Abstract
Objective
To determine the clinical course and outcomes of infants with chronic lung disease (CLD) and pulmonary hypertension (PH) who received prolonged sildenafil therapy.
Study design
Retrospective review of 25 patients < 2 years of age with CLD in whom sildenafil was initiated for the treatment of PH while hospitalized from January 2004 – October 2007. Hemodynamic improvement was defined by a 20% decrease in the ratio of pulmonary to systemic systolic arterial pressure or improvement in the degree of ventricular septal flattening by serial echocardiograms.
Results
Chronic sildenafil therapy (dose range: 1.5-8 mg/kg/d) was initiated at a median of 171 days of age (range: 14-673) for a median duration of 241 days (range: 28-950). Twenty-two patients (88%) achieved hemodynamic improvement after a median treatment duration of 40 days (range: 6-600). Eleven of the 13 patients with interval estimates of systolic pulmonary artery pressure by echocardiogram showed clinically significant reductions in PH. Five patients (20%) died during the follow up period. Adverse events leading to cessation or interruption of therapy occurred in 2 patients, one for recurrent erections, and the other had the medication held briefly due to intestinal pneumatosis.
Conclusions
These data suggest that chronic sildenafil therapy is well-tolerated, safe and effective for infants with PH and CLD.
Keywords: phosphodiesterase inhibitors, pediatrics, bronchopulmonary dysplasia, congenital diaphragmatic hernia, lung hypoplasia, chronic mechanical ventilation
Pulmonary hypertension (PH) complicates the course of chronic lung disease (CLD) in newborns and contributes to late morbidity and mortality during infancy, especially in the setting of bronchopulmonary dysplasia (BPD), congenital diaphragmatic hernia (CDH), persistent pulmonary hypertension of the newborn, (PPHN) and pulmonary hypoplasia.1-5 Infants with BPD and late PH have a mortality of 52% within 2 years after diagnosis, which is strongly associated with the severity of PH.5 Although recent advances in vascular biology have led to new therapeutic strategies for the treatment of chronic PH, few studies have investigated the efficacy of these strategies for infants with CLD.
Inhaled nitric oxide (iNO) has become the standard therapy for PH shortly after birth in term and near-term infants.6 However, its effectiveness during long-term treatment of PH beyond the immediate neonatal period remains unclear. Although several medications for the chronic treatment of PH have been studied in adults and older children, the utility of these medications in infants, especially with CLD, remains uncertain. Oral calcium channel blockers acutely improve pulmonary hemodynamics in some infants with BPD,7 but most patients are poorly responsive8 and the response to prolonged therapy is variable.9 Similarly, experience with other PH therapies, such as intravenous epoprostenol, endothelin receptor blockers,10-13 aerosolized prostacyclin analogues,14-18 iNO,19, 20 and other agents is limited in this population.
One possible strategy for chronic PH therapy is through augmentation of the nitric oxide/cyclic guanosine monophosphate (NO-cGMP) signaling pathway.21 Laboratory and clinical studies have demonstrated that most forms of PH are associated with disruption of endogenous NO production or activity.22 Normally, endothelium-derived NO activates soluble guanylate cyclase, thereby stimulating production of cGMP in pulmonary artery smooth muscle cells leading to vasodilation.23 The cGMP-specific type 5 phosphodiesterase (PDE-5), an enzyme found in high concentrations in pulmonary vascular smooth muscle, rapidly degrades cGMP, which could lead to impaired vasodilation and abnormal vascular growth and structure.24,25 PDE-5 inhibition preserves intracellular cGMP concentrations and provides an approach to augment cGMP–mediated vasodilation and suppression of smooth muscle proliferation in patients with PH.
Sildenafil, a highly selective PDE-5 inhibitor, has been shown to be beneficial in adults as both monotherapy26-31 and in combination with standard treatment regimens.32,33 Therefore, to examine the potential efficacy of long-term sildenafil therapy in infants with CLD, we reviewed our clinical experience with patients with CLD and PH treated with sildenafil.
METHODS
After institutional review board approval, we reviewed the medical records of all patients at our institution from January 2004 through October 2007 with a diagnosis of CLD (including BPD, CDH, PPHN, and pulmonary hypoplasia) who received their first dose of sildenafil therapy for PH as an inpatient before 2 years of age. The diagnosis of PH was based on echocardiographic criteria (as defined below). To more directly examine the effects of sildenafil therapy in pulmonary hypertension due to CLD, patients with complex congenital heart disease (any lesion other than atrial septal defect [ASD], persistent foramen ovale, or patent ductus arteriosus [PDA]) were excluded from analysis.
Sildenafil treatment was generally initiated at a dose of 0.5 mg/kg/dose every 8 hours, which was increased to achieve desired clinical effect (improved echocardiogram findings and/or improved clinical status) or a maximum dose of 2 mg/kg/dose every 6-8 hours. Other pulmonary hypertension therapy and supportive care were continued or initiated at the discretion of the primary care team. We generally target oxygen saturations in our older infants for ranges between 92-96% with the goal to avoid hypoxemia, and to minimize marked elevations of hyperoxia that may be toxic to the lung.
