Abstract
Salt induces oxidative stress in salt-sensitive (SS) animals and man. It is unknown in SS subjects if the low-sodium Dietary Approaches to Stop Hypertension (LS-DASH) reduces oxidative stress more than DASH, which is high in anti-oxidants. To assess the effects of DASH and LS-DASH on oxidative stress, 19 volunteers were studied after 3-weeks of a standardized usual low fruits and vegetables diet (ULFV), followed by 3-weeks on DASH (both diets ∼120 mmol Na+/day), then 3-weeks on low-sodium (LS)-DASH (60 mmol Na+/d). SS was defined as systolic blood pressure ≥5 mmHg lower on LS-DASH than DASH. In SS subjects (N=9), systolic blood pressure was lower on LS-DASH (111.0±2.0 mmHg) than DASH (118.0±2.2, p<0.01) and ULFV (122.3±2.7, p=0.002). In salt-resistant (SR) volunteers (N=10), systolic blood pressure was lower on DASH (113.0±1.6) than ULFV (119.0±1.8, p<0.05) but not LS-DASH (115.7±1.8). Urine F2-isoprostanes, a marker of oxidative stress, were lower in SS subjects on LS-DASH (1.69±0.24) than ULFV (3.09±0.50, p<0.05) and marginally lower than DASH (2.46±0.44, p<0.20). F2-isoprostanes were not different among the three diets in SR volunteers (2.18±0.29, 2.06±0.29, 2.27±0.53, respectively). Aortic augmentation index, a measure of vascular stiffness, was lower in SS subjects on LS-DASH than either DASH or ULFV, and lower on DASH than ULFV in SR volunteers. In SS but not SR subjects, LS-DASH is associated with lower values for F2-isorprostane and the aortic augmentation index. The results suggest that LS-DASH decreases oxidative stress, improves vascular function and lowers blood pressure in SS but not SR volunteers.
Keywords: Blood pressure, Dietary Approaches to Stop Hypertension (DASH), salt sensitive, oxidative stress, vascular function
Introduction
Several epidemiological and clinical studies demonstrated a clear relationship between salt intake and hypertension.1 However, the blood pressure (BP) response to changes in dietary salt are heterogeneous among individuals. Several studies have classified humans as salt-sensitive or salt-resistant based on blood pressure responses to differences in sodium balance.2,3 Although the pathophysiological and genetic factors underlying salt sensitivity are increasingly well understood, the mechanisms are not yet fully elucidated
The literature suggests that oxidative stress participates in the pathogenesis of hypertension. Studies in hypertensive patients have identified evidence for low antioxidant capacity4 and increased oxidative stress.5 In experimental models of salt-sensitive hypertension, high salt intake increases markers of vascular and systemic oxidative stress.2 Furthermore, high salt diets impair vascular dilatation by decreasing nitric oxide in both salt sensitive animals and humans.6,7 While several studies have linked increased oxidative stress to high salt intake in animals,8-11 the human data are more limited. In a study by Laffler and colleagues,12 acute (one day) sodium loading and depletion using an established protocol produced corresponding increases and decreases, respectively, in plasma F2-isoprostanes in salt sensitive hypertensives. In contrast, salt-resistant hypertensives manifested an increase in plasma F2-ispoprostanes with salt depletion.
In a study of hypertensive patients by Sanchez13 and coworkers, non-modulators (salt-sensitive) showed higher 24-hour urine isoprostane values than modulators (salt-resistant) on both high and low sodium diets for 10 days each. Unlike modulators, the non-modulators also failed to increase urinary nitrate/nitrate and cyclic GMP on the high as compared to low salt-diet. Thus, both the acute and intermediate term studies of sodium loading and depletion suggest that markers of oxidative stress differ in salt-sensitive and resistant subjects.
The DASH Eating Plan lowers blood pressure, especially when combined with low-salt intake.14 Furthermore, we reported that DASH improves anti-oxidant capacity and reduces oxidative stress in metabolic syndrome patients with elevated BP.15 It is not known whether low-sodium (LS)-DASH, differentially affects markers of oxidative stress and vascular function in salt-sensitive and salt-resistant subjects compared to the original DASH Eating Plan, which is already high in anti-oxidants and flavonoids. In our study, therefore, we investigated the effect of DASH and LS-DASH on markers of oxidative stress and vascular function in SS and SR volunteers.
Methods
The study protocol was approved by the Office of Research Protection and Integrity at Medical University of South Carolina (MUSC). Volunteers were recruited from the staff and clinics at MUSC and paid. Written informed consent was obtained from all volunteers prior to the initial screening examination.
Subjects in this report were part of a previous DASH study.16 The original study was designed to better delineate mechanisms by which DASH lowers blood pressure. Two groups of subjects were recruited and included lean normotensives without and obese hypertensives with the metabolic syndrome.17
Lean normotensive volunteers were 21–49 years of age and had body mass index (BMI) <25 kg/m2, waist circumference <102 cm for men and <88 cm for women, blood pressure consistently <130/85 mmHg on all three visits prior to the first study, fasting glucose <100 mg/dl, fasting triglycerides <125 mg/dl, HDL-cholesterol >40 mg/dl for men and >50mg/dl for women and/or total cholesterol / HDL <3.5. Obese hypertensives were also 21 – 49 years old, had blood pressures in the 130–159/85–99 mmHg range on the three screening visits, BMI >27 kg/m2 and waist circumference ≥40 inches for men and ≥35 inches for women. They also had at least one other risk factor including impaired fasting glucose [100–125 mg/dL], fasting triglycerides >150 mg/dl or HDL-cholesterol <50 for women and <40 for men.17
Participants were excluded for diabetes mellitus (fasting glucose ≥126 mg/dl or history of or treatment for diabetes), clinically evident target organ damage (>grade 2 KW change, left ventricular hypertrophy by electrocardiography, serum creatinine >1.5 mg/dL), or history of stroke, transient ischemic attack, myocardial infarction, angina pectoris, or chronic heart failure.
Volunteers discontinued all non-essential prescription and non-prescription medications and supplements at least 3 weeks before the baseline dietary intervention. Volunteers who required medications to maintain a blood pressure <160/<100 mmHg were ineligible for the study. Medications that were allowed included antihistamines for allergies, H2-receptor blockers and proton pump inhibitors for gastroesophageal reflux, and selective serotonin reuptake inhibitors for depression. Acetaminophen was allowed, whereas non-steroidal anti-inflammatory medications were not.
Study diets
Subjects followed four different diets for three weeks each. The first phase was a standardized usual low fruit and vegetable diet (ULFV) averaging one fruit and one vegetable (ULFV), ∼1700 mg potassium, 250 mg magnesium and 11 grams of fiber daily. Subjects were randomized to the usual diet supplemented with potassium, magnesium and fiber (ULFV-S) to match DASH, or DASH itself. Subjects then completed the complementary phase of the randomized diet for three weeks. Salt intake across the first three diets was ∼3000 mg/day. After finishing the first three diets, subjects consumed a low sodium (LS) DASH diet with target salt intake of 1500 mg/day. Nineteen of 30 volunteers completing the first three dietary periods also finished LS-DASH and are included in this report. All dietary phases were designed to maintain consistent intake of calcium, caffeine, and alcohol. DASH without additional low-fat dairy was selected, since it was difficult to increase consumption of these items in our earlier study of free-living volunteers.
