Abstract
The perifornical-lateral hypothalamic area (PF-LHA) has been implicated in the regulation of arousal. The PF-LHA contains wake-active neurons that are quiescent during nonREM sleep and in the case of neurons expressing the peptide hypocretin (HCRT), quiescent during both nonREM and REM sleep. Adenosine is an endogenous sleep factor and recent evidence suggests that adenosine via A1 receptors may act on PF-LHA neurons to promote sleep. We examined the effects of bilateral activation as well as blockade of A1 receptors in the PF-LHA on sleep-wakefulness in freely behaving rats.
The sleep-wake profiles of male Wistar rats were recorded during reverse microdialysis perfusion of artificial cerebrospinal fluid (aCSF) and two doses of adenosine A1 receptor antagonist, 1,3-dipropyl-8-phenylxanthine (CPDX; 5μM and 50μM) or A1 receptor agonist, N6-cyclopentyladenosine (CPA; 5μM and 50μM) into the PF-LHA for 2h followed by 4h of aCSF perfusion. CPDX perfused into the PF-LHA during lights-on phase produced arousal (F=7.035, p <0.001) and concomitantly decreased both nonREM (F=7.295, p<0.001) and REM sleep (F=3.456, p<0.004). In contrast, CPA perfused into the PF-LHA during lights-off phase significantly suppressed arousal (F = 7.891; p <0.001) and increased nonREM (F = 8.18; p <0.001) and REM sleep (F = 30.036; p = <0.001). These results suggest that PF-LHA is one of the sites where adenosine, acting via A1 receptors, inhibits PF-LHA neurons to promote sleep.
Keywords: Orexin, Hypocretin, Adenosine, Posterior-lateral hypothalamus, Sleep
INTRODUCTION
The perifornical-lateral hypothalamic area (PF-LHA) has been implicated in the regulation of behavioral arousal (Gerashchenko and Shiromani, 2004; Jones, 2005; McGinty and Szymusiak, 2003). The PF-LHA contains a heterogeneous population of neuronal groups as reflected by their state-dependent discharge properties as well as neurotransmitter phenotypes. These include cells expressing hypocretin or orexin (HCRT), melanin-concentrating hormone (MCH), Gamma-aminobutyric acid (GABA) and glutamate. Amongst various neuronal groups in the PF-LHA, HCRT neurons in particular have been extensively studied and have been implicated in the facilitation and/or maintenance of arousal (Alam et al., 2002; Koyama et al., 2003; Sakurai, 2007; Siegel, 2004; Takahashi et al., 2008). HCRT neurons exhibit wake-associated discharge and c-Fos protein immunoreactivity (Fos-IR) and are quiescent during nonREM and REM sleep (Espana et al., 2003; Estabrooke et al., 2001; Lee et al., 2005; Mileykovskiy et al., 2005). Local applications of HCRT at various projection targets including the basal forebrain, preoptic area and locus coeruleus promote waking (Bourgin et al., 2000; Methippara et al., 2000; Thakkar et al., 2001). Human narcoleptics exhibit HCRT cell loss (Peyron et al., 2000; Thannickal et al., 2000). Symptoms of narcolepsy including excessive sleepiness and cataplexy, are also exhibited by dogs with HCRT-2 receptor mutation (Lin et al., 1999), HCRT peptide knockout mice (Chemelli et al., 1999), rats with a destruction of HCRT-receptor expressing neurons in the PF-LHA (Gerashchenko et al., 2001) and HCRT/ataxin-3 transgenic mice with destruction of HCRT neurons (Hara et al., 2001). In contrast to HCRT neurons, GABAergic and MCH neurons in the PF-LHA have been implicated in sleep regulation. GABAergic and MCH neurons exhibit sleep-associated Fos-IR (Kumar et al., 2005; Modirrousta et al., 2005). MCH neurons discharge selectively during sleep, especially REM sleep and intracerebroventricular administration of MCH increases both nonREM and REM sleep (Hassani et al., 2009; Verret et al., 2003).
Adenosine is a ubiquitous neuromodulator that has been implicated in the regulation of sleep (Basheer et al., 2004; Benington and Heller, 1995; Datta and Maclean, 2007; Dunwiddie and Masino, 2001; McCarley, 2007). The neuronal production of adenosine is coupled with metabolic activity and is higher during waking as compared with sleep (Maquet, 1995). Adenosine or its agonists promote sleep and increase EEG slow wave activity, whereas its antagonists, e.g., caffeine and theophylline, are potent behavioral stimulants and suppress sleep (Benington et al., 1995; Bennett and Semba, 1998; Landolt et al., 1995; Methippara et al., 2005; Radulovacki et al., 1984). Adenosine acts via A1, A2A, A2B and A3 receptors; A1 and A2A receptors are known to mediate the sleep-promoting effects of adenosine (Basheer et al., 2007; Dunwiddie and Masino, 2001; Ribeiro et al., 2002; Scharf et al., 2008). The A1 subtype inhibits adenylate cyclase and is inhibitory, whereas the A2A subtype stimulates adenylate cyclase and produces excitatory effects in central nervous system. Recently we found that in the lateral preoptic area, A1 receptor activation produced arousal whereas A2A receptor activation produced sleep enhancement, suggesting that adenosine-induced sleep is site and receptor dependent (Methippara et al., 2005).