Cardiopulmonary hemodynamic variables were determined by echocardiogram and cardiac catheterization studies, which were performed as clinically indicated. All echocardiograms were performed with the patient receiving the level of cardiopulmonary support, including PH medications, prescribed by the primary care team. The frequency of echocardiogram studies was determined by the clinical team, but was based on disease severity or at 2-4 month intervals during long-term follow-up. Echocardiograms were officially read by a member of a dedicated team of 3 cardiologists whose interpretations were made independent of the care provided by the clinical team. Additionally, this team of cardiologists has established strict criteria by which they assess PH. Echocardiogram measurements included tricuspid regurgitant jet velocity (TRJV) and qualitative measures of pulmonary hypertension: right atrial (RA) enlargement, right ventricular (RV) dilation, right ventricular hypertrophy (RVH), and ventricular septal flattening. Shunt lesions and direction of blood flow were also recorded. Estimated systolic pulmonary artery pressure (sPAP) was calculated with no allowance for the right atrial pressure using the modified Bernoulli equation: (TRJV2 × 4). Systemic systolic blood pressure (ssBP) was recorded via blood pressure cuff unless the patient had an existing arterial catheter. PH was defined by an estimated sPAP/ssBP ≥ 0.5 by ECHO prior to any PH therapy being instituted. In the absence of a measurable TRJV, evidence of ventricular septal flattening was adequate for the diagnosis of PH.
The indications and methods of cardiac catheterization at our institution have been described.34 Cardiac catheterization measurements included mean pulmonary artery pressure (mPAP), mean systemic blood pressure (mBP), pulmonary (PVR) and systemic (SVR) vascular resistances, mean right atrial pressure (RAP), pulmonary capillary wedge pressure (PCWP), and pulmonary (Qp) and systemic (Qs) blood flows. Not all measurements were available in every patient. Measurements were recorded with and without iNO treatment when available. Reactivity was assessed by adding iNO to those not previously receiving it or withdrawing it in those who were receiving it. Patients being treated with iNO (dose range: 5 - 40 ppm) at the time of catheterization were assessed off iNO only if their hemodynamic status could tolerate the evaluation. Withdrawal of iNO in these patients was performed slowly to minimize rebound effect. A 20% or greater difference in either the mPAP/mBP or PVR/SVR was defined as a positive reactivity test. All measurements and evaluations were performed in Denver, Colorado (altitude 1600 meters).
The primary outcome was improvement in PH defined by ≥ 20% decrease in the ratio of pulmonary to systemic systolic arterial pressure or improvement in the degree of septal flattening assessed by serial echocardiograms. Patients without a specified degree of septal flattening at baseline must have had a normal septum on subsequent echocardiogram to be considered improved. Time to improvement was measured from the start of sildenafil therapy to the first echocardiogram demonstrating improvement. Secondary assessments included survival, the ability to wean off other PH therapy, especially iNO, and the ability to wean off mechanical ventilation. Safety was assessed by documented adverse events while on sildenafil and the discontinuation of sildenafil treatment for reasons other than improved clinical status.
Statistical Analysis
Descriptive statistics of patient characteristics are reported by the median (range) for non-normally distributed data and by mean ± standard deviation (SD) for normally distributed data. Changes from baseline to follow up assessments were analyzed by Student paired t-test. Time to clinical improvement from starting sildenafil treatment was summarized using Kaplan-Meier Product-Limit estimates. In all analyses, a P value ≤ 0.05 was considered to be significant. Statistical analyses were carried out using SAS 9.1 (SAS Institute Inc., Cary, NC).
RESULTS
Patient Population
Sildenafil therapy was initiated in 25 inpatients with CLD under the age of 2 years hospitalized between January 2004 and October 2007 at our tertiary care children’s hospital (Table I). Sixteen patients (64%) started sildenafil during their initial hospitalization after birth; treatment was started during re-hospitalization in the remaining patients. The median age at initiation of sildenafil therapy for infants with BPD was 184 days (range: 55-673), with no patient started prior to 40 weeks post-conceptual age.
Table 1.
Clinical Characteristics of Study Patients*
| Patients | 25 |
| Sex, male/female | 15/10 |
| Gestational age at birth, weeks | 28 (23 - 41) |
| Etiology of chronic lung disease, (%) | |
| Bronchopulmonary dysplasia | 18 (72%) |
| Congenital diaphragmatic hernia | 3 (12%) |
| Persistent pulmonary hypertension of the newborn | 3 (12%) |
| Pulmonary hypoplasia | 1 (4%) |
| Age at initiation of sildenafil, days | 171 (14 - 673) |
| Respiratory support at initiation of sildenafil, (%) | |
| Oxygen | 25 (100%) |
| CPAP | 1 (4%) |
| Mechanical ventilation | 18 (72%) |
| ECMO | 1 (4%) |
| Medications at initiation of sildenafil, (%) | |
| Nitric Oxide | 18 (72%) |
| Calcium Channel Blocker† | 1 (4%) |
| Bosentan | 2 (8%) |
| Milrinone | 4 (16%) |
| Diuretics | 19 (76%) |
| Systemic steroids | 12 (48%) |
CPAP = continuous positive airway pressure; ECMO = extracorporeal membrane oxygenation.
Values are median (range) unless otherwise noted.
For systemic hypertension.