To encourage dietary compliance, subjects met weekly with GCRC dietician. Sample menus of the standardized usual, DASH and LS-DASH diets were provided to each volunteer. Subjects were given a small digital camera (PenCam Vr, Aiptek, Irvine, CA) to photograph all food intake for 3 days prior to each weekly visit. Subjects were instructed to keep a food dairy for the same 3 days as well. A plastic ruler was given to the subjects to be placed in the picture field to facilitate more precise estimates of intake. To verify adherence with the study diets, subjects collected weekly 24-hour urines for measurement of sodium and potassium. Nutrient consumption and dietary compliance were monitored closely by the GCRC dietician from 3-day food records and photographs as well as the urine data. Each subject's isocaloric energy intake was estimated using the Harris Benedict equation,18 and calories were adjusted each week according to clinic weights to minimize weight changes.
The macronutrient targets for the three diets were ∼50% carbohydrate, 35% fat, and 15% protein. Target Na+ intake was 3000 mg daily for the usual low fruits and vegetable (ULFV) and DASH diets and 1500 mg for low sodium (LS)-DASH. Calcium intake was ∼700 mg daily for all diets. The Minnesota Nutrition Data Systems19 (Nutrition Coordinating Center, Minneapolis, MN) was used to analyze food records and pictures and estimate intake of multiple nutrients for each volunteer.
Hemodynamic measurements
BP and heart rate were measured in triplicate with subjects seated quietly for the 3 qualifying visits and weekly thereafter for the remainder of the study. BP was measured by a trained observer using a mercury sphygmomanometer and appropriately sized cuff. Systolic BP was defined by the first Korotkoff sound, diastolic BP by the disappearance of the last (fifth) Korotkoff sound. Heart rate was measured by palpating the radial pulse for 60 seconds between the second and third BP measurement.
H.D.I./Pulse Wave CR-2000 (Eagan, MN)20 was used to estimate small and larger artery elasticity.21 Central (aortic) blood pressures were estimated using the Sphygmacor device (SCOR–MX, West Ryde, NSW Australia).22 Aortic augmentation index, a measure of vascular stiffness, was calculated.22 Cardiac output, thoracic fluid content, and heart rate were measured by BioZ impedance cardiography (CardioDynamics, San Diego, CA). 23
Metabolic assay methods
Plasma insulin was measured by radioimmunoassay.24 The homeostatic model assessment of insulin resistance (HOMAir) was calculated.25 Triglycerides, total and HDL-cholesterol were measured.26 LDL-cholesterol was calculated.27
F2-isoprostanes
Urine F2-isoprostanes were measured using gas chromotography/negative ion chemical ionization (GC/NICI) mass spectrometry by Dr. Morrow as described in a previous collaboration.21
Study design and protocol
After following the ULFV diet for three weeks, subjects were randomized to either DASH or ULFV supplemented with potassium, magnesium and fiber to match DASH for three weeks then three weeks on the alternative diet. After finishing the first three diets, subjects followed LS-DASH as the fourth three week dietary period.
Subjects were admitted to the GCRC at 8:00 AM after three weeks on each diet and following an overnight fast. Intravenous access was obtained, and baseline hemodynamic data were recorded every 10 minutes for one hour. Blood was drawn for the assays described.
Statistical analysis
Sample size estimates
Changes in isoprostanes represent a secondary study outcome. A sample size of nine per group affords 90% power to detect a within group change of 20% ± 15% at p≤0.05 and 75% power to detect a within group change of 20% ± 20% using a two-sided, one-sample t-test. With two groups of nine each, the study had 70% power to detect differences of 40% between groups at p<0.05 using a two-sided, two-sample t-test.
Since the supplemented diet (ULFV-S) did not affect blood pressure in the previous report, that phase was excluded from the present analysis.16 Group comparisons for descriptive categorical variables, e.g., gender, were made using Chi-square tests. For descriptive continuous variables such as age, nutrient intake, and BP, two sample t-tests were used for between-group comparisons. Changes in BP, other hemodynamic variables, and selected biochemical measurements across the three dietary phases within and between groups were made using general linear models for repeated measures. All statistical analyses were performed with SAS Version 9.1. All statistical tests were two-sided, and p-values <0.05 were accepted as significant.
Results
Baseline characteristics
Nineteen volunteers completed the study. Salt sensitivity was defined by systolic blood pressure on LS-DASH ≥5 mmHg below values on DASH. By this definition, nine subjects were salt sensitive (SS) and 10 salt resistant (SR). Baseline characteristics of the two groups are provided in Table 1 including estimated glomerular filtration rates.28 SS subjects were older than SR volunteers, and their body mass indices and estimated glomerular filtration rates lower, although the latter two were not statistically significantly. The two groups were otherwise similar including baseline blood pressures.
Table 1.
Selected baseline descriptive characteristics of salt sensitive and salt resistant volunteers Values provided represent mean ± one standard error.
| Variables | Salt resistant | Salt Sensitive | P-value |
|---|---|---|---|
| Number | 10 | 9 | 1.00 |
| Age, years | 34.3 ± 2.5 | 44.1 ± 1.4 | 0.004 |
| Gender, F/M | 7/3 | 7/2 | 1.00 |
| Race, black/white | 5/5 | 2/7 | 0.35 |
| BMI, kg/m2 | 30.1 ± 2.7 | 26.5 ± 1.6 | 0.28 |
| Height, meters | 1.67 ± 0.03 | 1.67 ± 0.01 | 0.97 |
| Hip circumference, in | 42.5 ± 2 | 40.7 ± 1.1 | 0.24 |
| Abd circumference, in | 37.1 ± 2.4 | 35.9 ± 1.5 | 0.29 |
| Systolic BP, mm Hg | 120.9 ± 4.1 | 121.1 ± 4.4 | 0.97 |
| Diastolic BP, mm Hg | 76.1 ± 3.9 | 78.8 ± 4.0 | 0.64 |
| HR, beats/minute | 69.8 ± 2.8 | 70.2 ± 2.6 | 0.92 |
| Sodium, mmol/L | 138.2 ± 0.5 | 139.7 ± 0.7 | 0.12 |
| Chloride, mg/dL | 103.2 ± 0.7 | 104.0 ± 0.8 | 0.45 |
| Potassium, mmol/L | 4.2 ± 0.1 | 4.5 ± 0.2 | 0.17 |
| Calcium, mg/dL | 9.4 ± 0.1 | 9.3 ± 0.1 | 0.60 |
| Phosphorus, mg/dL | 3.6 ± 0.2 | 3.2 ± 0.2 | 0.14 |
| Glucose, mg/dL | 91.3 ± 3.7 | 88.6 ± 5.2 | 0.67 |
| eGFR ml/min/1.7 m2 | 104.7 ± 7.6 | 93.1 ± 8.3 | 0.32 |
| Total Chol, mg/dL | 187.5 ± 12.9 | 179.4 ± 8.1 | 0.61 |
| Triglycerides, mg/dL | 79.1 ± 14.4 | 71.4 ± 13.8 | 0.71 |
| Total HDL-C, mg/dL | 49.3 ± 3.1 | 50.9 ± 5.3 | 0.80 |
| LDL-C, mg/dL | 122.3 ± 8.6 | 114.3 ± 8.6 | 0.52 |
HR = heart rate; eGFR = estimated GFR.