Although many studies examining the role of adenosine as a homeostatic sleep factor have focused on the cholinergic basal forebrain (Alam et al., 1999; Basheer et al., 2004; Blanco-Centurion et al., 2006; Thakkar et al., 2003a; Thakkar et al., 2003b), evidence suggests that adenosine in the PF-LHA may also play a role in the homeostatic regulation of sleep. For example, immunohistochemical evidence suggests that A1 receptors are localized on HCRT neurons (Thakkar et al., 2002). An in vitro pharmacological study suggests that adenosine inhibits HCRT neurons and that this effects is mediated via A1 receptors (Liu and Gao, 2007). A recent in vivo study found that adenosine A1 receptor antagonist, when microinjected into the PF-LHA produced arousal and suppressed nonREM and REM sleep (Thakkar et al., 2008).
In this study we examined the effects of bilateral perfusion, using reverse microdialysis, of an adenosine A1 receptor agonist, N6-cyclopentyladenosine, and an A1 receptor antagonist, 1,3-dipropyl-8-phenylxanthine, into the PF-LHA on the sleep-wake profiles of rats. Unlike microinjection study (Thakkar et al., 2008), drug delivery via reverse microdialysis allowed us to examine the long-term effects of the continuous perfusion of pharmacological agents that were delivered without disturbing the animals. Furthermore, it also reduced the likelihood that treatment effects could be due to mechanical or inflammatory responses (Quan and Blatteis, 1989).
RESULTS
A. Site of drug delivery
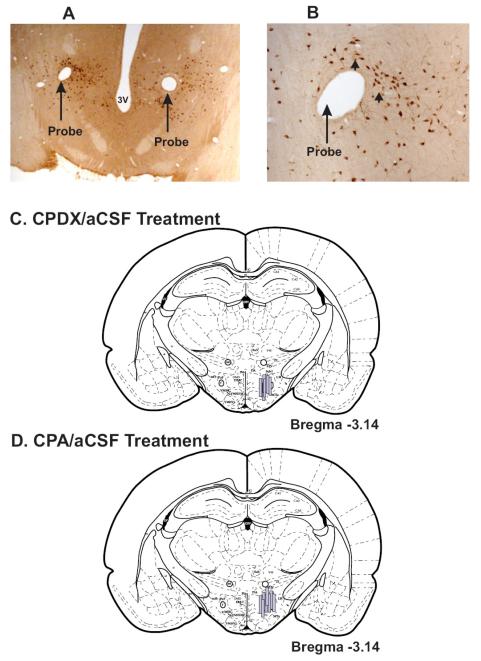
Locations of the microdialysis probes along with the outlines of membrane that were used for perfusing CPDX and CPA are shown in figure-1. The microdialysis probes were localized in the PF-LHA and adjoining areas between AP —2.8 to −3.3 (Paxinos and Watson, 1998). In earlier studies we found that perfusion of bicuculline or serotonin into the PF-LHA using the same drug delivery protocol (Alam et al., 2005; Kumar et al., 2007) affected c-Fos-IR in 500-750μm radius around the probe. Therefore, based on the probe locations, it is likely that the perfused CPDX and CPA affected areas including perifornical area, portions of dorsomedial and ventomedial hypothalamic area, lateral hypothalamic area, and ventral zona incerta.
Figure-1. Sites of CPDX and CPA perfusions.
A. Photomicrograph of a horizontal section (40x magnification) showing tracts of the microdialysis probes (large arrows) from an animal that was perfused with aCSF and CPDX. These probes were localized in the HCRT neuronal field (small arrows), which has been magnified (400x) in figure B.
C and D. Reconstruction diagrams (coronal sections) through the PF-LHA showing the outlines and locations of the microdialysis probes used for the delivery of aCSF/CPDX (C) and aCSF/CPA (D). Although, the rostro-caudal locations of the microdialysis probes varied ± 0.2mm, they are represented on one plane, which was most commonly encountered. In all these studies, aCSF and drugs were perfused bilaterally, however, the probes are shown on one side for a better anatomical comparison. Arc, arcuate hypothalamic nucleus; DMH, dorsomedial hypothalamic nucleus; f, fornix; mt, mammillothalamic tract; Mtu, Medial tuberal nucleus; PeF, perifornical nucleus; VMH, ventromedial hypothalamic nucleus, ZI, zona incerta.
