Abstract
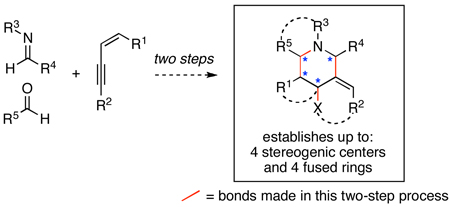
A two-step process is described for the union of aromatic imines, conjugated alkynes and aldehydes that results in a stereoselective synthesis of highly substituted piperidines. This synthetic process has been made possible by defining a unique regioselective functionalization of conjugated alkynes that establishes a suitably functionalized substrate for subsequent heterocycle-forming cationic annulation. Given the flexibility of the coupling process, heterocycles can be accessed through a process that establishes up to four stereogenic centers and four fused rings.
Substituted piperidines are structural motifs found in a variety of natural products and small molecules of biomedical relevance.1 Despite the significant advances made that define chemical methods suitable for the construction of this heterocyclic core, the stereocontrolled preparation of highly substituted piperidines remains a challenge in chemical synthesis.2 Of the many methods available, cationic annulation of suitably functionalized amines with aldehydes (via Pictet-Spengler or aza-Prins cyclization) has long been recognized as a useful pathway to a subset of functionalized piperidines.3, 4 Aside from problems associated with the control of these cationic annulation reactions, preparation of complex unsaturated amine substrates suitable for these heterocycle-forming processes can be cumbersome. Here, we describe a bimolecular bond construction for the