Abstract
Objective
To compare survival in older patients with acute ischemic stroke admitted to intensive care units (ICU) with those not requiring ICU care and to assess the impact of mechanical ventilation (MV) and percutaneous gastrostomy tubes (PEG) on long-term mortality.
Design
Multi-center retrospective cohort study.
Setting
Administrative data from the Centers for Medicare and Medicaid Services covering 93 metropolitan counties primarily in the Eastern half of the United States.
Patients
31,301 patients discharged with acute ischemic stroke in 2000.
Interventions
None
Measurements
Mortality from the time of index hospitalization up to the end of the follow-up period of 12 months. Information was also gathered on use of mechanical ventilation, percutaneous gastrostomy, sociodemographic variables and a host of comorbid conditions.
Main Results
26% of all patients with acute ischemic stroke required ICU admission. The crude death rate for ICU stroke patients was 21% at 30 days and 40% at 1-year follow-up. At 30 days, after adjustment of sociodemographic variables and comorbidities, ICU patients had a 29% higher mortality hazard compared to non-ICU patients. Mechanical ventilation was associated with a five-fold higher mortality hazard (hazard ratio 5.59, confidence interval 4.93–6.34). The use of PEG was not associated with mortality at 30 days. By contrast, at 1-year follow up in 30-day survivors, ICU admission was not associated with mortality hazard (hazard ratio 1.01; 95% confidence interval 0.93–1.09). Mechanical ventilation still had a higher risk of death (hazard ratio 1.88, 95% confidence interval 1.57–2.25), and PEG patients had a 2.59 fold greater mortality hazard (95% confidence interval 2.38–2.82).
Conclusions
Both short-term and long-term mortality in older patients with acute ischemic stroke admitted to ICUs is lower than previously reported. The need for MV and PEG are markers for poor long-term outcome. Future research should focus on the identification of clinical factors that lead to increased mortality in long-term survivors and efforts to reduce those risks.
Keywords: intensive care units, mortality, outcomes, stroke
INTRODUCTION
Approximately 700,000 people experience a new or recurrent stroke each year in the United States. After heart disease and cancer, stroke ranks third among all causes of death, accounting for about 1 in every 16 deaths in 2004. In 2001, $3.7 billion were paid to Medicare beneficiaries discharged from short-stay hospitals with stroke (1). These statistics have prompted a number of researchers to evaluate the role of intensive care unit (ICU) management of stroke patients in terms of its impact on survival and functional outcomes. The effect on health care expenditure is also of interest, since ICU patients account for 20–40% of all hospital costs (2). Accurate knowledge of such outcomes and resource consumption is important to clinicians, health care administrators and policy makers alike to help guide decision-making. However, very few studies have been carried out in this field in the United States.
Mortality rates in ICU patients with stroke range between 15–59% in the short-term (≤ 3 months) (3–6). The wide variation in reported death rates is related to a number of factors. Firstly, studies have been conducted in a number of countries, likely with differing practices in the intensive care unit management of stroke. Secondly, very small numbers of patients have been included, ranging from 58 to 138, mostly in single centers (3–5, 7, 8). Finally, there has been no uniformity in the type of stroke patient studied, with reports including either hemorrhagic stroke, ischemic stroke or both. Only one study has, thus far, addressed the issue of long-term survival; the study was conducted in Spain and reported a 1-year mortality of 66% in acute ischemic stroke patients admitted to ICU (4).
Mechanical ventilation (MV) and percutaneous gastrostomy tubes (PEG) in the setting of stroke have also been the subject of prior investigations, since their use is associated with a considerable increase in resource consumption. Mechanical ventilation has been demonstrated to be a proxy for stroke severity and coma (9); reported mortality rates in these patients have been much higher, ranging between 46 and 92% (10–21). Almost all previous studies have been case-series analyses. The largest report to date was that by Bushnell et al. who conducted a retrospective chart review of 131 mechanically ventilated stroke patients, reporting a 30-day mortality of 49% and a 6-month mortality of 61% (11).
