Abstract
Purpose
Thymomas and thymic carcinomas are rare intrathoracic malignancies that can be invasive and refractory to conventional treatment. Because these tumors both originate from the thymus, they are often grouped together clinically. However, whether the underlying biology of these tumors warrants such clustering is unclear, and the optimum treatment of either entity is unknown.
Experimental design
All thymic tumors were profiled for mutations in genes encoding components of the EGFR and KIT signaling pathways, assessed for EGFR and KIT expression by immunohistochemistry (IHC), and analyzed by array-based comparative genomic hybridization (aCGH). Previously untreated tumors were subjected to global gene expression arrays.
Results
We analyzed 45 thymic tumors (thymoma n=38 (type A: n=8, type B2: n=22, type B3: n=8), and thymic carcinoma n=7). One thymoma and one thymic carcinoma harbored KRAS mutations (G12A and G12V, respectively), and one thymoma had a G13V HRAS mutation. Three tumors displayed strong KIT staining. Two thymic carcinomas harbored somatic KIT mutations (V560del and H697Y). In cell viability assays, the V560del mutant was associated with similar sensitivities to imatinib and sunitinib, while the H697Y mutant displayed greater sensitivity to sunitinib. Genomic profiling revealed distinct differences between type A-B2 thymomas vs. type B3 and thymic carcinomas. Moreover, aCGH could readily distinguish squamous cell carcinomas of the thymus vs. the lung, which can often present a diagnostic challenge.
Conclusion
Comprehensive genomic analysis suggests that thymic carcinomas are molecularly distinct from thymomas. These data have clinical, pathological, and therapeutic implications for the treatment of thymic malignancies.
Keywords: Thymoma; Thymic Carcinoma; EGFR, RAS mutations; KIT mutations; mutational profiling; genomic analysis
Statement of translational relevance
Thymomas and thymic carcinomas are rare intrathoracic cancers that can be aggressive and refractory to conventional treatment. To identify potential targets for therapy, we performed a comprehensive molecular analysis of 45 thymic tumors. We found that molecular distinctions exist between different histologic types of thymic tumors. For example, compared to thymomas, thymic carcinomas display many more chromosomal gains and losses and exclusively harbor somatic mutations in the kinase encoded by KIT. Corresponding KIT mutants studied biochemically displayed sensitivity to the KIT inhibitors, imatinib and sunitinib. Some thymic malignancies harbor mutations in RAS genes, which have been previously associated with resistance to EGFR-directed therapies. These results have direct therapeutic implications for the treatment of thymic malignancies.
Introduction
Thymomas and thymic carcinomas are malignant intrathoracic tumors which represent about 0.2% to 1.5% of all malignancies [1]. In general, thymomas are tumors with a tendency toward local recurrence rather than metastasis. Thus, most thymomas are treated surgically followed possibly by radiation [2]. By contrast, thymic carcinomas have a high risk of relapse and death despite surgery, chemotherapy, and radiation [3]. The optimal treatments for thymic tumors are not well-defined.
Because thymomas and thymic carcinomas are rare and both arise from thymic epithelium, they are often grouped together clinically. At the pathologic level, tumors of the thymus are classified according to criteria put forth by the World Health Organization (WHO) in 2004 [4]. In this schema, thymic epithelial malignancies are classified into thymomas (types A, AB, B1, B2, B3) and thymic carcinoma. These classes are based upon the morphology of epithelial cells (with an increasing degree of atypia from type A to thymic carcinoma), the relative proportion of the non-tumoral lymphocytic component (decreasing from types B1 to B3), and resemblance to normal thymic architecture [4]. Clinically, the degree of invasion or tumor stage is generally thought to be an important indicator of overall survival [5]. The best prognostic factor, however, is whether the tumor can be completely resected at the time of operation [6].
Compared to more common epithelial cancers, current knowledge about the biology of thymic tumors is limited. Research has been hampered by the rarity of the tumor and a lack of established cell lines and animal models. Recently, selected genes (EGFR, KIT, and TP53) have been analyzed in small cohorts of patients [7–12]. Notably, a number of cases have been reported of advanced thymic tumors responding to new targeted agents [13–17]. These cases suggest that thymic malignancies may be comprised of clinically relevant subsets that can be defined at the molecular level.
In order to gain insights into the biology of thymomas and thymic carcinomas, we performed a comprehensive genomic analysis of 45 resected thymic tumors. We used array comparative genomic hybridization, mutational profiling to assess the status of specific genes, mRNA expression profiling, and immunohistochemical analyses of proteins implicated in thymic tumor pathogenesis. We found differences between thymomas and thymic carcinomas that have important clinical and therapeutic implications.
Materials and Methods
Patient samples
Tumors specimens from patients with resected thymoma or thymic carcinoma who underwent surgical resection at Memorial Sloan-Kettering Cancer Center (MSKCC) from January 1997 to December 2007 were obtained with patients’ consent under institutional tissue procurement protocols. All tumors were frozen either in the operative room or in the Pathology Department and stored at −80°C in institutional tumor banks.
Clinical stage was determined according to the Masaoka system [5]. Frozen specimens, as well as all additional pathologic material available from corresponding tumors, were reviewed by a single reference pathologist (W.D.T.) for tumor classification according to the 2004 WHO criteria [4]. For this study, we selected thymic tumors which, on matching frozen specimens, contained at least 50% epithelial cells. This cutoff was chosen to eliminate the need for microdissection of epithelial cells within tumor samples of mixed epithelial and lymphocytic subtypes, as reported previously [18]. Only B2 thymomas that met these criteria were included. We excluded from the study thymoma types AB and B1, because of the problem of extensive lymphocytes in the stroma, which could confound genomic analyses.
Mutational Profiling
DNA was extracted using standard methods. Mutational profiling was done using mass spectrometry-based genotyping (Sequenom, Sequenom, San Diego, CA), as previously described [19]. All samples were analyzed for a total of 74 Sequenom assays, designed to detect 101 known somatic mutations in genes of the EGFR signaling pathway: EGFR, KRAS, HRAS, NRAS, BRAF, PIK3CA, AKT1, ERBB2, and MEK1 (Supplemental Table 1) [20].
In addition, we performed direct dideoxynucleotide-based sequencing of select exons from genes known to be commonly mutated and for which Sequenom assays were not available: EGFR exon 19, KIT exons 9, 10, 11, 13, 14, 17, and all coding exons of TP53 and PTEN (See Supplemental Table 2 and Supplemental Methods).
Genomic profiling
DNA was digested and labeled by random priming using Bioprime reagents (Invitrogen, Carlsbad, CA) and Cy3- or Cy5-dUTP. Labeled DNA was hybridized to Agilent 244K comparative genomic hybridization (CGH) arrays (Agilent Technologies, Santa Clara, CA). Normal genomic DNA (Roche, Basel, Switzerland) was used as a reference for all samples. After washing, hybridized slides were scanned and images quantified using Feature Extraction 8.5 (Agilent Technologies). Data were interpreted using standard methodology (See Supplemental Methods).