B. Effects of CPDX on sleep-wakefulness
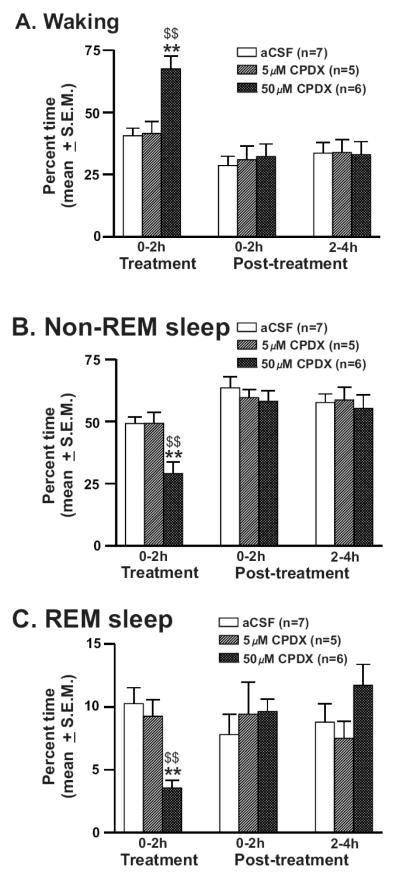
The effects of two doses of CPDX vs. aCSF perfused into the PF-LHA on sleep-wakefulness were studied in a group of 7 rats. The percent time (mean ± S.E.M.) spent in waking, nonREM, and REM sleep during the 6h recording period including 2h of treatment and 4h of post-treatment conditions for aCSF (n=7) and CPDX (5μM, n=5 and 50μM, n=6) treated rats are shown in figure-2. aCSF treated rats spent a significant portion of the recording time in sleep including nonREM (56.8 ± 1.6%) and REM sleep (8.9 ± 1.2%) during the 6h-recording period without significant differences between treatment and post-treatment periods. CPDX treated rats spent significantly more time in waking (F = 7.035; p <0.001) and less time in nonREM (F = 7.295; p <0.001) and REM sleep (F = 3.456; p = 0.004). Of the two doses used, 50μM CPDX-induced behavioral changes were significantly greater compared to both aCSF and 5μM CPDX treatments during the 2h of its perfusion. The sleep-wake profiles during the post-treatment period in the three groups were comparable.
Figure-2. Effects of CPDX on sleep-wakefulness.
Percent time (mean ± SEM) spent in waking (A), nonREM (B) and REM sleep (C) at 2h intervals during the 6h recording period including first 2h of treatment (aCSF, 5μM or 50μM CPDX perfusion into the PF-LHA) and 4h of post-treatment recordings (aCSF perfusion). In the presence of CPDX in the PF-LHA, spontaneously sleeping rats spent significantly more time in waking and less time in nonREM and REM sleep. *, as compared to aCSF control; $, as compared to 5μM CPDX. *, $, p<0.05; **, $$, p <0.01.
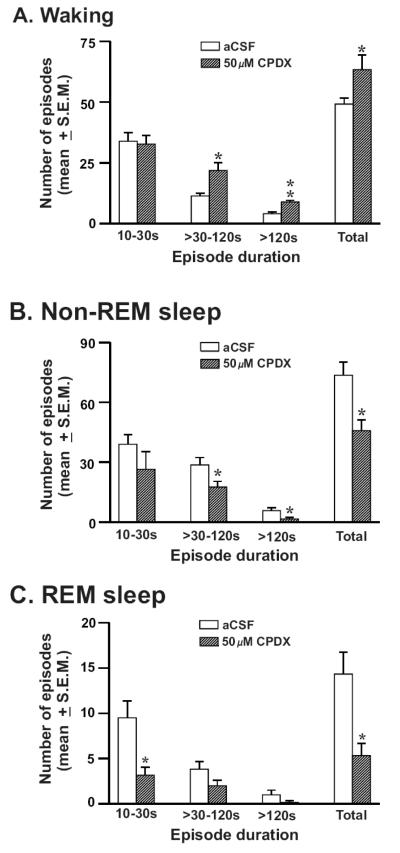
The frequencies of waking, nonREM and REM sleep episodes of various durations during aCSF vs. 50μM CPDX perfusion during the 2h treatment period are shown in figure-3. During CPDX perfusion, rats exhibited a significant increase in the frequency of waking episodes including medium (>30-120s) and long episodes (>120s). Animals exhibited significant reductions in number of medium and long episodes of nonREM sleep in response to CPDX. CPDX significantly reduced the frequency of REM sleep episodes. Fewer medium and long REM episodes were encountered during aCSF perfusion. During CPDX perfusion their frequencies also decreased but not significantly. The number of waking, nonREM and REM sleep episodes during post-treatment conditions after aCSF and 50μM CPDX perfusion were comparable.
Figure-3. Effects of CPDX on the frequency of sleep-wake episodes.
The number (mean ± SEM) of waking (A), nonREM (B) and REM sleep (C) episodes during the 2h recording period with aCSF and 50μM CPDX perfusion into the PF-LHA. 50μM CPDX produced a significant increase in waking and concomitant decreases in nonREM and REM sleep episodes during the treatment period. *, p<0.05.
