasma compartment for disposal by the liver [2], inhibit LDL oxidation [3], and are anti-inflammatory [4]. Most plasma HDL are spherical particles with a neutral lipid core surrounded by a surface monolayer of apolipoproteins, cholesterol and phospholipids [5]. Apo A-I and apo A-II are 64% and 20% of HDL-protein mass [5]. Some HDL particles contain only apo A-I (LpAI); most of the remainder contain both apo A-I and apo A-II (LpA-I/A-II) [6]. Formation of LpA-I/LpA-II in plasma occurs via LCAT-mediated fusion of nascent discoidal apo A-II HDL with small spherical LpA-I [7] or by displacement of apo AI by the more lipophilic apo A-II [8, 9, 10]. Intracellular formation has not been fully delineated. Apo E, a minor HDL protein, preferentially associates with large HDL [11, 12, 13]. The distributions of HDL apolipoproteins, which are exchangeable, are modified by LCAT, CETP, PLTP and hepatic lipase [14]. Indeed, HDL structural remodeling and apo A-I dissociation are essential to RCT [2, 15].
Apo A-I is antiatherogenic, promoting RCT, activating LCAT, and inducing regression of atherosclerotic lesions [16, 17, 18]. Whereas studies of mice over-expressing apo A-II reveal both anti- and pro-atherogenic activities [19, 20], a recent study showed that apo A-II level is inversely associated with risk for atherosclerosis [21]. Human apo A-II is distinguished by Cys6, which forms disulfide-linked dimers [22, 23]. Homodimeric apo A-II is more lipophilic than the monomer, giving it distinct properties [24]; >96% of human plasma apo A-II is dimeric. Apo A-II dimerization, which is slow in aqueous buffer [22], is catalyzed ~7500-fold by lipid surfaces [25]. Apo E, which occurs in three isoforms with different cysteine contents, mediates hepatic clearance of apo B-containing lipoproteins [26] and promotes RCT [27]. Apo E3 (Cys112, Arg158), the most common isoform, is synthesized by HepG2 cells, and in plasma of E3 homozygotes about 55% of the apo E3 is homodimeric or heterodimeric with apo A-II [28, 29].
Hepatocytes synthesize and secrete HDL apolipoproteins [30, 19]; ~20% of apo A-I is lipidated intracellularly with additional lipidation occurring after secretion [31, 32, 33, 34]. The corresponding intracellular lipidation itineraries of apo A-II and apo E have not been reported [10]. Given the mechanistic link between apo A-II lipidation and dimerization, we investigated the synergism between these processes in lipoprotein biogenesis in HepG2 and Huh7 cells and found that apolipoproteins A-I, A-II and apo E are uniquely distributed among intracellular and newly secreted lipoproteins.
2. Materials and Methods
2.1 Cell culture
Human hepatoma HepG2 cells (American Type Culture Collection, Manassas, VA) and Huh7 cells (kindly provided by Dr. Yumin Xu in Dr. Boris Yoffe’s laboratory, Baylor College of Medicine, Houston, TX) were cultured in MEM (InVitrogen, Carlsbad, CA) with 10% FBS, 1 mM sodium pyruvate and penicillin-streptomycin antibiotics (10 U/mL, 10 μg/mL respectively) (InVitrogen, Carlsbad, CA). Cells were plated and grown to about 80% confluency, washed twice with PBS, incubated with serum-free MEM, and then cells and media were harvested for analysis. Two time points were chosen, one relatively early (2 h) and one relatively late (24 h) after secretion. The early 2 h media is enriched in newly secreted lipoproteins, while by the time of collection of the late 24 h media extensive remodeling of the secreted lipoproteins has occurred [35, 36, 37, 32]. The longer time has been used in many previous studies of HepG2 lipoprotein secretion [38, 30, 39, 40]. Dimerization of apo A-II and E was blocked at the time of harvest by addition of 5 mM iodoacetamide or 10 mM N-ethyl maleimide (NEM) to alkylate any free cysteines. Medium was collected, centrifuged to pellet cell debris and the supernatant was transferred to fresh tubes. Cell dishes were placed on ice, cells were washed twice with ice-cold PBS, and lysed in NP-40 lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% NP-40 (Roche, Indianapolis, IN), pH 8.0) with 10 mM NEM and protease inhibitor cocktail (Roche, Indianapolis, IN). Control experiments showed that this lysis buffer did not affect HDL elution profiles by size exclusion chromatography (data not shown). Cell lysates were centrifuged to remove nuclear debris, and the supernatants transferred to fresh tubes. One-tenth volume 10X buffer (100 mM Tris-HCl pH 7.4, 1 M NaCl, 10 mM EDTA, 10 mM NaN3) was added, and media and lysate samples were stored at 4°C until further processing and analysis.
2.2 Ultracentrifugation
Lipidated and lipid-free apolipoproteins in cell media were separated essentially as described [31]. Media samples were adjusted to d = 1.25 g/ mL with solid KBr and lipoproteins floated by centrifugation. Three fractions were collected: T: top 3 mL, containing the lipid-associated apolipoproteins; M: middle 3 mL (M); B: bottom 6 mL, containing the lipid-free proteins (B). The middle fraction (M) contained less than 10% of the protein in the bottom (B) fraction and little if any of the lipidated proteins (data not shown). Buffer was exchanged for TBS and samples were concentrated with Centricon Plus-20 centrifugal filter devices, 5,000 MWCO (Millipore, Bedford, MA).
2.3 Size exclusion chromatography (SEC)
Lipoproteins and lipid-free proteins in media and cell lysate samples were separated by SEC on tandem Superose 6 FPLC columns, as described [41, 42]. Eluants (1 mL) were collected into tubes, which were pooled into 10 fractions each for media and lysate samples, as in Figure 2. Peak elution volumes for human plasma lipoproteins were VLDL, Fraction #1, LDL, Fraction #3 and HDL, Fraction #6; lipid-free apo-A-I eluted in fraction #8. Larger HDL eluted in Fraction 5, and lipid-poor HDL in Fraction 7 [42].
Figure 2. Size exclusion chromatography (SEC) profiles of HepG2 media and lysate samples.
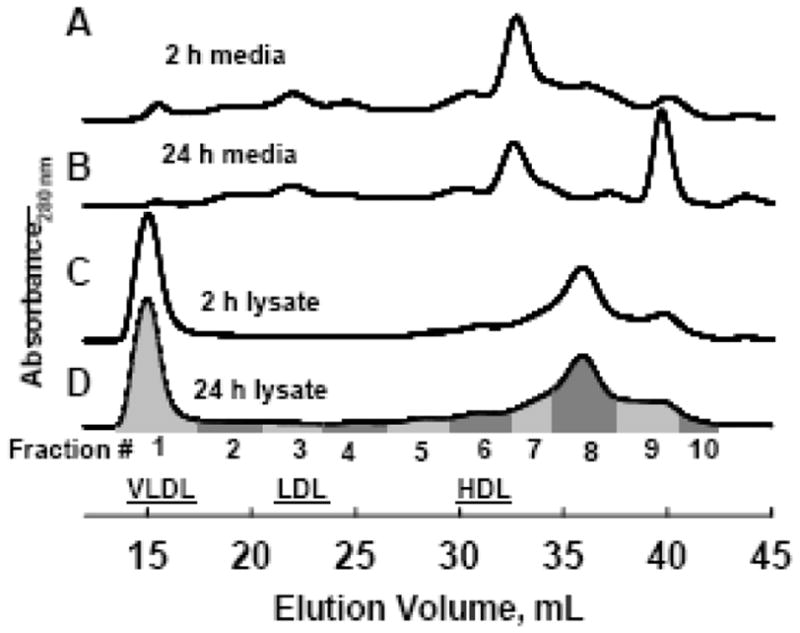
Samples were collected at 2 or 24 h. Eluants were pooled into 10 fractions as indicated by the gray bars, and used for subsequent Western blot analysis of apolipoprotein distribution as in Figure 3. Human plasma VLDL, LDL and HDL peak fractions are 1, 3 and 6, respectively.
