Abstract
EEG power and high frequency activity in the seizure onset zone has been increasingly considered for its relationship with seizures in animal and human studies of epilepsy. We examine the relationship between quantitative EEG measures and metabolic imaging in epilepsy patients undergoing intracranial EEG (icEEG) analysis for seizure localization. Patients with mesial temporal lobe epilepsy (MTLE) and neocortical epilepsy (NE) were studied. Metabolic imaging was performed with MR spectroscopic imaging using N-acetyl aspartate (NAA) and creatine (Cr). All data were acquired from the mesial temporal lobe such that a direct comparison of the same anatomical regions between the two groups could be performed. While no difference was seen in the total power recorded from the mesial temporal lobe, the MTLE group had significantly greater power in the high frequency bands. There was a significant positive exponential relationship between total icEEG power with NAA/Cr in MTLE, R= +0.84 p<0.001, which was not seen in NE. There was also a significant negative relationship between fractional gamma power with NAA/Cr in MTLE R= −0.66 p<0.02, also not seen in NE. These data argue that within the seizure onset zone, the tight correlation between total power and NAA/Cr suggests that total electrical output is powered by available mitochondrial function. These data are also consistent with the hypothesis that high frequency activity is an abnormal manifestation of tissue injury.
Keywords: intracranial EEG, gamma power, N-acetyl aspartate, human
Introduction
Over the past decade, much work in quantitative intracranial EEG has focused on developing and understanding measures for seizure localization and prediction (Mormann et al 2007, Iasemidis et al 2005). More recently there has been greater interest on high frequency activity, for its possible specificity to the seizure onset zone (Urrestarazu et al 2007, Bragin et al 2002), and manifestation of synaptic re-organization in the seizure onset region (Staba et al 2007, Worrell et al 2008). However, there is relatively less known in terms of the basic quantitative intracranial EEG measures and their relationships to parameters of tissue injury in different patient groups. Zaveri et al 2001 studied a group of 14 medial temporal lobe epilepsy (MTLE) patients with and without sclerosis to evaluate their neuropathology with respect to quantitative EEG. They reported that the intracranial EEG (icEEG) measures of total and delta power were significantly lower in those with sclerosis in comparison with MTLE patients without sclerosis. While this latter observation may seem counter-intuitive, it suggested that tissue loss intrinsically decreases total EEG power. Inter-ictal discharges have been related to a range of measures, including SPECT and metabolic MR imaging (Guillon et al 1997, Guye et al 2002), which as a clinical measure of brain irritability has been suggestive. More recently, Bartolomei et al 2008 studied n=17 MTLE patients with icEEG, finding that a time dependent ratio of high to low band power (denoted by “epileptogenicity index”) was greater in the seizure onset zone and also in MR negative patients.
In this report we develop further the notion of the role of different EEG power bands and how they may be influenced by tissue injury and metabolic dysfunction. We do this by examining n=26 epilepsy patients undergoing intracranial EEG study for seizure localization. All data were acquired from the medial temporal lobe as it is a common site of study and seizure onset. 14 of these patients were ultimately determined to suffer from neocortical epilepsy with or without dual pathology, and 12 from medial temporal lobe epilepsy. As these patients were studied in the context of an ongoing program of metabolic imaging, 22 of these patients also had MR spectroscopic (MRS) studies of NAA/Cr available as a measure of tissue function and injury (Vermathen et al 2002, Pan & Takahashi 2005). NAA synthesis is localized to neuronal mitochondria and known to correlate with ATP synthesis rates as shown in in vitro models of mitochondrial function (Bates et al 1996; Heales et al 1995). Such in vivo metabolic measurements have previously been correlated with a variety of cognitive performance scales, most pertinently being linked with memory (Chao et al 2005, Pan et al 2001). Comparison of these data from the two patient groups provides a consistent evaluation of how the medial temporal lobe behaves electrically and metabolically when it is or is not the site of seizure onset. We determined the interictal power values (total power and power in delta, theta, alpha, beta and gamma frequency bands) and Teager energy, a high frequency weighted evaluation of signal energy (Kaiser 1990).
