Abstract
This study was designed to establish whether 3,3′-Diindolylmethane (DIM) can inhibit cervical lesions, alter estrogen metabolism in favor of C-2 hydroxylation and enhance immune function in the K14-HPV16 transgenic mouse model. Mice were bred, genotyped, implanted with E2 pellets (0.25mg/90 day release) under anesthesia and divided into groups. Wild type and transgenic mice were given either AIN76A diet alone or with 2000ppm DIM for 12weeks. Blood and reproductive tracts were obtained. Blood was analyzed for estrogen metabolites and IFN-gamma. The cervical transformation zone was sectioned and stained for histology. Estradiol C-2 hydroxylation and serum IFN-gamma levels were significantly increased over controls in wild type and transgenic mice receiving DIM. In wild type mice without DIM, hyperplasia of the squamous epithelium was observed. Wild type mice fed DIM displayed a normal thin epithelium. In transgenic mice without DIM, epithelial cell projections into the stroma (papillae) were present. An additional degree of nuclear anaplasia in the stratum espinosum was observed. Dysplastic cells were present. Transgenic mice fed DIM displayed some mild hyperplasia of the squamous epithelium. DIM increases estrogen C-2 hydroxylation in this model. Serum INF-γ was increased indicating increased immune response in the DIM fed animals. Histopathology showed a marked decrease in cervical dsyplasia in both wild type and transgenic mice indicating that DIM delays or inhibits the progression from cervical dysplasia to cervical cancer. Using the K14-HPV16 transgenic mouse model, we have shown that DIM inhibits the development of E6/E7 oncogene induced cervical lesions.
Introduction
Although cervical cancer incidence and mortality rates have declined in the United States over the past three decades, the disease remains a serious national and international health issue. Incidence rates for Hispanic women are higher than those for non-Hispanic women. Even though the mortality rate for African American women has declined more rapidly than the rate for Caucasian women, the African American mortality rate continues to be more than double that of Caucasians (1).
The Human Papilloma Virus (HPV) is recognized as the major cause of cervical cancer. In 2006, an estimated 10,000 women in the United States were diagnosed with this type of cancer and nearly 4,000 will die from it. Cervical cancer strikes nearly half a million women each year worldwide, claiming a quarter of a million lives (2).
HPVs are divided into “low-risk” viruses and “high risk” (types that are more likely to lead to the development of cancer). Both types of HPV can cause the growth of abnormal cells, but generally only the high-risk types of HPV may lead to cancer. Sexually transmitted, high-risk HPVs include types 6,11,16,18, and a few other less frequent variants (3).
Early studies by Wattenberg, have shown that compounds present in cruciferous vegetables had potent anticancer activity (4). Chemical analysis indicated that the activity could be accounted for by two classes of compounds, the indole derivatives originating from glucobrassinin and the various isothiocyanates including phenylhexylisothiocyanate as gluconasturtin, and sulforaphane derived from glucoraphanin. Our focus is on the indoles. The initial compound released from glucobrassinin by the action of the enzyme myrosinase is indole-3-carbinol (I3C), a compound that has proved to be highly unstable both in vitro and in vivo (5).
Most early studies were carried out using I3C either as an additive in cell culture studies or orally in animal and human studies without any concern for its stability. In animal studies it was shown to have activity against breast tumor formation (6, 7) and against endometrial tumors (8). In cell culture studies and in human studies I3C/DIM increased 2-hydroxylation of estradiol and decrease 16α-hydroxylation (9, 10). This change was correlated with its anti-tumor activity.
I3C given orally is almost immediately converted to a number of condensation products in the stomach in varying proportions depending upon the exact pH (11). These include a dimer, 3,3′-diindolylmethane (DIM), trimers including the linear trimer (LTR [2-(indol-3-ylmethyl) indol-3-yl] indol-3-ylmethane]), and the cyclic trimer (CTR [5,6,11,12,17,18-hexahydrocyclonal [1,2-b: 4,5-b’:7,8-b]), the closed ring dimer Indolocarbazole (ICZ [Indolo[3,2-b]carbazole]), and ascorbigen the condensation product of I3C and ascorbic acid.(15) Although DIM predominates, variable amounts of the others are also formed depending on the exact pH in the stomach or in the media. At least one of the other compounds (LTR) has a pro-estrogenic response and would not be protective (12, 13). The chemical structures of I3C and the primary acid condensation product DIM are given in Figure 1.
Figure1. Chemical Structure of I3C/DIM.
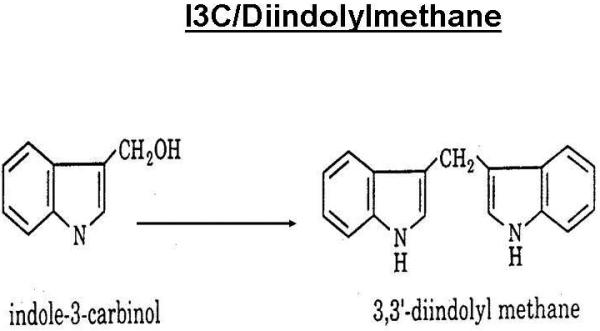
Indole-3-Carbinol is derived from the glucosinolate, glucobrassicin which is present in cruciferous vegetables. Upon contact with the acid environment of the stomach several acid condensation products are formed. The primary acid condensation product is 3,3′-diindolylmethane
Even in neutral conditions (pH 7.0-7.4) in tissue culture media I3C is rapidly converted to DIM (10). Attempts to measure plasma I3C after oral administration of I3C to mice showed only a transient minimal blood level of I3C for only a few minutes, when 250 mg/kg was given (6). DIM is a useful preventive for cervical-vaginal cancer as well as other cancers with a papillomavirus component. DIM alters the Phase I metabolism of estradiol in favor of C-2 hydroxylation pathways and away from C-4 or C-16 hydroxylation (14-18). C-16 hydroxylation produces 16α-hydroxyestrone, an endogenous carcinogen that is a promoter of HPV 16 and 18 proliferation (14, 15). DIM, by altering the directional pathway of estrogen metabolism, and probably through other non-estrogenic effects induces viral dormancy. DIM suppresses viral infection in children with recurrent respiratory papillomatosis, an HPV induced disease state (19, 20).