C. Effects of CPA on sleep-wakefulness
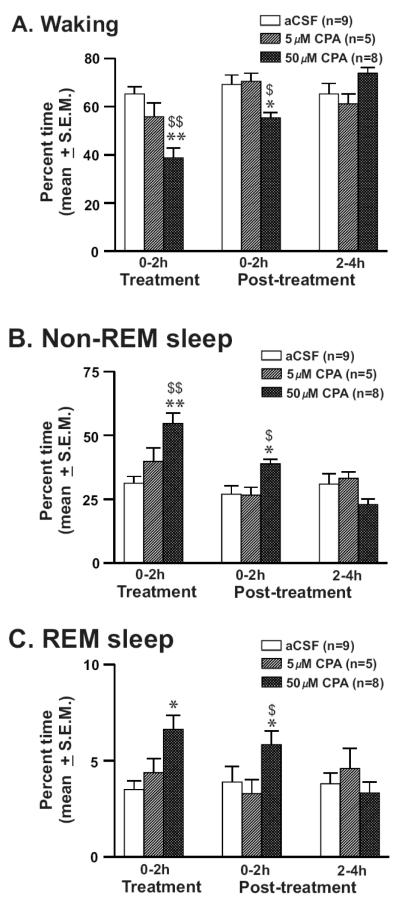
The effects of two doses of CPA vs. aCSF perfused into the PF-LHA on sleep-wakefulness were studied in a group of 9 rats. The sleep-wake profiles of rats microdialysed with aCSF (n=9) and CPA (5μM, n=5 and 50μM, n=8) on percent time (mean ± S.E.M.) spent in waking, nonREM, and REM sleep are shown in figure-4. aCSF treated rats were predominantly awake (66.6 ± 1.7%) and spent less time in nonREM (29.7 ± 1.4%) and REM sleep (3.7 ± 0.4%) during the 6h-recording period. There were no significant differences between 2hr treatment and 4hr post-treatment conditions. CPA perfusion significantly decreased the mean time spent in waking (F = 7.891; p <0.001) and increased the meantime spent in nonREM (F = 8.18; p <0.001) and REM sleep (F = 30.036; p = <0.001). Of the two doses used, 50μM CPA induced significantly greater suppression in waking and enhancement of nonREM and REM sleep, compared to both aCSF and 5μM CPA treatments during the 2h of its perfusion and the effects lasted up to the first 2h post-treatment. The sleep-wake profiles during the 3-4h post-treatment period in the three groups were not significantly different.
Figure-4. Effects of CPA on sleep-wakefulness.
Percent time (mean ± SEM) spent in waking (A), nonREM (B) and REM sleep (C) at 2h intervals during the 6h recording period including first 2h of treatment (aCSF, 5μM and 50μM CPA perfusion into the PF-LHA) and 4h of post-treatment recordings (aCSF perfusion). 50μM CPA suppressed waking and induced nonREM and REM sleep that lasted up to 2h post-treatment period. *, as compared to aCSF control; $, as compared to 5μM CPA. *, $, p<0.05; **, $$, p <0.01.
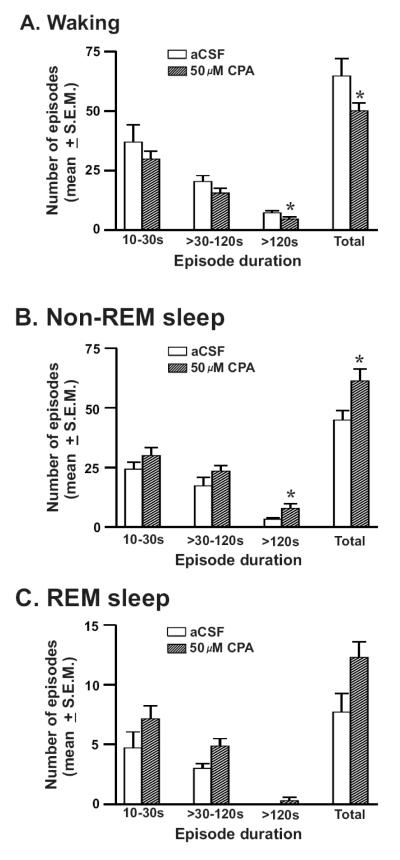
The frequencies of waking, nonREM and REM sleep episodes during aCSF and 50μM CPA perfusion during the 2h treatment period are shown in figure-5. The frequency of waking episodes decreased significantly in response to CPA due to a significant decrease in the number of long episodes (>120s). The number of nonREM sleep episodes in particular long episodes of nonREM sleep increased significantly. CPA perfusion also increased the frequency of REM sleep episodes, although not significantly (p=0.07). The frequency and duration of waking, nonREM and REM sleep episodes during post-treatment conditions after aCSF and 50μM CPA perfusion were comparable.
Figure-5. Effects of CPA on the frequency of sleep-wake episodes.
The frequency (mean ± SEM) of various durations of waking (A), nonREM (B) and REM sleep (C) episodes during aCSF and 50μM CPA perfusion into the PF-LHA. 50μM CPA produced a significant decrease in waking and concomitant increase in nonREM sleep episodes. *, p <0.05.
DISCUSSION
This study demonstrates that blockade of A1-receptor-mediated adenosinergic transmission in the PF-LHA by local perfusion of an adenosine A1 receptor antagonist produced arousal with concomitant reductions in nonREM and REM sleep in sleeping rats. Conversely, adenosine A1 receptor activation by its agonist suppressed waking and increased nonREM and REM sleep in awake animals. We note: a) that A1 receptor agonist and antagonist produced opposite and reversible behavioral effects; b) that the microdialysis drug administration method we used, unlike an earlier microinjection study, provides a better control on drug concentration and its continuous delivery in undisturbed animals and also reduces the likelihood that treatment effects could be due to mechanical or inflammatory responses (Quan and Blatteis, 1989; Thakkar et al., 2008); and c) that in this study the microdialysis probes used for delivering A1 receptor agonist and antagonist were localized in the PF-LHA (see figure-1) and based on our previous studies of Fos-IR in response to bicuculline and serotonin perfusions into the PF-LHA, it is likely that the perfused drugs affected neuronal population in ~500-750μm diameter field around the microdialysis probe (Alam et al., 2005; Kumar et al., 2007). We conclude, therefore, that the observed effects were physiological and that endogenous adenosine acting via A1 receptor plays a role in the regulation of sleep by inhibiting PF-LHA neurons.