Methods
Patients
Patients (age 18-55) from the Yale University Epilepsy Surgery Program undergoing intracranial EEG (icEEG) monitoring for surgical evaluation were invited to participate in the MR imaging protocols as approved by the Yale Human Investigations Committee (HIC). Only patients with medial temporal lobe contacts (strips and/or depths, Ad-Tech Medical, Racine, WI) were included in this analysis. There was a total of 26 patients, n=12 medial temporal lobe epilepsy (MTLE) patients and n=14 neocortical epilepsy with or without dual pathology patients (Table 1). MTLE patients were defined by location of electrical seizure onset. Patients with dual pathology (n=4) were classified with the neocortical group and were defined based on clinical, surgical and histologic criteria: n=3 patients #19, #20 and #21 who had a neocortical resection in addition to the standard medial temporal lobe resection; n=1 patient #16 who underwent medial temporal lobe resection with no effect on seizure frequency and showed heterotopic neurons in their pathology. 23 of the 26 patients (n=12 MTLE, n=11 neocortical epilepsy) had completed temporal lobe metabolic imaging studies. Of the 12 MTLE patients, 7 had MRI detected hippocampal atrophy and 5 were MRI negative. Of the 14 neocortical epilepsy patients, all 4 classified as dual pathology had MRS data; 10 were without dual pathology of which 6 had MRS data. Because all data were acquired from the medial temporal lobe, we also denote the MTLE group as “within-seizure-zone” group; the neocortical group as “outside-seizure-zone” group.
Table 1.
Patient characteristics
| Medial temporal lobe epilepsy group | ||||||
|---|---|---|---|---|---|---|
| Patient ID | Etiology | HA on MRI? | Medications | Years of epilepsy at time of evaluation | Operated? | |
| 1 | FCS, trauma | Yes | CBZ | 35 | No, bilateral | |
| 2 | trauma | Yes | OXC,LTG | 9 | Yes | |
| 3 | None | No | LTG, LEV | 12 | No, function | |
| 4 | None | No | LEV, VPA | 8 | No, function | |
| 5 | FCS | No | LEV,CBZ,VPA | 9 | No, lost to f/u | |
| 6 | None | Yes | CBZ,TPM,ZNS | 17 | Yes | |
| 7 | Perinatal hypoxia | Yes | LTG,OXC,CLZ | 37 | Yes | |
| 8 | trauma | No | CBZ,ZNS | 19 | No, function | |
| 9 | None | Yes | CBZ,VPA | 39 | No, bilateral | |
| 10 | FCS | No | DPH,OXC | 16 | No, function | |
| 11 | trauma | Yes | LEV,DPH | 4 | Yes | |
| 12 | FCS | Yes | ZNS | 8 | Yes | |
| Neocortical epilepsy group | ||||||
| Patient ID | Lobe of onset | Etiology | Dual pathology? | Meds | Yrs of epilepsy at time of surgery | Operated? |
| 13 | Temporal | Trauma | No | CBZ,LTG | 13 | Yes |
| 14 | Temporal | Unknown | No | DPH | 7 | Yes |
| 15 | Temporal | FCS, heterotopia | Yes | TPM,LEV | 7 | Yes |
| 16 | Multifocal | Trauma | No | CBZ,GBP | 10 | No |
| 17 | Temporal-parietal-frontal | Trauma | No | DPH, LTG, LEV | 11 | Yes |
| 18 | Temporal | Gliosis | Yes | CBZ,LEV, ZNS | 3 | Yes |
| 19 | Temporal-Occipital | Unknown | Yes | LTG | 31.5 | Yes |
| 20 | Temporal | Sclerosis; unknown | Yes | OXC,LTG | 23 | Yes |
| 21 | Frontal-temporal | Dysplasia of Taylor | No | LEV,OXC | 15 | Yes |
| 22 | Temporal | FCS | No | LTG, CBZ, PB | 38 | Yes |
| 23 | Occipital | Hypoxia | No | CBZ,GBP | 21 | Yes (Neuropace) |
| 24 | Parietal | Heterotopic | No | ZNS,LTG,CBZ, DZP | 7 | Yes |
| 25 | Temporal | Meningitis | No | TPM,LEV | 18 | Yes |
| 26 | Frontal | Polymicrog yria | No | OXC, LEV | 24 | No |
Meds: CBZ carbamazepine; OXC oxcarbazepine; LTG lamotrigine; LEV levetiracetam; VPA valproic acid; TPM topiramate; ZNS zonisamide; DPH phenytoin; CLZ clonazepam; PB Phenobarbital, GBP gabapentin In the MTLE table, explanations are provided if no surgery was performed
icEEG Data Acquisition
The intracranial electrodes in this study were placed under stereotactic guidance and included a combination of strip and depth electrodes. The subdural strip electrodes are 4mm diameter platinum disks (2.3mm exposed surface diameter) with an inter-contact (center-to-center) distance of 10mm. The depth electrodes are 2.3mm length platinum contacts with an inter-contact (center-to-center) distance of 10mm. Patients are monitored continuously using a commercial EEG acquisition and storage system (Biologic Systems Corp., Mundelein, Illinois). Up to 128 channels of EEG are recorded with 256 Hz sampling and stored in digital form along with a time synchronized video signal of the patient. Offline analysis is performed with custom software written in a mixture of high level languages and