HPV infection activates cervical intraepithelial neoplasia (CIN) which progresses through various stages to cervical cancer. E6 and E7 exons from viral DNA interfere with the host cells control of transcription and the cell cycle. Normal cell division is regulated by two tumor suppressor gene products, Rb (Retinoblastoma protein) that promotes smooth transition through the cell cycle, and P53, that stops cell division when DNA is damaged promoting DNA repair. The viral protein E7 binds to Rb and causes uncontrolled cell reproduction which is one of the definitions of a malignant cell. The E6 viral protein binds to P53 and the protein inactive. E6 also activates telomerase which promotes malignancy as the mutant cells continue to reproduce uncontrollably (21).
DIM disrupts several stages of HPV proliferation that influence the initiation and progression from cervical dysplasia to cervical cancer. Carter et al and Yuan et al have demonstrated that DIM specifically inhibits proliferation of viral transcripts E6 and E7 in human keratinocytes and in CaSki cells (22, 23). DIM can induce a G1 cell cycle arrest in MCF-7 cells that is accompanied by inhibition of cyclin-dependent kinase expression (24). DIM inhibits cell adhesion, spreading and invasion associated with the upregulation of PTEN (a tumor suppressor gene) and E-cadherin (a regulator of cell cell adhesion) in T-47-D human breast cancer cells (25). These mechanisms may also apply to the initiation and progression via increasing severity of CIN to cervical carcinoma. Additionally, DIM prevents PTEN loss in vivo in the K14HPV16 transgenic mouse model (26).
DIM elevates four key cytokines in-vivo: Interferon-Gamma (IFN-γ), Granulocyte Colony Stimulating Factor (G-CSF), Interleukin-12 (IL-12), and Interleukin-6 (IL-6). IFN-γ is an indicator of overall immune response (27, 28). G-CSF is responsible for White Blood Cell production in the body. IL-6 is responsible for the body’s direct anti-bacterial response. IL-12 stimulates the growth and function of T-Cells which help to fight off pathogens such as viral infections.
Reduction in the severity of CIN and in some cases the complete elimination of cervical dysplasia indicates that HPV16 oncogene expression becomes latent after continued DIM administration. The objective of this study is to monitor changes in estrogen metabolism, IFN-γ levels and cervical histology in mice after receiving DIM in the diet and to compare these objective biomarkers with respective controls.
Methods
The K14-HPV16 mouse model is well established and is frequently used in studies involving cervical dysplasia and cervical cancer (29). Cervical neoplasia and cancer are linked to persistent infection of “high-risk” HPV viral types, where E6 and E7 oncogenes have enhanced affinity for cellular proteins controlling a collection of functions necessary for neoplastic progression or growth and spread of malignancies. The K14-HPV16 mouse model is transgenic for the entire HPV16 early region under control of the human keratin-14 promoter, and expresses HPV16 E6 and E7 oncogenes in basal squamous epithelial cells.
Tumorigenesis in this model is hormone-dependent. Chronic estradiol treatment at 0.1-0.25 mg/90-day induces invasive squamous cancers in the vulva, vagina and outer cervix of K14-HPV16 mice. None of the tumors are metastatic. At low estradiol dose (0.05 mg/90-day) squamous cancers are almost exclusively localized to the transformation zone situated between the upper cervix and lower uterus. Epithelial dysplasia and metaplasia are observed after 4 months of estradiol treatment, leading to high-grade dysplasia and multifocal carcinomas by 6 months (30).
Breeder pairs were kindly provided by the NCI Mouse Repository (MMHCC, Frederick, MD). After arrival, the mice were quarantined for as long as deemed necessary by the veterinarian based on serological testing and results. The mice were housed four per cage. These cages have sterile Micro-isolator tops. They were fed irradiated AIN-76A diet and given sterile water via sterile water bottles. The animals were given daily health checks, which included a pain scale form.
Breeding began during quarantine. The hemizygote K14-HPV16 was bred with the FVB wild type to produce the first litter of pups. At weaning the pups were be genotyped. Those mice exhibiting the E6-E7 exon (K14-HPV16 positive) were bred with FVB wild type on order generate progeny for the experimental protocols. Once pregnancy was determined the females were separated into individual cages for parturation and rearing. All females (K14-HPV16 and FVB wild type) were trochar implanted with E2 pellets (0.25mg/90 day release, Innovative Research of America, Sarasota FL) at weaning under anesthesia. The Animal Resource Facility strictly follows the guidelines laid out by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International), the policies according to the Public Health Service Policy on Humane Care and Use of Laboratory Animals as well as Guidelines set forth by the Animal Welfare Act.
The animals were given AIN-76A mouse diet until they were divided into respective groups. Two groups received AIN-76A mouse diet without DIM added and two groups were given AIN-76A mouse 2000ppm of DIM. The diets were prepared by Land O’Lakes Purina Feed, LLC, TestDiet® division, Richmond, IN 47364.