selective union of conjugated alkynes with imines as a means to establish appropriate functionality for subsequent stereoselective cationic annulation. Specifically, the process described defines a two-step three-component coupling reaction between conjugated alkynes, imines and aldehydes for the stereoselective synthesis of piperidines containing up to four stereogenic centers and four fused rings.
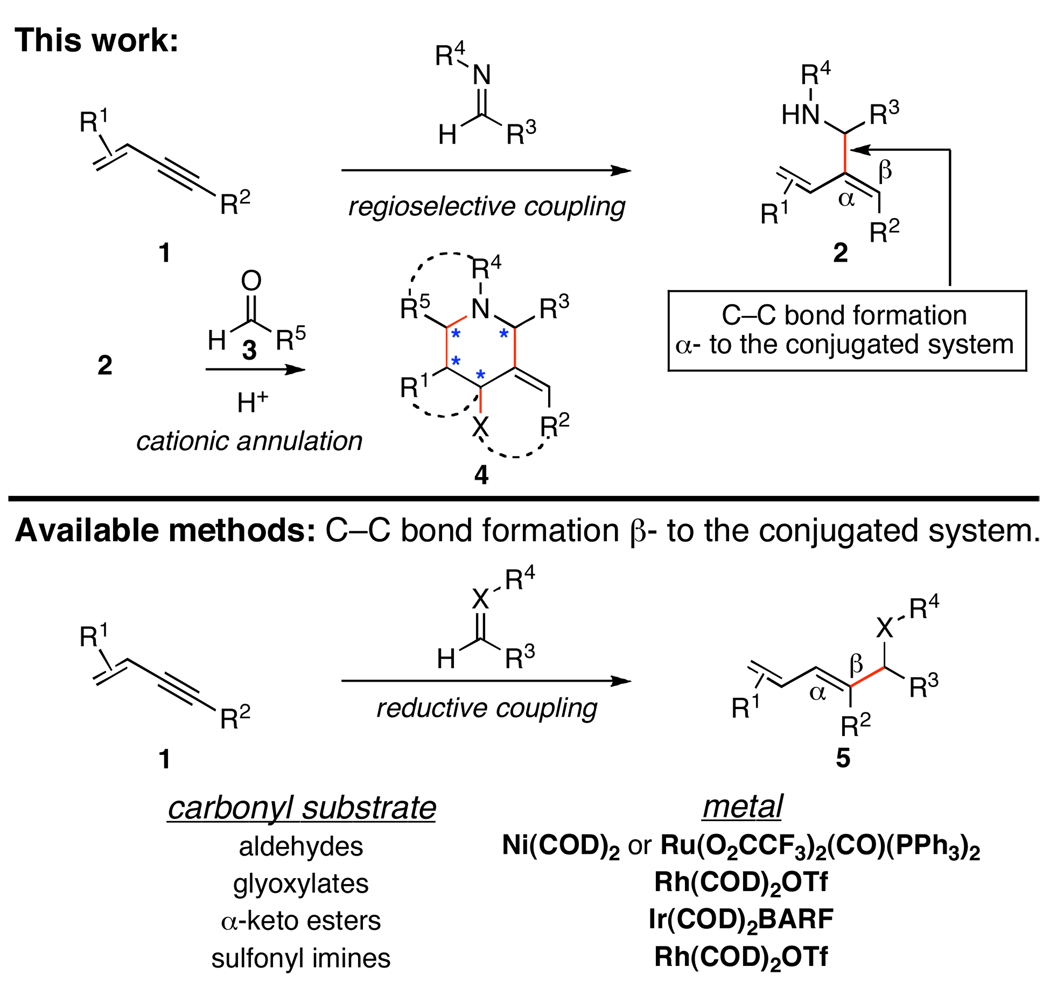
From the outset we sought a bimolecular alkyne–imine coupling that would enable the synthesis of allylic amines well suited for cationic annulation (1 → 2, and 2 + 3 → 4; Figure 1). With this goal in mind, we were aware of the numerous methods available for the metal catalyzed reductive coupling of conjugated alkynes with carbonyl electrophiles. Unfortunately, the selectivity of these coupling reactions is uniformly dictated by the presence of π-conjugation and delivers an isomeric product to that required for the heterocycle synthesis of interest here (2 vs. 5; Figure 1).5
Figure 1.
Regioselective union of conjugated alkynes with imines for heterocycle synthesis.
Recently, we have defined a Ti-mediated alkyne–imine coupling reaction for the synthesis of allylic amines and γ-lactams where a tethered alkoxide directs regioselective functionalization of an internal alkyne and overrides the influence of simple non-bonded steric interactions.6 The knowledge gleaned from this study served as a guiding principle for the design of the first step of