Abstract
Monoamine oxidase (MAO) B catalyzes the degradation of β-phenylethylamine (PEA), a trace amine neurotransmitter implicated in mood regulation. Although several studies have shown an association between low MAO B activity in platelets and behavioral disinhibition in humans, the nature of this relation remains undefined. To investigate the impact of MAO B deficiency on the emotional responses elicited by environmental cues, we tested MAO B knockout (KO) mice in a set of behavioral assays capturing different aspects of anxiety-related manifestations, such as the elevated plus maze, defensive withdrawal, marble burying and hole-board. Furthermore, MAO B KO mice were evaluated for their exploratory patterns in response to unfamiliar objects and risk-taking behaviors. In comparison to their wild-type (WT) littermates, MAO B KO mice exhibited significantly lower anxiety-like responses and shorter latency to engage in risk-taking behaviors and exploration of unfamiliar objects. To determine the neurobiological bases of the behavioral differences between WT and MAO B KO mice, we measured the brain-regional levels of PEA in both genotypes. Although PEA levels were significantly higher in all brain regions of MAO B KO in comparison to WT mice, the most remarkable increments were observed in striatum and prefrontal cortex, two key regions for the regulation of behavioral disinhibition. However, no significant differences in transcript levels of PEA’s selective receptor, trace amine-associated receptor 1 (TAAR1), were detected in either region. Taken together, these results suggest that MAO B deficiency may lead to behavioral disinhibition and decreased anxiety-like responses partially through regional increases of PEA levels.
Keywords: Monoamine Oxidase B, mice, behavioral disinhibition, anxiety, phenylethylamine
Monoamine oxidase (MAO) (EC 1.4.3.4) is the key enzyme catalyzing the oxidative deamination of monoamine neurotransmitters in the central nervous system. Although the two MAO isoenzymes, A and B, share about 70% homology and follow the same kinetic mechanism (Bortolato et al, 2008; Shih et al, 1999a), they exhibit significant differences in substrate and inhibitor specificities: whereas MAO A displays high affinity for serotonin (5-hydroxytryptamine, 5-HT) and norepinephrine (NE), and is inhibited by low doses of clorgyline, MAO B prefers β-phenylethylamine (PEA) and is selectively inhibited by small concentrations of l-deprenyl (Bortolato et al, 2008; Shih et al, 1999a). This neurochemical divergence implies that the two MAO isoenzymes likely exert different functions in the organization of brain activity and neurophysiological processes. Their specific roles in behavioral regulation, however, remain partially elusive. A powerful tool to elucidate the influence of MAO A and MAO B on behavior is afforded by mice carrying genetic knockout (KO) of either enzyme (Cases et al, 1995; Chen et al, 2004; Grimsby et al, 1997; Scott et al, 2008).
MAO A KO mice have been the focus of extensive phenotypic characterization (Bortolato et al, 2008; Shih et al, 1999a). Among other features, these mutants have high brain levels of 5-HT, DA and NE and exhibit a rather complex behavioral phenotype, including impulsive aggression, low exploratory activity and greater retention of aversive memories (Cases et al, 1995; Kim et al, 1997; Popova et al, 2001; Shih et al, 1999b). This set of behavioral abnormalities strikingly mirrors the nosographic characteristics of Brunner syndrome, a genetic disorder induced by a nonsense mutation of MAO A gene and featuring antisocial conduct and mental retardation (Brunner et al, 1993).
Conversely, the neurobiological and behavioral implications of MAO B deficiency remain poorly understood. MAO B KO mice display high brain levels of PEA, but not 5-HT, DA or NE. PEA has been implicated in the regulation of emotional responses, including exploratory activity, arousal and behavioral reinforcement (Sabelli and Javaid, 1995). Recently, several lines of investigations have ascertained that some of the actions of PEA are mediated by the activation of specific receptors, such as trace amine-associated receptor 1 (TAAR1) (Borowsky et al, 2001; Lindemann et al, 2005), which has been implicated in the regulation of DA signaling in the striatum (Lindemann et al, 2008; Wolinsky et al, 2007; Xie and Miller, 2009).
In keeping with these premises, MAO B KO mice exhibit decreases in behavioral parameters reflective of stress susceptibility (Bohus et al, 1987; Korte et al, 1996; Louvart et al, 2005), such as forced-swim immobility and locomotor habituation (Grimsby et al, 1997; Lee et al, 2004). Low MAO B platelet activity in humans has been consistently correlated with extraversion and novelty-seeking traits, yet a causal relationship between the two phenomena has not been established (Oreland, 1993).
This scenario suggests that MAO B deficiency may result in behavioral disinhibition, a temperamental tendency characterized by novelty- and sensation-seeking personality and negligence of potential or actual dangers (Iacono et al, 2003). To verify this possibility, in the present study we analyzed the behavioral performances enacted by MAO B KO mice in a number of models exploring different facets of responsiveness to contextual stimuli, including anxiety-like responses, exploratory activity and risk-taking behaviors.
Materials and methods
Animals
We used 4–5 months old, experimentally naïve male 129/Sv mice (n=166; 83/genotype), weighing 30–35 g. MAO B KO mice and WT littermates were generated as previously described (Grimsby et al, 1997). Animals were housed in group cages with ad libitum access to food and water. The room was maintained at 22°C, on a 12 h:12 h light:dark cycle, with lights off at 6:00 pm. Prior to behavioral testing, all animals were found to display equivalent physical and neurological characteristics. All experimental procedures were in compliance with the National Institute of Health guidelines and approved by the University of Southern California Animal Use Committees. To avoid potential carryover effects, each animal was used only once throughout the study. Litter effects were minimized by using mice from at least 3 different litters in each behavioral test.
Elevated plus-maze
The test was performed as previously described (Wall and Messier, 2000), under either dim (10 lux) or bright (300 lux) environmental light. Briefly, the apparatus was made from black Plexiglas with a light grey floor and consisted of two open (25 × 5 cm) and two closed arms (25 × 5 × 5 cm), which extended from a central platform (5 × 5 cm) at 60 cm from the ground. Mice (n = 17/genotype) were individually placed on the central platform facing an open arm, and their behavior was observed for 5 min by an experimenter unaware of the genotype. An arm entry was counted when all four paws were inside the arm. Behavioral measures included: time spent and entries into each partition of the elevated plus-maze; number of fecal boli.
Defensive withdrawal
We used a variation of the protocol described in Bortolato et al (2006). Mice (WT = 7; MAO B KO = 10) were individually placed inside a cylindrical aluminum chamber (7 cm diameter × 11 cm length) located along one of the four walls of a dimly-lit (10 lux) black Plexiglas open field (40 × 40 × 40 cm), with the open end facing the center. Mice were allowed to freely explore the environment for 15 min. Behaviors were recorded and monitored by an observer unaware of the genotype. Behavioral measures included: latency to exit the chamber; transitions between the chamber and open field; time spent in the chamber; head pokes out of the chamber; crossings (on a 4 × 4 square grid superimposed onto the video image of the open field); velocity (ratio of crossings to time spent in the open field).
Marble burying
Testing was performed using a modification of the methods described in Hirano et al (2005). Briefly, mice (WT = 20; MAO B KO = 13) were individually placed in a dimly-lit (10 lux) Makrolon cages (35 × 28 cm), with 5 cm of fine sawdust, for a 30-min acclimatization period. Subsequently, mice were briefly removed and 20 marbles (1 cm diameter) were placed in each cage, on top of the sawdust. Mice were then returned to the cages, and their behavior was videorecorded for the following 30 min. Measures included the number of buried marbles, and the number and total duration of digging bouts. A marble was considered buried if at least two thirds of its surface area was covered in sawdust. General activity was analyzed by counting the crossings of a grid (5 × 4 squares), as described above.
Hole-board
We used a grey Plexiglas platform (40 × 40 cm) raised to a height of 15 cm from the floor of a transparent Plexiglas box (40 × 40 × 40 cm) in a dimly-lit room (10 lux). The platform consisted of 16 equivalent square compartments (12 peripheral and 4 central), each featuring a central circular hole (2.5 cm diameter). Mice (WT = 8; MAO B KO = 12) were individually placed in the center and their behavior was recorded for 6 min. Behaviors were measured diachronically in 2-min intervals, and included the number of crossings between compartments, and the time spent and number of head pokes in the peripheral and central compartments.
Novel object exploration
We used a modified version of the protocol described in Bortolato et al (2009). Mice (WT = 7, MAO B KO = 8) were individually acclimatized to a dimly-lit (10 lux) grey Plexiglas cubic box (20 × 20 × 20 cm) for 15 min. Twenty-four h later, animals were exposed to two novel black plastic cylinders (8 cm tall × 3.5 cm in diameter), affixed to the floor and symmetrically placed at 6 cm from the two nearest walls. Mice were placed in a corner, facing the center and at equal distance from the two objects. Their start position was rotated and counterbalanced for each genotype throughout the test. Behaviors were videotaped for 15 min. Analysis included number and total duration of exploratory approaches, latency to the first exploration, and the number of crossings (measured as described in the Defensive Withdrawal section). Exploration was defined as sniffing or touching either of the two objects with the snout; sitting on the object was not considered exploration. The time spent in the central 4 squares and the object areas (defined as a 1.75 cm-wide annulus concentric to the cylinders) was also measured.
Novelty-induced grooming
Mice (WT = 8, MAO B KO = 7) were placed in a dimly-lit (10 lux) Makrolon cage (35 × 28 cm) for 30 min. Following their removal, five voluminous objects (different for size, shape and color) were attached to the bottom of one half of the cage (object area). Animals were returned to the empty half of the cage and left undisturbed for 20 min. Time spent in the object area and grooming bouts and duration were recorded and analyzed by an observer unaware of the genotype.