Genotyping the Mouse Model
Mouse tail clippings were digested using Proteinase K in TNES buffer at 55C overnight. DNA was isolated using 6M NaCl and chloroform-isoamyl alcohol according to established procedures. The DNA was amplified by using primers JA7 (5′-aga act gca atg ttt gac cca cag-3′) and JA4 (5′-tct gca aca aga cat aca tcg acc gg-3”). JA7 and JA4 are MMHCC designations. The primers were provided by Invitrogen (Carlsbad, CA). The Master mix reagents, Primer combinations and cycling conditions were provided in the FVB.Cg-Tg (KRT-HPV16)wt1Dh PCR protocol (MMHCC). The Indy Air Thermocycler was used to conduct high speed PCR (Idaho Technologies Inc., Salt Lake City Utah). The PCR products were visualized by ethidium bromide staining of 2.0% agarose gels. Each mouse was genotyped and identified as either wild type or transgenic and then grouped accordingly,
Tissue Procurement and Determination of Changes in Cervical Morphology
Mice were anesthetized and blood was obtained by cardiac puncture. Reproductive tracts and surrounding soft tissue and lymph nodes were dissected and immediately frozen at -80C. The entire reproductive tract was serially sectioned, and 10-15 sections were collected at 75 μm intervals for hematoxylin and eosin (H&E) staining using a Cryocut 1800 Cryostat (Reichert-Jung). Examination of serial sections was done with an inverted Nikon Te300 microscope with Hoffmann contrast optics for phase microscopy as well as bright field for H&E work. The microscope has 5X, 10X and 40X objectives.
A histopathological grading system for transgenic mouse cervical squamous carcinogenesis developed by Riley et al (31) was used to classify histological samples. This grading system is based on the established criteria for classification of human cervical neoplasia or malignancy accounting for differences between the mouse model and patients. Using this mouse cervical neoplasia grading system, CIN-I consists of a 2-fold increase in the basal/basaloid cell layers of cervical and vaginal squamous epithelia of transgenic compared with estrogen-treated nontransgenic mice. CIN II lesions contain cells with additional increases in nuclear size, degree of anaplasia, frequency, and distribution of dysplastic cells in the suprabasal layers of the squamous epithelium. Moreover, the basal aspect of the squamous epithelium is projected into papillary folds projecting into the underlying vaginal or cervical stroma. CIN III lesions contain abundant anaplastic cells, some with pronounced increases in nuclear size. CIS demonstrates a pronounced degree of remodeling and undulation of the epithelial-stromal border and most of the cellular features of well-differentiated squamous carcinoma, with retention of an intact basement membrane without evidence of microinvasion on serial sections.
Assessment of Immune Response using serum IFN-gamma Levels
Mouse IFN-γ was analyzed using serum by ELISA (eBioscience, San Diego, CA). The assay is straight forward and specific for the analyte. Appropriate controls were run in each assay and the samples were run in triplicate.
Quantification of DIM in serum
Extraction of DIM from Serum-100ul of serum was diluted with 1 ml of sodium acetate buffer (pH 4.8) and 20 μl of glucuronidase (110,200 U/ml; Sigma). The solution was incubated at 40°C for 24 h. The internal standard, 4,4-dichlorodiindolylomethane (dichloro-DIM generously provided by Dr. Stephen Safe) was then added and the sample is extracted with chloroform.
Twenty microliters of dry pyridine and 80 μl of N,O-bis(trimethylsilyl) trifluoroacetamide catalyzed with 1% trimethyl-chlorosilane (Pierce Chemical, Rockford, IL) were added, and the sample was heated to 100°C for 1 h. The sample is injected without further treatment (32). Gas Chromatography-Mass Spectrometry Conditions are identical to those used for the determination of estrogen metabolites.
Determination of estrogen metabolites in serum
Serum was diluted 1:1 with sodium acetate buffer (pH 4.65) and 20 μl of β-glucuronidase (110,200 units/ml, Sigma). The solution was incubated at 40 degrees C for 24 hours to deconjugate the steroids. After the addition of deuterated estradiol each sample was thoroughly vortexed and extracted with chloroform. The chloroform extract was evaporated to dryness under nitrogen. The residue was derivitized with 10 μl of dry pyridine and 40 μl of bis(trimethylsilyl)-trifluoroacetamide (BSTFA), vortexed, and allowed to react at room temperature overnight. One microliter of each sample was injected into the GC-MS without further treatment.
GC-MS Conditions-An Agilent Technologies 6980N gas chromatograph equipped with an Agilent 5973 mass selective detector, an Agilent 7683 injector and an HP G1701CA MSD Chemstation was used for the analysis of estradiol and selected metabolites. The injection port was equipped with a split/splitless capillary inlet system and a silanized glass insert. The temperature of the injection port was maintained at 300C, the interface at 270C and the ion source was maintained at 280C. The ionization energy was 70eV. The carrier gas was helium at a flow rate of 1 ml/minute. Separations were carried out using a Hewlett-Packard Ultra 2 capillary column with cross-linked 5% phenyl-methyl silicone (25m × 0.2mm × 0.33um film thickness). The oven temperature was increased from 60C to 260C at 40C/minute then at 1C/minute to 280C.
Under selected ion monitoring, the following mass ions and GC elution times of trimethylsilylated estrogens were monitored: estrone (E1) m/z 342, estradiol (E2) m/z 416,deuterated estradiol (d4E2) m/z 420, 2-hydroxyestrone (2-OHE1) m/z 430, 4-hydroxyestrone (4-OHE1) m/z 430, 16α-hydroxyestrone (16α-OHE1) m/z 286 and 430, and estriol (E3) m/z 504. [2,4,16α,16β-2H4]; estradiol was synthesized in our laboratory according to the method of Dehennin and co-workers and used as an internal standard. Each of the above steroids were quantified using a six point calibration curve that ranged from 1 to 100ng (33-36).
Results
Histopathology
DIM decreases cell proliferation and papillae invasion and prevents thickening of the cervical epithelium in both wild type and K14-HPV 16 transgenic mice. Histopathology of the cervico-uterine transformation zone in Wild Type and transgenic mice that have received either the normal mouse diet or the diet enriched with 2000ppm of DIM for 12 weeks are presented in Figure 2.
Figure 2. Cervical Epitheliun (5X) in Wild Type and Transgenic Mice With and Without DIM in the Diet.