the heterocycle synthesis described here. While targeting a bond construction that, as in our previous studies, would require stoichiometric quantities of Ti(Oi-Pr)4, the potential of such a process in complex heterocycle synthesis and the inability to generate the desired substitution in 2 with available catalytic methods drove our investigation of this chemistry.
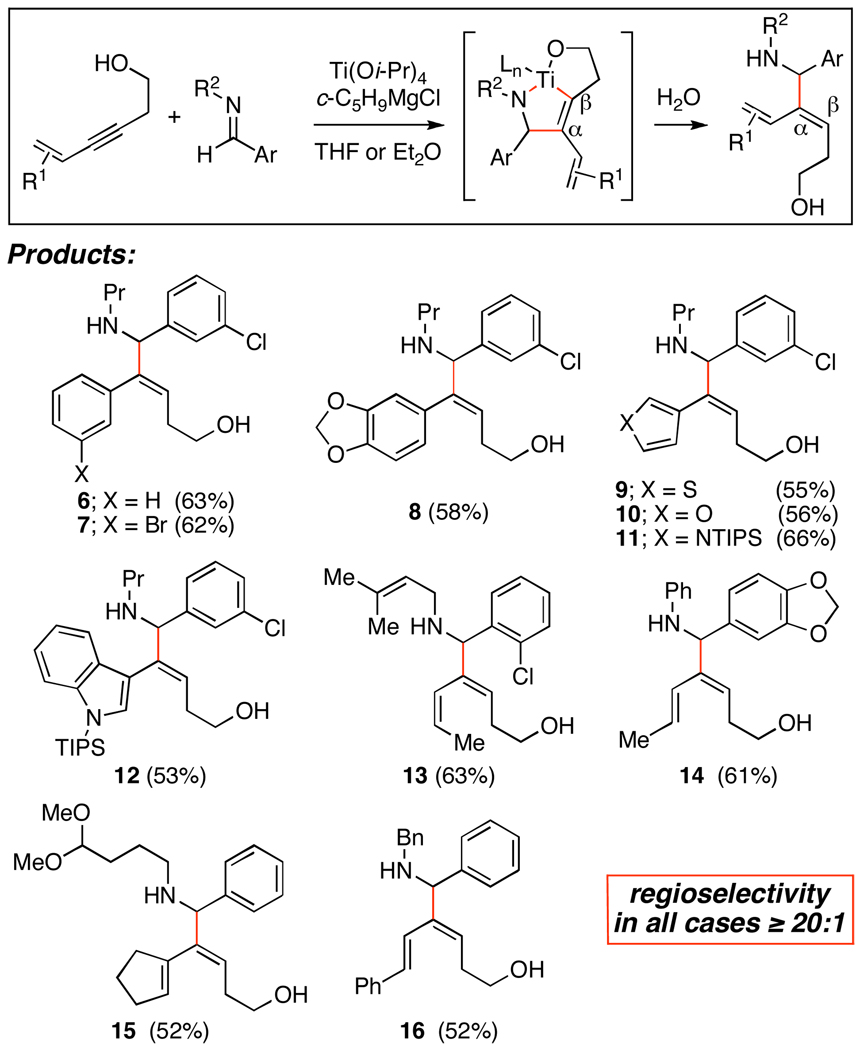
As illustrated in Figure 2, alkoxide-directed C–C bond formation provides an exceptionally powerful means to deliver the unique regioisomer required here, where C–C bond formation occurs α-to the conjugated π-system; in opposition to that expected from the electronic effects that dictate the regiochemical course of related reductive coupling reactions.5 The selective production of 6–12 highlights the effectiveness of this reductive coupling reaction with a variety of aromatic and heteroaromatic conjugated alkynes. While demonstrating the compatibility of the coupling reaction with electron rich aromatics, aromatic halides, thiophenes, furans, pyrroles and indoles, these reactions provided single regioisomers of coupled products in 52–66% yield.
Figure 2.
Alkoxide-directed union of conjugated alkynes with imines.
The successful formation of 13–16 demonstrates that this metal-mediated coupling process is equally effective with enyne containing substrates. Here, high regioselectivity is possible in a chemoselective coupling process, where no evidence was found for the functionalization of the conjugated alkene of the starting enyne.
