Abstract
The transcription factor, Nuclear Factor-κB (NF-κB), regulates many genes involved in host immunity and cell survival. Unregulated NF-κB activity has been linked to many chronic inflammatory diseases and is an important target for identification of inhibitors to better manage these disorders. We present a novel screening system to identify NF-κB inhibitors that combines sensitive fluorescence detection with medium- to high-throughput flow cytometry (HyperCyt®). To validate this approach, we quantified activation of NF-κB by standard flow cytometry and the HyperCyt® platform. Results were comparable with regard to EC50 values for TNFα-mediated activation; however, the HyperCyt® platform provided more sensitive signal detection and a greater linear range for detection. To demonstrate the usefulness of this screening tool, we identified a novel inhibitor of NF-κB activation from a resveratrol-based chemical library. Inhibition of NF-κB activation by analog 6q (IC50 = 19 μM) showed a 3.7-fold improvement over that of resveratrol (IC50 ~70 μM).
Keywords: Jurkat, NF-κB, GFP, reporter, resveratrol, luteolin, flow cytometry
Background
Nuclear Factor-κB (NF-κB) is a transcription factor that activates expression of many genes involved in inflammation [1] and apoptosis [2]. Because of this, the signaling pathways that lead to activation of this transcription factor are critical for maintaining host immunity and for cell survival. Conversely, unregulated or constitutively activated NF-κB has been linked to tumorigenesis and many chronic inflammatory diseases, such as Parkinson’s disease [3], inflammatory bowel disease [4], arthritis [5] and asthma [6]. The underlying mechanism as to how NF-κB becomes unregulated in many of these disease states remains unknown yet continues to be an area of active investigation.
Clinical management of the chronic inflammatory conditions resulting from dysregulation of NF-κB activity relies on identification of potent and safe inhibitors of NF-κB activation. To date, hundreds of inhibitors of the NF-κB pathway have been identified, including natural and synthetic molecules [7,8]. In most cases, the targets of these inhibitors are unknown owing to the complex nature of the NF-κB signaling pathway . In spite of the number of inhibitors identified, the majority have not translated into good lead compounds due to poor predicted bioavailability and/or measurable toxicities. This has severely impacted the number of compounds moving forward into clinical trials. Nevertheless, even with these hurdles there is still warranted interest in identifying new, safer inhibitors of NF-κB activation because of the central role this transcription factor plays in many human pathologies.
Typical screening regimens designed to detect the most potent inhibitors of NF-κB activation are very costly and labor intensive. In lieu of more traditional approaches, high throughput screening assays are becoming increasingly prevalent and provide the opportunity to screen many more compounds in a shorter period of time; a need of critical importance when considering some chemical libraries contain millions of compounds. To accommodate this need, we present here a novel NF-κB reporter cell line that can be used in conjunction with HyperCyt® flow cytometry for medium- to high-throughput screening regimens.
A number of other NF-κB reporter cell lines are commercially available but these rely on luciferase activity to monitor NF-κB activation. Although luciferase has a proven track record as a good reporter, sample processing is labor intensive and time consuming which limits the expandability to testing large numbers of potential inhibitors. We have chosen green fluorescent protein (GFP) as our reporter because of its high sensitivity and linearity and it is less cumbersome to measure since it does not require cell lysis or substrate hydrolysis; rather, fluorescence is directly measured using intact cells [9,10]. We have also chosen to use the Jurkat cell line as our host for the NF-an immortalized line of human [11]. This will ensure a robust NF-κB signaling response upon stimulation for maximal GFP expression. Moreover, Jurkat cells grow well as suspension cultures which will eliminate handling difficulties by flow cytometry procedures. Other NF-κB reporter systems use attachment-dependent cell lines, such as MEF, HeLa, HEK293, NIH3T3 and C2C12 cell lines, which tend to aggregate when detached and impede the cell stream passing through the flow cytometer’s fluidic channel.
In this study, we provide evidence that our NF-κB/GFP-Jurkat reporter cell line is a novel and useful tool that can be adapted to high-throughput screening of inhibitors of NF-κB activation through the use of flow cytometry. This advancement is anticipated to accelerate the discovery of unique inhibitors of the NF-κB pathway for future clinical applications.
