Abstract
Corticosteroid signaling mechanisms mediate a wide range of adaptive physiological responses, including those essential to reproduction. Here, we investigated the presence and relative abundance of corticosteroid receptors during the breeding season in the plainfin midshipman fish (Porichthys notatus), a species that has two male reproductive morphs. Only type I “singing” males acoustically court females and aggressively defend a nest site, whereas type II “sneaker” males steal fertilizations from nesting type I males. Cloning and sequencing first identified glucocorticoid (GR) and mineralocorticoid (MR) receptors in midshipman that exhibited high sequence identity with other vertebrate GRs and MRs. Absolute quantitative real time PCR then revealed higher levels of GR in the central nervous system (CNS) of type II males than type I males and females, while GR levels in the sound-producing, vocal muscle and the liver were higher in type I males than type II males and females. MR expression was also greater in the CNS of type II males than type I males or females, but the differences were more modest in magnitude. Lastly, plasma levels of cortisol, the main glucocorticoid in teleosts, were two to three fold greater in type II males compared to type I males. Together, the results suggest a link between corticosteroid regulation and physiological and behavioral variation in a teleost fish that displays male alternative reproductive tactics.
Keywords: qPCR, male alternative reproductive tactics, glucocorticoid receptor, mineralocorticoid receptor
1. Introduction
Among vertebrates, the hormonal regulation of responses to environmental stressors (both biotic and abiotic) is ancient and includes critical regulatory processes mediated by corticosteroid signaling mechanisms (Baker, 1997; Bridgham et al., 2006). Steroid receptors are ligand-activated transcription factors that mediate the actions of steroid hormones, including corticosteroids, on a diversity of functions ranging from sexual differentiation and adult reproductive behavior to immune responses, sodium reabsorption, and anti-inflammatory processes (Bentley, 1998; Kime, 1987). Steroid receptors, including those targeted by corticosteroids, are a subfamily of the greater superfamily of nuclear receptors that are composed of several domains important for transcriptional activation (Gronemeyer and Laudet, 1995). Vertebrates have two main classes of corticosteroid receptors, glucocorticoid (GR) and mineralocorticoid (MR) (Bentley, 1998). While both receptor classes have long been known for tetrapods, more recent studies have identified a single corticosteroid receptor in jawless fish (lamprey and hagfish), but distinct GR and MR in cartilaginous and teleost fishes (Bridgham et al., 2006; Greenwood et al., 2003).
Teleosts show a wide range of neuroendocrine phenotypes reflecting, in part, an equally divergent range of reproductive tactics (Bass and Forlano, 2008; Bass and Grober, 2009). Here, we investigated the presence and abundance of corticosteroid receptors in the plainfin midshipman (Porichthys notatus) that has two male morphs that follow either a displaying/courting or a sneak-satellite spawning tactic (Bass, 1996; Bass and Grober, 2009). Territorial, type I males build and guard nests (females offer no parental care) and acoustically court females with a long duration courtship “hum” (mins to > 1 h) involving the rapid contraction (~100 Hz at 16°C) of paired vocal muscles attached to the walls of the gas-filled swimbladder that are considered among the fastest known vertebrate muscles (Bass and Marchaterre, 1989; Brantley and Bass, 1994; Rome et al., 1996). Type I males also make shorter “growls” (sec-mins) and very brief “grunts” (msec) during intraspecific, aggressive encounters (Bass et al., 1999; Brantley and Bass, 1994). Sneak/ satellite spawning, type II males steal fertilizations from nesting type I males, are about 50% smaller than type I males and have a nine fold greater testes/ body mass ratio than type I males (Brantley and Bass, 1994). Type II males (and females) are known so far to only produce grunts in non-spawning contexts (Brantley and Bass, 1994).
The divergent vocal repertoires between the two male morphs are paralleled by a large suite of neural and non-neural mechanisms (Bass, 1996; Bass and Forlano, 2008; Bass and Remage-Healey, 2008). For example, the vocal muscles of type I males have several traits that are greater in magnitude than those of type II sneaker males (and females), ranging from overall mass to fiber number and diameter, the abundance of mitochondria in the sarcoplasm, and the ultrastructural dimensions of myofibrils (Bass and Marchaterre, 1989; Brantley et al., 1993a). The divergence in vocal behavior and muscle traits is indicative of an even larger number of morph-specific traits in the vocal control network that determines the temporal properties of natural vocalizations (Bass and Remage-Healey, 2008). In sum, the type I male morph exhibits a large suite of morphological and physiological traits mainly adapted to the performance of a metabolically demanding behavior, namely advertisement calling that may last for more than one hour (see above) (Bass et al., 1999; Brantley and Bass, 1994; Ibara et al., 1983).
Corticosteroids, as well as androgens and estrogen, have been proposed to influence the development and adult maintenance of alternative male morphs in midshipman fish and other teleosts (Forlano et al., 2006; Knapp, 2003; Remage-Healey and Bass, 2007). Cortisol, the predominant corticosteroid in teleosts (Kime, 1987), can modulate the activity pattern of a hindbrain-spinal vocal pattern generator that directly establishes the temporal features of midshipman vocalizations (Bass and Remage-Healey, 2008). Intramuscular, dorsal trunk injections of cortisol in type I males increases the duration of the vocal motor, occipital nerve volley that reflects the activity of the hindbrain-spinal pattern generator, but suppresses the duration of the motor volley in type II males (Remage-Healey and Bass, 2007).
Male morph-specific patterns of cortisol action on vocal as well as non-vocal behaviors might depend, in part, on differential patterns of expression of corticosteroid receptors. Hence, the aim of the present study was to more directly test the hypothesis that both glucocorticoid and mineralocorticoid receptors show male morph-specific patterns of expression in the central nervous system and peripheral tissues in midshipman fish. We also tested the corollary that plasma levels of cortisol would differ between the two male morphs. A prior study of only type I males and females showed that cortisol levels were significantly higher in females, but only during the non-breeding season when fish are collected from deep offshore sites (Sisneros et al., 2004).
2. Materials and Methods
2.1. Animals and Blood Sampling
Midshipman fish were collected from field-nest sites in northern California during the summer breeding season of May-August (see (Bass, 1996), shipped to Cornell University within 72h and maintained in seawater tanks until sacrificed within 24h for tissue collection. The size of the testis and vocal muscle provided characters to easily identify each sex and male morph upon visual inspection (Bass, 1996). This study included all three adult reproductive morphs: type I males (20.6 – 25.1 grams, 12–13.5 cm), type II males (3.94–5.88 grams, 8–8.5 cm) and females (11.12–17.5 grams, 10–11.5 cm). Tissue sampling and blood collection were carried out following deep anesthetization (0.025% benzocaine; Sigma, St. Louis, MO). All experimental protocols were approved by the Cornell University Institutional Animal Care and Use Committee. Blood was obtained for cortisol assays by cardiac puncture within three – four hours after collection from nest sites in a subset of type I (n=5) and type II males (n=6) (Brantley et al., 1993b; Sisneros et al., 2004); a previous study compared females to type I males (Sisneros et al., 2004). Blood plasma was collected after centrifugation and frozen for later analysis by radioimmunoassay (RIA) at the Diagnostic Laboratory, New York State College of Veterinary Medicine at Cornell, Ithaca, NY. Like most studies in teleosts (reviewed in Prunet et al., 2006), aldosterone was not detected in RIAs for blood samples collected as above from three additional type I males and hence further assays were not done, in part, because of limited availability.