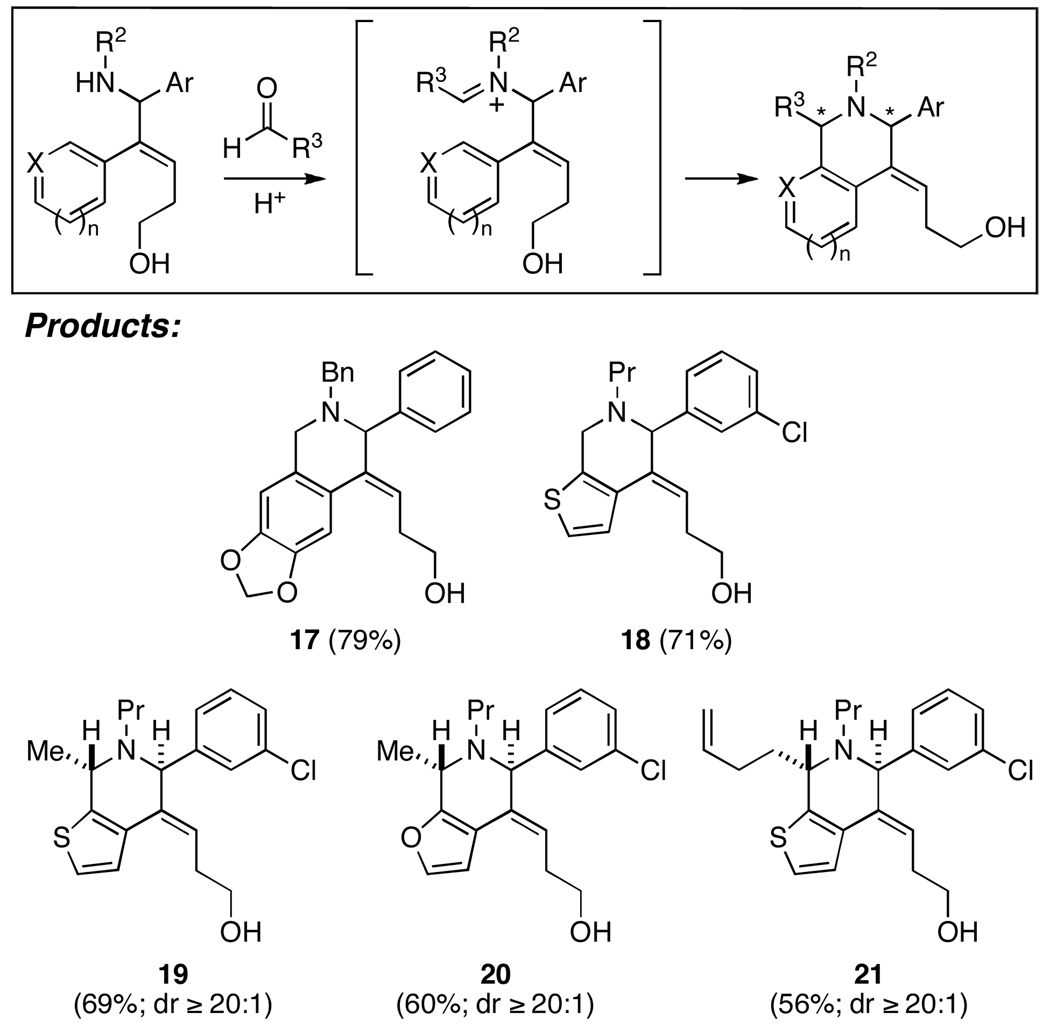
With a site-selective convergent coupling reaction in hand, we shifted our attention to exploring the unique reactivity of the allylic amine products derived from this coupling process. As anticipated based on well-precedented Pictet-Spengler chemistry,4e cationic annulation of substrates containing conjugated aromatics defines a concise pathway to polysubstituted fused bicyclic heterocycles (Figure 3). While the generation of 17 and 18 (via acid-promoted addition to 1,3,5-trioxane in EtOH or CH3CN) demonstrates the basic reactivity profile of interest with electron rich and heteroaromatic systems, the formation of 19–21 highlights that good levels of stereocontrol are possible in convergent annulation with aldehydes. In all cases, high selectivity for the formation of the 2,6-trans disubstituted piperidines was observed (dr ≥ 20:1).
Figure 3.
Cationic annylation via Pictet-Spengler-like cyclization.
Moving on, cationic annulation of the 1,3-diene-containing allylic amines (derived from reductive cross-coupling of enynes with aromatic imines) provides a unique opportunity for the rapid generation of complex heterocycles. Here, cationic annulation terminated by C–O bond formation, defines a concise pathway to bi-, tri-and tetracyclic piperidines with typically very high levels of stereoselection (Figure 4).7,8
Figure 4.
Cationic annulation via aza-Prins-initiated cyclization.
