Abstract
The decision to generate a productive immune response or tolerance often depends on the context in which T cells first see Ag. Using a classical system of tolerance induction, we examined the immunological consequence of Ag encountered in the presence of naïve or activated apoptotic cells. Naïve apoptotic cells induced tolerance when injected i.v.; however, previously activated apoptotic cells induced immunity. Further analysis revealed a key role for CD154, as tolerance resulted after i.v. injection of either naïve or activated apoptotic CD154−/− T cells, while co-injection of an agonistic anti-CD40 mAb with naïve apoptotic T cells induced robust immunity. DC fed activated apoptotic T cells in vitro produced IL-12p40 in a CD154-dependent manner, and the use of IL-12p40−/− mice or mAb-mediated neutralization of IL-12 revealed a link between CD154, IL-12, and the ability of activated apoptotic T cells to induce immunity rather than tolerance. Collectively these results show that CD154 expression on apoptotic T cells can determine the outcome of an immune response to Ag recognized within the context of the apoptotic cells, and suggest the balance between naïve and activated apoptotic T cells may dictate whether a productive immune response is encouraged.
INTRODUCTION
Cell death is important to homeostasis in the immune system and critical to maintaining important T cell populations. Developing thymocytes undergo apoptosis during selection in the thymus leading to the removal of self-reactive clones (1); while effector T cells undergo activated-induced apoptotic cell death following an immune response leaving behind a small memory pool of cells to respond to future challenges (2). Although the apoptosis occurring in these situations does not typically evoke an immunological response, the death of large numbers T cells in the periphery can induce a tolerogenic response (3). In this scenario, apoptotic T cells are cross-presented to the immune system resulting in the activation of CD8+ regulatory T cells that kill effector T cell targets (4, 5). This form of tolerance extends to naïve T cells of the same specificity, and suggests that this active immunoregulation is directed toward the Ag-reactive T cells for the purpose of controlling any self responses that might be generated when large numbers of T cells die and release potentially dangerous autoantigens and cytokines (3, 4). During certain infections there is also the death of large numbers of cells; however, in many cases productive immunity can develop. For example, large numbers of activated T cells can die by apoptosis during the initial steps of the immune response to Listeria monocytogenes (6, 7), but in this case the dead cells do not generate tolerance that would prevent essential T cell-mediated immunity from developing. The question then is what determines whether T cell apoptosis leads to tolerance or a productive immune response?
The role of apoptosis in the maintenance of immunological homeostasis and tolerance has been inferred from several experimental findings; for example, tolerance induction after injection of apoptotic cells and the capacity of APC to induce and maintain tolerance after dead cell phagocytosis (4, 8, 9). Furthermore, the therapeutic use of apoptotic cells has been extensively explored in the transplantation field (10–13). Interestingly, the combination of donor-specific transfusion of apoptotic cells and blockade of the CD40-CD154 pathway profoundly increases transplant survival (14–18). While the use of apoptotic cells to prolong the survival of organ transplants (13) and treat autoimmune diseases (19) is well-documented, the mechanism(s) by which tolerance versus immunity is induced to Ag presented within the context of dead cells remain(s) unclear.
It has recently become evident that there are a number of properties of apoptotic cells that can determine whether tolerance or immunity is induced. For example, apoptotic cells can release immunosuppressive (20) or immunogenic molecules (21) that modulate immunity. In addition, caspase activation and ROS production during apoptosis are critical factors that determine whether immunity or tolerance is induced (22). These observations together suggest that apoptotic cells have some intrinsic properties that can modulate immunity. There has also been some data suggesting that the activation status of the cell undergoing apoptosis can play a role in determining the type of immune response generated (23); however, the precise mechanism for this has not been determined. In the present study we examined the effect of T cell apoptosis on the immune system with the hypothesis that the activation state of the apoptotic T cells will dictate the outcome of the immune response. Using a well-established i.v. tolerance model, where hapten-modified apoptotic cells are injected intravenously (i.v.) into mice prior to active immunization (24–27), we compared the tolerogenic nature of naïve and activated apoptotic T cells. Our results demonstrate that, in contrast to naïve apoptotic cells, activated apoptotic CD4+ T cells did not induce tolerance because they express CD154. CD154 induces IL-12 production, which also promotes immunity rather than tolerance. Thus, the outcome of an encounter between apoptotic T cells and the immune system can depend on the activation state of the apoptotic T cells, implying that T cell death during an active T cell response may function to further promote immunity.
METHODS
Animals and reagents
C57Bl/6 and BALB/c mice were purchased from The National Cancer Institute. CD154−/− mice were obtained from Dr. Timothy Ratliff (Purdue University, West Lafayette, IN), CD40−/− mice were obtained from Dr. Gail Bishop (University of Iowa, Iowa City, IA), Kb−/− act-mOVA mice (28) were obtained from Dr. John Harty (University of Iowa), and OT-II mice were obtained from Dr. Yi Luo (University of Iowa). CD154−/−, CD40−/−, Kb−/− act-mOVA, and OT-II mice were backcrossed onto the C57Bl/6 background >10 generations. IL-12p40−/− mice were obtained from Dr. Kevin Legge (University of Iowa), and were backcrossed onto the BALB/c background >10 generations. Anti-CD4 (GK1.5) and -CD40 (FGK45) mAb were purified from hybridoma supernatants. Neutralizing anti-IL-12 mAb (15.1.2 and 15.6.7) (29) were obtained from Dr. Robert Fairchild (The Cleveland Clinic, Cleveland OH). Neutralizing anti-IL-23, PE-conjugated anti-CD80, and PE-conjugated anti-CD86 mAb were purchased from eBioscience (eBioscience, San Diego, CA). Rat IgG isotype control was obtained from Dr. Thomas Waldschmidt (University of Iowa). All animal procedures were performed according to National Institutes of Health guidelines and approved by the University of Iowa IACUC. In all in vivo experiments, groups consisted of 4 or more animals, and experiments were repeated at least 2 times with similar results before reporting.
Activation and trinitrophenyl (TNP) coupling of spleen cells
Spleen cells were isolated and activated with either PMA/ionomycin (20 ng/ml: 200 ng/ml; Sigma Chemicals, St. Louis, MO) or plate-bound anti-CD3/anti-CD28 mAb (1 μg/ml; eBioscience) for 16 h. Splenocyte activation was confirmed by measuring CD25 and CD69 upregulation using FITC-conjugated specific mAb (eBioscience) and flow cytometry. CD154 expression on naïve and activated CD4+ T cells was measured by adding either biotinylated anti-CD154 (MR1; 1 μg/ml; Miltenyi) or biotinylated isotype mAb (hamster IgG; eBioscience) to the cells while they were in culture being activated with PMA: ionomycin. After 16 h, the cells were washed, stained with FITC-conjugated anti-CD4 mAb (eBioscience), fixed with 2% paraformaldehyde and permeabilized with 0.05% saponin buffer, and incubated with streptavidin-PE. CD154 expression on CD4+ T cells was then determined by flow cytometry. Single cell suspensions of freshly isolated naïve or activated spleen cells were coupled with TNP as previously described (4). Briefly, 108 cells were incubated in 0.5 ml PBS and 0.5 ml 10 mM TNBS for 5 min at room temperature. After incubation, the cells were washed three times with PBS before use. Naïve or activated TNP-coupled spleen cells were then γ-irradiated (3000 R) to induce apoptosis. Necrotic cells were prepared by alternating five freeze/thaw cycles using liquid nitrogen/37°C water bath. TNP-coupled apoptotic (107) or equivalent necrotic cells were injected i.v. via the retro-orbital plexus.
Measurement of immune response – tolerogenicity and immunogenicity
In the i.v. tolerance system used herein, the tolerogenicity and immunogenicity of i.v. administered apoptotic cells was tested by slightly modifying the experimental design (22). To measure the tolerogenicity of the i.v. administered apoptotic cells, apoptosis was first induced in haptenated (TNP-coupled) splenocytes by γ-irradiation, and then the cells were immediately injected i.v. After 48 h, animals were immunized with 0.1 ml of 10 mM TNBS s.c. After another 4 d, the immune response was examined by challenging the animals with 33 μl of 10 mM TNBS in the right footpad and 33 μl of PBS in the left footpad. The DTH response to TNBS (TNP) is measured 24 h later by a masked observer. Values were expressed in micrometer (μm ± S.E.) and represent the difference between the TNBS challenged right footpad and PBS challenged left footpad of the same mouse. Background values represent the difference between the challenged and unchallenged footpad in naïve mice. To measure the immunogenicity of the i.v. administered apoptotic cells, the haptenated cells were γ-irradiated to induce apoptosis and injected i.v. as described above, but the mice were challenged 5 d later with the footpad injection of TNBS.