2.2. Cloning, Sequencing, and Alignment
RNA was isolated and pooled from type I male liver (n=3) from the breeding season using Trizol (Invitrogen, Carlsbad, CA) and reverse transcribed using Superscript III Reverse Transcriptase (Invitrogen, Carlsbad, CA) following the manufacturer’s protocols. PCR on cDNA from all tissues were conducted using degenerate primers as follows. Glucocorticoid receptor: forward primer (PACRFRK): 5'-CCN-GCN-TGY-CGN-TTY-CGN-AAR-TG (Tm=62.0°C); reverse primer (PLLFHQK): 5'-YTT-TTG-RTG-RAA-NAG-NAG-NGG (Tm=52.4°C). Mineralocorticoid receptor: forward primer (PYLTPSV): 5'-CCV-TAY-CTG-MCS-CCS-TCC-RTC (Tm=62.3°C); reverse primer (DLVLGMR): 5'-CCK-CAT-SCC-CAS-CAC-CAG-RTC (Tm=62.8°C). The primers were designed to amplify a highly conserved region based on an alignment of vertebrate corticosteroid receptors (see Fig. 1, Fig 2). Beta actin primers were designed from Acanthopagrus schlegelii (Genbank Accession No. AY491380); forward primer (GDGWTHT): 5’ GGT-GAT-GGT-GTG-ACC-CAC-ACA-GTG (Tm=61.9°C); reverse primer (KYSVWIG): 5’ TTT-ATG-AGA-CAG-ACC-TAG-CCT (Tm=54.3°C).
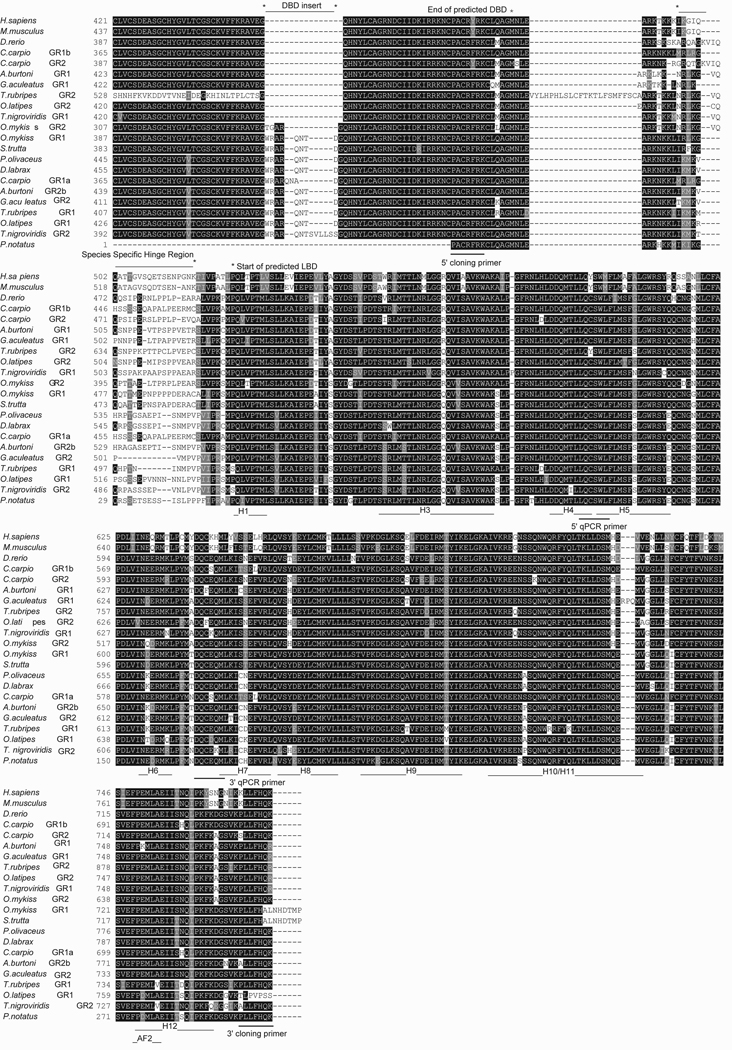
Figure 1.
An alignment of the ligand binding domain (LBD) for vertebrate glucocorticoid receptors (GR) reveals high sequence identity between multiple vertebrate species. From the 5’ end of the alignment, the areas highlighted are the final residues that code for the DNA binding domain (DBD), the species-specific hinge region, and the LBD for which activational domains (AF2) and helices (h1-12) are noted. Forward (5’) and reverse (3’) primers are indicated for both cloning and qPCR procedures.
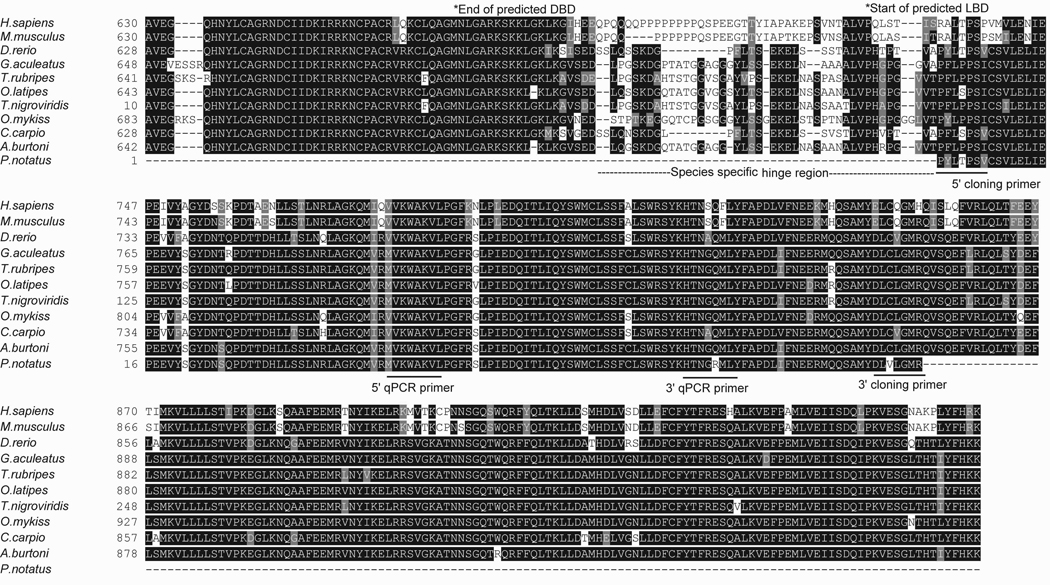
Figure 2.