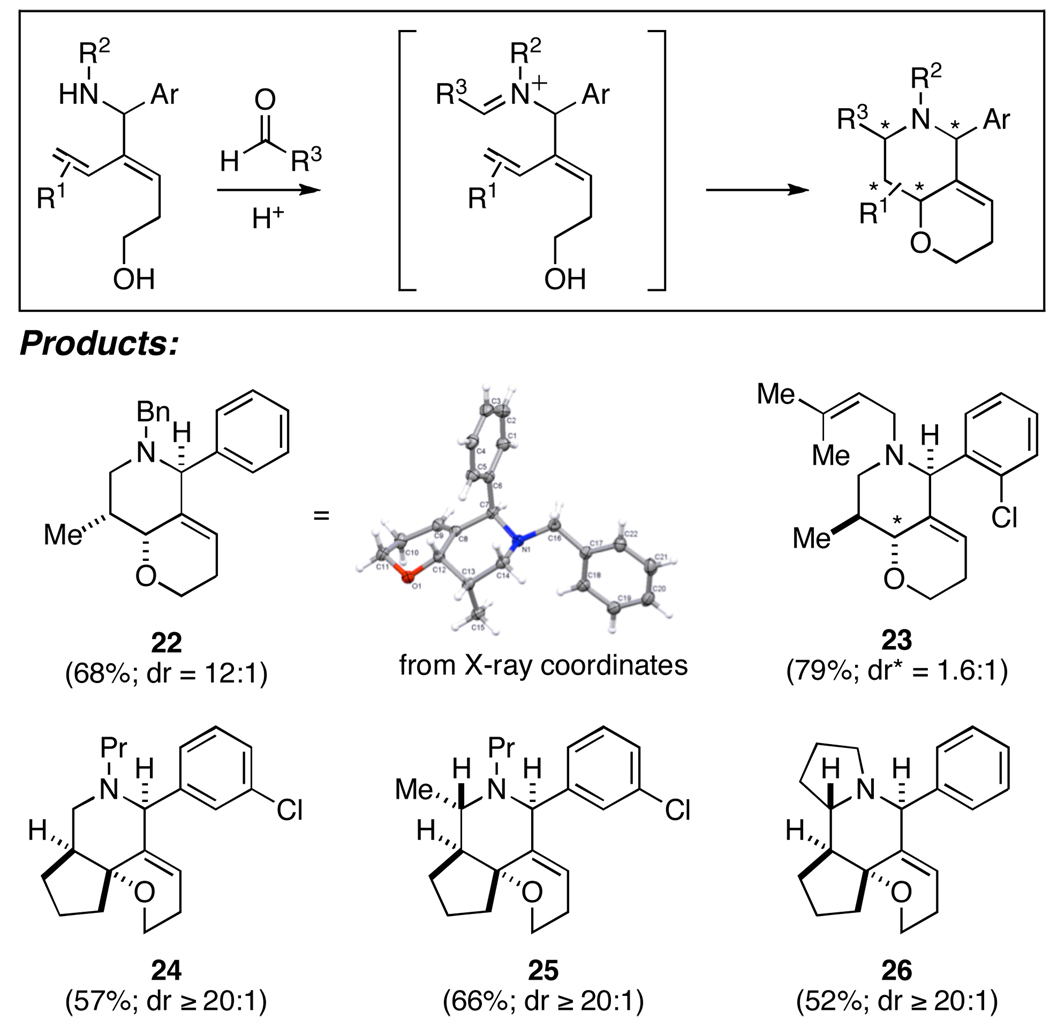
Our preliminary investigation of this reaction pathway indicated that the stereochemical course of the annulation reaction is complex. For example, cyclization of an enyne bearing a (Z)-disubstituted alkene leads to the generation of a bicyclic product 22 with good levels of stereoselection (dr = 12:1) while cyclization of a related isomeric enyne proceeds with very low levels of stereoselection (i.e. 23 is derived from the (E)- disubstituted alkene and is produced as a 1.6:1 mixture of stereoisomers). This observed lack of stereospecificity is consistent with stepwise cyclization terminated by diastereoselective addition to an intermediate allylic carbocation.9
In an effort to enhance diastereoselection we examined the cyclization of enynes where the alkene was constrained in a five membered ring. Perhaps due to the expected preference for formation of a cis-fused saturated aza-indane over the trans-fused isomer, all cationic annulation reactions with this subset of enynes proceeded with very high levels of diastereoselection (24–26; dr ≥ 20:1 in all cases). As observed earlier in the annulation reactions of substrates containing conjugated aromatics, in these more complex cyclization reactions, stereoselection was observed for the formation of the central piperidine bearing 2,6-trans-substitution (i.e. 25 and 26).