Nutritional Variables
Intake of fruits and vegetables on ULFV averaged ∼2/day in contrast to ∼8/day on DASH and LS-DASH (Table 2). Sodium intake on LS-DASH was less than DASH and ULFV. Potassium, fiber and magnesium intakes were higher on DASH and LS-DASH than ULFV. Calcium and alcohol intake were comparable across the three diets.
Table 2.
The nutrient goal and average intake in all subjects combined for ULFV, DASH, and LS-DASH. Values are expressed as mean ± SEM.
| Nutrients (units) | All Subjects | |||||
|---|---|---|---|---|---|---|
| Goal ULFV | Actual ULFV | Goal LS-DASH | Actual LS-DASH | Goal DASH | Actual DASH | |
| Fruit, N/day | 1-2 | 0.9 ± 0.8 | 1-2 | 5.2 ± 1.4 | 5-6 | 4.5 ± 1.5 |
| Veg, N/day | 1-2 | 1.1 ± 0.6 | 1-2 | 3 ± 1 | 3-4 | 3.3 ± 1.5 |
| Kcals/day | 2000 | 1998±63** | 2000 | 1983±65 | 2000 | 2234±60xx |
| Protein, % kcal | 15 | 16.1±0.4* | 15 | 15.6±0.5 | 15 | 14.8 ± 0.4 |
| Carb, % kcals | 50 | 46.3±1.0*** | 50 | 52.2±1.1+++ | 50 | 52.3± 1.1 |
| Fat, % kcals | 35 | 36.9±0.8*** | 35 | 31.4±0.9+++ | 35 | 32.2±0.9 |
| Alcohol, % kcals | 16 | 0.77±0.24 | 16 | 0.84±0.30 | 16 | 0.8 ± 0.27 |
| Chol, mg/d | 300 | 313±20** | 300 | 241±19++ | 300 | 240 ± 15 |
| Fiber, mg/d | 9 | 12.4±0.5*** | 31.0 | 26.5±1.1+++ | 31.0 | 29.4±1.1x |
| Na+, mg/d | 3000 | 3758±144 | 1500 | 1221±56+++ | 3000 | 3729±137xxx |
| K+, mg/d | 1700 | 1995±70*** | >4100 | 3999±154+++ | >4100 | 3916±109 |
| Ca++, mg/d | 700 | 747±43 | 700 | 682±40 | 700 | 794±33x |
| Mg++, mg/d | 175 | 212±7*** | 500 | 407±15+++ | 500 | 416±14 |
Veg, = vegetables; N/day = servings per day; Kcal = 1000 calories or 1 Calorie, % kcal = % of total daily calories; Carb = carbohydrate; Sat = saturated fat; MUFA = monounsaturated fat; PUFA = polyunsaturated fat; Chol = cholesterol.
p<0.05, for within group differences between ULFV and DASH.
p<0.01, for within group differences between ULFV and DASH.
p<0.001 for within group differences between ULFV and DASH.
p<0.05, for within group differences between ULFV and LS-DASH.
p<0.01, for within group differences between ULFV and LS-DASH.
p<0.001 for within group differences between ULFV and LS-DASH.
p<0.05, for within group differences between DASH and LS-DASH.
p<0.01, for within group differences between DASH and LS-DASH.
p<0.001 for within group differences between DASH and LS-DASH.
Blood pressure
In SS subjects, systolic blood pressures were lower on LS-DASH than on DASH and ULFV (Figure 1). Systolic blood pressure was lower on DASH than ULFV in SR subjects. SS subjects tended to have higher BMI at baseline and to lose more weight than SR individuals on LS-DASH compared to DASH. Baseline BMI was not associated with the change in systolic blood pressure between either DASH and LS-DASH (r=-0.27, p=0.26) or ULVF and LS-DASH (r=-0.13, p=0.59). The differences in BMI were marginally related to change in systolic blood pressure (r=0.39, p=0.10) between DASH and LS-DASH. Diastolic blood pressure was not different in SS or SR subjects across diets. Estimated central systolic (p=0.06) and diastolic blood pressures (p=0.06) were marginally lower on LS-DASH than ULFV in SS subjects.
Figure 1.
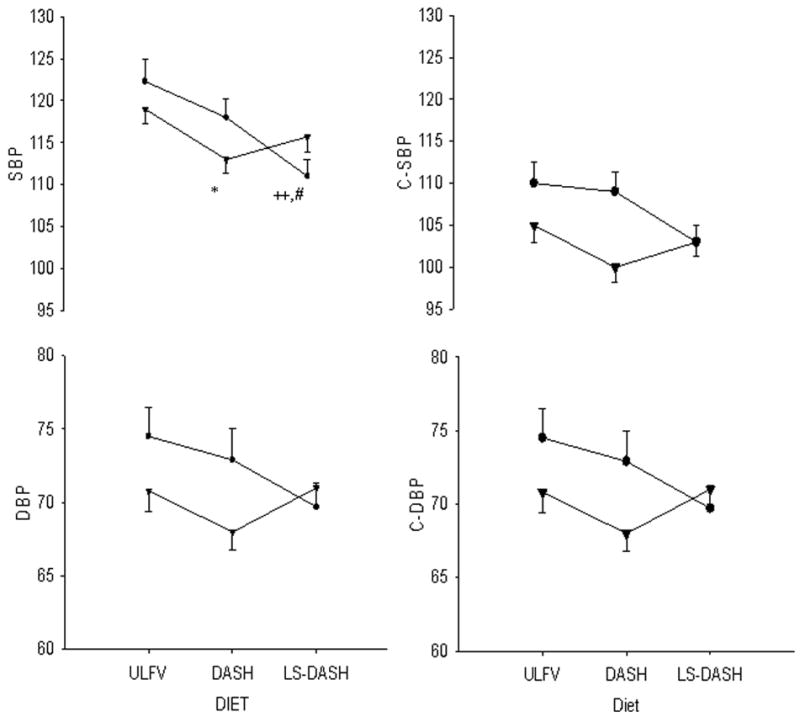
Peripheral (brachial, cuff [left side]) and estimated central (right side) systolic (SBP [top]) and diastolic blood pressures (DBP [bottom]) are provided for salt sensitive [●SS] and salt resistant [▲SR] subjects on the three dietary periods. Values reflect the means and standard errors of multiple baseline, supine readings after three weeks on each diet. ULFV = usual diet low in fruits and vegetables; DASH = high fruits and vegetables Dietary Approaches to Stop Hypertension. LS-DASH = low-sodium, high fruits and vegetables DASH. * p<0.05 DASH vs ULFV in SR; ++ p<0.01 LS-DASH vs ULFV in SS; # p<0.05 LS-DASH vs DASH in SS.
Nutrient differences between DASH and LS-DASH
Within group differences between LS-DASH and DASH were generally minimal except for sodium intake (Table 3). Between group differences were also generally minimal as shown.
Table 3.