An alignment of vertebrate mineralocorticoid receptors. The sequences begin at the 3’ end of the DNA binding domain (DBD), include the species-specific hinge region, and the full length ligand binding domain (LBD). The areas highlighted are the final residues that code for the DBD, the species-specific hinge region, and the LBD. Forward (5’) and reverse (3’) primers are indicated for both cloning and qPCR procedures.
Amplification was performed with Taq polymerase (Epicentre Biotechnologies, Madison, WI) using the following cycles: Stage 1 – 94°C for 2 min (1 cycle), Stage 2 – 94°C for 1 min, 55°C for 1 minute, 72°C for 3 min (35 cycles), and Stage 3 – 72°C extension for 10 minutes (1 cycle). Products were run on a 1% agarose gel and verified to be the approximate expected size: a 906 bp product for GR, a 366 bp product for MR, and a 564 bp product for beta actin. The primers contained restriction sites on the 5 prime ends to increase the efficiency of ligation into the Bluescript KS (+) plasmid, and the products were subcloned using DH5α competent cells and plated. Twenty individual colonies were grown in liquid culture, mini-prepped (Qiagen, Germantown, MD), and sequenced at the Cornell University Life Sciences Core Laboratory Center using T3 and T7 primers. The resulting sequences were subject to a BLAST analysis (NCBI) to verify identity and for alignment with other vertebrate sequences (CLustalW).
Sequences for both alignments included a broad range of vertebrates, as follows. Glucocorticoid receptor: Homo sapiens (GenBank Accession No. AY436590), Mus musculus (GenBank Accession No. nm_008173), Danio rerio (Genbank accession number NM_001020711.2), Cyprinus carpio GR1b (GenBank Accession No. AM697886), Cyprinus carpio GR2 (GenBank Accession No. AM183668), Astatotilapia burtoni isoform 1 (GenBank Accession No. AF263738), Gasterosteus aculeatus GR1 (Ensembl Identifier: ENSGACG00000018209), Takifugu rubripes GR2 (Ensembl Identifier: ENSTRUG00000007443), Oryzias latipes (Ensembl Identifier: ENSORLG00000006022), Tetroadon nigroviridis GR1 (Ensembl Identifier: ENSTNIG00000008946), Oncorhynchus mykiss GR2 (GenBank Accession No. NM_001124482), Oncorhynchus mykiss GR1 (GenBank Accession No. NM_001124730), Salmo trutta (GenBank Accession No. AY863149), Paralichthys olivaceus (GenBank Accession No. AB013444), Dicentrarchus labrax (GenBank Accession No. AY549305), Cyprinus carpio GR1a (GenBank Accession No. AJ879149), Astatotilapia burtoni isoform 2b (GenBank Accession No. AF263740). Mineralocorticoid receptor: Homo sapiens (GenBank Accession No. nm_000901.3), Mus musculus (GenBank Accession No. nm_001083906.1), Danio rerio (GenBank Accession No. nm_001100403.1), Gasterosteus aculeatus (Ensembl Identifier: ENSGACG00000017193), Takifugu rubripes (Ensembl Identifier: ENSTRUG000000014871), Oryzias latipes (Ensembl Identifier: ENSORLG00000007530), Tetroadon nigroviridis (Ensembl Identifier: ENSTNIG00000017732), Oncorhynchus mykiss (GenBank Accession No. NM_001124483.1), Cyprinus carpio (GenBank Accession No. AJ783704), Astatotilapia burtoni (GenBank Accession No. AF263741). A second isoform, GR2a, for A. burtoni was not included in the alignment because it shows a 100% sequence identify with the partial sequence of A. burtoni GR2b used in the analysis (see Greenwood et al., 2003).
2.3. Absolute-Quantitative Real Time PCR (qPCR)
The qPCR analysis was based on the analysis of tissues from individual fish (n=4–5 per morph/sex). RNA was isolated using Trizol (Invitrogen, Carlsbad, CA), treated twice with DNaseI (Invitrogen, Carlsbad, CA) to remove genomic DNA contamination, and reverse transcribed using Superscript III Reverse Transcriptase (Invitrogen, Carlsbad, CA) following the manufacturer’s protocols. As in previous biochemical and qPCR studies in midshipman (Forlano et al., 2005; Schlinger et al., 1999), the CNS was divided into three main regions for quantitative analysis: forebrain including olfactory bulb (Ob), telencephalon (Tel) and preoptic area (POA); a large middle region including midbrain tectum and tegmentum (Mid), diencephalon (Di) and cerebellum (cbl); and the remaining hindbrain-rostral spinal cord region (VH-SC) that houses the vocal pattern generator circuit (Bass et al., 1994). Samples were also taken from liver, testis, and vocal muscle (VM). Female gonad was not investigated due to limited availability and a major focus here being on divergence between the two male morphs.
Absolute qPCR was conducted on cDNA from all tissues using gene specific primer pairs that were chosen based on the following criteria: 1) amplify a product about 150 nucleotides in length, 2) isolate a single product with no dimer pairs, and 3) have a standard efficiency (R2) of no less than 98%. The following primers were designed from the nucleotide sequence of the midshipman genes. Glucocorticoid receptor: the forward primer 5’-GCT-GCA-GTG-CTC-GTG-GCT-TT (Tm=61.3°C) and reverse primer 5’-GCA-TCT-GCT-CGC-ACT-GGT-CA (Tm=60.3°C) were chosen as the primer pair (R2=0.984). Mineralocorticoid receptor: the forward primer 5’-GCA-TGG-TGA-AAT-GGG-CCA-AAG-TAC-TTC (Tm=60.3°C) and the reverse primer 5’-AAG-TAG-AGC-ATC-CGT-CC (Tm=60.6°C) were chosen as the primer pair (R2=0.981). Beta actin: the forward primer 5’-CCA-CGC-CAT-CCT-GCG-TCT (Tm=60.7°C) and the reverse primer 5’-GCT-CGA-AGT-CCA-GGG-CAA-CA (Tm=60.1°C) were chosen as the primer pair (R2=0.987). All real time reactions were run in triplicate along with no template controls and contained the following: 10 µl of 2X Power SYBR Green PCR Master Mix (Applied Biosystems ), 2 µl of forward and reverse primer at a concentration of 100 nM, 4 µl of H2O, and 2 µl of the appropriate cDNA. Reactions were run on an Applied Biosystems 7900 HT Sequence Detection System at the Cornell University Life Sciences Core Laboratory Center under the default manufacturer’s conditions (SDS 2.1 software) using 60°C as a melting temperature.