Baseline measurements
Echocardiogram findings at the time of sildenafil initiation are presented in Table II (available at www.jpeds.com). All patients had interventricular septal flattening at baseline, although the degree was not specified in 5 patients. Estimated sPAP was possible by detection of consistent TRJV in 17 patients (68%). Twelve of these patients (70%) had severe PH with systolic pulmonary/systemic artery pressures 0.67. Shunt lesions (ASD or PDA) were present at the time of sildenafil initiation in 19 (76%) patients, with 9 patients having bidirectional or right-to-left shunting of blood flow. Three patients had an ASD that was repaired prior to sildenafil treatment. Two other patients underwent coil occlusion of aorto-pulmonary collateral vessels prior to sildenafil treatment.
Table 2.
Baseline Echocardiogram Findings
| n (%) |
|
|---|---|
| Measureable tricuspid regurgitant jet | 17 (68) |
| Estimated sPAP, mm Hg, mean ± SD | 67 ± 20 |
| Estimated sPAP/ssBP, mean ± SD | 0.79 ± 0.24 |
| sPAP/ssBP ≥ 0.67 | 12 (48) |
| Septal flattening | 25 (100) |
| Mild | 8 (32) |
| Moderate | 7 (28) |
| Severe | 5 (20) |
| Degree not specified | 5 (20) |
| Right atrial dilation | 17 (68) |
| Right ventricular dilation | 17 (68) |
| Right ventricular hypertrophy | 18 (72) |
| Detectable shunt* | 19 (76) |
| Atrial level | 17 (68) |
| Patent ductus arteriosis | 4 (16) |
| Direction of shunt flow | |
| Left-to-right | 10 (40) |
| Bidirectional | 8 (32) |
| Right-to-left | 1 (4) |
sPAP = systolic pulmonary artery pressure; ssBP = systemic systolic blood pressure.
Two patients had both an atrial septal defect and a patent ductus arteriosus.
Cardiac catheterization was performed in 21 (84%) of subjects at the time of sildenafil initiation. Seventeen patients had iNO reactivity testing performed (Table III; available at www.jpeds.com), and 14 (82%) were found to be reactive. Eleven of the 17 patients (65%) were reactive to iNO as defined by at least a 20% reduction in mPAP/mBP, and 10 of 14 (71%) with documented resistance measurements were reactive to iNO by at least a 20% reduction in PVR/SVR. Interestingly, iNO caused a small but statistically significant increase in PCWP (8.7±2.1 vs. 9.5± 2.7 mm Hg; P < 0.03). Two of 16 patients with PCWP measurements off iNO had levels 12 mm Hg, and 4 of 20 patients with PCWP measurements on iNO had levels 12 mm Hg. The two patients with the highest PCWP measurements on iNO (16 and 17 mm Hg, respectively) did not have measurements performed off iNO. One patient with BPD was found to have severe pulmonary vein stenosis during cardiac catheterization and 5 patients were found to have aorto-pulmonary collaterals.
Table 3.
Baseline Pulmonary Vascular Reactivity (n = 17)*
| n |
Off iNO |
iNO |
p-value |
|
|---|---|---|---|---|
| mPAP, mm Hg | 17 | 36.6 ± 11.2 | 26.8 ± 5.4 | <0.001 |
| mBP, mm Hg | 17 | 55.8 ± 14.0 | 58.7 ± 13.2 | 0.137 |
| mPAP/mBP | 17 | 0.7 ± 0.16 | 0.5 ± 0.12 | <0.001 |
| RAP, mm Hg | 15 | 6.5 ± 2.0 | 6.9 ± 2.77 | 0.361 |
| PCWP, mm Hg | 15 | 8.7 ± 2.09 | 9.5 ± 2.67 | 0.028 |
| PVR, U * m2 | 15 | 5.6 ± 2.34 | 3.3 ± 1.78 | <0.001 |
| SVR, U * m2 | 14 | 10.5 ± 4.14 | 10.8 ± 3.99 | 0.834 |
| PVR/SVR | 14 | 0.6 ± 0.32 | 0.3 ± 0.16 | 0.004 |
| Qp, L/min/m2 | 15 | 5.0 ± 1.49 | 6.7 ± 4.26 | 0.085 |
| Qs, L/min/m2 | 15 | 4.9 ± 1.69 | 6.0 ± 4.03 | 0.127 |
| Qp/Qs | 15 | 1.1 ± 0.32 | 1.1 ± 0.27 | 0.212 |
Values are mean ± SD unless otherwise noted.
Not all measurements were available in some patients under each condition.
iNO = inhaled nitric oxide; mPAP = mean pulmonary artery pressure; RAP = mean right atrial pressure; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; SVR = systemic vascular resistance; mBP = mean systemic arterial pressure; Qp = pulmonary blood flow; Qs = systemic blood flow; Qp/Qs = shunt fraction. Comparisons by paired Student t-test.
Effects of Sildenafil Treatment
Outcomes for patients treated with sildenafil are shown in Table IV. The median duration of sildenafil use was 241 days (range: 28–950). Most patients remained on sildenafil throughout the follow-up period. During the course of treatment, two patients underwent ligation of a PDA, one patient had an ASD repaired, and two patients underwent coil occlusion of aorto-pulmonary collateral vessels.
Table 4.
