Abstract
The primary product of the renin gene is preprorenin. A signal peptide sorts renin to the secretory pathway in juxtaglomerular cells where it is released into the circulation to initiate the renin-angiotensin system cascade. In the brain, transcription of renin occurs from an alternative promoter encoding a mRNA starting with a new first exon (exon-1b). Exon-1b initiating transcripts skip over the classical first exon (exon-1a) containing the initiation codon for preprorenin. Exon-1b transcripts are predicted to use a highly conserved initiation codon within exon-2, producing renin which should remain intracellular because it lacks the signal peptide. To evaluate the roles of secreted and intracellular renin, we took advantage of the organization of the renin locus to generate a secreted renin-specific knockout which preserves intracellular renin expression. Expression of secreted renin mRNA was ablated in the brain and kidney, whereas intracellular renin mRNA expression was preserved in fetal and adult brain. We noted a developmental shift from the expression of secreted renin mRNA in the fetal brain to intracellular renin mRNA in the adult brain. Homozygous secreted renin knockout mice exhibited very poor survival at weaning. The survivors exhibited renal lesions, low hematocrit, an inability to generate a concentrated urine, decreased arterial pressure, and impaired aortic contraction. These results suggest that 1) preservation of intracellular renin expression in the brain is not sufficient to compensate for a loss of secreted renin, and 2) secreted renin plays a pivotal role in renal development and function, survival, and the regulation of arterial pressure.
Keywords: Gene Targeting, Transcription, Alternative Promoters, Hypertension
Introduction
Renin is the first and rate limiting enzyme in the renin-angiotensin system (RAS). Renin processes angiotensinogen (AGT) into Ang-I, which is further proteolytically cleaved by angiotensin converting enzyme (ACE) into Ang-II. In the canonical pathway, Ang-II derived from the circulation binds to AT1 and AT2 receptors in target tissues to exert its function to regulate cardiovascular and water/electrolyte homeostasis. In addition to Ang-II, ample evidence now supports the hypothesis that alternative angiotensin peptides, such as Ang-(1–7), may act as effector peptides in cardiovascular regulation.1,2 The relative contribution of Ang-II and Ang-(1–7) remains a source of debate and has added significant complexity to the RAS. A second level of complexity stems from the wealth of data showing that many, if not all components of the RAS are expressed in many tissues. The concept that tissue-specific RAS pathways exist in tissues, including the kidney, brain, heart, vasculature, and adrenal gland, resulting in local production and action of angiotensin peptides continues to gain experimental support.3 In the kidney, local Ang-II is thought to regulate blood flow and sodium reabsorption, whereas in the brain it stimulates thirst and sympathetic activity. That antihypertensive agents targeting the RAS are effective in hypertensive patients with normal or even low plasma renin activity have contributed to the argument that tissue RAS are physiologically relevant and important.
The classical renin protein is composed of a signal peptide that leads to its secretion and release into the systemic circulation, a pro-segment that protects the active site of the enzyme from interacting with angiotensinogen, and mature active renin. The canonical site of renin synthesis, storage and release is the juxtaglomerular (JG) cells of the kidney. It is thought that other sites of renin synthesis release primarily prorenin. The first exon of renin (termed exon-1a) transcribed in renal JG cells harbors the transcription start site and encodes the initiation codon for translation. We and others identified an alternative isoform of renin mRNA in the brain of mouse, rat and human.4,5 This isoform is derived from a different transcription start site and transcribes a unique first exon (termed exon-1b). Transcripts initiating at exon-1b skip over exon-1a and splice directly to exon-2, which contains an evolutionarily conserved in-frame ATG which may act as an alternative translation initiation codon.6 Exon-1b transcripts are predicted to encode a truncated form of renin that lacks 1) the signal peptide, and thus remains intracellular, and 2) the first third of the pro-segment, and thus is constitutively active. Previous studies have shown that this form of renin, which we have termed intracellular renin (icRen), is enzymatically active and functional both in vitro and in vivo.5–7 We hypothesize that secreted renin (sRen) and icRen isoforms play differential roles in cardiovascular regulation and fluid homeostasis. Functional studies examining the importance of renin in the brain have been very challenging because the level of renin protein is extremely low. Moreover, because there are no unique peptides in icRen (the sequence of icRen is a subset of sRen) there is no clear way to differentiate the products. We therefore employed a unique gene targeting strategy to generate knockouts (both null and conditional) of one isoform, while retaining expression of the other. Herein we report that we have established a null model of sRen with the preservation of icRen expression.
Methods
Generation of sRen Knockout Mice
Gene targeting was performed in mouse ES cells derived from a tyrosinase mutant line of C57BL/6J-Tyr(c-2J). Germ-line transmission was screened in the offspring from the chimeras bred with C57BL/6J. Details of vector construction are in the Supplemental Methods (available at http://hyper.ahajournals.org). All experimental procedures on mice were approved by the University of Iowa Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Gene Expression
RNA was extracted from mouse tissues using TriReagent (Invitrogen). Mouse renin mRNA was measured with an RNase protection assay as previously described.4 Specific primers were used to amplify the sRen and icRen mRNA. The forward primer for sRen and icRen mRNA anchors to exon-1a and exon-1b, respectively. A common reverse primer located in exon-3 was used for both. A primer set in exon-4 and exon-5 was utilized to detect total renin mRNA. Taqman probes were used for realtime-PCRs for sRen and total renin mRNA. Cyber green with Takara Taq polymerase was employed to measure icRen mRNA. Primers sequences are listed in Table S1.
