Abstract
There is currently much interest in investigating the neural substrates of metaphor processing. In particular, it has been suggested that the right hemisphere plays a special role in the comprehension of figurative (non-literal) language, and in particular metaphors. However, some studies find no evidence of right hemisphere involvement in metaphor comprehension (e.g. Lee & Dapretto, 2006; Rapp et al., 2004). We suggest that lateralization differences between literal and metaphorical language may be due to factors such as differences in familiarity (Schmidt et al., 2007), or difficulty (Bookheimer, 2002; Rapp et al., 2004) in addition to figurativeness. The purpose of this study was to separate the effects of figurativeness, familiarity, and difficulty on the recruitment of neural systems involved in language, in particular right hemisphere mechanisms. This was achieved by comparing neural activation using functional magnetic resonance imaging (fMRI) between four conditions: literal sentences, familiar and easy to understand metaphors, unfamiliar and easy to understand metaphors, and unfamiliar and difficult to understand metaphors. Metaphors recruited the right insula, left temporal pole and right inferior frontal gyrus in comparison with literal sentences. Familiar metaphors recruited the right middle frontal gyrus when contrasted with unfamiliar metaphors. Easy metaphors showed higher activation in the left middle frontal gyrus as compared to difficult metaphors, while difficult metaphors showed selective activation in the left inferior frontal gyrus as compared to easy metaphors. We conclude that the right hemisphere is involved in metaphor processing and that the factors of figurativeness, familiarity and difficulty are important in determining neural recruitment of semantic processing.
Keywords: right hemisphere, figurative language, metaphors, semantic processing, insula
The nature of semantic processing of non-literal (i.e. figurative) language has been debated over the years. Traditional views hold that metaphors and other non-literal language (e.g. irony, inferences, indirect requests) require qualitatively different processing strategies for comprehension, and therefore figurative language is inherently different than literal language (Grice, 1975). One crucial aspect of the debate over whether figurative language is qualitatively different from literal language focuses on the role of the right hemisphere in metaphor comprehension. Initially it was hypothesized that the right hemisphere has a unique role in metaphor comprehension: the right hemisphere theory of metaphor. This was due to the difficulties that patients with right hemisphere brain damage have specifically with comprehension of non-literal language. An early imaging study (Bottini et al., 1994) and an early divided visual field study (Anaki, Faust, & Kravetz, 1998), both with neurologically unimpaired subjects, each found a right hemisphere advantage for metaphor processing. However, more recent work (for a review, see Kacinik & Chiarello, 2007) has called into question the right hemisphere theory. Some neuroimaging research has failed to find right hemisphere recruitment during metaphor comprehension (Rapp, Leube, Erb, Grodd, & Kircher, 2004, Rapp, Leube, Erb, Grodd, & Kircher, 2007). Other researchers find right hemisphere recruitment for metaphor comprehension, but suggest that the right hemisphere involvement is not due to the figurative nature of metaphors. Some researchers attribute right hemisphere involvement to factors such as differences in familiarity (e.g. Schmidt, DeBuse, & Seger, 2007; Mashal, Faust, & Hendler, 2005), or complexity (Bookheimer, 2002). Giora (1997, 1999, 2003) suggests that it is meaning salience (determined by frequency, conventionality, xx and xx) rather than figurativeness which determines the laterality of semantic processing. Beeman's coarse semantic coding theory (Beeman et al., 1994, Beeman, 1998; Jung-Beeman, 2005) proposes that the left hemisphere specializes in processing only fine (close) semantic relationships while the right hemisphere is adept at both fine (close) and coarse (distant) semantic relationships. While all of these proposals are to some degree overlapping, it is Beeman's theory that we use as the basis for insight into two of the factors examined in this study: figurativeness and familiarity. In addition, we suggest that right hemisphere recruitment during metaphor processing may be affected by a third factor, difficulty.
Figurativeness
Since the role of figurativeness in right hemisphere language is still controversial we chose to include it in our study to investigate its role in the neural basis of metaphor as well as how it may overlap with familiarity and difficulty. Figurative language is non-literal, and includes metaphors, idioms, and irony. A right hemisphere role in figurative language processing is consistent with Beeman's (1998) coarse semantic coding theory. According to Beeman's theory, the right hemisphere is involved in processing metaphors because they tend to have more distant semantic relationships than literal language. For example, literal sentences such as All his money is from the lottery contain nouns that have many overlapping semantic features (money-lottery) while metaphors (Respect is a precious gem) are more likely to contain nouns with less semantic overlap (respect-gem).
The association of the right hemisphere with metaphor processing has been supported by research in various domains. For example, when presented with a metaphorical phrase like she gave him a hand, patients with right hemisphere brain lesions tend to select a picture representing the literal rather than the figurative meaning (Mackenzie, Begg, Brady, & Lees, 1997; Mackenzie, Begg, Lees, & Brady, 1999; Rinaldi, Marangolo, & Baldassarri, 2004; Winner & Gardner, 1977). Anaki et al. (1998) centrally presented word primes to participants, who then were required to make a lexical decision regarding words presented to their left or right visual fields. The target words were related either literally or metaphorically to the prime. A left visual field (right hemisphere) processing time advantage (faster overall reaction times) was obtained for the metaphorically related words, suggesting a right hemisphere advantage for processing the metaphorical meanings of words (see also Faust & Mashal, 2007; Mashal & Faust, 2008; and Schmidt et al., 2007 for related findings). However, other studies do not support the right hemisphere theory (e.g. Lee & Dapretto, 2006; Rapp et al., 2004; 2007). Thus the inclusion of the factor of figurativeness in the current study is critical.
Familiarity
The second factor that may affect right hemisphere recruitment during metaphor processing and that can be associated with the coarse semantic coding theory (Beeman, 1998) is familiarity. Schmidt et al. (2007) demonstrated that metaphor familiarity may be a reasonable index of the coarseness of semantic relationship within the sentence, and that familiarity can be examined independently of figurativeness. An unfamiliar metaphor is more likely to have distant semantic relationships than a familiar sentence, and thus be processed efficiently in the right hemisphere. There are a number of reasons why this is so. Metaphors which utilize close associates may occur more frequently and therefore be more likely to become familiar over time. It could also be that associations have formed between these words due to speakers' experience with the familiar metaphor over time (see Bowdle & Gentner, 2005 for discussion). For example, familiar metaphors such as Babies are angels contain nouns that have many overlapping semantic features (babies-angels). However, unfamiliar metaphors such as Dictionaries are microscopes of words contain nouns with less overlapping semantic features. Stimulus familiarity (based on stimulus norming data) was shown to differentially recruit the right and left hemispheres and to modulate right hemisphere recruitment for metaphor processing (Schmidt et al., 2007). Both unfamiliar literal sentences and unfamiliar metaphors showed a right hemisphere processing time advantage (faster overall reaction times), whereas familiar literal sentences and familiar metaphors and showed a left hemisphere processing time advantage in a divided visual field study.
Modulation of neural activation on the basis of familiarity has been examined within the domain of metaphors. Metaphors differ greatly in familiarity, ranging from conventional, or very familiar metaphors (babies are angels), to novel or highly unfamiliar metaphors (marriage is an alloy). In many cases, familiar metaphors do not recruit the right hemisphere whereas unfamiliar metaphors do (e.g. Mashal et al., 2005). The importance of metaphor familiarity can be seen by examining the published metaphor imaging literature to date (see Table 1). To the best of our knowledge, Table 1 includes all published functional neuroimaging studies of metaphor processing to date. These studies encompass a wide variety of tasks, including sentence (e.g. Ahrens et al., 2007) and word pair (e.g., Mashal, et al., 2005) processing. All studies which report right hemisphere activation used novel or unfamiliar metaphors or semantic relationships (items 1–8 on Table 1), while most studies not reporting right hemisphere involvement (items 9–14) do not use novel metaphors. This suggests that metaphor familiarity is a factor that is important in determining the role of the right hemisphere in metaphor comprehension. This view is supported by a recent transcranial magnetic stimulation (TMS) study which demonstrated the critical involvement of right posterior temporal cortex in the processing of novel metaphors (Pobric, Mashal, Faust, & Lavidor, 2008).
Table 1. Metaphor Imaging Literature.
All known functional neuroimaging studies of metaphor processing published to date. Studies are listed with stimulus types grouped together, based on our reading of each paper. In addition, studies that found activation in right hemisphere areas are listed first (1–8).