Expression profiling
RNA was extracted using standard methods. RNA was converted to double-strand cDNA using T7-promoter-tagged oligo d(T) primers and reverse transcriptase. RNA targets were synthesized from cDNA by in vitro transcription and then labeled with biotinylated UTP and CTP. Biotinylated cDNA was fragmented and hybridized for 16 hours at 45°C to HG-U133A 2.0 Affymetrix oligonucleotide arrays. Data were analyzed using standard methods (See Supplemental Methods).
As expression profiles may considerably be altered by induction chemotherapy, especially with anthracyclines, and/or radiotherapy, as previously reported for other tumor types [21], pretreated tumors were not analyzed by global gene expression arrays.
Immunohistochemistry
EGFR immunohistochemistry (IHC) was done using anti-EGFR mouse antibody clone 31G7 (1:1000) from Invitrogen (Camarillo, CA) and antigen retrieval by pepsin at 0.5%. KIT staining was performed using an anti-KIT rabbit polyclonal antibody from Dako (A4502; 1:2000, Carpinteria, CA) on a Ventana Symphony instrument (Ventana, Tucson, AZ). The staining intensity of the epithelial tumor cells was qualitatively evaluated from 0 (no staining) to 1+ (low staining), 2+ (moderate), and 3+ (elevated staining) by 2 investigators (N.G. and M.F.Z.), who were blinded to clinical and genomic data. For EGFR, only membrane staining was evaluated. For KIT, either cytoplasmic or membrane staining was considered positive. CD5 expression results in thymic carcinomas were retrieved from patients’ pathology reports, as this staining was done part of the routine pathology diagnosis using an anti-CD5 rabbit monoclonal antibody (Clone SP19, Ventana). Negative and positive controls were included in all studies.
Ba/F3-KIT mutant transformant assays
Ba/F3 cells harboring the KITV560del and KITH697Y mutations were generated using the QuikChange II XL site-directed Mutagenesis Kit (Stratagene Agilent Technologies), as previously described (See Supplemental Methods) [22]. For drug sensitivity assays, KITV560del and KITH697Y cells were incubated with 10 to 10,000 nM imatinib mesylate (provided by Novartis, Basel, Switzerland) or sunitinib malate (provided by Pfizer, New York, NY) in a 96-well plate in triplicate at the density of 1×105 cells/well in 100 µl of media for 48 hours (37°C, 5% CO2); 20 µl of the tetrazolium compound MTS [3-(4,5- dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt)] or Cell Titer Blue Reagent (Promega, Madison, WI) was then added and incubated with cells for 2 hours. For MTS assays, the absorbance at 490nm was recorded with a DTX880 multimode detector (Beckman Coulter, Fullerton, CA). For Cell Titer Blue, the fluorescence was read with a Spectra Max M5 detector (Molecular Devices, MDS, Toronto, Canada). Cell growth-inhibition was plotted as the ratio of the average quantity of formazan product (MTS) or resazurin reduction (Cell Titer Blue Reagent) in treated wells relative to non-treated controls. Assays were repeated at least 3 times in triplicate. The GI50 values for imatinib and sunitinib were calculated using a standard non-linear regression algorithm available in the GraphPad Prism software (GraphPad, La Jolla, CA), version 4.03.
Statistical methods
All patients were included in the statistical calculations. Follow-up was obtained in all cases, and was censored on June 30st, 2009. Categorical variables were compared using the Chi-square test. Survival was assessed using the Kaplan-Meier method. Results were considered significant at the 0.05 level. Statistical analyses were performed using the SPSS software program (Chicago, IL), version 17.0.
Results
Patient characteristics
A total of 98 thymic tumor specimens were stored in MSKCC tumor banks during the study period (Supplemental Figure 1). 64 cases corresponded to previously untreated tumors, which were resected upfront after diagnosis. 34 tumors were resected after induction treatment. For this study, we identified 45 eligible thymic tumor tissue specimens, 27 from previously untreated tumors and 18 from pre-treated tumors (Supplemental Figure 1). The clinical characteristics of patients with examined tumors are listed in Supplemental Table 3. 23 thymic tumors occurred in men, and 22 in women. The median age at diagnosis was 62 years. Tumor type was thymoma (types A, B2, B3) in 38 cases, and thymic carcinoma in 7 cases. At the time of analysis, all but 3 patients were alive. Median follow-up was 31.4 months (range 1.4–160.0 months). Five-year survival was 92%, and median survival was not reached.
Exploration of the EGFR signaling pathway
At least one patient with thymoma has been reported in the literature to have had a documented radiographic response to the EGFR tyrosine kinase inhibitor (TKI) erlotinib [16]. Because mechanisms of sensitivity and resistance to EGFR inhibitors have been well-defined, we first investigated the status of various EGFR pathway biomarkers.
Using IHC with an anti-EGFR antibody, we found low intensity staining in 6 (15%) cases, moderate staining in 10 (26%) cases, and high staining in 23 (51%) cases. 6 cases were not interpretable (Table 1). There was no strong correlation between EGFR staining and thymoma histological type. High EGFR staining was significantly associated with stage III-IV tumors (p=0.023, Chi-square test).
Table 1.
Results of immunohistochemical stainings for EGFR and KIT. The intensity of the epithelial tumor cell staining was qualitatively evaluated from 0 (no staining) to 1 (low staining), 2 (moderate), and 3 (elevated staining).
