Abstract
Objective
The association between cholesterol and endothelial dysfunction remains controversial. We tested the hypothesis that lipoprotein subclasses are associated with coronary endothelial dysfunction.
Methods and Results
Coronary endothelial function was assessed in 490 patients between November 1993 and February 2007. Fasting lipids and nuclear magnetic resonance (NMR) lipoprotein particle subclasses were measured. There were 325 females and 165 males with a mean age of 49.8±11.6 years. Coronary endothelial dysfunction (epicardial constriction >20% or increase in coronary blood flow <50% in response to intracoronary acetylcholine) was diagnosed in 273 patients, the majority of whom (64.5%) had microvascular dysfunction. Total cholesterol and LDL-C (low density lipoprotein cholesterol) were not associated with endothelial dysfunction. One-way analysis and multivariate methods adjusting for age, gender, diabetes, hypertension and lipid-lowering agent use were used to determine the correlation between lipoprotein subclasses and coronary endothelial dysfunction. Epicardial endothelial dysfunction was significantly correlated with total (p = 0.03) and small LDLp (LDL particles) (p<0.01) and inversely correlated with total and large HDLp (high density lipoprotein particles) (p <0.01).
Conclusions
Epicardial, but not microvascular, coronary endothelial dysfunction was associated directly with LDL particles and inversely with HDL particles, suggesting location-dependent impact of lipoprotein particles on the coronary circulation.
Keywords: nuclear magnetic resonance spectroscopy, lipoprotein particles, endothelial dysfunction, atherosclerosis
INTRODUCTION
The coronary endothelium is an active site of continuous vascular injury and repair. Endothelial integrity reflects the homeostatic balance between oxidative endothelial injury and endogenous reparatory processes1, 2. Endothelial dysfunction is an early central phase in the evolution of atherosclerosis and independently predicts future cardiovascular events2. Although endothelial function assessment has not yet gained routine use in clinical practice, there is a substantial body of evidence supporting its predictive value for cardiovascular events. Multivariate analysis of 10 studies with follow-up as long as 92 months, showed that endothelial dysfunction is associated with a 3- to 5-fold increase in events compared to normal patients2.
Coronary endothelial function can be assessed by the intracoronary administration of the endothelium dependent vasodilator acetylcholine3. Lack of coronary vasodilation and/or increase in coronary blood flow signifies epicardial and microvascular endothelial dysfunction, respectively. Both are regarded as markers of early atherosclerosis. Although total cholesterol, elevated LDL-C and low HDL-C are well established risk factors for CVD (cardiovascular disease), a consistent association with endothelial dysfunction has not been consistently demonstrated.
Lipoprotein lipid concentrations are not equivalent to lipoprotein particle concentrations. Patients with identical lipoprotein concentrations may have very different lipid particle concentrations and subsequently different cardiovascular risks. Lipoprotein lipid measurement therefore may not differentiate risk in patients as it does not directly measure the proportion of atherogenic subclasses and their attendant cardiovascular risk4, 5. Normocholesterolemic patients with high levels of small dense LDLp have increased CVD4, 6 while higher concentrations of large HDLp appear to have a protective effect7. Associations have been found between small LDLp and coronary artery calcification8, 9, the metabolic syndrome10, 11 and the degree of angiographic coronary stenosis12. Even with adjustment for LDL-C, LDLp are still strongly associated with CVD outcomes and incident coronary heart disease6, 13.
NMR analysis was developed in the early 1990’s and is a more feasible technique that correlates well with earlier centrifugation14. It allows for quantitation of both lipoprotein particle concentration and size unlike electrophoretic methods which determine only their size. The purpose of this study is to test the hypothesis that lipoprotein particles are associated with coronary endothelial dysfunction.
METHODS
This study was approved by the Institutional Review Board. Informed consent was obtained from all participants. The study group was comprised of 490 patients referred for invasive endothelial function testing for evaluation of chest pain. Patients withheld all potentially vasoactive medications for at least 24 hours prior to. Fasting blood samples were obtained on the same day of the angiogram and used for NMR analysis.
Study Protocol
Assessment of Coronary Endothelial Function
The methodology for coronary endothelial function analysis has been described previously2, 3, 15. Diagnostic coronary angiography was performed and patients with significant obstructive coronary disease (stenoses >30%) were excluded. A Doppler guide wire (FloWire®, Volcano Corp, Rancho Cordova, California) was positioned within a coronary infusion catheter (Ultrafuse®, SciMed Life Systems, Minneapolis, Minnesota) in the mid left anterior descending artery. Endothelium-independent microvascular coronary flow reserve was assessed by administration of intracoronary bolus injections of incremental doses (18–60 μg) of adenosine until either maximal hyperemia was achieved or the largest dose was given.
