Abstract
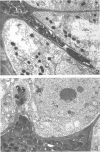
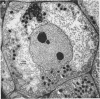
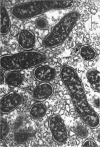
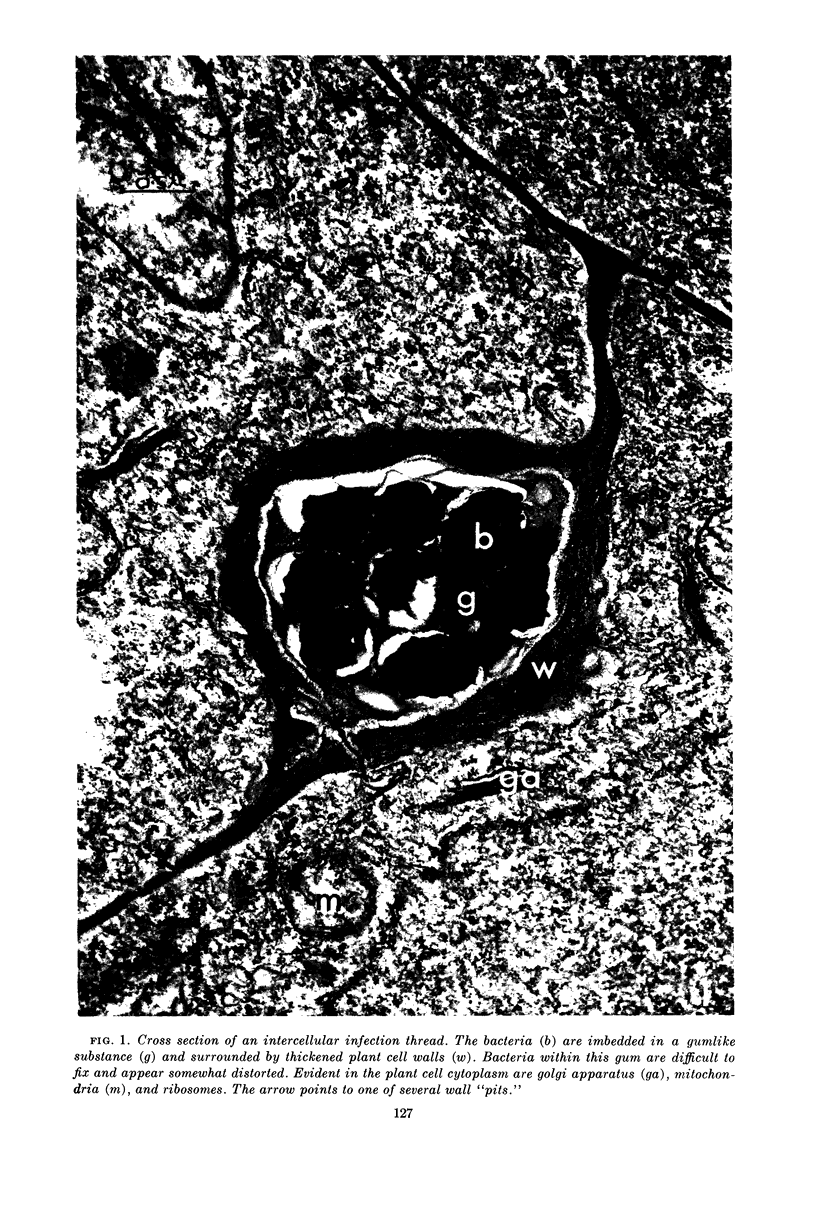
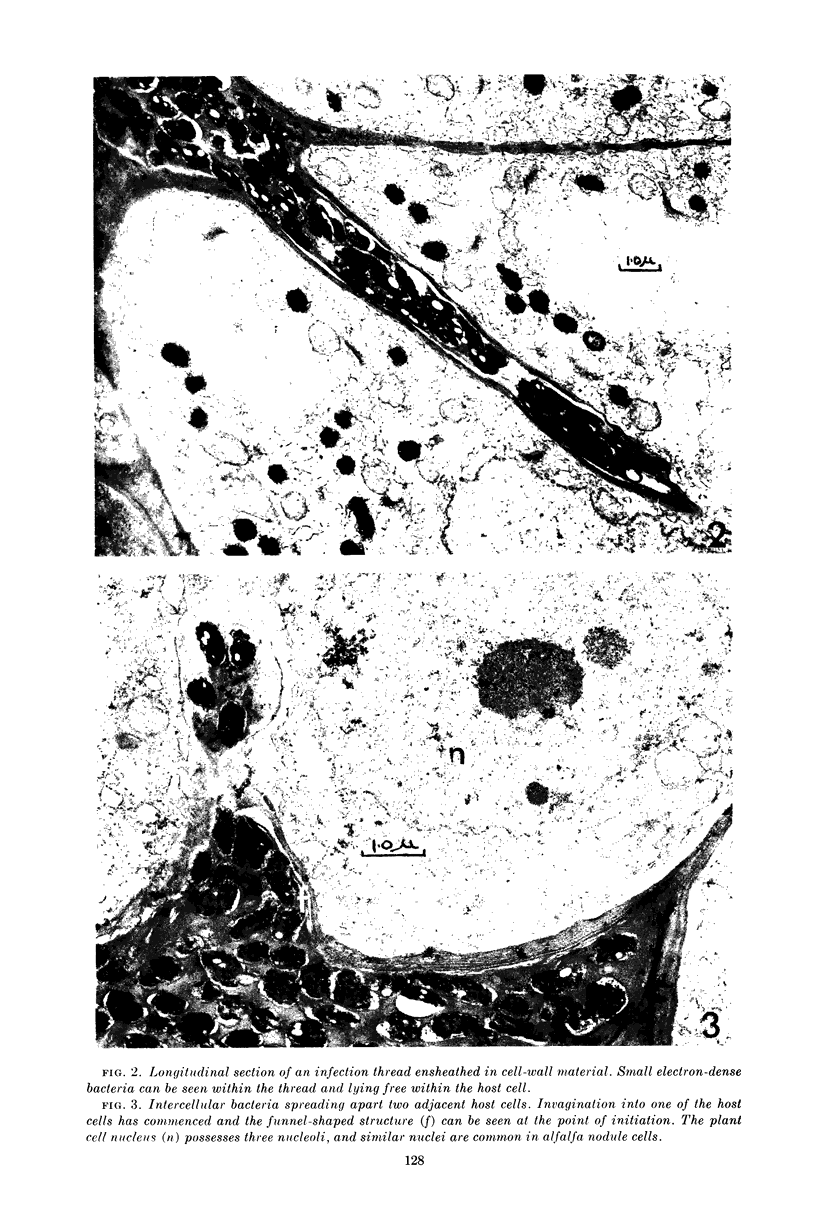
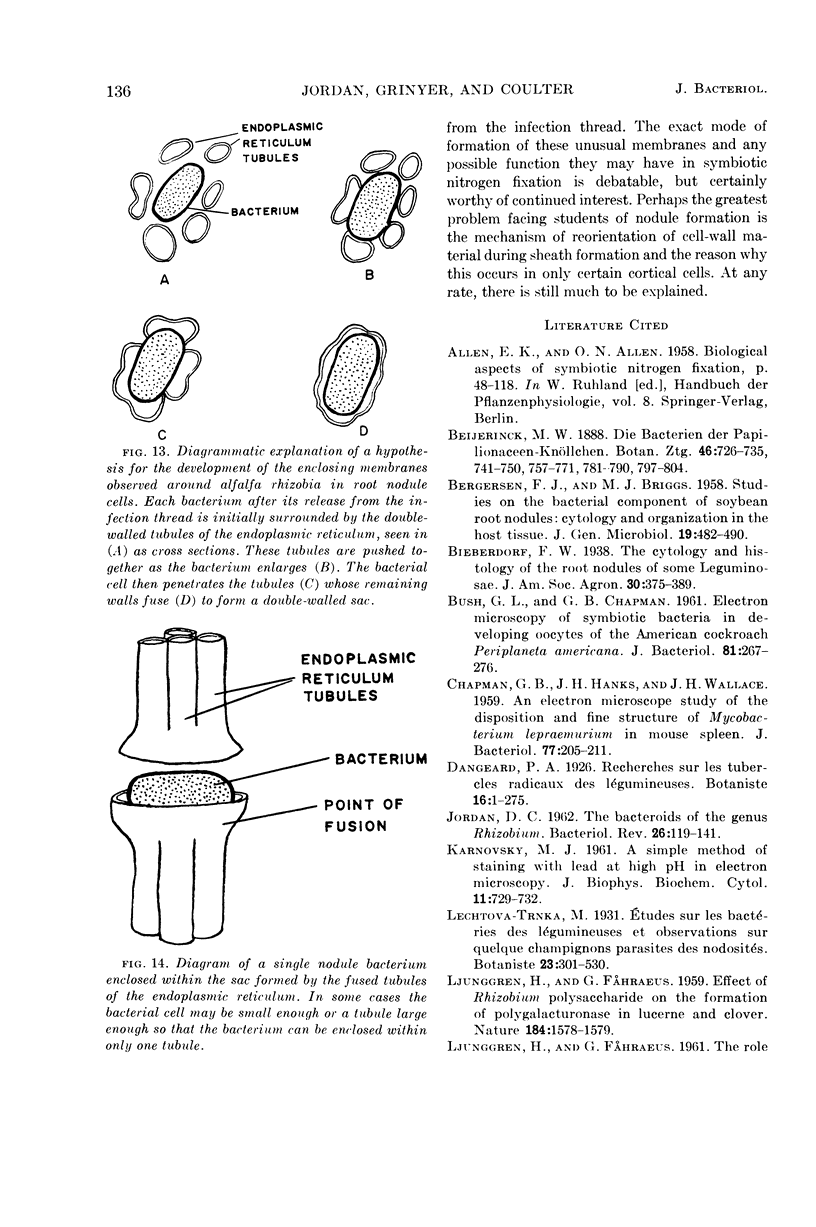
Jordan, D. C. (Ontario Agricultural College, Guelph, Canada), I. Grinyer, and W. H. Coulter. Electron microscopy of infection threads and bacteria in young root nodules of Medicago sativa. J. Bacteriol. 86:125–137. 1963.—Ultrathin sections of alfalfa nodules (Medicago sativa L.) revealed that initially the infection thread consisted of a naked mucoid strand, containing imbedded bacteria, migrating between the walls of the cortical cells of the host plant. At certain locations, the plant cell walls were forced into a funnel-shaped structure which elongated to form a tubular sheath containing bacterial cells and gum. Lateral and terminal vesicles formed on the ensheathed infection thread, and these burst to liberate the bacteria into the host cell cytoplasm, the