CD8+ T cell, CD4+ T cell, and CD11c+ DC isolation
CD8+ T cells were purified from splenocyte single cell suspensions by negative selection using a CD8+ T cell isolation kit (Miltenyi Biotec, Auburn, CA), per manufacturer’s instruction. CD4+ T cells were purified from the spleens of C57BL/6 or OT-II mice by negative selection using a CD4+ T cell isolation kit (Miltenyi Biotec), per manufacturer’s instruction. Enriched CD8+ T cells, CD4+ T cells, or CD4+ OT-II T cells were adoptively transferred i.v. via the retro-orbital plexus. CD11c+ DC were isolated from the spleens of C57Bl/6 mice injected with Ad5-Flt3L (30) by positive selection using CD11c (N418) MicroBeads (Myltenyi Biotec). Purified CD11c+ DC (0.5 × 106) were cultured with naïve or activated apoptotic cells (106) in complete RPMI (HyClone, Logan, UT) in a total volume of 1 ml for 48 h. As a negative or positive control, CD11c+ DC were either cultured alone or with 1 μg LPS, respectively. After culture, supernatants were collected, and analyzed for the presence of IL-12p40 by ELISA (eBioscience). In some cases, CD80 and CD86 expression was also measured on the DC by flow cytometry. Purity of the isolated populations was determined by flow cytometry using anti-CD4, -CD8, and -CD11c mAb, and was >90%.
In vivo proliferation
To measure the ability of Ag derived from naïve or activated apoptotic cells to stimulate T cell proliferation, C57Bl/6 mice were injected with 106 CFSE-labeled OT-II T cells i.v. 24 h before receiving 107 naïve or activated apoptotic act-mOVA spleen cells i.v. After 5 d, splenocytes from the recipient mice were isolated, and cell proliferation, as measured by CFSE dilution, was determined by flow cytometry.
OVA323-339 restimulation and intracellular INF-γ staining
C57Bl/6 mice were injected with 106 OT-II T cells i.v. 24 h before receiving 107 naïve or PMA/ionomycin-activated apoptotic (induced by γ-irradiation) act-mOVA spleen cells i.v. After 5 d, splenocytes from the recipient mice were isolated, and 2 × 106 cells were cultured in vitro alone or restimulated with OVA323-339 (3 μg/ml) for 5 h in the presence of Brefeldin A (3 μg/ml). The cells were collected and processed for IFN-γ intracellular staining using a PE-conjugated anti-IFN-γ mAb (eBioscience). The percentage of IFN-γ-producing OT-II T cells [as determined by PE-Cy5-conjugated anti-CD90.1 mAb (eBioscience) staining] in response to OVA323-339 restimulation (after subtracting the percentage of IFN-γ+ OT-II T cell from unstimulated cultures) was then reported.
Statistical analysis
Significant differences between groups were evaluated using a two-tailed Student’s t test (p < 0.05).
RESULTS
Activated apoptotic cells prime for immunity and do not induce tolerance
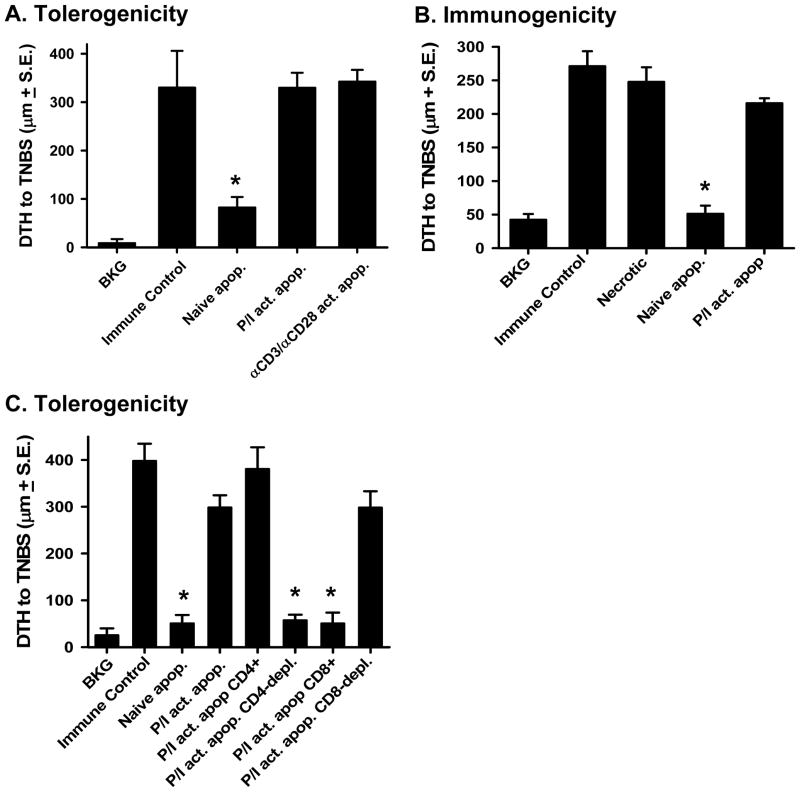
The activation state of immune cells undergoing apoptotic death can dramatically influence immune function (23), and activated apoptotic cells, but not naïve apoptotic cells, can mature and activate human DC in vitro (31). We therefore explored the role of activated apoptotic cells in a classical tolerance model (4, 5, 22), where hapten-modified apoptotic cells are injected i.v. to induce tolerance or measure immunity (supplemental Figure 1). Animals were given either haptenated (TNP-coupled) naïve or activated apoptotic cells (supplemental Figure 2) i.v. 48 h before s.c. immunization. Data in Figure 1A show that naïve apoptotic cells were tolerogenic, which is consistent with previous results (5), but the activated apoptotic cells (either PMA/ionomycin- or anti-CD3/anti-CD28-activated) were unable to induce tolerance (Figure 1A). Thus, T cell activation prior to the induction of apoptosis abrogates the tolerogenic effect of the apoptotic cells. Administration of apoptotic cells i.v. does not directly prime a CD4+ T cell-mediated immune response, while necrotic cells can stimulate potent CD4+ T cell-mediated immunity (5). Similar to necrotic cells, i.v.-administered activated apoptotic cells were also strongly immunogenic (Figure 1B). Since we used agents that specifically activate T cells, we then tested whether there was a specific subpopulation within the activated splenocytes responsible for overcoming tolerance. Purified CD4+ T cells, purified CD8+ T cells, and the correspondingly T cell-depleted splenocytes were then activated with PMA/ionomycin. The activated cells were haptenated, irradiated, and injected i.v. 48 h before s.c. immunization. The results show that tolerance was observed when activated apoptotic splenocytes were depleted of CD4+ cells or when purified activated apoptotic CD8+ T cells were injected i.v. (Figure 1C). However, activated apoptotic CD8-depleted splenocytes and purified activated apoptotic CD4+ T cells were able to abrogate tolerance as well as bulk splenocytes. These results indicate that it is the activated apoptotic CD4+ T cells that prevent tolerance induction.
Figure 1. Activated apoptotic cells prime for immunity and do not induce tolerance when injected i.v.
A. Tolerogenicity – C57Bl/6 mice were injected i.v. with naïve or activated (PMA/ionomycin- or anti-CD3/CD28-stimulated) apoptotic TNP-coupled splenocytes i.v. 48 h before TNBS immunization. After 4 d, mice were challenged with TNBS in the right and PBS in the left footpad. B. Immunogenicity - C57Bl/6 mice were injected i.v. with necrotic, naïve apoptotic, or activated apoptotic TNP-coupled splenocytes. Four days later, mice were challenged with TNBS in the right and PBS in the left footpad. C. Tolerogenicity - C57Bl/6 mice were injected i.v. with naïve or activated apoptotic TNP-coupled splenocytes i.v. 48 h before TNBS immunization. The activated populations included bulk splenocytes, purified CD4+ T cells, CD4-depleted splenocytes, purified CD8+ T cells, or CD8-depleted splenocytes. After 4 d, mice were challenged with TNBS in the right and PBS in the left footpad. In A.–C., measurements (μm ± S.E.) were taken 24 h later and represent the difference between right and left footpad. Immune control groups were injected with TNBS 4 d before footpad challenge, and background (BKG) groups were only footpad challenged. * p < 0.05 vs. immune control.