| Source | Experiment Design | Stimuli | Main findings |
|---|---|---|---|
| 1. Bottini et al., 1994 | n=6, PET, block design | Complex sentences: Novel metaphors, uncommon literal sentences. | Right frontal and temporal activations for metaphors compared to literal sentences. |
| 2. Sotillo et al., 2005 | n=24, ERP LORETA spatial analysis | Complex/poetry style phrases: Spanish unfamiliar metaphorical phrase followed by metaphorically related or unrelated word. | Higher N400 activation for metaphoric words localized to right MTG and STG. |
| 3. Mashal et al., 2005 | n=15, 1.5T fMRI block design, principal component analysis | Word pairs: Literal relationship, conventional metaphorical relationship, or novel metaphor relationship. | Novel metaphor processing: large network including left frontal and temporal areas, and right Wernicke's area, precuneus and insula. |
| 4. Mashal et al., 2007 | n=15, 1.5T fMRI block design, ROI analysis | Word pairs: Literal relationship, conventional metaphorical relationship, or novel metaphor relationship. | Bilateral inferior frontal gyrus, middle frontal gyrus and superior temporal activations for metaphors, including right Wernicke's area. |
| 5. Arzouan et al., 2007 | n=29, ERP LORETA spatial analysis | Word pairs: Literal relationship, conventional metaphorical relationship, or novel metaphor relationship. | Right temporal and superior frontal involvement in novel metaphor processing. |
| 6. Pobric et al., 2008 | n=12, rTMS | Word pairs: Literal relationship, conventional metaphorical relationship, or novel metaphor relationship. | rTMS of right posterior superior temporal sulcus disrupted processing of novel but not conventional metaphors. |
| 7. Stringaris et al., 2006 | n=12, 1.5 T fMRI, event related, nonparametric analysis | Simple sentence - word combinations: Conventional metaphors and literal sentences with related or unrelated words as targets. | Right ventrolateral prefrontal cortex activation to target words in the metaphoric but not literal condition. |
| 8. Ahrens et al., 2007 | n=8, 1.5TfMRI, block design | Simple sentences: Mandarin Chinese anomalous (novel) and conventional metaphors and literal sentences. | Bilateral middle frontal gyrus and precentral gyrus, left inferior frontal gyrus and fusiform, right superior frontal gyrus activations for anomalous metaphors compared to literal |
| 9. Stringaris et al., 2007 | n=11, 1.5T fMRI, event related, nonparametric analysis | Simple sentences: Conventional metaphors and literal sentences of the form 'Some X are Y.' | Left inferior frontal gyrus and thalamus active for metaphors, no right activation specific to metaphors. |
| 10. Eviatar & Just, 2006 | n=16, 3T fMRI event related, ROI | Simple Sentences: Conventional metaphoric, ironic and literal sentences, following two sentence 'story.' | Metaphors recruited mainly LH areas: inferior frontal and inferior temporal. |
| 11. Rapp et al., 2004 | n=15, 1.5T fMRI event-related design | Simple sentences: German metaphors and literal sentences with the form an X is a Y, may have been moderately familiar. | Virtually no right hemisphere activations when comparing metaphors to literal sentences or grey screen baseline. |
| 12. Rapp et al., 2007 | Reanalysis of Rapp et al., 2004 data. ROIs subjected to laterality index calculation | Simple sentences: German metaphors and literal sentences with the form an X is a Y, may have been moderately familiar. | No differences in laterality patterns between literal and metaphorical sentences. |
| 13. Mashal et al., 2008 | n=14, 1.5T fMRI | Sentences: novel metaphors, literal sentences, and unfamiliar nonsensical sentences. | Stronger left dorsolateral prefrontal and posterior middle temporal activation for novel metaphors than for literal and nonsensical sentences. |
| 14. Lee & Dapretto, 2006 | n=12, 3T fMRI | Word triads: Literal relationship or conventional metaphorical relationship. | Only left prefrontal and tempo-parietal activations, no right activations for metaphorical relationships. |
Difficulty
Beeman's coarse semantic coding theory leads to the prediction that both figurativeness and familiarity will influence right hemisphere recruitment. However, right hemisphere recruitment may also depend on more general task demands that are not specifically semantic. One possibility is that right hemisphere homologs of left language processing areas are simply recruited when processing is generally speaking more difficult or effortful (Just, Carpenter, Keller, Eddy, & Thulborn, 1996). Right hemisphere homologs may be recruited to “help” the left hemisphere, or the right hemisphere may have some independent function more likely to be required in difficult tasks. Linguistic stimuli that are more difficult to process may thus recruit the right hemisphere more than easier ones. Across a variety of semantic and nonsemantic language tasks, the right hemisphere is recruited when task difficulty is relatively high, as summarized in the sample of functional neuroimaging language studies in Table 2. Difficulty here is operationally defined as requiring more cognitive processing, and is reflected in longer reaction times in each task. For example, Seger, Desmond, Glover, and Gabrieli (2000) found right hemisphere activation when participants generated an unusual verb to a presented noun as compared to simply generating the first verb to come to mind. The right hemisphere was also recruited when subjects generated rather than read the last word of a sentence (Kircher, Brammer, Andreu, Williams, & McGuire, 2001). Incorrect responses in a lexical decision task recruited the right frontal lobes (Rissman, Eliassen, & Blumstein, 2003), as did more degraded stimuli (Sharp, Scott, & Wise, 2004).
Table 2. Right hemisphere task attributions by area in a sampling of imaging studies.
A sample of studies was selected, all of which employed some type of linguistic task. These studies demonstrate the fact that the right hemisphere is recruited for tasks of higher difficulty, as indexed by response time, since each task that recruited the RH had slower response times.
| RH Area | Task | Reference |
|---|---|---|
| Superior Frontal | semantic decision for degraded > clear auditory stimuli | Sharp et al., 2004 |
| unusual verb generation > first verb to come to mind | Seger et al., 2000 | |
| incorrect > correct stimuli in lexical decision | Rissman et al., 2003 | |
| Middle/ Inferior Frontal | unrelated > related stimuli in lexical decision | Rissman et al., 2003 |
| incorrect > correct stimuli in lexical decision | Rissman et al., 2003 | |
| Anterior Cingulate | generate > read (sentence ending in low cloze word) | Kircher et al., 2001 |
| synonym > rhyme judgment | Roskies, et al., 2001 | |
| Superior/Middle Temporal | decide or generate > read (sentence ending) | Kircher et al., 2001 |
| synonym > rhyme judgment | Roskies, et al., 2001 | |
| unusual verb generation > first verb to come to mind | Seger et al., 2000 | |
| unrelated > related stimuli in lexical decision at long SOA | Rossell et al., 2003 | |
| lexical decision > tone decision | Rissman et al., 2003 | |
Since a wide variety of tasks recruit the right hemisphere when the condition is more difficult (Table 2), it could be that right hemisphere activations can be explained simply based on task difficulty rather than on factors related to semantic processing. Coarse semantic relationships may recruit the right hemisphere, not because of the nature of the semantic relationship, but simply because it is more difficult to process coarse semantic relationships. The difficulty explanation for right hemisphere recruitment of figurative language and coarse semantic relationships is supported by a number of the tasks in Table 2. Some of these tasks may be more difficult because they involve processing less familiar semantic relationships. For example, unusual verb generation involves accessing unusual semantic associations. Processing unrelated word pairs as compared to related word pairs may also require accessing a broad semantic network. However other tasks clearly do not involve broad semantic networks; for example processing degraded stimuli (which is difficult on a perceptual, not semantic level). It is beyond the scope of this paper to speculate on the processes underlying the performance of these tasks, but it is clear that a wide variety of linguistic tasks which are likely to differ substantially in terms of underlying processing mechanisms still recruit similar areas in the right hemisphere for difficult conditions. Thus it is possible that the difficulty of the task alone is responsible for right hemisphere recruitment.
Working Memory
Beeman's coarse coding theory suggests that right hemisphere recruitment during metaphor processing is due to the presence of coarse semantic relationships in metaphors (Beeman, 1998). Such coarse semantic relationships may require extra-linguistic processes which are mediated in neural regions beyond semantic homologs. In particular, we reasoned that processing language that includes coarse semantic relationships may require working memory functions not typically associated with semantic processing in order to cope with the broad scope of possible meanings associated with each word. Right (but not left) middle and inferior frontal regions (BA10, BA46, BA47) have been specifically related to such working memory functions. Wager and Smith (2003), in a meta-analysis of working memory, report that these right hemisphere areas are specifically related to manipulation of information stored in working memory, an executive function. Manipulation is distinguished from other executive functions such as updating information in working memory and maintaining order information, and involves transforming the characteristics of stimuli in working memory by “shifting attention and inhibition of irrelevant stimulus dimensions” (Wager & Smith, 2003, p. 270). In the current context this would involve invoking unusual or distant semantic characteristics or meanings of a word and inhibiting irrelevant meanings. Thus we suggest that right middle and inferior frontal regions will be recruited based on familiarity and figurativeness but not difficulty. Both figurativeness and familiarity factors are based on the concept of coarse semantic coding, suggesting that they will recruit qualitatively and quantitatively similar regions. However, if figurativeness on its own plays a role in right hemisphere recruitment, then there should be some right hemisphere activations for figurativeness which do not overlap with the familiarity activations.
The Current Investigation
The factors of familiarity and difficulty may be just as important as figurativeness in determining the role of the right hemisphere in metaphor comprehension. The purpose of this study was to test this hypothesis and separate the effects of these three factors on the recruitment of neural systems involved in language, in particular right hemisphere mechanisms. This was achieved via contrasts between five sentence types (Table 3): nonword sentences (Nonwords) literal sentences (Literal), metaphors that were familiar and easy to understand (Easy-Familiar), metaphors that were unfamiliar but easy to understand (Easy-Unfamiliar), and unfamiliar metaphors that were difficult to understand (Difficult-Unfamiliar; see Methods for details on how metaphors were selected). We examined five contrasts between sentence types: one Semantic contrast, two Figurativeness contrasts, a Familiarity contrast, and a Difficulty contrast. The Semantic contrast compared all four English sentence types (both literal and metaphor) with the nonword sentences. The first Figurativeness contrast compared neural activation associated with Literal sentences to all three groups of metaphors; the second compared literal sentences with the Easy-Familiar metaphors. This allowed for a general comparison of figurativeness in the first contrast, whereas in the second contrast the literal and metaphorical sentences were similar in terms of difficulty and familiarity. The Familiarity contrast compared the Easy-Familiar metaphors to the Easy-Unfamiliar metaphors. Finally, the Difficulty contrast compared the Easy-Unfamiliar metaphors with the Difficult-Unfamiliar metaphors.
Table 3.