Gene copy number was determined for each tissue sampled from each individual using standard curve analysis for all gene primer sets, including housekeeping genes. The standards covered a linear range of 5×10^6 to 100 copy/µl. Briefly, the raw Ct values were converted to copy number with the standard curve produced using the SDS 2.1 (Applied Biosystems). Each target gene copy number was normalized using the beta actin (βA) copy number (n) from the same tissue sample (e.g., forebrain GRn/forebrain βAn). Beta actin was chosen, in part, because it had been previously used in mRNA expression studies of midshipman fish (Forlano et al., 2005). Initial cloning also investigated the use of glyceraldehyde 3-phosphate dehydrogenase (GADPH) as a reference gene, but GADPH primers did not perform consistently. By contrast, the βA gene-specific primers showed excellent qPCR efficiency (R2=0.99, data not shown), and variation in expression of βA was less than 5% between morphs within a given tissue. However, since variation in βA expression was much greater than 15% between tissues, we did not statistically compare expression between peripheral and central tissues. We recognize that βA may yet be regulated in a manner not apparent in the comparisons made for the current study of midshipman. A study of the cichlid A. burtoni showed that βA expression differed between reproductive (R) and non-reproductive (NR) males (Renn et al., 2008), although R and NR males are not comparable to type I and II midshipman, both of whom are reproductively active (Brantley and Bass, 1994).
2.4. Statistics
Normalized mRNA values and plasma cortisol levels were compared between morphs for each tissue sampled. One-way ANOVA and post-hoc Tukey-HSD tests were performed on all data sets using JMP 7.0 software. Welch-ANOVA tests were performed for each statistical comparison where unequal variance may influence the analysis.
3. Results
3.1. Cloning and Sequencing
In order to examine whether corticosteroids and their receptors play a role in the alternative reproductive tactics of the midshipman male morphs, we first designed primers that could amplify portions of the midshipman GR and MR genes. The resulting PCR products were subcloned and 20 independent clones of GR and MR were sequenced. For both GR and MR, the sequencing of multiple clones revealed that only one gene was amplified by each of the degenerate primer pairs, and gene-specific primers also revealed a single product as expected. The translated protein sequence can be viewed on Genbank on the NCBI webserver (Genbank Accession No. EF092836 for GR and EU926160 for MR).
We note that the reverse primer designed for cloning of the midshipman GR is based upon an amino acid sequence that is divergent between the two GR genes in the cichlid A. burtoni (GR1 final coding residue is an arginine – R, and GR2b final coding residue is a lysine – K; see GR alignment in Fig. 1). The coding nucleotides for these amino acids in the GR sequences of A. burtoni differ by one nucleotide in the middle position (GR1: lysine = aaa, GR2b: arginine = aga), and they are both amides (see Fig. 1). Thus, we realize that if this amino acid is divergent between potentially duplicated midshipman GR genes, it may not be surprising that only one receptor was amplified with this primer. This variation is, however, not consistent across teleosts; this amino acid is not divergent between the two GR genes in the common carp, C. carpio (see Fig. 1).
Comparisons of the midshipman receptors with other vertebrate GRs (alignment in Fig. 1 and sequence identity in Supp. Table 1) and MRs (alignment in Fig. 2 and sequence identity in Supp. Table 2) revealed high sequence identity. Steroid receptors are composed of several domains important for transcriptional activation: DNA binding domain (DBD-C domain), ligand binding and dimerization domain (LBD-E domain), N-terminal (A/B-domain), hinge region (D-domain), and C-terminal (F-domain) (Gronemeyer and Laudet, 1995). The aligned sequences in the GR included the entire LBD domain, with its predicted binding residues and a portion of the DBD domain sequence. The midshipman GR was most similar to the cichlid GR2 (Supp. Table 1) that has a nine amino acid insert in the DBD domain (Fig. 1). The midshipman GR and MR shared 52% sequence identity in the overlapping sequences of the DBD/ LBD domains.
3.2. Absolute qPCR
We hypothesized that the expression of GR and MR in the type I and type II males may differ from each other and reflect their different reproductive tactics. Indeed, robust and significant differences were found between the two male morphs, as well as between both male morphs and females, for both GR and MR in central and peripheral tissues. Welch-ANOVA verified both morph and gene specific differences for both the GR and MR (p<0.0001). The results for GR and MR are presented in graphical format in Figure 3, and in numerical detail in Supplemental Table 3 and Supplemental Table 4.
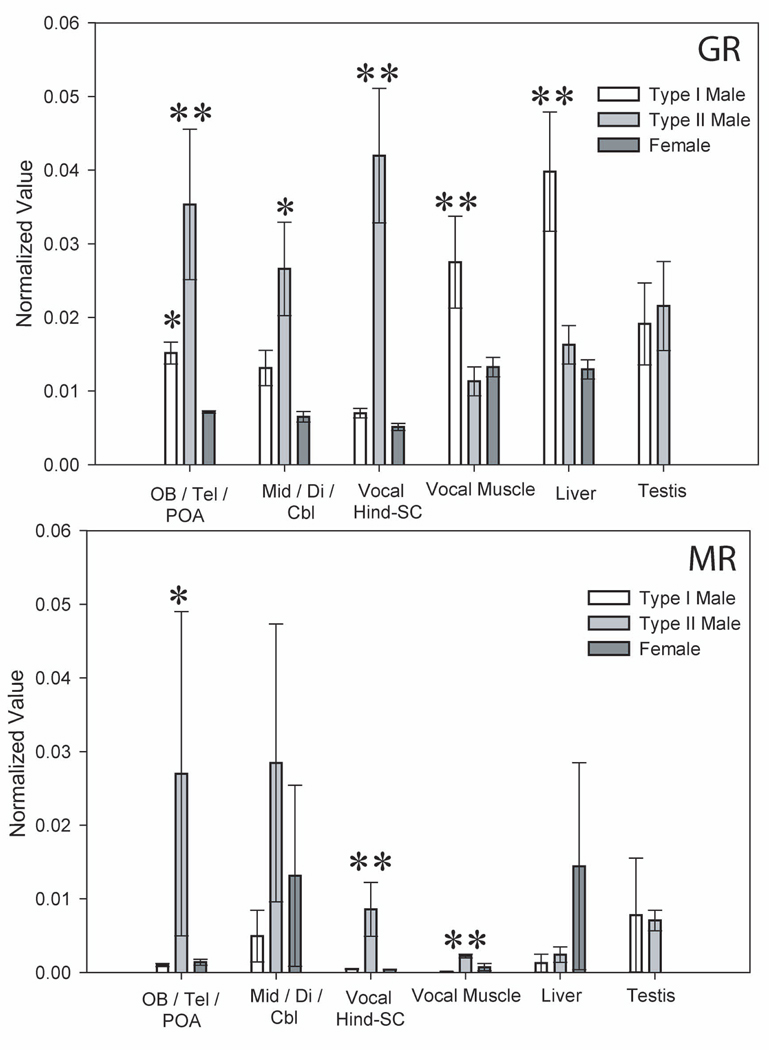
Figure 3.