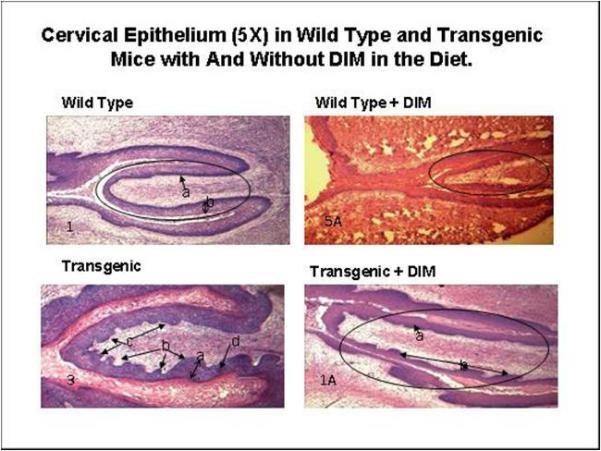
DIM decreases cell proliferation and papillae invasion and prevents thickening of the cervical epithelium in both wild type and K14-HPV 16 transgenic mice. After 12 weeks of DIM consumed in the diet.
The wild type specimen has an oval encircling the cervix. Thickened epithelium and mild hyperplasia (b) are apparent. The stratum basal was well demarcated (deep purple (a)). Papillae (projections of the collagen layer into the epithelium) were absent. The wild type plus DIM specimen shows a normal epithelial thickness. A few mild papillae were also present.
In the transgenic specimen, increased squamous hyperplasia (a) was observed. Epithelial papillomatosis (b) was apparent. Anaplastic squamous epithelial cells were observed at the stratum basale (c). Dysplastic cells (d) in the epithelium at the level of stratus espinosum and/or granular were observed. No glandular hyperplasia or keratin pearls were observed in any of the analyzed specimens. In the transgenic plus DIM specimen, the oval line represents the cervix. Increased squamous hyperplasia (a) and epithelial papillae (b) were present. There are 41% less epithelial papillae projecting into the stroma in the DIM treated transgenic mice when compared to transgenic not receiving DIM in the diet over a 12 week period. Dysplastic cells in the epithelium at the level of stratus espinosum and/or granular were not observed at this magnification. No glandular hyperplasia or keratin pearls were observed in any of the analyzed specimens.
The cervical region was examined at higher magnification (Figure 3). In the wild type specimen, hyperplasia of the squamous epithelium was observed (a). This corresponds to CIN-I in the histopathological grading system of Riley et al (32). The wild type plus DIM slide displayed a normal thin epithelium. In the transgenic specimen, epithelial cell projections into the stroma (papillations, arrow) were apparent. Cells with an additional degree of nuclear anaplasia (white arrow) were observed in the stratum espinosum (out of the stratum basale). Epithelial cells projecting into the stroma were displayed. Dysplastic cells were present (arrows). This corresponds to CIN-II/CIN-III in the histopathological grading system of Riley et al (32). The cervical transformation zone for the transgenic mouse with DIM displayed only mild hyperplasia of the squamous epithelium.
Figure 3. Cervical Epitheliun (40X) in Wild Type and Transgenic Mice With and Without DIM in the Diet.
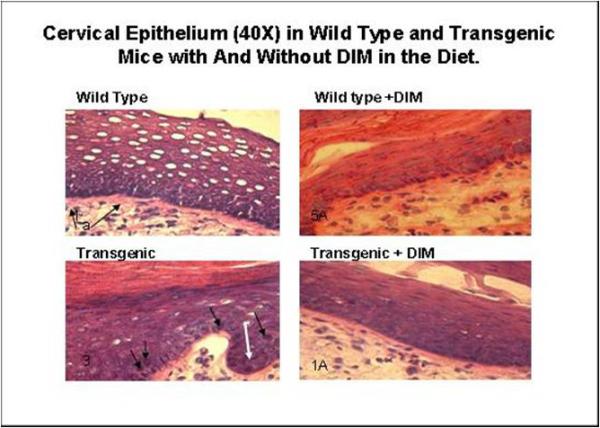
In the wild type specimen, hyperplasia of the squamous epithelium was observed (a). Wild type plus DIM displayed a normal thin epithelium. In the transgenic specimen epithelial cell projections into the stroma are apparent (papillation, arrow). Some cells exhibit an additional degree of nuclear anaplasia (white arrow) in the stratum espinosum (out of the stratum basale). Epithelial cells projected into the stroma are displayed. Dysplastic cells are present (arrows). The specimen for the transgenic mouse with DIM displayed some mild hyperplasia of the squamous epithelium.
Immune Response
Mean serum IFN-gamma concentrations were significantly higher (p<0.05) in both groups that were fed 2000ppm in the diet when compared to mice receiving diets that did not contain DIM (Figure 4). Xue et al (28) was able to show an increase in 4 out of 22 cytokines using a mouse membrane cytokine array using male C57BL/6 mice. They administered a single oral (30mg/kg) dose of DIM in a C57Bl/6 male mouse model (n=3) and serum was sampled at regular intervals over 24 hours. Each cytokine exhibited different kinetics with IFN-gamma levels increasing after 24 hours. C57BL/6 has certain immunophenotypes that distinguish it from other inbred strains like BALB/c. The K-14 HPV 16 model used here is transgenic and the FVB wildtype mouse may respond at greater doses of DIM. It is important to note that the study by Xue (28) used male mice. We are using estradiol implanted females. The induction of cytokines by DIM has not been attempted previously in this model.
Figure 4. Serum IFN-gamma levels in wild type and transgenic mice receiving 2000ppm DIM continuously in the diet.