Selected variables (mean ± SE) in salt-sensitive and salt-resistant subjects and DASH and low-salt DASH (DASH-Na+) that have linked to blood pressure.
| Nutrient intake | Salt Sensitive DASH | Salt Resistant DASH | Salt Sensitive DASH-Na+ | Salt Resistant DASH-Na+ |
|---|---|---|---|---|
| Na+, mg/d | 3688 ± 157+++ | 3706 ± 151 | 1268 ± 64 | 1195 ± 63+++ |
| K+, mg/d | 3720 ± 119 | 4143 ± 113* | 3532 ± 152** | 4471 ± 178 |
| Ca++, mg/d | 751 ± 38 | 836 ± 33 | 631 ± 40 | 738 ± 46 |
| Mg++, mg/d | 377 ± 15 | 442 ± 14** | 387 ± 17 | 419 ± 17 |
| Lycopene, mcg/d | 11788 ±2271+++ | 9142 ±1500 | 3976 ± 624 | 4654 ± 808++ |
| Selenium, mg/d | 136 ± 12+ | 121 ± 8 | 109 ± 6 | 110 ± 6 |
| Vitamin C, mg/d | 185 ± 9 | 221 ± 11* | 200 ± 16 | 233 ± 13 |
| Vitamin E, mg/d | 12.9 ± 1.0 | 15.6 ± 0.9 | 14.1 ± 1.4 | 11.9 ± 0.7++ |
| Alpha Vit E, mg/d | 10.2 ± 0.7 | 13.2 ± 0.8* | 11.5 ± 0.9 | 10.5 ± 0.7++ |
| Beta Vit E, mg/d | 0.39 ± 0.03 | 0.5 ±0.03* | 0.39 ± 0.02 | 0.42 ±0.03 |
| Folate, μg/d | 479 ± 22+ | 535 ± 19 | 406 ± 19** | 509 ± 28 |
| Arginine, g/d | 4.7 ± 0.2 | 5.2 ± 0.2 | 4.9 ± 0.2 | 4.9 ± 0.2 |
| Arachidonic, g/d | 0.12 ± 0.01 | 0.15 ± 0.02 | 0.15 ± 0.01 | 0.16 ± 0.02 |
| Linoleic Acid, g/d | 14.8 ± 0.9 | 15.8 ± 0.9 | 14.1 ± 0.9 | 14.0 ± 0.9 |
| Linolenic, g/d | 1.5 ± 0.1+ | 1.7 ± 0.1 | 1.2 ± 0.1* | 1.6 ± 0.1 |
| EPA, g/d | 0.045± 0.02 | 0.07 ± 0.02 | 0.10 ± 0.02 | 0.10 ± 0.03 |
| DHA, g/d | 0.11 ± 0.04+ | 0.20 ± 0.07 | 0.29 ± 0.07 | 0.24 ± 0.08 |
| Total trans fat, g/d | 4.4 ± 0.4++ | 3.8 ± 0.3 | 2.6 ± 0.2 | 2.7 ± 0.3+ |
| Insoluble Fiber, g/d | 17.1 ± 0.8 | 22.4 ± 0.7*** | 16.8 ± 0.9* | 20.1 ± 0.9+ |
| Soluble Fiber, g/d | 7.9 ± 0.4 | 9.8 ± 0.4** | 7.0 ± 0.4* | 8.7 ± 0.4+ |
p<0.05, for between group differences on comparable diets.
p<0.01, for between group differences on comparable diets.
p<0.001 for between group differences on comparable diets.
p<0.05, for within group differences between DASH and DASH-Na+.
p<0.01, for within group differences between DASH and DASH-Na+.
p<0.001 for within group differences between DASH and DASH-Na+.
Urine electrolytes
Urine Na+ was less on LS-DASH than DASH and ULFV in both groups. Urine potassium was higher on LS-DASH and DASH than the ULFV but not different between DASH and LS-DASH in both groups (Table 4). Urine magnesium was higher on DASH than ULFV in all volunteers combined but not different from LS-DASH. Urine magnesium was not different on LS-DASH than ULFV in SS and SR groups separately.
Table 4.
Selected hemodynamic and metabolic data in salt-sensitive and salt-resistant volunteers on the three dietary periods.
| SS ULFV | SS DASH | SS LS-DASH | SR ULFV | SR DASH | SR LS-DASH | |
|---|---|---|---|---|---|---|
| Weight, kg | 74.3 ± 5.3 | 74.7 ± 5.4 | 73.4 ± 5.4 | 83.7 ± 7.3 | 83.6 ± 7.0 | 83.2 ± 6.9 |
| BMI, kg/m2 | 26.5 ± 1.5 | 26.6 ± 1.5 | 26.2 ± 1.5 | 30.0 ± 2.6 | 30.0 ± 2.5 | 29.9 ± 2.5 |
| Cardiac output | 5.2 ± 0.1 | 4.8 ± 0.2 | 4.5 ± 0.2++ | 4.9 ± 0.2 | 4.9 ± 0.2 | 4.7 ± 0.2 |
| TSR, units | 1280 ± 39 | 1437 ± 80 | 1415 ± 72 | 1445 ± 80 | 1400 ± 102 | 1541 ± 112 |
| TFC, 1000/Zo | 35.3 ± 0.5 | 31.8 ± 0.7*** | 30.6 ± 0.3+++ | 35.7 ± 1.1 | 33.6 ± 1.5 | 32.9 ± 1.1 |
| HR, beats/min | 62.0 ± 1.0 | 62.1 ± 0.9 | 56.7 ± 1.0++, xx | 68.2 ± 2.9 | 63.4 ± 1.6 | 63.8 ± 1.9 |
| SAEI, ml/mmHg × 102 | 5.8 ± 0.4 | 7.0 ± 0.5 | 8.0 ± 0.5++ | 7.5 ± 0.4 | 9.0 ± 0.6 | 8.1 ± 0.6 |
| LAEI, ml/mmHg × 102 | 14.6 ± 0.4 | 14.8 ± 0.5 | 15.0 ± 0.4 | 15.4 ± 0.7 | 16.6 ± 0.7 | 15.0 ± 0.6 |
| Insulin, μU/mL | 7.6 ± 1.3 | 8.5 ± 2.0 | 7.9 ± 1.6 | 9.2 ± 1.6 | 9.2 ± 1.8 | 9.1 ± 2.2 |
| Glucose, mg/dL | 81 ± 3 | 83 ± 2 | 86 ± 2 | 86 ± 2 | 85 ± 3 | 85 ± 3 |
| HOMAir | 1.6 ± 0.3 | 1.8 ± 0.4 | 1.7 ± 0.4 | 2.0 ± 0.4 | 1.9 ± 0.4 | 1.9 ± 0.5 |
| Chol, mg/dL | 155 ± 9 | 160 ± 9 | 152 ± 6 | 179 ± 11 | 173 ± 10 | 161 ± 9 |
| TG, mg/dL | 63 ± 15 | 67 ± 15 | 73 ± 21 | 83 ± 17 | 69 ± 13 | 62 ± 14 |
| LDL, mg/dL | 101 ± 8 | 102 ± 9 | 95 ± 5 | 118 ± 7 | 115 ± 7 | 105 ± 6 |
| HDL, mg/dL | 42 ± 3 | 44 ± 4 | 42 ± 4 | 45 ± 3 | 44 ± 3 | 44 ± 3 |
| VLDL, mg/dL | 12 ± 3 | 13 ± 3 | 15 ± 4 | 17 ± 3 | 14 ± 3 | 12 ± 3 |
| uNa+, mmol/d | 93 ± 6 | 97 ± 10 | 34 ± 2***,XXX | 120 ± 11 | 104 ± 9 | 51 ± 6***,XXX |
| uK+, mmol/d | 28 ± 1 | 42 ± 4 | 40 ± 3 | 41 ± 4 | 53 ± 6 | 47 ± 6 |
| PRA, ngAI/mL/hr | 0.57 ± 0.14 | 0.75 ± 0.2 | 1.29 ± 0.36 | 0.57 ± 0.15 | 0.83 ± 0.22 | 1.35 ± 0.22+ |
| Plasma aldosterone | 36.8 ± 10.3 | 62.0 ± 18.0 | 79.3 ± 12.5+ | 23.0 ± 4.6 | 30.2 ± 6.5 | 50.8 ± 7.1++, x |
TSR =total systemic resistance, TVC = thoracic fluid content; PRA = plasma renin activity;
p<0.05, for within group differences between ULFV and DASH.
p<0.01, for within group differences between ULFV and DASH.
p<0.001 for within group differences between ULFV and DASH.
p<0.05, for within group differences between ULFV and LS-DASH.
p<0.01, for within group differences between ULFV and LS-DASH.
p<0.001 for within group differences between ULFV and LS-DASH.
p<0.05, for within group differences between DASH and LS-DASH.
p<0.01, for within group differences between DASH and LS-DASH.
p<0.001 for within group differences between DASH and LS-DASH.