2.4 Thiopropyl sepharose (TPS) chromatography
Columns of Thiopropyl Sepharose 6B (GE Healthcare, Piscataway, NJ), 1 mL bed volume, were used to bind the cysteine containing apo A-II to separate LpA-I from LpA-I/A-II. For study of cell lysates, HepG2 and Huh7 cells, incubated for 2 h in serum-free MEM were lysed as above, except that NEM was omitted, and the lysis buffer pH was adjusted to 7.4, to maintain cytosolic binding conditions. Binding of dimeric apo A-II to TPS requires its prior reduction with DTT to form the free –SH groups to which TPS binds. Thus, lysates were treated with 20 mM DTT to reduce all disulfide bonds and then dialysed against TBS containing 0.5 mM DTT to maintain the reduced –SH groups. The 1mL bed volume minicolumns had a calculated –SH capacity of ~ 15 μmol, corresponding to a 5 fold excess for the amount of DTT to be loaded. The contribution of cell lysate proteins to the total –SH concentration was estimated to be at least an order of magnitude less than that from the 0.5 mM DTT. Human plasma HDL samples were reduced with 20 mM DTT, dialyzed and processed through TPS columns in parallel with the cell lysates as controls for separation of LpA-I from LpA-I/A-II (Figure 4 and Table 2). Four to 6 mL of the reduced and dialyzed cell lysates or HDL were loaded onto the TPS columns. The initial flow-through was recycled through the column for 1 h. The subsequent flow-through was collected (Free fraction), the columns were washed with 5 bed volumes (5 mL) TBS (Wash fraction) and eluted with 5 bed volumes 20 mM DTT in TBS (Bound fraction). Aliquots of each fraction were delipidated, solubilized in SDS-PAGE sample buffer, and analyzed by SDS-PAGE and Western blotting.
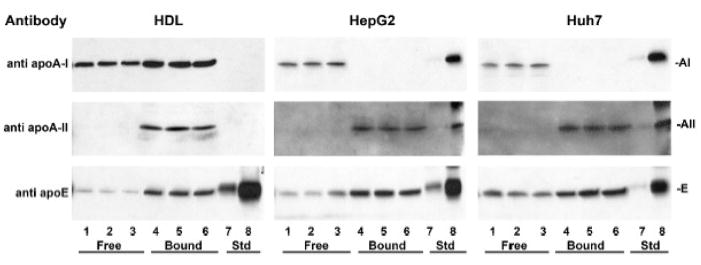
Figure 4. Intracellular apo A-I and apo A-II are on distinct particles.
Cell lysate samples from HepG2 (middle panels) and Huh7 cells (right panels) collected at 2 h were fractionated on TPS columns and probed by Western blot with antibodies to apo A-I, apo A-II and apo E. Lanes 1 – 3: Triplicate aliquots of column pass through fraction (Free). Lanes 4 – 6: Triplicate aliquots of bound fraction (Bound). Lanes 7, 8: standard apolipoproteins, 0.1 and 1 ng each, respectively. The efficiency and capacity of the TPS columns to bind reduced apo A-II and resolve LpA-I from LpA-I/A-II was demonstrated with human plasma HDL (left panels), processed in parallel with the cell lysates but loaded onto the TPS columns at more than ten times the lysate apolipoprotein concentration.
Table 2.
Intracellular apo A-I and apo A-II are on distinct particles. a
| HDL | HepG2 cell lysate | Huh7 cell lysate | ||||
|---|---|---|---|---|---|---|
| free | bound | free | bound | free | bound | |
| Apo A-I | 0.34 + 0.05 | 0.66 + 0.05 | 0.98 + 0.03 | 0.02 + 0.03 | 0.94 + 0.09 | 0.06 + 0.09 |
| Apo A-II | 0.06 + 0.04 | 0.94 + 0.04 | 0.01 + 0.01 | 0.99 + 0.01 | 0.08 + 0.11 | 0.92 + 0.11 |
| Apo E | 0.22 + 0.16 | 0.78 + 0.16 | 0.40 + 0.10 | 0.60 + 0.10 | 0.41 + 0.07 | 0.59 + 0.07 |
Plasma and cells lysates were fractionated on TPS columns, as in Figure 4. Western blot band intensity was quantified on a Storm 840 imaging system with ImageQuant TL software, and the relative amount of each apolipoprotein in the free and bound fractions was calculated from two sets of blots, each done in triplicate. Values are mean and standard deviation.
To determine the kinetics of LpAI/AII formation in hepatoma media, 60 mL media per time point was collected as above at 0.5, 1.0, 2.0 and 20 h. At the time of harvest, 2 mM paraoxon-ethyl (Sigma, St. Louis) was added to inhibit LCAT to stop further formation of LpAI/AII [43]. After 30 min, 1mM DTT was added to reduce disulfide bonds, and after another 30 min, samples were loaded onto 7 mL bed volume TPS columns. After two passes through the column, the flow through (Free fraction) was collected, the column was washed with 10 bed volumes TBS, and bound fraction was eluted with 5 bed volumes 20 mM DTT in TBS. Free and bound fractions were concentrated and aliquots analyzed by SDS-PAGE and Western blotting.
2.5 SDS-PAGE and Western blots
Lipoproteins and apolipoproteins in media and cell lysates were separated according to density by ultracentrifugation, SEC or TPS and analyzed for apolipoproteins A-I, A-II, B and E by SDS-PAGE (15% Tris-Glycine Ready Gels; BioRad, Hercules, CA) and Western blotting. Samples were loaded in non-reducing or reducing (plus β-mercaptoethanol, (+βMSH)) sample buffer, as indicated in the figure legends. After transfer to nitrocellulose, the Western blotting method was essentially that of the Amersham ECL-Plus Western Blotting Manual (Amersham Biosciences/ GE Healthcare, Piscataway, NJ). Band identification is based on molecular weight and reaction by Western blot with highly specific, high affinity antibodies. All SDS/PAGE gels and Western blots were done with both molecular weight standards and apolipoprotein standards on all the gels/blots. Intensity of bands detected by Western blotting was quantitated either by densitometry of X-ray exposures using Kodak 1D image analysis software, or directly on a Storm 840 imaging system (GE Healthcare, Piscataway, NJ) with ImageQuant TL software. Antibodies for immunoblots were HRP-conjugated goat anti-human apo A-I, apo A-II, apo B and apo E (Academy Biomedical, Houston, TX), or goat polyclonal (Academy Biomedical, Houston, TX) or mouse monoclonal (BioDesign/Meridian Life Sciences, Cincinnati, OH) antibodies with appropriate HRP-conjugated second antibodies (Chemicon/Millipore, Billerica, MA or Jackson Immunoresearch, West Grove, PA).
3. Results
3.1 Apolipoprotein Lipidation and Dimerization in Secreted Lipoproteins
In our first experiments ultracentrifugation was used to separate lipidated and lipid-free apolipoproteins as previously reported [31]. Immunoblot analysis of fractions from HepG2 cell media harvested after 2 and 24 h (Figure 1; Table 1) demonstrated that at 2 h, apo A-I was partially lipidated (85 %) while apo A-II and apo E were essentially fully lipidated (95%, 94%). Extent of dimerization of apo A-II and apo E was determined by SDS-PAGE analyses under non-reducing and reducing conditions. Most apo A-II in 2 h media is homodimer (92%) while most apo E is monomer (97%). By 24 h, apo E has undergone dimerization (36% A-II-E and 9% E2). At this later time, for all three apolipoproteins, a larger proportion was recovered in the lipid-free d>1.25 fraction (24 – 28%). This could be due to the low amount of lipid production by HepG2 cells [38, 30, 39, 40] and/or loss of apolipoproteins from lipoproteins during ultracentrifugation (see below) [44, 11, 45]. The minor lipid-free apo A-II monomer bands detected in 24 h media (Figure 1, Panel B, anti-A-II, lanes 7 and 8) have increased molecular weight, and may be glycosylated isoforms [46]. All apo B was lipidated at both 2 and 24 h.