| Tumor | Pre-treated | Pathology | Immunohistochemistry | |||
|---|---|---|---|---|---|---|
| Histology | Type | Stage | EGFR | KIT | ||
| 1 | no | Thymoma | A | 2 | NE | NE |
| 2 | no | Thymoma | A | 2 | NE | NE |
| 3 | no | Thymoma | A | 1 | 2 | 0 |
| 4 | no | Thymoma | A | 2 | 1 | 0 |
| 5 | no | Thymoma | A | 2 | 3 | 0 |
| 6 | yes | Thymoma | A | 3 | 3 | 0 |
| 7 | yes | Thymoma | A | 3 | NE | NE |
| 8 | yes | Thymoma | A | 3 | 3 | 0 |
| 9 | no | Thymoma | B2 | 3 | 2 | 0 |
| 10 | no | Thymoma | B2 | 4 | 1 | 0 |
| 11 | no | Thymoma | B2 | 2 | 3 | 0 |
| 12 | no | Thymoma | B2 | 2 | 2 | 0 |
| 13 | no | Thymoma | B2 | 1 | 1 | 0 |
| 14 | no | Thymoma | B2 | 2 | 2 | 0 |
| 15 | no | Thymoma | B2 | 3 | 3 | 0 |
| 16 | no | Thymoma | B2 | 2 | 1 | 0 |
| 17 | no | Thymoma | B2 | 1 | 3 | 0 |
| 18 | no | Thymoma | B2 | 2 | 2 | 0 |
| 19 | no | Thymoma | B2 | 2 | 1 | 0 |
| 20 | no | Thymoma | B2 | 1 | 1 | 0 |
| 21 | no | Thymoma | B2 | 2 | 3 | 0 |
| 22 | no | Thymoma | B2 | 2 | 2 | 0 |
| 23 | yes | Thymoma | B2 | 3 | 3 | 0 |
| 24 | yes | Thymoma | B2 | 3 | 3 | 0 |
| 25 | yes | Thymoma | B2 | 3 | 2 | 0 |
| 26 | yes | Thymoma | B2 | 3 | 3 | 0 |
| 27 | yes | Thymoma | B2 | 3 | 2 | 0 |
| 28 | yes | Thymoma | B2 | 4 | 3 | 0 |
| 29 | yes | Thymoma | B2 | 3 | 2 | 0 |
| 30 | yes | Thymoma | B2 | 4 | NE | NE |
| 31 | no | Thymoma | B3 | 1 | 3 | 0 |
| 32 | no | Thymoma | B3 | 3 | 3 | 0 |
| 33 | yes | Thymoma | B3 | 4 | 3 | 0 |
| 34 | yes | Thymoma | B3 | 4 | 3 | 0 |
| 35 | yes | Thymoma | B3 | 4 | NE | NE |
| 36 | yes | Thymoma | B3 | 4 | 3 | 0 |
| 37 | yes | Thymoma | B3 | 3 | 3 | 0 |
| 38 | yes | Thymoma | B3 | 3 | 3 | 0 |
| 39 | no | Thymic carcinoma | SCC | 2 | NE | NE |
| 40 | no | Thymic carcinoma | SCC | 4 | 3 | 3 |
| 41 | no | Thymic carcinoma | SCC | 3 | 3 | 0 |
| 42 | no | Thymic carcinoma | SCC | 3 | 3 | 0 |
| 43 | no | Thymic carcinoma | SCC | 2 | 3 | 0 |
| 44 | no | Thymic carcinoma | SCC | 3 | 3 | 3 |
| 45 | yes | Thymic carcinoma | SCC | 3 | 2 | 3 |
Legend: SCC: Squamous Cell Carcinoma; NE: not evaluable.
We then profiled tumors for the presence of mutations in genes encoding components of the EGFR signaling pathway that are known to be mutated at specific recurrent nucleotide positions in human cancers [20]. Somatic RAS mutations were found in 3 (7%) of 45 tumors (Table 2): a heterozygous G to C substitution at nucleotide position 35 in exon 1 of KRAS, resulting in a alanine for glycine amino acid substitution at position 12 (G12A) in a type B2 thymoma (case 28); a heterozygous G to T substitution at nucleotide position 35 in exon 1 of KRAS, resulting in a valine for glycine amino acid substitution at position 12 (G12V) in a thymic carcinoma (case 41); and a heterozygous G to T mutation at position 38 in exon 1 of HRAS, resulting in a valine for glycine amino acid substitution at position 13 (G13V), in a type A thymoma (case 3) (Figure 1). EGFR staining was high in the KRAS-mutant tumors and moderate in the HRAS-mutant tumor (Figure 1). We did not detect any mutations in the EGFR kinase domain or in any other tested EGFR signaling pathway genes.
Table 2.
Results of mutational analyses.
| Tumor | Pre-treated | Pathology | Genes | ||||
|---|---|---|---|---|---|---|---|
| Histology | Type | Stage | KRAS | HRAS | KIT | ||
| 1 | no | Thymoma | A | 2 | wt | wt | wt |
| 2 | no | Thymoma | A | 2 | wt | wt | wt |
| 3 | no | Thymoma | A | 1 | wt | G13V | wt |
| 4 | no | Thymoma | A | 2 | wt | wt | wt |
| 5 | no | Thymoma | A | 2 | wt | wt | wt |
| 6 | yes | Thymoma | A | 3 | wt | wt | wt |
| 7 | yes | Thymoma | A | 3 | wt | wt | wt |
| 8 | yes | Thymoma | A | 3 | wt | wt | wt |
| 9 | no | Thymoma | B2 | 3 | wt | wt | wt |
| 10 | no | Thymoma | B2 | 4 | wt | wt | wt |
| 11 | no | Thymoma | B2 | 2 | wt | wt | wt |
| 12 | no | Thymoma | B2 | 2 | wt | wt | wt |
| 13 | no | Thymoma | B2 | 1 | wt | wt | wt |
| 14 | no | Thymoma | B2 | 2 | wt | wt | wt |
| 15 | no | Thymoma | B2 | 3 | wt | wt | wt |
| 16 | no | Thymoma | B2 | 2 | wt | wt | wt |
| 17 | no | Thymoma | B2 | 1 | wt | wt | wt |
| 18 | no | Thymoma | B2 | 2 | wt | wt | wt |
| 19 | no | Thymoma | B2 | 2 | wt | wt | wt |
| 20 | no | Thymoma | B2 | 1 | wt | wt | wt |
| 21 | no | Thymoma | B2 | 2 | wt | wt | wt |
| 22 | no | Thymoma | B2 | 2 | wt | wt | wt |
| 23 | yes | Thymoma | B2 | 3 | wt | wt | wt |
| 24 | yes | Thymoma | B2 | 3 | wt | wt | wt |
| 25 | yes | Thymoma | B2 | 3 | wt | wt | wt |
| 26 | yes | Thymoma | B2 | 3 | wt | wt | wt |
| 27 | yes | Thymoma | B2 | 3 | wt | wt | wt |
| 28 | yes | Thymoma | B2 | 4 | G12A | wt | wt |
| 29 | yes | Thymoma | B2 | 3 | wt | wt | wt |
| 30 | yes | Thymoma | B2 | 4 | wt | wt | wt |
| 31 | no | Thymoma | B3 | 1 | wt | wt | Wt |
| 32 | no | Thymoma | B3 | 3 | wt | wt | wt |
| 33 | yes | Thymoma | B3 | 4 | wt | wt | wt |
| 34 | yes | Thymoma | B3 | 4 | wt | wt | wt |
| 35 | yes | Thymoma | B3 | 4 | wt | wt | wt |
| 36 | yes | Thymoma | B3 | 4 | wt | wt | wt |
| 37 | yes | Thymoma | B3 | 3 | wt | wt | wt |
| 38 | yes | Thymoma | B3 | 3 | wt | wt | wt |
| 39 | no | Thymic carcinoma | SCC | 2 | wt | wt | wt |
| 40 | no | Thymic carcinoma | SCC | 4 | wt | wt | V560del |
| 41 | no | Thymic carcinoma | SCC | 3 | G12V | wt | wt |
| 42 | no | Thymic carcinoma | SCC | 3 | wt | wt | wt |
| 43 | no | Thymic carcinoma | SCC | 2 | wt | wt | wt |
| 44 | no | Thymic carcinoma | SCC | 3 | wt | wt | H697Y |
| 45 | yes | Thymic carcinoma | SCC | 3 | wt | wt | wt |
EGFR, AKT1, BRAF, ERBB2, MEK1, NRAS, PIK3CA, PTEN, and TP53 were wild-type (wt) in all tumors.
Legend: SCC: Squamous Cell Carcinoma
Figure 1. RAS and KIT mutant thymic tumors.