process being similar to that proposed by certain of the earlier workers. In some cases, the ensheathed thread migrated entirely across a host cell without rupturing, and, in this case, the tip of the thread fused into the host cell wall opposite the point of entry. The bacteria, after their release from the thread, became bacteroidal, and ultimately each bacterial cell became surrounded by a double-layered membrane. It is suggested that this enclosing membrane may be derived from the tubules of the endoplasmic reticulum, although the possibility of the formation of these membranes de novo as a protective mechanism of the host plant cannot entirely be disregarded.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGERSEN F. J., BRIGGS M. J. Studies on the bacterial component of soybean root nodules: cytology and organization in the host tissue. J Gen Microbiol. 1958 Dec;19(3):482–490. doi: 10.1099/00221287-19-3-482. [DOI] [PubMed] [Google Scholar]
- BUSH G. L., CHAPMAN G. B. Electron microscopy of symbiotic bacteria in developing oocytes of the American cockroach, Periplaneta americana. J Bacteriol. 1961 Feb;81:267–276. doi: 10.1128/jb.81.2.267-276.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN G. B., HANKS J. H., WALLACE J. H. An electron microscope study of the disposition and fine structure of Mycobacterium lepraemurium in mouse spleen. J Bacteriol. 1959 Feb;77(2):205–211. doi: 10.1128/jb.77.2.205-211.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDAN D. C. The bacteroids of the genus Rhizobium. Bacteriol Rev. 1962 Jun;26:119–141. [PMC free article] [PubMed] [Google Scholar]
- KARNOVSKY M. J. Simple methods for "staining with lead" at high pH in electron microscopy. J Biophys Biochem Cytol. 1961 Dec;11:729–732. doi: 10.1083/jcb.11.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LJUNGGREN H., FAHRAEUS G. Effect of Rhizobium polysaccharide on the formation of polygalacturonase in lucerne and clover. Nature. 1959 Nov 14;184(Suppl 20):1578–1580. doi: 10.1038/1841578a0. [DOI] [PubMed] [Google Scholar]