Vascular Function
The aortic augmentation index (AIx) was lower in SS subjects on LS-DASH than DASH and ULFV (Figure 2). AIx was higher in SS than SR subjects on ULFV and DASH but not LS-DASH. AIx was lower on DASH than ULFV in SR subjects. Although estimated GFR tended to be lower in SS than SR subjects (Table 2), AIx was not related to estimated GFR on any of the three study diets or to differences in AIx between the dietary periods (all r-values <0.36, p-values >0.1).
Figure 2.
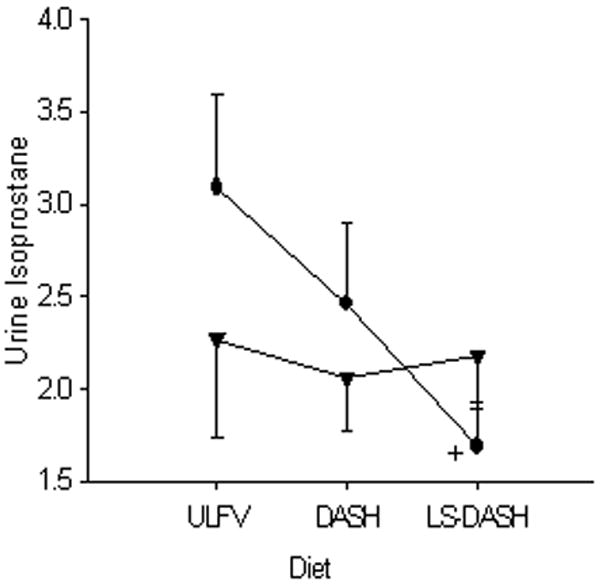
Urine F2-isoprostane values are shown for the salt sensitive [●SS] and salt resistant [▲SR] subjects on the three dietary periods. Urine isoprostanes were lower on LS-DASH than ULFV in SS subjects. + p<0.05 LS-DASH vs ULFV in SS.
In SS subjects, small artery elasticity index was higher on LS-DASH than ULFV but not different from DASH. Large artery elasticity index generally followed the same pattern, but differences were not significant. Total systemic resistance (TSR) was marginally lower in SS subjects on LS-DASH than ULFV (P=0.06), but not than DASH. No differences in small or large artery elasticity or TSR were observed in SR subjects among the study diets.
Cardiac output, thoracic fluid content and heart rate response to dietary intervention
Thoracic fluid content and cardiac output were lower in SS subjects on LS-DASH than ULFV but were not different from DASH (Table 4). Heart rate in SS subjects was lower on LS-DASH than DASH or ULFV. These values were not different among study diets in SR subjects.
Urine F2-isoprostanes
In SS subjects, urine F2-isoprostanes were lower on LS-DASH than ULFV but were not significantly lower than DASH (Figure 3). Urine isoprostanes were not different in SR subjects among the three diets. Estimated glomerular filtration rate was marginally related to the change in urine F2-isoprostanes between ULFV–DASH (r=0.44, p=0.06).
Figure 3.
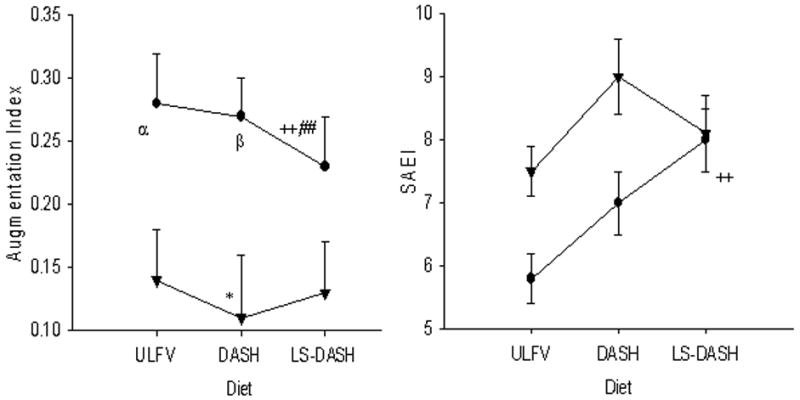
The aortic (central) augmentation index (AIx [upper panel]) was lower on LS-DASH than ULFV and DASH in SS subjects [●SS]. Among SR subjects [▲SR], AIx was lower on DASH than ULFV. Small artery elasticity (lower panel) was higher on LS-DASH than ULFV in SS subjects.
* p<0.05 DASH vs ULFV in SR; ++ p<0.01 LS DASH vs ULFV, ##, p<0.01 LS-DASH vs DASH; α p<0.05 SS vs SR on ULFV; β p<0.05 SS vs SR on DASH.
Plasma renin activity (PRA) and plasma aldosterone concentration
PRA was higher in SS volunteers on LS-DASH than ULFV (Table 4). Plasma aldosterone was higher on LS-DASH than ULFV in both groups and higher on LS-DASH than DASH in SR but not SS volunteers.
Changes in insulin, HOMA index and Lipid profile
Fasting insulin, HOMA, and lipids were not different among the study diets in either SS or SR volunteers (Table 4).
The study topic and key findings are summarized in Table 5.
Table 5. Summary.
| What is known about topic: |
| • An increase of oxidative stress with salt loading and/or a decrease with salt depletion is implicated in the salt-sensitive blood pressure responses of animals and man (5–12) |
| What this study adds: |
| • Compared to a usual diet, low sodium DASH reduced urinary F2-isoprotanes, a marker of oxidative stress, and improved vascular distensiblity in salt-sensitive but not in salt resistant human subjects |
Discussion
In this study, salt-sensitivity, defined by a reduction in systolic blood pressure of 5 mmHg or more on low sodium-DASH as compared to DASH was observed in nine of 19 subjects. Among the salt-sensitive subjects only, urine F2-isoprostanes, a marker of oxidative stress, declined during LS-DASH. Furthermore, the reduction of oxidative stress in salt-sensitive subjects was associated with improvement in two indices of vascular function, namely, small artery elasticity increased and the aortic augmentation index (AIx) decreased.