Plots showing quantitative real time PCR (qPCR) values for glucocorticoid receptor (GR, top) and mineralocorticoid receptor (MR, bottom) mRNA for each tissue sampled in the midshipman reproductive morphs (legend, top right). Values were normalized using the beta actin gene copy number from the same tissue (see Materials and Methods). Standard errors are shown and asterisks indicate significantly greater levels over one (single asterisk) or both (double asterisks) other morphs (see Supplemental Table 3 and Supplemental Table 4 for numerical data). Three CNS regions were sampled: forebrain including olfactory bulb (OB), telencephalon (Tel) and preoptic area (POA); large middle region including midbrain tectum and tegmentum (Mid), diencephalon (Di) and cerebellum (Cbl); and the remaining hindbrain (hind) and rostral spinal cord (SC) that houses the vocal pattern generator circuit (Bass et al., 1994).
For GR, type II males showed significant expression above that of both other morphs in forebrain and the vocal hindbrain-spinal cord (indicated by double asterisks in Fig. 3), but only above females in the middle CNS region containing midbrain, diencephalon, and cerebellum (single asterisk in Fig. 3) (one way ANOVA, p ≤ 0.013). Type I male forebrain GR levels were also significantly greater than those for females, (p = 0.0001). In the vocal muscle and liver, type I males showed significantly greater levels of GR expression above type II males and females (p ≤ 0.014). By contrast, there were no differences in levels of expression between the male morphs for the testis (p = 0.645).
For MR, type II males again showed higher levels than type I males and females in the vocal hindbrain-spinal cord (p = 0.002), but only above type I males in the forebrain (p = 0.033). There were no significant differences across all morphs in the middle CNS region. In the periphery, type II males exhibited significantly higher levels of MR over both type I males and females in the vocal muscle alone (p = 0.002).
In general, the expression levels of GR were significantly greater than those for MR within any one tissue for each morph (p ≤ 0.027). GR and MR expression levels were similar, however, for the middle CNS region in type II males and females, and the liver in females (p ≥ 0.061).
3.3. Cortisol levels
On average, RIA showed that plasma cortisol levels were more than twice as high in type II males compared to type I males: 134.96 ng/ml ± 21.05 (s.e.m) and 48.16 ng/ml ± 14.13 for, respectively, type II and type I males (p = 0.009; dF = 8, 1; F ratio = 11.72). The cortisol values likely represent basal rather than stress-induced conditions related to captivity. Previous studies of midshipman show no significant differences in cortisol levels for individuals sampled either acutely or 3–4 h post-capture as was done here (Knapp et al., 1999; Sisneros et al., 2004). Additionally, the cortisol levels for the blood samples collected here using cardiac puncture 3–4 h after collecting type I males from nests were similar to those determined for blood samples collected from the caudal vein of type I males at the nest site (Knapp et al., 1999).
4. Discussion
The principle findings of this investigation in the midshipman fish that has two male reproductive morphs are that (1) the GR and MR show high sequence identity with that of other vertebrate GRs and MRs and (2) there are robust morph-specific patterns in the abundance of GR and more modest differences in MR. In general, the CNS of type II males has higher levels of both GR and MR than the CNS of type I males and females. Type I males, however, show a striking increase in GR in the liver and vocal muscle as compared to type II males and females.
4.1. Vertebrate corticosteroid receptors
The consequences of corticosteroid activity are as diverse as they are necessary. Corticosteroids and their receptors have co-evolved to influence a multitude of physiological and behavioral parameters via discrete modifications in steroid signaling pathways. For example, successive mutations in key residues, such as those between GR and MR, have lead to the capacity for novel function in the face of substrate availability, termed “molecular exploitation” (Bridgham et al., 2006). Like tetrapods (Yao et al., 2008), two separate genes for GR and MR have now been identified in cartilaginous fish (Carroll et al., 2008) and several teleost species (Alsop and Vijayan, 2008; Schaaf et al., 2009) that now includes midshipman fish (this report). We report one GR for midshipman as reported in several other teleosts including zebrafish (Alsop and Vijayan, 2009; Schaaf et al., 2009). However, as discussed earlier (section 3.1), we cannot yet rule out the possibility that midshipman may yet have a duplicated GR gene as in some teleosts (Alsop and Vijayan, 2009; Bury and Sturm, 2007; Stolte et al., 2006).
Both GR and MR bind the same genomic response elements, two palindromic half-sites, but regulate separate cell processes through the interaction of their distinctive A/B domains with unique sets of transcription factors (Evans, 1988; Liu et al., 1996; Pearce, 1994). Although the midshipman MR sequence reported here is not as complete as the GR sequence, there are two interesting differences in their amino acid sequences. S810 in human MR is important in binding aldosterone (Bledsoe et al., 2005) and it is conserved in midshipman, but the residue is M in the midshipman GR. Similarly, Q642 in human GR is important in binding dexamethasone (Bledsoe et al., 2002) and it is conserved in the midshipman GR, but it is an L in MR. These differences may be responsible for the ligand selectivity of the midshipman GR and MR, which is currently under investigation (A. Arterbery, A. Bass, D. Deitcher, E. Fogerty and L. Kraus, in preparation).
As in humans, cortisol is considered the principal circulating glucocorticoid in teleosts (Bentley, 1998), including midshipman (Knapp et al., 1999; Sisneros et al., 2004; this report). While 11-deoxycorticosterone has been implicated in corticosteroid receptor ligand-induced activity in some teleosts (Milla et al., 2008), it has not yet been assayed in midshipman fish. Cortisol binds to induce the transcriptional activity of both GR and MR, however, MR has a 10-fold higher sensitivity and is also activated by aldosterone (Krozowski and Funder, 1983). Aldosterone levels are typically either much lower than those for cortisol, as in some tetrapods including humans (Baker et al., 2007), or close to non-detectable as in most teleosts so far studied (Prunet et al., 2006) that now includes midshipman (see Materials and Methods). Hence, MR is thought to be occupied by cortisol under basal conditions in both fish and tetrapods (Krozowski and Funder, 1983).
4.2. Divergent patterns of GR and MR expression
Collectively, qPCR and anatomical studies show that GRs and MRs are expressed throughout the body in both cartilaginous and teleost fishes (Bury et al., 2003; Schaaf et al., 2008; Sturm et al., 2005). In general, GR levels were higher than MR levels in both the CNS and all of the peripheral tissues assayed in midshipman fish. Comparable studies in skates (Leucoraja erinacea) show that MR and GR levels are similar in the brain, while contrasting patterns are seen in peripheral tissues (Carroll et al., 2008). In cichlid fish (Astatotilapia burtoni), MR levels are higher in the brain, while GR is higher in peripheral tissues (Greenwood et al., 2003).