Sample Stimuli
| Stimulus Category | Sentence |
|---|---|
| Pronounceable nonword sentences | Sla inseriot rull hake slub fuggler. |
| Erents hust fress ut hegrets. | |
| Literal sentences | The computers at my house are new. |
| All his money is from the lottery. | |
| The mirror is through that doorway. | |
| Many newspapers are successful. | |
| Easy-Familiar metaphors | Freedom is a breath of fresh air. |
| Respect is a precious gem. | |
| Books are treasure chests of information. | |
| A pond is nature's mirror. | |
| Easy-Unfamiliar metaphors | A shadow is a piece of night. |
| The dog's stomach is his master's alarm clock. | |
| Dictionaries are microscopes of words. | |
| Ritual is the prison of individuality. | |
| Difficult-Unfamiliar metaphors | Political success is a house of cards. |
| A smile is an ambassador. | |
| Skyscrapers are honeycombs for glass. | |
| The waltz is the nightingale of dance. |
We hypothesized that right hemisphere recruitment during metaphor comprehension is caused by a complex set of factors, which include difficulty, familiarity, and figurativeness. We hypothesized right hemisphere recruitment for all three factors, but with qualitatively different patterns of activation. Our specific predictions are based on Beeman's (1998) coarse coding theory, working memory findings in the literature, and the recruitment of the right hemisphere during difficult language tasks (e.g. Just et al., 1996).
First, we predicted that left hemisphere areas associated with semantic processing would be recruited in the Semantic contrast comparing nonword sentences with literal and metaphor sentences. Left hemisphere semantic areas include a large extent of temporal lobe association areas extending longitudinally from temporal-occipito-parietal junction to the temporal poles (Binder et al., 1997; Bright, Moss, & Tyler, 2004; Dronkers, Wilkins, VanValin, Redfern, & Jaeger, 2004; Grossman et al. 2004; Mummery, 2000). Left inferior frontal cortex (BA44, 45, 47) is also important for semantic processing (Blaxton et al., 1996; Demb et al., 1995; Gabrieli et al., 1996, Kapur et al., 1995; Klein, Milner, Zatorre, Meyer & Evans, 1995; Nathaniel-James, Fletcher, & Frith, 1997; Phelps, Hyder, Blamire & Schulman, 1997; Seger et al., 2000; Vandenberghe, Price, Wise, Josephs, & Frackowiak, 1996; Wagner, Desmond, Glover & Gabrieli, 1998). We also predict that the right homologs of these areas will be activated in other contrasts.
Second, we predicted that the Figurativeness contrast would recruit right hemisphere regions. In light of Beeman's coarse coding theory, we predict that some of these regions would be in right hemisphere homologs of left hemisphere semantic processing regions in the temporal lobe and inferior frontal gyrus. We predicted that semantic areas recruited by Figurativeness would also be recruited by Familiarity, since comprehension of both unfamiliar and figurative language depends on processing relatively distant semantic relationships.
Third, working memory findings in the literature (Wager & Smith, 2003) lead us to predict that both the Figurativeness and Familiarity contrasts would recruit additional right hemisphere regions that have been associated with working memory, in particular the middle frontal gyrus (BA10, 46, 47).
Fourth, we predicted that right hemisphere recruitment in the Difficulty contrast would be limited to right homologs of left hemisphere linguistic processing areas, since the right hemisphere can be characterized as “helping” the left hemisphere when processing becomes computationally difficult. In addition, left hemisphere language areas should show quantitatively greater activation for difficult stimuli as well (Just et al., 1996).
Method
Participants
Participants were ten members of the Stanford University community, five male and five female, with a mean age of 25 years (range: 19 – 42). An eleventh subject was eliminated from the analysis due to scanner malfunction. Participants were right handed by self-report, native speakers of English, had an education level equal to at least one year of college, met the criteria for MRI scanning (no metallic implants, no claustrophobia, head size compatible with the custom head coil), and were neurologically healthy (no known neurological or psychiatric injury or disease; not taking any psychoactive medication or drugs). Participants gave informed consent to participate in the study, which was approved by the institutional review boards of Stanford and Colorado State Universities in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Stimuli
Experimental stimuli consisted of three types of metaphorical sentences, literal sentences, and nonword sentences; see Table 3 for sample stimuli. The metaphors were taken from Katz et al. (1988), with normed values on 7-point scales based on (1) familiarity and (2) ease of interpretation. Metaphors were selected and divided into three lists such that the first two lists differed in terms of familiarity (t = 12.9, p < .001), but not difficulty (p > .05) while the second and third lists differed in terms of difficulty (t = 11.5, p < .001), but not familiarity (p > .05). The three resulting groups of metaphors were labeled Easy-Familiar (familiarity M = 4.99, SD=.64; ease of interpretation M = 5.87, SD=.53), Easy-Unfamiliar (familiarity M = 3.17, SD=.28; ease of interpretation M = 5.75, SD=.29) and Difficult-Unfamiliar (familiarity M = 3.04, SD=.27; ease of interpretation M = 4.67, SD=.36). It should be emphasized that while the metaphorical stimuli used varied along a familiarity dimension, none of them were highly familiar, conventional metaphors such as She has a warm heart. This exclusion was necessary since conventional metaphors involve lexicalized meanings of metaphorically used words, and are processed in a qualitatively different way (Bowdle & Gentner, 2005). Therefore, our “Familiar” stimuli were only relatively more familiar than the “Unfamiliar” stimuli; they were not highly familiar. Additional stimuli consisted of literal sentences and baseline sentences composed of pronounceable nonwords. The literal sentences were created using new combinations of the same words used in the three metaphor lists. The Easy-Familiar, Easy-Unfamiliar, Difficult-Unfamiliar, and Literal sentence types did not differ in terms of average word frequency per sentence, F(3,89) = 1.6, p > .05 (Kucera & Francis, 1967). In terms of syntactic structure, sentences were simple, active, declarative sentences, about half with a modifying phrase; the number of such phrases did not vary across sentence types, F(3,92) = 2.5, p > .05).
Nonwords formed by switching letters in words from the three metaphor lists were combined to form the nonword sentences. These were considered our baseline task. There were a total of 24 sentences of each type resulting in 120 sentences total. Sentences ranged in length from 3 to 12 words with a mean length of 6.0 words. A one-way ANOVA indicated that sentence length was not consistent across lists, F(4,115) = 4.2, p < .01. Post hoc Tukey HSD and Scheffe comparisons revealed that Easy-Unfamiliar metaphors were significantly longer (mean word count = 7.0) than Easy-Familiar metaphors (mean word count = 5.5, p < .01) or Difficult-Unfamiliar metaphors (mean word count = 5.5, p < .01) but not the Literal (mean word count = 6.0, p > .05) or nonword sentences (mean word count = 6.0, p > .05). This difference was not possible to avoid in the list construction, since within the corpus of Katz et al. (1988) sentences that were both unfamiliar and easy to understand were characteristically longer. However, this difference in length was not reflected in a difference in processing time, as shown in Behavioral Results, below. It should be kept in mind that this is a potential confound when interpreting the results. However, we suggest that a lack of difference in processing time across conditions is the most important determinant of comparable conditions in fMRI experiments, a view adopted by other researchers (e.g. Kable, Lease-Spellmeyer, & Chatterjee, 2002).
Procedure
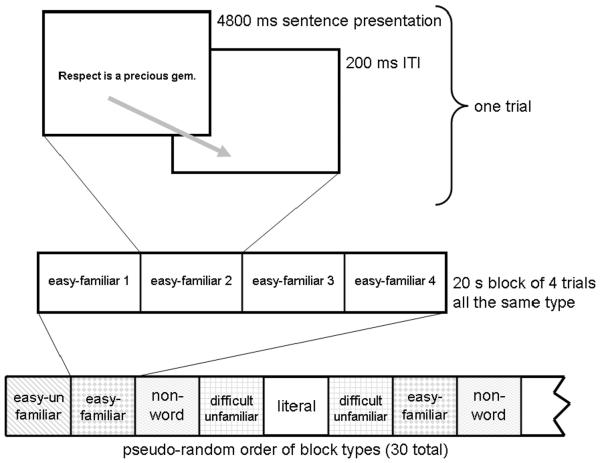
Prior to entering the scanner, participants were shown sample sentences and instructed that while in the scanner they should read each sentence and press the response key after each sentence as soon as they had understood it. For the nonword sentences, participants were asked to read the sentence without trying to pronounce the words and then press the response key. Participants were told to be prepared for a brief memory test based on the meanings of the sentences after they exited the scanner, to ensure that they would fully read the sentences. On each trial the entire sentence appeared at once in the center of the screen for 4800 ms, during which the participants silently read the sentence and made their button press response. The stimulus was followed by a blank screen for 200 ms, for a total of 5000 ms per sentence. The 120 sentences were randomly assigned to 20-second blocks of 4 sentences of the same type, with no repetition of stimuli across blocks. The resulting 30 blocks were arranged in pseudo random order with the constraints that there were no more than two consecutive metaphor blocks, literal and non-word blocks were not adjacent, and there were no consecutive blocks of the same metaphor type, as depicted in Fig. 1. A total scanning time of 10 minutes was required to complete the experiment. A block design was used in order to maximize statistical power.
Fig. 1. Experimental Design.
For each trial the sentence appeared in the center of the screen for 4800 ms, followed by a blank screen for 200 ms, for a total of 5000 ms per sentence. The 120 sentences were randomly assigned to 20-second blocks of 4 sentences of the same type. Blocks were arranged in pseudo random order with the constraints that there were no more than two consecutive metaphor blocks, literal and non-word blocks were not adjacent, and there were no consecutive blocks of the same metaphor type.
After exiting the scanner, participants completed a short written memory and comprehension test based on a subset of sentences they had seen in the scanner. Questions were to be answered based on the sentences and not general knowledge. For the metaphors, this required comprehension of the metaphor rather than just recognition.1 For example, to show memory of the metaphor A degree is a doorway, participants responded to the question Why is it good to get a degree? by selecting one of provides maturity, provides new opportunities or helps you to think better. Literal sentences were tested in a similar way, but required only recall, not comprehension of the sentence. Nonword sentences were tested for recognition as in Did you see this sentence? E blutch aw vot welond. A total of three questions per stimulus type was included in the test, for a total of fifteen questions (13 % of total stimuli). Since we only tested a subset of the stimuli, and did not directly test for understanding, a caveat of this study is that we can not be sure that they comprehended all the sentences in the way which we intended.