Renal Histology and Function
Kidneys were harvested and incubated in Pen-Fix for 3 days, paraffin-embedded and subjected to H&E staining. Following a 2-day acclimation period, urine was collected for 24 hours in metabolism cages designed for mice (Nalgene). Urine osmolarity was determined by freezing-point analysis (Fiske 2400). Urine sodium and potassium were determined by flame photometry (Instrumentation Laboratory 943). Animals were killed by CO2 asphyxia, decapitated, and blood was transferred into three 75 mm heparin-coated capillary tubes for hematocrit. Tubes were centrifuged at 12,600 × g for 3 minutes (BD Triac) before reading. Remaining blood (400 μL) was collected into 50 μL of 0.5 M EDTA, mixed, and placed on ice for 5 minutes. Plasma was stored at −80°C until analysis. Plasma aldosterone was determined by ELISA (Cayman Chemical, #10004377) using the manufacturer’s instructions.
Cardiovascular Studies
Under ketamine and xylazine anesthesia (85.5 mg/kg:12.5 mg/kg), by means of an anterior neck incision, left common carotid artery was implanted with radiotelemetry catheters (PA-C10, Data Sciences International). The radiotelemeter transmitter was kept subcutaneously into the right flank. After 10 days of recovery, direct arterial pressure and heart rate were recorded continuously during 10 days (sampling every 5 minutes with 10 seconds intervals). After the day/night recordings, the sampling frequency was increased to 2000 Hz and a two hour recording was made for analysis of the spontaneous baroreflex using the sequence method.8 The sympathetic and vagal effects to the heart were assessed by autonomic blockade with propranolol (5 mg/kg) and methyl-atropine (2 mg/kg), respectively. The intrinsic heart rate was calculated after simultaneous β-adrenergic and muscarinic blockade. Aortic function was measured ex vivo. Aortas were dissected and incubated in an organ bath while constrictor or dilator agents were added into the chamber at increasing doses as described.9
Statistical Analysis
All comparisons were between gender and age-matched wild-type and knockout mice. Data are plotted as mean±SEM and were analyzed with ANOVA. P<0.05 is considered statistically significant.
Results
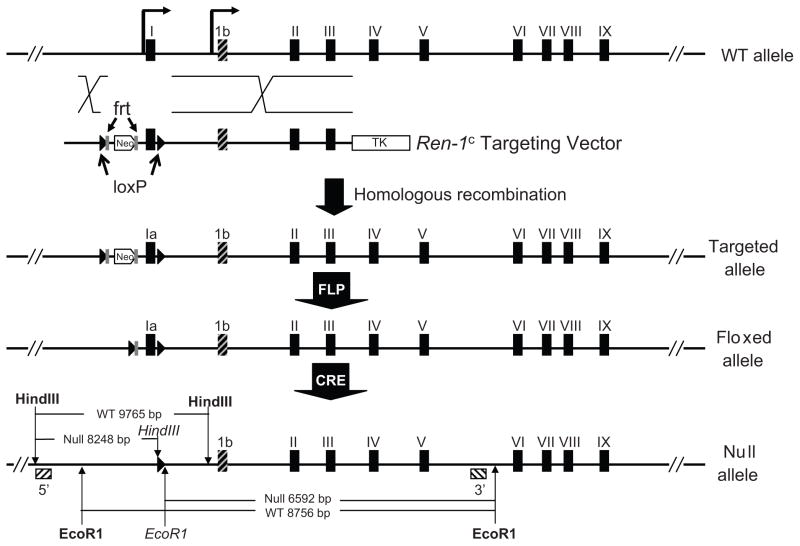
We employed a unique gene targeting approach to preserve expression of icRen while deleting sRen. In order to accomplish this, it was necessary to retain the common portions of the gene encoding renin (exons 2–9). Therefore we specifically ablated exon-1a of mouse renin gene along with the classical promoter while preserving exon-1b as well as its discrete transcriptional regulatory elements in the genome of C57BL/6J (Figure 1). The use of C57BL/6 ES cells allowed us to work in a genetic background containing only a single allele of the renin gene (Ren-1c). Recall the 129 strain carries two alleles of renin (Ren-2 and Ren-1d). The introduction of Cre-LoxP system confers this model the capability to cripple sRen expression in specific tissues or cell types. ES cell clones were screened via PCR genotyping, and chimeric mice were generated from ES clone FV115. Founder mice harboring the floxed exon-1a allele along with the neomycin cassette were bred with mice expressing FLPase in the early embryo. The resulting mice containing the floxed exon-1a allele (lacking the neomycin gene) were then bred with E2A-Cre mice expressing Cre-recombinase in the early embryo to generate a null allele of sRen. A series of PCR assays were sequentially performed to verify the fidelity of gene targeting, to screen for germline transmission, the floxed allele, and ultimately the null allele (data not shown). Southern blotting using probes outside the homology arms in the targeting construct confirmed the generation and transmission of the sRen null allele in +/− and −/− mice (Figure 2).