Methods
Reagents
TNFα was obtained by R&D Systems (Minneapolis, MN) and was used in all experiments to activate hrGFP expression in recombinant Jurkat clones. Hygromycin B, Streptomyces sp. (Calbiochem, La Jolla, CA) was used for the selection of recombinant Jurkat clones. Luteolin was purchased from Sigma (St. Louis, MO). Resveratrol was purchased from A.G. Scientific Inc. (San Diego, CA) and analog 6q was synthesized in the lab [12].
Cell culture
Human Jurkat T-lymphocytes were obtained from American Type Culture Collection (Manassas, VA) and grown in RPMI-1640 (Thermo Scientific HyClone®, Logan, UT) supplemented with 10% (v/v) Fetal Bovine Serum (FBS) (Irvine Scientific, Santa Ana, CA), 1 mM sodium pyruvate, 2 mM L-glutamine, 100 μg/ml streptomycin sulfate, and 100 units/ml penicillin. Cells were cultured at 37°C with 5% CO2 and passaged twice weekly.
Transfection and expansion of transformed Jurkat cells
Jurkat cells were grown in complete medium and subcultured 24 h prior to electroporation. Cells were washed in Phosphate Buffered Saline (PBS), pH 7.0, and then suspended in HeBs electroporation buffer (20 mM Hepes, 137 mM NaCl, 5 mM KCl, 0.7 mM Na2HPO4, 6 mM dextrose, pH 7.04) at a density of 1.25 × 107 cells/ml (800 μL final volume). Cells were electroporated with 40 μg PathDetect® cis-reporter plasmid pNF-κB/hrGFP (Stratagene, La Jolla, CA) using a Gene Pulser® II system (Bio-Rad, Hercules, CA) with the following parameters: Gene Pulser® cuvette with a 0.4 cm electrode gap, 0.40 kV, 960 μF, ∞ Ω and a field strength of 1.0 kV/cm. After electroporation, cells were immediately placed in complete culture media and incubated for 48 h at 37°C, 5% CO2 for recovery. Cells were then split into complete media containing 1.5 mg/ml Hygromycin B. The optimal concentration of Hygromycin B was determined by performing a death curve with untransfected Jurkat cells (data not shown). Individual clones were isolated by single cell deposition using the Cytomation MoFlo High Speed Sorter (Beckman Coulter, Inc. Fullerton, CA) and cultured in complete medium containing Hygromycin B.
Measurement of hrGFP expression and flow cytometry
Recombinant Jurkat clones were cultured in 24 well-plates with a seeding density of 2 × 106 cells/ml. Cells were then incubated without or with the indicated concentrations of TNFα or, alternatively, incubated with TNFα (20 ng/ml) together with either resveratrol, analog 6q, luteolin, or vehicle alone (dimethylsulfoxide, DMSO, at 0.1% final concentration) in complete medium for 24 h. Cells were harvested and centrifuged at 800 rpm for 10 min at 4° C. Cells were suspended in PBS at a density of 6 × 106 cells/ml and flow cytometry was performed using a FACScan flow cytometer (λexcitation 488nm; λemission 585nm) to record GFP fluorescence or added to wells of a 96-well plate and used for HyperCyt® flow cytometry.
Cytotoxicity assay
Cells were grown in 96-well plates with a seeding density of 1 × 106 cells/ml in complete media containing the indicated concentrations of resveratrol, analog 6q, luteolin, or vehicle alone (DMSO at 0.1% final concentration). After a 24 h incubation, WST-1 (Roche Molecular Biochemicals, Indianapolis, IN) was added to the cultures to a final concentration of 10% (v/v). Following an additional incubation at 37°C for 60 min, absorbance was recorded with a Genesys 10 uv spectrophotometer (Rochester, NY) (450 nm; reference wavelength, 690 nm).
Data analysis
All data aquired from the HyperCyt® flow cytometry system was analyzed using IDLeQuery© analysis software. All experimental protocols were done in at least triplicates and error bars represent standard deviations of mean values.