Outcomes of Patients Treated with Sildenafil (n = 25)
| Duration of sildenafil treatment, median days (range) | 241 (28 - 950) |
|
n (%) |
|
| Clinical Improvement by Echocardiogram* | 22 (84) |
| Improved degree of septal flattening | 18 (72) |
| Normalized septal flattening | 11 (44) |
| ≥ 20% decrease in pulmonary/systemic artery pressure, (n=13) | 11 (85) |
| Pulmonary/systemic artery pressure ≤ 0.33 (n=13) | 3 (23) |
| Weaned off Sildenafil | 2 (8) |
| In process of weaning off sildenafil at last contact | 6 (24) |
| Sildenafil discontinued due to side effects† | 1 (4) |
| Weaned off iNO after sildenafil initiated, (n = 18) | 15 (83) |
| Addition of other medications for PH during sildenafil treatment | 5 (20) |
| CCB | 1 (4) |
| Bosentan | 3 (12) |
| Epoprostenol | 2 (8) |
| Death during treatment with sildenafil | 5 (20) |
| Support withdrawn, respiratory futility (2 BPD, 1 pulmonary hypoplasia) | 3 (12) |
| Support withdrawn, neurological devastation (CDH) | 1 (4) |
| Sepsis (BPD) | 1 (4) |
All patients who failed to improve had no measureable tricuspid regurgitant jet and mild septal flattening at baseline.
Stopped after 950 days due to frequent erections. An additional patient’s sildenafil was briefly held secondary to pneumatosis intesitinalis which developed shortly after sildenafil initiation.
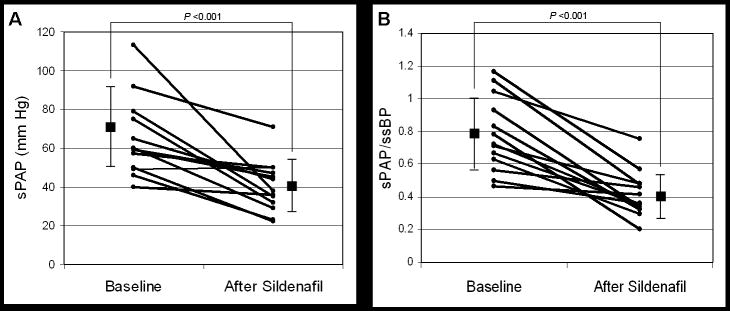
Twenty-two patients (88%) showed clinical improvement by echocardiogram after a median treatment duration of 40 days (range: 6-600, Figure 1). Thirteen patients (52%) had interval estimates of sPAP by echocardiogram available for evaluation at a median of 58 days (range: 25-334) after sildenafil therapy (Figure 2). For this group, there was a significant decrease in both the absolute sPAP (64.9 ± 20.3 vs. 40.2 ± 13.2 mm Hg, P < 0.001) and sPAP/ssBP (0.78 ± 0.23 vs. 0.41 ± 0.14, P < 0.001) after treatment with sildenafil. Eleven of the 13 patients (85%) showed at least a 20% improvement in sPAP/ssBP. The 2 patients who did not show improvement by this measure did have interval improvements in the degree of interventricular septal flattening.
Figure 1.
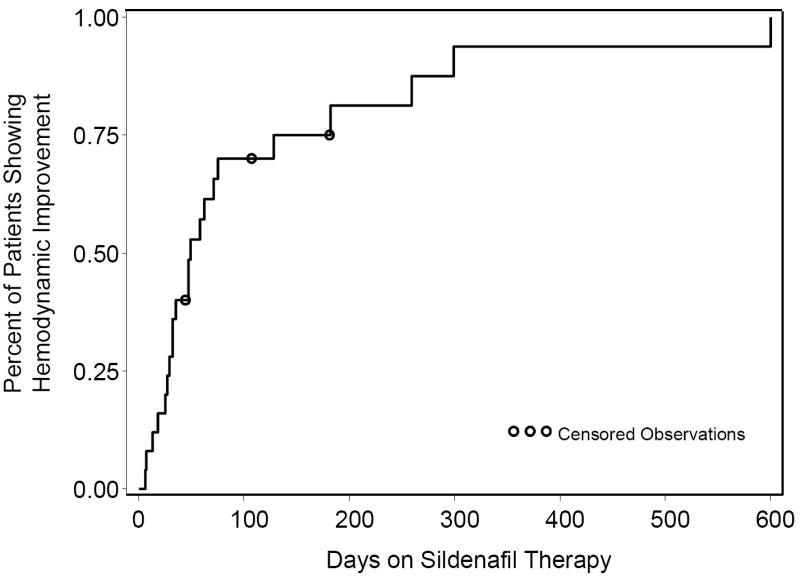
Kaplan-Meier Estimates of Time to Clinical Improvement on Sildenafil Therapy. Circles represent censored follow-up. Hemodynamic improvement was defined by at least a 20% improvement sPAP/ssBP or improved degree of septal flattening by echocardiogram.
Figure 2.
Changes in systolic pulmonary artery pressures (A) and pulmonary/systemic systolic artery pressure (B) as determined by echocardiogram in response to prolonged sildenafil therapy. Median duration of treatment between studies was 58 days (range: 25 - 334). Individual data plotted together with mean ± SD; n = 13 patients.
Eighteen patients (72%) exhibited improvement in the degree of interventricular septal flattening. Septal flattening completely resolved in 11 of these patients (44%), 4 of whom had moderate or severe septal flattening at baseline. Of the 7 patients without septal improvement, 4 exhibited improvement by interval estimates of sPAP. Two of the three patients not acutely reactive to iNO during baseline cardiac catheterization had interval improvement by echocardiogram during sildenafil treatment after 47 and 299 days, respectively. The three patients who failed to show improvement were followed for periods of 43, 106, and 180 days, respectively, and had mild septal flattening by echocardiogram prior to sildenafil initiation without a measureable TRJV. One of these patients required mechanical ventilation and iNO prior to sildenafil initiation, and subsequently weaned off these modalities without worsening of PH.