The mechanisms underlying these novel observations in salt-sensitive humans are unknown, but previous studies provide clues. Reactive oxygen species contribute to impaired endothelial function in salt sensitive rats 12,29,30 and decreased endothelium-dependent dilation in normotensive rats on high salt diets.31 One suggested mechanism is a decrease in nitric oxide synthase activity.32,33 Another possibility is that high salt diets increase superoxide production which interacts with nitric oxide to form peroxynitrite, thereby, reducing available nitric oxide.34-36 In fact, salt loading in animal models of salt-sensitive hypertension raises blood pressure and decreases nitric oxide by increasing oxidative stress.33 Decreased bioavailability of nitric oxide is implicated in the pathogenesis of human hypertension.37,38 In humans with salt-sensitive hypertension, salt loading was associated with decreased plasma and urinary levels of nitric oxide metabolites.8,39
The AIx appears to be a more sensitive marker of changes in arterial stiffness than pulse wave velocity among individuals less than 50 years,40 which would include our volunteers. AIx correlates with both Framingham coronary heart disease risk41 and with endothelial function.40 In this study, salt sensitive subjects had values for AIx which were double those in salt-resistant volunteers, a difference that is probably not explained by age alone.40 The improvement of arterial stiffness in salt sensitive subjects on LS-DASH was associated with reduction of blood pressure and oxidative stress, which raises the possibility that salt sensitivity, oxidative stress and arterial stiffness are inter-related. The low sodium-DASH also improved small artery elasticity, another marker of endothelial function,42,43 which likely contributed to the decline in AIx. In other words, rapid reflection of pressure waves from small arteries contribute to the second component of pulse-pressure, which is captured in AIx.22,44 Moreover, small artery elasticity is reduced in otherwise healthy individuals at risk for atherosclerosis.45,46
Heart rate declined significantly in salt-sensitive subjects on low sodium-DASH, and, to a lesser degree in salt-resistant volunteers. This observation may reflect greater sympathetic activity on ULFV and DASH than LS-DASH. Previous studies also suggest greater sympathetic activity on higher than lower sodium intake in salt-sensitive subjects.47-49 Furthermore, salt-sensitive hypertension is associated with alterations in autonomic cardiovascular control.50,51 In fact, impaired sympathetic inhibition has been described during high sodium intake in salt-sensitive hypertensive humans,2 and a defect in reflex cardiovascular control was proposed as a potential mechanism.48
Thoracic fluid content decreased on low-sodium DASH in our salt sensitive subjects. The literature raises two possible explanations. First, as noted, evidence suggests that sympathetic activity declines in salt-sensitive subjects with sodium restriction. An increase in sympathetic activity is associated with redistribution of blood volume toward the cardiopulmonary space,52 and reduced sympathetic activity could reverse this phenomenon. Secondly, the decline in oxidative stress may have reduced body fluid volume including thoracic fluid content.53-55 Oxidative stress enhances activity of both the Na/K+/2Cl− co-transporter and the luminal Na+/H+ exchanger.56-58 Moreover, the decreased availability of nitric oxide in salt-sensitive subjects on high salt diets, could raise the renal threshold for pressure natriuresis and secondarily increase fluid retention.58-60 The decrease in oxidative stress among salt-sensitive subjects on LS-DASH may have reversed these phenomena.
The limitations of this study include a comparatively small sample size with adequate power only to detect comparatively large changes. While the definition of salt sensitivity has been used in other studies, it is, nonetheless arbitrary. The dietary periods were not randomized, and there were no washout periods between the various dietary phases. Yet, all subjects followed the same dietary sequence. When these subjects were divided based on differences in systolic blood pressure between DASH and LS-DASH, differences in urine F2-isoprostanes and vascular function were seen. And, the findings are consistent with previous published studies in humans and animals.
In summary, LS-DASH, compared to a standardized usual diet, reduced oxidative stress and improved vascular function in salt-sensitive but not salt-resistant volunteers in this study. The data further suggest that LS-DASH reduced sympathetic activity and facilitated sodium-volume excretion in this group. Thus, LS-DASH emerges as a useful intervention for not only lowering blood pressure but also reducing oxidative stress and improving vascular-endothelial function in salt-sensitive subjects. While DASH is also beneficial in salt-resistant subjects, additional benefits of low sodium DASH were not observed within the limited time period encompassed by this study.
Acknowledgments
The authors thank Kelley Martin, RD, MS, General Clinical Research Nutritionist, for her extraordinary efforts in assisting volunteers to comply with the study diets. We also thank the entire General Clinical Research Center staff for their dedication to the integrity of the research protocol as well as Kim Edwards for administrative support.
This research was supported by grants from the National Institutes of Health HL58794, HL04290 [BME]; GM15431, DK48831 [JDM]; MD00267 from the National Center for Minority Health and Disparities, and the General Clinical Research Center (RR-01070) from the Division of Research Resources.
Contributor Information
Yaser Al-Solaiman, Departments of Medicine and Pharmacology, Medical University of South Carolina, Charleston, SC.
Ammar Jesri, Departments of Medicine and Pharmacology, Medical University of South Carolina, Charleston, SC.
Yumin Zhao, Departments of Medicine and Pharmacology, Medical University of South Carolina, Charleston, SC.
Jason D. Morrow, Departments of Medicine and Pharmacology, Medical University of South Carolina, Charleston, SC Departments of Medicine and Pharmacology, Vanderbilt University, Nashville, TN.
Brent M. Egan, Departments of Medicine and Pharmacology, Medical University of South Carolina, Charleston, SC
References
- 1.Muntzel M, Drüeke T. A comprehensive review of the salt and blood pressure relationship. Am J Hypertens. 1992;5:1S–42S. doi: 10.1093/ajh/5.4s.1s. [DOI] [PubMed] [Google Scholar]