Endothelium-dependent vasoreactivity was assessed by intracoronary administration of acetylcholine selectively infused at increasing concentrations (10−6, 10−5, 10−4 mol/L) for 3 minutes at each concentration. Hemodynamic parameters were measured and angiography was performed after each infusion of acetylcholine. At the end of the procedure, the change in coronary artery diameter in response to a 100ug bolus of intracoronary nitroglycerin (Abbott Laboratories, Abbott Park, Illinois) was assessed.
Epicardial endothelial dysfunction was defined as a decrease in coronary artery diameter >20% in response to maximum dose of intracoronary acetylcholine and microvascular endothelial dysfunction as ≤ 50% increase in coronary blood flow (CBF)16. Endothelial dysfunction was defined as the presence of either epicardial or microvascular dysfunction.
Nuclear Magnetic Resonance Spectroscopy
Fasting blood samples were obtained from the study subjects prior to diagnostic coronary angiography. Patients had no alcohol for 24 hours before the test. They had no fatty foods in their last evening meal taken before 6PM the day before the test. Lipoprotein particle concentrations and size were measured in our laboratory with a 400 MHz NMR analyzer using an automated commercial NMR spectroscopic assay (NMR LipoProfile®, LipoScience, Inc., Raleigh, NC)14. Concentrations of VLDL and LDL (including IDL) subclasses in nmol/L units and HDL subclasses in μmol/L units were obtained from the measured amplitudes of the distinct lipid methyl group NMR signals they emit.
The estimated diameters of the 9 measured subclasses are as follows: large VLDL (>60 nm), medium VLDL (35–60 nm), small VLDL (27–35 nm), IDL (23–27 nm), large LDL (21.2–23.0 nm), small LDL (18.0–21.2 nm), large HDL (8.8–13.0 nm), medium HDL (8.2–8.8 nm), and small HDL (7.3–8.2 nm). Total LDL and HDL particle concentrations are the sum of the IDL, large LDL, and small LDL subclass concentrations and large, medium, and small HDL subclass concentrations, respectively. Weighted-average VLDL, LDL, and HDL particle sizes were calculated by summing the diameter of each subclass multiplied by its relative mass percentage as estimated by the amplitude of its methyl NMR signal. Inter-assay reproducibility, determined from replicate analyses of plasma pools, is indicated by the following coefficients of variation: <0.6% for LDL size, <5% for total LDL and HDL particle concentrations, <6% for large HDL particle concentration, and <15% for small LDL particle concentration14.
Statistical Analysis
Statistical analysis was performed by a statistician who was blinded to the clinical data. Continuous variables were presented as mean +/− standard deviation (SD) and dichotomous variables as frequencies and percentages. Baseline characteristics of patients with and without endothelial dysfunction were compared using a two-sample t test for continuous variables and by the Pearson chi-square statistic for categorical variables. Single predictor and multivariable logistic regression models were used to calculate the effect of individual lipoprotein subclasses on endothelial function. Adjustments were made for age, gender, hypertension, diabetes mellitus and statin use in multivariable logistic regression models. Associations with a p value <0.05 were considered to be statistically significant. Analysis was performed using JMP (version 7, SAS Institute, Inc., Cary, NC).
RESULTS
The study population consisted of 490 patients undergoing endothelial function assessment between November 17, 1993 and February 16, 2007. Table 1 shows the baseline characteristics of the study population. The mean age was 49.8 years (range 19 to 78 years). There was a predominance of females (325 females and 165 males) and Caucasians (444 white and 46 non-whites). The distribution of conventional risk factors was: diabetes mellitus 7.6% (n=37), hypertension 42% (n= 206), current smoking 12.7% (n= 62) and hyperlipidemia 55.3% (n=271). Hyperlipidemia was defined as total cholesterol > 200mg/dl, triglycerides >150mg/dl, LDL-C >100mg/dl or current use of a lipid-lowering agent which was being used by 35.3% (n=173) of patients. The mean lipid values were: total cholesterol 190 ± 45mg/dl, triglycerides 139 ± 88 mg/dl, HDL-C 53 ±17mg/dl and LDL-C 109 ± 37 mg/dl.