Results
The Jurkat cells used in this study were stably transfected with a pNF-κB/hrGFP cis-reporter plasmid. This plasmid contains five tandem repeats of the NF-κB consensus binding sequence placed immediately upstream from the humanized renilla GFP (hrGFP) coding sequence. hrGFP was developed from Renilla renifomis and modified from the more common enhanced GFP (EGFP) so as to use human codons for translation in mammalian expression systems. In contrast to EGFP used in other reporter systems, hrGFP has lower cytotoxicity [13]. This important attribute avoids undesirable alterations in gene expression profile that often arise from the high cytotoxicity of EFGP. Moreover, hrGFP expression results in markedly high-level fluorescence that can be easily quantified by flow cytometry. Clonal populations of stably transfected cells were obtained from single cell isolates using a high speed fluorescence-activated cell sorter.
Comparison of standard flow cytometry and HyperCyt® measurements
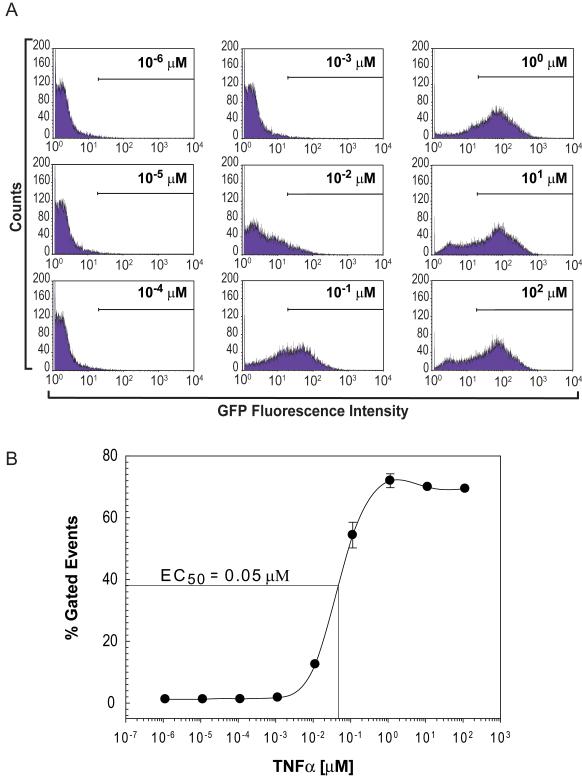
To assess if our NF-κB/hrGFP reporter is functional in Jurkat cells, we activated the NF-κB signaling pathway by stimulating cells with various amounts of TNFα and measured hrGFP fluorescence by standard flow cytometry (Fig. 1). We found a dose-dependent relationship between the concentration of TNFα applied to cells and hrGPF fluorescence as measured by individual cell counts (gated events surpassing a fluorescence intensity set at 2 × 101). From these data, we calculate an EC50 value of 0.05 μM for TNFα-mediate activation of the NF-κB signaling pathway in Jurkat cells. Moreover, full activation of the NF-κB pathway in the reporter cell line (Fig. 1A, panels G-I) resulted in an increase in hrGFP fluorescence by two orders of magnitude from baseline values demonstrating a large dynamic range for quantification.
Figure 1. Dose-dependent TNFα-activation of NF-κB/hrGFP expression in Jurkat cells: Quantification by flow cytometry.
Jurkat cells, stably transfected with pNF-κB/hrGFP reporter plasmid, were incubated without or with the indicated concentrations of TNFα for 24 h. A) Cells were harvested and standard flow cytometry measurements were made (hrGFP fluorescence was measured at λexcitation 488nm; λemission 585nm). Shown are one-parameter histogram analyses for each concentration of TNFα used. Minimum gate was set at a fluorescence intensity of 2 × 101 to exclude autofluorescence values of unstimulated cells. B) Graph represents percent of gated events exceeding the 2 × 101 minimum fluorescence threshold for each concentration of TNFα used in (A). Error bars represent standard deviations of triplicate values.