Wire-beam bridge
The apparatus consisted of a 30-cm high Plexiglas platform and a 50-cm high Plexiglas wall, oppositely placed at 30 cm distance. The platform was surmounted by an 8-cm deep enclosure, with a square (13 × 13 cm) opening facing the wall and placed right above the edge of the platform. Following 24 h of isolation and food deprivation, mice (WT = 6; MAO B KO = 5) were individually placed in the enclosure, under dim (10 lux) light conditions, and returned to their cage after 10 min. The edge of the platform and the wall were then connected by a horizontal, unrailed bridge (1.25 × 30 cm), made in black aluminum wire. The bridge consisted of 2 parallel beams (0.01 cm thick) perpendicularly connected by 24 equally distanced cross-ties (1.25 cm long). It was modestly flexible, with a downward deflection of 1 cm per 100-g load at the center point. A circular plastic dish (6 cm in diameter) containing 6 food pellets (approx. 20 g) was attached onto the end of the bridge adjacent to the wall. Mice were placed in the enclosure and their behavior was recorded for 10 min. Behavioral measures included the latencies to access the bridge (with all 4 paws on it) and to reach the food, as well as the sniffing frequency (calculated as the ratio between the sniffing approaches to the bridge prior to the actual access to it and the latency to access the bridge).
PEA level determination
PEA levels were determined as indicated by Grimsby et al (1997). Briefly, brain regions of WT (n=6) and MAO B KO (n=7) mice were identified following the stereotaxic atlas by Franklin and Paxinos (1997), excised and homogenized in 0.5 N perchloric acid solution with [H2] PEA as an internal standard. PEA was extracted with diethyl ether, derivatized with pentafluoropropionic anhydride and analyzed using a gas chromatograph directly interfaced with a mass-selective detector (Hewlett-Packard, Palo Alto, CA). Base peaks at 104 and 107 m/z were used for detection of PEA and the internal standard respectively.
TAAR1 receptor mRNA level determination
RNA was extracted from the striatum and prefrontal cortex of WT and MAO B KO mice (n=4/genotype) using Trizol (Invitrogen, Carlsbad, CA). Two micrograms of total RNA were used in first-strand cDNA synthesis with M-MLV reverse transcriptase (Promega, Madison, WI) according the manufacturer instructions. The transcript was amplified with specific primers (TAAR1-F: 5′-ATGCATCTTTGCCACGCTATC-3′, TAAR1-R: 5′-TCAAGGCTCTTCTGAACC-3′) by using iQTM SYBR® Green Supermix (Bio-Rad, Hercules, CA). PCR reactions were performed using 1 μl of 20× diluted cDNA with one cycle of 94°C for 3 min, followed by 40 cycles of amplification at 94°C for 30 sec, 62°C (annealing temperature) for 30 sec, and 72°C for 30 sec. TAAR1 mRNA expression was normalized to 18S rRNA levels (Boda et al 2009). Relative comparison of gene expression between WT and MAO B KO mice was determined as in Rieu and Powers (2009).
Statistical analysis
Statistical analyses on behavioral parameters were performed with one-way or two-way ANOVAs, as appropriate, followed by Tukey’s test with Spjøtvoll-Stoline correction for post-hoc comparisons (Kirk 1982). Normality and homoscedasticity of data distribution were verified by using the Kolmogorov-Smirnov and Bartlett’s test. Non-parametric comparisons were performed by Mann-Whitney test. Significance threshold was set at P = 0.05.
Results
Elevated plus-maze
In previous reports, we documented that MAO B KO and WT mice do not display significant differences in anxiety-related parameters in the elevated plus maze (such as the number of entries and the time spent in the open arms), under light conditions analogous to those kept in the housing room (300 lux) (Grimsby et al, 1997). Nevertheless, cogent evidence has shown that high levels of environmental illuminance exacerbate the anxiogenic properties of the open arms of the elevated plus-maze (Dawson and Tricklebank, 1995). These premises prompted us to speculate that dim light conditions (10 lux) may be more appropriate to unravel subtle behavioral alterations displayed by MAO B KO mice in this paradigm. Indeed, under these conditions, MAO B KO mice did exhibit significantly more open arm entries (Fig. 1a) [F(1,18) = 4.48, P<0.05] and spent a longer time in the open arms (Fig. 1b) [F(1,18) = 4.69, P<0.05] in comparison to their WT counterparts. MAO B KO mice also spent a significantly shorter time on the center platform (Fig. 1d) [F(1,18) = 4.89, P<0.05]. Conversely, the two genotypes exhibited comparable entries in the center (Fig. 1c) [F(1, 18) = 1.63, NS], as well as both entries [F(1,18) = 0.51, NS] and time spent [F(1,18) =0.08, NS] in the closed arms (Fig 1e–f). The reduction in anxiety-like responses in MAO B KO mice was also confirmed by their lower number of fecal boli excreted during the testing session (Fig. 1g) [F(1, 18) = 7.24, P<0.05]. The behavioral variability between genotypes did not reflect differences in activity, as shown by the equivalent number of total entries [F(1, 18) = 1.89, NS] (Fig. 1h).
Figure 1.
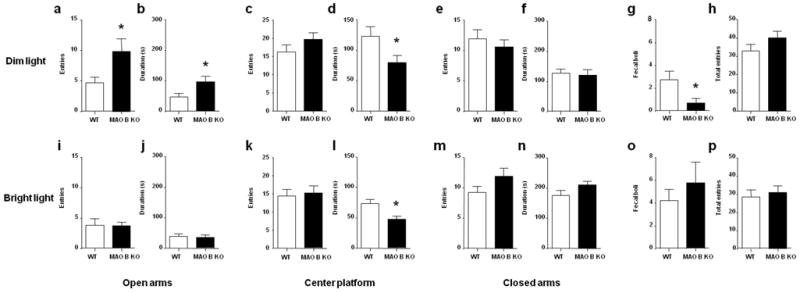
MAO B KO mice exhibit significant reductions in anxiety-like behaviors in the elevated plus maze under dim (a-H), but not bright (i-p) light conditions. All values are represented as means ± SEM. *P < 0.05, compared to wild type (WT) controls. For more details, see result section.
In a second set of experiments performed under bright light conditions (300 lux) with a different set of animals, we confirmed the lack of substantial behavioral differences between WT and MAO B KO mice (Fig. 1i – p). However, the latter spent a shorter time on the center platform (Fig. 1l) [F(1, 14) = 8.66, P < 0.05], in a fashion similar to their homogenotypic counterparts tested under dim light.
Defensive withdrawal
To further characterize the ethological significance of the behavioral alterations displayed by MAO B KO mice in the elevated plus-maze, we tested them in another well-validated model of anxiety, the defensive withdrawal paradigm. This conflict test harnesses the conflict between the natural tendency of rodents to explore a novel open arena and to retreat into an enclosed chamber, according to their degree of threat perception (Takahashi et al, 1989). MAO B KO mice showed a significantly reduced latency to withdraw from the chamber (Fig. 2a) [U(7,10) = 10, P< 0.05] and a marked reduction, albeit not significant, in time spent inside the chamber (Fig. 2b) [F(1,15) = 3.17, P< 0.10]. The number of transitions between the chamber and the open arena was also higher for MAO B KO mice (Fig. 2c) [F(1,15) = 7.4, P< 0.05]. However, no differences between MAO B KO and WT mice were detected in head pokes {(WT = 23.6 ± 3.6; MAO B KO = 22.3 ± 7.1) [F(1,15) = 0.03, NS]}, velocity in the open field (defined as the ratio between the number of crossings and the time spent in the arena) {(WT = 0.15 ± 0.06; MAO B KO = 0.22 ± 0.03) [F(1, 15) = 1.49, NS]}, and fecal boli {(WT = 3.1 ± 1.0; MAO B KO = 1.6 ± 0.5) [F(1,15) = 1.81, NS]}.
Figure 2.
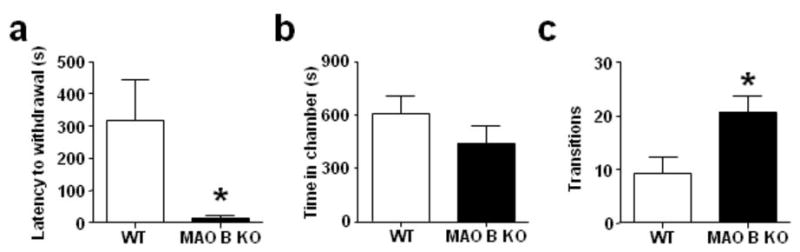
MAO B KO mice display decreased anxiety-like behaviors in the defensive withdrawal paradigm. All values are represented as means ± SEM. *P < 0.05, compared to WT mice. For more details, see result section.
Marble burying
To further substantiate the hypothesis that MAO B KO mice show decreased anxiety-like behaviors, we used the marble burying paradigm, as this model has been recently shown to capture aspects of environmental reactivity different from those assessed in the conflict-based assays (Thomas et al, 2009). Unlike their WT counterparts, MAO B KO mice buried a remarkably low number of marbles (Fig. 3a) [U(20,13) = 72, P < 0.05], but displayed equivalent cage activity (Fig. 3b) [F(1,24) = 0.27, NS]. The reduction in buried marbles was paralleled by a significant decrease in digging bouts (Fig. 3c) [F(1,26) = 7.63, P< 0.05] and duration (Fig. 3d) [U(19,10) = 34, P< 0.01]. In spite of their dramatic reduction in digging and burrowing behaviors, MAO B KO mice approached and actively explored the marbles, with an average frequency of 4.38 ± 0.01 exploratory bouts/min. This parameter, however, could not be efficiently compared across genotypes, as WT mice engaged in significantly more marble-burying behavior.