Table 1.
Baseline characteristics
| Variable | Normal (n =179) | Endothelial Dysfunction (n= 273) | p |
|---|---|---|---|
| Age | 49.0 ±11.5 | 50.6 ±11.7 | 0.14 |
| Male gender | 52 (29.1) | 104 (38.1) | 0.05 |
| Postmenopausal female | 75 (41.9) | 109 (40.0) | 0.90 |
| Body mass index, kg/m2 | 28.0 ± 6.1 | 29.7 ± 6.2 | 0.001 |
| Diabetes mellitus | 6 (3.4) | 30 (11.0) | 0.002 |
| Glycosylated hemoglobin, % | 5.2 ± 0.5 | 5.5 ± 1.0 | <0.001 |
| hs-crp (mg/L) | 0.8 ± 1.5 | 1.4 ± 3.3 | 0.14 |
| Hyperlipidemia | 92 (51.4) | 158 (57.9) | 0.40 |
| Lipid Lowering agent use | 53 (29.6) | 104 (38.2) | 0.06 |
| Hypertension | 74 (41.3) | 123 (45.1) | 0.72 |
| Antihypertensive use | 42 (23.5) | 79 (28.9) | 0.30 |
| ACE inhibitor use | 31 (17.3) | 45 (16.5) | 0.80 |
| Current smokers | 21 (11.7) | 40 (14.7) | 0.40 |
Values expressed as mean ± standard deviation or n (%)
ACE angiotensin converting enzyme
Coronary endothelial dysfunction was diagnosed in 273 patients. There were 251 patients with microvascular dysfunction, 177 patients with epicardial dysfunction and 156 patients with both. The test was normal in 179 patients. Results were missing or incomplete in 38 patients. Diabetes mellitus and body mass index were the only clinical variables associated with endothelial dysfunction. Univariate analysis showed that coronary endothelial dysfunction was associated with HDL-C (p=0.02) but not with any other conventional lipid parameter.
We then analyzed the lipid and lipoprotein associations by the type of coronary endothelial dysfunction (microvascular versus epicardial). Despite lack of significant associations with conventional lipid levels (Table 1), there were statistically significant positive associations with total and small LDLp and an inverse correlation with large HDLp (Table 2).
Table 2.
Distribution of lipids and lipoprotein particles by coronary endothelial function.
| Lipid fraction | Normal n = 179 | Coronary Endothelial Dysfunction n = 273 | p |
|---|---|---|---|
| Total cholesterol (mg/dl) | 188 ± 44 | 191 ± 44 | 0.57 |
| LDL-C (mg/dl) | 106 ± 33 | 112 ± 38 | 0.24 |
| HDL-C (mg/dl) | 55 ± 18 | 51 ± 15 | 0.02 |
| Triglycerides (mg/dl) | 127 ± 76 | 146 ± 96 | 0.05 |
| Lipoprotein (a) (mg/dl) | 3.5 ± 7.3 | 2.5 ± 5.5 | 0.20 |
| Total LDLp (nmol/L) | 1078 ± 390 | 1158 ± 393 | 0.03 |
| Mean LDLp size (nm) | 20.7 | 20.9 | 0.03 |
| Total Small LDLp (nmol/L) | 687 ± 431 | 791 ± 434 | <0.01 |
| Large LDLp (nmol/L) | 353 ± 198 | 324 ± 193 | 0.09 |
| IDLp (nmol/L) | 37 ± 40 | 44 ± 42 | 0.04 |
| Total HDLp (μmol/L) | 29.5 ± 6.8 | 29.0 ± 6.5 | 0.45 |
| Large HDLp (μmol/L) | 6.6 ± 3.7 | 5.7 ± 3.7 | <0.01 |
Values expressed as mean ± SD
Endothelial dysfunction was defined as either epicardial or microvascular dysfunction or both.
These associations persisted when adjusted for age, gender, hypertension and statin use. When adjusted for diabetes, small LDLp remained significantly associated with coronary endothelial dysfunction (r2 = 0.04, p=0.03) and persisted with further adjustment for triglyceride concentrations (r2 = 0.02, p = 0.03). However, the inverse association with large HDLp was weakened (r2 = 0.03, p=0.09). When mean LDLp size was included in the model, the relationship between endothelial dysfunction and small LDLp was reduced (p = 0.16) but the relationship with diabetes again persisted (p = 0.01) and was independent of statin use (p = 0.19). There was no statistically significant association between any particle and microvascular dysfunction in either univariate or multivariate analysis. The associations were not affected by gender. The presence of both epicardial and microvascular dysfunction did not change the association with LDLp or HDLp.