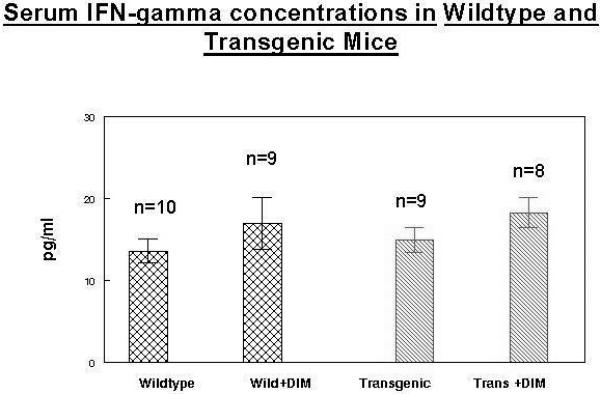
Mean serum IFN-gamma concentrations were significantly higher (p<0.05) in both groups that were fed 2000ppm in the diet when compared to mice receiving diets that did not contain DIM
Mean Body weights for each group for 15 weeks are presented in Figure 5. Mean body weights were similar for all groups.
Figure 5. Mean Body Weights for Wild type with and without 2000ppm DIM in the diet and transgenics with and without 2000ppm DIM in the diet.
Mean body weights were similar for all groups.
Food consumption
Food consumption was monitored in each group (Figure 6). These measurements were begun after weaning and E2 pellet implantation. Animals in both DIM groups consumed slightly less food (about 5%) over the monitoring period. This may be due to taste differences or possibly other factors. All groups had dietary consumption levels that were consistent with animal feeding recommendations for mice on custom purified diet (3-6g/animal/day) (37).
Figure 6. Mean Food Consumption for Wild type with and without 2000ppm DIM in the diet and transgenics with and without 2000ppm DIM in the diet.
Animals in both DIM groups consumed slightly less food (about 5%) over the monitoring period.
DIM concentrations in serum were quantified by GC-MS using the method of Sepkovic et al (32). DIM levels in serum were not different in either of the groups receiving DIM in the diet. No DIM was found in the groups receiving mouse chow alone (Figure 7).
Figure 7. DIM concentrations in in wild type and transgenic mice receiving 2000ppm DIM continuously in the diet.
DIM concentrations in serum were quantified by GC-MS using the method of Sepkovic et al (32)
Estrogen metabolites were quantified by stable isotope dilution GC-MS in the blood of wild type and transgenic mice with and without DIM administered in the diet for 12 weeks (Table 1). The results are presented as ng/100ul serum. 2-Hydroxyestrone concentrations are significantly increased after DIM administration in both wild type and in HPV transgenic female mice. In contrast, there was no increase in 16-OHE1 concentrations. 2-OHE1/16-OHE1 ratios reflect increased C-2 hydroxylation in the DIM fed groups. 4-OHE1 concentrations were unchanged in both wild type and transgenic groups.
Table 1. Comparison of estrogen metabolism in Wildtype and HPV Transgenic mice with and without 2000ppm DIM in the diet.
| E1 | E2 | 2OHE1 | 16OHE1 | 4OHE1 | E3 | C2/C16 Ratio | ||
|---|---|---|---|---|---|---|---|---|
| Wildtype | ||||||||
| n=9 | Mean | 4.7 | 0.8 | 0.9 | 1.9 | 0.0 | 1.5 | 0.5 |
| SD | 5.0 | 0.5 | 1.0 | 1.5 | 0.1 | 1.5 | ||
| Wildtype with DIM | * | |||||||
| n=7 | Mean | 4.8 | 0.2 | 3.2 | 1.1 | 0.0 | 0.1 | 2.8 |
| SD | 4.3 | 0.2 | 3.7 | 1.0 | 0.0 | 0.3 | ||
| Transgenic | ||||||||
| n=10 | Mean | 3.6 | 0.3 | 0.3 | 1.3 | 0.0 | 0.7 | 0.2 |
| SD | 4.2 | 0.3 | 0.3 | 0.9 | 0.0 | 0.7 | ||
| Transgenic with DIM | * | |||||||
| n=10 | Mean | 1.0 | 1.6 | 1.0 | 0.9 | 0.0 | 0.9 | 1.1 |
| SD | 1.2 | 2.6 | 1.1 | 1.1 | 0.1 | 1.0 | ||
DIM increased C-2 hydroxylation of estradiol in both the wildtype mouse and in the HPV transgenic mouse by 28%, (p< 0.05) and 33%, (p<0.05) respectively.
Discussion
The objective of this study was to monitor changes in estrogen metabolism, IFN-γ levels and histopathology in mice after receiving DIM in the diet and to compare these objective biomarkers with respective controls. Using this model we have shown that immune responses (IFN-γ levels) are significantly increased in both wild type and HPV transgenic mice after DIM administration in the diet for 12 weeks. Even though the increases in IFN-gamma after DIM administration were modest, Garcia-Pineres et al (38, 39) have shown that IFN-gamma levels as well as the levels of other cytokines are increased after vaccination with HPV type 16 L1 virus like particles (VLPs). Our goal is to test the efficacy of vaccines in the mouse model. These vaccines contain L1 VLPs.
Marked changes in cervical histology were observed in the DIM fed groups when compared to their respective controls. Estrogen metabolites were quantified by stable isotope dilution GC-MS in the blood of wild type and transgenic mice with and without DIM administered in the diet for 12 weeks. The results are presented as ng/100ul serum. 2-Hydroxyestrone concentrations are significantly increased after DIM administration in both wild type and in HPV transgenic female mice. The C-2/C-16α-hydroxylation ratio is also increased in both groups.
Yuan et al (23) has shown that 16α-OHE1 increased expression of HPV E6/E7oncogenes whereas I3C/DIM and the estrogen metabolite 2-hydroxyestrone (2-OHE1) abrogated the estrogen-increased expression of HPV oncogenes in Caski cells. This study provides evidence of suppressed E6/E7 oncogene expression by DIM in vivo through comparison of cervical histology in DIM fed transgenic mice with respective controls. This confirms the results of a study by Jin et al, (40) where dietary administration of 2000ppm I3C) for 24 weeks prevented cervical cancer in K14-HPV16 transgenic mice and reduced cervical dysplasia in FVB/n non-transgenic controls.