Figure 3.
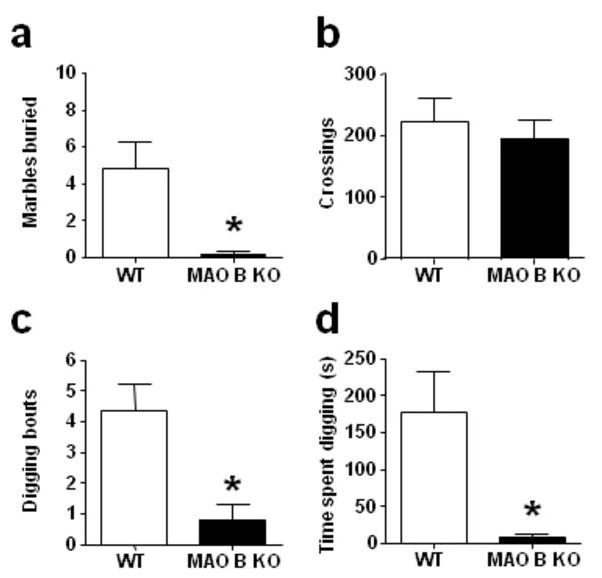
MAO B KO mice exhibit a reduction in marble-burying and digging behaviors. All values are represented as means ± SEM. *P < 0.05, ** P < 0.01 compared to WT controls. For more details, see result section.
Hole-board
Following the detection of reduced anxiety-related manifestations in MAO B KO mice, we assessed the potential impact of these alterations on the exploratory activity by testing the animals in the hole-board paradigm (Casarrubea et al, 2008). The total number of head dips was comparable between MAO B KO and WT (Fig. 4a) [F(1, 18) = 0.28, NS]. Nevertheless, MAO B KO mice performed significantly more head dips in the center than WT mice (Fig. 4b) [F(1, 17) = 4.52, P< 0.05], a behavior that has been interpreted as reflective of anxiolysis (Hranilovic et al, 2005). Peripheral head dips were comparable between genotypes (Fig. 4c) [F(1, 18) = 2.07, NS]. No other significant differences were found in either locomotor activity [F(1, 16) = 0.27, NS] or time spent in the central quadrants {(WT = 29.2 ± 6.6; MAO B KO = 76.5 ± 29.8) [F(1, 16) = 1.11, NS]. Although the latency to initial head dip was equivalent across genotypes, (Fig. 4d) [F(1, 18) = 0.06, NS] MAO B KO mice exhibited a significantly decreased latency to the first head dip in one of the central holes (Fig. 4e) [U(8, 12) = 17, P< 0.05]. Latency to the first peripheral head dip was statistically equivalent in WT and MAO B KO mice (Fig. 4f) [U(8, 11) = 36, NS].
Figure 4.
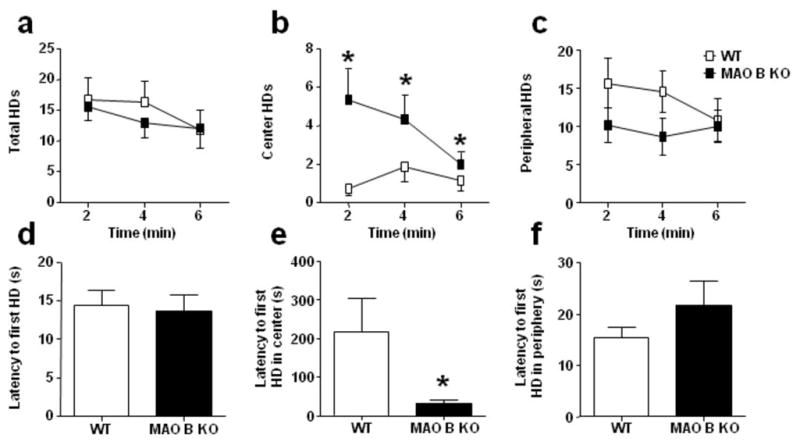
MAO B KO mice explore central holes with longer duration and shorter latency in the hole-board test. All values are represented as means ± SEM. *P < 0.05, compared to WT controls. For more details, see result section.
Novel object exploration
During both the acclimation phase and the exposure to novel objects MAO B KO and WT mice exhibited similar magnitude and patterns of locomotor activity (data not shown). MAO B KO mice approached the objects with a significantly reduced latency (Fig. 5a) [U(7, 8) = 6, P< 0.01] and explored them for a significantly longer time (Fig. 5b) [F(1, 13) = 5.17, P< 0.05]. In contrast, the number of exploratory approaches was analogous between genotypes {(WT = 80.9 ± 19.3; MAO B KO = 95.6 ± 8.3) [F(1, 13) = 0.96, NS]}. Statistical analysis also showed a trend for MAO B KO mice to spend longer time in object areas {(WT = 161.1 ± 28.5; MAO B KO = 263.6 ± 36.9) [F(1, 13) = 3.97, P< 0.10]}. These results suggest that MAO B KO mice may be characterized by a stronger drive (or a reduced timidity) to explore unfamiliar objects.
Figure 5.
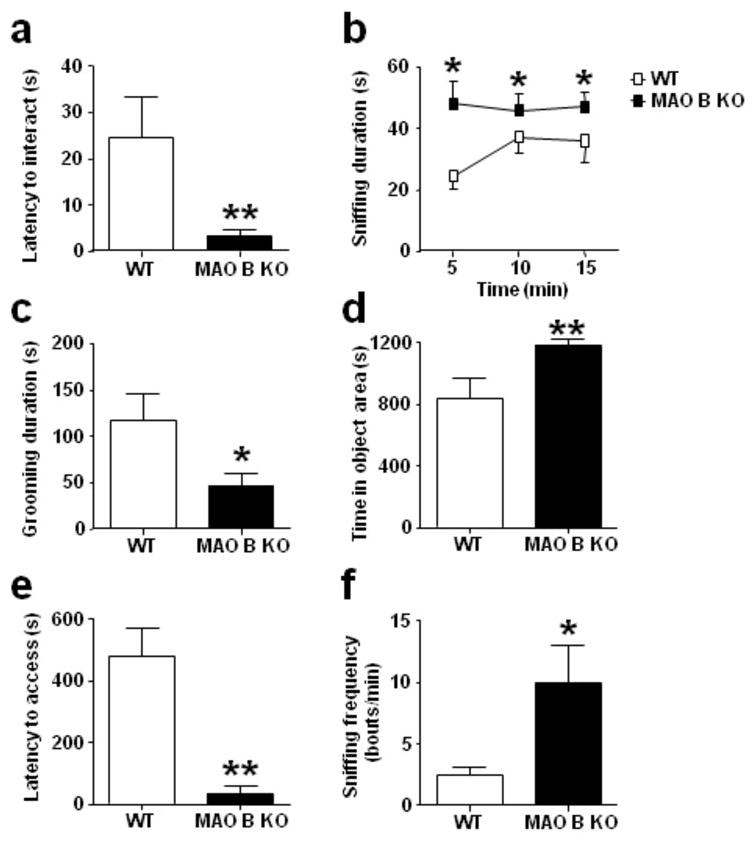
MAO B KO mice display higher levels of exploration targeting novel objects and risk-taking behavior in the wire-beam bridge test. All values are represented as means ± SEM. *P < 0.05, ** P < 0.01 compared to WT littermates. For more details, see result section.
Novelty-induced grooming
Previous studies (Kalueff and Tuohimaa, 2004) and preliminary observations conducted by our group have shown that 129/Sv mice display poor spontaneous grooming activity in response to novel environmental stimuli. Thus, we optimized our protocol to detect novelty-induced grooming and avoidance by placing five voluminous objects in one half of the cage. MAO B KO mice spent significantly less time grooming (Fig. 5c) [F(1, 12) = 4.93, P< 0.05], but engaged in a similar number of grooming bouts {(WT = 7.71 ± 1.69; MAO B KO = 5.71 ± 1.26) [F(1, 12) = 0.78, NS]} in comparison to their WT counterparts. Furthermore, MAO B KO mice spent a significantly longer time in the object area (Fig. 5d) [U (8, 7) = 6, P< 0.01]. These results confirm that MAO B KO mice display less environmental and object-related neophobia than WT mice.
Wire-beam bridge test
Low levels of platelet MAO activity have been strongly associated with features of the behavioral disinhibition spectrum including impulsivity, sensation-seeking, and risk-taking (Hirschfeld-Becker et al, 2003; Ruchkin et al 2005). In order to capture these elements, we measured the animal’s proclivity to cross an unrailed flexible bridge suspended over a 30-cm deep gap, to reach a food reward. MAO B KO mice exhibited a significantly shorter latency to access the bridge (Fig. 5e) [U(6, 5) = 1.5, P< 0.01]] and latency to touch the food {(WT = 483.83 ± 81.64; MAO B KO = 48.00 ± 23.66) [U(6, 5) = 1, P< 0.01]} to obtain the food reward in comparison to their WT counterparts. In the time prior to accessing the novel bridge, MAO B KO mice engaged in a significantly higher sniffing frequency towards it (Fig. 5f) [U(6, 5) = 2, P < 0.05] compared to WT mice. These results provide further support that MAO B KO mice display greater impulsivity, sensation-seeking and risk-taking behaviors than WT mice.