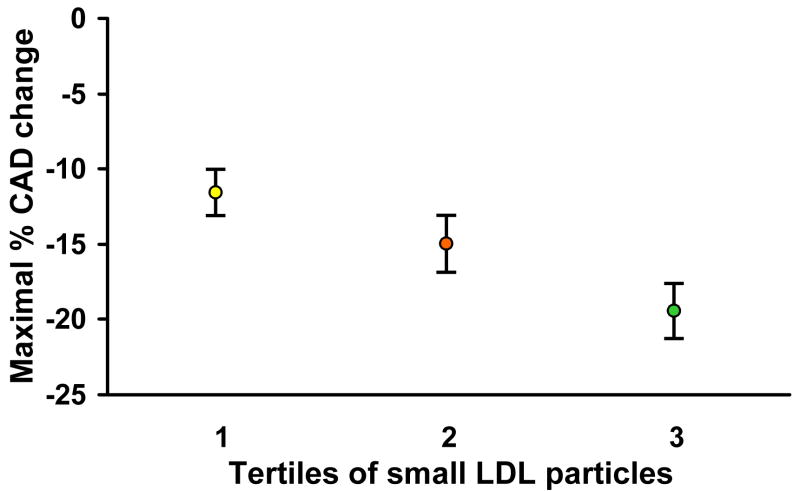
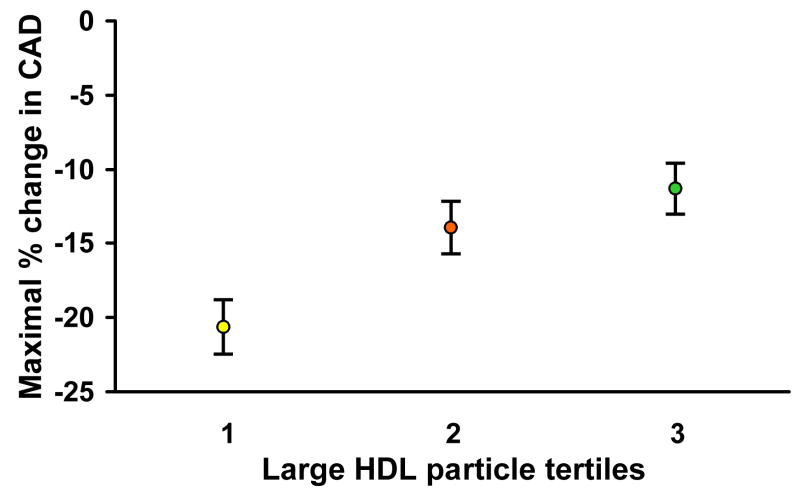
Hemodynamic data are shown in Table 3. Analysis of coronary epicardial function using the maximal increase in coronary artery diameter to acetylcholine relative to tertiles of small LDLp and large HDLp was then performed. With increasing tertiles of small LDLp, there was a statistically significant, progressive decline in epicardial response to intracoronary acetylcholine (Figure 1). Conversely, the maximal change in coronary artery diameter increased with increasing tertiles of large HDLp as shown in Figure 2.
Table 3.
Hemodynamic Data
| Hemodynamic data | Normal n = 273 | Coronary Endothelial Dysfunction n = 179 | p |
|---|---|---|---|
| Mean arterial pressure, mmHg | 100± 15 | 100 ±16 | 0.50 |
| Heart rate, bpm | 72 ±13 | 70 ± 13 | 0.07 |
| CFR to adenosine | 3.0 ± 0.7 | 3.0 ± 0.7 | 0.20 |
| Baseline coronary artery diameter, mm | 2.3 ± 0.6 | 2.2 ± 0.6 | 0.04 |
| Baseline CBF, ml/min | 54.6 ± 35 | 50.8±30 | 0.50 |
| % change in coronary artery diameter to acetylcholine | −5.3 ± 16 | −22.8 ± 24 | <0.001 |
| % change in CBF to acetylcholine | 111.7 ± 101 | 26.1 ± 83 | <0.001 |
Values expressed as mean ± SEM.
CFR: coronary flow reserve, CBF: coronary blood flow
Figure 1.