It should also be noted that the DIM plasma levels observed in mice in this study approximate those observed in humans given a single 1,000 mg dose of DIM (13). In the study by Jin et al (40), which utilized the same transgenic mouse model as that described herein, a solid diet containing 2,000 ppm of I3C was fed to the animals over 24 weeks without any overt signs of toxicity. As mice consume 3 g of solid food per week, the 2000 ppm I3C-enriched diet is equivalent to a dose of 2 g I3C/kg diet or 2 mg I3C/gram diet. This amount of dietary I3C could be ingested by humans who consume a diet heavily enriched in cruciferous vegetables (41, 42). This dose is comparable to the dose of DIM per day in this study.
Several pharmacokinetic studies using DIM in animals and in humans have been conducted. In mice (female CD-1 strain), Anderton et al 2004 (12) studied I3C and DIM disposition upon oral administration of a dose equivalent to 7.5 mg I3C (250 mg I3C/kg body weight). Mice were sacrificed at 0 to 24 h following dosing, after which I3C and DIM plasma and tissue (liver, kidney, lung, heart, and brain) concentrations were determined. I3C was found to be rapidly absorbed and eliminated from plasma and tissues, with plasma levels falling below detection limits by 1 h. In contrast, DIM levels in plasma were highest 2 hours after drug administration, peaking at 800ng/ml (3.2 μM). These plasma DIM levels are quite similar to those found in our own mouse model (85ng DIM/100 μl plasma or 850 ng/ml).
Reed et al (13) performed a phase I trial in women with indole-3-carbinol (I3C), the precursor to DIM, where subjects received doses of I3C ranging from 400 to 1,200 mg. The only I3C-derived product found in plasma was DIM, with CMAX and AUC values that increased from 61 ng/ml (0.25 μM) and 329 mg/h/liter after the lower 400 mg I3C dose to 607 ng/ml (2.5 μM) and 3,376 mg/h/liter following the 1,000 mg dose, with no further increases noted at the higher dose. Albeit being variable, the overall DIM plasma profiles within each dosing group were qualitatively similar, with a TMAX at 2 h and DIM levels decreasing to 20ng/ml or less by 24 h.
In a subsequent study, Reed et al (43) again examined the pharmacokinetics of DIM (as well as its safety and tolerability) in a single ascending-dose study but employing lower doses (50-300 mg) of the agent, which was given as nutritional-grade, absorption-enhanced BioResponse DIM (BR-DIM). No DIM-related adverse effects were reported at doses up to 200 mg, although at the 300-mg dose, two of six subjects reported mild nausea, headache and/or vomiting. Analysis of plasma samples taken over the next 24 h showed that only one of the three subjects given the 50-mg dose had detectable plasma DIM concentrations. The 100-mg dose of BR-DIM resulted in a mean CMAX of 32 ng/ml and a mean AUC of 128 mg/h/liter, whereas the 200-mg dose gave a mean CMAX of 104 ng/mL and a mean AUC of 553 ng/h/ml. The 300 mg BR-DIM dose gave CMAX and AUC values essentially identical to those observed at the 200 mg dose, indicating that maximum intestinal absorption of the agent had been reached. These pharmacokinetic studies were undertaken using single dose administration of DIM. In contrast, our study employs continuous administration of DIM contained in the mouse diet.
Taken together, the data presented here show that DIM inhibits E6/E7 oncogene expression in the HPV transgenic model through the alteration of estradiol metabolism toward C2-hydroxylation, the enhancement of immune function and by inhibiting oncogene induced changes in cervical histology. Yuan et al (23) have previously demonstrated that E6/E7 oncogene expression is suppressed by both DIM and 2-OHE1 in CaSki cells. We have confirmed these results in vivo. In the mouse model, we have shown that DIM inhibits the development of E6/E7 oncogene induced cervical lesions.
Riby et al (27 have shown that DIM activates and potentiates IFN-gamma signaling in MCF-7 cells and Xue et al (28) has shown that DIM stimulates murine immune function. This is the first time that estrogen metabolites and IFN-γ levels have been quantified in the K14-HPV16 mouse model after DIM consumption. The three objective biomarkers employed in this study were all altered to indicate what may be viral latency. Latent viruses may permit the development of protective antibodies following HPV vaccine administration.
There is precedent for giving vaccines after viral infection. Herpes zoster results from reactivation of latent varicella-zoster virus (VZV) within the sensory ganglia. Live attenuated varicella-zoster virus (VZV) vaccine has been administered to individuals who have had Herpes zoster with excellent results (44). Hope-Simpson proposed that immunity to VZV plays a pivotal role in the pathogenesis of herpes zoster, and subsequent observations support the thesis that cell-mediated immunity to VZV is a major determinant of the risk and severity of herpes zoster (45). VZV vaccine boosts cell-mediated immunity by increasing IFN-gamma (46). DIM increases cell-mediated immunity through the elevation of IFN-gamma as well as other cytokines mentioned above.