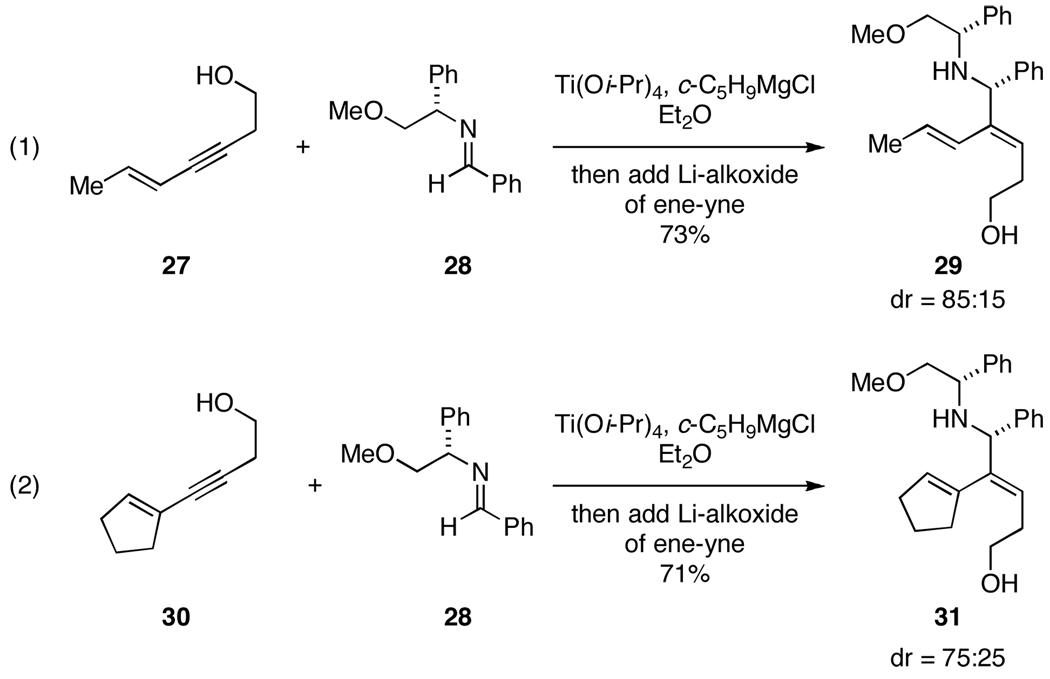
This regioselective reductive cross-coupling reaction between enynes and aromatic imines can be rendered asymmetric. As depicted in Figure 5, use of a phenylglycine-modified aromatic imine (28) leads to moderate stereoselection in the formation of the functionalized allylic amines 29 (dr = 85:15) and 31 (dr = 75:25).10
Figure 5.
Preliminary studies in asymmetric synthesis.