Fifteen of 18 patients (83%) receiving iNO therapy at the time of sildenafil initiation were weaned off iNO after a median treatment duration of 32 days (range: 1-334). Ten of 18 patients (56%) weaned off mechanical ventilation after a median of 21 days (range: 1-316) of sildenafil therapy. Other medications used to treat PH were added in seven (28%) patients (Table V; available at www.jpeds.com). However, only 5 patients had these medications added specifically due to insufficient clinical improvement on sildenafil therapy (Table IV). At the time of last contact, 18 (72%) remained on sildenafil as single therapy for PH, 6 of whom were having dose reductions with plans to discontinue sildenafil. During follow-up, 2 patients weaned off sildenafil after 29 and 314 days, and no longer required PH therapy.
Table 5.
Patients Treated with Additional PH Medications After Initiation of Sildenafil
| Patient | Medication(s) Added | Reason for Medication | Days After Sildenafil Initiation | Days of Medication Use | Remained on drug at latest follow-up |
|---|---|---|---|---|---|
| 1 | Bosentan | Sildenafil intolerance | 950 | 155 | Yes |
| 2 | CCB | Lack of echocardiogram improvement | 75 | 269 | Yes |
| 3 | Epoprostenol | Lack of echocardiogram improvement | 18 | 10 | No |
| 4 | CCB | Systemic hypertension | 66 | 66 | Yes |
| 5 | Bosentan | Lack of echocardiogram improvement | 108 | 490 | No |
| 6 | Bosentan | Lack of echocardiogram improvement | 129 | 112 | Yes |
| 7 | Epoprostenol | Lack of echocardiogram improvement | 16 | 28 | No |
| Bosentan | Lack of echocardiogram improvement | 56 | 191 | Yes |
PH = pulmonary hypertension; CCB = calcium channel blocker
Five (20%) patients died at a median age of 213 days (range: 70-440) and a median of 135 days (range: 25–241) after sildenafil initiation (Table IV). Although all of these patients were mechanically ventilated and treated with iNO at the initiation of sildenafil treatment, none died from refractory PH and right heart failure. Three of the patients died from severe refractory obstructive airways disease that progressed despite aggressive mechanical ventilation. One patient with BPD died suddenly of presumed sepsis. Another patient with CDH developed meningitis, and support was withdrawn secondary to neurological devastation. In each of these cases, serial echocardiogram assessments revealed progressive improvement in PH.
One patient discontinued sildenafil after 950 days secondary to complaints of frequent erections, and was subsequently treated with bosentan. Another patient temporarily discontinued sildenafil shortly after initiation secondary to the development of pneumatosis intestinalis. This patient safely restarted sildenafil and continued treatment for 688 days without other documented adverse events.
DISCUSSION
To determine the tolerance, safety, and potential efficacy of sildenafil in the treatment of PH in young infants with CLD, we evaluated the clinical course and outcomes of 25 patients with CLD who began treatment with sildenafil for late PH during a hospitalization prior to the age of 2 years. We found that, as part of an aggressive program to treat PH in infants with CLD, sildenafil therapy was associated with improvement in PH by echocardiogram in most (88%) patients without significant rates of adverse events. Although the time to improvement was variable, many patients were able to wean off mechanical ventilator support and other PH therapies, especially iNO, during the course of sildenafil treatment without worsening of PH. However, several (28%) were treated with additional or alternate PH medications. Five (20%) patients died during the course of sildenafil treatment, although none specifically from refractory PH. No severe adverse effects with sildenafil treatment were observed, with only one patient discontinuing sildenafil use after 950 days secondary to frequent erections.
Previous studies have shown that patients with CLD and late PH represent a very high-risk population with increased morbidities and mortality.3-5 However, the natural course of disease in these patients is poorly understood. A recent study of patients with BPD and PH reported survival rates of 64% at 6 months, 61% at one year and 52% at two years after the diagnosis of PH.5 Although it is difficult to directly compare study populations, patients with BPD in our study group had a survival rate of 83% and the entire study group experienced 80% survival during a median follow-up period of 8 months after sildenafil initiation. These results suggest that sildenafil therapy as part of an overall program to aggressively treat lung disease and PH in infants with CLD may improve outcomes.
Sildenafil has been shown to improve hemodynamics and other outcome measures in adults with PH.31 Furthermore, in a small study of older children with idiopathic PH and PH secondary to congenital heart disease, long-term sildenafil use showed improved and sustained hemodynamics and exercise tolerance.35 Current reports of sildenafil in infants have been limited to its use for the acute treatment of PPHN36 and CDH,37 acute PH treatment after cardiac surgery,38, 39 and to assist in weaning off iNO.40 This study represents the largest evaluation of prolonged sildenafil use in infants with CLD to date, and reinforces the findings of previous studies.