Figure 1. Lipidation and dimerization of apolipoproteins in HepG2 media by density ultracentrifugation.
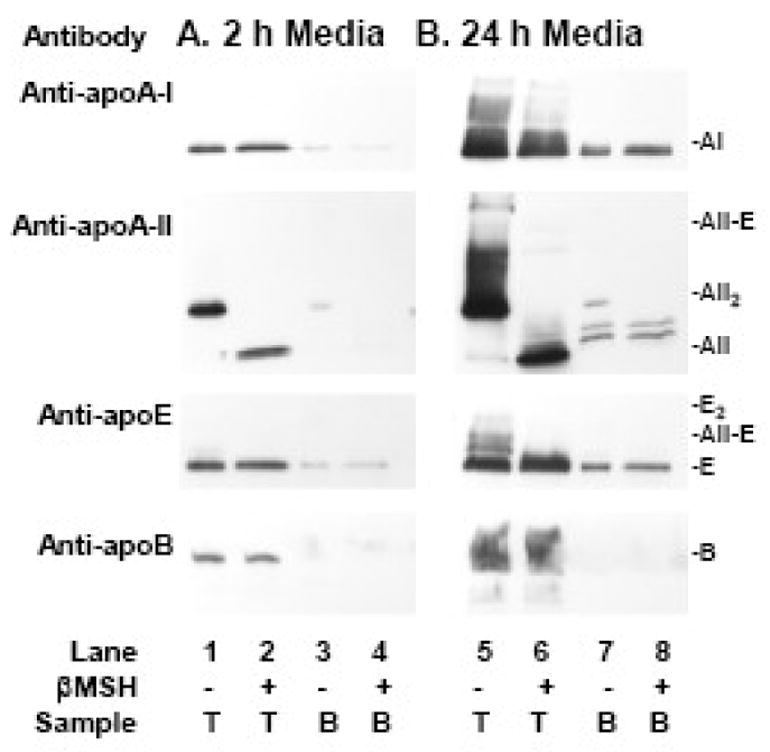
Western blot analysis of the dimerization and lipidation of apolipoproteins A-I, A-II, E, and B in HepG2 media collected at 2 h (Panel A) and 24 h (Panel B). Media was separated into lipidated and non-lipidated species by density ultracentrifugation. Lanes 1, 2, 5, 6: T: top, d < 1.25; Lanes 3, 4, 7, 8: B: bottom d>1.25. Sample buffer was non-reducing (βMSH − ) or reducing (βMSH +). Migration of apolipoprotein standards is indicated in the right margin.
Table 1.
Lipidation and dimerization of apolipoproteins in HepG2 media determined by density ultracentrifugation. a
| Apo A-II b | Apo E b | Apo A-I | Apo-B | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| total | A-II | (A-II)2 | A-II-E | total | E | A-II-E | E2 | |||
| 2 h Media | ||||||||||
| Lipidated | 95+2 | 4+3 | 92+3 | 4+2 | 94+3 | 97+1 | 3+1 | 0+1 | 85+8 | 96+3 |
| Lipid free | 6+1 | 2+2 | 88+8 | 10+9 | 6+3 | 98+3 | 0+0 | 2+3 | 15+8 | 5+3 |
| 24 h Media | ||||||||||
| Lipidated | 76+1 | 7+6 | 78+8 | 15+7 | 72+1 | 55+5 | 36+1 | 9+5 | 74+10 | 96+0 |
| Lipid free | 24+1 | 57+14 | 37+14 | 6+3 | 28+1 | 98+1 | 1+1 | 1+1 | 26+10 | 5+0 |
Percent distribution of apolipoproteins in the lipidated (top) and lipid-free (bottom) fractions after density ultracentrifugation at d = 1.25 with KBr. Values were determined by densitometry of Western blots, as in Figure 1. Values are mean and range for two independent experiments, each done in duplicate.
For apo A-II and E, the percent of monomer, dimer and heterodimer in the lipidated and lipid-free fractions is shown.
3.2 Apolipoprotein Distribution among HDL Subfractions
Although flotation separates lipidated from lipid-free proteins it provides little information about the lipoprotein subfractions into which apolipoproteins are distributed. To further analyze apolipoprotein lipidation, media and lysates were analyzed by SEC, which resolves lipoprotein particles and lipid-free proteins [41]. Importantly, this method obviates ultracentrifugal artifacts that strip apolipoproteins from HDL [44, 11]. SEC of samples from 2 and 24 h HepG2 media (Figure 2) and the attendant immunoblots of the collected fractions (Figure 3A, 3B) reveal distinct distributions of apolipoproteins A-I, A-II and E. Apo A-I in 2 h media was distributed equally (43% each) between Fractions 6 and 7, which correspond to average plasma-sized HDL and small lipid-poor HDL, with 14% on larger HDL (Fraction 5). By 24 h, media apo A-I on larger HDL increased (Fractions 5 and 6) consistent with maturation of HDL species during the longer incubation time. Essentially all apo A-II in 2 h media is homodimer and most (~69%) elutes in Fraction 6 while 23% elutes on smaller lipid-poor particles (Fraction 7) and 8 % on larger HDL (Fraction 5). At 24 h, Apo A-II homodimer has a lipoprotein distribution like that at 2 h, but the apo A-II-apo E heterodimers formed by this time are on larger HDL (Fractions 4, 5 and 6). Apo E in 2 h media is monomeric, and associated with larger HDL as well as LDL (Fractions 2 – 6). By 24 h, apo E homodimers and apo A-II-apo E heterodimers are detected and these are associated with larger HDL particles (Fractions 4 – 6), while only monomeric apo E is present in LDL Fraction 3. In human plasma also the dimeric apo E content of HDL particles is relatively high while monomeric apo E is predominantly on apo B-containing lipoproteins [29, 45]. Media apo B appeared in Fraction 1 (VLDL) and to a lesser extent in Fraction 3 (LDL) (data not shown).
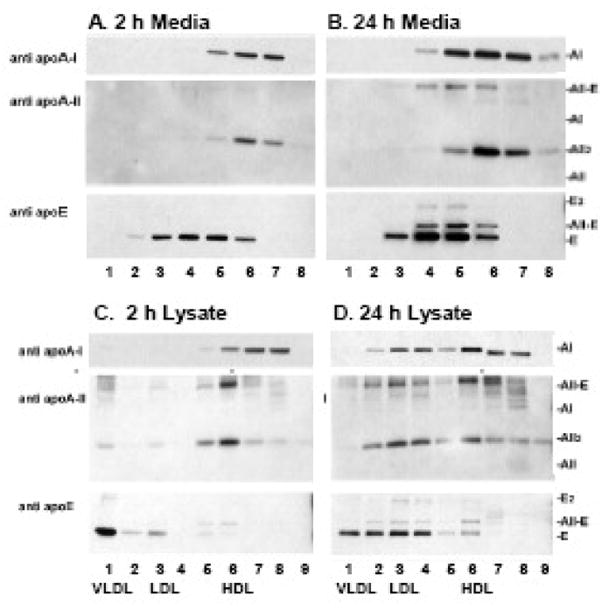
Figure 3. Lipidation and dimerization of apolipoproteins in HepG2 media and cell lysates by SEC.
Two and 24 h media and cell lysate SEC fractions, pooled as in Figure 2, analyzed by SDS-PAGE/ Western blot. Antibody used for the immunoblot is indicated to the left of each panel; mobility of standard apolipoproteins is indicated to the right. Lane numbers correspond to SEC fraction numbers. Samples were loaded in non-reducing sample buffer. In the cell lysates panels C and D, lane 8 contained a large amount of cell protein, and this resulted in some non-specific staining in the region between the migration of AII-E and A-I standards.