Functional MRI acquisition
Imaging was performed with a custom-built whole head coil in a 3.0 Tesla MRI Signa LX Horizon Echospeed (General Electric Medical Systems). Head movement was minimized for participants using a “bite-bar” formed with the participant's dental impression. In addition to the functional scans, three anatomical scans were performed: a coronal T1-weighted localizer scan, a three-dimensional high-resolution T1-weighted spoiled gradient echo scan with 124 contiguous 1.5 mm slices [minimum full echo time (TE), 30 degrees flip angle, 24 cm field of view, 256 × 256 acquisition matrix], and a inplane anatomical T1-weighted spin-echo scan with 22 contiguous 5 mm axial slices [minimum full TE; 500 ms TR, 24 cm field of view, 256 × 256 acquisition matrix]. Functional scanning was performed using a T2* sensitive gradient echo spiral in-out pulse sequence (Glover & Lai, 2001; Preston et al., 2004) [30 ms TE; 1500 ms TR; 65 degree flip angle; 24 cm field of view; 64 × 64 acquisition matrix] of the same 22 contiguous 5 mm axial slices as the inplane images. Scanning order was ascending and slices were acquired in an interleaved sequence.
Stimuli were presented using a magnet-compatible projector (Resonance Technology, Inc., Van Nuys, CA) that back-projects visual images onto a screen mounted above the participant's head. E-prime software (Psychology Software Tools, Pittsburgh, PA) running on a personal computer was used to generate visual stimuli and control experimental parameters. Responses were obtained using a magnet compatible response system. The scanner was started 8 seconds before the behavioral task began in order to allow for steady stage magnetization to be achieved; the resulting initial 4 scans were discarded.
Image Processing
Individual participant image preprocessing and analysis was performed using BrainVoyager 2000 4.9, and the group whole brain analyses, ROI analyses, and signal change analyses were performed using BrainVoyager QX 1.0.9 and 1.7 (Brain Innovation, Maastricht, The Netherlands). The functional data were first subjected to preprocessing, consisting of scan time correction, then motion correction, and then temporal filtering. The scan time correction used a cubic spline interpolation and was corrected to the first slice. The 3D motion correction was rigid body, and used trilinear interpolation images which were realigned to the first image of the first scan. The temporal filtering used a high pass filter in frequency space and linear trend removal. A fast Fourier transform was performed, and frequencies of less than 3 cycles across the time course of the scan were cut off.
Each participant's high resolution anatomical image was normalized to the Talairach and Tournoux (1988) brain template. The normalization process in BrainVoyager consists of two steps, an initial rigid body translation into the AC-PC plane, followed by an elastic deformation into the standard space performed on 12 individual subvolumes. The resulting set of transformations was applied to the participant's functional image volumes to form volume time course representations to be used in subsequent statistical analyses. Finally, the volume time course representations were spatially smoothed with a Gaussian kernel, full width at half maximum of 6.0 mm.
Contrasts
A total of five contrasts were examined. The first contrast compared all four experimental sentence types with the baseline non-word sentences. Subsequently the four experimental sentences types were used in four additional contrasts to allow the examination of three characteristics of semantic stimuli. Figurativeness was examined by contrasting (a) the Literal sentences with all three types of metaphorical sentences and (b) the Literal sentences with the Easy-Familiar metaphors. The Easy-Familiar metaphors were most likely to match the literal sentences on the dimensions of ease and familiarity. Familiarity was examined by contrasting the Easy-Familiar metaphors with the Easy-Unfamiliar metaphors. Difficulty was examined by contrasting the Easy-Unfamiliar metaphors with the Difficult-Unfamiliar metaphors. The subtraction logic employed in these contrasts is based on the assumption that neural mechanisms common to both conditions in a contrast will be equivalent, so that when the two conditions are subtracted from each other, the remaining activation reflects only brain regions unique to the two conditions being subtracted (Friston, Ashburner, Kiebel, Nichols, & Penny, 2007). For example, we assumed that the Literal and Figurative conditions each employ common sentence processing mechanisms, and that the only differences between the two sets of stimuli is the figurative nature of the later. Thus any positive brain activations resulting from the Figurative > Literal contrast reflect processing due only to the figurative nature of the stimuli while negative activations reflect processing due only to the literal nature of the stimuli. Such subtractions are used widely in fMRI research to compare two conditions with each other; however we offer the caveat that there are inherent issues with interpreting data derived from subtractions. This should be kept in mind when interpreting these data.
Statistical Analysis
Activation across the brain in the five block types (Nonword, Literal, Easy-Familiar, Easy-Unfamiliar, Difficult-Unfamiliar) were compared using BrainVoyager QX 1.0.9. The hemodynamic response for each condition was modeled by convolving a prototypical hemodynamic response function with the time course of the conditions. Then conditions were compared using the general linear model in Brain Voyager with separate subject predictors and subjects treated as random effects, using the five contrasts described above. The threshold was set at p < .001, uncorrected for multiple comparisons, which corresponded to a t-value threshold of 4.5. This is a commonly used threshold in fMRI research (e.g. Badre & D'Esposito, 2007). Gyral locations and Brodmann area designations of regions of significant activation were confirmed using Talairach Daemon (Lancaster, Summerln, Rainey, & Fox, 1997).
ROI Analyses
ROI templates were created in BrainVoyager QX 1.0.9 and 1.7. They were based on selected functionally activated clusters from the whole brain analysis. Selection was based on findings from the metaphor processing literature as indicated for each ROI in Table 4. The ROI is identified via a region growing process which starts with a central voxel indicated by mouse click, and which spreads to suprathreshold adjacent voxels, stopping at the boundaries of the functional cluster. The ROI general linear model (ROI GLM) tool of BrainVoyager QX 1.0.9 was used to analyze contrasts separately within each ROI. This tool calculates the average time course of all the voxels in the ROI for each subject, compares the time course across subjects and conditions in an ANOVA with subjects treated as random effects, and computes t and p values for contrasts of interest between specified conditions.
Table 4. Whole Brain Analysis.
All activations for the three contrasts that are significant at t = 4.5, p < .001, uncorrected are depicted.. Areas identified in the whole brain analysis that are also listed as findings in the metaphor processing literature were subsequently selected as ROIs. These are listed in the References column and listed at the bottom of the table. Activations used as ROIs are indicated in bold typeface. BA = Brodmann area, x, y, z = coordinates from the atlas of Talairach and Tournoux (1988), R= right hemisphere, L = left hemisphere.
| Activated Brain Area | BA | # Voxels | x | y | z | References |
|---|---|---|---|---|---|---|
| All Sentences (Literal, Easy-Familiar, Easy-Unfamiliar, Difficult-Unfamiliar) > Nonword Sentences | ||||||
| L middle frontal gyrus to insula | 46–13 | 4140 | −34 | 18 | 13 | |
| R middle frontal gyrus | - | 201 | 27 | −3 | 24 | |
| L hippocampus | 92 | −34 | −10 | −13 | ||
| Literal Sentences > Metaphors (Easy-Familiar, Easy-Unfamiliar, Difficult-Unfamiliar) | ||||||
| no significant activations at p < .001 | ||||||
| Metaphors (Easy-Familiar, Easy-Unfamiliar, Difficult-Unfamiliar) > Literal Sentences | ||||||
| L precentral gyrus | 4 | 251 | −64 | −8 | 27 | |
| R insula | 13 | 160 | 36 | −4 | 8 | 3 |
| L temporal pole | 38 | 367 | −57 | 21 | −9 | 7 |
| L inferior parietal lobe | - | 105 | −42 | −38 | 27 | |
| R precuneus | 7 | 155 | 13 | −51 | 40 | |
| L lingual gyrus | 18 | 915 | −18 | −72 | −1 | |
| Literal Sentences > Easy-Familiar Metaphors | ||||||
| no significant activations at p < .001 | ||||||
| Easy-Familiar Metaphors > Literal Sentences | ||||||
| R precuneus | 7 | 37 | 6 | −65 | 34 | |
| L precentral gyrus | 3 | 300 | −64 | −9 | 24 | |
| L precuneus | 18 | 1042 | −13 | −78 | 22 | |
| R cuneus | 18 | 197 | 15 | −85 | 22 | |
| L posterior cingulate | 30 | 27 | −24 | −65 | 13 | |
| L middle occipital gyrus | 18 | 15 | −31 | −80 | 7 | |
| L lingual gyrus | 18/19 | 570 | −27 | −73 | 1 | |
| R inferior frontal gyrus BA47 | 47 | 302 | 60 | 28 | −4 | 2,3,4,5,6,7 |
| L fusiform/lingual gyrus | 19 | 23 | −29 | −61 | −4 | |
| L parahippocampal gyrus | 35 | 89 | −15 | −32 | −10 | |
| R inferior frontal gyrus | 47 | 15 | 14 | 12 | −26 | |
| Familiar Metaphors (Easy-Familiar) > Unfamiliar Metaphors (Easy-Unfamiliar) | ||||||
| R middle frontal gyrus BA8 | 8 | 170 | 49 | 18 | 38 | 3,4,5,7 |
| L middle occipital gyrus | 18 | 52 | −29 | −99 | 7 | |
| L middle occipital gyrus | 18 | 276 | −24 | −89 | 2 | |
| R occipital lobe | 13 | 25 | −52 | 1 | ||
| Unfamiliar Metaphors (Easy-Unfamiliar)> Familiar Metaphors (Easy-Familiar) | ||||||
| L cingulate gyrus | 24 | 28 | −12 | −11 | 30 | |
| L hippocampus | - | 23 | −29 | −36 | −2 | |
| Easy Metaphors (Easy-Unfamiliar) > Difficult Metaphors (Difficult-Unfamiliar) | ||||||
| L precuneus | 7 | 47 | −24 | −54 | 49 | |
| L middle frontal gyrus BA46 | 46 | 597 | −46 | 37 | 22 | 3,4,5,7 |
| L/R anterior cingulate | 24 | 519 | 1 | 20 | 18 | |
| L anterior cingulate | 24 | 36 | −5 | 30 | 17 | |
| L anterior cingulate | 32 | 2466 | −10 | 40 | 3 | |
| L caudate head | - | 748 | −15 | 19 | 5 | |
| R anterior cingulate | 32 | 827 | 17 | 34 | 1 | |
| L putamen | - | 1378 | −28 | 0 | −6 | |
| Difficult Metaphors (Difficult-Unfamiliar) > Easy Metaphors (Easy-Unfamiliar) | ||||||
| L superior parietal lobe | 7 | 52 | −33 | −73 | 46 | |
| L inferior frontal gyrus BA9 | 9 | 31 | −65 | 11 | 34 | 2,3,4,5,6,7 |
Results
Behavioral Performance
The dependent measure was defined as latency between presentation of the sentence and the participants' button press that indicated they had finished reading the sentence. A one-way ANOVA revealed that despite the different sentence lengths, there was no difference across conditions in sentence reading time, F(4, 36) < 1. The mean response time was 1949 ms (SD = 627 ms). The high variability in response times suggests that there was not enough power to detect any potential differences with only ten subjects. Participant responses to the recognition test suggested that they remembered and understood at least a subset of the sentences; the mean rate of correct responses was 77% (SD = 19%). There were no significant differences in correct responses between the five sentence types, F(4,36) = 1.2, p > .05. Overall the behavior findings are consistent with the notion that participants complied with task instructions and understood the different sentence types.