Figure 1. Generation of sRen Knock-out Mouse Model.
Cre-LoxP strategy was used to target exon-1a of mouse Ren-1c gene using the targeting vector shown. Homologous recombinant founder mice containing the targeted allele were bred with FLpase transgenics to generate the floxed allele. The null allele was obtained by breeding with E2A-Cre transgenic mice. The frt (gray rectangle) and loxP (black triangles) sites are indicated. Exon 1b is shaded black and gray. The final null allele is shown along with the expected sizes from EcoR1 and HindIII digestion and use of the 5′ and 3′ probes (crosshatched boxes). The restriction site indicated in bold are those present in genomic DNA whereas those in italics are unique to the null allele and engineered at the loxP site.
Figure 2. Southern Blotting of Null and Littermate Controls.
5′ and 3′ Southern probes were employed to confirm correct targeting of Ren-1c exon1a. HindIII digestion produces a 9765 bp wildtype (WT) band and an 8248 bp null band with the 5′ probe. EcoRI digestion generates an 8756 bp WT band and a 6592 bp null band using the 3′ probe. The position of the probes were shown in Figure 1.
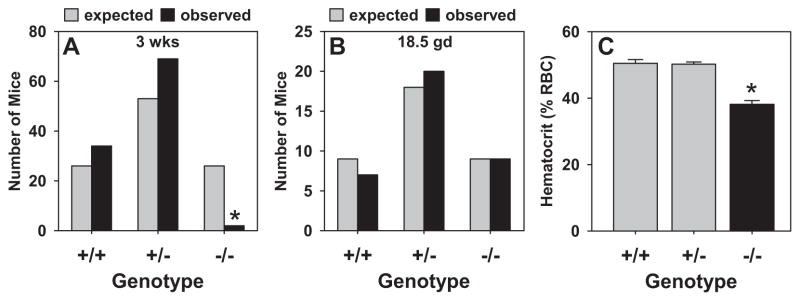
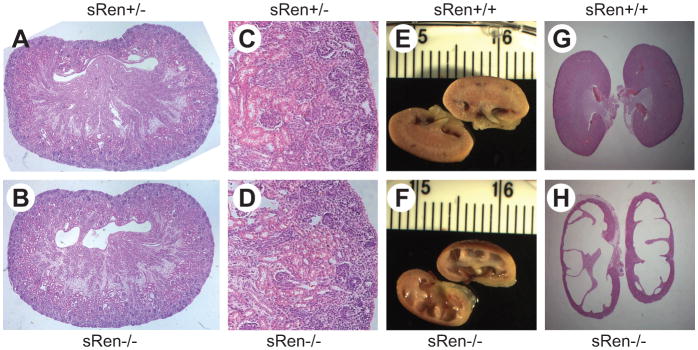
Intercrosses of +/− mice were performed to obtain −/− homozygotes. An analysis of the first 105 offspring from these crosses at 3 weeks of age revealed significant pre-weaning lethality as only 2 −/− mice (out of 26 expected) survived to 3 weeks of age (Figure 3A). On the contrary, live sRen−/− mice were obtained at birth (Figure S1), and an analysis of fetuses collected at prenatal day 18.5 revealed a normal ratio of +/+, +/− and −/− offspring (Figure 3B). This implicates a defect in survival between birth and weaning. Consistent with other models of RAS gene deficiency, sRen−/− mice exhibited a significantly reduced hematocrit (Figure 3C).10 Although kidneys from sRen−/− newborns appeared structurally normal (Figure 4A–D), kidneys from surviving adult sRen−/− mice exhibited severe renal atrophy compared with sRen+/+ controls (Figure 4E–H). Also consistent with previous models, sRen−/− mice exhibited increased urine output and an inability to generate a concentrated urine (Table 1).11 Probably as a compensatory mechanism, plasma aldosterone was elevated by 35% (Table 1). We conclude that secreted renin is required for survival and its loss causes severe defects in the structure and function of the kidney after birth.
Figure 3. Genetics.
A–B. Genotype frequencies of 3 week old (A) and 18.5 gd fetal (B) offspring of +/− X +/− crosses. *, P<0.05 by χ2. C. Hematocrit of mice with the indicated genotypes. *, P<0.05 vs +/+.
Figure 4. Renal Histology.
A–D. Saggital (A, B) and coronal (C, D) sections through kidney from newborn sRen+/− (A, C) and sRen−/− (B, D) mice. Images were photographed through 4× lens (A–B) and 10× lens (C–D). E–H. Cross-section (E, F) and hematoxylin and eosin staining (G, H) of sRen+/+ (E, G) and sRen−/− (F, H) kidney from surviving adult mice.
Table 1.