3.3 Intracellular Apolipoprotein Distribution Among Lipoprotein Subfractions
Cell lysates were harvested at the same time as the 2 and 24 h media, fractionated by SEC and analyzed for apolipoproteins (Figures 2 and 3C, 3D). The distribution of apolipoproteins in the 2 and 24 h lysates differs: the 2 h samples are enriched in newly synthesized intracellular apolipoproteins, while by 24 h the cell lysates contain more lipoproteins that were secreted, processed in the media and endocytosed back into the cell [35, 36]. In 2 h lysates, intracellular apo A-I is 45% lipid-free (Fraction 8), 45% is on small lipid-poor HDL (Fraction 7) and 10% is on average plasma-sized HDL (Fraction 6). In 24 h lysates some apo A-I is on larger LDL sized particles (Figure 3D, Fractions 3 and 4). In 2 h lysates, all apo A-II was dimeric, present as a mix of apo A-II homodimer and A-II-E heterodimer, and eluted in the HDL Fraction 6 (Figure 3C). Thus both dimerization and lipidation of apo A-II occur rapidly after synthesis, prior to secretion. In 24 h lysates, some intracellular apo A-II is in the LDL Fractions 2 – 4 (Figure 3D). Apo E distributed differently among intracellular and secreted lipoproteins. Apo E in 2 h lysates is mostly a monomer in the VLDL Fraction 1 (Figure 3C), whereas in media at 2 and 24 h it is on LDL and large HDL, but no longer detected on VLDL. At 24 h, intracellular apo E is present on VLDL (Fraction 1) and LDL (Fractions 2 – 4), with a small fraction on HDL (Fraction 6). The majority of intracellular apo B in HepG2 cells was in the nascent VLDL fraction (Fraction 1), with a lesser amount in LDL Fractions 2–4 in both the 2 and 24 h lysates (data not shown).
These results clearly demonstrate that apo A-II and apo E lipidation and dimerization follow different, very distinct pathways, and that lipidation of these apolipoproteins both inside hepatocytes and after secretion differs from the lipidation of apo A-I.
3.4 Do apo A-I and apo A-II occur on a common lipoprotein particle before secretion?
Most intracellular apo A-II is lipidated (Figure 3C, Fraction 6) while most apo A-I is lipid-poor (Fraction 7) or lipid-free (Fraction 8), indicating that there is little if any intracellular LpA-I/A-II. To analyze this further, we separated intracellular apo A-I-only particles from apo A-II containing particles by TPS chromatography. As a control, we separated normal human plasma LpA-I and LpA-I/A-II by using TPS (Figure 4, HDL panels). LpA-I flows through the column (Free fraction, lanes 1–3), while LpA-I/A-II binds, and is eluted with 20 mM DTT (Bound fraction, lanes 4 – 6). The relative amounts of apo A-I in the free and bound fractions, 34 + 5% and 66 + 5% (Table 2) agree with published values [8, 1].
TPS analysis of 2 h cell lysates from two different human hepatoma cell lines, HepG2 and Huh7, reveals that essentially all intracellular apo A-I elutes in the flow-through, Free fraction (Figure 4, HepG2 and Huh7 panels, lanes 1 – 3) while all the apo A-II is retained in the Bound fraction (lanes 5 – 7). Quantification of band intensity by phosphorimaging indicates that 98 + 3% and 94 + 9% of the apo A-I in HepG2 and Huh7 cell lysates eluted in the Free fraction, while 99 + 1% and 92 + 11% of the apo A-II was retained in the Bound fraction (Table 2 ). Thus in both HepG2 and Huh7 cells, intracellular apo A-I and apo A-II are present on distinct particles, and do not co-associate on nascent HDL prior to secretion. Rather, as shown below, HDL particles containing both apo A-I and apo A-II (LpA-I/A-II) form after secretion.
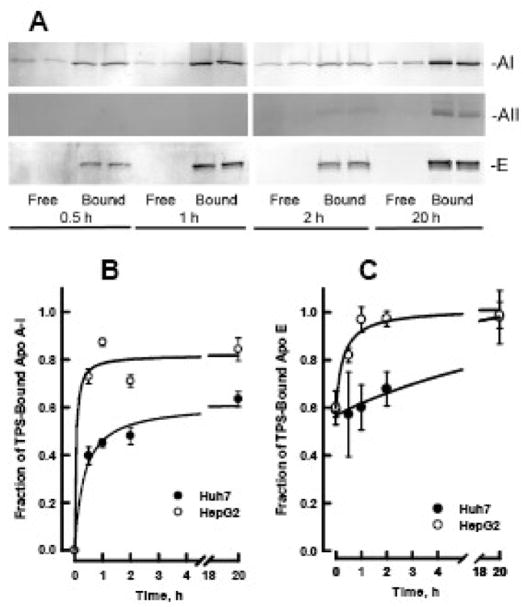
TPS analysis of hepatoma cell media reveals a time-dependent increase in secreted apo A-I associated with LpA-I/A-II, with more LpA-I/A-II appearing in the media of HepG2 than in that of Huh7 cells (Figure 5). Immunoblots of the Free and Bound fractions of media collected at 0.5, 1.0, 2.0 and 20 h show apo AI in both fractions, while apo A-II is detectable only in the Bound fractions (Figure 5A). The rate of LpA-I/A-II formation was calculated by quantification of band intensity by phosphorimaging (Figure 5B). LpA-I/A-II formation in hepatoma media is rapid: within 30 min of secretion, 40% of apo A-I in Huh7 media and 73% of apo A-I in HepG2 media is present on LpA-I/A-II. By 20 h, 84% of the apo A-I in HepG2 media and 63% of that in Huh7 media is associated with LpA-I/A-II.
Figure 5. Time course for formation of LpAI/AII in media of hepatoma cells.
A. Western blot of TPS flow through (Free) and bound (Bound) fractions of HepG2 media collected at 0.5, 1.0, 2.0 and 20 h. Each sample was loaded in duplicate lanes. Membranes were probed with antibodies to apo A-I (top), apo A-II (middle) and apo E (bottom). Similar blots were obtained with media of Huh7 cells (data not shown). B. Rate of formation of LpA-I/A-II in HepG2 (open circles) and Huh7 (closed circles) media. C. The fraction of media apo E that binds to TPS as a function on time. Amounts of each apolipoprotein in the free and bound fractions was determined by phosphorimaging of the Western blots. The data are mean and SEM (n=4).
3.5 Impaired Binding of Apo E to TPS
As expected for the cysteine-containing E2 and E3 isoforms, most human plasma HDL apo E is in the bound fraction (Figure 4, lanes 4 – 6 and Table 2). However, a small amount was also found in the free fraction (lanes 1 – 3). In cell lysates, where apo E is present on nascent VLDL (Figure 3C, apo E panel), most apo E was in the bound fractions for both HepG2 and Huh7 cell lysates (Figure 5 and Table 2), but as with HDL apo E, some eluted in the flow-through fractions. The phenotype of HepG2 cell apo E is E3/E3 [29], one cysteine per protein molecule that should be retained on TPS. However, a fraction of the cysteines in the lipoprotein bound apo E may not be accessible to TPS. After secretion, the amount of apo E in the free fraction decreases with time so that by 20 h all the media apo E binds TPS (Figure 5A and 5C). This change in apo E binding to TPS occurs more rapidly in HepG2 media than in Huh7 media. Changes in apo E conformation on triglyceride rich VLDL that affect receptor binding have been reported [47].