Neural Activations
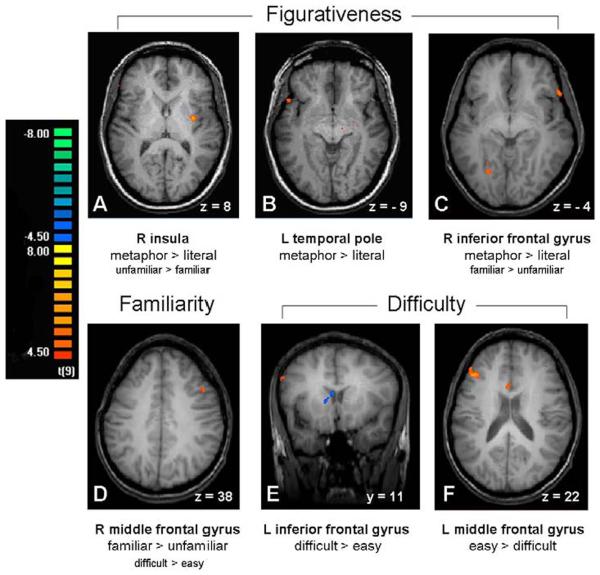
The results of the whole brain analysis are reported in Table 4. All sentence types contrasted with nonword sentences showed activation in the left middle frontal gyrus and insula and the right middle frontal gyrus. As illustrated in Fig. 2A and 2B, Metaphors (Easy-Familiar, Easy-Unfamiliar and Difficult-Unfamiliar) showed selective activation in the left temporal pole and right insula when contrasted with Literal sentences and Easy-Familiar metaphors alone showed activation in the right inferior frontal gyrus in comparison to Literal sentences (Fig. 2C). The familiarity contrast (Easy-Familiar > Easy-Unfamiliar metaphors) showed a right middle frontal activation (Fig. 2D). A number of activations were found for easy metaphors (Easy-Unfamiliar > Difficult-Unfamiliar), including left middle frontal gyrus (Fig.2F) and bilateral medial frontal activations. In addition, the left inferior frontal gyrus was more active for difficult than for easy metaphors as seen in Fig.2E. Across comparisons, right hemisphere activity was seen in addition to left. The right middle frontal gyrus was affected by familiarity, the right anterior cingulate gyrus by difficulty, and the right insula and inferior frontal gyrus by figurativeness.
Fig. 2.
Primary activations for each of the three principal contrasts in the whole brain analysis. Activations shown at p < .001, uncorrected. Brain figures shown in neurological convention (left = left). The primary contrast from the whole brain analysis is listed under each brain area. Each of these areas was then used in a region of interest (ROI) analysis. Additional contrasts that showed significant activation in the ROI analysis are depicted in smaller type below the primary contrast. Figs. 2A, 2B and 2C. Figurativeness contrast. Selected Metaphor > Literal activations are depicted. Fig. 2A displays the right insula; the left insula was also active for this contrast in the ROI analysis. Figs. 2B and 2C depict the left temporal pole and right inferior frontal gyrus, respectively. Figs. 2C and 2D. Familiarity contrast. Middle frontal gyrus activation (Fig. 2D) from the Familiar > Unfamiliar contrast. The inferior frontal area (Fig. 2C) also showed activation for this contrast in the ROI analysis. Figs. 2E and 2F. Difficulty contrast. Left inferior frontal and left middle frontal activations from the Easy (orange; 2F) > Difficult (blue; 2E) contrast.
Six ROIs were selected from the whole brain analysis based on areas of interest from the literature, as depicted in bold typeface in Table 4. The voxel size of each ROI is also shown in this table. Those selected included all those within the lateral frontal, temporal, and insular language areas in both hemispheres. Follow up analyses were performed on these ROIs to determine if any of these areas were significantly active in additional contrasts. The right insula ROI, in addition to being significantly more active for all three Metaphor types than Literal stimuli, was also more active for Easy-Unfamiliar than Easy-Familiar metaphors, t(9) = 2.4, p = .04; see Fig. 2A. The left temporal pole ROI, in addition to being significantly more active for all three Metaphor types than Literal stimuli, was also more active for Easy-Familiar metaphors in contrast with Literal sentences, t(9) = 3.5, p = .007; see Fig. 2B. The right inferior frontal gyrus BA47 ROI, in addition to being significantly more active for Easy-Familiar metaphors than Literal sentences, was also more active for all three Metaphor types than Literal stimuli, t(9) = 2.9, p = .02; and affected by familiarity (more active for Easy-Familiar than Easy-Unfamiliar metaphors, t(9) = 2.6, p = .03; see Fig. 2C). The right middle frontal gyrus BA8 ROI, in addition to being affected by familiarity (significantly more active for Easy-Familiar than Easy-Unfamiliar metaphors), was also affected by difficulty (more active for Difficult-Unfamiliar metaphors than Easy-Unfamiliar metaphors, t(9) = 2.3, p < .05; see Fig.2D.)
For each ROI defined, the opposite hemisphere homolog (left or right) was also defined as an ROI to facilitate examination of lateralized differences (Rapp et al., 2007). Note that the homolog consisted of exactly the same voxels, but with the x coordinate changed in sign. As a result, we found that two left homolog of right hemisphere ROIs showed significant activations. The left insula showed a similar pattern of activation to the right insula (Fig. 2A), with greater activity for the three Metaphor sentences types than Literal sentences, t(9) = 3.5, p = .007. The inferior frontal gyrus BA47 area, significantly more active for Easy-Familiar metaphors than Literal sentences on the right, was also more active on the left for all three Metaphor types than Literal stimuli, t(9) = 2.2, p = .05 (Fig.2C).
Discussion
This study investigated neural activations associated with reading metaphorical sentences which varied along three dimensions. We separately evaluated the effects of figurativeness (Figs. 2A, and 2C), familiarity (Fig. 2D), and difficulty (Figs. 2E and 2F) on brain activation. Each of these contrasts recruited distinct regions, supporting our hypothesis that all three factors are important for characterizing the neural basis of metaphor. The areas activated in these contrasts included classic left hemisphere semantic processing areas including the inferior frontal gyri and anterolateral temporal cortex (Bright, et al., 2004; Gabrieli et al., 1996; Grossman et al. 2004; Seger et al., 2000). Right hemisphere areas were also active for each of the contrasts, contrary to findings suggesting that the right hemisphere is not involved in metaphor comprehension (e.g. Gagnon et al., 2003; Lee & Dapretto, 2006; Rapp et al., 2004, 2007; Stringaris et al., 2007; Tompkins, 1990).
Our first prediction was confirmed; we report a large left hemisphere activation for all sentences compared to the nonword sentences in well established semantic areas in the left inferior frontal gyrus (Blaxton et al., 1996; Demb et al., 1995; Gabrieli et al., 1996, Kapur et al., 1995; Klein, Milner, Zatorre, Meyer & Evans, 1995; Nathaniel-James, Fletcher, & Frith, 1997; Phelps, Hyder, Blamire & Schulman, 1997; Seger et al., 2000; Vandenberghe, Price, Wise, Josephs, & Frackowiak, 1996; Wagner, Desmond, Glover & Gabrieli, 1998).
Figurativeness and Familiarity
Our second prediction was that right hemisphere homologs of left hemisphere semantic areas would be identified in the figurativeness and familiarity contrasts due to their dependence on course coding mechanisms (Beeman, 1998). The results generally conformed to this prediction. Activations in the figurative contrast were located in the right inferior frontal gyrus, right insula, and left temporal pole; activity was modulated by familiarity in right middle frontal gyrus BA8 and right inferior frontal gyrus BA47 (in the ROI analysis of this area originally defined by the whole brain figurativeness contrast).