Plasma Aldosterone and Renal Function in sRen−/− and sRen+/+ Mice
| Genotype | PAC (pg/mL) | UOsm (mOsm/kg) | UOsm (mOsm/d) | UV (mL/d) | UNa(mM) | UK(mM) | UNa(mEq/d) | UK(mEq/d) |
|---|---|---|---|---|---|---|---|---|
| +/+ (n=3) | 694±70 | 3295±496 | 4.4±0.6 | 1.4±0.3 | 359±18 | 231±57 | 0.5±0.1 | 0.4±0.1 |
| −/− (n=4) | 933±51* | 634±117* | 5.0±0.5 | 9.0±2* | 85±17* | 62±12* | 0.7±0.1 | 0.5±0.1 |
PAC, Plasma aldosterone concentration; UOsm, Urine osmolality; UV, Urine volume; UNa, Urinary sodium; UK, Urinary potassium.
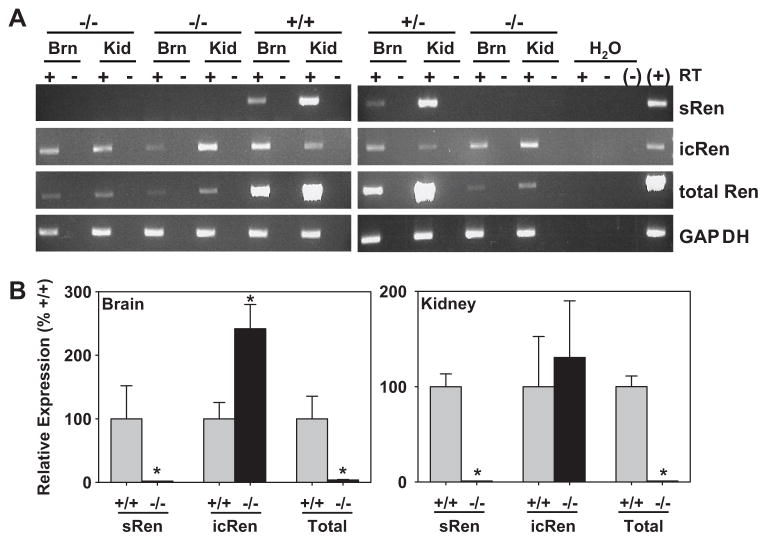
We next sought to obtain evidence for the preservation of icRen mRNA expression in the sRen-null mice. We used primer sets capable of individually detecting sRen vs icRen mRNA, and total renin (sRen + icRen mRNA). There was a marked reduction in total renin mRNA in the kidney and brain from 18.5 day gestation fetuses from sRen−/− as compared with +/+ and +/−littermates (Figure 5A). This was attributed to a loss of sRen mRNA as there was clear preservation of icRen mRNA in sRen−/− mice. These data were confirmed by real time Q-PCR (Figure 5B). It is notable that at this stage of fetal development, renin mRNA in the brain is mainly attributable to sRen mRNA as the decrease in total renin mRNA completely paralleled the decrease in sRen mRNA in sRen−/− mice. On the contrary, icRen mRNA was preserved and even increased in the brain of sRen−/− fetuses (Figure 5B). Similar results for total renin and sRen mRNA were observed in the kidney, consistent with the highly predominant transcription from the classical promoter in renal JG cells.
Figure 5. Expression Profile of Renin Isoforms in Fetuses.
A. RT-PCR of the indicated renin isoform mRNAs and GAPDH in brain (Brn) and kidney (Kid) of 18.5 gd fetuses genotypes as indicated. +/− reflects presence or absence of reverse transcriptase (RT) in the reaction. B. Realtime Q-PCR assays on ren-ex1a, ren-ex1b and total renin mRNA in the brain (left) and kidney (right) as indicated. +/+, gray bars; −/−, black bars. *, P<0.05. N=4 for all samples except N=3 for ren-ex1b in brain.
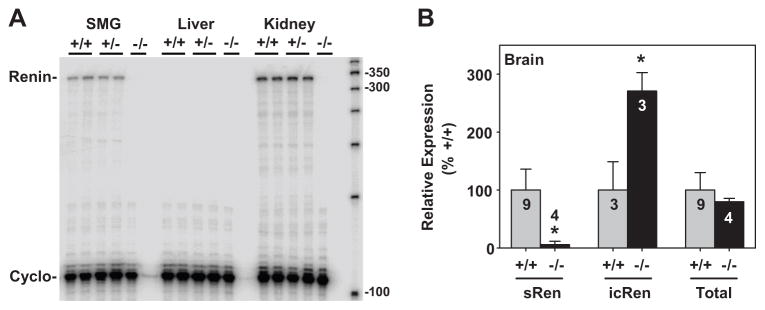
In adult survivors, total renin mRNA was completely ablated in the submandibular gland and kidney of sRen−/− mice (Figure 6A). As expected, there was no expression of renin mRNA in the liver. Q-PCR revealed that the levels of sRen mRNA were completely ablated, whereas the levels of icRen mRNA were increased in the brain of adult sRen−/− mice (Figure 6B). Interestingly, however, there was no apparent change in the level of total renin mRNA in the brain from adult sRen−/− mice suggesting a developmental shift in utilization of renin mRNA isoforms. Our data suggests that during late embryonic development, sRen mRNA is the major isoform expressed in the brain, whereas in adults, icRen mRNA is the predominant form expressed in the brain.