The patterns of GR and MR expression observed in midshipman fish may reflect any number of physiological/ behavioral states (Korte et al., 2005; Sapolsky et al., 2000), including a wide range of factors linked to the midshipman’s seasonal and daily migrations inside and outside of the intertidal zone (Bass, 1996). However, as noted in the Introduction, the current study investigated patterns of GR and MR expression in the context of the midshipman’s two male morphs that show an extreme divergence in a wide range of traits coupled to morph-specific vocal and spawning tactics during the breeding season. Hence, we mainly focus the remainder of this section of the discussion on the potential causal relationships between patterns of receptor expression and well-established morph-specific, reproductive-related traits in midshipman fish, fully recognizing that alternative explanations may exist.
4.2.1. Peripheral tissues: vocal muscle, liver, and testis
The vocal repertoire of type I males is far more dynamic than that of type II males and females (see Introduction). In particular, the type I male’s production of long duration advertisement hums throughout the night is likely to require intense metabolic support. Elevated GR levels in both the vocal muscle and the liver of type I males could contribute to the physiological support for prolonged vocal muscle activation. Increased GR in peripheral tissues, especially muscle, liver, and adipose tissue (Vegiopoulos and Herzig, 2007) has been associated with producing new substrate for gluconeogenesis, thereby providing glucose as an energy source (Bentley, 1998; Einstein et al., 2004). The far more modest, but significantly elevated levels of MR in the type II muscle may yet support vocal function in type II males that has been little studied.
The vocal muscle of type I male midshipman fish exhibits a distinct suite of morph-specific traits that now include the highest GR levels, all of which may support the physiological demands of courtship calling during the breeding season (Bass and Forlano, 2008; Bass and Remage-Healey, 2008; Walsh et al., 1995). The vocal muscles of midshipman fish and other members of their family (Batrachoididae) are among the fastest contracting vertebrate muscles (Cohen and Winn, 1967; Rome et al., 1996). As such, the vocal muscles provide exquisite models for elucidating the potential role of corticosteroids and their cognate receptors in supporting the molecular and mechanical requirements of high speed contraction (Rome et al., 1996) that, at least in the case of midshipman, can continue non-stop for an hour or more.
Midshipman male morphs also differ in their profile of circulating steroids (Brantley et al., 1993b; Knapp et al., 1999; Sisneros et al., 2004). The lack of any significant divergence in testis GR and MR expression between the two male morphs suggests that corticosteroids do not play a critical role in any functions of the testis related to morph-specific patterns of steroid biosynthesis (e.g., size; see Bass 1996).
4.2.2. CNS
The GR/MR expression patterns of peripheral tissues can be more directly linked to functional outcomes because of each tissue’s more singular function, such as sound production by the vocal muscle (see above). The same logic can be applied to the midshipman’s hindbrain-spinal cord region that is dominated by a neural circuit dedicated to vocal patterning (Bass and Baker, 1990; Bass et al., 1994). Neurophysiological studies of the vocal hindbrain-spinal cord have led to several cogent hypotheses regarding the functional significance of divergent patterns of mRNA and protein abundance (Bass and Remage-Healey, 2008). Cortisol has morph-specific effects on vocal activity that is dependent, in part, on its direct actions on the vocal hindbrain-spinal cord (Remage-Healey and Bass, 2004, 2007). The robust male dimorphisms in GR and MR expression in the vocal hindbrain-spinal cord parallel the morph-specific neurophysiological effects of cortisol (Fig. 3). Consistent with the qPCR results, we predict that anatomical studies will show a higher density of GRs and MRs in the vocal hindbrain-spinal nuclei of type II males compared to type I males, as shown for aromatase mRNA and protein (Forlano and Bass, 2005; Schlinger et al., 1999).
High and low GR mRNA levels in the mammalian CNS have been associated with, respectively, an increased and decreased display of anxiety-like behaviors (Brinks et al., 2007). Similar receptor-behavioral comparisons can be made for midshipman fish. Type II males are chronically exposed to intense aggression from nest-holding type I males (Brantley and Bass, 1994). While type I males will consistently attack type II males, the reverse is not the case; type II males successfully escape their attacks and soon return to a nest to attempt further sneak-spawnings. Compared to the territorial type I males, the anxiety-like escape phenotype of type II males is paralleled by higher CNS levels of GR/MR and increased plasma cortisol levels. This phenotype may extend to other species with alternative male tactics. The divergent cortisol levels between midshipman male morphs resemble those of the type II-like and type I-like males in sunfish (Lepomis macrochirus) that have alternative reproductive tactics much like those of midshipman (Knapp and Neff, 2007).
For type II male midshipman that exhibit the highest CNS levels of corticosteroid receptor expression among the reproductive morphs, elevated receptor expression may reflect a constitutively higher basal cortisol level that provides long-term support for their reproductive behavior and physiology. The latter relationship may reflect, more generally, a positive feedback mechanism between GR/MR and cortisol, as reported for the peripheral tissues of some teleosts (Takahashi et al., 2006; Vijayan et al., 2003). Consistent with this interpretation, type I males showed comparatively reduced levels of both GR/MR and cortisol.
4.3 Conclusions
Vertebrates exhibit a wide range of corticosteroid-related behavioral phenotypes (Korte et al., 2005; Sapolsky et al., 2000). The results presented here suggest a strong relationship between peripheral and central GR/MR regulation and a suite of neural, endocrinological and behavioral traits that diverges widely between two male reproductive morphs. We propose that the co-elevation of cortisol and receptor mRNA in type II male midshipman reflects an adaptive response that supports a stable reproductive behavior, in this case sneak/satellite spawning. The opposite relationship between corticosteroid levels and receptors has been shown in mammals (including humans), where chronically elevated glucocorticoid levels are often correlated with reduced GR expression in the brain and depression (Pariante, 2006). A more complete understanding of the molecular mechanisms leading to alternative male reproductive phenotypes like those shown by midshipman fish can begin to contribute to a more complete understanding of the benefits and costs of therapies aimed at the maintenance of GR function and expression in the face of elevated systemic cortisol levels (Pariante, 2006). More generally, vertebrates with alternative behavioral phenotypes (see Rhen and Crews, 2002) can now provide naturally-occurring models for identifying molecular mechanisms that promote the successful transitioning from disease to adaptive behavioral and physiological states.