PEA brain-regional levels
We previously determined that MAO B KO mice feature high brain levels of PEA (Grimsby et al, 1997). To identify the regional determinants of the influence of PEA on the spectrum of behavioral alterations in MAO B KO mice, we examined PEA levels in several regions associated with anxiety and emotional reactivity. MAO B KO mice displayed higher PEA levels in the frontal cortex [MAO B KO/WT ratio: 4.4; F(1,11) = 145.44; P < 0.001], striatum [MAO B KO/WT ratio: 11.5; F(1,11) = 55.20; P < 0.001], hippocampus [MAO B KO/WT ratio: 2.6; F(1,11) = 20.94; P < 0.001], thalamus [MAO B KO/WT ratio: 1.9; F(1,11) = 7.42; P < 0.05], and cerebellum [MAO B KO/WT ratio: 3.7; F(1,11) = 11.67; P < 0.01] compared to their WT counterparts (Table 1).
Table 1.
MAO B KO mice exhibit significantly higher PEA levels in all regions tested.
| PEA levels | ||
|---|---|---|
| Region | WT (N = 7) | MAO B KO (N = 6) |
| Cortex | 1.68 ± 0.28 | 7.4 ± 0.40*** |
| Striatum | 2.24 ± 0.37 | 25.8 ± 3.42*** |
| Hippocampus | 3.52 ± 0.87 | 9.18 ± 0.87*** |
| Thalamus | 6.55 ± 0.68 | 12.5 ± 2.24* |
| Cerebellum | 2.13 ± 0.56 | 7.81 ± 1.69** |
All values are represented as means ± SEM.
P < 0.05,
P < 0.01,
P < 0.001 compared to WT littermates.
TAAR1 transcript brain-regional levels
As the most dramatic increase in PEA regional levels featured by MAO B KO mice was observed in striatum (1150 % in comparison to WT) and frontal cortex (440% in comparison to WT), we tested the expression of the transcript of its selective receptor, TAAR1, in these regions. Striatal TAAR1 mRNA was comparable between MAO B KO and WT mice (Relative expression: WT: 1 ± 0.24; MAO B KO: 1.37± 0.35) [F(1, 6) = 1.16; NS]. Similarly, no significant difference was identified between both genotypes in mRNA expression in frontal cortex (Relative expression: WT: 1 ± 0.32; MAO B KO: 1.54 ± 0.62) [F(1, 6) = 3.03; NS].
Discussion
The major result of the present study is that MAO B deficiency in mice leads to behavioral disinhibition in several models of contextual anxiety and risk-taking behavior, as well as enhanced novelty-seeking responses with respect to unfamiliar objects. These findings are in substantial agreement with previous cross-sectional investigations, documenting that low platelet MAO B enzymatic activity is correlated with several facets of behavioral disinhibition (Buchsbaum et al, 1976; Oreland, 1993; von Knorring et al, 1984), including novelty- and sensation-seeking personality (Fowler et al, 1980a; Reist et al, 1990; Ruchkin et al, 2005), poor impulse control (Paaver et al, 2007; Skondras et al, 2004), and proclivity to engage in risky behaviors (Blanco et al, 1996).
Previous studies indicate that low platelet MAO B activity is highly heritable (Oxenstierna et al, 1986) and may influence behavior since the neonatal stage (Sostek et al, 1981), suggesting that this index may be a genetic determinant for uninhibited personality (Blanco et al, 1996; Oreland and Hallman, 1995). Notably, MAO B deficiency in Norrie disease patients was reported to result in no overt physical or mental alterations (Lenders et al, 1996). However, it should be observed that the severe degree of sensory and perceptual impairments induced by Norrie disease (early-onset blindness and progressive hearing loss) likely masked alterations in environmental reactivity in these subjects.
The role of MAO B in emotional regulation is further supported by a host of clinical studies, showing that chronic administration of l-deprenyl exerts mood-enhancing and anxiolytic effects in depression (Mendlewicz and Youdim, 1980; Quitkin et al, 1984; Robinson et al, 2007) and other disorders (Goad et al, 1991; Tariot et al, 1987; Tolbert and Fuller, 1996). Interestingly, l-deprenyl (both in acute and chronic administration) elicits only minor or no anxiolytic-like effects in rodents (Commissaris et al, 1995; De Angelis and Furlan, 2000; Nowakowska et al, 2001). The most likely explanation for the apparent discrepancy between these reports and our results lies in the genetic nature of MAO B inactivation examined in this study, which cannot be completely recapitulated by the outcomes of chronic exposure to enzyme inhibitors (Whitaker-Azmitia et al, 1994).
In rodents, emotional reactivity and novelty-seeking behavior is measured as a function of the exploratory activity towards unfamiliar environments and objects (Oreland, 1993; Robinet et al, 1998; Fornai et al, 1999). Accordingly, the validity of novelty-induced tasks as animal models of anxiety (Pellow et al, 1985; Takahashi et al, 1989) is based on the opposition between exploratory drive and neophobia-derived avoidance (Dellu et al, 2000). This contrast can be influenced by certain environmental manipulations, such as the variation of light intensity in the experimental room (Dawson and Tricklebank, 1995).
In a dimly lit elevated plus maze, MAO B KO mice displayed a significant reduction in anxiety-like behavior, signified by an increase in open arm time and entries, as well as a reduction of defecation frequency (Tarantino and Bucan, 2000). Conversely, a bright illuminance level (300 lux) failed to elicit significant differences between MAO B KO and WT mice in these anxiety-related parameters, possibly due to “floor effects”.
Dim light conditions have been extensively used to capture fine modifications in anxiety-like behaviors (Bortolato et al, 2006; Bourin et al, 2001; Genn et al, 2003; Löw et al, 2000; Rubino et al, 2008). In the present study, low environmental luminosity provided an optimal setting to reveal the reduction in anxiety-like behaviors in MAO B KO mice, suggesting that the deficiency of this enzyme results in subtle, context-dependent changes in anxiety regulation. This contention is also supported by the observation that the behavioral abnormalities in MAO B KO mice could not be observed in their home cages (data not shown), but only in the presence of novel objects or contexts.
In the defensive withdrawal test, MAO B KO mice exhibited reductions in latency to exit the chamber and in transitions between the chamber and the open arena, independent of differences in locomotor activity. Both parameters are highly dependable indices to measure defensive behaviors (Arborelius and Nemeroff, 2002), and their reduction is considered reflective of reduced fearfulness or deficits in threat detection. This interpretation is also supported by the significant decline in time spent on the elevated plus-maze central platform, which has been suggested to indicate potential impairments in decision-making or impulse-control processes (Rodgers et al, 1992; Trullas and Skolnick, 1993).
The reduction in anxiety-related responses in MAO B KO mice was also confirmed by the nearly complete abrogation of their marble-burying behavior. This murine assay has been validated to capture different aspects of anxiety-like behaviors than conflict-based paradigms (Thomas et al, 2009), in a fashion sensitive to anxiolytic and antidepressant drugs (Borsini et al, 2002; Broekkamp et al, 1986; Nicolas et al, 2006; Njung’e and Handley, 1991). Marble burying has been shown to reflect digging activity, but is independent from locomotion or exploration (Gyertyan, 1995; Thomas et al, 2009). Accordingly, MAO B KO mice dug significantly less than WT counterparts, but displayed a comparable number of crossings.
Our findings on the patterns of exploratory activity in the hole-board test are also supportive of reduced anxiety-like behaviors in MAO B KO mice. Although overall locomotor activity was comparable between genotypes, MAO B KO mice manifested a lower level of avoidance towards the central holes, indicating a reduction in thigmotactic behavior (Hranilovic et al, 2005) and novelty-related aversion (Brown and Nemes, 2008). Since animals were initially placed in the center of the hole board, it may be also proposed that the increased number of central head dips may also reflect an enhancement in perseverative behavior, following exploration of the first central hole. Nevertheless, this possibility is partially tempered by the equivalent latency to the first peripheral head dip between the two genotypes, which was accompanied by a similar locomotor activity (and number of head dips in the external zone) in the first 2-min period of testing. This phenomenon likely indicates that MAO B KO mice did not exhibit an initial tendency to neglect holes in the external zone of the hole board.
The possibility that the behavior enacted by MAO B KO mice may be reflective of low neophobia is also supported by their reaction to unfamiliar objects. Indeed, in comparison to their WT counterparts, MAO B KO mice exhibited a stronger inclination to explore novel objects (with higher duration and lower latency), as well as lower levels of novelty-induced grooming and reduced avoidance of object-laden areas. Alternatively, these responses may also signify poor impulse control and higher drive towards risk-taking behaviors in MAO B KO mice. In rodents, impulsivity is generally studied by means of go/no-go tests, which measure the capacity to withhold behavioral reactions (Dalley et al, 2008). However, as these tasks are based on operant responses, they cannot be dependably used in MAO B KO mice, due to alterations in mnemonic acquisition in these mice (Bortolato et al, in preparation). To obviate this pitfall, we tested MAO B KO mice in the wire beam bridge task, an assay devised to verify their inclination to engage in risk-taking behaviors (such as walking on a novel, flexible bridge placed above a 30-cm deep gap) to reach a rewarding goal. The prevalence of motivational drives over the ability to adjust behavioral responses to contextual elements is considered a key feature of impulsiveness (Jentsch and Taylor, 1999; Bechara et al, 2000). Upon exposure to the novel bridge, MAO B KO mice exhibited a significantly shorter latency to both access and cross the bridge to reach the food reward compared to WT mice. This divergence likely signifies enhanced risk-taking behavior in MAO B KO mice, and may reflect alterations in decision-making processes and emotional regulation in this genotype (Llewellyn, 2008). Interestingly, MAO B KO mice engaged in a significantly higher frequency of sniffing bouts towards the bridge than WT mice, showing that their shorter latency to bridge access was not reflective of inadequate exploration of the bridge itself or lower risk assessment.