Ours is the first large-scale study aiming to establish the long-term outcome of older patients hospitalized with acute ischemic stroke in the United States. Our objective was to compare the survival of patients with acute ischemic stroke admitted to intensive care units with those not requiring ICU care (non-ICU patients) using a multi-center retrospective cohort study design. Our study is also the first with sufficient power to also analyze the separate effects of mechanical ventilation and percutaneous gastrostomy tube insertion on long-term outcome.
MATERIALS AND METHODS
Population and Sampling
We identified Medicare beneficiaries 65 years of age and older discharged with acute ischemic stroke during 2000 in 11 metropolitan regions of the country. Patients were included in the sample if they had an International Classification of Diseases, 9th edition (ICD-9) diagnosis code of 434 or 436 in the first position on the discharge diagnosis list from an acute care hospitalization, which has been found to accurately identify acute ischemic stroke in 89–90% of cases (22). If a patient had more than one acute ischemic stroke discharge over the study period, one discharge was randomly selected. This approach obviated the need for analyses accounting for repeated observations on the same patient. However, the number of patients with repeated strokes is small, with less than 3% of patients having a second stroke during 2000.
We obtained HMO data from a large national managed care organization and FFS (Fee For Service) data from the Centers for Medicare and Medicaid Services (CMS). HMO data included patients who were enrolled in 11 Medicare Plus Choice plans serving 93 metropolitan counties primarily in the eastern half of the United States (N = 1,939 patients with acute ischemic stroke in 422 hospitals). Comparable data were obtained for all FFS patients (N=29,850) discharged with acute ischemic stroke in the same counties. This study was approved by the Institutional Review Board of the University of Wisconsin.
Data Extraction
We utilized enrollment data and final institutional and physician/supplier claims for all study patients from one year prior to their index hospital admission date up to the time of their death or until 12 months after the date of their index hospitalization. Both HMO and FFS patients had claims submitted using standard CMS forms. For HMO patients, we also obtained enrollment and disenrollment data and all claims submitted to the HMO from out-of-network facilities. For both FFS and HMO patients, we obtained the Medicare denominator file to determine age, sex, race, zip code, Medicaid enrollment, and date of death. This file was used to exclude FFS beneficiaries who were missing Medicare Part A or Part B coverage, had end-stage renal disease, received railroad retirement benefits, or were enrolled in an HMO at any point from one year prior to their index hospitalization to the time of their death.
Variables
The main dependent variable was mortality at any time following the index hospital admission date up to the end of the follow-up period. Explanatory variables included ICU admission and two validated indicator variables representing disease severity, namely mechanical ventilation (CPT 94656, 94657; ICD-9 96.7x) (9) and placement or revision of a gastrostomy tube (CPT 43750, 43760, 43761, 43832, 43246; ICD-9 43.11) (23). An ICU admission was identified using the Centers for Medicare and Medicaid Services revenue codes indicating billing for an ICU stay (revenue codes 020X, where X ranges from 0 to 9). This includes the following types of ICUs: general, surgical, medical, pediatric, psychiatric, intermediate ICU, burn care, trauma, and other intensive care.
We included individual and neighborhood sociodemographic characteristics as potential confounder variables. Individual characteristics comprised age, gender, race and an indicator identifying beneficiaries with low to modest income who are fully enrolled in Medicaid, or received some help with Medicare cost-sharing through Medicaid. Zip+4 information were used to link patient data to the corresponding Census 2000 block group and obtain neighborhood socioeconomic characteristics, including percent over 24 years of age with college degree and percent below poverty line (24).