In conclusion, we have described a two-step three-component coupling process for the synthesis of stereodefined and highly substituted piperidines. Site-selective union of readily available conjugated alkynes with aromatic imines provides a means of accessing allylic amine products not readily available with other reductive cross-coupling methods (where C–C bond formation occurs α-to the conjugated π-system). As a result of the unique selectivity of this alkyne–imine cross-coupling reaction, subsequent cationic annulation with carbonyl electrophiles defines a powerful pathway to highly substituted piperidines. The complex annulation processes discovered are typically highly selective, generating up to four stereogenic centers (dr is typically ≥ 20:1) and four fused rings. Due to the rapid access to structural complexity afforded by this chemical process and ubiquitous presence of substituted piperidines in natural products and small molecules of biomedical relevance, we anticipate that this two-step three-component coupling process will be of great utility in chemical synthesis. The ease of use, low cost, and unique reactivity profile of Ti(Oi-Pr)4 combine to define a powerful system of increasing utility in defining unique and highly regioselective bond constructions in organic chemistry.11, 12
Supplementary Material
Acknowledgment
We gratefully acknowledge financial support of this work by the American Cancer Society (RSG-06-117-01), Boehringer Ingelheim, Eli Lilly & Co. and the National Institutes of Health – NIGMS (GM80266 and GM80266-04S1).
Footnotes
Supporting Information Available Experimental procedures and tabulated spectroscopic data for new compounds (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.a) Watson PS, Jiang B, Scott B. Org. Lett. 2000;2:3679–3681. doi: 10.1021/ol006589o. [DOI] [PubMed] [Google Scholar]; b) Källström S, Leino R. Bioorg. Med. Chem. 2008;16:601–635. doi: 10.1016/j.bmc.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 2.For recent reviews, see: Escolano C, Amat M, Bosch J. Chem. Eur. J. 2006;12:8198–8207. doi: 10.1002/chem.200600813. Cossy J. Chem. Rec. 2005;5:70–80. doi: 10.1002/tcr.20035. Buffat MGP. Tetrahedron. 2004;60:1701–1729. Weintraub PM, Sabol JS, Kane JM, Borcherding DR. Tetrahedron. 2003;59:2953–2989.
- 3.a) Bohlmann F, Winterfeldt E, Overwien H, Pagel H. Chem. Ber. 1962;95:944–948. [Google Scholar]; b) Grob CA, Wohl RA. Helv. Chem. Acta. 1966;49:2175–2189. [Google Scholar]; c) Cope AC, Burrows WD. J. Org. Chem. 1966;29:3099–3103. [Google Scholar]
- 4.For recent reviews of cationic annulation reactions in heterocycle synthesis, see: Speckamp WN, Hiemstra H. Tetrahedron. 1985;41:4367–4416. Blumenkopf TA, Overman LE. Chem. Rev. 1986;86:857–873. Overman LE. Aldrichimica Acta. 1995;28:107–120. Royer J, Bonin M, Micouin L. Chem. Rev. 2004;104:2311–2352. doi: 10.1021/cr020083x. Cox ED, Cook JM. Chem. Rev. 1995;95:1797–1842.
- 5. Huang W-S, Chan J, Jamison TF. Org. Lett. 2000;2:4221–4223. doi: 10.1021/ol006781q. Patel SJ, Jamison TF. Angew. Chem. Int. Ed. 2003;42:1364–1367. doi: 10.1002/anie.200390349. Mahandru GM, Liu G, Montgomery J. J. Am. Chem. Soc. 2004;126:3698–3699. doi: 10.1021/ja049644n. Miller KM, Luanphaisarnnont T, Molinaro C, Jamison TF. J. Am. Chem. Soc. 2004;126:4130–4131. doi: 10.1021/ja0491735. Miller KM, Jamison TF. Org. Lett. 2005;7:3077–3080. doi: 10.1021/ol051075g. Kong J-R, Cho C-W, Krische MJ. J. Am. Chem. Soc. 2005;125:11269–11276. doi: 10.1021/ja051104i. Sa-ei K, Montgomery J. Org. Lett. 2006;8:4441–4443. doi: 10.1021/ol061579u. Komanduri V, Krische MJ. J. Am. Chem. Soc. 2006;128:16448–16449. doi: 10.1021/ja0673027. Cho C-W, Krische MJ. Org. Lett. 2006;8:3873–3876. doi: 10.1021/ol061485k. Ngai M-Y, Barchuk A, Krische MJ. J. Am. Chem. Soc. 2007;129:280–281. doi: 10.1021/ja0670815. Cho C-W, Skucas E, Krische MJ. Organometallics. 2007;26:3860–3867. Hong Y-T, Cho C-W, Skucas E, Krische MJ. Org. Lett. 2007;9:3745–3748. doi: 10.1021/ol7015548. Patman RL, Chaulagain MR, Williams VM, Krische MJ. J. Am. Chem. Soc. 2009;131:2066–2067. doi: 10.1021/ja809456u. A reviewer suggested that we cite the following additional papers that describe unrelated reductive coupling reactions of isolated alkynes with activated imines: Skucas E, Kong JR, Krische MJ. J. Am. Chem. Soc. 2007;129:7242–7243. doi: 10.1021/ja0715896. Barchuk A, Ngai M, Krische MJ. J. Am. Chem. Soc. 2007;129:8432–8433. doi: 10.1021/ja073018j. Ngai M, Barchuk A, Krische MJ. J. Am. Chem. Soc. 2007;129:12644–12645. doi: 10.1021/ja075438e.