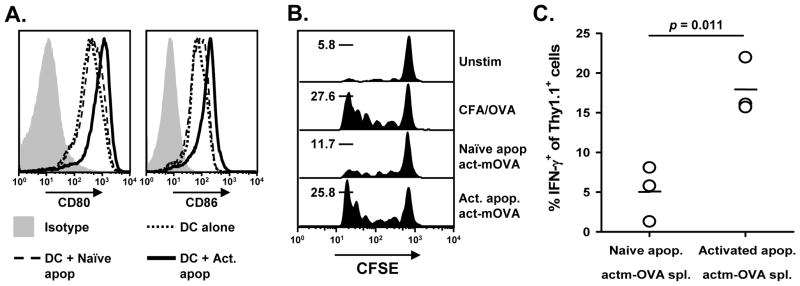
Apoptotic cells induce tolerance following i.v. injection by being engulfed by splenic DC and cross presented to the immune system in a tolerogenic form. Recent work by Ren et al. found that DC exposed to apoptotic cells (dexamethasone-treated splenocytes) inhibited T cell expansion (32). Thus, one potential difference between naïve and activate apoptotic cells could be that DC that have eaten the naïve apoptotic cells are unable to stimulate CD4+ T cell proliferation, whereas DC that have engulfed activated apoptotic cells become properly licensed to induce a proliferative response. We tested this possibility by feeding CD11c+ DC either naïve or activated splenocytes, and then measured CD80 and CD86 expression on the DC 48 h later. DC fed activated apoptotic cells upregulated CD80 and CD86 expression, whereas DC fed naïve apoptotic cells expressed these molecules at a similar level as DC cultured alone (Figure 2A).
Figure 2. Activated apoptotic cells, but not naïve apoptotic cells, license DC to prime CD4+ T cells.
A. CD11c+ DC were isolated and cultured with naïve or activated (PMA/ionomycin) apoptotic cells for 48 h. CD80 and CD86 expression was then measured by flow cytometry. B. C57Bl/6 mice received 106 CFSE-labeled OT-II T cells 24 h before receiving 107 naïve or activated apoptotic Kb−/− act-mOVA splenocytes i.v. After 5 d, splenocytes from the recipient mice were isolated, and cell proliferation, as measured by CFSE dilution, was determined by flow cytometry. The percentage of cells that underwent 5 divisions is indicated for each group (n = 3). C. Alternatively, splenocytes from the recipient mice were isolated and cultured in vitro alone or restimulated with OVA323-339 (3 μg/ml) for 5 h in the presence of Brefeldin A (3 μg/ml). The cells were collected and processed for IFN-γ intracellular staining. The percentage of IFN-γ-producing OT-II T cells in response to OVA323-339 restimulation (after subtracting the percentage of IFN-γ+ OT-II T cell from unstimulated cultures) is depicted.
We then tested the ability of the i.v. administered naïve or activated apoptotic cells to prime CD4+ T cells in vivo. To do this, CFSE-labeled OT-II T cells were adoptively transferred into C57Bl/6 mice 24 h before injecting naïve or activated apoptotic Kb−/− act-mOVA splenocytes (28). After 5 d, splenocytes from the recipient mice were isolated, and OT-II T cell proliferation was assessed by measuring CFSE dilution. There was robust OT-II proliferation when the mice were given activated apoptotic act-mOVA cells (comparable to that seen in mice immunized with CFA/OVA), but the proliferation was much reduced when naïve apoptotic cells were administered (Figure 2B). We also took splenocytes from the recipient mice and cultured them in vitro for 48 h alone or with OVA323-339. The cells were processed to detect OT-II T cell production of IFN-γ by flow cytometry, which revealed that the mice primed with naïve apoptotic cells contained significantly less IFN-γ producing OT-II T cells after in vitro restimulation compared to mice primed with activated apoptotic cells (Figure 2C). Similar to the experiments with haptenated splenocytes, the i.v. administration of naïve apoptotic act-mOVA splenocytes to C57BL/6 mice induced tolerance to ovalbumin, but the delivery of activated apoptotic act-mOVA splenocytes lead to immunity (supplemental Figure 3). Thus, the tolerance observed after i.v. administration of naïve apoptotic cells relates to the failure of the naïve apoptotic cells to stimulate CD4+ T cell proliferation and attain full effector function [i.e. inability to produce proinflammatory cytokines (IFN-γ)]. Collectively, the results in Figures 1 and 2 show that the activation status of cells prior to inducing apoptosis has dramatically different consequences on the response of the immune system.
CD154 expression abolishes the ability of activated apoptotic cells to induce tolerance
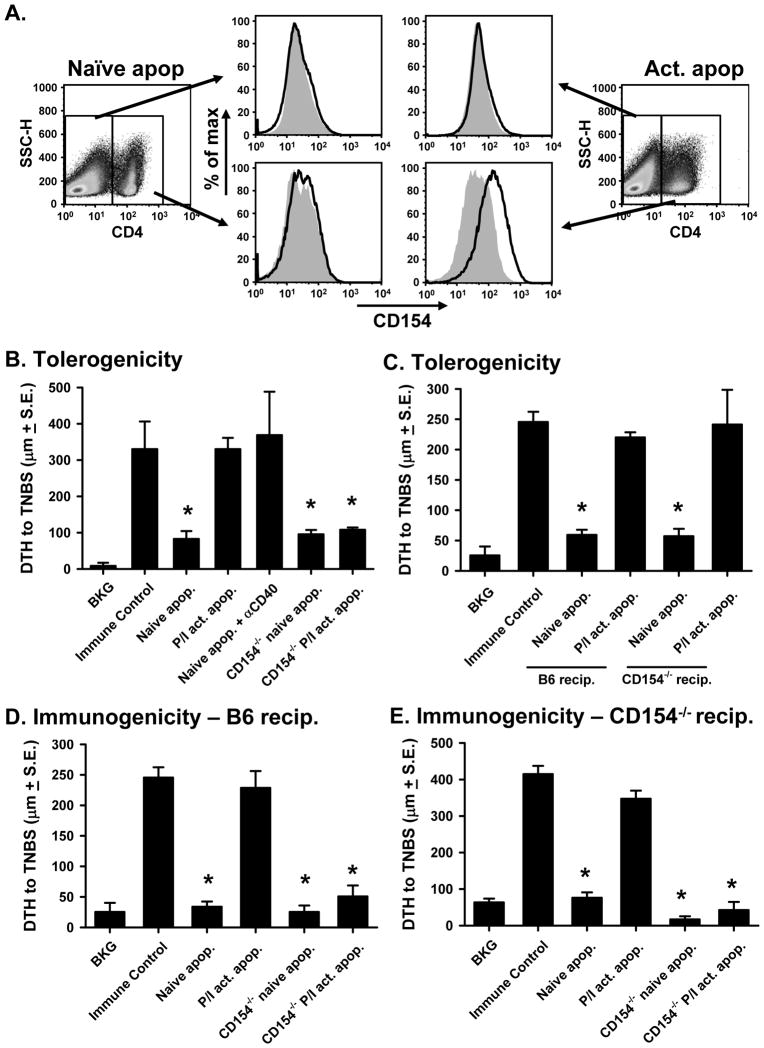
Naïve and activated T cells differ in their expression of cell surface markers; specifically, activated cells have increased levels of co-stimulatory molecules and their ligands (33, 34), prompting us to test whether this could account for the different immunological outcome following injection of naïve and activated apoptotic cells. One important costimulatory protein expressed early after T cell activation is CD154 (35–37), which is readily upregulated on CD4+ T cells within PMA/ionomycin-activated splenocytes (Figure 3A). CD154 is involved in the activation and licensing of DC through CD40 ligation (38, 39), and we have previously shown that the combination of agonistic anti-CD40 mAb with naïve apoptotic cells primes for immunity (4). This observation was verified in Figure 3B, where co-injection of naïve apoptotic cells and anti-CD40 mAb induced immunity instead of tolerance. We then tested the role of CD40-CD154 interactions in the present system by using splenocytes from CD154+/+ and CD154−/− mice. Injection of naïve CD154+/+ and CD154−/− apoptotic cells prior to immunization induced tolerance, while the injection of the activated (CD154+/+) apoptotic cells did not (Figure 3B). Interestingly, activated apoptotic CD154−/− splenocytes (supplemental Figure 4) administered i.v. into C57Bl/6 recipients induced tolerance rather than immunity. The relevant CD154 expression was on the activated apoptotic cells and not in the recipient mice because i.v. treatment of CD154−/− recipients with naïve or activated apoptotic CD154+/+ cells led to similar results (Figure 3C). Importantly, the CD154−/− activated apoptotic cells were unable to prime for immunity when injected i.v. into either C57Bl/6 (Figure 3D) or CD154−/− recipients (Figure 3E), suggesting the essential role of CD154 expression on the activated apoptotic cells for promoting immunity. Collectively, the data in Figure 3 demonstrate that activation-induced expression of CD154 on apoptotic lymphocytes is instrumental in instructing the immune system’s response to Ag associated with the apoptotic cells.