- 2.Campese VM. Salt sensitivity in hypertension: renal and cardiovascular implications. Hypertension. 1994;23:531–550. doi: 10.1161/01.hyp.23.4.531. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–490. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 4.Russo C, Olivieri O, Girelli D, Faccini G, Zenari ML, Lombardi S, Corrocher R. Anti-oxidant status and lipid peroxidation in patients with essential hypertension. J Hypertens. 1998;16:1267–1271. doi: 10.1097/00004872-199816090-00007. [DOI] [PubMed] [Google Scholar]
- 5.Lacy F, O'Connor DT, Schmid-Schonbein GW. Plasma hydrogen peroxide production in hypertensives and normotensive subjects at genetic risk of hypertension. J Hypertens. 1998;16(3):291–303. doi: 10.1097/00004872-199816030-00006. [DOI] [PubMed] [Google Scholar]
- 6.Chen PY, Gladish RD, Sanders PW. Vascular smooth muscle nitric oxide synthase anomalies in Dahl/Rapp salt-sensitive rats. Hypertension. 1998;31:918–924. doi: 10.1161/01.hyp.31.4.918. [DOI] [PubMed] [Google Scholar]
- 7.Bragulat E, de la Sierra A, Antonio MT, Coca A. Endothelial dysfunction in salt-sensitive essential hypertension. Hypertension. 2001;37:444–448. doi: 10.1161/01.hyp.37.2.444. [DOI] [PubMed] [Google Scholar]
- 8.Zhou MS, Adam AG, Jaimes EA, Raij L. In salt-sensitive hypertension, increased superoxide production is linked to functional upregulation of angiotensin II. Hypertension. 2003;42:945–951. doi: 10.1161/01.HYP.0000094220.06020.C8. [DOI] [PubMed] [Google Scholar]
- 9.Jaimes EA, Zhou MS, Pearse DD, Puzis L, Raij L. Upregulation of cortical COX-2 in salt-sensitive hypertension: role of angiotensin II and reactive oxygen species. Am J Physiol. 2008;29:F385–392. doi: 10.1152/ajprenal.00302.2007. [DOI] [PubMed] [Google Scholar]
- 10.Tian N, Moore RS, Braddy S, Rose RA, Gu JW, Hughson MD, Manning RD., Jr Interactions between oxidative stress and inflammation in salt-sensitive hypertension. Am J Physiol. 2007;293:H3388–3395. doi: 10.1152/ajpheart.00981.2007. [DOI] [PubMed] [Google Scholar]
- 11.Swei A, Lacy F, DeLano FA, Schmid-Schonbein GW. Oxidative stress in the Dahl hypertensive rat. Hypertension. 1997;30:1628–1633. doi: 10.1161/01.hyp.30.6.1628. [DOI] [PubMed] [Google Scholar]
- 12.Laffer CL, Bolterman RJ, Romero JC, Elijovich F. Effect of salt on isoprostanes in salt-sensitive essential hypertension. Hypertension. 2006;47:434–440. doi: 10.1161/01.HYP.0000202480.06735.82. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez R, Fischer P, Cuniberti L, Masnatta LD, Ramirez AJ. Vascular oxidative stress is associated with insulin resistance in hyper-reninemic nonmodulating essential hypertension. J Hypertension. 2007;25:2434–2440. doi: 10.1097/HJH.0b013e3282f03597. [DOI] [PubMed] [Google Scholar]
- 14.Sacks M, Svetkey LP, Vollmer WM, Appel LJ, Bray FA, Harsha D, Obarzanek E, Conlin PR, Miller ER, 3rd, Simons-Morton DG, Karanja N, Lin PH, DASH-Sodium Collaborative Research Group Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 15.Lopes HF, Martin KL, Nashar K, Morrow JD, Goodfriend TL, Egan BM. DASH diet lowers blood pressure and lipid-induced oxidative stress in obesity. Hypertension. 2003;41:422–430. doi: 10.1161/01.HYP.0000053450.19998.11. [DOI] [PubMed] [Google Scholar]
- 16.Al-Solaiman Y, Jesri A, Mountford WK, Lackland DT, Zhao Y, Egan BM. DASH lowers blood pressure in obese hypertensives beyond potassium, magnesium and fiber. doi: 10.1038/jhh.2009.58. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 18.Harris J, Benedict F. A biometric study of human basal metabolism. Proc Natl Acad Sci USA. 1918;4:370–373. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schakel S, Buzzard I, Gebhardt S. Procedures for estimating nutrient values for food composition databases. J Food Comp Anal. 1997;10:102–114. [Google Scholar]
- 20.McVeigh GE, Bratteli CW, Morgan DJ, Alinder CM, Glasser SP, Finkelstein SM, Cohn JN. Age-related abnormalities in arterial compliance identified by pressure pulse contour analysis: Aging and arterial compliance. Hypertension. 1999;33:1392–1398. doi: 10.1161/01.hyp.33.6.1392. [DOI] [PubMed] [Google Scholar]
- 21.Stojiljkovic MP, Zhang D, Lopes HF, Lee CG, Goodfriend TL, Egan BM. Hemodynamic effects of lipids in humans. Am J Physiol. 2001;280:R1674–1679. doi: 10.1152/ajpregu.2001.280.6.R1674. [DOI] [PubMed] [Google Scholar]
- 22.Lemogoum D, Flores G, Van den Abeele W, Ciarka A, Leeman M, Dagaute JP, van de Borne P, Van Bortel L. Validity of pulse pressure and augmentation index as surrogate measures of arterial stiffness during beta-adrenergic stimulation. J Hypertens. 2004;22:511–517. doi: 10.1097/00004872-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Van DeWater JM, Miller TW, Vogel RL, Mount BE, Dalton ML. Impedance Cardiography. The next vital sign technology? Chest. 2003;123:2028–2033. doi: 10.1378/chest.123.6.2028. [DOI] [PubMed] [Google Scholar]
- 24.Besch W, Woltanski KP, Keilacker H, Diaz-Alonso JM, Schulz B, Amendt P, Kohnert KD, Ziegler M. Measurement of insulin in human sera using a new RIA kit, 1: insulin determination in the absence of insulin antibodies–conventional assay and micro modification. Exp Clin Endocrinol. 1987;90:264–270. doi: 10.1055/s-0029-1210700. [DOI] [PubMed] [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 26.De Bruin TW, Brouwer CB, Gimpel JA, Erkelens DW. Postprandial decrease in HDL cholesterol and HDL apo A-I in normal subjects in relation to triglyceride metabolism. Am J Physiol. 1991;260:E492–E498. doi: 10.1152/ajpendo.1991.260.3.E492. [DOI] [PubMed] [Google Scholar]
- 27.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 28.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendrickson S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 29.Manning RD, Jr, Meng S, Tian N. Renal and vascular oxidative stress and salt-sensitivity of arterial pressure. Acta Physiol Scand. 2003;179:243–250. doi: 10.1046/j.0001-6772.2003.01204.x. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki H, Swei A, Zweifach BW, Schmid-Schonbein GW. In vivo evidence for microvascular oxidative stress in spontaneously hypertensive rats. Hydroethidine microfluorography Hypertension. 1995;25:1083–1089. doi: 10.1161/01.hyp.25.5.1083. [DOI] [PubMed] [Google Scholar]
- 31.Lenda DM, Sauls BA, Boegehold MA. Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt. Am J Physiol. 2000;279:H7–H14. doi: 10.1152/ajpheart.2000.279.1.H7. [DOI] [PubMed] [Google Scholar]
- 32.Satoh M, Fujimoto S, Haruna Y, Arakawa S, Horike H, Komai N, Sasaki T, Tsujioka K, Makino H, Kashihara N. NAD(P)H oxidase and uncoupled nitric oxide synthase are major sources of glomerular superoxide in rats with experimental diabetic nephropathy. Am J Physiol. 2005;288:F1144–F1152. doi: 10.1152/ajprenal.00221.2004. [DOI] [PubMed] [Google Scholar]