Figure 6. Renin Expression Levels in Adults.
A. RNase protection assay of submandibular gland (SMG) liver and kidney RNA from adult mice of the indicated genotypes. The renin and cyclophilin mRNA protected products are shown. B. Realtime Q-PCR assays on ren-ex1a, ren-ex1b and total renin mRNA in the brain as indicated. +/+, gray bars; −/−, black bars. *, P<0.05. The sample number is indicated for each bar.
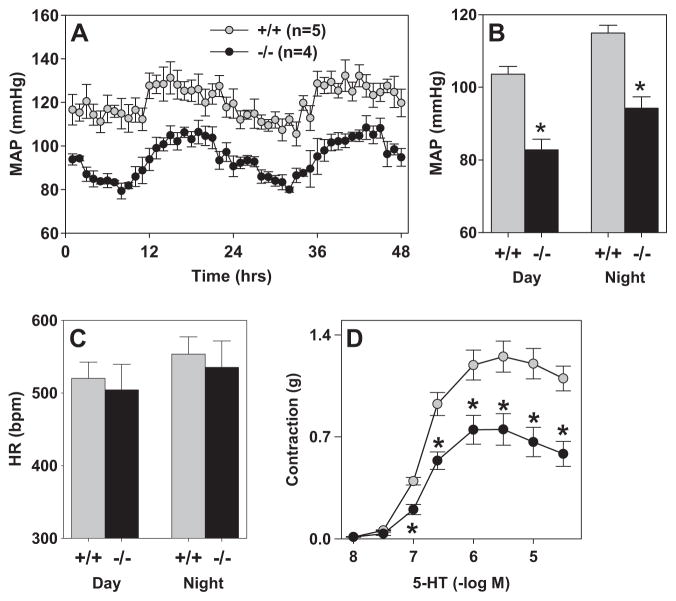
We next measured mean arterial blood pressure and heart rate via radiotelemetry. sRen null mice which survived to adulthood displayed a normal circadian rhythm (Figure 7A), but exhibited a significant decrease in mean arterial blood pressure compared to age and gender matched wild-type littermates during both the daytime and nighttime hours (Figure 7B). Baseline heart rate was unchanged in both groups (Figure 7C). Baroreflex function in sRen−/− was essentially normal except there was a decrease in the number of baroreflex sequences (Table 2). This may reflect a decrease in the blood pressure variability. The autonomic regulation of heart rate was also normal in sRen−/− although there was a trend toward an increase in the vagal tone to the heart as observed after muscarinic blockade with methyl-atropine. The intrinsic heart rate was similar in both groups.
Figure 7. Blood Pressure, Heart Rate and Aortic Function.
A. Hourly MAP over a 48-hr period of +/+ (gray) and −/− (black). B-C. Summary of 10-days of blood pressure (B) and heart rate recording (C) of +/+ and −/− split into day and night time periods. *, P<0.05. N=5 for +/+ and 4 for −/−. D. Contractile response of aorta to 5-HT. N=4 each for +/+ and −/−. *, P<0.02.
Table 2.
Cardiovascular Parameters in sRen−/− and sRen+/+ Mice
| Genotype | No. Sequences | Gain | BEI | iHR (bpm) | ΔHR Atropine (bpm) | ΔHR Propranolol (bpm) |
|---|---|---|---|---|---|---|
| +/+ (n=4) | 278±31 | 2.9±0.2 | 0.08±0.01 | 477±4 | +62±18 | −57±37 |
| −/− (n=4) | 154±15 | 3.5±0.3 | 0.06±0.01 | 470±40 | +132±26 | −48±18 |
| P Value | 0.01 | 0.09 | 0.28 | 0.87 | 0.07 | 0.82 |
BEI, baroreflex effective index; iHR, intrinsic heart rate.
Aorta from sRen−/− mice exhibited normal responses to acetylcholine and nitroprusside after pre-constriction with PGF2α, and exhibited normal contractile responses to PGF2α, phenylephrine and KCl (Figure S2). However, an impaired constrictor response to 5-HT was noted in the aorta from sRen−/− mice (Figure 7D).
Discussion
This is the first study designed to examine the differential roles, if any, of sRen and icRen in the regulation of cardiovascular function. The long term goal of this project is test the provocative hypothesis that the unique expression of icRen in the brain plays a role in blood pressure and water and electrolyte homeostasis.12 As a first step toward this goal, we used a novel gene targeting strategy to fully ablate sRen while preserving icRen mRNA. The novel findings from our study are that complete loss of sRen, even with preservation of icRen, causes lethality, and in adult survivors, severe renal abnormalities, anemia, an inability to concentrate urine, and hypotension. This suggests that sRen is an essential component required during early neonatal life and is a critical regulator of arterial pressure in adults. Our data also suggests that preservation of icRen is not sufficient to rescue defects caused by loss of sRen.