4. Discussion
Lipidation of Apolipoproteins A-II and E are Distinct from that of Apo A-I
Our data showing most intracellular apo A-I is lipid-free or on small nascent HDL are consistent with that of others [30, 31, 32, 33, 34]. In elegant pulse-chase studies Chisholm et al reported only 20% of newly synthesized intracellular apo A-I is lipidated prior to secretion, and secretion begins within 30 minutes of synthesis. [31]. We found that in 2 h lysates 45% of intracellular apo A-I was lipid-free, suggesting additional lipidation occurs over this time period. Importantly, in contrast to apo A-I, all intracellular apo A-II and apo E were lipidated within 2 h of synthesis. Although some intracellular apo A-II in 2 h lysates occurred with small, nascent HDL, most was on plasma-sized HDL (Fraction 6, Figure 3C). Most intracellular apo E in 2 h lysates was associated with VLDL. Apo E in rat hepatoma cells has also been reported to bind to maturing VLDL [48]. After secretion, remodeling in the media redistributes the apolipoproteins among lipoproteins; still each apolipoprotein retains a distinctive pattern of lipid association both intracellularly, as we report here for the first time, and post secretion [39, 12]. The patterns of lipid association among apolipoproteins in HepG2 media at 24 h simulate those of human plasma HDL, in which most apo A-II is on mid-size HDL, apo A-I associates with a broader range of particle sizes, and most apo E resides on larger HDL [29, 13].
LpA-I/A-II forms after secretion
LpA-I/A-II formation was investigated in two hepatoma cell lines. Though widely used to study lipoprotein secretion, HepG2 cell media apo B-lipoproteins are lipid-poor [38, 30, 39, 40]. In contrast, Huh7 cells [49, 50] are better producers of VLDL [51, 52]. In both cell lines, TPS analysis revealed no detectable intracellular LpA-I/A-II (Figure 4). After secretion, LpA-I/A-II forms rapidly, with faster kinetics in HepG2 than in Huh7 media (Figure 5). Thus, as proposed by Ikewaki et al [8], LpA-I/A-II forms after secretion of the nascent apo A-II HDL.
Remodeling of Secreted Lipoproteins is Apolipoprotein-Specific
The nascent HDL particles secreted by HepG2 cells are discoidal, with low neutral lipids, similar to those found in plasma of LCAT-deficient patients [53, 54] and are remodeled to plasma-like spherical HDL particles by LCAT activity [55]. HepG2 cells secrete many of the proteins and lipids of lipoprotein metabolism, including LCAT, CETP, PLTP and hepatic lipase [38, 56, 57, 58]. Our data confirm the remodeling of secreted lipoproteins to more mature forms in HepG2 media. Apo A-I is hepatically secreted as both lipid-free protein and as lipidated, nascent discoidal HDL particles (this work and [30, 31, 32, 33]). In vitro, these apo A-I particles and model nascent HDL (rHDL) are converted to spherical HDL by LCAT activity [55, 59, 60, 7]. In contrast, discoidal apo A-II rHDL, which are poor LCAT substrates [59], are not converted to spherical apo A-II HDL [59, 61]. Instead, LCAT catalyzes fusion of the apo A-II rHDL discs with small spherical apo A-I rHDL to form spherical LpA-I/A-II HDL [7]. A similar conversion is observed in vivo; human discoidal apo A-II rHDL infused into rabbits, which lack apo A-II, rapidly fuses with endogenous spherical LpA-I to form LpA-I/A-II [10]. Requirement of LCAT for the in vivo formation of spherical LpA-I/A-II is suggested by the absence of these spherical particles in LCAT deficient patients, where all the apo A-II is confined to pre-β discoidal HDL [13]. Our in vitro data show intracellular apo E is associated with nascent VLDL, while apo E in 2 h media is associated with LDL and HDL. Thus, the apo E on secreted VLDL particles rapidly redistributes to a pattern like that of human plasma [29].
Apo A-II and Apo E have Distinct Dimerization Kinetics
Lipid-free apo A-II dimerizes slowly, a process that is greatly accelerated by binding to lipids [25]. In HepG2 cells, we found no monomeric intracellular apo A-II, suggesting that following its synthesis apo A-II undergoes rapid, sequential lipidation and dimerization. In contrast, intracellular apo E on VLDL (fraction 1, Figure 3C) is primarily monomeric (this work and [29]). Apo E dimerization is much slower than that of apo A-II with no dimer detected in media at 2 h, when apo E is associated with LDL and HDL. Apo A-II-apo E heterodimers are present after the longer 24 h incubation, during which lipoprotein remodeling may occur. Apo E is required for HDL remodeling in dog plasma, where it supports the conversion of HDL3 to HDL1, a large, cholesterol-enriched, receptor-competent HDL [62]; during remodeling, HDL acquire apo E [63].
Apolipoprotein Dimerization and Lipidation are Correlated
Apolipoproteins A-I, A-II and E belong to a gene family of soluble proteins that have amphipathic helical regions that bind to lipids with an affinity, i.e., free energy, that is expected to be apolipoprotein-specific. According to linear free energy relationships, apo A-II homodimerization is expected to increase its free energy of binding by a factor of two. Whereas the free energy of association of monomeric apo A-II with HDL is −7.3 kcal/mol, the corresponding free energy value for dimeric apo A-II is not known and required measuring dimeric lipid-free apo A-II concentrations that were below our level of detection [64]. Our data are consistent with this. Nearly all homo- and heterodimeric apo A-II and apo E are lipidated. In contrast, a greater fraction of monomeric apo A-II and apo E, as well as obligatory monomer apo A-I, are found to be lipid-free after ultracentrifugation (Table 1). The lipid affinities of monomers and dimers may contribute in a currently unknown way to their distinct distributions between lipoproteins species.
A Refined Model for Hepatocyte HDL Formation and Secretion
Our data are consistent with current models of apo A-I in HDL biogenesis and remodeling [31, 32, 37], and support the following refined model that includes apo A-II and apo E (Figure 6). Synthesis and initial lipidation of apolipoproteins occurs in the endoplasmic reticulum (ER) [32]. Further lipidation occurs in the Golgi, so that half of the apo A-I is secreted on small nascent HDL, while the remainder is secreted as lipid-free apo A-I (Figure 3 and [31, 32]). In contrast, all of apo A-II is lipidated prior to secretion, to a particle size that approaches that of human plasma HDL. Apo A-II is secreted as a lipid-associated dimer on nascent HDL particles that do not contain apo A-I (Figures 3 and 4). LCAT catalyzes fusion of the apo A-II nascent HDL discs with small spherical apo A-I HDL to form LpA-I/A-II spherical HDL [7]. Apo E in the Golgi associates with maturing VLDL particles (Figure 3 and [48]). Apo E secreted on VLDL is rapidly remodeled (double arrow) into LDL-sized particles and moves to HDL, where it subsequently forms homodimers and heterodimers with apo A-II. These secreted lipoproteins may undergo retroendocytosis, after which some apo A-I and apo A-II transfer to LDL-sized particles, while apo E transfers to HDL and is resecreted [37, 36].
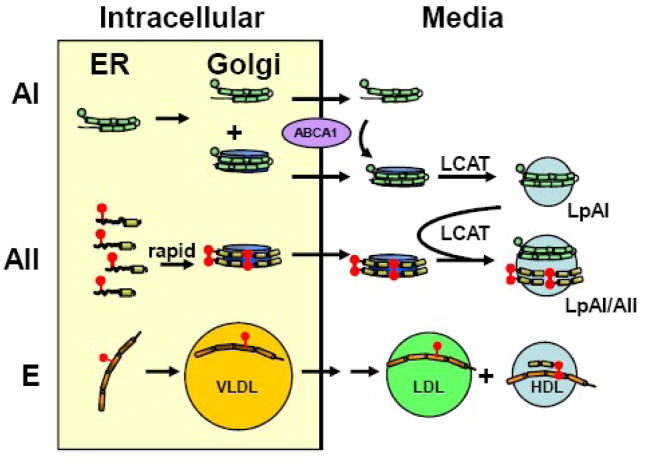
Figure 6. Model for hepatic HDL formation and secretion.