Figure 3. Tolerogenicity of i.v.-injected apoptotic cells is determined by CD154 expression.
A. C57Bl/6 splenocytes were activated for 16 h with PMA/ionomycin before measuring CD154 expression on CD4+ T cells. B. Tolerogenicity – C57Bl/6 mice were injected i.v. with CD154+/+ or CD154−/− naïve or activated apoptotic TNP-coupled splenocytes i.v. 48 h before TNBS immunization. Some of the mice were injected with naïve apoptotic TNP-coupled splenocytes and agonistic anti-CD40 mAb (FGK45; 300 μg). After 4 d, mice were challenged with TNBS in the right and PBS in the left footpad. C. Tolerogenicity – C57Bl/6 or CD154−/− mice were injected i.v. with CD154+/+ naïve or activated apoptotic TNP-coupled splenocytes i.v. 48 h before TNBS immunization. After 4 d, mice were challenged with TNBS in the right and PBS in the left footpad. D & E. Immunogenicity - C57BL/6 (D) and CD154−/− (E) mice were injected i.v. with CD154+/+ or CD154−/− naïve or activated apoptotic TNP-coupled spleen cells. Four days later, mice were challenged with TNBS in the right and PBS in the left footpad. In B.–E., measurements (μm ± S.E.) were taken 24 h after footpad challenge and represent the difference between right and left footpad. In all cases, immune control groups were injected with TNBS 4 d before footpad challenge, and background (BKG) groups were only footpad challenged. * p < 0.05 vs. immune control.
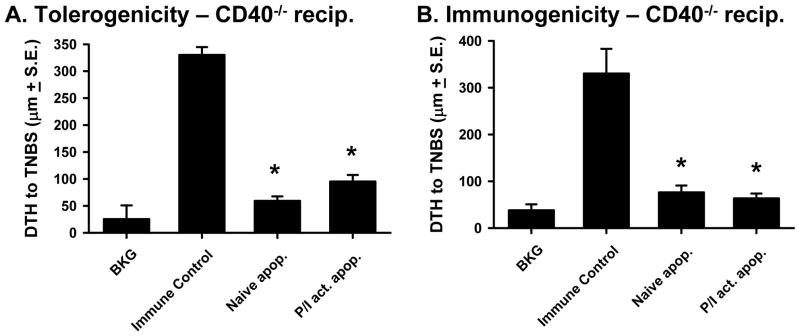
Because CD40 is the only receptor known to bind to CD154 (40), it was predicted that CD154-expressing, activated apoptotic cells would be tolerogenic when injected into CD40−/− recipient mice before immunization, and would also be unable to directly prime for immunity. This proved to be the case as both naïve and activated C57Bl/6 apoptotic splenocytes injected i.v. into CD40−/− recipients prior to immunization induced tolerance (Figure 4A), and neither apoptotic cell population directly primed for immunity (Figure 4B). Together with the data in Figure 3, these results suggest that the tolerogenic or immunogenic properties of apoptotic cells can be determined by CD154 expression, which can simulate CD40-expressing cells in the recipient.
Figure 4. CD40−/− mice are tolerized by naïve and activated apoptotic cells.
A. Tolerogenicity – CD40−/− mice were injected i.v. with CD154+/+ naïve or activated (PMA/ionomycin) apoptotic TNP-coupled splenocytes i.v. 48 h before TNBS immunization. After 4 d, mice were challenged with TNBS in the right and PBS in the left footpad. B. Immunogenicity – CD40−/− mice were injected i.v. with naïve or activated apoptotic TNP-coupled spleen cells. Four days later, mice were challenged with TNBS in the right and PBS in the left footpad. In both A. & B., measurements (μm ± S.E.) were taken 24 h later and represent the difference between right and left footpad. Immune control groups were injected with TNBS 4 d before footpad challenge, and background (BKG) groups were only footpad challenged. * p < 0.05 vs. immune control.
Activated apoptotic T cells stimulate immunity by inducing IL-12 from DC
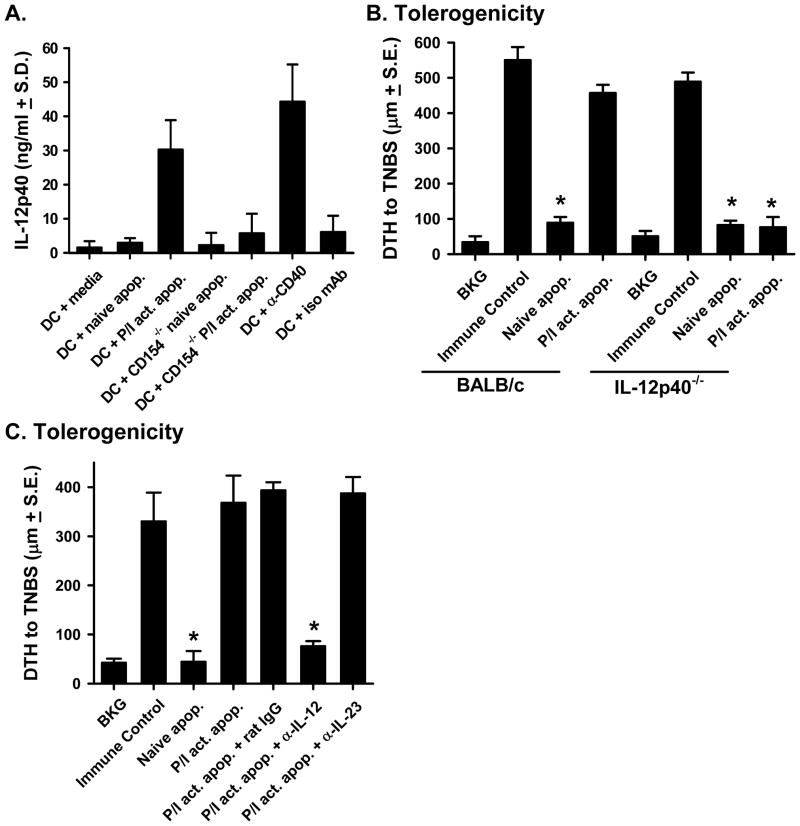
Data in Figure 1 demonstrated that naïve and activated apoptotic cells stimulated CD4+ T cell proliferation through the DC; however, only activated apoptotic cells induced effector cytokine production (IFN-γ). These results suggest that activated apoptotic cells might stimulate DC function to promote immunity. One DC-derived cytokine important for stimulating CD4+ T cell function is IL-12 (41–43), so we initially examined the ability of naïve or activated apoptotic cells to stimulate DC to produce this cytokine. Naïve or activated apoptotic spleen cells were added to DC for 48 h, and the culture supernatants were analyzed for IL-12p40. When the DC were cultured with CD154+/+ activated apoptotic cells, significant IL-12p40 was produced (Figure 5A). In contrast, DC cultured with naïve apoptotic cells (either CD154+/+ or CD154−/−) or DC cultured with CD154−/− activated apoptotic cells did not produce significant IL-12p40 above background levels (DC + media). These results demonstrate that CD154 expression on the activated apoptotic cells induce DC production of IL-12p40.