Left panels: Hematoxylin and Eosin (H&E) stainings (original magnification, ×40). Middle panels: sequencing chromatograms. Righ panels: immunohistochemical studies (IHC) with anti-EGFR or anti-KIT antibodies. The asterisks indicate the mutations.
Exploration of KIT signaling pathway
Patients with thymic tumors have exhibited radiographic responses to KIT inhibitors like imatinib [13–15]. Moreover, KIT staining by IHC has been reported to be frequent in thymic carcinoma [7, 8, 23, 24]. Thus, we next investigated the status of KIT.
Three (7%) of 45 tumors displayed staining with an anti-KIT antibody (the same antibody used to assess KIT expression in GastroIntestinal Stromal Tumors (GISTs)), all of which were thymic carcinomas (Table 1). Direct sequencing of exons that encode the KIT kinase and transmembrane domains revealed that 2 (4%) of the 45 tumors, both thymic carcinomas, harbored KIT mutations (Table 2). One tumor, CD5-positive, contained a heterozygous deletion of nucleotides 1678 to 1680 in exon 11 of KIT, resulting in a deletion of valine at position 560 (V560del) (Figure 1). The other mutation, found in a CD5-negative tumor, was a heterozygous C to T mutation at position 2089 in exon 14 of KIT, resulting in a tyrosine for histidine amino acid substitution at position 697 (H697Y) (Figure 1). Both KIT mutations were somatic. KIT mutations were mutually exclusive with RAS mutations.
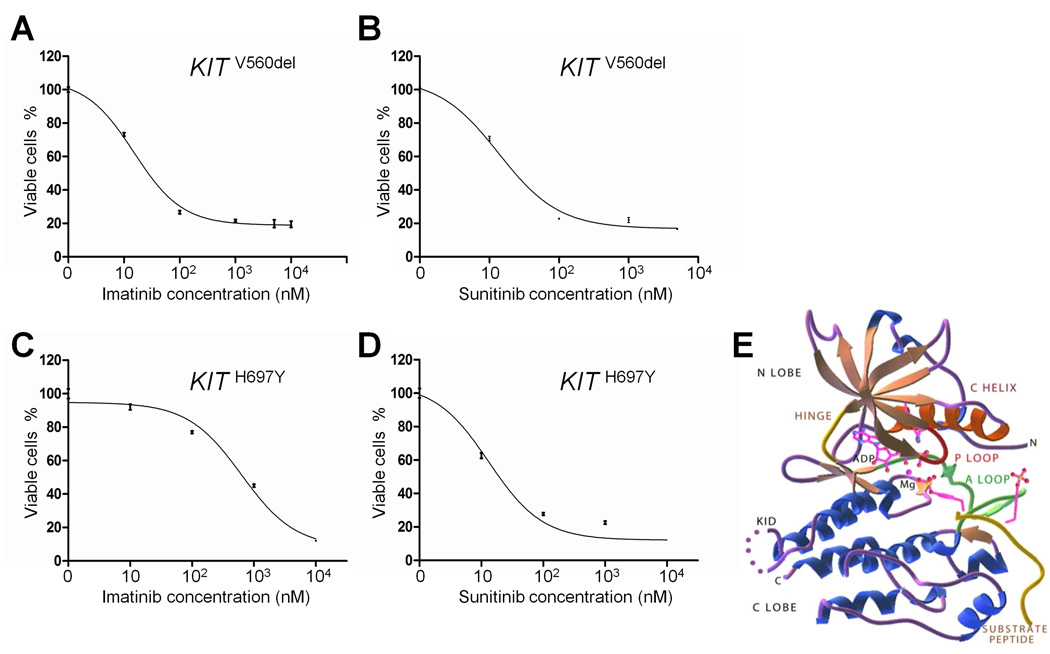
The KIT V560 deletion occurs in the juxta-membrane domain of the KIT protein. Similar types of mutations (i.e. KIT V560G, KIT del557-558) have been found in GISTs sensitive to imatinib [25], and this exact mutation was previously observed in a case of thymic carcinoma responding to imatinib [13]. The KIT H697Y has not been previously reported in any cancer (COSMIC). The H697 residue is part of the KIT “kinase insertion domain” [26], a region that does not show amino-acid sequence homology with other kinases (Figure 2E). The region was not visualized in the published crystal structure of KIT [26]. However, this residue is conserved through KIT orthologs (Supplemental Figure 2), suggesting that it is a critical residue for KIT protein function.
Figure 2. Characterization of KIT mutants.
(A, B) The growth of Ba/F3-KITV560del cells was potently inhibited by imatinib and sunitinib with GI50s of 15.0nM, and 13.6 nM, respectively. (C, D) On the contrary, Ba/F3-KITH697Y cells were more sensitive to sunitinib (GI50 of 13.2 nM), than to imatinib (GI50 of 631.4 nM). (E) Crystal structure of KIT (from [26]). The KIT V560 deletion occurs in the juxta-membrane domain of the KIT protein. The H697 residue is located in the KIT “kinase insertion domain” (KID).
To confirm that the V560del mutation is associated with imatinib sensitivity, and to study the H697Y mutation, we generated stable Ba/F3 transfectants expressing the mutant alleles. Ba/F3 cells are a murine pro-B cell line that are dependent on IL-3 for growth and which can be rendered IL-3-independent after the introduction of transforming kinase oncogenes [27]. Ba/F3-KITV560del and Ba/F3-KITH697Y cells grew in the absence of IL-3, demonstrating that these mutations confer gain-of-function (data not shown). The growth of Ba/F3-KITV560del cells was potently inhibited by imatinib and sunitinib with GI50s of 15.0nM and 13.6 nM, respectively (Figures 2A and 2B). The growth of Ba/F3-KITH697Y cells was more potently inhibited by sunitinib (GI50 of 13.2 nM) than by imatinib (GI50 of 631.4 nM) (Figures 2C and 2D). Although the latter GI50 was still below 1000nM for imatinib, a cut-off reported for resistance to this drug in GISTs [22, 28], these data suggest that sunitinib may be a more effective KIT inhibitor than imatinib in KIT-mutant thymic carcinomas.