We next measured TNFα-mediated activation of the NF-κB/hrGFP reporter system using a high-throughput assay format; this being the HyperCyt® Autosampler. The HyperCyt® platform is designed for rapid high-throughput analysis of hundreds of experimental points by interfacing a flow cytometer and autosampler [14]. With this robotic configuration, cells are aspirated from microplate wells and delivered to the flow cytometer for quantification. Briefly, a sampling probe moves from one well to the next aspirating cell suspensions with a peristaltic pump. Between wells the pump runs continuously drawing an air bubble into the sample line to demarcate individual samples. The samples are delivered in a continuous stream to the flow cytometry for time-resolved data collection. As shown in figure 2, and consistent with data obtained from standard flow cytometry measurements, treatment of cells with increasing amounts of TNFα corresponded to an increase in mean GFP-fluorescence intensity. The calculated EC50 value of 0.15 μM using the HyperCyt® was comparable to that determined by standard flow cytometry.
Figure 2. Dose-dependent TNFα-activation of NF-κB/hrGFP expression in Jurkat cells: Quantification by the HyperCyt® platform.
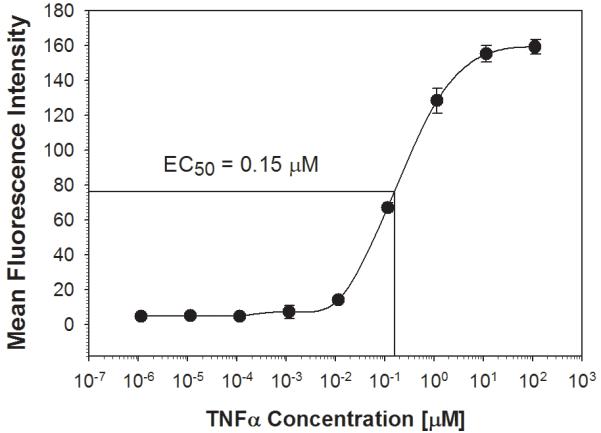
Stably transfected Jurkat cells were plated at 6 × 106 cells/ml in a 96-well plate and incubated without or with the indicated concentrations of TNFα as in figure 1. The HyperCyt® Autosampler was used to measure hrGFP fluorescence. The graph represents mean fluorescence intensity of time-gated cells at each concentration of TNFα and error bars represent standard deviations of triplicate values.
Validation of the HyperCyt® platform for identification of inhibitors of NF-κB activation
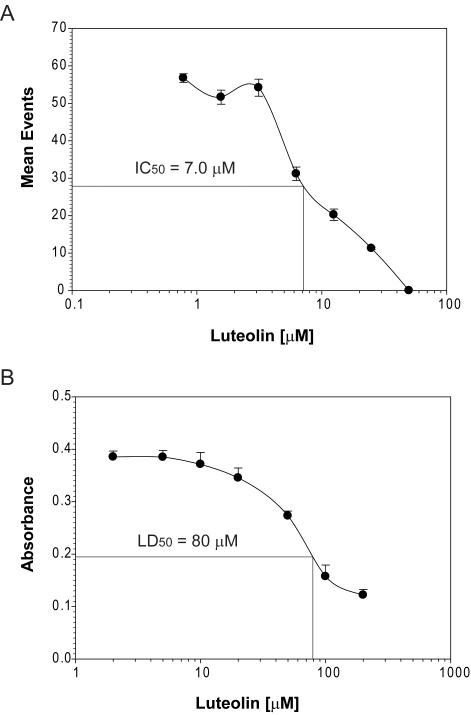
Identifying inhibitors of NF-κB signaling has been pursued using standard luciferase reporter assays; however, these assays are time-consuming and labor intensive, making them less useful for high-throughput analyses. We propose that our newly developed NF-κB/hrGFP reporter system would be more useful for medium- and high-throughput assays for inhibitor identification because of its utility in the HyperCyt® platform. To examine this potential, we used HyperCyt® to confirm the inhibition of NF-κB activation by the natural product, luteolin. Luteolin is a documented anti-inflammatory compound [15] and potent inhibitor of activation of NF-κB signaling [16]. Jurkat reporter cells were incubated with TNFα (20 ng/ml) in the absence or presence of the indicated concentrations of luteolin. hrGFP fluorescence was then quantified using the HyperCyt® flow cytometry platform. As shown in figure 3A, luteolin inhibited TNFα activation of NF-κB in a dose-dependent manner. The IC50 value was calculated as 7.0 μM which is in agreement with prior measurements [17].