RAS Deficiency, Lethality and Renal Defects
The increased lethality and renal structural abnormalities observed in adult sRen null mice is consistent with other models of RAS gene ablation including renin null mutants lacking the entire renin gene.11,13–15 That the mice are born in normal numbers, exhibit normal renal histology at birth, but then succumb within a few weeks is also consistent with other RAS deficiencies. Similarly, anemia has been reported in mice lacking ACE.10 This implies that Ang II generated in the extracellular spaces in tissues or in the systemic circulation is required for continued development of the kidney after birth and is a major regulator of arterial pressure in adults. Mice lacking sRen were impaired in their ability to generate concentrated urine although their total 24 hr sodium and potassium excretion was essentially normal. The modest elevation in aldosterone is likely a consequence of these renal defects. Although icRen mRNA is the predominant form of renin mRNA in the brain of adult mice, it represents only a very small fraction (estimated less than 1%) of the total renin mRNA in the kidney. Consequently, it is not surprising that preservation of this small fraction of icRen mRNA in the kidney was insufficient to rescue the defects caused by loss of sRen. Indeed, it remains unclear what minimum level of renal renin is required to retain viability and renal structure and function and whether this renin needs to be secreted.
Complementation studies have been used to identify the important sites of RAS expression in order to rescue defects observed in RAS knockout mice. For example, we previously reported that the human renin and human angiotensinogen genes could fully complement lethality and renal defects in angiotensinogen deficient mice.16 Because the transgenes were expressed in many tissues and plasma Ang II was elevated, it provided a proof-of-principle that genetic means could be used to replace endogenous Ang II with transgenic Ang II. Other genetic data from our laboratory showing that exclusive targeting of renal angiotensinogen and renal Ang II is insufficient to rescue lethality in angiotensinogen deficient mice implicated the importance of circulating Ang II for the maintenance of renal structure.17 This is supported by complementation studies from other investigators where over-expression of RAS components in adipose tissue or liver resulted in Ang II release in the circulation which rescued renal defects.18,19 More contradictory to this hypothesis was data from Lochard et al. who reported that brain-specific over-production of Ang II normalized blood pressure and corrected some renal defects in angiotensinogen deficient mice.20 The Ang II level in the brain was elevated by approximately 6-fold, and was presumably generated in the extracellular space because the construct was cleverly designed to secrete Ang II. Interestingly, the increase in brain Ang II did not translate into an increase in circulating Ang II in their transgenic mice. It should be noted however, that they did not repeat these measurements when their transgene was bred on the angiotensinogen-deficient genetic background. Consequently, the mechanism by which brain-specific Ang II improved renal structure and function remains unclear. In our study, preservation, or even an increase in icRen expression in the brain was clearly ineffective at preventing renal abnormalities.
Evidence for An Intracellular Renin in the Brain
Our data showing preservation of icRen mRNA expression in the sRen null mice provides additional support for the concept that an independent renin mRNA is transcribed at the renin locus. The increase in icRen mRNA in the brain of sRen null mice, and the developmental shift in the expression from sRen in the fetal brain to icRen in the adult brain suggest that independent regulatory elements and a novel promoter mediate expression of icRen mRNA. This conclusion is strengthened when one considers that the ablation of sRen was generated by deletion of exon-1a and 500 bp of surrounding DNA including the classical renin promoter. Sequence analysis of the region directly upstream of mouse exon-1b reveals the absence of a classical TATA-box but the presence of a potential CCAAT box at approximately −50. A promoter prediction program also identified a potential promoter sequence approximately 700 bp upstream of exon-1b (within intron 1 of the renin locus).21 The same algorithm predicted a promoter upstream of exon 1a, but did not identify a promoter in the second intron. Understanding the transcriptional elements and physiological signals regulating expression of icRen will require additional investigation. It may be important to consider whether the low levels of icRen mRNA in the brain reflect the activity of a very weak promoter or are due to other factors. For example, Mercure et al. previously showed that whereas truncation of the renin prosegment resulted in increased renin activity, it also caused a marked decrease in secretion and expression of renin.22 Therefore it is unclear if the very truncation which eliminates the signal peptide and part of the prosegment also causes decreased expression.
Lee-Kirsch first identified transcripts initiating at exon-1b in the brain of mouse, rat and human.5 AtT-20 cells transfected with exon-1b cDNAs resulted in renin activity in cell lysates, but not medium, and in vitro translated icRen was not processed in microsomal membranes. These results were consistent with the production of active prorenin and retention of the protein intracellularly. We then identified the presence of the exon-1b mRNA in the brain of transgenic mice carrying a highly regulated genomic construct encoding the human renin gene and validated its presence in human fetal brain RNA.4 The exon-1b form of renin mRNA was the predominant form in the brain of these mice and the protein was localized throughout the hypothalamus.23 The hypertension in double transgenic mice carrying this highly regulated human renin gene was reduced after intracerebroventricular losartan, providing indirect evidence supporting the function of icRen. More direct evidence for the function of icRen is derived from studies specifically over-expressing this form of the protein. We reported that brain-specific expression of either sRen or icRen in transgenic mice resulted in a similar increase in blood pressure.6 Peters et al. reported that expression of icRen in transgenic rats is retained in the cytoplasm and results in increased aldosterone production.7 Clausmeyer et al. reported the presence of icRen mRNA in the adrenal gland, and its synthesis in the heart was stimulated after myocardial infarction.24,25 Therefore, icRen may not be exclusive to the brain.