MATLAB (The Math Works Inc., Natick, MA). Intracranial EEG epochs, one hour in duration, at least 6 hours removed from a seizure, are selected for analysis. The epoch is selected from either day 2 or day 3 of the monitoring (that is, 3 or 4 days after surgery to place intracranial electrodes). The measurements are made during wakefulness, typically between either 9-10AM or 4-5PM. The icEEG is examined for artifacts which are marked at a 1-sec resolution. Power and Teager energy of artifact free icEEG segments is obtained for each electrode contact studied and averaged over the epoch. Delta power was assigned 0–4 Hz, theta 4–8 Hz, alpha 8–13 Hz, beta 13–24 Hz, and gamma 24–50 Hz bands. The total power is calculated as the signal power between 0.1-50Hz, and power in the frequency bands is calculated after Fourier transform of the data and as the average of the power within the frequency bands defined above. As a result, direct summation of the frequency band powers does not match the total power; however after scaling of the bands to the number of frequencies in each band they do. Because typically one to three contacts were available in the medial temporal lobe for each patient, data from all such contacts were averaged for each subject prior to further analysis. Given the variety of final locations of contacts, we did not further subdivide our patient group based on location of EEG contacts. The majority of contacts studied were located in the pes and body of the hippocampus. We did consider evaluating spike activity with the power measures. However, the objective detection of spike activity is technically problematic given the variability in the electrode spatial coverage, in contrast to the relative consistency of the quantitative power measures and was not a main goal of this investigation.
Magnetic resonance
1H spectroscopic imaging: All data were acquired using a whole body 4T Varian Inova imaging spectrometer with a volume 1H TEM coil. Scout images were acquired with an inversion recovery gradient echo. The triply obliqued hippocampal slice was defined along the planum temporale prescribed from an off-sagittal slice. After scout imaging, 3D localized shimming was performed over the bilateral hippocampi using first, second and third order shims. The spectroscopic image was optimized to collect data on N-acetyl aspartate, creatine and choline using a 3D localized adiabatic LASER sequence with two dimensions of phase encoding, with TR/TE 2s/72ms (Hetherington et al 2007). The spectroscopic image had a nominal voxel size of 0.64cc (24×24 encodes, FOV 192×192mm, acquisition time 19min).
Data analysis
To provide reproducible volume selection along the hippocampal plane between subjects, a semi-automated single voxel selection and reconstruction routine was used (Hetherington et al 2007). Briefly, data are analyzed by tracing the boundaries of the hippocampi, calculating a midline, and then reconstructing 4 pixels along the length of the hippocampus centered over the mid-line. The positions are selected by centering pixel #2 at the level of the aqueduct and reconstructing two pixels anterior and pixel posterior by translating in 9mm increments along the midline. The spatial reconstruction utilizes a cosine filter to confine the point-spread function with an effective sampling volume of 1cc. The 1H data are fit in the spectral domain with the resonance areas determined as ratios. Because the location of electrodes varies slightly between subjects, to provide a consistent MR comparison, the spectroscopic data are averaged over hippocampal voxels #1 through #4.
Statistics
Group differences were evaluated using a two-tailed students t-test, p=0.05 significance. Since the statistical distribution of the total power measurements was determined not to be gaussian, and because the cross-modal measurements of electrical power and NAA/Cr did not necessarily correlate linearly, the correlation analysis between total power and NAA/Cr was performed using log transformed data. Similar to that proposed by Bartolomei 2008 (who used a comparison between high and low band power as an index of epileptogenicity), we examined the fractional high band power (i.e., a unitless ratio of gamma/total power or beta/total power) that may characterize tissue dysfunction. The fraction gamma power is assessed between the two patient groups, and in correlation with NAA/Cr. A p-value of less than 0.05 was used as the criterion for statistical significance.