Acknowledgments
Grant Support: NIH Grant 1R01CA136847
References
- 1.Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, Altekruse SF, Feuer EJ, Huang L, Mariotto A, Miller BA, Lewis DR, Eisner MP, Stinchcomb DG, Edwards BK, editors. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: 19752006. [Google Scholar]
- 2.Division of STD Prevention . Prevention of genital HPV infection and sequelae: Report of an external consultants’ meeting. Centers for Disease Control and Prevention; Atlanta, GA: 1999. [Google Scholar]
- 3.Munoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. New England Journal of Medicine. 2003;348(6):518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 4.Loub WD, Wattenberg LW, Davis DW. Aryl hydrocarbon hydroxylase induction in rat tissues by naturally occurring indoles of cruciferous plants. J Natl Cancer Inst. 1975;54:985–8. [PubMed] [Google Scholar]
- 5.De Kruif CA, Marsman JW, Venrkamp JC, Falke J. Structure elucidation of acid reaction products of indole-3carbinol: detection in vivo and enzyme induction in vitro. Chem Biol Interact. 1991;80:303–15. doi: 10.1016/0009-2797(91)90090-t. [DOI] [PubMed] [Google Scholar]
- 6.Bradlow HL, Michnovicz JJ, Telang NT, Osborne MP. Effect of dietary indole-3-carbinol on estradiol metabolism and spontaneous mammary tumors in mice. Carcinogenesis. 1991;12:1571–1574. doi: 10.1093/carcin/12.9.1571. [DOI] [PubMed] [Google Scholar]
- 7.Grubbs CJ, Steele VE, Casebolt, et al. Chemoprevention of chemically induced mammary tumors. Anticancer Research. 1995;15:709–716. [PubMed] [Google Scholar]
- 8.Kojima T, Tanaka T, Mori H. Chemoprevention of Spontaneous Endometrial Cancer in Female Donryku Rats by Dietary Indole-3-carbinol. Cancer Research. 1994;54:1446–1449. [PubMed] [Google Scholar]
- 9.Telang NT, Kurihara H, Wong GY, et al. Preneoplastic transformation in mouse mammary tissue: identification and validation of intermediate biomarkers for chemoprevention. Anticancer Res. 1991;11:1021–1027. [PubMed] [Google Scholar]
- 10.Michnovicz JJ, Bradlow HL. Induction of estradiol metabolism by dietary indole-3-carbinol in humans. J Natl Cancer Inst. 1990;50:947–950. doi: 10.1093/jnci/82.11.947. [DOI] [PubMed] [Google Scholar]
- 11.Preobrazhenskaya MN, Bukhman VM, Korolev AM, Efimov SA. Ascorbigen and other indole-derived compounds from Brassica vegetables and their analogs as anticarcinogenic and immunomodulating agents. Pharmacol Ther. 1993;60:301–13. doi: 10.1016/0163-7258(93)90012-3. [DOI] [PubMed] [Google Scholar]
- 12.Anderton MJ, Manson MM, Verschoyle RD, et al. Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clin Cancer Res. 2004;10:5233–41. doi: 10.1158/1078-0432.CCR-04-0163. [DOI] [PubMed] [Google Scholar]
- 13.Reed GA, Arneson DW, Putnam W, III, et al. Single- and Multiple-Dose Administration of Indole-3-carbinol to Women: Pharmacokinetics Based on 3,3′-Diindolylmethane. Cancer Epidemiol Biomarkers & Prev. 2006;15:2477–2481. doi: 10.1158/1055-9965.EPI-06-0396. [DOI] [PubMed] [Google Scholar]
- 14.Auborn KJ, Woodworth C, DiPaolo JA, Bradlow HL. The interaction between HPV infection and estrogen metabolism in cervical carcinogenesis. Int. J. Cancer. 1991;49:867–869. doi: 10.1002/ijc.2910490611. [DOI] [PubMed] [Google Scholar]
- 15.Schneider J, Kinne D, Fracchia A, Pierce V, et al. Abnormal oxidative metabolism of estradiol in women with breast cancer. Proc. Soc. Acad. Sci. 1982;79:3047–3051. doi: 10.1073/pnas.79.9.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fishman J, Schneider J, Hershcope RJ, Bradlow HL. Increased estrogen 16 alpha-hydroxylase activity in women with breast and endometrial cancer. J. Steroid Biochem. Mol. Biol. 1984;20:1077–1081. doi: 10.1016/0022-4731(84)90021-9. [DOI] [PubMed] [Google Scholar]
- 17.Steinberg BM, Auborn KJ. Papillomavirsuses in head and neck disease: Pathophysiology and possible regulation. J. Cell Biochem. 1993;17F:155–164. doi: 10.1002/jcb.240531022. [DOI] [PubMed] [Google Scholar]
- 18.Michnovicz JJ, Adlercreutz H, Bradlow HL. Changes in levels of urinary estrogen metabolites after oral indole-3-carbinol treatment in humans. J Natl Cancer Inst. 1997;89(10):718–23. doi: 10.1093/jnci/89.10.718. [DOI] [PubMed] [Google Scholar]
- 19.Auborn K, Abramson A, Bradlow HL, et al. Estrogen Metabolism and Respiratory Papillomatosis: A Pilot Study on Dietary Prevention. Anticancer Res. 1998;18:4569–4574. [PubMed] [Google Scholar]
- 20.Coll DA, Rosen CA, Auborn K, et al. Treatment of recurrent respiratory papillomatosis with Indole-3-Carbinol. Am J. Otolaryngol. 1997;18:283–285. doi: 10.1016/s0196-0709(97)90012-0. [DOI] [PubMed] [Google Scholar]
- 21.Riley RR, Duensing S, Brake T, et al. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res. 2003;15(16):4862–71. 63. [PubMed] [Google Scholar]
- 22.Carter TH, Liu K, Ralph W, Jr, et al. Diindolylmethane alters gene expression in human keratinocytes in vitro. J Nutr. 2002;132(11):3314–24. doi: 10.1093/jn/132.11.3314. [DOI] [PubMed] [Google Scholar]
- 23.Yuan F, Chen D, Liu K, Sepkovic DW, et al. Anti-estrogen effect of Indole-3-carbinol in cervical cancer cells: Implication for prevention of cervical cancer. Anticancer Res. 1999;19:1673–1680. [PubMed] [Google Scholar]
- 24.Cram EJ, Liu BD, Bjeldanes LF, Firestone GL. Indole-3-carbinol inhibits CDK6 expression in human MCF-7 breast cancer cells by disrupting Sp1 transcription factor interactions with a composite element in the CDK6 gene promoter. J. Biol. Chem. 2001;276(25):22332–22340. doi: 10.1074/jbc.M010539200. [DOI] [PubMed] [Google Scholar]