Figure 3. Luteolin inhibits TNFα-stimulated hrGFP expression in a dose dependent manner and demonstrates cytotoxicity only at high concentrations in Jurkat cells.
(A) NF-κB-dependent hrGFP expression in stably transfected Jurkat cells was stimulated with 20 luteolin for 24 h. Cells were harvested and subjected to HyperCyt® flow cytometric analysis. Cells treated with TNFα alone (control) resulted in a mean value of 56. (B) Cells were incubated with luteolin as in (A). Following a 24 h incubation, WST-1 reagent was added to cultures and incubated for one additional hour. Absorbance was then measured by spectrophotometry (460 nm, reference wavelength 690 nm). Cells incubated without luteolin (control) generated a value of 0.39 ± 0.01 absorbance units. Error bars represent standard deviations of triplicate values.
When screening for inhibitors of NF-κB activation, it is also important to test for cytotoxicity in parallel to rule out potential false-positives. Toward this end, we incubated Jurkat reporter cells with various concentrations of luteolin and measured cell viability by assessing their metabolic state using the WST-1 assay. Luteolin showed some cytotoxicity at high concentrations (Fig. 3B, LD50 = 80 μM); however, these cytotoxic concentrations are much greater than those required to inhibit NF-κB activation.
Identification of a novel inhibitor of NF-κB activation using the HyperCyt® platform
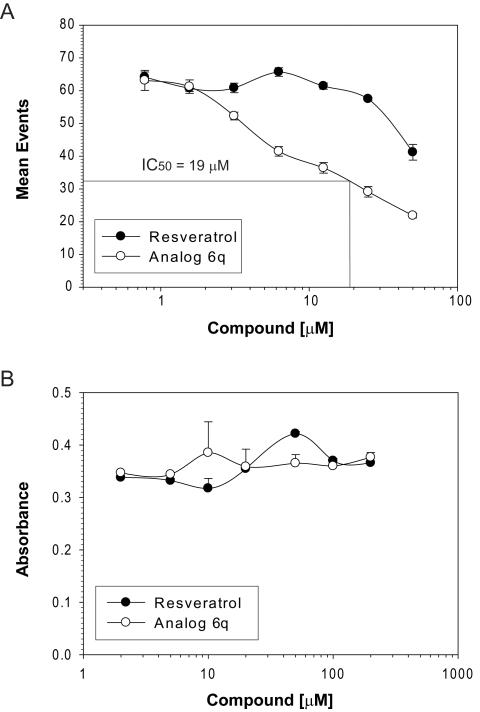
The natural product, resveratrol, is a now well established inhibitor of NF-κB activation [18-20]. Reasoning that the chemical structure of resveratrol can be modified to improve upon its capacity toward NF-κB inhibition, we have constructed a chemical library of resveratrol analogs [12] with the objective of identifying novel, more potent inhibitors than the parent compound. In a preliminary screen of this chemical library, analog 6q appeared to show improved properties as an anti-inflammatory (unpublished observation). To confirm this observation and quantify the inhibitory activity of 6q for NF-κB activation, we compared resveratrol and 6q in parallel using the HyperCyt® platform. Reporter cells were incubated with 6q at the indicated concentrations, followed by measuring hrGFP fluorescence with the HyperCyt® flow cytometry system. As expected, resveratrol inhibited NF-κB activation in a dose-dependent manner. Extrapolation of the data indicates an IC50 value of ~70 μM (Fig. 4A). Importantly, analog 6q inhibited NF-κB activation with an IC50 value of 19 μM, a 3.7-fold improvement over the parent compound.
Figure 4. Identification of an improved inhibitor of NF-κB activation that shows little or no cytotoxicity in Jurkat cells.