- 33.Chen PY, Gladish RD, Sanders PW. Vascular smooth muscle nitric oxide synthase anomalies in Dahl/Rapp salt-sensitive rats. Hypertension. 1998;31:918–924. doi: 10.1161/01.hyp.31.4.918. [DOI] [PubMed] [Google Scholar]
- 34.Boegehold MA. Effect of dietary salt on arteriolar nitric oxide in striated muscle of normotensive rats. Am J Physiol. 1993;264:H1810–H1816. doi: 10.1152/ajpheart.1993.264.6.H1810. [DOI] [PubMed] [Google Scholar]
- 35.Guzik TJ, West NE, Pillai R, Taggart DP, Channon KM. Nitric oxide modulates superoxide release and peroxynitrite formation in human blood vessels. Hypertension. 2002;39:1088–1094. doi: 10.1161/01.hyp.0000018041.48432.b5. [DOI] [PubMed] [Google Scholar]
- 36.Bragulat E, de la Sierra A, Antonio MT, Coca A. Endothelial dysfunction in salt-sensitive essential hypertension. Hypertension. 2001;37:444–448. doi: 10.1161/01.hyp.37.2.444. [DOI] [PubMed] [Google Scholar]
- 37.Berry C, Brosnan MJ, Fennell J, Hamilton CA, Dominiczak AF. Oxidative stress and vascular damage in hypertension. Curr Opin Nephrol Hypertens. 2001;10:247–255. doi: 10.1097/00041552-200103000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Panza JA, Quyyumi AA, Brush JE, Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 39.Fujiwara N, Osanai T, Kamada T, Katoh T, Takahashi K, Okumura K. Study on the relationship between plasma nitrite and nitrate level and salt sensitivity in human hypertension: modulation of nitric oxide synthesis by salt intake. Circulation. 2000;101:856–861. doi: 10.1161/01.cir.101.8.856. [DOI] [PubMed] [Google Scholar]
- 40.McEniery CM, Wallace S, Mackenzie IS, McDonnell B, Yasmin, Newby DE, Cockcroft JR, Wilkinson IB. Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension. 2006;48:602–608. doi: 10.1161/01.HYP.0000239206.64270.5f. [DOI] [PubMed] [Google Scholar]
- 41.Stamatelopoulos KS, Kalpakos D, Protogerou AD, Papamichael CM, Ikonomidis I, Tsitsirikos M, Revela I, Papaioannou TG, Lekakis JP. The combined effect of augmentation index and carotid intima-media thickness on cardiovascular risk in young and middle-aged men without cardiovascular. J Hum Hypertens. 2006;20:273–279. doi: 10.1038/sj.jhh.1001978. [DOI] [PubMed] [Google Scholar]
- 42.McVeigh GE, Allen PB, Morgan DR, Hanratty CG, Silke B. Nitric oxide modulation of blood vessel tone identified by arterial waveform analysis. Clin Sci. 2001;100:387–393. [PubMed] [Google Scholar]
- 43.Kals J, Kampus P, Kals M, Teesalu R, Zilmer K, Pulges A, Zilmer M. Arterial elasticity is associated with endothelial vasodilatory function and asymmetric dimethylargnine level in healthy subjects. Scand J Clin Lab Invest. 2007;67:536–544. doi: 10.1080/00365510701203470. [DOI] [PubMed] [Google Scholar]
- 44.Finkelstein SM, Cohn JN. First- and third-order models for determining arterial compliance. J Hypertens. 1992;1:S11–S14. [PubMed] [Google Scholar]
- 45.McVeigh GE, Burns DE, Finkelstein SM, McDonald KM, Mock JE, Feske W, Carlyle PF, Flack J, Grimm R, Cohn JN. Reduced vascular compliance as a marker for essential hypertension. Am J Hypertens. 1991;4:245–251. doi: 10.1093/ajh/4.3.245. [DOI] [PubMed] [Google Scholar]
- 46.Glasser SP, Arnett DK, McVeigh GE, Finkelstein SM, Bank AJ, Morgan DJ, Cohn JN. Vascular compliance and cardiovascular disease:a risk factor or a marker. Am J Hypertens. 1997;10:1175–1189. doi: 10.1016/s0895-7061(97)00311-7. [DOI] [PubMed] [Google Scholar]
- 47.Piccirillo G, Bucca C, Durante M, Santagada E, Munizzi MR, Cacciafesta M, Marigliano V. Heart rate and blood pressure variabilities in salt-sensitive hypertension. Am J Hypertens. 2000;13:873–883. doi: 10.1161/01.hyp.28.6.944. [DOI] [PubMed] [Google Scholar]
- 48.Campese VM, Romoff MS, Leviatan J, Saglikes Y, Friedler RM, Massry SG. Abnormal relationship between sodium intake and sympathetic nervous system activity in salt sensitive patients with essential hypertension. Kidney Int. 1982;21:371–378. doi: 10.1038/ki.1982.32. [DOI] [PubMed] [Google Scholar]
- 49.Grassi G, Cattaneo BM, Seravalle G, Lanfranchi A, Mancia G. Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension. 1998;31:68–72. doi: 10.1161/01.hyp.31.1.68. [DOI] [PubMed] [Google Scholar]
- 50.Coruzzi P, Parati G, Brambilla L, Brambilla V, Gualerzi M, Novarini A, Castiglioni P, Di Rienzo M. Effects of salt sensitivity on neural cardiovascular regulation in essential hypertension. Hypertension. 2005;46:1321–1326. doi: 10.1161/01.HYP.0000189183.50301.5c. [DOI] [PubMed] [Google Scholar]
- 51.Trimarco B, Lembo G, Ricciardelli B, De Luca N, Rendina V, Condorelli G, Volpe M. Salt-induced plasticity in cardiopulmonary baroreceptor reflexes in salt-resistant hypertensive patients. Hypertension. 1991;18:483–493. doi: 10.1161/01.hyp.18.4.483. [DOI] [PubMed] [Google Scholar]
- 52.Blaustein MP, Zhang J, Chen L, Hamilton BP. How does salt retention raise blood pressure? Am J Physiol. 2006;90:R514–R523. doi: 10.1152/ajpregu.00819.2005. [DOI] [PubMed] [Google Scholar]
- 53.Hamlyn JM. Increased levels of a humoral digitalis-like factor in deoxycorticosterone acetate-induced hypertension in the pig. J Endocrinol. 1989;122:409–420. doi: 10.1677/joe.0.1220409. [DOI] [PubMed] [Google Scholar]
- 54.Takada T, Nakagawa M, Ura N, Kaide J, Yoshida H, Shimamoto K. Endogenous immunoreactive ouabain-like and digoxin-like factors in reduced renal mass hypertensive rats. Hypertens Res. 1998;21:193–199. doi: 10.1291/hypres.21.193. [DOI] [PubMed] [Google Scholar]
- 55.Juncos R, Hong NJ, Garvin JL. Differential effects of superoxide on luminal and basolateral Na+/H+ exchange in the thick ascending limb. Am J Physiol. 2006;290:R79–R83. doi: 10.1152/ajpregu.00447.2005. [DOI] [PubMed] [Google Scholar]
- 56.Taylor NE, Cowley AW., Jr Effect of renal medullary H2O2 ion salt-induced hypertension and renal injury. Am J Physiol. 2005;289:R1573–1579. doi: 10.1152/ajpregu.00525.2005. [DOI] [PubMed] [Google Scholar]
- 57.Taylor NE, Glocka P, Liang M, Cowley AW., Jr NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension. 2006;47:692–698. doi: 10.1161/01.HYP.0000203161.02046.8d. [DOI] [PubMed] [Google Scholar]
- 58.Hu L, Manning RD., Jr Role of nitric oxide in regulation of long-term pressure-natriuresis relationship in Dahl rats. Am J Physiol. 1995;268:H2375–2383. doi: 10.1152/ajpheart.1995.268.6.H2375. [DOI] [PubMed] [Google Scholar]
- 59.Campese VM, Tawadrous M, Bigazzi R, Bianchi S, Mann AS, Oparil S, Raij L. Salt intake and plasma natriuretic peptide and nitric oxide in hypertension. Hypertension. 1996;28:335–340. doi: 10.1161/01.hyp.28.3.335. [DOI] [PubMed] [Google Scholar]
- 60.Fenoy FJ, Ferrer P, Carbonell L, Garcia-Salom M. Role of nitric oxide on papillary blood flow and pressure natriuresis. Hypertension. 1995;25:408–414. doi: 10.1161/01.hyp.25.3.408. [DOI] [PubMed] [Google Scholar]