Results
Group comparisons of quantitative EEG
Table 2 displays the icEEG power and energy estimates for the two groups. Although there is wide variability in both groups, a significant difference (p<0.05) in the gamma power and Teager energy was seen between the MTLE (“within-seizure-zone” 1.14±0.72, 1.16±0.37 gamma power and Teager energy respectively) group versus the neocortical (“outside-seizure-zone” 0.53±0.36, 0.63±0.36) group, with greater energies in the MTLE group. Delta power was fractionally the largest contributor to total power in both patient groups (68.8%±5.4 MTLE; 72.8%±13.3 neocortical), but there was no group difference in total and delta power. Notably, with the within-seizure-zone group, an important explanation for variability in the power measures was seen depending on the presence or absence of hippocampal tissue loss. Even in this small group where 7 of 12 MTLE patients had clinical MR-detected hippocampal atrophy, comparison of the EEG parameters shows a strong dependence with atrophy: the total power of the atrophic patients was 0.021±0.009, smaller than the non-atrophic patients at 0.070±0.041, p=0.056, consistent with the report of Zaveri et al 2001.
Table 2.
Quantitative EEG
| Total power ×1000 |
Delta | Theta | Alpha ×1000 |
Beta ×1000 |
Gamma* ×1000 |
Teager* ×1000 |
Delta/Tot Pwr |
Beta/ TotPwr ×100* |
Gamma/ TotPwr ×100* |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MTLE (n=12) | Total | 41±36 | 0.68±0.65 | 0.16±0.12 | 40±31 | 10.7±8.4 | 1.1±0.7 | 1.2±0.7 | 0.69±0.05 | 4.20±1.99 | 1.25±0.65 |
| With atrophy (n=7) | 21±9 | 0.37±0.20 | 0.09±0.05 | 27±15 | 8.0±4.9 | 1.0±0.7 | 1.0±0.4 | 0.67±0.04 | 5.03±2.12 | 1.57±0.67 | |
| Without atrophy (n=5) | 70±41 | 1.12±0.83 | 0.24±0.14 | 59±40 | 14.5±10.9 | 1.3±0.8 | 1.4±1.0 | 0.71±0.07 | 3.04±1.09 | 0.80±0.24 | |
| Non-MTLE (n=14) | Total | 64±77 | 0.73±0.85 | 0.14±0.11 | 29±22 | 5.3±3.3 | 0.5±0.4 | 0.6±0.4 | 0.73±0.13 | 2.49±2.00 | 0.70±0.71 |
| With dual (n=4) | 33±35 | 0.30±0.21 | 0.10±0.07 | 27±16 | 6.1±4.1 | 0.6±0.4 | 0.6±0.4 | 0.63±0.85 | 4.19±2.74 | 1.00±0.73 | |
| Without dual (n=10) | 77±86 | 0.91±0.96 | 0.15±0.12 | 29±24 | 5.0±3.1 | 0.5±0.3 | 0.6±0.4 | 0.77±0.13 | 1.81±1.32 | 0.59±0.69 |
p<0.05 between the MTLE and non-MTLE groups
Note that total power was assessed in the time domain; thus direct summation of the frequency bands do not match total power (see text). For ease of presentation as indicated, the values are multiplied by 100 or 1000.
We also evaluated the fractional high frequency powers by taking the ratio of beta/total power and gamma/total power. This normalization process did not substantially reduce the variation in the neocortical group; however, the observations remained similar. Both of the high frequency measures were significantly increased in the MTLE group (0.042±0.020, 0.013±0.007 fractional beta and fractional gamma power respectively) compared to the neocortical group (0.025±0.020, 0.007±0.007) at p<0.05. Similar to the above, subdividing the MTLE group showed that the atrophic patients had greater fractional gamma power than the non-atrophic patients (0.016±0.007 atrophic, 0.008±0.002 non-atrophic) significant at p<0.025.
MRS measures of NAA/Cr
In the within-seizure-zone MTLE group, the quantitative power measures were correlated with mitochondrial function as measured by the medial temporal lobe measurements of NAA/Cr (Figure 1). An analysis with the log transform on total power is significant with R=+0.84 (R2=0.70) and p<0.001. This relationship was not seen with the outside-seizure-zone neocortical group (Figure 1). In the MTLE group, consideration of the separate frequency bands with NAA/Cr showed that the relationship was best seen with the lower frequencies (delta, theta, alpha) and was less evident with the higher frequencies (data not shown). This was consistent with the observations that the lower frequency bands comprise the largest contributions to total power.
Figure 1.