In a previous report, we described that MAO B KO mice exhibited lower immobility in the forced swim test than WT counterparts (Grimsby et al, 1997). In mutant mice, this behavior has been associated with either enhanced (Parks et al, 1998) or reduced anxiety-like behavior (Bale and Vale, 2003; Tschenett et al, 2003). Our present findings help define the conceptual framework for the interpretation of the stress response exhibited by MAO B KO mice, suggesting that their behavior in the forced swim paradigm may reflect their enhanced ability to counteract the stress induced by novel contextual factors and hazardous situations. Substantial evidence has indeed shown that the increased mobility in forced swim test is associated with a lower vulnerability to stress-induced anhedonia and depression (Strekalova et al, 2004; Trzctńska et al, 1999), as well as decreased neophobia (Gundersen et al, 2009). Accordingly, decreased grooming activity has been shown to be a dependable criterion to measure increased inclination to cope with stress (Kalueff and Tuohimaa, 2004). The higher resistance to stress in MAO B KO mice is also corroborated by their significantly lower levels of hyperthermia (Bouwknecht et al, 2007) induced by 2 h of physical restraint, in comparison with WT littermates (WT: ΔT: 1.09 ± 0.13 °C; MAO B KO: ΔT: 0.12 ± 0.22 °C; P<0.01) (unpublished data).
MAO B KO mice feature a significant elevation in whole-brain levels of PEA, but not other monoamines (Grimsby et al, 1997). This premise suggests that this trace amine may play a key role in the behavioral alterations induced by MAO B genetic deficiency. Indeed, PEA has been shown to enhance mood and sensory functions in joint administration with MAO B inhibitors (which prevent its degradation) (Sabelli et al, 1994; Sabelli et al, 1995). Furthermore, the synthetic PEA analog amphetamine is known to increase novelty-seeking behaviors and reduce impulse control in both rodents and humans (Evenden and Ryan, 1996; Leyton et al, 2002; Williamson et al, 1997).
In apparent contrast with our findings, acute administration of PEA has been shown to induce anxiety in rodents (Lapin, 1990; 1993). However, congenital, chronic exposure to high PEA levels may reduce anxiety-spectrum responses in MAO B KO mice, probably via the progressive recruitment of processes opposing the anxiogenic effects of this trace amine. Similarly, MAO B KO mice also fail to exhibit other alterations reminiscent of the effects induced by acute PEA administration, such as hyperlocomotion (Mantegazza and Riva, 1963), stereotyped behavior (Moja et al, 1976) and anorexia (Dourish and Boulton, 1981). Final verification of the involvement of PEA in the behavioral performance of MAO B KO mice would require pharmacological manipulations to reduce their PEA levels, such as the inhibition of its synthesis. The accomplishment of this objective, however, is hindered by the lack of substrate-specificity of the only PEA-synthesizing enzyme as yet characterized in mice, aromatic l-amino acid decarboxylase (EC4.1.1.28) (Kubovcakova et al, 2004; Zucchi et al, 2006). Indeed, this enzyme catalyzes key reactions in the synthesis of all the other major neurotransmitter systems, such as DA, NE and 5-HT (Allen et al, 2009), and its inhibition results in a number of non-specific effects on several brain functions (Fisher et al, 1999).
To partially circumvent these limitations, in the present study we have examined the differential expression of PEA levels in several brain regions associated with emotional reactivity. Although the increase in PEA brain levels involves several brain regions of MAO B KO mice, the most marked enhancements in PEA levels were observed in striatum and prefrontal cortex, two regions extensively implicated in behavioral disinhibition (Johansson et al, 2000; Winstanley et al, 2006).
The involvement of striatum and prefrontal cortex in behavioral disinhibition has been linked to the functional activity of DAergic system (Pattij et al, 2007), suggesting that DA may be implicated in the behavioral alterations in MAO B KO mice. This possibility is supported by a host of studies underscoring the key role of DA in behavioral disinhibition (Black et al, 2002; Megens et al, 1992; van Gaalen et al, 2006) and anxiolysis (Shabanov et al, 2005; Picazo et al, 2009). Previous studies have shown that PEA induces modification of the DA signaling, which may play a role in the behavioral responses mediated by this trace amine (Kuroki et al, 1990; Sotnikova et al, 2004). Interestingly, while MAO B KO mice do not feature alterations of striatal DA synthesis, uptake and release, they do exhibit alterations in DA receptors in this region (Chen et al, 1999). Furthermore, structural changes in other monoamine systems (such as 5-HT) may also be induced by MAO B deficiency (Whitaker-Azmitia et al, 1994; Oreland et al, 2007).
Emerging evidence points to a role of TAAR1 receptor in the PEA-mediated modulation of DAergic signaling (Xie and Miller, 2009; Lindemann et al, 2008). Although no apparent compensatory changes in TAAR1 receptor expression were detected in either striatum or frontal cortex, the present data cannot allow any final conclusion on the functional contribution of this receptor to the behavioral spectrum of MAO B KO mice. While pharmacological studies with TAAR1 receptor antagonists may help elucidate this issue, these agents are currently unavailable (Sotnikova et al, 2009).
In summary, this study documents that MAO B deficiency in mice results in behavioral disinhibition and reduced neophobia. These results complement previous findings on the correlation between low MAO B platelet activity and novelty-seeking personality, suggesting a potential causal link between the two phenomena. Nevertheless, both the interpretation of the behavioral phenotype in MAO B KO mice and its translational validity should be considered with caution, in view of several limiting considerations. First, although the array of behavioral abnormalities in MAO B KO mice supports the idea that these animals do display behavioral disinhibition, some of the reported alterations - such as the increased exploration of novel objects or central holes in the hole-board paradigm, or the decreased marble burying - may also reflect other disturbances in perceptual, attentional, emotional and cognitive regulation. Further investigations on the impact of MAO B deficiency in these behavioral domains (and in female mice) are necessary to elucidate this possibility and further refine our understanding of the complex phenotype exhibited by MAO B KO mice. Second, the results observed in MAO B KO mice may not be directly applicable to clinical manifestations, in view of the predominance of this isoenzyme in the human brain (Fowler et al, 1980b; Oreland and Gottfries, 1986; Kalaria et al, 1988), which contrasts with its relatively poor expression in rodents (Saura et al, 1996). Third, while dopamine is degraded by MAO A in mice (Cases et al, 1995; Fornai et al, 1999), it is mainly metabolized by MAO B in primates (Garrick and Murphy, 1980), suggesting that the reported alterations in murine phenotype may only partially reproduce the behavioral outcomes of MAO B deficiency in humans. Irrespective of these considerations, these findings strongly support the role of MAO B in the modulation of the neural pathways underlying behavioral disinhibition and emotional reactivity towards contextual stimuli, and warrant further investigations on the function of this enzyme in the regulation of anxiety-related endophenotypes.
Acknowledgments
This work was supported by NIMH grants, R01MH39085-24A1, R01MH67968, R37MH39085 (MERIT Award), and the Boyd and Elsie Welin Professorship. We thank Eric Ka-Wai Hui, Takeshi Kumazawa, Roberto Frau and Lauren Burgeno for their valuable contributions in the execution of the experiments.
Footnotes
Financial disclosures: All authors report no biomedical financial interests or potential conflicts of interest.