Figure 5. The inability of activated apoptotic cells to induce tolerance relates to IL-12 production.
A. Splenic CD11c+ DC were isolated and cultured alone, with naïve or activated apoptotic CD154+/+ splenocytes, with naïve or activated apoptotic CD154−/− splenocytes, or with an agonistic anti-CD40 mAb (FGK45) or isotype control mAb. After 48 h, the amount of IL-12p40 in the culture supernatant was measured by ELISA. B. Tolerogenicity – BALB/c or IL-12p40−/− mice were injected i.v. with naïve or activated (PMA/ionomycin) apoptotic TNP-coupled splenocytes i.v. 48 h before TNBS immunization. After 4 d, mice were challenged with TNBS in the right and PBS in the left footpad. C. Tolerogenicity – C57BL/6 were injected i.v. with naïve or activated apoptotic TNP-coupled splenocytes i.v. 48 h before TNBS immunization. Some of the mice were injected with activated apoptotic TNP-coupled splenocytes and anti-IL-12 mAb (200 μg each of 15.1.2 and 15.6.7), anti-IL-23 mAb (10 μg), or isotype control mAb (400 μg). After 4 d, mice were challenged with TNBS in the right and PBS in the left footpad. In A. & B., measurements (μm ± S.E.) were taken 24 h after footpad challenge and represent the difference between right and left footpad. Immune control groups were injected with TNBS 4 d before footpad challenge, and background (BKG) groups were only footpad challenged. * p < 0.05 vs. immune control.
We further examined the role of activated apoptotic cell-induced IL-12 using two complementary in vivo methods. In the first approach, naïve and activated apoptotic cells were injected i.v. into BALB/c or IL-12p40−/− mice, and the mice were immunized 48h later. When the DTH response was measured 4 d later, we found that both naïve and activated apoptotic cells induced tolerance (Figure 5B). Since IL-12p40 can form a heterodimer with either IL-12p35 or IL-23p19 to form bioactive IL-12 or IL-23, respectively (44), our second approach used neutralizing mAb to specifically assess the importance of these cytokines. C57BL/6 mice were given naïve or activated apoptotic cells 48 h before immunization. In addition, some of the mice that received activated apoptotic cells also received either neutralizing anti-IL-12 (29) or anti-IL-23 mAb. Specific neutralization of IL-12, but not IL-23, restored tolerance in mice given activated apoptotic cells (Figure 5C). Together, these results show that IL-12 production is required for activated apoptotic cells to induce immunity, and suggest a link between CD154, IL-12, and the ability of activated apoptotic cells to stimulate immunity.
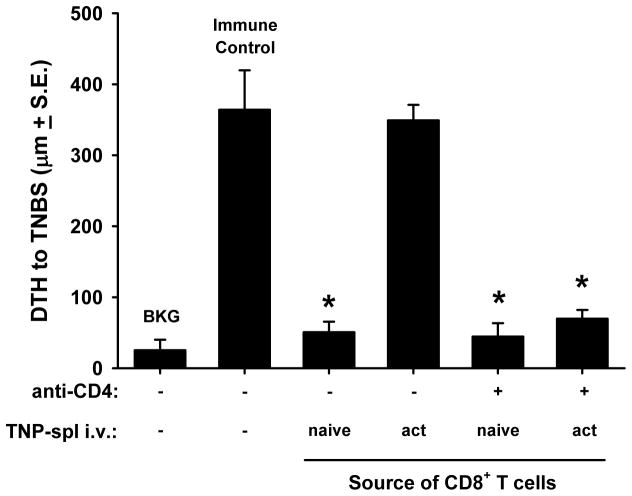
Eliminating CD4+ T cell help restores infectious tolerance mediated by CD8+ T cells in mice given activated apoptotic cells
We have previously shown that CD8+ T cells from mice given naïve apoptotic cells can transfer tolerance to naïve recipients (“infectious tolerance”) (5). In addition, we know that these CD8+ regulatory T cells (Treg) are induced because of the lack of CD4+ T cell-mediated “help”; as demonstrated by depletion of CD4+ T cells converting immunogenic necrotic cells into a tolerogen (5). One prediction would be that activated apoptotic cells would not induce CD8+ Treg (because they stimulate CD4+ T cell help), and that removal of CD4+ T cells would again reveal a CD8+ Treg population. To test this hypothesis, naïve or activated apoptotic cells were injected into intact or CD4+ T cell-depleted mice. After 7 d, CD8+ T cells were purified from the spleens of these mice and transferred into naïve recipients. These mice were immediately immunized, challenged after 4 d, and immunity was measured 24 h later. Consistent with our previous reports, CD8+ T cells from mice previously given naïve, apoptotic cells transferred tolerance to naïve mice (4, 5); however, CD8+ T cells from mice previously given activated, apoptotic cells did not (Figure 6). Interestingly, CD8+ T cells from CD4+ T cell-depleted mice given activated apoptotic cells treated mice now transferred tolerance to naïve recipients. While the removal of CD4+ T cells did not alter generation of the CD8+ Treg following naïve apoptotic cells delivery, the depletion of CD4+ helper T cells led to the conversion of the immunogenic CD154-expressing activated apoptotic T cells into a tolerizing signal capable of stimulating the development of CD8+ Treg. Thus, the tolerogenic or immunogenic nature of naïve or activated apoptotic cells correlates with the activation of CD4+ T cells.
Figure 6. Activated apoptotic cells induce immunity by stimulating CD4-mediated help.
C57Bl/6 mice or C57Bl/6 mice depleted of CD4+ cells (anti-CD4 GK1.5 (100 μg/d/mouse for 3 d) were injected i.v. with naïve or activated (PMA/ionomycin) apoptotic TNP-coupled splenocytes. Seven days later, spleens were removed and CD8+ T cells were isolated by negative selection. Purified CD8+ T cells were then transferred to naïve C57Bl/6 mice, which were immediately immunized with TNBS. Four days later, mice were challenged with TNBS in the right and PBS in the left footpad. Measurements (μm ± standard error) were taken 24 h later and represent the difference between right and left footpad. Immune control groups were injected with TNBS 4 d before footpad challenge, and background (BKG) groups were only footpad challenged. * p < 0.05 vs. immune control.
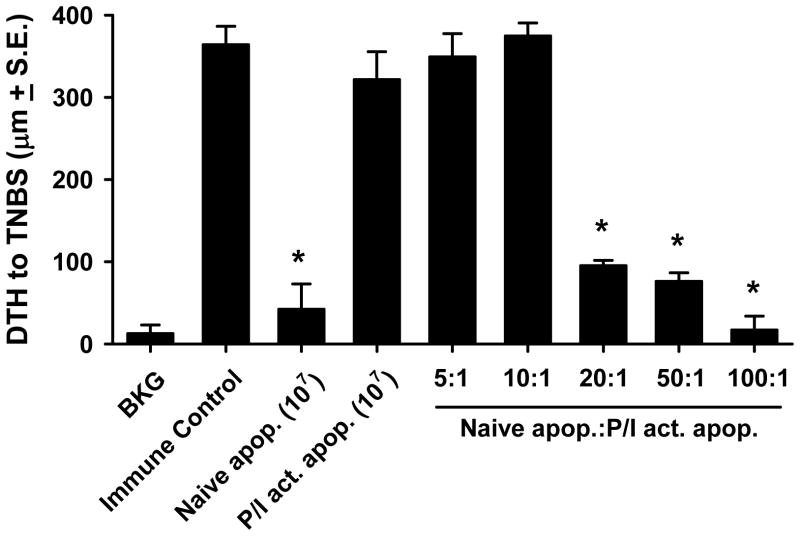
Activated apoptotic cells are dominant over naïve apoptotic cells in instructing the immune system
The data presented demonstrate that naïve apoptotic cells induce immune tolerance when administered i.v.; in sharp contrast, activated apoptotic cells are potent stimulators of immunity when given by the same route. As we have shown both populations of apoptotic cells interact with DC to stimulate their respective effects, the question then arose as to what would be the result if both populations of apoptotic cells (i.e. naïve and activated) were simultaneously presented to the immune system. This was addressed with the experiment in Figure 7. Mice received 107 apoptotic cells i.v. 48 h before immunization, but with each injection the ratio of naïve:activated apoptotic cells was varied. The results show that with ratios of 5:1 and 10:1 naïve:activated apoptotic cells, the effect of activated apoptotic cells was dominant (i.e. no tolerance). Only when the naïve:activated apoptotic cells ratio was 20:1 or higher was tolerance observed. Thus, activated apoptotic cells show a dominant effect on the outcome of an immune response taking place in the presence of dead cells.