Apolipoprotein synthesis and its initial lipidation occur in the endoplasmic reticulum (ER). In the Golgi, apo A-II particles are further lipidated and approach the size of human plasma HDL, while apo A-I particles are smaller, with about half of the intracellular apo A-I remaining lipid-free. After secretion, lipid-free apo A-I acquires lipid by interaction with ABCA1, and nascent discoidal apo A-I HDL matures to spherical LpA-I by action of LCAT and other remodeling proteins. Apo A-II, which dimerizes shortly after lipidation, is secreted as a lipid-associated dimer on particles that do not contain apo A-I. Shortly after secretion, LCAT promotes fusion of these particles with spherical LpA-I to form LpA-I/A-II. Monomeric apo E in the Golgi associates with maturing VLDL particles which are rapidly remodeled after secretion (double arrow) to LDL sized particles and some apo E transfers to HDL, where it forms homodimers and heterodimers with apo A-II. Apolipoproteins are represented by green (A-I), yellow (A-II) and orange (E) helixes; cysteine groups are red balls; nascent HDL are blue discs, and spherical HDL, blue spheres.
Physiological Significance
The unique distributions of apolipoproteins A-I, A-II and E among nascent and maturing lipoprotein particles suggest apolipoprotein-specific HDL formation and processing. Lipidated and lipid-free apo A-I are expected to support cellular cholesterol efflux via ABCG1 and ABCA1 respectively [32, 65, 66], and if HepG2 and Huh7 cells are valid models of hepatocytes in vivo, one could surmise that lipid-free apo A-I is rapidly utilized by ABCA1 or renally disposed because plasma lipid-free apo A-I concentrations are very low (<0.05%) [42]. Lp A-I/A-II are formed by the LCAT promoted fusion of small spherical apo A-I HDL with nascent discoidal apo A-II-HDL [7, 10]. Whereas plasma levels of apo A-I are determined by catabolism, apo A-II levels are determined by synthetic rate. This is a major factor regulating the distribution of apo A-I among HDL subclasses LpA-I and LpA-I/A-II in normolipidemic humans [67, 8]. Plasma residence time for apo A-II is longer than that for apo AI [68], consistent with its higher lipophilicity, and with the lability of apo A-I association with HDL [41, 69, 42, 2]. Apo A-II also modulates the substrate properties of HDL. The rate of hepatic lipase catalyzed fatty acid release from HDL containing apo A-II is twice that of HDL devoid of apo A-II [70]. Apo E HDL also have distinct properties; apo E facilitates RCT by allowing CE-rich-core expansion of HDL [27]. Understanding the roles of apolipoprotein-specific HDL metabolism will provide the basis for development of better therapies to promote RCT.
Acknowledgments
This work was supported by grants-in-aid from the National Institutes of Health HL-30914 and HL-56865 to H.J.P. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart Lung and Blood Institute nor the National Institutes of Health. We thank Amber Chen for technical assistance, Dr. Dan Liao for bringing to our attention the human hepatoma Huh7 cell line, and Dr. Yumin Xu in Dr. Boris Yoffe’s laboratory (Baylor College of Medicine) for giving us a flask of these cells.
Abbreviations
- βMSH
β-mercaptoethanol
- LpA-I and LpA-I/A-II
HDL containing only apo A-I or both apo A-I and apo A-II respectively
- NEM
N-ethyl maleimide
- NP-40
Nonidet P40
- RCT
reverse cholesterol transport
- SEC
size exclusion chromatography
- TPS
thiopropyl-sepharose
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rader DJ. Molecular regulation of HDL metabolism and function: implications for novel therapies. J Clin Invest. 2006;116:3090–3100. doi: 10.1172/JCI30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curtiss LK, Valenta DT, Hime NJ, Rye KA. What is so special about apolipoprotein AI in reverse cholesterol transport? Arterioscler Thromb Vasc Biol. 2006;26:12–19. doi: 10.1161/01.ATV.0000194291.94269.5a. [DOI] [PubMed] [Google Scholar]
- 3.Datta G, Epand RF, Epand RM, Chaddha M, Kirksey MA, Garber DW, Lund-Katz S, Phillips MC, Hama S, Navab M, Fogelman AM, Palgunachari MN, Segrest JP, Anantharamaiah GM. Aromatic residue position on the nonpolar face of class a amphipathic helical peptides determines biological activity. J Biol Chem. 2004;279:26509–26517. doi: 10.1074/jbc.M314276200. [DOI] [PubMed] [Google Scholar]
- 4.Navab M, Anantharamaiah GM, Fogelman AM. The role of high-density lipoprotein in inflammation. Trends Cardiovasc Med. 2005;15:158–161. doi: 10.1016/j.tcm.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Havel RJ, Goldstein JL, Brown MS. Lipoproteins and Lipid Transport. 1980:393–494. [Google Scholar]
- 6.Cheung MC, Albers JJ. Characterization of lipoprotein particles isolated by immunoaffinity chromatography. Particles containing A-I and A-II and particles containing A-I but no A-II. J Biol Chem. 1984;259:12201–12209. [PubMed] [Google Scholar]
- 7.Clay MA, Pyle DH, Rye KA, Barter PJ. Formation of spherical, reconstituted high density lipoproteins containing both apolipoproteins A-I and A-II is mediated by lecithin:cholesterol acyltransferase. J Biol Chem. 2000;275:9019–9025. doi: 10.1074/jbc.275.12.9019. [DOI] [PubMed] [Google Scholar]
- 8.Ikewaki K, Zech LA, Kindt M, Brewer HB, Jr, Rader DJ. Apolipoprotein A-II production rate is a major factor regulating the distribution of apolipoprotein A-I among HDL subclasses LpA-I and LpA-I:A-II in normolipidemic humans. Arterioscler Thromb Vasc Biol. 1995;15:306–312. doi: 10.1161/01.atv.15.3.306. [DOI] [PubMed] [Google Scholar]
- 9.Edelstein C, Halari M, Scanu AM. On the mechanism of the displacement of apolipoprotein A-I by apolipoprotein A-II from the high density lipoprotein surface. Effect of concentration and molecular forms of apolipoprotein A-II. J Biol Chem. 1982;257:7189–7195. [PubMed] [Google Scholar]
- 10.Hime NJ, Drew KJ, Wee K, Barter PJ, Rye KA. Formation of high density lipoproteins containing both apolipoprotein A-I and A-II in the rabbit. J Lipid Res. 2006;47:115–122. doi: 10.1194/jlr.M500284-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Castro GR, Fielding CJ. Evidence for the distribution of apolipoprotein E between lipoprotein classes in human normocholesterolemic plasma and for the origin of unassociated apolipoprotein E (Lp-E) J Lipid Res. 1984;25:58–67. [PubMed] [Google Scholar]
- 12.McCall MR, Forte TM, Shore VG. Heterogeneity of nascent high density lipoproteins secreted by the hepatoma-derived cell line, Hep G2. J Lipid Res. 1988;29:1127–1137. [PubMed] [Google Scholar]
- 13.Asztalos BF, Schaefer EJ, Horvath KV, Yamashita S, Miller M, Franceschini G, Calabresi L. Role of LCAT in HDL remodeling: investigation of LCAT deficiency states. J Lipid Res. 2007;48:592–599. doi: 10.1194/jlr.M600403-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.McKeone BJ, Massey JB, Knapp RD, Pownall HJ. Apolipoproteins C-I, C-II, and C-III: kinetics of association with model membranes and intermembrane transfer. Biochemistry. 1988;27:4500–4505. doi: 10.1021/bi00412a042. [DOI] [PubMed] [Google Scholar]