References
- Allen GF, Land JM, Heales SJ. A new perspective on the treatment of aromatic L-amino acid decarboxylase deficiency. Mol Genet Metab. 2009;97(1):6–14. doi: 10.1016/j.ymgme.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Nemeroff C. Preclinical models of anxiety. In: Stein D, Hollander E, editors. Textbook of anxiety disorders. American Psychiatric Publishing Inc; Washington, DC: 2002. pp. 29–42. [Google Scholar]
- Bale TL, Vale WW. Increased depression-like behaviors in corticotropin-releasing factor receptor-2-deficient mice: sexually dichotomous responses. J Neurosci. 2003;23 (12):5295–301. doi: 10.1523/JNEUROSCI.23-12-05295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123(Pt 11):2189–202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Black KJ, Hershey T, Koller JM, Videen TO, Mintun MA, Price JL, Perlmutter JS. A possible substrate for dopamine-related changes in mood and behavior: prefronal and limbic effects of a D3-preferring dopamine agonist. Proc Natl Acad Sci USA. 2002;99 (26):17113–8. doi: 10.1073/pnas.012260599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C, Orensanz-Munoz L, Blanco-Jerez C, Saiz-Ruiz J. Pathological gambling and platelet MAO activity: a psychobiological study. Am J Psychiatry. 1996;153(1):119–121. doi: 10.1176/ajp.153.1.119. [DOI] [PubMed] [Google Scholar]
- Boda E, Pini A, Hoxha E, Parolisi R, Tempia F. Selection of reference genes for quantitative real-time RT-PCR studies in mouse brain. J Mol Neurosci. 2009;37(3):238–53. doi: 10.1007/s12031-008-9128-9. [DOI] [PubMed] [Google Scholar]
- Bohus B, Benus RF, Fokkema DS, Koolhaas JM, Nyakas C, van Oortmerssen GA, et al. Neuroendocrine states and behavioral and physiological stress responses. Prog Brain Res. 1987;72:57–70. doi: 10.1016/s0079-6123(08)60196-x. [DOI] [PubMed] [Google Scholar]
- Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, Boyle N, Pu X, Kouranova E, Lichtblau H, Ochoa FY, Branchek TA, Gerald C. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci USA. 2001;98(16):8966–71. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsini F, Podhorna J, Marazziti D. Do animal models of anxiety predict anxiolytic-like effects of antidepressants? Psychopharmacology (Berl) 2002;163(2):121–141. doi: 10.1007/s00213-002-1155-6. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Campolongo P, Mangieri RA, Scattoni ML, Frau R, Trezza V, et al. Anxiolytic-like properties of the anandamide transport inhibitor AM404. Neuropsychopharmacology. 2006;31(12):2652–2659. doi: 10.1038/sj.npp.1301061. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Chen K, Shih JC. Monoamine oxidase inactivation: from pathophysiology to therapeutics. Adv Drug Deliv Rev. 2008;60(13–14):1527–1533. doi: 10.1016/j.addr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Frau R, Piras A, Luesu W, Bini V, Diaz G, et al. Methamphetamine Induces Long-term Alterations in Reactivity to Environmental Stimuli: Correlation with Dopaminergic and Serotonergic Toxicity. Neurotox Res. 2009;15(1):369–393. doi: 10.1007/s12640-009-9024-2. [DOI] [PubMed] [Google Scholar]
- Bourin M, Nic Dhonnchadha BA, Claude Colombel M, Dib M, Hascoët M. Cyamemazine as an anxiolytic drug on the elevated plus maze and light/dark paradigm in mice. Behav Brain Res. 2001;124(1):87–95. doi: 10.1016/s0166-4328(01)00238-8. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Spiga F, Staub DR, Hale MW, Shekhar A, Lowry CA. Differential effects of exposure to low-light or high-light open-field on anxiety-related behaviors: relationship to c-Fos expression in serotonergic and non-serotonergic neurons in the dorsal raphe nucleus. Brain Res Bull. 2007;72(1):32–43. doi: 10.1016/j.brainresbull.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekkamp CL, Rijk HW, Joly-Gelouin D, Lloyd KL. Major tranquillizers can be distinguished from minor tranquillizers on the basis of effects on marble burying and swim-induced grooming in mice. Eur J Pharmacol. 1986;126(3):223–229. doi: 10.1016/0014-2999(86)90051-8. [DOI] [PubMed] [Google Scholar]
- Brown GR, Nemes C. The exploratory behaviour of rats in the hole-board apparatus: is head-dipping a valid measure of neophilia? Behav Processes. 2008;78(3):442–448. doi: 10.1016/j.beproc.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262(5133):578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Coursey RD, Murphy DL. The biochemical high-risk paradigm: behavioral and familial correlates of low platelet monoamine oxidase activity. Science. 1976;194(4262):339–341. doi: 10.1126/science.968488. [DOI] [PubMed] [Google Scholar]
- Casarrubea M, Sorbera F, Crescimanno G. Structure of rat behavior in hole-board: I) multivariate analysis of response to anxiety. Physiol Behav. 2008;96(1):174–179. doi: 10.1016/j.physbeh.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268(5218):1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, He M, Sibille E, Thompson A, Sarnyai Z, Baker H, Shippenberg T, Toth M. Adaptive changes in postsynaptic dopamine receptors despite unaltered dopamine dynamics in mice lacking monoamine oxidase B. J Neurochem. 1999;73(2):647–55. doi: 10.1046/j.1471-4159.1999.0730647.x. [DOI] [PubMed] [Google Scholar]
- Chen K, Holschneider DP, Wu W, Rebrin I, Shih JC. A spontaneous point mutation produces monoamine oxidase A/B knock-out mice with greatly elevated monoamines and anxiety-like behavior. J Biol Chem. 2004;279(38):39645–39652. doi: 10.1074/jbc.M405550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commissaris RL, Humrich J, Johns J, Geere DG, Fontana DJ. The effects of selective and non-selective monoamine oxidase (MAO) inhibitors on conflict behavior in the rat. Behav Pharmacol. 1995;6(2):195–202. [PubMed] [Google Scholar]
- Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav. 2008;90(2):250–60. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Dawson GR, Tricklebank MD. Use of the elevated plus maze in the search for novel anxiolytic agents. Trends Pharmacol Sci. 1995;16(2):33–36. doi: 10.1016/s0165-6147(00)88973-7. [DOI] [PubMed] [Google Scholar]
- De Angelis L, Furlan C. The anxiolytic-like properties of two selective MAOIs, moclobemide and selegiline, in a standard and an enhanced light/dark aversion test. Pharmacol Biochem Behav. 2000;65(4):649–653. doi: 10.1016/s0091-3057(99)00237-3. [DOI] [PubMed] [Google Scholar]
- Dellu F, Contarino A, Simon H, Koob GF, Gold LH. Genetic differences in response to novelty and spatial memory using a two-trial recognition task in mice. Neurobiol Learn Mem. 2000;73(1):31–48. doi: 10.1006/nlme.1999.3919. [DOI] [PubMed] [Google Scholar]
- Dourish CT, Boulton AA. The effects of acute and chronic administration of beta-phenylethylamine on food intake and body weight in rats. Prog Neuropsychopharmacol. 1981;5 (4):411–414. doi: 10.1016/0364-7722(81)90093-x. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Thrivikraman KV, Su Y, Nemeroff CB, Montkowski A, Landgraf R, et al. Endocrine and behavioral effects of airpuff-startle in rats. Psychoneuroendocrinology. 1996;21(4):391–400. doi: 10.1016/0306-4530(96)00006-6. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1996;128(2):161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Fisher A, Biggs CS, Eradiri O, Starr MS. Dual effects of L-3,4-dihydroxyphenylalanine on aromatic L-amino acid decarboxylase, dopamine release and motor stimulation in the reserpine-treated rat: evidence that behaviour is dopamine independent. Neuroscience. 2000;95(1):97–111. doi: 10.1016/s0306-4522(99)00406-6. [DOI] [PubMed] [Google Scholar]
- Fornai F, Chen K, Giorgi FS, Gesi M, Alessandri MG, Shih JC. Striatal dopamine metabolism in monoamine oxidase B-deficient mice: a brain dialysis study. J Neurochem. 1999;73(6):2434–2440. doi: 10.1046/j.1471-4159.1999.0732434.x. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, von Knorring L, Oreland L. Platelet monoamine oxidase activity in sensation seekers. Psychiatry Res. 1980a;3(3):273–279. doi: 10.1016/0165-1781(80)90057-8. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Wiberg A, Oreland L, Marcusson J, Winblad B. The effect of age on the activity and molecular properties of human brain monoamine oxidase. J Neural Transm. 1980b;49:1–20. doi: 10.1007/BF01249185. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Academic Press; San Diego, CA: 1997. [Google Scholar]
- Garrick NA, Murphy DL. Species differences in the deamination of dopamine and other substrates for monoamine oxidase in brain. Psychopharmacology (Berl) 1980;72(1):27–33. doi: 10.1007/BF00433804. [DOI] [PubMed] [Google Scholar]
- Genn RF, Tucci SA, Thomas A, Edwards JE, File SE. Age-associated sex differences in response to food deprivation in two animal tests of anxiety. Neurosci Biobehav Rev. 2003;27(1–2):155–61. doi: 10.1016/s0149-7634(03)00017-4. [DOI] [PubMed] [Google Scholar]
- Goad DL, Davis CM, Liem P, Fuselier CC, McCormack JR, Olsen KM. The use of selegiline in Alzheimer’s patients with behavior problems. J Clin Psychiatry. 1991;52(8):342–345. [PubMed] [Google Scholar]
- Grimsby J, Toth M, Chen K, Kumazawa T, Klaidman L, Adams JD, et al. Increased stress response and beta-phenylethylamine in MAOB-deficient mice. Nat Genet. 1997;17(2):206–210. doi: 10.1038/ng1097-206. [DOI] [PubMed] [Google Scholar]