Figure 7. Activated apoptotic cells are dominant over naïve apoptotic cells in the induction of immunity versus tolerance.
C57Bl/6 mice were injected i.v. with naïve or activated (PMA/ionomycin) apoptotic TNP-coupled splenocytes i.v. 48 h before TNBS immunization. Some groups of mice received a mixture of naïve and activated apoptotic TNP-coupled splenocytes (107 cells total) at the indicated ratios. After 4 d, mice were challenged with TNBS in the right and PBS in the left footpad. Measurements (μm ± S.E.) were taken 24 h later and represent the difference between right and left footpad. Immune control groups were injected with TNBS 4 d before footpad challenge, and background (BKG) groups were only footpad challenged. * p < 0.05 vs. immune control.
DISCUSSION
The concept that physiological cell death (i.e. apoptosis) was tolerogenic and pathological cell death (i.e. necrosis) was immunogenic was historically based on the nature of the death process and the molecular signals produced by the dying cells. As investigation of cell death became more sophisticated, it was concluded that the differences between tolerogenic and immunogenic cell death extended well beyond the distinct molecular differences of apoptotic and necrotic cells. A number of molecular mechanisms have been suggested to explain how apoptotic cells actively participate in determining whether tolerance or immunity ensues, such as the intrinsic antigenicity of the cells (21), the nature of the cell death stimulus (45), the cell death pathway engaged (21, 22, 46), the production of ROS (22), and the release of immunosuppressive cytokines such as IL-10 (20) and TGFβ (47). In addition, apoptotic cells express phosphatidylserine, which signals them for phagocytosis shortly after the apoptotic process has begun (48), ensuring that the apoptotic cells are rapidly cleared before damage-associated molecular patterns (DAMPs) (49, 50) can be released. In line with this idea, we have observed that the tolerogenic ability of naïve apoptotic splenocytes can be eliminated if the apoptotic cells are cultured for 18 h before i.v. injection (P.G., T.S.G, T.A.F., unpublished data), suggesting that these late apoptotic/secondary necrotic cells are now releasing DAMPs that override their normally tolerogenic nature. Necrosis was believed to be largely immunogenic because of the uncontrolled release of a number of DAMPs. For example, necrotic cell expression and/or release of heat shock proteins or HMGB1 can strongly stimulate the immune system through the interact with a number of pattern recognition receptors (21, 51, 52). HMGB1 can also be released from apoptotic cells (53, 54); however, the HMGB1 is oxidized in a caspase-dependent manner that maintains the tolerogenic effect of the apoptotic cells (22). In addition, necrosis can activate the NLRP3 inflammasome, resulting in IL-1β and Il-18 release (55). While many of the original parameters thought to distinguish tolerogenic/apoptotic/physiological cell death from immunogenic/necrotic/pathological cell death are still valid, it is important to keep in mind that immune response to dead cells can be influenced by many more molecular differences than initially thought.
The experiments described in this report were designed to investigate the impact of the cellular activation state of apoptotic lymphocytes on the immune system, with the intention of elucidating the mechanism that instructs the immune system to either ignore or respond to Ag recognized in the context of these apoptotic cells. Our results demonstrate the critical role of CD154 expression in determining the tolerogenic/immunogenic nature of the apoptotic cells administered. Consequently, our data are the first, to our knowledge, to show that i.v.-administered naïve apoptotic cells induce tolerance because they lack of CD154 expression, which is needed to stimulate IL-12 production from DC. Although CD154 expression has been reported on multiple cells of the immune system, including CD8+ T cells, B cells, granulocytes, and platelets, CD154 expression is the best characterized on CD4+ T cells (38–40, 56–58). CD154-expressing CD4+ T cells play critical roles in activating DC (38, 57, 59), as DC maturation via CD40 ligation results in costimulatory molecule expression and activates the DC to produce IL-12 (41, 42, 60, 61) – an important cytokine needed to prime T cells during infection. The use of agonistic anti-CD40 Ab with apoptotic cells (62) or CD154+ apoptotic cell lines (63) to mature DC and abrogate the tolerogenic potential of the apoptotic cells has also been previously reported. Recent studies with human PBMC demonstrated that bone marrow-derived DC cultured with naïve apoptotic cells did not activate these DC, as measured by the lack of CD80, CD86 and MHC II expression and production of different pro-inflammatory cytokines (31). In contrast, DC activation readily occurred after incubation with PBMC that had been activated prior to apoptosis induction. While broadly consistent with our data, this report failed to define the molecular mechanism behind the stimulation of human DC with activated apoptotic PBMC.
Though our data describe the importance of CD40-CD154 interactions between activated apoptotic cells and DC to abolish the tolerogenic effects of the apoptotic cells, it is important to note that presence or absence of this ligand-receptor interaction is probably not the only mechanism governing the immunogenic or tolerogenic ability of the apoptotic cells. For example, inhibition of inducible NO synthase (iNOS) by the arginine analog, NG-monomethyl-L-arginine, can reverse the ability of DC that have eaten apoptotic cells to inhibit T cell proliferation (32). It was further determined that the iNOS production by the DC was tied to IFN-γ responsiveness. IFN-γ is a potent inducer of iNOS from DC (64), but it can also stimulate DC to produce indoleamine 2,3-dioxygenase (IDO) (65). While it was not the focus of the present study, it would thus be interesting to compare DC production of iNOS and IDO following incubation with naïve or activated apoptotic cells in future studies.
One intriguing observation from our studies was that the immunogenic nature of the activated apoptotic cells correlated with the direct priming of CD4+ T cells, providing them with the ability to provide CD4+ T cell “help”. Based on the number of recent publications, it is clear the CD4+ T cell priming is essential for the induction of robust CD8+ T cell responses (66–68). It addition, activation of CD8+ T cells in the absence of CD4+ T cell help alters their programming, leading to TRAIL-mediated AICD after a second encounter with Ag (69). Previous experiments from our laboratory demonstrated the importance of CD4+ T cell priming and TRAIL-expressing CD8+ regulatory T cells in i.v. tolerance (5). We found that these TRAIL-expressing CD8+ regulatory T cells were similar to the “helpless” CD8+ T cells generated in the absence of CD4+ T cell help (69), and the delivery of necrotic cells i.v. failed to induce tolerance and the CD8+ regulatory T cells because CD4+ T cell priming occurred. Likewise, the present data shows that CD8+ T cells from mice given activated apoptotic cells were unable to transfer tolerance, suggesting that these mice received the necessary CD4+ T cell “help” after priming with the CD154-expressing activated apoptotic cells for immunity. In line with these results, we have examined splenic CD8+ T cells from mice given either naïve or activated apoptotic cells for TRAIL expression, and found that only the CD8+ T cells from mice given naïve apoptotic cells, but not activated apoptotic cells, had increased TRAIL expression (supplemental Figure 5). While it is clear from our previous and current data that the administration of either necrotic or activated apoptotic Ag-coupled cells induces immunity instead of tolerance, the molecular cues displayed by the necrotic or activated apoptotic cells that stimulate immunity are most likely different.
Why or when would it be beneficial for an apoptotic T cell to express CD154 as it is dying in vivo? One possible scenario would be during an acute infection, where the high number of CD154-expressing dead/dying T cells may help maintain a high threshold of inflammation required to clear the pathogen. This environment would also facilitate the priming of “helped” CD8+ T cells and any subsequent memory responses (66, 70, 71). Then, as CD154 expression decreases in relation with the contraction of the effector response, the tolerogenic effects of the apoptotic cells should re-emerge. The generation of “helpless” CD8+ T cells would be favored at this stage of the immune response, which may be essential in the maintenance of tolerance (5, 69). By contrast, during chronic infections, persistent cross-presentation of Ag from CD154-expressing apoptotic T cells may participate in the maintenance of the chronic low-level inflammation, and may even facilitate the emergence of autoimmune reactions. Indeed, overexpression of CD154 has been correlated with a number of autoimmune diseases, including systemic lupus erythematosis, rheumatoid arthritis, and inflammatory bowel disease(72). Thus, we may want to think of the apoptotic death of activated lymphocytes (those associated with Ag/pathogen) as “inflammatory apoptosis” in comparison to the “silent apoptosis” of naïve lymphocytes (those without Ag) (73).