Gene expression profiling
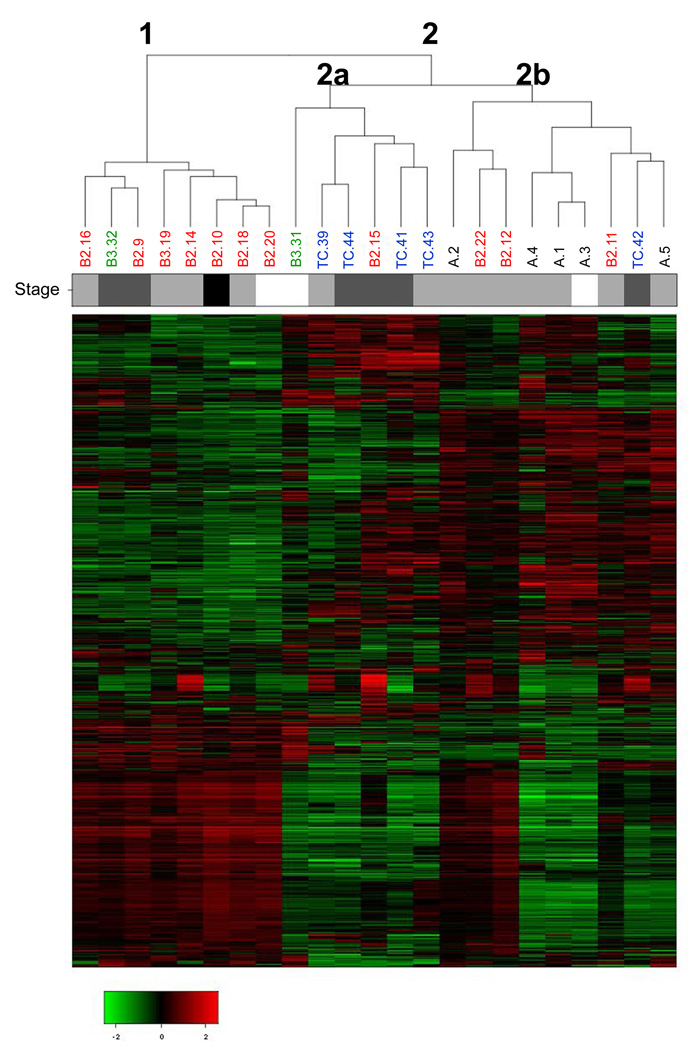
We next performed mRNA expression profiling of 23 out of the 27 previously untreated thymic tumors (those with adequate high quality RNA for hybridization to Affymetrix U133A oligonucleotide arrays). Normal human thymus tissue was unavailable for analysis. Unsupervised hierarchical cluster analysis of gene expression data revealed 2 major groups (Figure 3): cluster 1 (n=8) and cluster 2 (n=15). Cluster 1 was associated with thymoma type B2 histology, while cluster 2 was associated with thymoma type A and thymic carcinoma (p=0.023, Chi-square test). Gene Ontology based pathway analysis revealed that most of the genes that were more highly expressed in cluster 1 vs. cluster 2 were related to the immune system (Supplemental Tables 4, 5 and 6). This result may be due to the lymphocytic infiltrates found in type B2 thymomas compared to the epithelial predominance of type A thymomas and thymic carcinomas, although clustering of the B2 thymomas was not correlated with differences in epithelial tumor cell content of the analyzed specimens. Cluster 2 could be further separated into 2 groups, clusters 2A (n=6) and 2B (n=9), which were associated with thymic carcinoma and thymoma type A, respectively (p=0.036, Chi-square test). Overall, the hierarchical clustering analysis correlated well with the histologic WHO classification. There were no associations with clinical stage, survival, EGFR/KIT staining or mutation. We did not identify genes encoding kinases as being overexpressed in any type or cluster of tumors, including RAS- and KIT-mutant tumors.
Figure 3. Gene expression analysis of 23 thymic tumors.
Expression levels are colored red for values above the median, and green for values below the median. Type is indicated for each sample (TC: thymic carcinoma). For stage, white corresponds to stage 1, light gray to stage 2, dark gray to stage 3, and dark to stage 4.
Genomic profiling
To identify potential recurrent copy number changes at the DNA level, we performed array-based comparative genomic hybridization using Agilent 244K arrays and DNA from the 45 thymic tumor specimens. A hierarchical clustering algorithm generated 2 distinct clusters (Figure 4A). Cluster 1 (n=19) was associated with thymic carcinoma and type B3 thymoma and characterized by multiple chromosomal aberrations, whereas cluster 2 (n=26) was associated with type A and B2 thymomas (p<0.001, Chi-square test) and showed infrequent copy number alterations. There was no association between clinical stage (p=0.065, Chi-square test) or neoadjuvant treatment (p=0.311, Chi-square test) and these genomic clusters. Cluster 1 further subdivided in 2 major clusters, cluster 1a (n=9) with only type B3 thymomas and thymic carcinomas, characterized by chromosome 1q gain, and cluster 1b (n=7), with thymomas and thymic carcinomas sharing chromosome 6 loss (Figure 4A). Cluster 1 was significantly associated with high EGFR expression at IHC, as compared with cluster 2 (p=0.001, Chi-square test).
Figure 4. Genomic profiles of 45 thymic tumors.
(A) Unsupervised clustering analysis. Gains are indicated in red, and losses in green, by genomic position along the 22 chromosomes. (B) Genomic profiles and recurrent copy number alterations in type A and B thymomas, and in thymic carcinomas. Gains are indicated in red and losses in blue.
We separately analyzed thymoma types A, B, and thymic carcinoma, to identify recurrent gene copy number alterations (CNAs) (Figure 4B and Supplemental Table 7). Only thymic carcinoma exhibited CNAs that were not reported as copy number variations. No recurrent CNAs occurred in KRAS, HRAS, or KIT, or in genes differentially expressed in the different tumor types.
Comparison of carcinoma of the thymus vs. squamous cell carcinoma of the lung
Thymic carcinomas often display squamous cell histology and invade the lungs, pleura, and mediastinum [3]. As primary squamous cell carcinomas of the lung share many of these properties, mediastinal squamous cell carcinomas often present a diagnostic challenge. Thus, we asked whether molecular analyses could be used to discriminate these 2 entities. We directly compared the genomic profiles of the 7 thymic carcinoma tumors with those from 6 primary squamous cell carcinomas of the lung, that were matched for histological grade (Supplemental Table 8). CD5 was expressed in 4 (57%) of the 7 thymic carcinomas (Supplemental Table 8). All tumors, including CD5-negative cases, demonstrated classic diagnostic, clinical, pathological, and surgical features of the disease. Copy number data analysis revealed that compared to squamous lung cancers, chromosomal aberrations were more frequent and occurred in different and larger loci in thymic carcinomas (Supplemental Figure 3). Thymic carcinomas exhibited gains in chromosomes 1, 3q, 5, 8, 12, 15, 17q, 18, and 20, and losses in chromosomes 1p, 2p, 3p, 6, 7, 13q, 14, 16q, and 17p losses. Lung carcinomas displayed chromosome 2q, 3q, 5p, 7p, 8, 11q, 14p, 15p, 17p, and 18p gains, and chromosome 1q, 3p, 4p, 5q, 6q, 9p, 13q, 15p, 16q, and 17q losses (Supplemental Figure 3).
Discussion
In order to gain further insight into the biology of thymic tumors, we performed a comprehensive molecular analysis of 45 resected thymic tumors. To our knowledge, this study represents the first and one of the largest of its kind. Previous studies have focused on only a small number of tumors [29] and employed only a limited number of analytical methods [29–31]. Our analysis has three main new findings.