At the time of sildenafil initiation, most study patients underwent successful cardiac catheterization without adverse events. In addition to documentation of PH severity and reactivity to iNO, catheterization identified other previously unrecognized abnormalities that altered patient management including hemodynamically significant shunt lesions, pulmonary vein stenosis, and left ventricular diastolic dysfunction. In patients who underwent iNO reactivity testing, we found a statistically significant increase in PCWP measurements on iNO. Because iNO may increase pulmonary blood flow to the left heart, iNO treatment may unmask subtle left ventricular dysfunction that may contribute to pulmonary hypertension in these patients.41 Based on these findings, we recommend patients with CLD and PH undergo cardiac catheterization prior to the initiation of chronic PH medications for prolonged therapy. Interestingly, of the 3 infants who did not show acute pulmonary vasoreactivity to iNO, 2 demonstrated improvement in PH during long-term sildenafil therapy. Thus, the role of reactivity testing in determining PH therapy in this population remains unclear.
The pharmacokinetics and optimal dosing for sildenafil in young infants remains somewhat uncertain. Patients treated in this study were started at a dose of 1.5 mg/kg/day in 3 divided doses that was steadily increased over 1-2 weeks until the desired clinical response was achieved or to a maximum dose of 8 mg/kg/day in 4 divided doses. Avoidance of systemic hypotension was achieved with this dosing regimen. Most patients were treated with the maximum dose which was adjusted as patients gained weight during the follow-up period. Optimal criteria and timing to wean from sildenafil therapy also remain unclear. The general practice in this study was to begin weaning after at least two echocardiograms showing resolution of PH, and weaning occurred over weeks to months. Whether this strategy is too conservative and leads to unnecessarily prolonged therapy is currently unknown.
There are several potential limitations to this study. Although we and many clinicians rely on echocardiogram findings to assess hemodynamic improvement and response to therapy in this population, there are inherent limitations to this methodology because there is no data-derived definition of PH. Thus, defining the levels of pulmonary artery pressure to identify the presence and severity of PH and to guide therapy remains uncertain. However, severity of late PH in the BPD population does appear to correlate with survival.5 Because there was no control group, clinical improvement cannot be directly attributed to sildenafil therapy. Other factors, such as aggressive management of respiratory disease or time, may have affected outcomes. Due to the retrospective design of this study, all possible side-effects were not necessarily documented resulting in an underestimation of adverse events. For example, the potential adverse contribution of sildenafil treatment to retinopathy of prematurity remains a concern. However, prematurely born patients in our study were not started on sildenafil therapy until at least 40 weeks post-conceptual age, generally after patients may have developed or received therapy for retinopathy of prematurity. Furthermore, although neurological and ophthalmologic evaluations were not routinely performed in all subjects, the data available did not suggest worsening after sildenafil therapy However, any prospective study of sildenafil therapy in this population should include neurological and ophthalmologic follow-up to evaluate potential adverse outcomes.
In summary, we report the outcomes of infants with PH and CLD who were treated with long-term sildenafil therapy. We found that chronic sildenafil therapy, as part of an aggressive treatment program to treat underlying lung disease and PH, was well-tolerated, had few adverse events, and was related to progressive improvement in PH in most patients. This study provides the basis for large scale clinical trials to evaluate the efficacy and safety of long-term sildenafil use in infants with PH and CLD.
Acknowledgments
The authors would like to thank the Clinical Informatics Department at The Children’s Hospital for their assistance with the preparation of this manuscript.
This study supported by Thrasher Foundation, NIH NCCR 5 K23 RR021021 and NHLBI 1RO1 HL085703.
ABBREVIATIONS
- CLD
chronic lung disease
- PH
pulmonary hypertension
- BPD
bronchopulmonary dysplasia
- CDH
congenital diaphragmatic hernia
- PPHN
persistent pulmonary hypertension of the newborn
- iNO
inhaled nitric oxide
- NO
nitric oxide
- cGMP
cyclic guanosine monophosphate
- PDE-5
type 5 phosphodiesterase
- ASD
atrial septal defect
- PDA
patent ductus arteriosus
- TRJV
tricuspid regurgitant jet velocity
- RA
right atrial
- RV
right ventricular
- RVH
right ventricular hypertrophy
- sPAP
systolic pulmonary artery pressure
- ssBP
systemic systolic blood pressure
- mPAP
mean pulmonary artery pressure
- mBP
mean systemic blood pressure
- PVR
pulmonary vascular resistance
- SVR
systemic vascular resistances
- RAP
mean right atrial pressure
- PCWP
pulmonary capillary wedge pressure
- Qp
pulmonary blood flow
- Qs
systemic blood flow
Footnotes
No reprints will be available from the authors.