Supplementary Material
Acknowledgments
The authors thank Erik Brincks and Lyse Norian for helpful discussions.
Footnotes
This work was supported by the National Institutes of Health Grant CA109446 (TSG).
References
- 1.Owen JJ, Jenkinson EJ. Apoptosis and T-cell repertoire selection in the thymus. Ann N Y Acad Sci. 1992;663:305–310. doi: 10.1111/j.1749-6632.1992.tb38673.x. [DOI] [PubMed] [Google Scholar]
- 2.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- 3.Herndon JM, Stuart PM, Ferguson TA. Peripheral deletion of antigen-specific T cells leads to long-term tolerance mediated by CD8+ cytotoxic cells. J Immunol. 2005;174:4098–4104. doi: 10.4049/jimmunol.174.7.4098. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson TA, Herndon J, Elzey B, Griffith TS, Schoenberger S, Green DR. Uptake of apoptotic antigen-coupled cells by lymphoid dendritic cells and cross-priming of CD8+ T cells produce active immune unresponsiveness. J Immunol. 2002;168:5589–5595. doi: 10.4049/jimmunol.168.11.5589. [DOI] [PubMed] [Google Scholar]
- 5.Griffith TS, Kazama H, VanOosten RL, Earle JK, Jr, Herndon JM, Green DR, Ferguson TA. Apoptotic cells induce tolerance by generating helpless CD8+ T cells that produce TRAIL. J Immunol. 2007;178:2679–2687. doi: 10.4049/jimmunol.178.5.2679. [DOI] [PubMed] [Google Scholar]
- 6.Carrero JA, Vivanco-Cid H, Unanue ER. Granzymes drive a rapid listeriolysin O-induced T cell apoptosis. J Immunol. 2008;181:1365–1374. doi: 10.4049/jimmunol.181.2.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merrick JC, Edelson BT, Bhardwaj V, Swanson PE, Unanue ER. Lymphocyte apoptosis during early phase of Listeria infection in mice. Am J Pathol. 1997;151:785–792. [PMC free article] [PubMed] [Google Scholar]
- 8.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Min W, Huang X, Gorczynski R, Cattral M. Fas ligand-transfected dendritic cells induce apoptosis of antigen-specific T cells. Transplant Proc. 2001;33:234. doi: 10.1016/s0041-1345(00)01990-4. [DOI] [PubMed] [Google Scholar]
- 10.Bittencourt MC, Perruche S, Contassot E, Fresnay S, Baron MH, Angonin R, Aubin F, Herve P, Tiberghien P, Saas P. Intravenous injection of apoptotic leukocytes enhances bone marrow engraftment across major histocompatibility barriers. Blood. 2001;98:224–230. doi: 10.1182/blood.v98.1.224. [DOI] [PubMed] [Google Scholar]
- 11.Waller EK, Ship AM, Mittelstaedt S, Murray TW, Carter R, Kakhniashvili I, Lonial S, Holden JT, Boyer MW. Irradiated donor leukocytes promote engraftment of allogeneic bone marrow in major histocompatibility complex mismatched recipients without causing graft-versus-host disease. Blood. 1999;94:3222–3233. [PubMed] [Google Scholar]
- 12.del Rosario ML, Zucali JR, Kao KJ. Prevention of graft-versus-host disease by induction of immune tolerance with ultraviolet B-irradiated leukocytes in H-2 disparate bone marrow donor. Blood. 1999;93:3558–3564. [PubMed] [Google Scholar]
- 13.Kleinclauss F, Perruche S, Cahn JY, Tiberghien P, Saas P. Administration of donor apoptotic cells: an alternative cell-based therapy to induce tolerance? Transplantation. 2003;75:43S–45S. doi: 10.1097/01.TP.0000067951.90241.54. [DOI] [PubMed] [Google Scholar]
- 14.Yamada And A, Sayegh MH. The CD154-CD40 costimulatory pathway in transplantation. Transplantation. 2002;73:S36–39. doi: 10.1097/00007890-200201151-00012. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Larregina AT, Shufesky WJ, Perone MJ, Montecalvo A, Zahorchak AF, Thomson AW, Morelli AE. Use of the inhibitory effect of apoptotic cells on dendritic cells for graft survival via T-cell deletion and regulatory T cells. Am J Transplant. 2006;6:1297–1311. doi: 10.1111/j.1600-6143.2006.01308.x. [DOI] [PubMed] [Google Scholar]
- 16.Markees TG, Phillips NE, Gordon EJ, Noelle RJ, Shultz LD, Mordes JP, Greiner DL, Rossini AA. Long-term survival of skin allografts induced by donor splenocytes and anti-CD154 antibody in thymectomized mice requires CD4+ T cells, interferon-gamma, and CTLA4. J Clin Invest. 1998;101:2446–2455. doi: 10.1172/JCI2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markees TG, Phillips NE, Noelle RJ, Shultz LD, Mordes JP, Greiner DL, Rossini AA. Prolonged survival of mouse skin allografts in recipients treated with donor splenocytes and antibody to CD40 ligand. Transplantation. 1997;64:329–335. doi: 10.1097/00007890-199707270-00026. [DOI] [PubMed] [Google Scholar]
- 18.Parker DC, Greiner DL, Phillips NE, Appel MC, Steele AW, Durie FH, Noelle RJ, Mordes JP, Rossini AA. Survival of mouse pancreatic islet allografts in recipients treated with allogeneic small lymphocytes and antibody to CD40 ligand. Proc Natl Acad Sci U S A. 1995;92:9560–9564. doi: 10.1073/pnas.92.21.9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyake Y, Asano K, Kaise H, Uemura M, Nakayama M, Tanaka M. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J Clin Invest. 2007;117:2268–2278. doi: 10.1172/JCI31990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao Y, Herndon JM, Zhang H, Griffith TS, Ferguson TA. Antiinflammatory effects of CD95 ligand (FasL)-induced apoptosis. J Exp Med. 1998;188:887–896. doi: 10.1084/jem.188.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kepp O, Tesniere A, Schlemmer F, Michaud M, Senovilla L, Zitvogel L, Kroemer G. Immunogenic cell death modalities and their impact on cancer treatment. Apoptosis. 2009;14:364–375. doi: 10.1007/s10495-008-0303-9. [DOI] [PubMed] [Google Scholar]
- 22.Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29:21–32. doi: 10.1016/j.immuni.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spetz AL, Sorensen AS, Walther-Jallow L, Wahren B, Andersson J, Holmgren L, Hinkula J. Induction of HIV-1-specific immunity after vaccination with apoptotic HIV-1/murine leukemia virus-infected cells. J Immunol. 2002;169:5771–5779. doi: 10.4049/jimmunol.169.10.5771. [DOI] [PubMed] [Google Scholar]
- 24.Gershon RK, Kondo K. Infectious immunological tolerance. Immunology. 1971;21:903–914. [PMC free article] [PubMed] [Google Scholar]
- 25.Pierres A, Bromberg JS, Sy MS, Benacerraf B, Greene MI. Mechanisms of regulation of cell-mediated immunity. VI. Antigen density dependence of the induction of genetically restricted suppressor cells. J Immunol. 1980;124:343–348. [PubMed] [Google Scholar]
- 26.Sy MS, Miller SD, Kowach HB, Claman HN. A splenic requirement for the generation of suppressor T cells. J Immunol. 1977;119:2095–2099. [PubMed] [Google Scholar]
- 27.Battisto JR, Bloom BR. Dual immunological unresponsiveness induced by cell membrane coupled hapten or antigen. Nature. 1966;212:156–157. doi: 10.1038/212156a0. [DOI] [PubMed] [Google Scholar]
- 28.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. 2003;3:1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 29.D’Andrea A, Rengaraju M, Valiante NM, Chehimi J, Kubin M, Aste M, Chan SH, Kobayashi M, Young D, Nickbarg E, et al. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.VanOosten RL, Griffith TS. Activation of tumor-specific CD8+ T Cells after intratumoral Ad5-TRAIL/CpG oligodeoxynucleotide combination therapy. Cancer Res. 2007;67:11980–11990. doi: 10.1158/0008-5472.CAN-07-1526. [DOI] [PubMed] [Google Scholar]