First, mutational analysis of genes encoding components of the EGFR signaling pathway led to the identification of RAS mutations in 3 (7%) out of 45 thymic epithelial tumors. Previously, investigators have collectively assessed the status of only one RAS gene (i.e. KRAS) in 17 tumors [16, 32, 33], but did not identify any mutation. In our study, 2 RAS mutant tumors were thymomas (type A and B2) which are low-grade, and the other tumor was a thymic carcinoma, which is high-grade. KRAS mutations predict for primary resistance to anti-EGFR directed therapies (i.e. gefitinib/erlotinib in lung cancer [34] and cetuximab in colon cancer [35]). HRAS mutations occur much more rarely in epithelial cancers [36]. Cells expressing activated or mutant HRAS (T24 cell line [37]) have also been reported to be resistant to gefitinib [38, 39]. These findings could then have therapeutic implications for the treatment of thymic tumors. Although RAS mutations are rare in thymic tumors, further assessment of RAS is needed and should probably be included in any therapeutic trial considering anti-EGFR therapy for thymic malignancies.
None of the 45 tumors harbored EGFR kinase domain mutations, which are associated with sensitivity to gefitinib/erlotinib. Thus far, only 2 such mutations have been found in a total of 122 tumors collectively analyzed [7, 9, 10, 16, 33]. Coupled with the data on RAS, these findings could explain why responses to EGFR TKIs have been rare in unselected thymic tumors [33, 40]. As both RAS mutant tumors were strongly positive for EGFR staining, the data also suggest that IHC staining for EGFR is not useful as an eligibility marker for anti-EGFR therapies.
The second finding is the presence of KIT mutations in 2 of 45 thymic tumors. One was a V560 deletion in exon 11, and the other was a novel H697Y mutation in exon 14. In contrast to RAS mutations, KIT mutations were found exclusively in thymic carcinomas. Previously, out of 106 thymic tumors collectively tested, only 3 cases of KIT mutation have been reported in the literature [7, 8, 11, 13, 15, 41, 42]; all these mutations also occurred in thymic carcinomas. Interestingly, one was the same KIT V560 deletion found here. This mutation is associated with sensitivity to KIT inhibitors, based upon multiple lines of evidence: 1) we showed in vitro that the growth of mutant-bearing Ba/F3 cells is readily inhibited by treatment with imatinib and sunitinib; 2) the patient whose tumor harbored this mutation responded to treatment with imatinib [13], and 3) this mutation has also been found in an imatinib-sensitive GIST [43]. Another KIT-mutant case of thymic carcinoma reported in the literature harbored an L576P mutation in exon 11 [7]. This mutation has also previously been described in GIST and melanoma and has been biologically characterized as being sensitive to imatinib [44]. The third mutation reported is a D820E mutation in exon 17, exhibited by a thymic carcinoma responding to sorafenib [42]. The D820Y KIT mutation is known to be associated with imatinib resistance and sorafenib sensitivity in GIST [22]. Finally, we show here that the KIT H697Y mutation, identified in exon 14 (which was not sequenced in previous studies) is associated in vitro with greater sensitivity to sunitinib vs. imatinib.
In our series, using the same antibody for KIT IHC as used by others, 3 of 7 thymic carcinomas were positive, but only 2 harbored KIT mutations. The rarity of KIT mutations in unselected thymic tumors and the greater sensitivity of the H697Y mutant to sunitinib could explain the absence of responses observed in 2 recent phase II trials with imatinib, where patients were selected either by histologic type (B3 thymomas and thymic carcinomas) [41], or using KIT staining by IHC [45]. Overall, including our cases, 7% (5 out of 70 collectively genotyped) of thymic carcinomas exhibited KIT mutations. Similar to melanomas where KIT mutations are found in only 10% of tumors [46], we would recommend for future trials in thymic carcinomas with KIT inhibitors enrolling only those patients with KIT mutant tumors. We would also favor the use of sunitinib over imatinib.
Our genomic profiling data confirm previously reported analyses using lower resolution techniques of CGH [18, 30, 31]. Losses of chromosomes 1q, 3p, 17p and gains of chromosome 1q are found both in type B2-B3 thymomas and thymic carcinomas. Chromosome 6q, 13q, 16q losses and chromosome 4p, 17q and 18 gains are common in type B3 thymomas and thymic carcinomas [18, 30]. As shown in Figure 4, the frequency, extent, and number of genomic aberrations increase from type A thymoma to thymic carcinoma. However, the clustering of a previous cohort of 65 tumors analyzed by CGH led to the identification of 2 groups, one sharing chromosome 1q gain and one with no recurrent pattern of chromosome imbalances [30]. The first group could be further subdivided into 2 clusters, one with chromosome 6q and 16q losses, and one with different genomic alterations [30]. In our cohort, cluster 1 subdivided in cluster 1a with chromosome 1q gain and cluster 1b with chromosome 6 and, to a lesser extent, chromosome 16q losses (Figure 4). The significance of these genomic alterations is unknown.
By using high resolution techniques of molecular profiling, our objective was also to identify genes or chromosomal regions that would have been altered both at the genomic and expression levels, i.e. overexpressed and amplified or underexpressed and deleted. However, we did not identify such regions, especially involving genes encoding proteins with therapeutic significance, such as tyrosine and serine-threonine kinases. The lack of findings could be due to the low number of samples analyzed. We also were not able to compare expression data from tumors with that from matched normal thymus samples, which was unavailable, as in other studies [29, 47]. Finally, especially for type B2 thymomas, results may have been confounded by the presence of lymphocytic infiltrates, although we did not identify any differences in the profiles depending on the epithelial tumor cell content.
One study previously reported results from gene expression profiling in thymomas [29, 47]. Only 4 tumors were actually analyzed: 2 non invasive tumors (one stage I type A thymoma, and one stage II type B3 thymoma), and 2 invasive tumors (2 stage IVa type B3 thymomas). Among several genes, the authors identified C-JUN, KIAA0022, and GPI-80 as being significantly correlated with tumor stage. We compared each tumor type one to another for differentially expressed genes (data not shown), but we did not identify these genes in our analyses.
Collectively, our expression and genomic clustering results indicate that tumors of the thymus defined histopathologically according to the current 2004 WHO classification do have different molecular features. Expression profiling separates type B2 thymomas, containing biologically active lymphocytes, from other thymic tumors, which are mostly epithelial. Furthermore, genomic profiling clearly discriminates thymic carcinomas and type B3 thymomas from type A and type B2 thymomas. From a classification perspective, our analysis then supports the distinctions of type A, type B2, type B3, and thymic carcinoma. Consistent with the notion of subtypes, only the latter tumor type harbors KIT mutations. Collectively, these results imply that the molecular basis for thymomas and thymic carcinomas is unique.
Finally, our data provides the first direct molecular comparison of thymic carcinoma with lung squamous cell carcinoma further demonstrating that these tumors are molecularly distinct from squamous cell carcinomas of the lung. Genomic profiling data are consistent with previous indirect comparisons between the 2 entities [4], with 1q, 17q, and18 gains and 3p, 6, 16q, and 17p losses in thymic carcinomas, and 3q, 11q, and 8q gains, and 3p, 5q, 9p, 13q losses in lung carcinomas. We were also able to identify additional differential alterations present in thymic tumors (chromosome 12 gains, and chromosome 2p, 6, 7, 14 losses). Consistent with these findings, we did not identify mutations in TP53 in thymic carcinomas, although such mutations are frequent (55%) in lung squamous cell carcinomas [48].