The right inferior frontal gyrus activation (BA47) was associated with both figurativeness and familiarity, in line with our third prediction that both factors would recruit similar right hemisphere regions due to the need to manipulate items in working memory during coarse semantic coding. This area is close to the right homologs of traditional language areas, and is known to be involved in manipulation of data stored in working memory, specifically on the right (Wager & Smith, 2003). Such manipulation is required when processing figurative or unfamiliar language since in both cases a broad range of meanings must be manipulated in order for meaning selection to take place, suggesting why this left hemisphere area was recruited during metaphor tasks. Finally, since there were no right hemisphere activations unique to the figurativeness contrast, we cannot support the view that right hemisphere recruitment is due primarily to figurativeness.
The right middle frontal gyrus (BA8) familiarity activation includes an area documented in a language imaging meta-analysis (Vigneau et al., 2006), in which it is identified as being central to semantic processing in its left hemisphere homolog. While Vigneau and colleagues did not investigate the right hemisphere, it is reasonable to expect homolog recruitment in various tasks (Just et al., 1996). The right middle frontal gyrus is primarily recruited for controlled semantic retrieval, semantic priming (Vigneau et al., 2006) and selection of responses or meanings (Thomson-Schill, D'Esposito, Aguirre, & Farah, 1997, Wagner, Pare-Blagoev, Clark, & Poldrack, 2001), as well as being part of a semantic working memory loop connected with the angular gyrus. It is furthermore identified as a supramodal semantic area encompassing both word and picture semantic processing (Vandenberghe et al., 1996). Semantic selection is likely to be of increased importance in sentences where multiple meanings are possible, for example in less familiar sentences. Our earlier findings (Schmidt et al., 2007) suggest that sentence familiarity may be a reasonable index of the coarseness of semantic relationship within the sentence. Thus for less familiar sentences, selection of appropriate meanings may be moredifficult, since less frequent meanings need to be retrieved, evaluated, and selected. Consequently the right homolog of this important left hemisphere semantic area is recruited.
One apparent contradiction is our finding of greater activation for Easy-Familiar metaphors than Easy-Unfamiliar metaphors in the right middle and inferior frontal gyri. However, these results need to be interpreted in light of the fact that we avoided using highly familiar or conventional metaphors, so that the range of familiarity across our stimuli extends from moderately familiar to very unfamiliar. For example, the following only moderately familiar metaphors were included in the Easy-Familiar condition: Indecision is a whirlpool; Education is a lantern; A pond is nature's mirror; The degree is a doorway; Dictators are the stranglers of liberty. Mason and Just (2004) observed a similar pattern in an inference processing tasks: inferences with an intermediate degree of casual relatedness to an antecedent condition resulted in more right hemisphere activation than either low or high degrees of relatedness. Their findings were reported across several brain regions including the right inferior frontal gyrus.
Difficulty
Current findings were in line with our fourth prediction that difficulty would result in increased activation in both left hemisphere language areas and their right hemisphere homologs. The difficulty contrast resulted in left hemisphere activations, with some regions being more active for difficult than easy (left inferior frontal) and some more active for easy than difficult (left middle frontal) stimuli. Right hemisphere homologs of left hemisphere language areas are also increasingly recruited when sentences become progressively difficult (Just et al., 1996), and are progressively more active as contextual complexity increases (Xu, Kemeny, Park, Frattali, & Braun, 2005). The ROI analysis revealed that the right middle frontal gyrus BA8 area, originally defined by the whole brain familiarity contrast was also affected by difficulty (Difficult > Easy). Thus difficulty did result in increased left hemisphere activation, and right hemisphere activations in metaphor processing were in part due to the difficulty of processing, not just relatively complex factors such as coarse coding.
The Right Hemisphere and Metaphor Processing
The current results speak to ongoing debate regarding the role of the right hemisphere in metaphor comprehension (e.g. Eviatar & Just, 2006; Mashal, Faust, Hendler, & Jung-Beeman, 2007; Rapp et al., 2004; Schmidt et al., 2007; Stringaris et al., 2006; 2007). These results support the findings and conclusions of Ahrens et al. (2007) who suggest that both left and right hemispheres play a role in metaphor comprehension, at least non-conventional metaphors. The view that the right hemisphere is not involved in metaphor comprehension (e.g. Rapp et al., 2007) is not supported. A number of researchers (Eviatar & Just, 2006; Lee & Dapretto, 2006; Stringaris et al., 2007) found no evidence of right hemisphere involvement in metaphor processing, but used only conventional metaphors. Stringaris et al., 2006 who did find right hemisphere activations also used conventional metaphors; however they tested the relationship between them and a final target word, where the relationship between the two could not be described as conventional. Rapp et al. (2004; 2007) found no right hemisphere involvement for metaphors that did not turn up in an internet search and were thus considered unfamiliar. Thus the authors define familiarity based on an internet search. However, even a slightly different wording could result in a null result in an internet search, and no norming data were reported. Additionally, these metaphors may have only recruited the left hemisphere due to the fact that they had short, simple sentence structures, and the task required participants to categorize the stimuli (a non-linguistic task) rather than evaluate them on a semantic basis (Ahrens et al., 2007). Similar arguments could be used with some of the lesions studies which report that patients with right hemisphere stroke can often understand metaphors correctly (e.g. Giora, Zaidel, Soroker, Batori, & Kasher, 2000; Rinaldi et al., 2004; Zaidel, Kasher, Soroker, & Batori, 2002), providing an argument against right hemisphere involvement in metaphor comprehension. Each of these studies employed only highly familiar or conventional metaphors, which may not recruit the right hemisphere to the degree that less familiar metaphors do (Schmidt et al., 2007).
Both the figurativeness and familiarity findings are consistent with theories of right hemisphere semantic processing. Beeman's coarse coding theory (1998) explains the proficiency of the right hemisphere in metaphor processing by suggesting that metaphors in general, and unfamiliar or unconventional metaphors more specifically, contain more distant semantic relationships, and take advantage of a right hemisphere proficiency in processing such relationships. For example, a familiar metaphor such as Babies are angels contains nouns that have many overlapping semantic features (babies-angels). However, an unfamiliar metaphor such as Dictionaries are microscopes contains nouns with less overlapping semantic features (dictionary-microscope). Giora's graded salience hypothesis (Giora, 1997, 1999, 2003) suggests that the right hemisphere is more likely to be recruited for processing the less salient meanings of words. Salience is determined by various factors, such as familiarity and conventionality, but not difficulty or figurativeness per se. That the right hemisphere activations based on figurativeness were also associated with familiarity is consistent with the idea that both of these factors depend on the same underlying mechanisms and that any right hemisphere recruitment is not primarily due to figurativeness per se, but to a broader concept such as salience.
The Insula and Metaphor Processing
Across the whole brain and ROI analyses, we found greater activity in the right and left insula in contrasts comparing Metaphors with Literal sentences and Unfamiliar metaphors with Familiar metaphors (Fig. 2A). Insula activation has now been reported in several studies of metaphor and/or unfamiliar language, but not highlighted or discussed. Kuperberg and colleagues (2000) found bilateral insula activation for pragmatically anomalous (literal) sentences (The parents couldn't sleep because the baby would phone) as contrasted with normal sentences. Ahrens et al. (2007) found neural recruitment of the bilateral insula for anomalous Chinese metaphors (Their financial capital has a lot of rhythm) as contrasted with literal sentences. Mashal and colleagues found activation in an insular area for novel and conventional metaphorical word pairs when compared to unrelated word pairs (Mashal et al., 2007). Using principal components analysis, they also found this area to be part of a network involved in processing both types of metaphors (Mashal et al., 2005).
Our results, in conjunction with these other studies, suggest that the insula may have an important role in processing less familiar language. What might this role be? It has been suggested that the insula performs an important function in speech and language processing by coordinating the language areas of the frontal and temporal lobes. It is indeed almost adjacent to the classic Broca's and Wernicke's areas. Metaphor processing may require more integration between the temporal lobes, where lexical access occurs, and the frontal lobes, where meaning selection takes place (Bennett & Netsell, 1999). Conversely, a number of studies have reported insula recruitment for practiced language tasks using relatively familiar stimuli (Kircher et al., 2001; Petersen, Fox, Posner, Mintun, and Raichle, 1988; Raichle et al., 1994; Seger et al., 2000; for discussion see Bennett & Netsell, 1999). This apparent contradiction can be resolved by noting that the practiced, less effortful tasks recruit more posterior parts of the insula, while metaphors and other unusual language recruit more anterior parts of the insula, as in our study and other studies of figurative language (Ahrens et al., 2007;. Kuperberg et al., 2000; Mashal et al., 2007).
Limitations and Future Directions
There are several limitations to the current work. Overall, figurativeness, familiarity, and difficulty ratings of metaphors are highly correlated (Katz et al., 1988), such that highly figurative language tends to be unfamiliar and difficult to understand. However, we were able to examine stimuli chosen to differ significantly in familiarity and difficulty ratings, and thus we were able to separate metaphor familiarity and difficulty at least within our experimental paradigm. Another limitation of our study is that due to scanning time limitations, we did not include literal sentences that varied in difficulty and familiarity in the same ways as the metaphors. Future studies are needed to address this shortcoming. Another potential limitation is that participants may not have comprehended the metaphors as we intended; due to scanning limitations we were unable to have an online evaluation of comprehension. Although we tested for comprehension, this test occurred after the scanning session was complete and included only a subset of the stimuli. For some subjects performance on this task was substantially below 100%, indicating that they may not have comprehended the metaphors. However, the original metaphorical sentences were not reproduced on the comprehension test, so good performance on the test required subjects to not only choose the answer matching the metaphorical meaning of the sentence, but to remember the metaphor and/or its meaning from the time of scanning to the time of test. The comprehension test was not performed until approximately 30 minutes after the scan, and was performed in a different context (outside the scanner, rather than inside). Therefore, we cannot be sure of whether the poor performance on the test was due to an initial failure to comprehend the metaphors, or merely a failure to retain the meaning.