- 25.Meng Q, Goldberg ID, Rosen EM, Fan S. Inhibitory effects of Indole-3-carbinol on invasion and migration in human breast cancer cells. Breast Cancer Res. Treat. 2000;63(2):147–52. doi: 10.1023/a:1006495824158. [DOI] [PubMed] [Google Scholar]
- 26.Mei Q, Anderson AE, Chen DZ, et al. Indole-3-carbinol prevents PTEN loss in cervical cancer in vivo. Mol Med. 2005;11(112):59–63. doi: 10.2119/2006-00007.Auborn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riby JE, Xue L, Chatterji U, et al. Activation and Potentiation of Interferon-(gamma) Signaling by 3,3′-Diindolylmethane in MCF-7. Breast Cancer Cells, Mol Pharmacol. 2006;69(2):430–9. doi: 10.1124/mol.105.017053. [DOI] [PubMed] [Google Scholar]
- 28.Xue L, Pestka J, Maoxiang L, et al. 3,3′-Diindolylmethane stimulates murine immune function in vitro and in vivo. Journal of Nutritional Biochemistry. 2008;19(5):336–44. doi: 10.1016/j.jnutbio.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arbeit JM, Munger K, Howley PM, Hanahan D. Progressive squamous epithelial neoplasia in K14-human papillomavirus type 16 transgenic mice. J Virol. 1994;68:4358–4368. doi: 10.1128/jvi.68.7.4358-4368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arbeit JM, Howley PM, Hanahan D. Chronic estrogen-induced cervical and vaginal squamous carcinogenesis in human papillomavirus type 16 transgenic mice. PNAS. 1996;93:2930–2935. doi: 10.1073/pnas.93.7.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riley RR, Duensing S, Brake T, Münger K, et al. Dissection of Human Papillomavirus E6 and E7 Function in Transgenic Mouse Models of Cervical Carcinogenesis. Cancer Research. 2003;63:4862–4871. [PubMed] [Google Scholar]
- 32.Sepkovic DW, Bradlow HL, Bell M. Quantitative Determination Of 3,3′-Diindolylmethane In The Urine Of Individuals Receiving Indole-3-Carbinol. Nutrition and Cancer. 2002;41(12):57–63. doi: 10.1080/01635581.2001.9680612. [DOI] [PubMed] [Google Scholar]
- 33.Sepkovic DW, Bradlow HL, Michnovicz J, et al. Catechol Estrogen Production in Rat Microsomes After Treatment with Indole 3 Carbinol, Ascorbigen, or β napthaflavone: A Comparison of Stable Isotope Dilution Gas Chromatography Mass Spectrometry and Radiometric Methods. Steroids. 1994;59:318–323. doi: 10.1016/0039-128x(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 34.Dehennin L, Reiffsteck A, Scholler R. Simple methods for the synthesis of twenty different highly enriched deuterium labelled steroids suitable as internal standards for isotope dilution mass spectrometry. Biochemical Mass Spectrometry. 1980;7:493–499. [Google Scholar]
- 35.Raju U, Sepkovic D, Dixon JM, et al. Estrone and estradiol metabolism in vivo in human breast cysts. Steroids. 2000;65:883–888. doi: 10.1016/s0039-128x(00)00184-7. [DOI] [PubMed] [Google Scholar]
- 36.Jellnick PH, Makin HLJ, Sepkovic DW, Bradlow HL. Influence of indol carbinols and Growth Hormone on the metabolism of 4-Androstenedione by Rat Liver Microsomes. J. Steroid Biochem. Molec Biol. 1993;46:791–798. doi: 10.1016/0960-0760(93)90320-v. [DOI] [PubMed] [Google Scholar]
- 37.Guide for the Care and Use of Laboratory Animals. Institute of Laboratory Animal Resources. Commission on Life Sciences . National Research Council. National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- 38.García-Piñeres A, Hildesheim A, Dodd L, et al. Cytokine and chemokine profiles following vaccination with human papillomavirus type 16 L1 Virus-like particles. Clin Vaccine Immunol. 2007;14(8):984–9. doi: 10.1128/CVI.00090-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.García-Piñeres AJ, Hildesheim A, Dodd L, et al. Gene expression patterns induced by HPV-16 L1 virus-like particles in leukocytes from vaccine recipients. J Immunol. 2009;182(3):1706–29. doi: 10.4049/jimmunol.182.3.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin L, Qi M, Chen DZ, Anderson A, et al. Indole-3-carbinol prevents cervical cancer in human papilloma virus type 16 transgenic mice. Cancer Research. 1999;59:3991–3997. [PubMed] [Google Scholar]
- 41.Scones K, Heaney RK, Fenwick GR. An estimate of the mean daily intake of glucosinolates from cruciferous vegetables in the UK. J. Sci. Food Agric. 1984;35:712–720. [Google Scholar]
- 42.Lewis J, Fenwick GR. Glucosinolate content of Brassica vegetables: analysis of twenty-four cultivars of calabrese (green sprouting broccoli, Brassica oleracea L. var. botrytis subvar. cymosa Lam.) Food Chem. 1987;25:259–268. [Google Scholar]
- 43.Reed GA, Sunega JM, Sullivan DK, et al. Single-dose pharmacokinetics and tolerability of absorption-enhanced 3,3′-diindolylmethane in healthy subjects. Cancer Epidemiol Biomarkers Prev. 2008;17(10):2619–24. doi: 10.1158/1055-9965.EPI-08-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oxman N, Levin MJ, Johnson GR, et al. A Vaccine to Prevent Herpes Zosterand Postherpetic Neuralgia in Older Adults. New Engl. J. Med. 2005;52:2201–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 45.Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58:9–20. doi: 10.1177/003591576505800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levin MJ, Smith JG, Kaufhold RM, et al. Decline in varicella-zoster virus (VZV)-specific cell-mediated immunity with increasing age and boosting with a high-dose VZV vaccine. J Infect Dis. 2003;188(9):1336–44. doi: 10.1086/379048. [DOI] [PubMed] [Google Scholar]