Studies regarding adenosinergic control of sleep have largely been focused on the basal forebrain neurons (see introduction). The findings of the present study that application of an A1 receptor agonist and an antagonist in the PF-LHA induced and suppressed sleep, respectively, indicate that A1 receptor-mediated hypnogenic responses to adenosine are not confined to the basal forebrain, but can be observed in other brain regions including the PF-LHA (Oishi et al., 2008). These findings are consistent and complimentary to the previous report that microinjection of A1 receptor antagonist into the PF-LHA produced arousal and suppressed spontaneous nonREM/REM sleep as well as significantly increased the latency of nonREM sleep during recovery after sleep deprivation (Thakkar et al., 2008). We found that A1 receptor antagonist and agonist altered the number of medium and long episodes. The number of short waking episodes, which potentially reflects state initiations, was unaffected. This suggests that the majority of neurons in the PF-LHA affected by adenosine are involved in arousal maintenance.
The PF-LHA contains a predominant population of neurons that are involved in cortical activation and behavioral arousal. These include wake/REM-active and wake-active neurons that are quiescent during nonREM sleep and during both nonREM and REM sleep, respectively (see introduction). The extracellular release of adenosine has been linked with the metabolic state of the cell. Although the extracellular levels of adenosine in the PF-LHA during spontaneous or prolonged waking remain unknown, it is likely that the activation of PF-LHA neurons, including HCRT neurons, during arousal contributes to adenosine release locally. In addition, a recent study in transgenic mice in which purinergic gliotransmission was inhibited by selective expression of a dominant negative SNARE domain under doxycycline control, supports glia as a potent source of adenosine since these mice exhibited significantly reduced accumulation of sleep pressure and attenuated responses to adenosinergic A1 receptor antagonist (Halassa et al., 2009). The accumulated adenosine, irrespective of its source, would eventually inhibit wake-promoting neurons including HCRT neurons via A1 receptor to regulate sleep.
In a complimentary study we found that A1 receptor activation significantly suppressed the extracellular discharge activity of the recorded PF-LHA neurons (unpublished data). This finding along with the behavioral changes as observed in this study suggests that adenosine exerts its sleep-promoting effect in the PF-LHA via A1 receptor mediated inhibition of its neurons. That PF-LHA neurons are under adenosinergic influences is supported by an earlier in vitro study showing that adenosine via A1 receptor exerts inhibitory influences on HCRT neurons, most potently via presynaptic inhibition of the glutamatergic input or excitatory postsynaptic potential (Liu and Gao, 2007).
Anatomically, HCRT neurons project extensively to major arousal systems including the basal forebrain. Interestingly, a recent study found that as compared to the control group, adenosine levels in the basal forebrain did not increase with 6h of sleep deprivation in rats in which HCRT receptor bearing PF-LHA neurons were lesioned with HCRT-2-saporin conjugate. This finding suggests that the activation of neuronal population in the PF-LHA could be driving basal forebrain release of adenosine during arousal (Murillo-Rodriguez et al., 2008).
It is pertinent to note that in the PF-LHA, HCRT neurons constitute only a subset of wake-active neurons and that HCRT neurons are intermingled with various other neuronal groups including MCH and GABAergic neurons that have been implicated in the regulation of sleep (Alam et al., 2005; Estabrooke et al., 2001; Hassani et al., 2009; Kumar et al., 2005; Verret et al., 2003). However, evidence suggests that the net physiological output of the PF-LHA is wake promoting. For example, glutamic acid microinjections into the PF-LHA produce arousal and suppress both nonREM and REM sleep, whereas, rats with neurotoxic lesion of this area exhibit increased nonREM and REM sleep (Alam and Mallick, 2008; Gerashchenko et al., 2001). Although the relative distribution of A1 receptors on various neuronal groups, in particular on GABAergic and MCH neurons, remains unknown it is likely that behavioral changes observed in this study were predominantly mediated via blockade or activation of wake-promoting HCRT and other neurons of unknown neurotransmitter phenotypes. Alternatively, it is possible that adenosine promotes sleep in part: a) through the indirect disinhibition of sleep-active neurons within the PF-LHA; and /or b) by activating sleep-promoting neurons, e.g., MCH and GABAergic neurons via A2A receptors. Although the distribution of A2A receptors in the PF-LHA is not well characterized, the perfused concentrations of agonist and antagonist used in this study may affect A2A receptors as well (Fredholm et al., 2001).
In conclusion, the findings of this study support that PF-LHA is one of the sites where adenosine acting via A1 receptors, in part, inhibits wake-promoting neurons to promote sleep.
EXPERIMENTAL PROCEDURE
Experiments were conducted on 16 freely behaving Sprague-Dawley male rats. All the experiments were conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals and were approved by Veterans Administration Greater Los Angeles Healthcare System’s Institutional Animal Care and Use Committee. The rats were housed individually and maintained on 12:12 h light:dark cycle (lights on at 6:00AM) with food and water available ad libitum.
A. Surgical Implantation
The details of the surgical procedure and experimental protocol are described in earlier studies (Alam et al., 2005). In brief, rats (n=16) were stereotaxically implanted with a) EEG (electroencephalogram) and EMG (electromyogram) electrodes for the monitoring of the sleep-wake cycles, and b) a bilateral microdialysis guide cannulae (23G stainless steel tube) using the stereotaxic coordinates, A, −2.9 to −3.1; L, 1.4 to 1.6; H, 4.5 to 5.5 (Paxinos and Watson, 1998) such that their tips rested 3 mm above the dorsal aspect of the PF-LHA and were blocked with stylets. Experiments were started at least 10 days after surgery and after acclimatization of the animals with the recording environment during the recovery period.