The authors deport no conflicts of Interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hislop AA, Haworth SG. Pulmonary vascular damage and the development of cor pulmonale following hyaline membrane disease. Pediatr Pulmonol. 1990;9:152–61. doi: 10.1002/ppul.1950090306. [DOI] [PubMed] [Google Scholar]
- 2.Abman S, Sondheimer H. Pulmonary circulation and cardiovascular sequelae of BPD. In: Weir EK, A S, Reeves JT, editors. Diagnosis and Treatment of Pulmonary Hypertension. New York: Futura; 1992. pp. 155–80. [Google Scholar]
- 3.Goodman G, Perkin RM, Anas NG, Sperling DR, Hicks DA, Rowen M. Pulmonary hypertension in infants with bronchopulmonary dysplasia. J Pediatr. 1988;112:67–72. doi: 10.1016/s0022-3476(88)80125-2. [DOI] [PubMed] [Google Scholar]
- 4.Dillon PW, Cilley RE, Mauger D, Zachary C, Meier A. The relationship of pulmonary artery pressure and survival in congenital diaphragmatic hernia. J Pediatr Surg. 2004;39:307–12. doi: 10.1016/j.jpedsurg.2003.11.010. discussion -12. [DOI] [PubMed] [Google Scholar]
- 5.Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics. 2007;120:1260–9. doi: 10.1542/peds.2007-0971. [DOI] [PubMed] [Google Scholar]
- 6.Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, et al. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med. 2000;342:469–74. doi: 10.1056/NEJM200002173420704. [DOI] [PubMed] [Google Scholar]
- 7.Brownlee JR, Beekman RH, Rosenthal A. Acute hemodynamic effects of nifedipine in infants with bronchopulmonary dysplasia and pulmonary hypertension. Pediatr Res. 1988;24:186–90. doi: 10.1203/00006450-198808000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Mourani PM, Ivy DD, Gao D, Abman SH. Pulmonary Vascular Effects of Inhaled Nitric Oxide and Oxygen Tension in Bronchopulmonary Dysplasia. Am J Respir Crit Care Med. 2004;170:1006–13. doi: 10.1164/rccm.200310-1483OC. [DOI] [PubMed] [Google Scholar]
- 9.Barst RJ, Maislin G, Fishman AP. Vasodilator therapy for primary pulmonary hypertension in children. Circulation. 1999;99:1197–208. doi: 10.1161/01.cir.99.9.1197. [DOI] [PubMed] [Google Scholar]
- 10.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 11.Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358:1119–23. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 12.Barst RJ, Langleben D, Frost A, Horn EM, Oudiz R, Shapiro S, et al. Sitaxsentan therapy for pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;169:441–7. doi: 10.1164/rccm.200307-957OC. [DOI] [PubMed] [Google Scholar]
- 13.Rosenzweig EB, Ivy DD, Widlitz A, Doran A, Claussen LR, Yung D, et al. Effects of long-term bosentan in children with pulmonary arterial hypertension. J Am Coll Cardiol. 2005;46:697–704. doi: 10.1016/j.jacc.2005.01.066. [DOI] [PubMed] [Google Scholar]
- 14.Ghofrani HA, Wiedemann R, Rose F, Olschewski H, Schermuly RT, Weissmann N, et al. Combination therapy with oral sildenafil and inhaled iloprost for severe pulmonary hypertension. Ann Intern Med. 2002;136:515–22. doi: 10.7326/0003-4819-136-7-200204020-00008. [DOI] [PubMed] [Google Scholar]
- 15.Olschewski H, Simonneau G, Galie N, Higenbottam T, Naeije R, Rubin LJ, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347:322–9. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- 16.Mikhail G, Gibbs J, Richardson M, Wright G, Khaghani A, Banner N, et al. An evaluation of nebulized prostacyclin in patients with primary and secondary pulmonary hypertension. Eur Heart J. 1997;18:1499–504. doi: 10.1093/oxfordjournals.eurheartj.a015478. [DOI] [PubMed] [Google Scholar]
- 17.Goldsmith DR, Wagstaff AJ. Inhaled iloprost: in primary pulmonary hypertension. Drugs. 2004;64:763–73. doi: 10.2165/00003495-200464070-00009. discussion 74-5. [DOI] [PubMed] [Google Scholar]
- 18.Ivy DD, Doran AK, Smith KJ, Mallory GB, Jr, Beghetti M, Barst RJ, et al. Short- and long-term effects of inhaled iloprost therapy in children with pulmonary arterial hypertension. J Am Coll Cardiol. 2008;51:161–9. doi: 10.1016/j.jacc.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivy DD, Parker D, Doran A, Kinsella JP, Abman SH. Acute hemodynamic effects and home therapy using a novel pulsed nasal nitric oxide delivery system in children and young adults with pulmonary hypertension. Am J Cardiol. 2003;92:886–90. doi: 10.1016/s0002-9149(03)00910-x. [DOI] [PubMed] [Google Scholar]
- 20.Channick RN, Newhart JW, Johnson FW, Williams PJ, Auger WR, Fedullo PF, et al. Pulsed delivery of inhaled nitric oxide to patients with primary pulmonary hypertension: an ambulatory delivery system and initial clinical tests. Chest. 1996;109:1545–9. doi: 10.1378/chest.109.6.1545. [DOI] [PubMed] [Google Scholar]
- 21.Abman SH. Recent advances in the pathogenesis and treatment of persistent pulmonary hypertension of the newborn. Neonatology. 2007;91:283–90. doi: 10.1159/000101343. [DOI] [PubMed] [Google Scholar]