It is critical to control for pre-existing differences in comorbidity between comparison groups, namely those admitted to the ICU and non-ICU patients. We identified 30 comorbid conditions that incorporated information from the initial hospitalization, all hospitalizations and all physician claims during the prior year using methods proposed by Elixhauser, et al. (25), and Klabunde, et al. (26). Of these 30 conditions, we included the 13 comorbidities present in over 5% of our sample as indicator variables. An “other comorbidity count” was generated for the remaining conditions present in less than 5% of our sample. We also coded the following: hospitalization during the year prior to the index hospitalization, dementia (27), stroke during the year prior to the index hospitalization (28), and concurrent cardiac events (acute myocardial infarction, unstable angina pectoris, coronary artery bypass graft, and cardiac catheterization) (29). Additionally, the Centers for Medicare and Medicaid Services’ hierarchical condition categories (CMS-HCC) score for the year prior to admission was calculated for each subject and included in models as a comprehensive risk adjustment measure (30).
Analysis
Analyses were conducted using Stata version 9.0. A two sided p value of < 0.05 was considered to be statistically significant. Baseline descriptive data were compared with ANOVA for continuous variables and the Chi-squared test for categorical variables. Kaplan-Meier survival curves were constructed to describe crude differences in mortality between comparison groups. The log-rank test was used to assess the significance of any differences observed.
The probability of death in patients who were admitted to the ICU was estimated using logistic regression. Unadjusted and adjusted probabilities were calculated for 30 days from the time of admission and, in 30-day survivors, for 12 months from the date of admission. These probabilities were estimated separately according to combinations of the presence/absence of mechanical ventilation and percutaneous gastrostomy tubes. This was achieved by including two-way interaction terms between mechanical ventilation and percutaneous gastrostomy tube indicator variables in the logistic regression models. 95% predicted probabilities and confidence intervals were constructed using the “prvalue” command in Stata, using the delta method to construct confidence intervals.
The association between ICU admission, mechanical ventilation, percutaneous gastrostomy and survival rate was evaluated using Cox proportional hazards regression. Since these variables showed evidence of non-proportional mortality hazard ratios over time, analyses were carried out separately for deaths occurring within 30 days of admission and those occurring between 30 days (conditional upon survival to 30 days) and the end of the follow up period, which was 12 months after the date of index hospitalization. Non-proportionality of hazards was assessed using Schoenfeld residuals. All modeling was carried out using robust estimates of variance that allowed for clustering of patients within hospitals.
RESULTS
Study Population
A total of 31,301 patients were included in the study. 26% of patients were admitted to an intensive care unit at some point during their index hospitalization. These ICU patients were slightly older than their non-ICU counterparts, with patients younger than 80 years being more likely to be admitted to the ICU than those over the age of 80 (Table 1). ICU patients were more likely to be male (39.2% vs. 36.8%, p<0.000), to be enrolled in a managed care organization (6.8% vs. 6.0%, p=0.01) and live in an area with a lower attained level of education (23% in block group with a college degree vs. 25%, p<0.000). Medicaid eligibility, Caucasian race and the poverty level of the area in which patients lived were not related to the likelihood of ICU admission; however, African Americans were less likely (12.9% vs. 14.3%, p=0.001), and other minorities were more likely to be admitted to the ICU (5% vs. 3.2%, p<0.000). As expected, the likelihood of MV and PEG placement was higher in ICU than in non-ICU patients. Specifically, 10.7% of ICU patients were mechanically ventilated compared to 0.8% of non-ICU patients (p<0.000), and 11.1% had placement or revision of a percutaneous gastrostomy in the ICU compared to 5.6% outside of the ICU (p<0.000). At the end of their hospitalization, non-ICU patients were slightly more likely to be discharged home (30% vs. 28%, p<0.000).
Table 1.