B. Experimental protocol
At least 24h before the experiment, the stylets of the microdialysis guide cannulae were replaced by microdialysis probes (semi-permeable membrane tip length, 1mm; outer diameter, 0.22 mm; molecular cut off size, 50 kDa; Eicom, Japan), fixed with dental acrylic and flushed with artificial cerebrospinal fluid (aCSF; composition in mM, 145 NaCl, 2.7 KCl, 1.3 MgSO4, 1.2 CaCl2, and 2 Na2HPO4; pH, 7.2) at a flow rate of 2 μl/min for 4h. The time taken by the aCSF solution to travel from the reservoir to the tips of the probes were precisely calculated.
1. Effects of adenosine A1 receptor agonist/antagonist on sleep-wake cycle
Studies suggest that maximum number of PF-LHA neurons exhibit Fos-IR during lights-off period when rats spent significantly more time in waking (Espana et al., 2003; Estabrooke et al., 2001; Kumar et al., 2007). Since in vitro studies suggest that adenosine exerts inhibitory influences on HCRT neurons via A1 receptor (Liu and Gao, 2007), the effects of adenosine A1 receptor agonist, N6-cyclopentyladenosine (CPA), on sleep-wakefulness were examined during lights-off period between 8.00PM to 3.00AM. In contrast, the effects of A1 receptor antagonist, 1,3-dipropyl-8-phenylxanthine (CPDX) on sleep-wakefulness were examined during lights-on period, when rats are predominantly asleep. Both CPA and CPDX were dissolved in distilled water and 0.1N NaOH, respectively, at mM concentrations and then the stock solutions were diluted to μM concentrations for perfusion. The pH of the perfusing solution was adjusted to 7.2. The EEG and EMG of each animal were recorded during perfusion of aCSF and 2 doses of CPA (5μM and 50μM) or CPDX (5μM or 50μM) for 2h followed by 4h of aCSF perfusion. The order of aCSF or drug delivery was randomized and the treatments were spaced by at least 24h. In few cases, the probes either got blocked or leaked and therefore, animals did not receive all three treatments. In our earlier studies we found that microdialysis probes remained functional for 5-6 days after implantation(Alam et al., 1999; Kumar et al., 2007), therefore, each animal was used for 5-6 days in this study. The effects of CPA and CPDX were examined in different group of animals. At the end of the experiments, rats were sacrificed and the locations of the microdialysis probes were histologically confirmed.
After a lethal dose of pentobarbital (100 mg/kg, i.p.) rats were injected with heparin (500U, i.p.), and perfused transcardially with 30-50 ml of 0.1 M phosphate buffer (pH 7.2) followed by 500 ml of 4% paraformaldehyde in phosphate buffer containing 15% saturated picric acid solution. The brains were removed and equilibrated in 10%, 20% and finally 30% sucrose. Horizontal sections were freeze-cut at 30μm thickness. Alternate sections from the series of sections spanning the probe tract were immunostained for HCRT-1 protein (Alam et al., 2005; Kumar et al., 2007).
C. Data analyses
A single person who was unaware of the experimental conditions scored sleep-waking states in 10 sec epochs as waking, non-rapid eye movement (nonREM) sleep and REM sleep, according to the method described earlier (Alam and Mallick, 1990). The sleep-wake data were compared at 2h intervals; 2 hr of aCSF/drug treatments and 4h of post-treatment with aCSF perfusion. The level of significance amongst different treatment conditions was determined by using One Way Repeated Measures ANOVAs followed by pair-wise multiple comparisons using Holm-Sidak method. We found strongest effects of CPDX and CPA during the 2h treatment period and therefore, the frequencies of sleep-wake episodes during aCSF vs. effective doses of CPDX or CPA during treatment period were further compared using paired t-test.
ACKNOWLEDGEMENTS
This work was supported by the US Department of Veterans Affairs Medical Research Service and US National Institutes of Health grants, NS-050939 (Alam); MH47480, HL60296 (McGinty); and MH63323 (Szymusiak).