The maximal change in coronary artery diameter (CAD) in response to intracoronary acetylcholine decreased with increasing concentrations of total small LDL particles. Tertile 1: LDL particle concentration < 504.1 nmol/L; tertile 2: 504.1–865 nmol/L; tertile 3: ≥ 865 nmol/L.
*Values displayed as mean ± standard error of the mean
Figure 2.
The maximal change in coronary artery diameter (CAD) in response intracoronary acetylcholine increased with increasing concentrations of large HDL particles. Tertile 1: HDL particle concentration < 4.1 μmol/L; tertile 2: 4.1 to 7.7 μmol/L; tertile 3: ≥7.7 μmol/L.
*Values displayed as mean ± standard error of the mean
DISCUSSION
This is the first study to report the relationship between early coronary atherosclerosis and NMR-derived lipoprotein particles in humans. The major findings were: 1) traditional cholesterol measures (total and LDL-C) are not associated with endothelial dysfunction, 2) epicardial dysfunction is positively correlated with small LDLp and inversely correlated with large HDLp, 3) the association of endothelial dysfunction with LDLp is independent of the presence of conventional risk factors and, 4) there is a differential effect on the epicardial versus the microvascular circulation.
Coronary Endothelial Dysfunction and LDL particles
The mean lipid levels in our study cohort would be considered low to intermediate without particular need for intervention by current guidelines17. Thus, risk assessment based solely on conventional lipids would have failed to identify those with early coronary atherosclerosis which was present in over 60% of the study population. However, their risk was discriminated by LDLp (Figure 1), underscoring a potential biological link and addition of incremental value to the standard lipid profile.
The lack of the predictive value of total LDL for cardiovascular events was recently demonstrated in several landmark clinical primary prevention trials18–20 which demonstrated significantly stronger associations between LDLp and CVD outcomes than with standard lipids. Epidemiological data suggests that approximately 20–25% of patients have discordance between LDL-C) and LDLp in the blood21. In the EPIC (European Prospective Investigation into Cancer and Nutrition)-Norfolk study6, a prospective study of apparently healthy individuals with moderately elevated LDL-C, LDLp was a better predictor of future cardiovascular events than LDL-C. In the large community-based Framingham Offspring cohort4, LDLp were again more sensitive indicators of cardiovascular risk than either LDL-C or non-HDL-C.
LDLp have also been shown to be superior in several secondary prevention trials12, 21 and in patients with acute coronary syndromes. For example, in the PROVE-IT TIMI 22 trial22, the mean LDL-C level (106mg/dl) was similar to that in our patient cohort. Despite a relatively low mean LDL-C of 62mg/dl in the aggressively treated arm, there remained a continuous incidence of major cardiovascular events. These observations lead us to speculate, like other investigators, that total LDL-C may not be most appropriate target for treatment. It is not yet known to what extent patients in a secondary prevention setting display LDL discordance.
Our study underscores the importance of measuring lipoprotein particles and provides preliminary evidence that high concentrations of small LDLp should raise the clinician’s suspicion of the presence of early atherosclerosis. Direct causality between both entities is still speculative. Growing evidence implicates small LDLp as the key mediator of atherosclerosis via oxidized LDL23. Small LDLp are more susceptible to oxidation24, 25, impair acetylcholine-induced vasodilatation, reduce endothelial nitric oxide activity23, 26, increase oxidative stress27 and are less readily cleared from the circulation26. These studies used gradient gel electrophoresis for measurement of lipoprotein particles but we anticipate similar results would be obtained using NMR spectroscopy given the high correlation between both techniques14, 28. Further studies are needed to determine if small LDLp could be a used as a surrogate for endothelial function assessment.
In the current study, the association between coronary endothelial dysfunction and LDLp was independent of diabetes mellitus. Similarly, Woodman et al described independent associations between oxidized and small LDLp size and peripheral endothelial dysfunction in diabetics23. Cardiovascular risk in diabetic patients with LDL-C <100mg/dl may be underestimated by their LDL-C values. LDLp are superior to non-HDL cholesterol4 and may also be useful risk stratification markers in intermediate cardiovascular risk populations. This could facilitate earlier treatment with agents such as statins and fibrates which decrease LDLp and may even shift them to larger, more buoyant subtypes29.
Statins and ACE inhibitors were not associated with better endothelial function and did not affect the correlation with LDLp. Only one-third of patients were on these medications, in varying doses and for different durations. Differences between the groups would therefore be difficult to detect. Patients were also relatively young and had minimal atherosclerosis. The study personnel were not involved in treatment decisions prior to the patients’ enrollment in the study.