Baseline Characteristics of Patients Admitted with Acute Ischemic Strokea
| Characteristic | Entire Sample N=31,301 |
ICU N=8,185 |
Non-ICU N=23,116 |
p -value |
|---|---|---|---|---|
| Sociodemographic | ||||
| Average age in years (standard deviation) | 79.9 (7.6) | 80.2 (7.6) | 79.1 (7.3) | 0.000 |
| 65–69 years | 9.7 | 10.7 | 9.4 | |
| 70–74 years | 16.6 | 17.9 | 16.2 | |
| 75–79 years | 22.6 | 24.6 | 21.9 | |
| 80–84 years | 22.0 | 21.7 | 22.1 | |
| >85 years | 29.0 | 25.1 | 30.4 | 0.000 |
| Male | 37.5 | 39.2 | 36.8 | 0.000 |
| Caucasian | 82.4 | 82.1 | 82.5 | 0.455 |
| African-American | 14.0 | 12.9 | 14.3 | 0.001 |
| Other minority | 3.7 | 5.0 | 3.2 | 0.000 |
| Medicaid | 17.4 | 17.1 | 17.5 | 0.375 |
| HMO member | 6.2 | 6.8 | 6.0 | 0.010 |
| Percent in block group below the poverty line (standard deviation) | 11 (11) | 12 (11) | 11 (11) | 0.12 |
| Percent adults 25 and older in block group with college degree (standard deviation) |
24 (17) | 23 (17) | 25 (18) | 0.000 |
| Index hospitalization | ||||
| Length of stay in days (standard deviation) | 6.0 (5.4) | 7.3 (6.1) | 5.5 (5.1) | 0.000 |
| Use of Mechanical Ventilation n (%) | 1,071 (3.4) | 876 (10.7) | 195 (0.8) | 0.000 |
| Use of Percutaneous Gastrostomy Tube n (%) | 2,195 ( 7.0) | 907 (11.1) | 1,288 (5.6) | 0.000 |
| Discharged from index hospital stay to: | ||||
| Home | 29 | 28 | 30 | 0.000 |
| Home with home health | 17 | 16 | 17 | |
| Rehabilitation center | 19 | 22 | 19 | |
| Skilled nursing facility | 31 | 30 | 31 | |
| Hospice/Other | 4 | 5 | 4 |
Values represent percents unless otherwise specified.
Differences in comorbidities were noted in the comparison groups (Table 2). Specifically, risk adjustment HCC scores were higher in ICU patients (mean score 2.53 ± 1.39 vs. 2.30 ± 1.30, p<0.000). Comorbidities that were significantly more likely in ICU patients were cardiac arrhythmias, congestive heart failure, chronic pulmonary disease, fluid and electrolyte disorders, valvular heart disease and concurrent cardiac events. Non-ICU patients, on the other hand, were more likely to have had a prior stroke, peripheral vascular disease, hypothyroidism, depression and dementia. The remainder of the specific disease entities that were evaluated had no impact on the likelihood of ICU admission.
Table 2.
Baseline Characteristics of Patients Admitted with Acute Ischemic Stroke - Prior Medical Historya
| Characteristic | Entire Sample N=31,301 |
ICU N=8,185 |
Non-ICU N=23,116 |
p -value |
|---|---|---|---|---|
| Prior medical history | ||||
| HCC score prior to index hospital discharge | 2 (1.3) | 3 (1.4) | 2 (1.3) | 0.000 |
| Prior hospitalization | 40 | 40 | 40 | 0.944 |
| Prior stroke | 7 | 6 | 8 | 0.001 |
| Cardiac arrhythmias | 40 | 49 | 37 | 0.000 |
| Congestive heart failure | 25 | 28 | 23 | 0.000 |
| Chronic pulmonary disease | 20 | 22 | 19 | 0.000 |
| Diabetes mellitus | 23 | 23 | 22 | 0.846 |
| Diabetes mellitus, complicated | 8 | 7 | 8 | 0.264 |
| Hypertension | 75 | 75 | 75 | 0.711 |
| Fluid and electrolyte disorders | 25 | 27 | 24 | 0.000 |
| Valvular disease | 16 | 18 | 15 | 0.000 |
| Peripheral vascular disorders | 15 | 13 | 15 | 0.000 |
| Hypothyroidism | 14 | 13 | 14 | 0.035 |
| Solid tumor without metastasis | 13 | 13 | 13 | 0.975 |
| Deficiency anemias | 16 | 17 | 16 | 0.202 |
| Depression | 9 | 8 | 9 | 0.000 |
| Dementia | 24 | 19 | 25 | 0.000 |
| Concurrent cardiac event | 2 | 4 | 2 | 0.000 |
| Other comorbidity count | 0 (0.7) | 0 (0.7) | 0 (0.7) | 0.003 |
Values represent percents unless otherwise specified.