- Gundersen BB, Blendy JA. Effects of the histone deacetylase inhibitor sodium butyrate in models of depression and anxiety. Neuropharmacology. 2009 doi: 10.1016/j.neuropharm.2009.04.008. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyertyan I. Analysis of the marble burying response: marbles serve to measure digging rather than evoke burying. Behav Pharmacol. 1995;6(1):24–31. [PubMed] [Google Scholar]
- Hirano K, Kimura R, Sugimoto Y, Yamada J, Uchida S, Kato Y, et al. Relationship between brain serotonin transporter binding, plasma concentration and behavioural effect of selective serotonin reuptake inhibitors. Br J Pharmacol. 2005;144(5):695–702. doi: 10.1038/sj.bjp.0706108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld-Becker DR, Biederman J, Calltharp S, Rosenbaum ED, Faraone SV, Rosenbaum JF. Behavioral inhibition and disinhibition as hypothesized precursors to psychopathology: implications for pediatric bipolar disorder. Biol Psychiatry. 2003;53(11):985–99. doi: 10.1016/s0006-3223(03)00316-0. [DOI] [PubMed] [Google Scholar]
- Hranilovic D, Cicin-Sain L, Bordukalo-Niksic T, Jernej B. Rats with constitutionally upregulated/downregulated platelet 5HT transporter: differences in anxiety-related behavior. Behav Brain Res. 2005;165(2):271–277. doi: 10.1016/j.bbr.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. Int J Psychophysiol. 2003;48 (2):147–78. doi: 10.1016/s0167-8760(03)00052-7. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146(4):373–90. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Johansson AK, Hansen S. Increased alcohol intake and behavioral disinhibition in rats with ventral striatal neuron loss. Physiol Behav. 2000;70(5):453–63. doi: 10.1016/s0031-9384(00)00284-5. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Mitchell MJ, Harik SI. Monoamine oxidases of the human brain and liver. Brain. 1988;111(Pt 6):1441–51. doi: 10.1093/brain/111.6.1441. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Tuohimaa P. Grooming analysis algorithm for neurobehavioural stress research. Brain Res Brain Res Protoc. 2004;13(3):151–158. doi: 10.1016/j.brainresprot.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Shih JC, Chen K, Chen L, Bao S, Maren S, et al. Selective enhancement of emotional, but not motor, learning in monoamine oxidase A-deficient mice. Proc Natl Acad Sci U S A. 1997;94(11):5929–5933. doi: 10.1073/pnas.94.11.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk RE. ExperimentaI design: procedures for the behavioral sciences. 2. Brooks Cole; Monterey, CA: 1982. [Google Scholar]
- Korte SM, De Kloet ER, Buwalda B, Bouman SD, Bohus B. Antisense to the glucocorticoid receptor in hippocampal dentate gyrus reduces immobility in forced swim test. Eur J Pharmacol. 1996;301(1–3):19–25. doi: 10.1016/0014-2999(96)00064-7. [DOI] [PubMed] [Google Scholar]
- Kubovcakova L, Krizanova O, Kvetnansky R. Identification of the aromatic L-amino acid decarboxylase gene expression in various mice tissues and its modulation by immobilization stress in stellate ganglia. Neuroscience. 2004;126(2):375–80. doi: 10.1016/j.neuroscience.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Kuroki T, Tsutsumi T, Hirano M, Matsumoto T, Tatebayashi Y, Nishiyama K, Uchimura H, Shiraishi A, Nakahara T, Nakamura K. Behavioral sensitization to beta-phenylethylamine (PEA): enduring modifications of specific dopaminergic neuron systems in the rat. Psychopharmacology (Berl) 1990;102(1):5–10. doi: 10.1007/BF02245736. [DOI] [PubMed] [Google Scholar]
- Lapin IP. Beta-phenylethylamine (PEA): an endogenous anxiogen? Three series of experimental data. Biol Psychiatry. 1990;28(11):997–1003. doi: 10.1016/0006-3223(90)90065-a. [DOI] [PubMed] [Google Scholar]
- Lapin IP. Anxiogenic effect of phenylethylamine and amphetamine in the elevated plus-maze in mice and its attenuation by ethanol. Pharmacol Biochem Behav. 1993;44 (1):241–3. doi: 10.1016/0091-3057(93)90305-d. [DOI] [PubMed] [Google Scholar]
- Lee M, Chen K, Shih JC, Hiroi N. MAO-B knockout mice exhibit deficient habituation of locomotor activity but normal nicotine intake. Genes Brain Behav. 2004;3(4):216–227. doi: 10.1111/j.1601-1848.2004.00074.x. [DOI] [PubMed] [Google Scholar]
- Lenders JW, Eisenhofer G, Abeling NG, Berger W, Murphy DL, Konings CH, et al. Specific genetic deficiencies of the A and B isoenzymes of monoamine oxidase are characterized by distinct neurochemical and clinical phenotypes. J Clin Invest. 1996;97(4):1010–1019. doi: 10.1172/JCI118492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A. Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology. 2002;27(6):1027–1035. doi: 10.1016/S0893-133X(02)00366-4. [DOI] [PubMed] [Google Scholar]
- Lindemann L, Hoener MC. A renaissance in trace amines inspired by a novel GPCR family. Trends Pharmacol Sci. 2005;26(5):274–81. doi: 10.1016/j.tips.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Lindemann L, Meyer CA, Jeanneau K, Bradaia A, Ozmen L, Bluethmann H, Bettler B, Wettstein JG, Borroni E, Moreau JL, Hoener MC. Trace amine-associated receptor 1 modulates dopaminergic activity. J Pharmacol Exp Ther. 2008;324(3):948–56. doi: 10.1124/jpet.107.132647. [DOI] [PubMed] [Google Scholar]
- Llewellyn DJ. The psychology of risk taking: toward the integration of psychometric and neuropsychological paradigms. Am J Psychol. 2008;121(3):363–76. [PubMed] [Google Scholar]
- Louvart H, Maccari S, Darnaudery M. Prenatal stress affects behavioral reactivity to an intense stress in adult female rats. Brain Res. 2005;1031(1):67–73. doi: 10.1016/j.brainres.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Löw K, Crestani F, Keist R, Benke D, Brünig I, Benson JA, Fritschy JM, Rülicke T, Bluethmann H, Möhler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290(5489):131–4. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Mantegazza P, Riva P. Amphetamine-like activity of β-phenylethylamine after a monoamine oxidase inhibitor in vivo. J Pharm Pharmacol. 1963;15:472–478. [Google Scholar]
- Megens AA, Niemegeers CJ, Awouters FH. Behavioral disinhibition and depression in amphetaminized rats: a comparison of risperidone, ocaperidone and haloperidol. J Pharmacol Exp Ther. 1992;260(1):160–7. [PubMed] [Google Scholar]
- Mendlewicz J, Youdim MB. Antidepressant potentiation of 5-hydroxytryptophan by L-deprenil in affective illness. J Affect Disord. 1980;2(2):137–146. doi: 10.1016/0165-0327(80)90013-0. [DOI] [PubMed] [Google Scholar]
- Moja EA, Stoff DM, Gillin JC, Wyatt RJ. Dose-response effects of beta-phenylethylamine on stereotyped behavior in pargyline-pretreated rats. Biol Psychiatry. 1976;11 (6):731–742. [PubMed] [Google Scholar]
- Nicolas LB, Kolb Y, Prinssen EP. A combined marble burying-locomotor activity test in mice: a practical screening test with sensitivity to different classes of anxiolytics and antidepressants. Eur J Pharmacol. 2006;547(1–3):106–115. doi: 10.1016/j.ejphar.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Njung’e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol Biochem Behav. 1991;38(1):63–67. doi: 10.1016/0091-3057(91)90590-x. [DOI] [PubMed] [Google Scholar]
- Nowakowska E, Kus K, Chodera A, Rybakowski J. Investigating potential anxiolytic, antidepressant and memory enhancing activity of deprenyl. J Physiol Pharmacol. 2001;52(4 Pt 2):863–873. [PubMed] [Google Scholar]
- Oreland L. Monoamine oxidase in neuropsychiatric disorders. In: Yasuhara H, Parvez S, Sandler M, Oguchi K, Nagatsu T, editors. Monoamine oxidase: basic and clinical aspects. VSP press; Utrecht, The Netherlands: 1993. pp. 219–247. [Google Scholar]
- Oreland L, Gottfries CG. Brain and brain monoamine oxidase in aging and in dementia of Alzheimer’s type. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10(3–5):533–40. doi: 10.1016/0278-5846(86)90023-0. [DOI] [PubMed] [Google Scholar]
- Oreland L, Hallman J. The correlation between platelet MAO activity and personality: short review of findings and a discussion on possible mechanisms. Prog Brain Res. 1995;106:77–84. doi: 10.1016/s0079-6123(08)61204-2. [DOI] [PubMed] [Google Scholar]
- Oreland L, Nilsson K, Damberg M, Hallman J. Monoamine oxidases: activities, genotypes and the shaping of behaviour. J Neural Transm. 2007;114(6):817–22. doi: 10.1007/s00702-007-0694-8. [DOI] [PubMed] [Google Scholar]
- Oxenstierna G, Edman G, Iselius L, Oreland L, Ross SB, Sedvall G. Concentrations of monoamine metabolites in the cerebrospinal fluid of twins and unrelated individuals--a genetic study. J Psychiatr Res. 1986;20(1):19–29. doi: 10.1016/0022-3956(86)90020-8. [DOI] [PubMed] [Google Scholar]
- Paaver M, Nordquist N, Parik J, Harro M, Oreland L, Harro J. Platelet MAO activity and the 5-HTT gene promoter polymorphism are associated with impulsivity and cognitive style in visual information processing. Psychopharmacology (Berl) 2007;194(4):545–554. doi: 10.1007/s00213-007-0867-z. [DOI] [PubMed] [Google Scholar]
- Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci USA. 1998;95(18):10734–9. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattij T, Janssen MC, Vanderschuren LJ, Schoffelmeer AN, van Gaalen MM. Involvement of dopamine D1 and D2 receptors in the nucleus accumbens core and shell in inhibitory response control. Psychopharmacology (Berl) 2007;191(3):587–98. doi: 10.1007/s00213-006-0533-x. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14(3):149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Picazo O, Chuc-Meza E, Anaya-Martinez V, Jimenez I, Aceves J, Garcia-Ramirez M. 6-Hydroxydopamine lesion in thalamic reticular nucleus reduces anxiety behaviour in the rat. Behav Brain Res. 2009;197(2):317–22. doi: 10.1016/j.bbr.2008.08.047. [DOI] [PubMed] [Google Scholar]