Supplementary Material
Acknowledgements
We would like to thank the Cornell Statistical Consulting Unit, Cornell Life Sciences Core Laboratories Center, and NIH; Kevin Rohmann for technical advice, Margaret Marchaterre for field assistance, Cornell Diagnostics Laboratory for the cortisol assays, Aaron Rice for statistical advice and two anonymous reviewers for many helpful comments on the manuscript. Support from a Cornell Diversity Fellowship and an NIH Institutional Training Grant (GM007469) to ASA and an NSF Research Grant (IOB 0516748) to AHB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alsop D, Vijayan M. The zebrafish stress axis: Molecular fallout from the teleost-specific genome duplication event. Gen. Comp. Endocrinol. 2008 doi: 10.1016/j.ygcen.2008.09.011. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Alsop D, Vijayan M. The zebrafish stress axis: molecular fallout from the teleost-specific genome duplication event. Gen. Comp. Endocrinol. 2009;161:62–66. doi: 10.1016/j.ygcen.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Baker ME. Steroid receptor phylogeny and vertebrate origins. Mol. Cell. Endocrinol. 1997;135:101–107. doi: 10.1016/s0303-7207(97)00207-4. [DOI] [PubMed] [Google Scholar]
- Baker ME, Chandsawangbhuwana C, Ollikainen N. Structural analysis of the evolution of steroid specificity in the mineralocorticoid and glucocorticoid receptors. BMC Evol. Biol. 2007;7:24. doi: 10.1186/1471-2148-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH. Shaping brain sexuality. Am. Sci. 1996;84:352–363. [Google Scholar]
- Bass AH, Bodnar DA, Marchaterre MA. Complementary explanations for existing phenotypes in an acoustic communication system. In: Hauser M, Konishi M, editors. Neural Mechanisms of Communication. Cambridge, MA: MIT Press; 1999. pp. 493–514. [Google Scholar]
- Bass AH, Forlano PH. Neuroendocrine mechanisms of alternative reproductive tactics: The chemical language of social plasticity. In: Oliveira RF, Taborsky M, Brockmann J, editors. Alternative Reproductive Tactics - an Integrative Approach. Cambridge Univ. Press; 2008. pp. 109–131. [Google Scholar]
- Bass AH, Grober MS. Reproductive Plasticity in fish: Evolutionary liability in the patterning of neuroendocrine and behavioral traits underlying divergent sexual phenotypes. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R, editors. Hormones, Brain, and Behavior. Academic Press; 2009. [Google Scholar]
- Bass AH, Marchaterre MA. Sound-generating (sonic) motor system in a teleost fish (Porichthys notatus): sexual polymorphism in the ultrastructure of myofibrils. J. Comp. Neurol. 1989;286:141–153. doi: 10.1002/cne.902860202. [DOI] [PubMed] [Google Scholar]
- Bass AH, Remage-Healey L. Central pattern generators for social vocalization: androgen-dependent neurophysiological mechanisms. Horm. Behav. 2008;53:659–672. doi: 10.1016/j.yhbeh.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P. Comparative Vertebrate Endocrinology. Cambridge, MA: Cambridge Univ. Press; 1998. [Google Scholar]
- Brantley RK, Bass AH. Alternative male spawning tactics and acoustic signals in the plainfin midshipman fish Porichthys notatus Girard (Teleostei, Batrachoididae) Ethology. 1994;96:213–232. [Google Scholar]
- Brantley RK, Tseng J, Bass AH. The ontogeny of inter- and intrasexual vocal muscle dimorphisms in a sound-producing fish. Brain Behav. Evol. 1993a;42:336–349. doi: 10.1159/000114170. [DOI] [PubMed] [Google Scholar]
- Brantley RK, Wingfield JC, Bass AH. Sex steroid levels in Porichthys notatus, a fish with alternative reproductive tactics, and a review of the hormonal bases for male dimorphism among teleost fishes. Horm. Behav. 1993b;27:332–347. doi: 10.1006/hbeh.1993.1025. [DOI] [PubMed] [Google Scholar]
- Bridgham JT, Carroll SM, Thornton JW. Evolution of hormone-receptor complexity by molecular exploitation. Science. 2006;312:97–101. doi: 10.1126/science.1123348. [DOI] [PubMed] [Google Scholar]
- Brinks V, van der Mark M, de Kloet R, Oitzl M. Emotion and cognition in high and low stress sensitive mouse strains: a combined neuroendocrine and behavioral study in BALB/c and C57BL/6J mice. Front. Behav. Neurosci. 2007;1:8. doi: 10.3389/neuro.08.008.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bury NR, Sturm A. Evolution of the corticosteroid receptor signalling pathway in fish. Gen. Comp. Endocrinol. 2007;153:47–56. doi: 10.1016/j.ygcen.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Bury NR, Sturm A, Le Rouzic P, Lethimonier C, Ducouret B, Guiguen Y, Robinson-Rechavi M, Laudet V, Rafestin-Oblin ME, Prunet P. Evidence for two distinct functional glucocorticoid receptors in teleost fish. J. Mol. Endocrinol. 2003;31:141–156. doi: 10.1677/jme.0.0310141. [DOI] [PubMed] [Google Scholar]
- Carroll SM, Bridgham JT, Thornton JW. Evolution of hormone signaling in elasmobranchs by exploitation of promiscuous receptors. Mol. Biol. Evol. 2008;25:2643–2652. doi: 10.1093/molbev/msn204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein M, Greenlee M, Rouen G, Sitlani A, Santoro J, Wang C, Pandit S, Mazur P, Smalera I, Weaver AP, Zeng YY, Ge L, Kelly T, Paiva T, Geissler W, Mosley RT, Williamson J, Ali A, Balkovec J, Harris G. Selective glucocorticoid receptor nonsteroidal ligands completely antagonize the dexamethasone mediated induction of enzymes involved in gluconeogenesis and glutamine metabolism. J. Steroid Biochem. Mol. Biol. 2004;92:345–356. doi: 10.1016/j.jsbmb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano PM, Bass AH. Seasonal plasticity of brain aromatase mRNA expression in glia: divergence across sex and vocal phenotypes. J. Neurobiol. 2005;65:37–49. doi: 10.1002/neu.20179. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Deitcher DL, Bass AH. Distribution of estrogen receptor alpha mRNA in the brain and inner ear of a vocal fish with comparisons to sites of aromatase expression. J. Comp. Neurol. 2005;483:91–113. doi: 10.1002/cne.20397. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Schlinger BA, Bass AH. Brain aromatase: new lessons from non-mammalian model systems. Front. Neuroendocrinol. 2006;27:247–274. doi: 10.1016/j.yfrne.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Greenwood AK, Butler PC, White RB, DeMarco U, Pearce D, Fernald RD. Multiple corticosteroid receptors in a teleost fish: distinct sequences, expression patterns, and transcriptional activities. Endocrinology. 2003;144:4226–4236. doi: 10.1210/en.2003-0566. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H, Laudet V. Transcription factors 3: nuclear receptors. Protein Profile. 1995;2:1173–1308. [PubMed] [Google Scholar]