Crude mortality rates
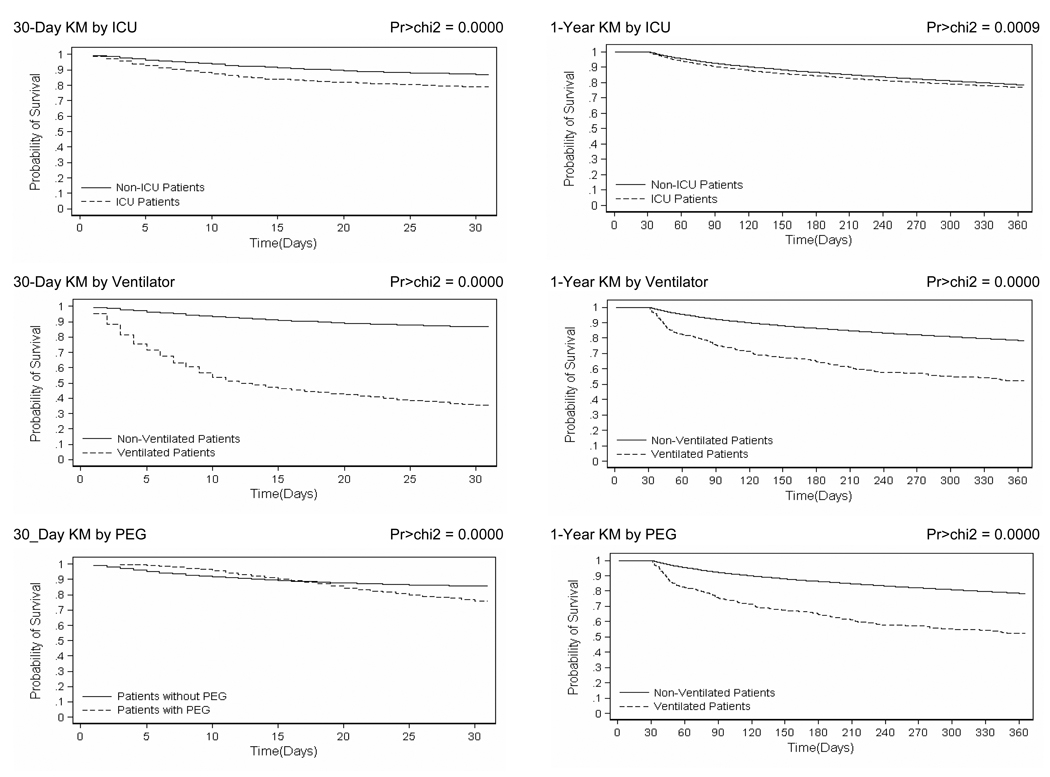
Figure 1 shows the Kaplan-Meier survival curves for patients admitted with ischemic stroke at 30 days and at 12 months, conditional upon 30-day survival. Absolute mortality rates are presented in Table 3. The overall death rate was 15% at 30 days and 34% at one-year follow-up. Crude 30-day mortality was significantly higher among ICU patients (21 % vs. 13%; log-rank test p<0.000), ventilated patients (65% vs. 14%; log-rank test p<0.000) and those with PEG tubes (24% vs. 15%; log-rank test p<0.000). The same effects were seen at 1 year for 30-day survivors; however, at 1 year, the effect of ICU admission was less pronounced (40% vs. 32%; log rank test p=0.0009) while that of mechanical ventilation (82% vs. 32%; log rank test p<0.000) and PEG placement (65% vs. 32%; log rank test p<0.000) were considerably more marked.