The correlation between lipoprotein particles and endothelial function was only observed in the epicardial vasculature; the microvasculature was unaffected. We have previously observed this microvascular sparing effect in young smokers30. We speculate that since atherosclerotic plaque formation is a macrovascular phenomenon, correlations would only be seen in the epicardial coronaries.
Large HDL Particles are Inversely Associated with Coronary Endothelial Dysfunction
Our findings extend previous observations that the protective effect of HDL is strongest with larger HDLp. We did not find positive correlation between small HDLp and endothelial dysfunction as reported in other studies31. This may have been because the mean HDL-C level in our study population was relatively high (53mg/dl). Nevertheless, the reverse correlations between HDLp and coronary endothelial function support the significance of our findings.
We postulate several potential mechanisms for our observation that large HDLp and not small HDLp were inversely associated with endothelial dysfunction. Large HDLp may be less cleared from the systemic circulation and therefore mediate more hepatic recycling of cholesterol. Conversely, small HDLp may be cleared more from the circulation and deposit more in arterial walls, with increasing inflammatory consequences, in a similar manner to small LDLp. In the recent ILLUMINATE trial32, the use of the cholesteryl ester transfer protein (CETP) inhibitor, torcetrapib, increased HDLp but caused hypertension and increased cardiovascular complications. More recent studies have shown that these deleterious effects are a direct effect of the torcetrapib molecule itself and not to elevated HDLp {Vergeer, 2008 #1; Forrest, 2008 #2}. In fact, increasing levels of HDLp with novel CETP inhibitors have not been associated with these adverse effects and have been shown, as in our current study, to be associated with improved endothelial function {Hermann, 2009 #140}.
Our study limitations include the lack of generalization of our findings to an asymptomatic population. The study population was referred and highly selected by exclusion of patients with significant coronary artery disease. Although it is difficult to identify specific risk factors that cause endothelial dysfunction within this relatively narrow cohort, diabetes, increased small LDLp and reduced large HDLp still had significant associations, attesting to their strength as risk factors. This unique group of patients allows us to assess the role of novel risk factors in assessing early asymptomatic coronary atherosclerosis. The correlations that we describe do not prove causality or provide mechanistic explanations but do support our hypothesis that small LDLp play a role in early atherogenesis independent of LDL-C and other conventional risk factors.
CONCLUSIONS
In a population with relatively low levels of LDL-C, NMR evaluation of lipoprotein patricles is able to discriminate potential atherosclerotic risk better than conventional risk factors. Future studies are needed to consolidate a compelling case for routine measurement of lipoprotein particles and entice physicians in their quest for greater efficacy in cardiovascular risk assessment.
Supplementary Material
Acknowledgments
a) Funding sources: The study was supported by funds from grants NIH K24 HL-69840, NIH R01 HL-63911, HL-77131.
b) We thank the Center for Translational Science Activity (CTSA) Service Center and Mr. Ryan J. Lennon M.S. for statistical support of this project.
c) We thank Dr. James Otvos for technical review of the manuscript.
Footnotes
d) Disclosures: The authors would like to thank Dr. James Otvos and others at LipoScience® for graciously placing the NMR instrument in our laboratory (Cardiovascular Laboratory Medicine) at the Mayo Clinic for use in this and other studies.