- Ibara RM, Penny LT, Ebeling AW, van Dykhuizen G, Calliet G. The mating call of the plainfin midshipman fish, Porichthys notatus. In: Noakes DGL, Lindquis DG, Helfmann GS, Ward JA, editors. Predators and prey in fishes. The Hague, Netherlands: Junk Press; 1983. pp. 205–212. [Google Scholar]
- Kime DE. Fundamentals of Comparative Vertebrate Endocrinology. NY, NY: Plenum Press; 1987. [Google Scholar]
- Knapp R. Endocrine mediation of vertebrate male alternative reproductive tactics: the next generation of studies. Integr. Comp. Biol. 2003;43:658–668. doi: 10.1093/icb/43.5.658. [DOI] [PubMed] [Google Scholar]
- Knapp R, Neff BD. Steroid hormones in bluegill, a species with male alternative reproductive tactics including female mimicry. Biol. Lett. 2007;3:628–631. doi: 10.1098/rsbl.2007.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp R, Wingfield JC, Bass AH. Steroid hormones and paternal care in the plainfin midshipman fish (Porichthys notatus) Horm. Behav. 1999;35:81–89. doi: 10.1006/hbeh.1998.1499. [DOI] [PubMed] [Google Scholar]
- Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci. Biobehav. Rev. 2005;29:3–38. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Krozowski ZS, Funder JW. Renal mineralocorticoid receptors and hippocampal corticosterone-binding species have identical intrinsic steroid specificity. Proc. Natl. Acad. Sci. USA. 1983;80:6056–6060. doi: 10.1073/pnas.80.19.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Wang J, Yu G, Pearce D. Steroid receptor transcriptional synergy is potentiated by disruption of the DNA-binding domain dimer interface. Mol. Endocrinol. 1996;10:1399–1406. doi: 10.1210/mend.10.11.8923466. [DOI] [PubMed] [Google Scholar]
- Milla S, Terrien X, Sturm A, Ibrahim F, Giton F, Fiet J, Prunet P, Le Gac F. Plasma 11-deoxycorticosterone (DOC) and mineralocorticoid receptor testicular expression during rainbow trout Oncorhynchus mykiss spermiation: implication with 17alpha, 20beta-dihydroxyprogesterone on the milt fluidity? Reprod. Biol. Endocrinol. 2008;6:19. doi: 10.1186/1477-7827-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM. The glucocorticoid receptor: part of the solution or part of the problem? J. Psychopharmacol. 2006;20:79–84. doi: 10.1177/1359786806066063. [DOI] [PubMed] [Google Scholar]
- Pearce D. A mechanistic basis for distinct mineralocorticoid and glucocorticoid receptor transcriptional specificities. Steroids. 1994;59:153–159. doi: 10.1016/0039-128x(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Prunet P, Sturm A, Milla S. Multiple corticosteroid receptors in fish: from old ideas to new concepts. Gen. Comp. Endocrinol. 2006;147:17–23. doi: 10.1016/j.ygcen.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Rapid, hierarchical modulation of vocal patterning by steroid hormones. J. Neurosci. 2004;24:5892–5900. doi: 10.1523/JNEUROSCI.1220-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Plasticity in brain sexuality is revealed by the rapid actions of steroid hormones. J. Neurosci. 2007;27:1114–1122. doi: 10.1523/JNEUROSCI.4282-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn SC, Aubin-Horth N, Hofmann HA. Fish and chips: functional genomics of social plasticity in an African cichlid fish. J Exp Biol. 2008;211:3041–3056. doi: 10.1242/jeb.018242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen T, Crews D. Variation in reproductive behaviour within a sex: neural systems and endocrine activation. J. Neuroendocrinol. 2002;14:517–531. doi: 10.1046/j.1365-2826.2002.00820.x. [DOI] [PubMed] [Google Scholar]
- Rome LC, Syme DA, Hollingworth S, Lindstedt SL, Baylor SM. The whistle and the rattle: the design of sound producing muscles. Proc. Natl. Acad. Sci. USA. 1996;93:8095–8100. doi: 10.1073/pnas.93.15.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, Champagne D, van Laanen IH, van Wijk DC, Meijer AH, Meijer OC, Spaink HP, Richardson MK. Discovery of a Functional Glucocorticoid Receptor {beta}-Isoform in Zebrafish. Endocrinology. 2008;149:1591–1599. doi: 10.1210/en.2007-1364. [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, Chatzopoulou A, Spaink HP. The zebrafish as a model system for glucocorticoid receptor research. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009 doi: 10.1016/j.cbpa.2008.12.014. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Greco AC, Bass AH. Aromatase activity in hindbrain vocal control region: Divergence between "singing" and "sneaking" males. Proc. R. Soc. Lond., Ser. B: Biol. Sci. 1999;266:131–136. [Google Scholar]
- Sisneros JA, Forlano PM, Knapp R, Bass AH. Seasonal variation of steroid hormone levels in an intertidal-nesting fish, the vocal plainfin midshipman. Gen. Comp. Endocrinol. 2004;136:101–116. doi: 10.1016/j.ygcen.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Stolte EH, van Kemenade BM, Savelkoul HF, Flik G. Evolution of glucocorticoid receptors with different glucocorticoid sensitivity. J. Endocrinol. 2006;190:17–28. doi: 10.1677/joe.1.06703. [DOI] [PubMed] [Google Scholar]
- Sturm A, Bury N, Dengreville L, Fagart J, Flouriot G, Rafestin-Oblin ME, Prunet P. 11-deoxycorticosterone is a potent agonist of the rainbow trout (Oncorhynchus mykiss) mineralocorticoid receptor. Endocrinology. 2005;146:47–55. doi: 10.1210/en.2004-0128. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Sakamoto T, Hyodo S, Shepherd BS, Kaneko T, Grau EG. Expression of glucocorticoid receptor in the intestine of a euryhaline teleost, the Mozambique tilapia (Oreochromis mossambicus): effect of seawater exposure and cortisol treatment. Life. Sci. 2006;78:2329–2335. doi: 10.1016/j.lfs.2005.09.050. [DOI] [PubMed] [Google Scholar]
- Vegiopoulos A, Herzig S. Glucocorticoids, metabolism and metabolic diseases. Mol. Cell. Endocrinol. 2007;275:43–61. doi: 10.1016/j.mce.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Vijayan MM, Raptis S, Sathiyaa R. Cortisol treatment affects glucocorticoid receptor and glucocorticoid-responsive genes in the liver of rainbow trout. Gen. Comp. Endocrinol. 2003;132:256–263. doi: 10.1016/s0016-6480(03)00092-3. [DOI] [PubMed] [Google Scholar]
- Walsh PW, Mommsen TP, Bass AH. Biochemical and molecular aspects singing in batrachoidid fishes. In: Hochachka PW, Mommsen TP, editors. Biochemistry and Molecular biology of Fishes. Amsterdam: Elsevier; 1995. pp. 279–289. [Google Scholar]
- Yao M, Hu F, Denver RJ. Distribution and corticosteroid regulation of glucocorticoid receptor in the brain of Xenopus laevis. J. Comp. Neurol. 2008;508:967–982. doi: 10.1002/cne.21716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.