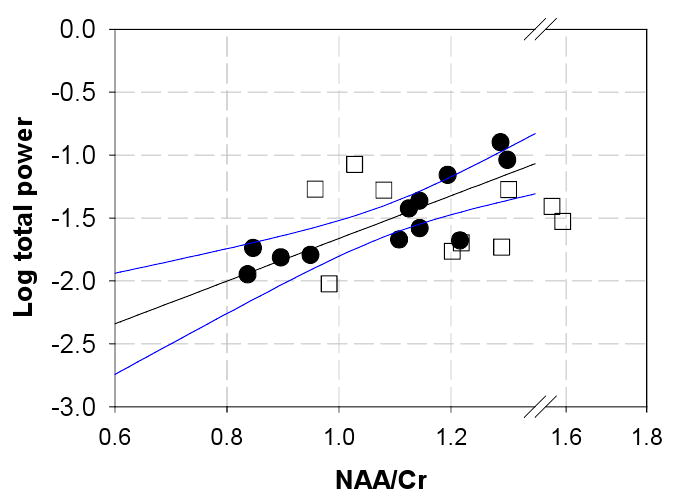
Relationship between medial temporal lobe measures of NAA/Cr and total power measured from MTLE patients (filled circles) and neocortical patients (open squares). The MTLE group data exhibits a relationship that is significant with R=+0.84, p<0.001, while no specific relationship was seen with the neocortical group. The blue lines indicate the confidence interval of the regression, calculated over the available range of the MTLE group. The plot is extended with an axis break in order to show all of the neocortical data,
Due to the question as to whether abnormal tissue metabolism was linked to the higher frequency activity, we examined how NAA/Cr correlated with fractional gamma power. In the MTLE group, Figure 2A shows a significant negative linear correlation between NAA/Cr with fractional gamma power R=−0.66 (R2 =0.44) p<0.02, with the higher fractional gamma at lower NAA/Cr. In the neocortical group, NAA/Cr was uncorrelated to fractional gamma power. It is possible that in the MTLE group, this negative relationship is a manifestation of the correlation of NAA/Cr with total power, since fractional gamma is calculated as gamma/total power. However, if this were the dominant feature, then we would expect to see comparable relationships of NAA/Cr with the other fractional frequency bands—which was not seen.
Figure 2.
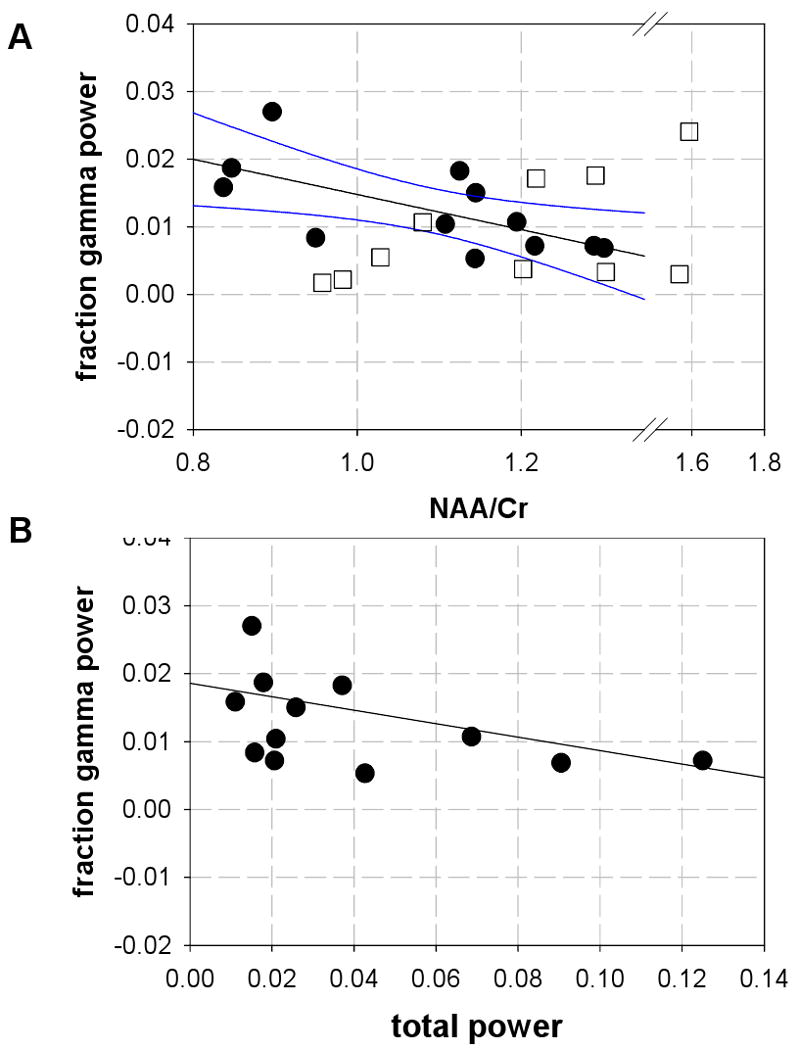
(A) Relationship between medial temporal lobe measures of NAA/Cr and fractional gamma power measured from MTLE patients (filled circles MTLE; open squares, neocortical patients). The MTLE group data exhibits a linear relationship that is significant with R=−0.66, p<0.02, while no specific relationship was seen with the neocortical group. The blue lines indicate the confidence interval of the regression. The plot is extended with an axis break in order to show all of the neocortical data,
(B) Replot of the EEG data from MTLE group. There is a significant rank correlation between total power and fraction gamma, R = −0.61 p<0.05.