e) Conflicts of interests: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted fnor publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ford M, Allison T, Lerman A. New Approaches to the Concept of Primary Prevention of Atherosclerosis. Current Treatment Options in Cardiovascular Medicine. 2008;1 doi: 10.1007/s11936-008-0008-y. [DOI] [PubMed] [Google Scholar]
- 2.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 3.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 4.Cromwell WC, Otvos JD, Keyes MJ, Pencina MJ, Sullivan L, Vasan RS, Wilson PW, D’Agostino RB. LDL particle number and risk of future cardiovascular disease in the Framingham Offspring Study - Implications for LDL management. Journal of Clinical Lipidology. 2007;1:583–592. doi: 10.1016/j.jacl.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mora S, Szklo M, Otvos JD, Greenland P, Psaty BM, Goff DC, Jr, O’Leary DH, Saad MF, Tsai MY, Sharrett AR. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2007;192:211–217. doi: 10.1016/j.atherosclerosis.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 6.El Harchaoui K, van der Steeg WA, Stroes ES, Kuivenhoven JA, Otvos JD, Wareham NJ, Hutten BA, Kastelein JJ, Khaw KT, Boekholdt SM. Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study. Journal of the American College of Cardiology. 2007;49:547–553. doi: 10.1016/j.jacc.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 7.van der Steeg WA, Holme I, Boekholdt SM, Larsen ML, Lindahl C, Stroes ES, Tikkanen MJ, Wareham NJ, Faergeman O, Olsson AG, Pedersen TR, Khaw KT, Kastelein JJ. High-density lipoprotein cholesterol, high-density lipoprotein particle size, and apolipoprotein A–I: significance for cardiovascular risk: the IDEAL and EPIC-Norfolk studies. Journal of the American College of Cardiology. 2008;51:634–642. doi: 10.1016/j.jacc.2007.09.060. [DOI] [PubMed] [Google Scholar]
- 8.Colhoun HM, Otvos JD, Rubens MB, Taskinen MR, Underwood SR, Fuller JH. Lipoprotein subclasses and particle sizes and their relationship with coronary artery calcification in men and women with and without type 1 diabetes. Diabetes. 2002;51:1949–1956. doi: 10.2337/diabetes.51.6.1949. [DOI] [PubMed] [Google Scholar]
- 9.Mackey RH, Kuller LH, Sutton-Tyrrell K, Evans RW, Holubkov R, Matthews KA. Hormone therapy, lipoprotein subclasses, and coronary calcification: the Healthy Women Study. Archives of internal medicine. 2005;165:510–515. doi: 10.1001/archinte.165.5.510. [DOI] [PubMed] [Google Scholar]
- 10.Kuller LH, Grandits G, Cohen JD, Neaton JD, Prineas R. Lipoprotein particles, insulin, adiponectin, C-reactive protein and risk of coronary heart disease among men with metabolic syndrome. Atherosclerosis. 2007;195:122–128. doi: 10.1016/j.atherosclerosis.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kathiresan S, Otvos JD, Sullivan LM, Keyes MJ, Schaefer EJ, Wilson PW, D’Agostino RB, Vasan RS, Robins SJ. Increased small low-density lipoprotein particle number: a prominent feature of the metabolic syndrome in the Framingham Heart Study. Circulation. 2006;113:20–29. doi: 10.1161/CIRCULATIONAHA.105.567107. [DOI] [PubMed] [Google Scholar]
- 12.Rosenson RS, Otvos JD, Freedman DS. Relations of lipoprotein subclass levels and low-density lipoprotein size to progression of coronary artery disease in the Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC-I) trial. The American journal of cardiology. 2002;90:89–94. doi: 10.1016/s0002-9149(02)02427-x. [DOI] [PubMed] [Google Scholar]
- 13.Mora S, Otvos J, Buring JE, Ridker PM. A Prospective Comparison of NMR-Measured LDL Particle Number, Apolipoprotein B100, and Standard Lipids with Incident CHD in 27,673 Initially Healthy Women. Circulation. 2007;116:787–788. [Google Scholar]
- 14.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clinics in laboratory medicine. 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Deanfield J, Donald A, Ferri C, Giannattasio C, Halcox J, Halligan S, Lerman A, Mancia G, Oliver JJ, Pessina AC, Rizzoni D, Rossi GP, Salvetti A, Schiffrin EL, Taddei S, Webb DJ. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. Journal of hypertension. 2005;23:7–17. doi: 10.1097/00004872-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 17.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 18.Kuller L, Arnold A, Tracy R, Otvos J, Burke G, Psaty B, Siscovick D, Freedman DS, Kronmal R. Nuclear magnetic resonance spectroscopy of lipoproteins and risk of coronary heart disease in the cardiovascular health study. Arterioscler Thromb Vasc Biol. 2002;22:1175–1180. doi: 10.1161/01.atv.0000022015.97341.3a. [DOI] [PubMed] [Google Scholar]