- 6.McLaughlin M, Takahashi M, Micalizio GC. Angew. Chem. Int. Ed. 2007;46:3912–3914. doi: 10.1002/anie.200605060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.For examples of annulation reactions between iminium ions and 1,3-dienes, see: Larsen SD, Grieco PA. J. Am. Chem. Soc. 1985;107:1768–1769. Grieco PA, Kaufman MD. J. Org. Chem. 1999;64:6041–6048. Liu J, Sung RP, Peters SD. Org. Lett. 2004;6:3989–3992. doi: 10.1021/ol048353g.
- 8.For an interesting example of a tandem aza-Prins reaction followed by intramolecular C–O bond formation in the context of total synthesis, see: Lee HM, Nieto-Oberhuber C, Shair MD. J. Am. Chem. Soc. 2008;130:16864–16866. doi: 10.1021/ja8071918.
- 9.Resubjecting each diastereomer of 23 to the reaction conditions did not lead to any observable interconversion. As such, the mixture of products obtained in this annulation reaction is deemed likely to result from a stepwise process entailing formation of a secondary allylic cation followed by C–O bond formation, not stereospecific cationic cyclization followed by acid-promoted isomerization of the product.
- 10.The relative stereochemistry of the products 29 and 31 was assigned in analogy to previously described alkoxide-directed reductive cross-coupling reactions of chiral imine 28 (see ref. 6, and literature cited therein). For the initial report that describes use of a phenylglycine-based imine in diastereoselective reductive coupling of aromatic imines with terminal alkynes, see: Fukuhara K, Okamoto S, Sato F. Org. Lett. 2003;5:2145–2148. doi: 10.1021/ol034599u.
-
11.It is instructive to compare the cost associated with running reductive cross-coupling chemistry based on stoichiometric Ti(Oi-Pr)4 with reported processes that employ substoichiometric quantities of reported Ni, Rh, Ir and Ru catalysts. For a hypothetical reductive cross-coupling reaction run on a 1 mole scale, the following costs are incurred for the purchase of the typically employed mol % of each reactive metal species:
1 mole Ti(Oi-Pr)4 = $18;
10 mol% Ni(COD)2 = $591;
5 mol% [Rh(COD)2]OTf = $4,391;
5 mol% [Ir(COD)2]BARF = $6,465;
5 mol% Ru(O2CCF3)2(CO)(PPh3)2 = $11,612
- 12.While the removal of toxic metal impurities in reaction products is a problem that surfaces in many applications of metal-catalyzed reactions in process chemistry (i.e. Pd-, Ni- or Ru-), the biproducts of this Ti(Oi-Pr)4-mediated coupling reaction include i-PrOH, Mg salts, simple hydrocarbons and TiO2 – a non-toxic species encountered daily in toothpaste, sunscreen and paint.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.