(A) NF-κB-dependent hrGFP expression in stably transfected Jurkat cells was stimulated with 20 ng/ml TNFα. In parallel, cells were incubated for 24 h without or with the indicated concentrations of resveratrol or its structural analog, 6q. Cells were harvested and subjected to HyperCyt® flow cytometric analysis. Cells treated with TNFα alone (control) resulted in a mean value of 64. (B) Cells were incubated with resveratrol or 6q as in (A). Following a 24 h incubation, WST-1 reagent was added to cultures, incubated for one additional hour and absorbance measured by spectrophotometry as in figure 3. Cells incubated without luteolin (control) generated a value of 0.37 ± 0.01 absorbance units. Error bars represent standard deviations of triplicate values.
We also tested for cytotoxicity following incubation of reporter cells with resveratrol or 6q. Both resveratrol and analog 6q showed little or no cytoxicity, even when cells were incubated with concentrations as high as 200 μM (Fig. 4B).
Discussion
NF-κB is a vital transcription factor that when activated mobilizes the innate immune system to protect against harmful agents. Under quiescent conditions, NF-κB is a heterodimer combining two of the five members of this gene family, the most prevalent of which is a dimer consisting of p50 and p65. This heterodimer is retained in the cytosol by complexing with a set of inhibitor proteins designated IκB. In response to a variety of extracellular stimuli, IκB becomes phosphorylated by IκB kinase (IKK). Once IκB is phosphorylated it dissociates from NF-κB and is sent to the proteosome for further degregation. Active NF-κB, in an unbound state, is then able to translocate to the nucleus where it binds to promoter regions of DNA initiating a cascade of gene transcription involved in inflammation. Besides controlling acute inflammatory responses, NF-κB activity has also been implicated in sustaining chronic inflammatory events, apoptosis and cell proliferation [2,21]. Because of these pleiotropic roles, it is now thought that NF-κB activation is a convergence point for multiple signal transduction pathways. As a result of its position at the focal point of many critical cellular functions, dysregulation of NF-κB activity can lead to numerous disease states. For this reason, inhibition of the NF-κB activation has become an attractive target for therapeutic intervention and a major focus for drug development in industry and academics [21,22]. However, it also must be kept in mind that NF-κB activation is also responsible for triggering peripheral immune responses that are critical for maintaining health. Therefore, in approaching NF-κB as a therapeutic target, there needs to be a balance between treating human disease and unfavorable side-effects. With this in mind, the goal should be to identify therapeutic compounds that reduce NF-κB activation as opposed to complete inhibition, thus preserving peripheral immune capacity while limiting chronic inflammatory events associated with disease.
We report here the construction and validation of a novel reporter system using Jurkat cells for screening chemical libraries to identify new inhibitors of the NF-κB signaling pathway. Previous screening regimens have relied upon labor-intensive luciferase based assays. This new reporter system combines high hrGFP signal intensity, direct measurement without a need for cell lysis or substrate addition, and the HyperCyt® flow cytometry platform, all necessary elements for medium- to high-throughput screening capabilities. Moreover, since Jurkat cells grow well in suspension culture, there are markedly easier to handle for flow cytometric measurements as compared with adherent cells. Consistent single cell suspensions are difficult to attain with adherent cells; formation of multicell aggregates often impede the fluid stream in the sheath of the flow cytometer. Such difficulties will likely compromise large scale, high-throughput screening regimens. To validate this new screening tool, we compared hrGFP fluorescence, following activation of NF-κB signaling with TNFα, between standard flow cytometry and the HyperCyt® platform. As expected, with traditional flow cytometry we readily quantified increases in hrGFP fluorescence resulting from TNFα stimulation and obtained sufficient data to calculate an EC50 value of 0.05 μM. In the HyperCyt® system, TNFα stimulation also showed a dose-dependent response in hrGFP fluorescence with a calculated EC50 value of 0.15 μM. These values confirm that the HyperCyt® platform is able to quantify a biological response with similar measure as standard flow cytometry. In addition, the signal detected by the HyperCyt® platform for maximal hrGFP expression was found to increase ~35.5-fold over that measured for unstimulated cells, whereas standard flow cytometry only detected a ~15-fold increase from baseline values. The HyperCyt® platform is thus more sensitive than conventional flow cytometry and capable of detecting hrGFP fluorescence over a greater linear range.