Discussion
Power measures in MTLE and neocortical non-MTL epilepsy
The total EEG power in these two patient groups demonstrated wide variability such that they were not significantly different. When examining the contributions from high frequencies, the MTLE group showed greater power than the neocortical group, both in absolute values (gamma power and Teager energy) and fractionally (fractional beta and gamma power) (Table 2). Given these group differences in high frequency powers and the possible significance of gamma activity with pathology, a Spearman rank correlation was calculated between fractional gamma and total power. Within the MTLE group this rank correlation was significant (R = −0.61 p<0.05, Figure 2B) while it was not significant in the neocortical outside-seizure-zone group. These data are consistent with the hypothesis that higher frequency activity characterizes regions of seizure onset (Bragin et al 2002; Bartolomei et al 2008).
A recent report from Bettus et al 2008 has similarly evaluated intracranial medial temporal lobe EEG data from MTLE and non-MTLE patients, finding that fractional theta power was lower in the MTLE group. While we did not observe this result in the present data, the difference may have several explanations. In particular, we included dual pathology patients within the neocortical group, as the nature of electrical activity in this group may reflect propagated seizures rather than onset. Additionally, several EEG differences are present in terms of frequency band definition, sampling distribution, electrode size and referential vs. bipolar montage. For example, the power spectral density of bipolar signals will be different from those of referential signals (Zaveri 2006). With the present referential icEEG data, elimination of the n=4 dual pathology patients from the neocortical group results in a borderline significant difference with lower fractional delta power in the MTLE compared to neocortical group. Altogether, this suggests that fractional power bands can be substantively informative with regards to specific location of seizure onset.
The present data are also consistent with the findings of Zaveri et al 2001, who evaluated EEG power in sclerotic and non-sclerotic MTLE patients. Zaveri found less total and delta band power in sclerotic patients in comparison to those without sclerosis, while there was no difference in the gamma and beta powers. In the present data, the differences in total and low band power are suggestive, with smaller values in the atrophic group (0.021±0.009, 0.37±0.20 respectively total and delta band) in comparison to the non-atrophic group (0.070±0.041, 1.12±0.83) although in this small group, the differences were of borderline significance.
Relationships with NAA/Cr
As a cross modal comparison between NAA/Cr with total electrical power, the relationship of Figure 1 has a R2 that is fairly high at 0.70, meaning that a majority of data scatter in these two parameters is due to each other (a R2 = 1 means that the two parameters are wholly dependent on each other). In this MTLE group, it is likely that tissue volume loss is influencing this relationship, as suggested by Zaveri et al 2001. However, it is important to note that as a unitless ratio, the value of NAA/Cr is not biased heavily by tissue volumetry. This latter view has been re-iterated by a number of studies. While the atrophic hippocampus typically does have lower NAA/Cr and is generally regarded as clearly injured, the ratio is not specifically determined by volumetric loss, and likely more reflects mitochondrial (dys)function (Sawrie et al 2001, Brazdil et al 2008, Vielhaber et al 2008). Thus as a whole, the tight relationship of Figure 1 is consistent with a view that total electrical activity is powered by mitochondrial function. Figure 2A shows that under conditions of low NAA/Cr, electrical output is biased towards higher frequency activity. Predictably, as we verified, lower total power correlates with higher fraction gamma in the MTLE group, as seen with a significant Spearman rank correlation (Figure 2B). Thus, the seizure onset zone (with or without tissue loss) is characterized by mitochondrial dysfunction, which appears to underlie much of the interindividual variation in total electrical power; with the lower power and lower NAA/Cr, there is also greater high frequency power. Finally however given that R2 = 0.44 in Figure 2A, it is possible that mitochondrial dysfunction does not underlie all of the variation in fraction gamma power so that other independent factors may also be contributing.