- Popova NK, Gilinsky MA, Amstislavskaya TG, Morosova EA, Seif I, De Maeyer E. Regional serotonin metabolism in the brain of transgenic mice lacking monoamine oxidase A. J Neurosci Res. 2001;66(3):423–427. doi: 10.1002/jnr.1234. [DOI] [PubMed] [Google Scholar]
- Quitkin FM, Liebowitz MR, Stewart JW, McGrath PJ, Harrison W, Rabkin JG, et al. l-Deprenyl in atypical depressives. Arch Gen Psychiatry. 1984;41(8):777–781. doi: 10.1001/archpsyc.1984.01790190051006. [DOI] [PubMed] [Google Scholar]
- Reist C, Haier RJ, DeMet E, Chicz-DeMet A. Platelet MAO activity in personality disorders and normal controls. Psychiatry Res. 1990;33(3):221–227. doi: 10.1016/0165-1781(90)90039-8. [DOI] [PubMed] [Google Scholar]
- Rieu I, Powers SJ. Real-Time Quantitative RT-PCR: Design, Calculations, and Statistics. Plant Cell. 2009;21(4):1031–3. doi: 10.1105/tpc.109.066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinet PM, Rowlett JK, Bardo MT. Individual differences in novelty-induced activity and the rewarding effects of novelty and amphetamine in rats. Behavioural Processes. 1998;44(1):1–9. doi: 10.1016/s0376-6357(98)00022-9. [DOI] [PubMed] [Google Scholar]
- Robinson DS, Gilmor ML, Yang Y, Moonsammy G, Azzaro AJ, Oren DA, et al. Treatment effects of selegiline transdermal system on symptoms of major depressive disorder: a meta-analysis of short-term, placebo-controlled, efficacy trials. Psychopharmacol Bull. 2007;40(3):15–28. [PubMed] [Google Scholar]
- Rodgers RJ, Lee C, Shepherd JK. Effects of diazepam on behavioural and antinociceptive responses to the elevated plus-maze in male mice depend upon treatment regimen and prior maze experience. Psychopharmacology (Berl) 1992;106(1):102–110. doi: 10.1007/BF02253596. [DOI] [PubMed] [Google Scholar]
- Rubino T, Vigano’ D, Realini N, Guidali C, Braida D, Capurro V, Castiglioni C, Cherubino F, Romualdi P, Candeletti S, Sala M, Parolaro D. Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology. 2008;33(11):2760–71. doi: 10.1038/sj.npp.1301664. [DOI] [PubMed] [Google Scholar]
- Ruchkin VV, Koposov RA, af Klinteberg B, Oreland L, Grigorenko EL. Platelet MAO-B, personality, and psychopathology. J Abnorm Psychol. 2005;114(3):477–82. doi: 10.1037/0021-843X.114.3.477. [DOI] [PubMed] [Google Scholar]
- Sabelli H, Fahrer R, Medina RD, Ortiz Fragola E. Phenylethylamine relieves depression after selective MAO-B inhibition. J Neuropsychiatry Clin Neurosci. 1994;6(2):203. doi: 10.1176/jnp.6.2.203. [DOI] [PubMed] [Google Scholar]
- Sabelli HC, Javaid JI. Phenylethylamine modulation of affect: therapeutic and diagnostic implications. J Neuropsychiatry Clin Neurosci. 1995;7(1):6–14. doi: 10.1176/jnp.7.1.6. [DOI] [PubMed] [Google Scholar]
- Saura J, Bleuel Z, Ulrich J, Mendelowitsch A, Chen K, Shih JC, Malherbe P, Da Prada M, Richards JG. Molecular neuroanatomy of human monoamine oxidases A and B revealed by quantitative enzyme radioautography and in situ hybridization histochemistry. Neuroscience. 1996;70(3):755–74. doi: 10.1016/s0306-4522(96)83013-2. [DOI] [PubMed] [Google Scholar]
- Scott AL, Bortolato M, Chen K, Shih JC. Novel monoamine oxidase A knock out mice with human-like spontaneous mutation. Neuroreport. 2008;19(7):739–743. doi: 10.1097/WNR.0b013e3282fd6e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabanov PD, Lebedev AA, Meshcherov ShK, Strel’tsov VF. The effects of neurochemical lesioning of dopaminergic terminals in early ontogenesis on behavior in adult rats. Neurosci Behav Physiol. 2005;35(5):535–44. doi: 10.1007/s11055-005-0089-y. [DOI] [PubMed] [Google Scholar]
- Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annu Rev Neurosci. 1999a;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih JC, Ridd MJ, Chen K, Meehan WP, Kung MP, Seif I, et al. Ketanserin and tetrabenazine abolish aggression in mice lacking monoamine oxidase A. Brain Res. 1999b;835(2):104–112. doi: 10.1016/s0006-8993(99)01478-x. [DOI] [PubMed] [Google Scholar]
- Skondras M, Markianos M, Botsis A, Bistolaki E, Christodoulou G. Platelet monoamine oxidase activity and psychometric correlates in male violent offenders imprisoned for homicide or other violent acts. European Archives of Psychiatry and Clinical Neuroscience. 2004;254(6):380–386. doi: 10.1007/s00406-004-0518-x. [DOI] [PubMed] [Google Scholar]
- Sostek AJ, Sostek AM, Murphy DL, Martin EB, Born WS. Cord blood amine oxidase activities relate to arousal and motor functioning in human newborns. Life Sci. 1981;28 (22):2561–2568. doi: 10.1016/0024-3205(81)90599-3. [DOI] [PubMed] [Google Scholar]
- Sotnikova TD, Budygin EA, Jones SR, Dykstra LA, Caron MG, Gainetdinov RR. Dopamine transporter-dependent and -independent actions of trace amine beta-phenylethylamine. J Neurochem. 2004;91(2):362–73. doi: 10.1111/j.1471-4159.2004.02721.x. [DOI] [PubMed] [Google Scholar]
- Sotnikova TD, Caron MG, Gainetdinov RR. Trace amine associated receptors (TAARs) as emerging therapeutic targets. Mol Pharmacol. 2009 doi: 10.1124/mol.109.055970. IN PRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology. 2004;29(11):2007–17. doi: 10.1038/sj.npp.1300532. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Kalin NH, Vanden Burgt JA, Sherman JE. Corticotropin-releasing factor modulates defensive-withdrawal and exploratory behavior in rats. Behav Neurosci. 1989;103(3):648–654. doi: 10.1037//0735-7044.103.3.648. [DOI] [PubMed] [Google Scholar]
- Tarantino LM, Bucan M. Dissection of behavior and psychiatric disorders using the mouse as a model. Hum Mol Genet. 2000;9(6):953–965. doi: 10.1093/hmg/9.6.953. [DOI] [PubMed] [Google Scholar]
- Tariot PN, Cohen RM, Sunderland T, Newhouse PA, Yount D, Mellow AM, et al. L-deprenyl in Alzheimer’s disease. Preliminary evidence for behavioral change with monoamine oxidase B inhibition. Arch Gen Psychiatry. 1987;44(5):427–433. doi: 10.1001/archpsyc.1987.01800170041007. [DOI] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology. 2009;204(2):361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert SR, Fuller MA. Selegiline in treatment of behavioral and cognitive symptoms of Alzheimer disease. Ann Pharmacother. 1996;30(10):1122–1129. doi: 10.1177/106002809603001012. [DOI] [PubMed] [Google Scholar]
- Trullas R, Skolnick P. Differences in fear motivated behaviors among inbred mouse strains. Psychopharmacology (Berl) 1993;111(3):323–331. doi: 10.1007/BF02244948. [DOI] [PubMed] [Google Scholar]
- Trzctńska M, Tonkiss J, Galler JR. Influence of prenatal protein malnutrition on behavioral reactivity to stress in adult rats. Stress. 1999;3(1):71–83. doi: 10.3109/10253899909001113. [DOI] [PubMed] [Google Scholar]
- Tschenett A, Singewald N, Carli M, Balducci C, Salchner P, Vezzani A, Herzog H, Sperk G. Reduced anxiety and improved stress coping ability in mice lacking NPY-Y2 receptors. Eur J Neurosci. 2003;18(1):143–8. doi: 10.1046/j.1460-9568.2003.02725.x. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, Brueggeman RJ, Bronius PF, Schoffelmeer AN, Vanderschuren LJ. Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology (Berl) 2006;187(1):73–85. doi: 10.1007/s00213-006-0396-1. [DOI] [PubMed] [Google Scholar]
- von Knorring L, Oreland L, Winblad B. Personality traits related to monoamine oxidase activity in platelets. Psychiatry Res. 1984;12(1):11–26. doi: 10.1016/0165-1781(84)90134-3. [DOI] [PubMed] [Google Scholar]
- Wall PM, Messier C. Ethological confirmatory factor analysis of anxiety-like behaviour in the murine elevated plus-maze. Behav Brain Res. 2000;114(1–2):199–212. doi: 10.1016/s0166-4328(00)00229-1. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Zhang X, Clarke C. Effects of gestational exposure to monoamine oxidase inhibitors in rats: preliminary behavioral and neurochemical studies. Neuropsychopharmacology. 1994;11(2):125–132. doi: 10.1038/npp.1994.42. [DOI] [PubMed] [Google Scholar]
- Williamson S, Gossop M, Powis B, Griffiths P, Fountain J, Strang J. Adverse effects of stimulant drugs in a community sample of drug users. Drug Alcohol Depend. 1997;44 (2–3):87–94. doi: 10.1016/s0376-8716(96)01324-5. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Cardinal RN, Robbins TW. Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cereb Cortex. 2006;16(1):106–14. doi: 10.1093/cercor/bhi088. [DOI] [PubMed] [Google Scholar]
- Wolinsky TD, Swanson CJ, Smith KE, Zhong H, Borowsky B, Seeman P, Branchek T, Gerald CP. The Trace Amine 1 receptor knockout mouse: an animal model with relevance to schizophrenia. Genes Brain Behav. 2007;6(7):628–39. doi: 10.1111/j.1601-183X.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- Xie Z, Miller GM. Trace Amine-Associated Receptor 1 as a Monoaminergic Modulator in Brain. Biochem Pharmacol. 2009 doi: 10.1016/j.bcp.2009.05.031. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchi R, Chiellini G, Scanlan TS, Grandy DK. Trace amine-associated receptors and their ligands. Br J Pharmacol. 2006;149(8):967–78. doi: 10.1038/sj.bjp.0706948. [DOI] [PMC free article] [PubMed] [Google Scholar]