- 31.Johansson U, Walther-Jallow L, Smed-Sorensen A, Spetz AL. Triggering of dendritic cell responses after exposure to activated, but not resting, apoptotic PBMCs. J Immunol. 2007;179:1711–1720. doi: 10.4049/jimmunol.179.3.1711. [DOI] [PubMed] [Google Scholar]
- 32.Ren G, Su J, Zhao X, Zhang L, Zhang J, Roberts AI, Zhang H, Das G, Shi Y. Apoptotic cells induce immunosuppression through dendritic cells: critical roles of IFN-gamma and nitric oxide. J Immunol. 2008;181:3277–3284. doi: 10.4049/jimmunol.181.5.3277. [DOI] [PubMed] [Google Scholar]
- 33.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khanolkar A, V, Badovinac P, Harty JT. CD8 T cell memory development: CD4 T cell help is appreciated. Immunol Res. 2007;39:94–104. doi: 10.1007/s12026-007-0081-4. [DOI] [PubMed] [Google Scholar]
- 35.Armitage RJ, Fanslow WC, Strockbine L, Sato TA, Clifford KN, Macduff BM, Anderson DM, Gimpel SD, Davis-Smith T, Maliszewski CR, et al. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357:80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- 36.Nonoyama S, Penix LA, Edwards CP, Lewis DB, Ito S, Aruffo A, Wilson CB, Ochs HD. Diminished expression of CD40 ligand by activated neonatal T cells. J Clin Invest. 1995;95:66–75. doi: 10.1172/JCI117677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 38.Grewal IS, Flavell RA. The role of CD40 ligand in costimulation and T-cell activation. Immunol Rev. 1996;153:85–106. doi: 10.1111/j.1600-065x.1996.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 39.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 40.Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi JP, van Kooten C, Liu YJ, Rousset F, Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 41.Wong KL, Lew FC, MacAry PA, Kemeny DM. CD40L-expressing CD8 T cells prime CD8α+ DC for IL-12p70 production. Eur J Immunol. 2008;38:2251–2262. doi: 10.1002/eji.200838199. [DOI] [PubMed] [Google Scholar]
- 42.Gorbachev AV, DiIulio NA, Fairchild RL. IL-12 augments CD8+ T cell development for contact hypersensitivity responses and circumvents anti-CD154 antibody-mediated inhibition. J Immunol. 2001;167:156–162. doi: 10.4049/jimmunol.167.1.156. [DOI] [PubMed] [Google Scholar]
- 43.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 44.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 45.Ferguson TA, Green DR, Griffith TS. Cell death and immune privilege. Int Rev Immunol. 2002;21:153–172. doi: 10.1080/08830180212058. [DOI] [PubMed] [Google Scholar]
- 46.Hunt JS, Vassmer D, Ferguson TA, Miller L. Fas ligand is positioned in mouse uterus and placenta to prevent trafficking of activated leukocytes between the mother and the conceptus. J Immunol. 1997;158:4122–4128. [PubMed] [Google Scholar]
- 47.Chen W, Frank ME, Jin W, Wahl SM. TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity. 2001;14:715–725. doi: 10.1016/s1074-7613(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 48.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 49.Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, Devera ME, Liang X, Tor M, Billiar T. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007;220:60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 50.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 51.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 52.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 53.Bell CW, Jiang W, Reich CF, 3rd, Pisetsky DS. The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol. 2006;291:C1318–1325. doi: 10.1152/ajpcell.00616.2005. [DOI] [PubMed] [Google Scholar]
- 54.Jiang W, Bell CW, Pisetsky DS. The relationship between apoptosis and high-mobility group protein 1 release from murine macrophages stimulated with lipopolysaccharide or polyinosinic-polycytidylic acid. J Immunol. 2007;178:6495–6503. doi: 10.4049/jimmunol.178.10.6495. [DOI] [PubMed] [Google Scholar]
- 55.Li H, Ambade A, Re F. Cutting edge: Necrosis activates the NLRP3 inflammasome. J Immunol. 2009;183:1528–1532. doi: 10.4049/jimmunol.0901080. [DOI] [PubMed] [Google Scholar]
- 56.Vogel LA, Noelle RJ. CD40 and its crucial role as a member of the TNFR family. Semin Immunol. 1998;10:435–442. doi: 10.1006/smim.1998.0145. [DOI] [PubMed] [Google Scholar]
- 57.Grewal IS, Flavell RA. The CD40 ligand. At the center of the immune universe? Immunol Res. 1997;16:59–70. doi: 10.1007/BF02786323. [DOI] [PubMed] [Google Scholar]
- 58.Noelle RJ. CD40 and its ligand in host defense. Immunity. 1996;4:415–419. doi: 10.1016/s1074-7613(00)80408-2. [DOI] [PubMed] [Google Scholar]
- 59.Diehl L, Den Boer AT, van der Voort EI, Melief CJ, Offringa R, Toes RE. The role of CD40 in peripheral T cell tolerance and immunity. J Mol Med. 2000;78:363–371. doi: 10.1007/s001090000126. [DOI] [PubMed] [Google Scholar]
- 60.Shu U, Kiniwa M, Wu CY, Maliszewski C, Vezzio N, Hakimi J, Gately M, Delespesse G. Activated T cells induce interleukin-12 production by monocytes via CD40-CD40 ligand interaction. Eur J Immunol. 1995;25:1125–1128. doi: 10.1002/eji.1830250442. [DOI] [PubMed] [Google Scholar]
- 61.Yamane H, Kato T, Nariuchi H. Effective stimulation for IL-12 p35 mRNA accumulation and bioactive IL-12 production of antigen-presenting cells interacted with Th cells. J Immunol. 1999;162:6433–6441. [PubMed] [Google Scholar]
- 62.Chen Z, Clark S, Birkeland M, Sung CM, Lago A, Liu R, Kirkpatrick R, Johanson K, Winkler JD, Hu E. Induction and superinduction of growth arrest and DNA damage gene 45 (GADD45) alpha and beta messenger RNAs by histone deacetylase inhibitors trichostatin A (TSA) and butyrate in SW620 human colon carcinoma cells. Cancer Lett. 2002;188:127–140. doi: 10.1016/s0304-3835(02)00322-1. [DOI] [PubMed] [Google Scholar]
- 63.Propato A, Cutrona G, Francavilla V, Ulivi M, Schiaffella E, Landt O, Dunbar R, Cerundolo V, Ferrarini M, Barnaba V. Apoptotic cells overexpress vinculin and induce vinculin-specific cytotoxic T-cell cross-priming. Nat Med. 2001;7:807–813. doi: 10.1038/89930. [DOI] [PubMed] [Google Scholar]
- 64.Lu L, Bonham CA, Chambers FG, Watkins SC, Hoffman RA, Simmons RL, Thomson AW. Induction of nitric oxide synthase in mouse dendritic cells by IFN-gamma, endotoxin, and interaction with allogeneic T cells: nitric oxide production is associated with dendritic cell apoptosis. J Immunol. 1996;157:3577–3586. [PubMed] [Google Scholar]
- 65.Williams CA, Harry RA, McLeod JD. Apoptotic cells induce dendritic cell-mediated suppression via interferon-gamma-induced IDO. Immunology. 2008;124:89–101. doi: 10.1111/j.1365-2567.2007.02743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 67.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 68.Bennett SR, Carbone FR, Karamalis F, Miller JF, Heath WR. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 70.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 71.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cron RQ. CD154 transcriptional regulation in primary human CD4 T cells. Immunol Res. 2003;27:185–202. doi: 10.1385/IR:27:2-3:185. [DOI] [PubMed] [Google Scholar]
- 73.Albert ML. Death-defying immunity: do apoptotic cells influence antigen processing and presentation? Nat Rev Immunol. 2004;4:223–231. doi: 10.1038/nri11308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.