- 22.Klinger JR. The nitric oxide/cGMP signaling pathway in pulmonary hypertension. Clin Chest Med. 2007;28:143–67. doi: 10.1016/j.ccm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Ignarro LJ, Harbison RG, Wood KS, Kadowitz PJ. Activation of purified soluble guanylate cyclase by endothelium-derived relaxing factor from intrapulmonary artery and vein: stimulation by acetylcholine, bradykinin and arachidonic acid. J Pharmacol Exp Ther. 1986;237:893–900. [PubMed] [Google Scholar]
- 24.Hanson KA, Burns F, Rybalkin SD, Miller JW, Beavo J, Clarke WR. Developmental changes in lung cGMP phosphodiesterase-5 activity, protein, and message. Am J Respir Crit Care Med. 1998;158:279–88. doi: 10.1164/ajrccm.158.1.9711042. [DOI] [PubMed] [Google Scholar]
- 25.Deruelle P, Grover TR, Storme L, Abman SH. Effects of BAY 41-2272, a soluble guanylate cyclase activator, on pulmonary vascular reactivity in the ovine fetus. Am J Physiol Lung Cell Mol Physiol. 2005;288:L727–33. doi: 10.1152/ajplung.00409.2004. [DOI] [PubMed] [Google Scholar]
- 26.Mikhail GW, Prasad SK, Li W, Rogers P, Chester AH, Bayne S, et al. Clinical and haemodynamic effects of sildenafil in pulmonary hypertension: acute and mid-term effects. Eur Heart J. 2004;25:431–6. doi: 10.1016/j.ehj.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Ghofrani HA, Schermuly RT, Rose F, Wiedemann R, Kohstall MG, Kreckel A, et al. Sildenafil for long-term treatment of nonoperable chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2003;167:1139–41. doi: 10.1164/rccm.200210-1157BC. [DOI] [PubMed] [Google Scholar]
- 28.Michelakis ED, Tymchak W, Noga M, Webster L, Wu XC, Lien D, et al. Long-term treatment with oral sildenafil is safe and improves functional capacity and hemodynamics in patients with pulmonary arterial hypertension. Circulation. 2003;108:2066–9. doi: 10.1161/01.CIR.0000099502.17776.C2. [DOI] [PubMed] [Google Scholar]
- 29.Kothari SS, Duggal B. Chronic oral sildenafil therapy in severe pulmonary artery hypertension. Indian Heart J. 2002;54:404–9. [PubMed] [Google Scholar]
- 30.Sastry BK, Narasimhan C, Reddy NK, Raju BS. Clinical efficacy of sildenafil in primary pulmonary hypertension: a randomized, placebo-controlled, double-blind, crossover study. J Am Coll Cardiol. 2004;43:1149–53. doi: 10.1016/j.jacc.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 31.Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–57. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 32.Ghofrani HA, Rose F, Schermuly RT, Olschewski H, Wiedemann R, Kreckel A, et al. Oral sildenafil as long-term adjunct therapy to inhaled iloprost in severe pulmonary arterial hypertension. J Am Coll Cardiol. 2003;42:158–64. doi: 10.1016/s0735-1097(03)00555-2. [DOI] [PubMed] [Google Scholar]
- 33.Bhatia S, Frantz RP, Severson CJ, Durst LA, McGoon MD. Immediate and long-term hemodynamic and clinical effects of sildenafil in patients with pulmonary arterial hypertension receiving vasodilator therapy. Mayo Clin Proc. 2003;78:1207–13. doi: 10.4065/78.10.1207. [DOI] [PubMed] [Google Scholar]
- 34.Mourani PM, Sontag MK, Younoszai A, Ivy DD, Abman SH. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics. 2008;121:317–25. doi: 10.1542/peds.2007-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humpl T, Reyes JT, Holtby H, Stephens D, Adatia I. Beneficial effect of oral sildenafil therapy on childhood pulmonary arterial hypertension: twelve-month clinical trial of a single-drug, open-label, pilot study. Circulation. 2005;111:3274–80. doi: 10.1161/CIRCULATIONAHA.104.473371. [DOI] [PubMed] [Google Scholar]
- 36.Shah PS, Ohlsson A. Sildenafil for pulmonary hypertension in neonates. Cochrane Database Syst Rev. 2007:CD005494. doi: 10.1002/14651858.CD005494.pub2. [DOI] [PubMed] [Google Scholar]
- 37.Noori S, Friedlich P, Wong P, Garingo A, Seri I. Cardiovascular effects of sildenafil in neonates and infants with congenital diaphragmatic hernia and pulmonary hypertension. Neonatology. 2007;91:92–100. doi: 10.1159/000097125. [DOI] [PubMed] [Google Scholar]
- 38.Stocker C, Penny DJ, Brizard CP, Cochrane AD, Soto R, Shekerdemian LS. Intravenous sildenafil and inhaled nitric oxide: a randomised trial in infants after cardiac surgery. Intensive Care Med. 2003;29:1996–2003. doi: 10.1007/s00134-003-2016-4. [DOI] [PubMed] [Google Scholar]
- 39.Schulze-Neick I, Hartenstein P, Li J, Stiller B, Nagdyman N, Hubler M, et al. Intravenous sildenafil is a potent pulmonary vasodilator in children with congenital heart disease. Circulation. 2003;108(Suppl 1):167–73. doi: 10.1161/01.cir.0000087384.76615.60. [DOI] [PubMed] [Google Scholar]
- 40.Namachivayam P, Theilen U, Butt WW, Cooper SM, Penny DJ, Shekerdemian LS. Sildenafil prevents rebound pulmonary hypertension after withdrawal of nitric oxide in children. Am J Respir Crit Care Med. 2006;174:1042–7. doi: 10.1164/rccm.200605-694OC. [DOI] [PubMed] [Google Scholar]
- 41.Mourani PM, Ivy DD, Rosenberg AA, Fagan TE, Abman SH. Left ventricular diastolic dysfunction in bronchopulmonary dysplasia. J Pediatr. 2008;152:291–3. doi: 10.1016/j.jpeds.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]