ABBREVIATIONS
- aCSF
Artificial cerebrospinal fluid
- CPA
N6-cyclopentyladenosine, an A1 receptor agonist
- CPDX
1,3-dipropyl-8-phenylxanthine, an A1 receptor antagonist
- Fos-IR
c-Fos protein immunoreactivity
- GABA
Gamma-aminobutyric acid
- HCRT
Hypocretin
- MCH
Melanin-concentrating hormone
- NonREM
Non-rapid eye movement sleep
- PF-LHA
Perifornical-lateral hypothalamic area
- TBS
Tris buffer saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE REFERENCES
- Alam MA, Mallick BN. Glutamic acid stimulation of the perifornical-lateral hypothalamic area promotes arousal and inhibits non-REM/REM sleep. Neurosci Lett. 2008;439:281–6. doi: 10.1016/j.neulet.2008.05.042. [DOI] [PubMed] [Google Scholar]
- Alam MN, Mallick BN. Differential acute influence of medial and lateral preoptic areas on sleep-wakefulness in freely moving rats. Brain Res. 1990;525:242–8. doi: 10.1016/0006-8993(90)90870-h. [DOI] [PubMed] [Google Scholar]
- Alam MN, Szymusiak R, Gong H, King J, McGinty D. Adenosinergic modulation of rat basal forebrain neurons during sleep and waking: neuronal recording with microdialysis. J Physiol. 1999;521(Pt 3):679–90. doi: 10.1111/j.1469-7793.1999.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MN, Gong H, Alam T, Jaganath R, McGinty D, Szymusiak R. Sleep-waking discharge patterns of neurons recorded in the rat perifornical lateral hypothalamic area. J Physiol. 2002;538:619–31. doi: 10.1113/jphysiol.2001.012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MN, Kumar S, Bashir T, Suntsova N, Methippara MM, Szymusiak R, McGinty D. GABA-mediated control of hypocretin- but not melanin-concentrating hormone-immunoreactive neurones during sleep in rats. J Physiol. 2005;563:569–82. doi: 10.1113/jphysiol.2004.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73:379–96. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Basheer R, Bauer A, Elmenhorst D, Ramesh V, McCarley RW. Sleep deprivation upregulates A1 adenosine receptors in the rat basal forebrain. Neuroreport. 2007;18:1895–9. doi: 10.1097/WNR.0b013e3282f262f6. [DOI] [PubMed] [Google Scholar]
- Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–60. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- Benington JH, Kodali SK, Heller HC. Stimulation of A1 adenosine receptors mimics the electroencephalographic effects of sleep deprivation. Brain Res. 1995;692:79–85. doi: 10.1016/0006-8993(95)00590-m. [DOI] [PubMed] [Google Scholar]
- Bennett HJ, Semba K. Immunohistochemical localization of caffeine-induced c-Fos protein expression in the rat brain. J Comp Neurol. 1998;401:89–108. doi: 10.1002/(sici)1096-9861(19981109)401:1<89::aid-cne6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Blanco-Centurion C, Xu M, Murillo-Rodriguez E, Gerashchenko D, Shiromani AM, Salin-Pascual RJ, Hof PR, Shiromani PJ. Adenosine and sleep homeostasis in the Basal forebrain. J Neurosci. 2006;26:8092–100. doi: 10.1523/JNEUROSCI.2181-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgin P, Huitron-Resendiz S, Spier AD, Fabre V, Morte B, Criado JR, Sutcliffe JG, Henriksen SJ, de Lecea L. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci. 2000;20:7760–5. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Datta S, Maclean RR. Neurobiological mechanisms for the regulation of mammalian sleep-wake behavior: reinterpretation of historical evidence and inclusion of contemporary cellular and molecular evidence. Neurosci Biobehav Rev. 2007;31:775–824. doi: 10.1016/j.neubiorev.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Espana RA, Valentino RJ, Berridge CW. Fos immunoreactivity in hypocretin-synthesizing and hypocretin-1 receptor-expressing neurons: effects of diurnal and nocturnal spontaneous waking, stress and hypocretin-1 administration. Neuroscience. 2003;121:201–17. doi: 10.1016/s0306-4522(03)00334-8. [DOI] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–62. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, AP IJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–52. [PMC free article] [PubMed] [Google Scholar]
- Gerashchenko D, Kohls MD, Greco M, Waleh NS, Salin-Pascual R, Kilduff TS, Lappi DA, Shiromani PJ. Hypocretin-2-saporin lesions of the lateral hypothalamus produce narcoleptic-like sleep behavior in the rat. J Neurosci. 2001;21:7273–83. doi: 10.1523/JNEUROSCI.21-18-07273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerashchenko D, Shiromani PJ. Different neuronal phenotypes in the lateral hypothalamus and their role in sleep and wakefulness. Mol Neurobiol. 2004;29:41–59. doi: 10.1385/MN:29:1:41. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–9. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–54. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Hassani OK, Lee MG, Jones BE. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc Natl Acad Sci U S A. 2009;106:2418–22. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol Sci. 2005;26:578–86. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Takahashi K, Kodama T, Kayama Y. State-dependent activity of neurons in the perifornical hypothalamic area during sleep and waking. Neuroscience. 2003;119:1209–19. doi: 10.1016/s0306-4522(03)00173-8. [DOI] [PubMed] [Google Scholar]
- Kumar S, Szymusiak R, Methippara MM, Seema R, Suntsova N, McGinty D, Alam MN. GABAergic and glutamatergic neurons in the perifornical lateral hypothalamic area exhibit differential Fos expression after sleep deprivation vs. recovery sleep. Sleep. 2005;29:A146. [Google Scholar]
- Kumar S, Szymusiak R, Bashir T, Rai S, McGinty D, Alam MN. Effects of serotonin on perifornical-lateral hypothalamic area neurons in rat. Eur J Neurosci. 2007;25:201–12. doi: 10.1111/j.1460-9568.2006.05268.x. [DOI] [PubMed] [Google Scholar]