Perspectives
There are many lingering questions surrounding the intracellular RAS concept (reviewed in 12). Is icRen localized in a cellular compartment where it can generate angiotensin peptides? What happens to these peptides if and once they are generated? Of obvious importance would be to determine if this occurs in neurons, and if so, what the function of intracellular Ang II is in neurons. Although our data clearly suggest that icRen cannot substitute for loss of sRen, it does not rule out a physiological function for icRen. By taking advantage of the fact that sRen and icRen mRNAs are derived from independent transcription start sites, we have generated the first of a series of models designed to dissect the physiological significance of sRen and icRen. Unfortunately, because the sRen and icRen proteins are essentially identical in sequence (icRen is a subset of secreted prorenin), antibodies specific to each isoform cannot be generated. This coupled with the low level of renin expression in the brain has hampered studies addressing its function. Consequently, the generation of the complementary model described herein, that is, ablation of icRen with preservation of sRen, should provide a unique experimental platform from which to assess the function of this protein. As icRen is the primary isoform of renin mRNA expressed in the adult brain and its expression is largely, but not exclusively brain-specific, an icRen null should function, essentially, as a brain-specific renin knockout. The generation of this model is currently in progress.
Supplementary Material
Acknowledgments
We thank Dr. Nobuyuki Takahashi and Dr. Feng Li for their technical advice in performing subcutaneous saline injections to newborn mice, and Henry Keen for bioinformatics.
Funding: Sigmund: NIH grants HL048058, HL061446, and HL084207, and the Roy J. Carver Trust
Xu: American Heart Association Pre-doctoral Fellowship (0910035G), Grobe: American Physiological Society Physiological Genomics Fellowship
Footnotes
Disclosures: Di Xu- AHA Predoctoral Fellowship
Giulianna R. Borges - none
Justin L. Grobe – American Physiological Society Postdoctoral Fellowship
Christopher J. Pelham - none
Baoli Yang - none
Curt D. Sigmund – NIH Grants, Roy J. Carver Trust
References
- 1.Sakai K, Sigmund CD. Molecular evidence of tissue Renin-Angiotensin systems: a focus on the brain. Curr Hypertens Rep. 2005;7:135–140. doi: 10.1007/s11906-005-0088-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moriguchi A, Tallant EA, Matsumura K, Reilly TM, Walton H, Ganten D, Ferrario CM. Opposing actions of angiotensin-(1–7) and angiotensin II in the brain of transgenic hypertensive rats. Hypertension. 1995;25:1260–1265. doi: 10.1161/01.hyp.25.6.1260. [DOI] [PubMed] [Google Scholar]
- 3.Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system--an endocrine and paracrine system. Endocrinology. 2003;144:2179–2183. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- 4.Sinn PL, Sigmund CD. Identification of Three Human Renin mRNA Isoforms Resulting from Alternative Tissue-Specific Transcriptional Initiation. Physiol Genomics. 2000;3:25–31. doi: 10.1152/physiolgenomics.2000.3.1.25. [DOI] [PubMed] [Google Scholar]
- 5.Lee-Kirsch MA, Gaudet F, Cardoso MC, Lindpaintner K. Distinct renin isoforms generated by tissue-specific transcription initiation and alternative splicing. Circ Res. 1999;84:240–246. doi: 10.1161/01.res.84.2.240. [DOI] [PubMed] [Google Scholar]
- 6.Lavoie JL, Liu X, Bianco RA, Beltz TG, Johnson AK, Sigmund CD. Evidence supporting a functional role for intracellular renin in the brain. Hypertension. 2006;47:461–466. doi: 10.1161/01.HYP.0000203308.52919.dc. [DOI] [PubMed] [Google Scholar]
- 7.Peters J, Wanka H, Peters B, Hoffmann S. A renin transcript lacking exon 1 encodes for a non-secretory intracellular renin that increases aldosterone production in transgenic rats. J Cell Mol Med. 2008;12:1229–1237. doi: 10.1111/j.1582-4934.2008.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borges GR, Salgado HC, Silva CA, Rossi MA, Prado CM, Fazan R., Jr Changes in hemodynamic and neurohumoral control cause cardiac damage in one-kidney, one-clip hypertensive mice. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1904–R1913. doi: 10.1152/ajpregu.00107.2008. [DOI] [PubMed] [Google Scholar]