Conclusions
The view that the right hemisphere is not involved in metaphor comprehension (Rapp et al., 2004; 2007) is not supported by our data, since we obtained a number of right hemisphere activations for metaphor processing. On the other hand, the opposite view, that the right hemisphere has a unique role in metaphor processing due to the figurative nature of metaphors is not supported either, as we found that both left and right hemisphere activations were affected by the familiarity and difficulty of the metaphors.
Our finding that figurativeness alone cannot account for right hemisphere recruitment during metaphor processing is in contrast to theories that assume figurativeness is a distinct characteristic (e.g. Grice, 1975). The quality of being figurative may not be the crucial factor in determining neural activation during metaphor processing. Indeed, it is the case that a large proportion of language has a nonliteral quality to it (Lakoff & Johnson, 1989), implying that whether or not language is figurative should not be the primary distinction determining the cognitive and neural organization of language. The existence of conventional metaphors (He has a warm heart; She stood up to her boss; That relationship isn't going anywhere) are so pervasive in our language, that it has even been argued that they form the basis of our cognition (Lakoff & Johnson, 1989).
For right hemisphere recruitment during language, the present results confirm the importance of two factors other than figurativeness: familiarity and difficulty. Familiarity was closely linked with figurativeness; there were a number of overlaps between familiarity and figurativeness activations, including right inferior frontal and insula regions. This is consistent with the coarse coding theory (Beeman, 1998) explanation for right hemisphere metaphor processing. Difficulty alone did not account for right hemisphere activation during figurative language, but difficulty and familiarity together accounted for activation in the right middle frontal region. Collectively, the present findings reveal a pattern of figurative language processing in which the factors of metaphor familiarity and difficulty are nearly as important as figurativeness in determining patterns of neural recruitment.
Acknowledgments
This work was supported in part by NIMH grant R03MH63784 and NIH T32 NS007413.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This is true for all of the metaphorical sentences except one, which due to an oversight only tested for recall of the sentence.
References
- Ahrens K, Liu H, Lee C, Gong S, Fang S, Hsu Y. Functional MRI of conventional and anomalous metaphors in Mandarin Chinese. Brain & Language. 2007;100:163–171. doi: 10.1016/j.bandl.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Anaki D, Faust M, Kravetz S. Cerebral hemispheric asymmetries in processing lexical metaphors. Neuropsychologia. 1998;36:353–362. doi: 10.1016/s0028-3932(97)00110-3. [DOI] [PubMed] [Google Scholar]
- Arzouan Y, Goldstein A, Faust M. Dynamics of hemispheric activity during metaphor comprehension: electrophysiological measures. Neuroimage. 2007;36:222–31. doi: 10.1016/j.neuroimage.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Badre D, D'Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. Journal of Cognitive Neuroscience. 2007;19:2082–2099. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- Beeman M. Coarse semantic coding and discourse comprehension. In: Beeman M, Chiarello C, editors. Right hemisphere language comprehension: Perspectives from cognitive neuroscience. Erlbaum; Mahwah, NJ: 1998. pp. 255–284. [Google Scholar]
- Beeman M, Friedman RB, Grafman J, Perez E, Diamond S, Lindsay MB. Summation priming and coarse semantic coding in the right hemisphere. Journal of Cognitive Neuroscience. 1994;6:26–45. doi: 10.1162/jocn.1994.6.1.26. [DOI] [PubMed] [Google Scholar]
- Bennett S, Netsell RW. Possible roles of the insula in speech and language processing: Directions for research. Journal of Medical Speech-Language Pathology. 1999;7:255–272. [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. The Journal of Neuroscience. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaxton TA, Bookheimer SY, Zeffiro TA, Figlozzi CM, Gaillard WD, Theodore WH. Functional mapping of human memory using PET: comparisons of conceptual and perceptual tasks. Canadian Journal of Experimental Psychology. 1996;50:42–56. doi: 10.1037/1196-1961.50.1.42. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: New approaches to understanding the cortical organization of semantic processing. Annual Review of Neuroscience. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Bottini G, Corcoran R, Sterzi R, Paulesu E, Schenone P, Scarpa P, et al. The role of the right hemisphere in the interpretation of figurative aspects of language: A positron emission tomography activation study. Brain. 1994;117:1241–1253. doi: 10.1093/brain/117.6.1241. [DOI] [PubMed] [Google Scholar]
- Bowdle BF, Gentner D. The career of metaphor. Psychological Review. 2005;112:193–216. doi: 10.1037/0033-295X.112.1.193. [DOI] [PubMed] [Google Scholar]
- Bright P, Moss H, Tyler LK. Unitary vs. multiple semantics: PET studies of word and picture processing. Brain & Language. 2004;89:417–432. doi: 10.1016/j.bandl.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Brownell HH, Simpson TL, Bihrle AM, Potter HH, Gardner H. Appreciation of metaphoric alternative word meanings by left and right brain-damaged patients. Neuropsychologia. 1990;28:375–383. doi: 10.1016/0028-3932(90)90063-t. [DOI] [PubMed] [Google Scholar]
- Coulson S, Van Petten C. Conceptual integration and metaphor: An event-related potential study. Memory & Cognition. 2002;30:958–968. doi: 10.3758/bf03195780. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. Journal of Comparative Neurology. 2005;493:154–66. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- de Bonis M, Epelbaum C, Deffez V, Feline A. The comprehension of metaphors in schizophrenia. Psychopathology. 1997;30:149–154. doi: 10.1159/000285041. [DOI] [PubMed] [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JDE. Semantic encoding and retrieval in the left inferior frontal cortex: A functional MRI study of task difficulty and process specificity. Journal of Neuroscience. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Jr., Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Eviatar Z, Just MA. Brain correlates of discourse processing: An fMRI investigation of irony and conventional metaphor comprehension. Neuropsychologia. 2006;44:2348–2359. doi: 10.1016/j.neuropsychologia.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust M, Mashal N. RH advantage in processing novel metaphoric expressions: A DVF Study. Neuropsychologia. 2007;45:860–870. doi: 10.1016/j.neuropsychologia.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE, Penny WD, editors. Statistical parametric mapping: The analysis of functional brain images. Elsevier; Amsterdam: 2007. [Google Scholar]
- Gabrieli JDE, Desmond JE, Demb JB, Wagner AD, Stone MV, Vaidya CJ, Glover GH. Function magnetic resonance imaging of semantic memory processes in the frontal lobes. Psychological Science. 1996;7:278–283. [Google Scholar]
- Gagnon L, Goulet P, Giroux F, Joanette Y. Processing of metaphoric and nonmetaphoric alternative meanings of words after right- and left-hemispheric lesion. Brain & Language. 2003;87:217–226. doi: 10.1016/s0093-934x(03)00057-9. [DOI] [PubMed] [Google Scholar]
- Giora R. Understanding figurative and literal language: The graded salience hypothesis. Cognitive Linguistics. 1997;8:183–206. [Google Scholar]
- Giora R. On the priority of salient meanings: Studies of literal and figurative language. Journal of Pragmatics. 1999;31:919–929. [Google Scholar]
- Giora R. On our mind: Salience, context and figurative language. Oxford University Press; New York: 2003. [Google Scholar]
- Giora R, Zaidel E, Soroker N, Batori G, Kasher A. Differential effects of right-and left-hemisphere damage on understanding sarcasm and metaphor. Metaphor and Symbol. 2000;15:63–83. [Google Scholar]
- Glover GH, Lai S. Self-navigated spiral fMRI: Interleaved versus single-shot. Magnetic Resonance Medicine. 1998;3:361–368. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- Glucksberg S. Understanding figurative language: From metaphors to idioms. Oxford University Press; New York: 2001. [Google Scholar]
- Grice HP. Logic and conversation. In: Cole P, Morgan JL, editors. Syntax and Semantics: Vol. 3. Speech Acts. Academic Press; New York: 1975. pp. 41–58. [Google Scholar]
- Grossman M, McMillan C, Moore P, Ding L, Glosser G, Work M, Gee J. What's in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer's disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127:628–649. doi: 10.1093/brain/awh075. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O'Brien LM, Kennedy DN, Filipek PA, et al. Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain. 2005;128:213–26. doi: 10.1093/brain/awh330. [DOI] [PubMed] [Google Scholar]
- Jung-Beeman M. Bilateral brain processes for comprehending natural language. Trends in Cognitive Science. 2005;11:512–518. doi: 10.1016/j.tics.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR. Brain activation modulated by sentence comprehension. Science. 1996;274:114–6. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- Kable J, Lease-Spellmeyer J, Chatterjee A. The neural substrate of action event knowledge. Journal of Cognitive Neuroscience. 2002;14:785–794. doi: 10.1162/08989290260138681. [DOI] [PubMed] [Google Scholar]
- Kacinik NA, Chiarello C. Understanding metaphors: Is the right hemisphere uniquely involved? Brain & Language. 2007;100:188–207. doi: 10.1016/j.bandl.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Kapur S, Craik FIM, Jones C, Brown GM, Houle S, Tulving E. Functional role of the prefrontal cortex in retrieval of memories: A PET study. NeuroReport. 1995;6:1881–1884. doi: 10.1097/00001756-199510020-00014. [DOI] [PubMed] [Google Scholar]