- Landolt HP, Dijk DJ, Gaus SE, Borbely AA. Caffeine reduces low-frequency delta activity in the human sleep EEG. Neuropsychopharmacology. 1995;12:229–38. doi: 10.1016/0893-133X(94)00079-F. [DOI] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–20. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–76. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Liu ZW, Gao XB. Adenosine inhibits activity of hypocretin/orexin neurons by the A1 receptor in the lateral hypothalamus: a possible sleep-promoting effect. J Neurophysiol. 2007;97:837–48. doi: 10.1152/jn.00873.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquet P. Sleep function(s) and cerebral metabolism. Behav Brain Res. 1995;69:75–83. doi: 10.1016/0166-4328(95)00017-n. [DOI] [PubMed] [Google Scholar]
- McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med. 2007;8:302–30. doi: 10.1016/j.sleep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- McGinty D, Szymusiak R. Hypothalamic regulation of sleep and arousal. Front Biosci. 2003;8:s1074–83. doi: 10.2741/1159. [DOI] [PubMed] [Google Scholar]
- Methippara MM, Alam MN, Szymusiak R, McGinty D. Effects of lateral preoptic area application of orexin-A on sleep-wakefulness. Neuroreport. 2000;11:3423–6. doi: 10.1097/00001756-200011090-00004. [DOI] [PubMed] [Google Scholar]
- Methippara MM, Kumar S, Alam MN, Szymusiak R, McGinty D. Effects on sleep of microdialysis of adenosine A1 and A2a receptor analogs into the lateral preoptic area of rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1715–23. doi: 10.1152/ajpregu.00247.2005. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–98. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modirrousta M, Mainville L, Jones BE. Orexin and MCH neurons express c-Fos differently after sleep deprivation vs. recovery and bear different adrenergic receptors. Eur J Neurosci. 2005;21:2807–16. doi: 10.1111/j.1460-9568.2005.04104.x. [DOI] [PubMed] [Google Scholar]
- Murillo-Rodriguez E, Liu M, Blanco-Centurion C, Shiromani PJ. Effects of hypocretin (orexin) neuronal loss on sleep and extracellular adenosine levels in the rat basal forebrain. Eur J Neurosci. 2008;28:1191–8. doi: 10.1111/j.1460-9568.2008.06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi Y, Huang ZL, Fredholm BB, Urade Y, Hayaishi O. Adenosine in the tuberomammillary nucleus inhibits the histaminergic system via A1 receptors and promotes non-rapid eye movement sleep. Proc Natl Acad Sci U S A. 2008;105:19992–7. doi: 10.1073/pnas.0810926105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain: in stereotaxic coordinates. Academic Press; 1998. Vol. [DOI] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–7. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Quan N, Blatteis CM. Microdialysis: a system for localized drug delivery into the brain. Brain Res Bull. 1989;22:621–5. doi: 10.1016/0361-9230(89)90080-4. [DOI] [PubMed] [Google Scholar]
- Radulovacki M, Virus RM, Djuricic-Nedelson M, Green RD. Adenosine analogs and sleep in rats. J Pharmacol Exp Ther. 1984;228:268–74. [PubMed] [Google Scholar]
- Ribeiro JA, Sebastiao AM, de Mendonca A. Adenosine receptors in the nervous system: pathophysiological implications. Prog Neurobiol. 2002;68:377–92. doi: 10.1016/s0301-0082(02)00155-7. [DOI] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–81. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Scharf MT, Naidoo N, Zimmerman JE, Pack AI. The energy hypothesis of sleep revisited. Prog Neurobiol. 2008 doi: 10.1016/j.pneurobio.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM. Hypocretin (orexin): role in normal behavior and neuropathology. Ann Rev Psychol. 2004;55:125–148. doi: 10.1146/annurev.psych.55.090902.141545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Lin JS, Sakai K. Neuronal activity of orexin and non-orexin waking-active neurons during wake-sleep states in the mouse. Neuroscience. 2008;153:860–70. doi: 10.1016/j.neuroscience.2008.02.058. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Ramesh V, Strecker RE, McCarley RW. Microdialysis perfusion of orexin-A in the basal forebrain increases wakefulness in freely behaving rats. Arch Ital Biol. 2001;139:313–28. [PubMed] [Google Scholar]
- Thakkar MM, Winston S, McCarley RW. Orexin neurons of the hypothalamus express adenosine A1 receptors. Brain Res. 2002;944:190–4. doi: 10.1016/s0006-8993(02)02873-1. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Delgiacco RA, Strecker RE, McCarley RW. Adenosinergic inhibition of basal forebrain wakefulness-active neurons: a simultaneous unit recording and microdialysis study in freely behaving cats. Neuroscience. 2003a;122:1107–13. doi: 10.1016/j.neuroscience.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Winston S, McCarley RW. A1 receptor and adenosinergic homeostatic regulation of sleep-wakefulness: effects of antisense to the A1 receptor in the cholinergic basal forebrain. J Neurosci. 2003b;23:4278–87. doi: 10.1523/JNEUROSCI.23-10-04278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM, Engemann SC, Walsh KM, Sahota PK. Adenosine and the homeostatic control of sleep: effects of A1 receptor blockade in the perifornical lateral hypothalamus on sleep-wakefulness. Neuroscience. 2008;153:875–80. doi: 10.1016/j.neuroscience.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–74. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verret L, Goutagny R, Fort P, Cagnon L, Salvert D, Leger L, Boissard R, Salin P, Peyron C, Luppi PH. A role of melanin-concentrating hormone producing neurons in the central regulation of paradoxical sleep. BMC Neurosci. 2003;4:19. doi: 10.1186/1471-2202-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]