The data from neocortical patients argues that outside of the seizure focus, the electrical output is less dependent on mitochondrial function. However, the neocortical epilepsy group is heterogeneous, comprised of patients with seizure onset from frontal, temporal and occipital lobes. It is possible that with larger sample numbers for each epilepsy type, we may similarly find relationships of NAA/Cr with total power. Nonetheless, it is clear that the in vivo electrophysiology of this neocortical group demonstrates a different influence from metabolic function in comparison to the MTLE group.
Acknowledgments
This work supported by NIH EB000473, NS054038 and the C.G. Swebilius Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartolomei F, Chauvel P, Wendling F. Epileptogenicity of brain structures in human temporal lobe epilepsy: a quantified study from intracerebral EEG. Brain. 2008;131:1818–1830. doi: 10.1093/brain/awn111. [DOI] [PubMed] [Google Scholar]
- Bettus G, Wendling F, Guye M, Valton L, Regis J, Chauvel P, Bartolomei F. Enhanced EEG functional connectivity in mesial temporal lobe epilepsy. Epilepsy Res. 2008;81:58–68. doi: 10.1016/j.eplepsyres.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Bates TE, Strangward M, Keelan J, Davey GP, Munro PM, Clark JB. Inhibition of N-acetylaspartate production: implications for 1H MRS studies in vivo. Neuroreport. 1996;7(8):1397–400. [PubMed] [Google Scholar]
- Bragin A, Mody I, Wilson CL, Engel J., Jr Local generation of fast ripples in epileptic brain. J Neurosci. 2002;22:2012–21. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brázdil M, Mareček R, Fojtíková D, Mikl M, Kuba R, Krupa P, Rektor I. Correlation study of optimized voxel-based morphometry and (1)H MRS in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. Hum Brain Mapp. 2008 doi: 10.1002/hbm.20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Schuff N, Kramer JH, Du AT, Capizzano AA, O'Neill J, Wolkowitz OM, Jagust WJ, Chui HC, Miller BL, Yaffe K, Weiner MW. Reduced medial temporal lobe N-acetylaspartate in cognitively impaired but nondemented patients. Neurology. 2005;64(2):282–9. doi: 10.1212/01.WNL.0000149638.45635.FF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillon B, Duncan R, Biraben A, Bernard AM, Vignal JP, Chauvel P. Correlation between interictal regional cerebral blood flow and depth-recorded interictal spiking in temporal lobe epilepsy. Epilepsia. 1998;39(1):67–76. doi: 10.1111/j.1528-1157.1998.tb01276.x. [DOI] [PubMed] [Google Scholar]
- Guye M, Le Fur Y, Confort-Gouny S, Ranjeva JP, Bartolomei F, Régis J, Raybaud CA, Chauvel P, Cozzone PJ. Metabolic and electrophysiological alterations in subtypes of temporal lobe epilepsy: a combined proton magnetic resonance spectroscopic imaging and depth electrodes study. Epilepsia. 2002;43(10):1197–209. doi: 10.1046/j.1528-1157.2002.05102.x. [DOI] [PubMed] [Google Scholar]
- Heales SJ, Davies SE, Bates TE, Clark JB. Depletion of brain glutathione is accompanied by impaired mitochondrial function and decreased N-acetyl aspartate concentration. Neurochem Res. 1995;20(1):31–8. doi: 10.1007/BF00995149. [DOI] [PubMed] [Google Scholar]
- Hetherington HP, Kuzniecky RI, Vives K, Devinsky O, Pacia S, Luciano D, Vasquez B, Haut S, Spencer DD, Pan JW. Thalamic and Hippocampal Dysfunction in TLE Measured by 1H MRS. Neurology. 2007;69:2256–2265. doi: 10.1212/01.wnl.0000286945.21270.6d. [DOI] [PubMed] [Google Scholar]