- Katz AN, Paivio A, Marschark M, Clark JM. Norms for 204 literary and 260 nonliterary metaphors on 10 psychological dimensions. Metaphor and Symbolic Activity. 1988;3:191–214. [Google Scholar]
- Kircher TT, Brammer M, Andreu NT, Williams SC, McGuire PK. Engagement of right temporal cortex during processing of linguistic content. Neuropsychologia. 2001;39:798–809. doi: 10.1016/s0028-3932(01)00014-8. [DOI] [PubMed] [Google Scholar]
- Klein D, Milner B, Zatorre RJ, Meyer E, Evans AC. The neural substrates underlying word generation: a bilingual functional-imaging study. Proceedings of the National Academy of Sciences USA. 1995;92:2899–903. doi: 10.1073/pnas.92.7.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera & Francis WN. Computational Analysis of Present-Day American English. Brown University Press; Providence: 1967. [Google Scholar]
- Kuperberg GR, McGuire PK, Bullmore ET, Brammer MJ, Rabe-Hesketh S, Wright I, et al. Common and distinct neural substrates for pragmatic, semantic, and syntactic processing of spoken sentences: an fMRI study. Journal of Cognitive Neuroscience. 2000;12:321–41. doi: 10.1162/089892900562138. [DOI] [PubMed] [Google Scholar]
- Lakoff G, Johnson M. Metaphors We Live By. University of Chicago Press; Chicago: 1980. [Google Scholar]
- Lancaster JL, Summerln JL, Rainey L, Freitas CS, Fox PT. The Talairach Daemon, a database server for Talairach Atlas Labels. NeuroImage. 1997;5:S633. [Google Scholar]
- Lee SS, Dapretto M. Metaphorical vs. literal word meanings: fMRI evidence against a selective role of the right hemisphere. NeuroImage. 2006;29:536–544. doi: 10.1016/j.neuroimage.2005.08.003. [DOI] [PubMed] [Google Scholar]
- MacKay G, Shaw A. A comparative study of figurative language in children with autistic spectrum disorders. Child Language Teaching & Therapy. 2004;20:13–32. [Google Scholar]
- Mackenzie C, Begg T, Brady M, Lees KR. The effects on verbal communication skills of right hemisphere stroke in middle age. Aphasiology. 1997;11:929–945. [Google Scholar]
- Mackenzie C, Begg T, Lees KR, Brady M. The communication effects of right brain damage on the very old and the not so old. Journal of Neurolinguistics. 1999;12:79–93. [Google Scholar]
- Mashal N, Faust M. Right hemisphere sensitivity to novel metaphoric relations: application of the signal detection theory. Brain and Language. 2008;104:103–112. doi: 10.1016/j.bandl.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Mashal N, Faust M, Hendler T. Processing conventional vs. novel metaphors by the two cerebral hemispheres: Application of principle component analysis to fMRI data. Neuropsychologia. 2005;43:2084–2100. doi: 10.1016/j.neuropsychologia.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Mashal N, Faust M, Hendler T, Jung-Beeman M. An fMRI investigation of the neural correlates underlying the processing of novel metaphoric expressions. Brain & Language. 2007;100:115–126. doi: 10.1016/j.bandl.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Mashal N, Faust M, Hendler T, Jung-Beeman M. An fMRI study of processing novel metaphoric sentences. Laterality. 2008;16:1–25. doi: 10.1080/13576500802049433. [DOI] [PubMed] [Google Scholar]
- Mason RA, Just MA. How the brain processes causal inferences in text: A theoretical account of generation and integration component processes utilizing both cerebral hemispheres. Psychological Science. 2004;15:1–7. doi: 10.1111/j.0963-7214.2004.01501001.x. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RSJ, Hodges JR. A voxel-based morphometry study of semantic dementia: Relationship between temporal lobe atrophy and semantic memory. Annals of Neurology. 2000;47:36–45. [PubMed] [Google Scholar]
- Nathaniel-James DA, Fletcher P, Frith CD. The functional anatomy of verbal initiation and suppression using the Hayling Test. Neuropsychologia. 1997;35:559–66. doi: 10.1016/s0028-3932(96)00104-2. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Hyder F, Blamire AM, Shulman RG. FMRI of the prefrontal cortex during overt verbal fluency. Neuroreport. 1997;8:561–565. doi: 10.1097/00001756-199701200-00036. [DOI] [PubMed] [Google Scholar]
- Pobric G, Mashal N, Faust M, Lavidor M. The role of the right cerebral hemisphere in processing novel metaphoric expressions: a transcranial magnetic stimulation study. Journal of Cognitive Neuroscience. 2008;20:170–181. doi: 10.1162/jocn.2008.20005. [DOI] [PubMed] [Google Scholar]
- Preston AR, Thomason ME, Ochsner KN, Cooper JC, Glover GH. Comparison of spiral-in/out and spiral-out BOLD fMRI at 1.5T and 3T. Neuroimage. 2004;21:291–301. doi: 10.1016/j.neuroimage.2003.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Fiez JA, Videen TO, MacLeod AM, Pardo JV, Fox PT, Petersen SE. Practice-related changes in human brain functional anatomy during nonmotor learning. Cerebral Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- Rapp AM, Leube DT, Erb M, Grodd W, Kircher TTJ. Neural correlates of metaphor processing. Cognitive Brain Research. 2004;20:395–402. doi: 10.1016/j.cogbrainres.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Rapp AM, Leube DT, Erb M, Grodd W, Kircher TTJ. Laterality in metaphor processing: lack of evidence from functional magnetic resonance imaging for the right hemisphere theory. Brain & Language. 2007;100:142–149. doi: 10.1016/j.bandl.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Rinaldi MC, Marangolo P, Baldassarri F. Metaphor comprehension in right brain-damaged patients with visuo-verbal and verbal material: a dissociation (re)considered. Cortex. 2004;40:479–490. doi: 10.1016/s0010-9452(08)70141-2. [DOI] [PubMed] [Google Scholar]
- Rissman J, Eliassen J, Blumstein SE. An event related fMRI investigation of implicit semantic priming. Journal of Cognitive Neuroscience. 2003;15:116–1175. doi: 10.1162/089892903322598120. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Camou SL, Reite ML, Rogers SJ. Planum temporale volume in children and adolescents with autism. Journal of Autism and Developmental Disorders. 2005;35:479–86. doi: 10.1007/s10803-005-5038-7. [DOI] [PubMed] [Google Scholar]
- Roskies AL, Fiez JA, Balota DA, Raichle ME, Petersen SE. Task-dependent modulation of regions in the left inferior frontal cortex during semantic processing. Journal of Cognitive Neuroscience. 2001;13:829–843. doi: 10.1162/08989290152541485. [DOI] [PubMed] [Google Scholar]
- Rossell SL, Price CJ, Nobre AC. The anatomy and time course of semantic priming investigated by fMRIs and ERPs. Neuropsycholgia. 2003;41:550–564. doi: 10.1016/s0028-3932(02)00181-1. [DOI] [PubMed] [Google Scholar]
- Schmidt GL, DeBuse CJ, Seger CA. Right hemisphere metaphor processing? Characterizing the lateralization of semantic processes. Brain & Language. 2007;100:127–141. doi: 10.1016/j.bandl.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Seger CA, Desmond JE, Glover GH, Gabrieli JDE. Function magnetic resonance imaging evidence for right-hemisphere involvement in processing unusual semantic relationships. Neuropsychology. 2000;14:361–369. doi: 10.1037//0894-4105.14.3.361. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Scott SK, Wise RJS. Monitoring and the controlled processing of meaning: Distinct prefrontal systems. Cerebral Cortex. 2004;14:1–10. doi: 10.1093/cercor/bhg086. [DOI] [PubMed] [Google Scholar]
- Sotillo M, Carretié L, Hinojosa JA, Tapia M, Mercado F, López-Martín S, Albert J. Neural activity associated with metaphor comprehension: spatial analysis. Neuroscience Letters. 2005;373:5–9. doi: 10.1016/j.neulet.2004.09.071. [DOI] [PubMed] [Google Scholar]
- Stringaris A, Medford N, Giora R, Giampietro CV, Brammer JM, David SA. How metaphors influence semantic relatedness judgments: The role of the right frontal cortex. NeuroImage. 2006;33:784–793. doi: 10.1016/j.neuroimage.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Stringaris AK, Medford NC, Giampietro V, Brammer MJ, David AS. Deriving meaning: Distinct neural mechanisms for metaphoric, literal, and non-meaningful sentences. Brain & Language. 2007;100:150–162. doi: 10.1016/j.bandl.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proceedings of the National Academy of Science USA. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins CA. Knowledge and strategies for processing lexical metaphor after right or left hemisphere brain damage. Journal of Speech and Hearing Research. 1990;33:307–316. doi: 10.1044/jshr.3302.307. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RS. Functional anatomy of a common semantic system for words and pictures. Nature. 1996;383:254–256. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, et al. Meta-analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–32. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith E,E. Neuroimaging studies of working memory: a meta-analysis. Cognitive, Affective and Behavioral Neuroscience. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Desmond JE, Glover GH, Gabrieli JDE. Prefrontal cortex and recognition memory: Functional MRI evidence for context-dependent retrieval processes. Brain. 1998;121:1985–2002. doi: 10.1093/brain/121.10.1985. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning, left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Winner E, Gardner H. The comprehension of metaphor in brain-damaged patients. Brain. 1977;100:717–729. doi: 10.1093/brain/100.4.717. [DOI] [PubMed] [Google Scholar]
- Xu J, Kemeny S, Park G, Frattali C, Braun A. Language in context: Emergent features of word, sentence, and narrative comprehension. NeuroImage. 2005;15:1002–15. doi: 10.1016/j.neuroimage.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Zaidel E, Kasher A, Soroker N, Batori G. Effects of right and left hemisphere damage on performance of the “Right Hemisphere Communication Battery.”. Brain & Language. 2002;80:510–535. doi: 10.1006/brln.2001.2612. [DOI] [PubMed] [Google Scholar]