- 9.Halabi CM, Beyer AM, de Lange WJ, Keen HL, Baumbach GL, Faraci FM, Sigmund CD. Interference with PPARγ Function in Smooth Muscle Causes Vascular Dysfunction and Hypertension. Cell Metabolism. 2008;7:215–226. doi: 10.1016/j.cmet.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole J, Ertoy D, Lin H, Sutliff RL, Ezan E, Guyene TT, Capecchi M, Corvol P, Bernstein KE. Lack of angiotensin II-facilitated erythropoiesis causes anemia in angiotensin-converting enzyme-deficient mice. J Clin Invest. 2000;106:1391–1398. doi: 10.1172/JCI10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliverio MI, Kim HS, Ito M, Le T, Audoly L, Best CF, Hiller S, Kluckman K, Maeda N, Smithies O, Coffman TM. Reduced growth, abnormal kidney structure, and type 2 (AT2) angiotensin receptor-mediated blood pressure regulation in mice lacking both AT1A and AT1B receptors for angiotensin II. Proc Natl Acad Sci USA. 1998;95:15496–15501. doi: 10.1073/pnas.95.26.15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grobe JL, Xu D, Sigmund CD. An intracellular renin-angiotensin system in neurons: fact, hypothesis, or fantasy. Physiology. 2008;23:187–193. doi: 10.1152/physiol.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi N, Lopez ML, Cowhig JE, Jr, Taylor MA, Hatada T, Riggs E, Lee G, Gomez RA, Kim HS, Smithies O. Ren1c homozygous null mice are hypotensive and polyuric, but heterozygotes are indistinguishable from wild-type. J Am Soc Nephrol. 2005;16:125–132. doi: 10.1681/ASN.2004060490. [DOI] [PubMed] [Google Scholar]
- 14.Kim HS, Maeda N, Oh GT, Fernandez LG, Gomez RA, Smithies O. Homeostasis in mice with genetically decreased angiotensinogen is primarily by an increased number of renin-producing cells. J Biol Chem. 1999;274:14210–14217. doi: 10.1074/jbc.274.20.14210. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi N, Li F, Hua K, Deng J, Wang CH, Bowers RR, Bartness TJ, Kim HS, Harp JB. Increased energy expenditure, dietary fat wasting, and resistance to diet-induced obesity in mice lacking renin. Cell Metab. 2007;6:506–512. doi: 10.1016/j.cmet.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davisson RL, Kim HS, Krege JH, Lager DJ, Smithies O, Sigmund CD. Complementation of reduced survival, hypotension and renal abnormalities in angiotensinogen deficient mice by the human renin and human angiotensinogen genes. J Clin Invest. 1997;99:1258–1264. doi: 10.1172/JCI119283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding Y, Stec DE, Sigmund CD. Genetic evidence that lethality in angiotensinogen-deficient mice is due to loss of systemic but not renal angiotensinogen. J Biol Chem. 2001;276:7431–7436. doi: 10.1074/jbc.M003892200. [DOI] [PubMed] [Google Scholar]
- 18.Massiera F, Bloch-Faure M, Ceiler D, Murakami K, Fukamizu A, Gasc JM, Quignard-Boulange A, Negrel R, Ailhaud G, Seydoux J, Meneton P, Teboul M. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 2001;15:2727–2729. doi: 10.1096/fj.01-0457fje. [DOI] [PubMed] [Google Scholar]
- 19.Ishida J, Sugiyama F, Tanimoto K, Taniguchi K, Syouji M, Takimoto E, Horiguchi H, Murakami K, Yagami K, Fukamizu A. Rescue of angiotensinogen-knockout mice. Biochem Biophys Res Commun. 1998;252:610–616. doi: 10.1006/bbrc.1998.9707. [DOI] [PubMed] [Google Scholar]
- 20.Lochard N, Silversides DW, Van Kats JP, Mercure C, Reudelhuber TL. Brain-specific restoration of angiotensin II corrects renal defects seen in angiotensinogen-deficient mice. J Biol Chem. 2002;278:2184–2189. doi: 10.1074/jbc.M209933200. [DOI] [PubMed] [Google Scholar]
- 21.Knudsen S. Promoter2.0: for the recognition of PolII promoter sequences. Bioinformatics. 1999;15:356–361. doi: 10.1093/bioinformatics/15.5.356. [DOI] [PubMed] [Google Scholar]
- 22.Mercure C, Thibault G, Lussier-Cacan S, Davignon J, Schiffrin EL, Reudelhuber TL. Molecular analysis of human prorenin prosegment variants in vitro and in vivo. J Biol Chem. 1995;270:16355–16359. doi: 10.1074/jbc.270.27.16355. [DOI] [PubMed] [Google Scholar]
- 23.Morimoto S, Cassell MD, Sigmund CD. The Brain Renin-Angiotensin System in Transgenic Mice Carrying a Highly Regulated Human Renin Transgene. Circ Res. 2002;90:80–86. doi: 10.1161/hh0102.102272. [DOI] [PubMed] [Google Scholar]
- 24.Clausmeyer S, Reinecke A, Farrenkopf R, Unger T, Peters J. Tissue-specific expression of a rat renin transcript lacking the coding sequence for the prefragment and its stimulation by myocardial infarction. Endocrinology. 2000;141:2963–2970. doi: 10.1210/endo.141.8.7623. [DOI] [PubMed] [Google Scholar]
- 25.Clausmeyer S, Sturzebecher R, Peters J. An alternative transcript of the rat renin gene can result in a truncated prorenin that is transported into adrenal mitochondria. Circ Res. 1999;84:337–344. doi: 10.1161/01.res.84.3.337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.