We next determined if we are able to quantify the activity of known NF-κB inhibitors and identify a novel inhibitor from our small molecule library using our NF-κB/hrGFP reporter system. For this we examined two natural products with established anti-inflammatory activities, luteolin and resveratrol. Luteolin is a tetrahydroxyflavone that is commonly found in celery, green pepper, and chamomile tea [23]. Luteolin prevents NF-κB signaling by inhibiting TNFα-mediated activation of IκB kinase [15,16] which in turn blocks IκB phosphorylation/degradation. Resveratrol is a polyphenolic phytoalexin and is found in relatively high concentrations in certain varietals of grapes. Resveratrol has reported anti-cancer, anti-aging, and anti-inflammatory activities [24-26]. It is known to inhibit TNFα-stimulation of NF-κB activation [12,27] by targeting IκB kinase activity [28,29]. Using the Jurkat-based reporter system, we found that both luteolin and resveratrol reduced TNFα-stimulated NF-κB activation in a dose-dependent manner with IC50 values of 7.0 and ~70 μM, respectively. To determine if our NF-κB/hrGFP reporter system is capable of identifying novel inhibitors of NF-κB signaling, we measured particular compounds in our resveratrol-based chemical library to identify inhibitors with improved activity when measured against the parent compound. This chemical library consists of a series of substituted trans-stilbenes based upon the structure of resveratrol [12]. We found one analog, 6q, which demonstrated improved inhibitory activity with a calculated IC50 value of 19 μM, ~3.7-fold greater than that measured for resveratrol and a value that approaches luteolin.
The results presented here provide proof-of-principle that our Jurkat NF-κB/hrGFP reporter system is capable of providing precise, quantitative data to identify novel inhibitors of NF-κB activation using the high-throughput format of HyperCyt® technology. Because of its large dynamic range, hrGFP detection by HyperCyt® presents the opportunity to identify potential therapeutic compounds that reduce NF-κB activity as opposed to its complete inhibition. With this approach we can expect to better preserve the peripheral immune response to stave off normal infections while reducing unregulated NF-κB activity that is responsible for the many diseases associated with this central transcription factor.
Acknowledgements
We thank Drs. David L. Vander Jagt and Lorraine Deck for providing access to the resveratrol analog library. This work was supported by the National Institutes of Health Grant AG027794 (to R.A.O.). Data was generated in the Flow Cytometry Shared Resource Center supported by the University of New Mexico Health Sciences Center and the University of New Mexico Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–34. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 2.Van Antwerp DJ, et al. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–9. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 3.Hunot S, et al. Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with parkinson disease. Proc Natl Acad Sci U S A. 1997;94:7531–6. doi: 10.1073/pnas.94.14.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Segain JP, et al. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut. 2000;47:397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simmonds RE, Foxwell BM. Signalling, inflammation and arthritis: NF-kappaB and its relevance to arthritis and inflammation. Rheumatology (Oxford) 2008;47:584–90. doi: 10.1093/rheumatology/kem298. [DOI] [PubMed] [Google Scholar]
- 6.Hart LA, et al. Activation and localization of transcription factor, nuclear factor-kappaB, in asthma. Am J Respir Crit Care Med. 1998;158:1585–92. doi: 10.1164/ajrccm.158.5.9706116. [DOI] [PubMed] [Google Scholar]
- 7.Egan LJ, Toruner M. NF-kappaB signaling: pros and cons of altering NF-kappaB as a therapeutic approach. Ann N Y Acad Sci. 2006;1072:114–22. doi: 10.1196/annals.1326.009. [DOI] [PubMed] [Google Scholar]