- Iasemidis LD, Shiau DS, Pardalos PM, Chaovalitwongse W, Narayanan K, Prasad A, Tsakalis K, Carney PR, Sackellares JC. Long-term prospective on-line real-time seizure prediction. Clin Neurophysiol. 2005;116(3):532–44. doi: 10.1016/j.clinph.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Kaiser J. On a simple algorithm to calculate the energy of a signal. Proceedings of ICASSP. 1990:381–384. [Google Scholar]
- Mormann F, Andrzejak R, Elger C, Lehnertz K. Seizure prediction: the long and winding road. Brain. 2007;130:314–333. doi: 10.1093/brain/awl241. [DOI] [PubMed] [Google Scholar]
- Pan JW, Takahashi K. Inter-dependence of NAA and high energy phosphates in healthy human brain. Ann Neurol. 2005;57(1):92–97. doi: 10.1002/ana.20317. [DOI] [PubMed] [Google Scholar]
- Pan JW, Krupp LB, Elkins LE, Coyle PK. Cognitive dysfunction lateralizes with NAA in multiple sclerosis. Appl Neuropsychol. 2001;8(3):155–60. doi: 10.1207/S15324826AN0803_4. [DOI] [PubMed] [Google Scholar]
- Sawrie SM, Martin RC, Knowlton R, Faught E, Gilliam F, Kuzniecky R. Relationships among hippocampal volumetry, proton magnetic resonance spectroscopy, and verbal memory in temporal lobe epilepsy. Epilepsia. 2001;42(11):1403–7. doi: 10.1046/j.1528-1157.2001.018301.x. [DOI] [PubMed] [Google Scholar]
- Spencer SS, Goncharova II, Duckrow RB, Novotny EJ, Zaveri HP. Interictal spikes on intracranial recording: behavior, physiology, and implications. Epilepsia. 2008;49(11):1881–92. doi: 10.1111/j.1528-1167.2008.01641.x. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Frighetto L, Behnke EJ, Mathern GW, Fields T, Bragin A, Ogren J, Fried I, Wilson CL, Engel J., Jr Increased fast ripple to ripple ratios correlate with reduced hippocampal volumes and neuron loss in temporal lobe epilepsy patients. Epilepsia. 2007;48(11):2130–8. doi: 10.1111/j.1528-1167.2007.01225.x. [DOI] [PubMed] [Google Scholar]
- Urrestarazu E, Chander R, Dubeau F, Gotman J. Interictal high-frequency oscillations (100-500 Hz) in the intracerebral EEG of epileptic patients. Brain. 2007;130(Pt 9):2354–66. doi: 10.1093/brain/awm149. [DOI] [PubMed] [Google Scholar]
- Vermathen P, Ende G, Laxer KD, Walker JA, Knowlton RC, Barbaro NM, Matson GB, Weiner MW. Temporal lobectomy for epilepsy: recovery of the contralateral hippocampus measured by (1)H MRS. Neurology. 2002;59(4):633–636. doi: 10.1212/wnl.59.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielhaber S, Niessen HG, Debska-Vielhaber G, Kudin AP, Wellmer J, Kaufmann J, Schönfeld MA, Fendrich R, Willker W, Leibfritz D, Schramm J, Elger CE, Heinze HJ, Kunz WS. Subfield-specific loss of hippocampal N-acetyl aspartate in temporal lobe epilepsy. Epilepsia. 2008;49(1):40–50. doi: 10.1111/j.1528-1167.2007.01280.x. [DOI] [PubMed] [Google Scholar]
- Worrell GA, Gardner AB, Stead SM, Hu S, Goerss S, Cascino GJ, Meyer FB, Marsh R, Litt B. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131(Pt 4):928–37. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaveri HP, Duckrow RB, de Lanerolle NC, Spencer SS. Distinguishing subtypes of temporal lobe epilepsy with background hippocampal activity. Epilepsia. 2001;42(6):725–30. doi: 10.1046/j.1528-1157.2001.00500.x. [DOI] [PubMed] [Google Scholar]
- Zaveri HP, Duckrow RB, Spencer SS. On the use of bipolar montages for the time-series analysis of intracranial electroencephalograms. Clinical Neurophysiology. 2006;117:2102–2108. doi: 10.1016/j.clinph.2006.05.032. [DOI] [PubMed] [Google Scholar]
- Zubler F, Seeck M, Landis T, Henry F, Lazeyras F. Contralateral medial temporal lobe damage in right but not left temporal lobe epilepsy: a (1)H magnetic resonance spectroscopy study. J Neurol Neurosurg Psychiatry. 2003;74(9):1240–4. doi: 10.1136/jnnp.74.9.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]