- 15.Guha M, Gao X, Jayaraman S, Gursky O. Correlation of Structural Stability with Functional Remodeling of High-Density Lipoproteins: The Importance of Being Disordered. Biochemistry. 2008;47:11393–11397. doi: 10.1021/bi8014746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tangirala RK, Tsukamoto K, Chun SH, Usher D, Pure E, Rader DJ. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein A-I in mice. Circulation. 1999;100:1816–1822. doi: 10.1161/01.cir.100.17.1816. [DOI] [PubMed] [Google Scholar]
- 17.Temel RE, Walzem RL, Banka CL, Williams DL. Apolipoprotein A-I is necessary for the in vivo formation of high density lipoprotein competent for scavenger receptor BI-mediated cholesteryl ester-selective uptake. J Biol Chem. 2002;277:26565–26572. doi: 10.1074/jbc.M203014200. [DOI] [PubMed] [Google Scholar]
- 18.Marcel YL, Kiss RS. Structure-function relationships of apolipoprotein A-I: a flexible protein with dynamic lipid associations. Curr Opin Lipidol. 2003;14:151–157. doi: 10.1097/00041433-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Blanco-Vaca F, Escola-Gil JC, Martin-Campos JM, Julve J. Role of apoA-II in lipid metabolism and atherosclerosis: advances in the study of an enigmatic protein. J Lipid Res. 2001;42:1727–1739. [PubMed] [Google Scholar]
- 20.Kalopissis AD, Pastier D, Chambaz J. Apolipoprotein A-II: beyond genetic associations with lipid disorders and insulin resistance. Curr Opin Lipidol. 2003;14:165–172. doi: 10.1097/00041433-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Birjmohun RS, Dallinga-Thie GM, Kuivenhoven JA, Stroes ES, Otvos JD, Wareham NJ, Luben R, Kastelein JJ, Khaw KT, Boekholdt SM. Apolipoprotein A-II Is Inversely Associated With Risk of Future Coronary Artery Disease. Circulation. 2007;116:2029–2035. doi: 10.1161/CIRCULATIONAHA.107.704031. [DOI] [PubMed] [Google Scholar]
- 22.Blanco-Vaca F, Via DP, Yang CY, Massey JB, Pownall HJ. Characterization of disulfide-linked heterodimers containing apolipoprotein D in human plasma lipoproteins. J Lipid Res. 1992;33:1785–1796. [PubMed] [Google Scholar]
- 23.Connelly PW, Maguire GF, Vezina C, Hegele RA, Little JA. Identification of disulfide-linked apolipoprotein species in human lipoproteins. J Lipid Res. 1993;34:1717–1727. [PubMed] [Google Scholar]
- 24.Lund-Katz S, Murley YM, Yon E, Gillotte KL, Davidson WS. Comparison of the structural and functional effects of monomeric and dimeric human apolipoprotein A-II in high density lipoprotein particles. Lipids. 1996;31:1107–1113. doi: 10.1007/BF02524284. [DOI] [PubMed] [Google Scholar]
- 25.Gillard BK, Chen YS, Gaubatz JW, Massey JB, Pownall HJ. Plasma factors required for human apolipoprotein A-II dimerization. Biochemistry. 2005;44:471–479. doi: 10.1021/bi048591j. [DOI] [PubMed] [Google Scholar]
- 26.Mahley RW, Huang Y. Apolipoprotein E: from atherosclerosis to Alzheimer's disease and beyond. Curr Opin Lipidol. 1999;10:207–217. doi: 10.1097/00041433-199906000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Mahley RW, Huang Y, Weisgraber KH. Putting cholesterol in its place: apoE and reverse cholesterol transport. J Clin Invest. 2006;116:1226–1229. doi: 10.1172/JCI28632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weisgraber KH, Mahley RW. Apoprotein (E--A-II) complex of human plasma lipoproteins. I. Characterization of this mixed disulfide and its identification in a high density lipoprotein subfraction. J Biol Chem. 1978;253:6281–6288. [PubMed] [Google Scholar]
- 29.Weisgraber KH, Shinto LH. Identification of the disulfide-linked homodimer of apolipoprotein E3 in plasma. Impact on receptor binding activity. J Biol Chem. 1991;266:12029–12034. [PubMed] [Google Scholar]
- 30.Thrift RN, Forte TM, Cahoon BE, Shore VG. Characterization of lipoproteins produced by the human liver cell line, Hep G2, under defined conditions. J Lipid Res. 1986;27:236–250. [PubMed] [Google Scholar]
- 31.Chisholm JW, Burleson ER, Shelness GS, Parks JS. ApoA-I secretion from HepG2 cells: evidence for the secretion of both lipid-poor apoA-I and intracellularly assembled nascent HDL. J Lipid Res. 2002;43:36–44. [PubMed] [Google Scholar]
- 32.Maric J, Kiss RS, Franklin V, Marcel YL. Intracellular lipidation of newly synthesized apolipoprotein A-I in primary murine hepatocytes. J Biol Chem. 2005;280:39942–39949. doi: 10.1074/jbc.M507733200. [DOI] [PubMed] [Google Scholar]
- 33.Chau P, Nakamura Y, Fielding CJ, Fielding PE. Mechanism of prebeta-HDL formation and activation. Biochemistry. 2006;45:3981–3987. doi: 10.1021/bi052535g. [DOI] [PubMed] [Google Scholar]
- 34.Tsujita M, Wu CA, Abe-Dohmae S, Usui S, Okazaki M, Yokoyama S. On the hepatic mechanism of HDL assembly by the ABCA1/apoA-I pathway. J Lipid Res. 2005;46:154–162. doi: 10.1194/jlr.M400402-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Hara S, McCall MR, Forte TM. Re-uptake of nascent low-density lipoproteins by HepG2 cells. Biochim Biophys Acta. 1993;1168:199–204. doi: 10.1016/0005-2760(93)90125-s. [DOI] [PubMed] [Google Scholar]
- 36.Heeren J, Grewal T, Laatsch A, Rottke D, Rinninger F, Enrich C, Beisiegel U. Recycling of apoprotein E is associated with cholesterol efflux and high density lipoprotein internalization. J Biol Chem. 2003;278:14370–14378. doi: 10.1074/jbc.M209006200. [DOI] [PubMed] [Google Scholar]
- 37.Heeren J, Beisiegel U, Grewal T. Apolipoprotein E recycling: implications for dyslipidemia and atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:442–448. doi: 10.1161/01.ATV.0000201282.64751.47. [DOI] [PubMed] [Google Scholar]
- 38.Erickson SK, Fielding PE. Parameters of cholesterol metabolism in the human hepatoma cell line, Hep-G2. J Lipid Res. 1986;27:875–883. [PubMed] [Google Scholar]
- 39.McCall MR, Nichols AV, Morton RE, Blanche PJ, Shore VG, Hara S, Forte TM. Transformation of HepG2 nascent lipoproteins by LCAT: modulation by HepG2 d > 1.235 g/ml fraction. J Lipid Res. 1993;34:37–48. [PubMed] [Google Scholar]
- 40.Hara S, McCall MR, Forte TM. Re-uptake of nascent low-density lipoproteins by HepG2 cells. Biochim Biophys Acta. 1993;1168:199–204. doi: 10.1016/0005-2760(93)90125-s. [DOI] [PubMed] [Google Scholar]
- 41.Pownall HJ. Remodeling of human plasma lipoproteins by detergent perturbation. Biochemistry. 2005;44:9714–9722. doi: 10.1021/bi050729q. [DOI] [PubMed] [Google Scholar]
- 42.Pownall HJ, Hosken BD, Gillard BK, Higgins CL, Lin HY, Massey JB. Speciation of Human Plasma High-Density Lipoprotein (HDL): HDL Stability and Apolipoprotein A-I Partitioning. Biochemistry. 2007;46:7449–7459. doi: 10.1021/bi700496w. [DOI] [PubMed] [Google Scholar]