- 8.Gilmore TD, Herscovitch M. Inhibitors of NF-kappaB signaling: 785 and counting. Oncogene. 2006;25:6887–99. doi: 10.1038/sj.onc.1209982. [DOI] [PubMed] [Google Scholar]
- 9.Chalfie M, et al. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–5. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 10.Tsien RY. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–44. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- 11.Abraham RT, Weiss A. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat Rev Immunol. 2004;4:301–8. doi: 10.1038/nri1330. [DOI] [PubMed] [Google Scholar]
- 12.Heynekamp J, et al. Substituted trans-Stilbenes, Including Analogs of the Natural Product Resveratrol, Inhibit the TNFα-induced Activation of Transcription Factor NF-κB. J. Med. Chem. 2006;49:7182–89. doi: 10.1021/jm060630x. [DOI] [PubMed] [Google Scholar]
- 13.Hanazono Y, et al. Green fluorescent protein retroviral vectors: low titer and high recombination frequency suggest a selective disadvantage. Hum Gene Ther. 1997;8:1313–9. doi: 10.1089/hum.1997.8.11-1313. [DOI] [PubMed] [Google Scholar]
- 14.Young SM, et al. High-throughput screening with HyperCyt flow cytometry to detect small molecule formylpeptide receptor ligands. J Biomol Screen. 2005;10:374–82. doi: 10.1177/1087057105274532. [DOI] [PubMed] [Google Scholar]
- 15.Chen CY, et al. Luteolin suppresses inflammation-associated gene expression by blocking NF-kappaB and AP-1 activation pathway in mouse alveolar macrophages. Life Sci. 2007;81:1602–14. doi: 10.1016/j.lfs.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JS, Jobin C. The flavonoid luteolin prevents lipopolysaccharide-induced NF-kappaB signalling and gene expression by blocking IkappaB kinase activity in intestinal epithelial cells and bone-marrow derived dendritic cells. Immunology. 2005;115:375–87. doi: 10.1111/j.1365-2567.2005.02156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen HQ, et al. Luteolin protects dopaminergic neurons from inflammation-induced injury through inhibition of microglial activation. Neurosci Lett. 2008;448:175–9. doi: 10.1016/j.neulet.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 18.Nam NH. Naturally occurring NF-kappaB inhibitors. Mini Rev Med Chem. 2006;6:945–51. doi: 10.2174/138955706777934937. [DOI] [PubMed] [Google Scholar]
- 19.Manna SK, et al. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;164:6509–19. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- 20.Faith SA, et al. Resveratrol suppresses nuclear factor-kappaB in herpes simplex virus infected cells. Antiviral Res. 2006;72:242–51. doi: 10.1016/j.antiviral.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Magne N, et al. NF-kappaB modulation and ionizing radiation: mechanisms and future directions for cancer treatment. Cancer Lett. 2006;231:158–68. doi: 10.1016/j.canlet.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 22.Haefner B. Targeting NF-kappaB in anticancer adjunctive chemotherapy. Cancer Treat Res. 2006;130:219–45. doi: 10.1007/0-387-26283-0_10. [DOI] [PubMed] [Google Scholar]
- 23.Shimoi K, et al. Intestinal absorption of luteolin and luteolin 7-O-beta-glucoside in rats and humans. FEBS Lett. 1998;438:220–4. doi: 10.1016/s0014-5793(98)01304-0. [DOI] [PubMed] [Google Scholar]
- 24.Aggarwal BB, et al. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004;24:2783–840. [PubMed] [Google Scholar]
- 25.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Sovak M. Grape extract, resveratrol, and its analogs: a review. J. Med. Food. 2001;4:93–104. doi: 10.1089/109662001300341752. [DOI] [PubMed] [Google Scholar]
- 27.Das DK, Maulik N. Resveratrol in cardioprotection: a therapeutic promise of alternative medicine. Mol Interv. 2006;6:36–47. doi: 10.1124/mi.6.1.7. [DOI] [PubMed] [Google Scholar]
- 28.Redell MS, Tweardy DJ. Targeting transcription factors for cancer therapy. Curr Pharm Des. 2005;11:2873–87. doi: 10.2174/1381612054546699. [DOI] [PubMed] [Google Scholar]
- 29.Kundu JK, Surh YJ. Molecular basis of chemoprevention by resveratrol: NF-kappaB and AP-1 as potential targets. Mutat Res. 2004;555:65–80. doi: 10.1016/j.mrfmmm.2004.05.019. [DOI] [PubMed] [Google Scholar]