In the future, we plan to include cases of type AB and B1 thymomas, together with more tumors of all subtypes, to validate our current findings and to determine if proposals to simplify the current WHO thymoma classification also have biological relevance [49, 50]. We also plan to perform more comprehensive mutational profiling of thymic malignancies. In the meantime, we recommend that despite occurring infrequently, thymic carcinomas should be treated separately from thymomas for clinical and therapeutic considerations.
Supplementary Material
Acknowledgments
We thank the MSKCC Department of Pathology Tumor Procurement Service and Bhuvanesh Singh from the Department of Surgery for assistance in the collection of tumor samples; Igor Dolgalev, Thomas Landers, and Sabrena Thomas from the Geoffrey Beene Translational Oncology Core Facility for their technical support; Eden C. Payabyab for assembling the tissue microarray; Yixuan Gong and Juliann Chmielecki for their helpful assistance.
Financial support
This study was supported by the HOPP Lung Cancer Research Fund (WP), the Rosalind Warren Memorial Fund (WP), the MSKCC Geoffrey Beene Cancer Research Center (WP), and the Anbinder Fund (MSKCC Sequenom Facility). We would like to acknowledge services provided by the Genomics Core Facility, which is partially supported by an NCI CC56 award (P30-CA008748). Nicolas Girard is a recipient of travel grants from the College des Professeurs de Pneumologie (CEP)/AstraZeneca and from the National Federation of French Comprehensive Cancer Center/Fondation de France.
References
- 1.de Jong WK, Blaauwgeers JL, Schaapveld M, Timens W, Klinkenberg TJ, Groen HJ. Thymic epithelial tumours: a population-based study of the incidence, diagnostic procedures and therapy. Eur J Cancer. 2008;44:123–130. doi: 10.1016/j.ejca.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Girard N, Mornex F, van Houtte P, Cordier JF, van Schil P. Thymoma: a focus on current therapeutic management. J Thorac Oncol. 2008;4:119–126. doi: 10.1097/JTO.0b013e31818e105c. [DOI] [PubMed] [Google Scholar]
- 3.Eng TY, Fuller CD, Jagirdar J, Bains Y, Thomas CR. Thymic carcinoma: state of the art review. Int J Radiat Oncol Biol Phys. 2004;59:654–664. doi: 10.1016/j.ijrobp.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 4.WHO histological classification of tumours of the thymus. Travis WB, Brambilla A, Muller-Hermelinck HK, Harris CC. World Health Organization Classification of Tumours. Lyon: IARC Press; Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. 2004:146.
- 5.Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up study of thymomas with special reference to their clinical stages. Cancer. 1981;48:2485–2492. doi: 10.1002/1097-0142(19811201)48:11<2485::aid-cncr2820481123>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.Blumberg D, Port JL, Weksler B, et al. Thymoma: a multivariate analysis of factors predicting survival. Ann Thorac Surg. 1995;60:908–913. doi: 10.1016/0003-4975(95)00669-c. [DOI] [PubMed] [Google Scholar]
- 7.Yoh K, Nishiwaki Y, Ishii G, et al. Mutational status of EGFR and KIT in thymoma and thymic carcinoma. Lung Cancer. 2008;62:316–320. doi: 10.1016/j.lungcan.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Tsuchida M, Umezu H, Hashimoto T, et al. Absence of gene mutations in KIT-positive thymic epithelial tumors. Lung Cancer. 2008;62:321–325. doi: 10.1016/j.lungcan.2008.03.035. [DOI] [PubMed] [Google Scholar]
- 9.Meister M, Schirmacher P, Dienemann H, et al. Mutational status of the epidermal growth factor receptor (EGFR) gene in thymomas and thymic carcinomas. Cancer Lett. 2007;248:186–191. doi: 10.1016/j.canlet.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki E, Sasaki H, Kawano O, et al. Expression and mutation statuses of epidermal growth factor receptor in thymic epithelial tumors. Jpn J Clin Oncol. 2006;36:351–356. doi: 10.1093/jjco/hyl028. [DOI] [PubMed] [Google Scholar]
- 11.Pan CC, Chen PC, Chiang H. KIT (CD117) is frequently overexpressed in thymic carcinomas but is absent in thymomas. J Pathol. 2004;202:375–381. doi: 10.1002/path.1514. [DOI] [PubMed] [Google Scholar]
- 12.Tateyama H, Eimoto T, Tada T, et al. p53 protein expression and p53 gene mutation in thymic epithelial tumors. An immunohistochemical and DNA sequencing study. Am J Clin Pathol. 1995;104:375–381. doi: 10.1093/ajcp/104.4.375. [DOI] [PubMed] [Google Scholar]
- 13.Ströbel P, Hartmann M, Jakob A, et al. Thymic carcinoma with overexpression of mutated KIT and the response to imatinib. N Engl J Med. 2004;350:2625–2626. doi: 10.1056/NEJM200406173502523. [DOI] [PubMed] [Google Scholar]
- 14.Li XF, Chen Q, Huang WX, Ye YB. Response to sorafenib in cisplatin-resistant thymic carcinoma: a case report. Med Oncol. 2009;26:157–160. doi: 10.1007/s12032-008-9100-0. [DOI] [PubMed] [Google Scholar]
- 15.Chuah C, Lim TH, Lim AS, et al. Dasatinib induces a response in malignant thymoma. J Clin Oncol. 2006;24:e56–e58. doi: 10.1200/JCO.2006.08.8963. [DOI] [PubMed] [Google Scholar]
- 16.Christodoulou C, Murray S, Dahabreh J, et al. Response of malignant thymoma to erlotinib. Ann Oncol. 2008;19:1361–1362. doi: 10.1093/annonc/mdn388. [DOI] [PubMed] [Google Scholar]
- 17.Farina G, Garassino MC, Gambacorta M, La Verde N, Gherardi G, Scanni A. Response of thymoma to cetuximab. Lancet Oncol. 2007;8:449–450. doi: 10.1016/S1470-2045(07)70141-9. [DOI] [PubMed] [Google Scholar]
- 18.Zettl A, Ströbel P, Wagner K, et al. Recurrent genetic aberrations in thymoma and thymic carcinoma. Am J Pathol. 2000;157:257–266. doi: 10.1016/S0002-9440(10)64536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chitale D, Gong Y, Taylor BS, et al. An integrated genomic analysis of lung cancer reveals loss of DUSP4 in EGFR-mutant tumors. Oncogene. 2009 doi: 10.1038/onc.2009.135. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding L, Getz G, Wheeler DA, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korn EL, McShane LM, Troendle JF, Rosenwald A, Simon R. Identifying pre-post chemotherapy differences in gene expression in breast tumours: a statistical method appropriate for this aim. Br J Cancer. 2002;86:1093–1096. doi: 10.1038/sj.bjc.6600216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo T, Agaram NP, Wong GC, et al. Sorafenib inhibits the imatinib-resistant KITT670I gatekeeper mutation in gastrointestinal stromal tumor. Clin Cancer Res. 2007;13:4874–4881. doi: 10.1158/1078-0432.CCR-07-0484. [DOI] [PubMed] [Google Scholar]