- 43.Yvan-Charvet L, Pagler TA, Wang N, Senokuchi T, Brundert M, Li H, Rinninger F, Tall AR. SR-BI inhibits ABCG1-stimulated net cholesterol efflux from cells to plasma HDL. J Lipid Res. 2008;49:107–114. doi: 10.1194/jlr.M700200-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Kunitake ST, Kane JP. Factors affecting the integrity of high density lipoproteins in the ultracentrifuge. J Lipid Res. 1982;23:936–940. [PubMed] [Google Scholar]
- 45.Borghini I, James RW, Blatter MC, Pometta D. Distribution of apolipoprotein E between free and A-II complexed forms in very-low- and high-density lipoproteins: functional implications. Biochim Biophys Acta. 1991;1083:139–146. doi: 10.1016/0005-2760(91)90034-f. [DOI] [PubMed] [Google Scholar]
- 46.Remaley AT, Wong AW, Schumacher UK, Meng MS, Brewer HB, Jr, Hoeg JM. O-linked glycosylation modifies the association of apolipoprotein A-II to high density lipoproteins. J Biol Chem. 1993;268:6785–6790. [PubMed] [Google Scholar]
- 47.Bradley WA, Gianturco SH. ApoE is necessary and sufficient for the binding of large triglyceride-rich lipoproteins to the LDL receptor; apoB is unnecessary. J Lipid Res. 1986;27:40–48. [PubMed] [Google Scholar]
- 48.Gusarova V, Seo J, Sullivan ML, Watkins SC, Brodsky JL, Fisher EA. Golgi-associated maturation of very low density lipoproteins involves conformational changes in apolipoprotein B, but is not dependent on apolipoprotein E. J Biol Chem. 2007;282:19453–19462. doi: 10.1074/jbc.M700475200. [DOI] [PubMed] [Google Scholar]
- 49.Nakabayashi H, Taketa K, Yamane T, Miyazaki M, Miyano K, Sato J. Phenotypical stability of a human hepatoma cell line, HuH-7, in long-term culture with chemically defined medium. Gann. 1984;75:151–158. [PubMed] [Google Scholar]
- 50.Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982;42:3858–3863. [PubMed] [Google Scholar]
- 51.Higashi Y, Itabe H, Fukase H, Mori M, Fujimoto Y, Takano T. Transmembrane lipid transfer is crucial for providing neutral lipids during very low density lipoprotein assembly in endoplasmic reticulum. J Biol Chem. 2003;278:21450–21458. doi: 10.1074/jbc.M301376200. [DOI] [PubMed] [Google Scholar]
- 52.Higashi Y, Itabe H, Fukase H, Mori M, Fujimoto Y, Sato R, Imanaka T, Takano T. Distribution of microsomal triglyceride transfer protein within sub-endoplasmic reticulum regions in human hepatoma cells. Biochim Biophys Acta. 2002;1581:127–136. doi: 10.1016/s1388-1981(02)00157-9. [DOI] [PubMed] [Google Scholar]
- 53.McCall MR, Forte TM, Shore VG. Heterogeneity of nascent high density lipoproteins secreted by the hepatoma-derived cell line, Hep G2. J Lipid Res. 1988;29:1127–1137. [PubMed] [Google Scholar]
- 54.McCall MR, Nichols AV, Blanche PJ, Shore VG, Forte TM. Lecithin:cholesterol acyltransferase-induced transformation of HepG2 lipoproteins. J Lipid Res. 1989;30:1579–1589. [PubMed] [Google Scholar]
- 55.McCall MR, Nichols AV, Morton RE, Blanche PJ, Shore VG, Hara S, Forte TM. Transformation of HepG2 nascent lipoproteins by LCAT: modulation by HepG2 d > 1.235 g/ml fraction. J Lipid Res. 1993;34:37–48. [PubMed] [Google Scholar]
- 56.Kaser S, Foger B, Ebenbichler CF, Kirchmair R, Gander R, Ritsch A, Sandhofer A, Patsch JR. Influence of leptin and insulin on lipid transfer proteins in human hepatoma cell line, HepG2. Int J Obes Relat Metab Disord. 2001;25:1633–1639. doi: 10.1038/sj.ijo.0801807. [DOI] [PubMed] [Google Scholar]
- 57.Swenson TL, Simmons JS, Hesler CB, Bisgaier C, Tall AR. Cholesteryl ester transfer protein is secreted by Hep G2 cells and contains asparagine-linked carbohydrate and sialic acid. J Biol Chem. 1987;262:16271–16274. [PubMed] [Google Scholar]
- 58.Siggins S, Jauhiainen M, Olkkonen VM, Tenhunen J, Ehnholm C. PLTP secreted by HepG2 cells resembles the high-activity PLTP form in human plasma. J Lipid Res. 2003;44:1698–1704. doi: 10.1194/jlr.M300059-JLR200. [DOI] [PubMed] [Google Scholar]
- 59.Jonas A, Sweeny SA, Herbert PN. Discoidal complexes of A and C apolipoproteins with lipids and their reactions with lecithin: cholesterol acyltransferase. J Biol Chem. 1984;259:6369–6375. [PubMed] [Google Scholar]
- 60.Nichols AV, Blanche PJ, Gong EL, Shore VG, Forte TM. Molecular pathways in the transformation of model discoidal lipoprotein complexes induced by lecithin:cholesterol acyltransferase. Biochim Biophys Acta. 1985;834:285–300. doi: 10.1016/0005-2760(85)90001-3. [DOI] [PubMed] [Google Scholar]
- 61.Forte TM, Bielicki JK, Goth-Goldstein R, Selmek J, McCall MR. Recruitment of cell phospholipids and cholesterol by apolipoproteins A-II and A-I: formation of nascent apolipoprotein-specific HDL that differ in size, phospholipid composition, and reactivity with LCAT. J Lipid Res. 1995;36:148–157. [PubMed] [Google Scholar]
- 62.Koo C, Innerarity TL, Mahley RW. Obligatory role of cholesterol and apolipoprotein E in the formation of large cholesterol-enriched and receptor-active high density lipoproteins. J Biol Chem. 1985;260:11934–11943. [PubMed] [Google Scholar]
- 63.Gordon V, Innerarity TL, Mahley RW. Formation of cholesterol- and apoprotein E-enriched high density lipoproteins in vitro. J Biol Chem. 1983;258:6202–6212. [PubMed] [Google Scholar]
- 64.Pownall HJ, Hickson D, Gotto AM., Jr Thermodynamics of lipid-protein association. The free energy of association of lecithin with reduced and carboxymethylated apolipoprotein A-II from human plasma high density lipoprotein. J Biol Chem. 1981;256:9849–9854. [PubMed] [Google Scholar]
- 65.Oram JF, Heinecke JW. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol Rev. 2005;85:1343–1372. doi: 10.1152/physrev.00005.2005. [DOI] [PubMed] [Google Scholar]
- 66.Wang N, Tall AR. Regulation and mechanisms of ATP-binding cassette transporter A1-mediated cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2003;23:1178–1184. doi: 10.1161/01.ATV.0000075912.83860.26. [DOI] [PubMed] [Google Scholar]
- 67.Rader DJ, Schaefer JR, Lohse P, Ikewaki K, Thomas F, Harris WA, Zech LA, Dujovne CA, Brewer HB., Jr Increased production of apolipoprotein A-I associated with elevated plasma levels of high-density lipoproteins, apolipoprotein A-I, and lipoprotein A-I in a patient with familial hyperalphalipoproteinemia. Metabolism. 1993;42:1429–1434. doi: 10.1016/0026-0495(93)90194-s. [DOI] [PubMed] [Google Scholar]
- 68.Ikewaki K, Zech LA, Brewer HB, Jr, Rader DJ. ApoA-II kinetics in humans using endogenous labeling with stable isotopes: slower turnover of apoA-II compared with the exogenous radiotracer method. J Lipid Res. 1996;37:399–407. [PubMed] [Google Scholar]
- 69.Pownall HJ. Detergent-mediated phospholipidation of plasma lipoproteins increases HDL cholesterophilicity and cholesterol efflux via SR-BI. Biochemistry. 2006;45:11514–11522. doi: 10.1021/bi0608717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mowri HO, Patsch JR, Gotto AM, Jr, Patsch W. Apolipoprotein A-II influences the substrate properties of human HDL2 and HDL3 for hepatic lipase. Arterioscler Thromb Vasc Biol. 1996;16:755–762. doi: 10.1161/01.atv.16.6.755. [DOI] [PubMed] [Google Scholar]