- 19.Blake GJ, Otvos JD, Rifai N, Ridker PM. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation. 2002;106:1930–1937. doi: 10.1161/01.cir.0000033222.75187.b9. [DOI] [PubMed] [Google Scholar]
- 20.Mackey RH, Kuller LH, Sutton-Tyrrell K, Evans RW, Holubkov R, Matthews KA. Lipoprotein subclasses and coronary artery calcium in postmenopausal women from the healthy women study. The American journal of cardiology. 2002;90:71i–76i. doi: 10.1016/s0002-9149(02)02636-x. [DOI] [PubMed] [Google Scholar]
- 21.Otvos JD, Collins D, Freedman DS, Shalaurova I, Schaefer EJ, McNamara JR, Bloomfield HE, Robins SJ. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation. 2006;113:1556–1563. doi: 10.1161/CIRCULATIONAHA.105.565135. [DOI] [PubMed] [Google Scholar]
- 22.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. The New England journal of medicine. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 23.Woodman RJ, Watts GF, Playford DA, Best JD, Chan DC. Oxidized LDL and small LDL particle size are independently predictive of a selective defect in microcirculatory endothelial function in type 2 diabetes. Diabetes Obes Metab. 2005;7:612–617. doi: 10.1111/j.1463-1326.2005.00478.x. [DOI] [PubMed] [Google Scholar]
- 24.Anderson TJ, Meredith IT, Charbonneau F, Yeung AC, Frei B, Selwyn AP, Ganz P. Endothelium-dependent coronary vasomotion relates to the susceptibility of LDL to oxidation in humans. Circulation. 1996;93:1647–1650. doi: 10.1161/01.cir.93.9.1647. [DOI] [PubMed] [Google Scholar]
- 25.Chait A, Brazg RL, Tribble DL, Krauss RM. Susceptibility of small, dense, low-density lipoproteins to oxidative modification in subjects with the atherogenic lipoprotein phenotype, pattern B. Am J Med. 1993;94:350–356. doi: 10.1016/0002-9343(93)90144-e. [DOI] [PubMed] [Google Scholar]
- 26.Vakkilainen J, Makimattila S, Seppala-Lindroos A, Vehkavaara S, Lahdenpera S, Groop PH, Taskinen MR, Yki-Jarvinen H. Endothelial dysfunction in men with small LDL particles. Circulation. 2000;102:716–721. doi: 10.1161/01.cir.102.7.716. [DOI] [PubMed] [Google Scholar]
- 27.Lavi S, Yang EH, Prasad A, Mathew V, Barsness GW, Rihal CS, Lerman LO, Lerman A. The interaction between coronary endothelial dysfunction, local oxidative stress, and endogenous nitric oxide in humans. Hypertension. 2008;51:127–133. doi: 10.1161/HYPERTENSIONAHA.107.099986. [DOI] [PubMed] [Google Scholar]
- 28.Hartman S, Jaffe AS, McConnell JP. Independent Evaluation of Nuclear Magnetic Resonance Lipoprotein Subclass Analysis. ACC. 2006 [Google Scholar]
- 29.Sirtori CR, Calabresi L, Pisciotta L, Cattin L, Pauciullo P, Montagnani M, Manzato E, Bittolo Bon G, Fellin R. Effect of statins on LDL particle size in patients with familial combined hyperlipidemia: a comparison between atorvastatin and pravastatin. Nutr Metab Cardiovasc Dis. 2005;15:47–55. doi: 10.1016/j.numecd.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Lavi S, Prasad A, Yang EH, Mathew V, Simari RD, Rihal CS, Lerman LO, Lerman A. Smoking is associated with epicardial coronary endothelial dysfunction and elevated white blood cell count in patients with chest pain and early coronary artery disease. Circulation. 2007;115:2621–2627. doi: 10.1161/CIRCULATIONAHA.106.641654. [DOI] [PubMed] [Google Scholar]
- 31.Johansson J, Carlson LA, Landou C, Hamsten A. High density lipoproteins and coronary atherosclerosis. A strong inverse relation with the largest particles is confined to normotriglyceridemic patients. Arterioscler Thromb. 1991;11:174–182. doi: 10.1161/01.atv.11.1.174. [DOI] [PubMed] [Google Scholar]
- 32.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B. Effects of torcetrapib in patients at high risk for coronary events. The New England journal of medicine. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 33.Duriez P, Bordet R, Berthelot P. The strange case of Dr HDL and Mr HDL: does a NO’s story illuminate the mystery of HDL’s dark side uncovered by Dr HDL’s drug targeting CETP? Medical hypotheses. 2007;69:752–757. doi: 10.1016/j.mehy.2007.01.059. [DOI] [PubMed] [Google Scholar]
- 34.Lavie CJ, Milani RV. Shedding light on high-density lipoprotein cholesterol: the post-ILLUMINATE era. Journal of the American College of Cardiology. 2008;51:56–58. doi: 10.1016/j.jacc.2007.08.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.