- 23.Henley JD, Cummings OW, Loehrer PJ., Sr. Tyrosine kinase receptor expression in thymomas. J Cancer Res Clin Oncol. 2004;130:222–224. doi: 10.1007/s00432-004-0545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakagawa K, Matsuno Y, Kunitoh H, Maeshima A, Asamura H, Tsuchiya R. Immunohistochemical KIT (CD117) expression in thymic epithelial tumors. Chest. 2005;128:140–144. doi: 10.1378/chest.128.1.140. [DOI] [PubMed] [Google Scholar]
- 25.Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 26.Mol CD, Lim KB, Sridhar V, et al. Structure of a c-kit product complex reveals the basis for kinase transactivation. J Biol Chem. 2003;278:31461–31464. doi: 10.1074/jbc.C300186200. [DOI] [PubMed] [Google Scholar]
- 27.Daley GQ, Baltimore D. Transformation of an interleukin 3-dependent hematopoietic cell line by the chronic myelogenous leukemia-specific P210bcr/abl protein. Proc Natl Acad Sci USA. 1988;85:9312–9316. doi: 10.1073/pnas.85.23.9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol. 2008;26:5352–5359. doi: 10.1200/JCO.2007.15.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasaki H, Ide N, Fukai I, Kiriyama M, Yamakawa Y, Fujii Y. Gene expression analysis of human thymoma correlates with tumor stage. Int J Cancer. 2002;101:342–347. doi: 10.1002/ijc.10624. [DOI] [PubMed] [Google Scholar]
- 30.Penzel R, Hoegel J, Schmitz W, et al. Clusters of chromosomal imbalances in thymic epithelial tumours are associated with the WHO classification and the staging system according to Masaoka. Int J Cancer. 2003;105:494–498. doi: 10.1002/ijc.11101. [DOI] [PubMed] [Google Scholar]
- 31.Lee GY, Yang WI, Jeung HC, et al. Genome-wide genetic aberrations of thymoma using cDNA microarray based comparative genomic hybridization. BMC Genomics. 2007;8:305. doi: 10.1186/1471-2164-8-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki M, Chen H, Shigematsu H, et al. Aberrant methylation: common in thymic carcinomas, rare in thymomas. Oncol Rep. 2005;14:1621–1624. [PubMed] [Google Scholar]
- 33.Kurup A, Burns M, Dropcho S, Pao W, Loehrer PJ. Phase II study of gefitinib treatment in advanced thymic malignancies. Journal of Clinical Oncology, ASCO Annual Meeting Proceedings. 2005;23:7068. [abstract] [Google Scholar]
- 34.Pao W, Wang TY, Riely GJ, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 36.Quinlan MP, Settleman J. Isoform-specific ras functions in development and cancer. Future Oncol. 2009;5:105–116. doi: 10.2217/14796694.5.1.105. [DOI] [PubMed] [Google Scholar]
- 37.Fasano O, Aldrich T, Tamanoi F, Taparowsky E, Furth M, Wigler M. Analysis of the transforming potential of the human H-ras gene by random mutagenesis. Proc Natl Acad Sci U S A. 1984;81:4008–4012. doi: 10.1073/pnas.81.13.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin B, Ariyama H, Baba E, et al. Activated Src and Ras induce gefitinib resistance by activation of signaling pathways downstream of epidermal growth factor receptor in human gallbladder adenocarcinoma cells. Cancer Chemother Pharmacol. 2006;58:577–584. doi: 10.1007/s00280-006-0219-4. [DOI] [PubMed] [Google Scholar]
- 39.Inoue R, Matsuyama H, Yano S, Yamamoto Y, Iizuka N, Naito K. Gefitinib-related gene signature in bladder cancer cells identified by a cDNA microarray. Anticancer Res. 2006;26:4195–4202. [PubMed] [Google Scholar]
- 40.Bedano PM, Perkins S, Burns M, et al. A phase II trial of erlotinib plus bevacizumab in patients with recurrent thymoma or thymic carcinoma. Journal of Clinical Oncology, ASCO Annual Meeting Proceedings. 2008;26:19087. [abstract] [Google Scholar]
- 41.Giaccone G, Smit EF, van Groeningen C, Hogedoorn PC. Phase II study of imatinib in patients with WHO B3 and C thymomas. Journal of Clinical Oncology, ASCO Annual Meeting Proceedings. 2008;26:14665. [abstract] [Google Scholar]
- 42.Bisagni G, Rossi G, Cavazza A, Sartori G, Gardini G, Boni C. Long lasting response to the multikinase inhibitor bay 43-9006 (Sorafenib) in a heavily pretreated metastatic thymic carcinoma. J Thorac Oncol. 2009;4:773–775. doi: 10.1097/JTO.0b013e3181a52e25. [DOI] [PubMed] [Google Scholar]
- 43.Antonescu CR, Besmer P, Guo T, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res. 2005;11:4182–4190. doi: 10.1158/1078-0432.CCR-04-2245. [DOI] [PubMed] [Google Scholar]
- 44.Antonescu CR, Busam KJ, Francone TD, et al. L576P KIT mutation in anal melanomas correlates with KIT protein expression and is sensitive to specific kinase inhibition. Int J Cancer. 2007;121:257–264. doi: 10.1002/ijc.22681. [DOI] [PubMed] [Google Scholar]
- 45.Salter JT, Lewis D, Yiannoutsos C, Loehrer PJ, Risley L, Chiorean EG. Imatinib for the treatment of thymic carcinoma. Journal of Clinical Oncology, ASCO Annual Meeting Proceedings. 2008;26:8116. [abstract] [Google Scholar]
- 46.Carvajal RD, Chapman PB, Wolchok JD, et al. A phase II study of imatinib mesylate (IM) for patients with advanced melanoma harboring somatic alterations of KIT. Journal of Clinical Oncology, ASCO Annual Meeting Proceedings. 2009;27:9001. [abstract] [Google Scholar]
- 47.Sasaki H, Ide N, Sendo F, et al. Glycosylphosphatidyl inositol-anchored protein (GPI-80) gene expression is correlated with human thymoma stage. Cancer Sci. 2003;94:809–813. doi: 10.1111/j.1349-7006.2003.tb01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujita T, Kiyama M, Tomizawa Y, Kohno T, Yokota J. Comprehensive analysis of p53 gene mutation characteristics in lung carcinoma with special reference to histological subtypes. Int J Oncol. 1999;15:927–934. doi: 10.3892/ijo.15.5.927. [DOI] [PubMed] [Google Scholar]
- 49.Suster S, Moran CA. Thymoma, atypical thymoma, and thymic carcinoma. A novel conceptual approach to the classification of thymic epithelial neoplasms. Am J Clin Pathol. 1999;111:826–833. doi: 10.1093/ajcp/111.6.826. [DOI] [PubMed] [Google Scholar]
- 50.Marchevsky AM, Gupta R, McKenna RJ, et al. Evidence-based pathology and the pathologic evaluation of thymomas: the World Health Organization classification can be simplified into only 3 categories other than thymic carcinoma. Cancer. 2008;112:2780–2788. doi: 10.1002/cncr.23492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.