Figure 1.
Table 3.
Crude Mortality Rates
| Entire Sample N=31,301a |
ICU N=8,185 |
Non-ICU N=23,116 |
||||
|---|---|---|---|---|---|---|
| Mortality | nb | % | n | % | n | % |
| 30-Days | ||||||
| Mechanical Ventilation | ||||||
| Yes (N=1,071) | 696 | 65 | 557 | 64 | 139 | 71 |
| No (N=30,230) | 4,099 | 14 | 1,191 | 16 | 2,908 | 13 |
| PEG | ||||||
| Yes (N=2,195) | 526 | 24 | 196 | 22 | 330 | 26 |
| No (N=29,106) | 4,269 | 15 | 1,552 | 21 | 2,717 | 12 |
| Total | 4,795 | 15 | 1,748 | 21 | 3,047 | 13 |
| 1-year | ||||||
| Mechanical Ventilation | ||||||
| Yes (N=1,071) | 874 | 82 | 709 | 81 | 165 | 85 |
| No (N=30,230) | 9,752 | 32 | 2,539 | 35 | 7,213 | 31 |
| PEG | ||||||
| Yes (N=2,195) | 1,422 | 65 | 584 | 64 | 838 | 65 |
| No (N=29,106) | 9,204 | 32 | 2,664 | 37 | 6,540 | 30 |
| Total | 10,626 | 34 | 3,248 | 40 | 7,378 | 32 |
N - Denotes total number of patients in group/subgroup
n - Denotes number deceased
Adjusted hazard ratios
The results of Cox multivariable regression analysis are presented in Table 4. At 30 days, after adjustment for sociodemographic variables, comorbidities, the use of MV and PEG, the mortality hazard remained 29% higher in ICU patients (hazard ratio [HR] 1.29, 95% CI 1.18–1.40; p<0.000). The use of mechanical ventilation was associated with more than a 5-fold increase in mortality hazard (HR 5.59, 95% CI 4.93–6.34; p<0.000), while PEG insertion or revision had no impact (HR 0.96, 95% CI 0.85–1.07).
Table 4.
Mortality Risk Ratios in Patients with Acute Ischemic Stroke (N=31,301)
| 30-Day Mortality Risk Ratio | One-Year Mortality Risk Ratio in 30-Day survivors |
|||||
|---|---|---|---|---|---|---|
| Characteristic | HR | 95 % CI | p | HR | 95 % CI | p |
| ICU S tay | 1.29 | (1.18, 1.4) | 0.000 | 1.01 | (0.93, 1.09) | 0.869 |
| Mechanical Ventilation | 5.59 | (4.93, 6.34) | 0.000 | 1.88 | (1.57, 2.25) | 0.000 |
| Percutaneous Gastrostomy | 0.96 | (0.85, 1.07) | 0.434 | 2.59 | (2.38, 2.82) | 0.000 |
| Sociodemographic | ||||||
| 70–74 years | 1.09 | (0.92, 1.3) | 0.324 | 1.19 | (1.03, 1.37) | 0.019 |
| 75–79 years | 1.46 | (1.25, 1.7) | 0.000 | 1.52 | (1.33, 1.74) | 0.000 |
| 80–84 years | 1.85 | (1.59, 2.15) | 0.000 | 2.04 | (1.78, 2.34) | 0.000 |
| >85 years | 3.07 | (2.66, 3.54) | 0.000 | 3.05 | (2.67, 3.48) | 0.000 |
| Female | 1.03 | (0.95, 1.11) | 0.456 | 0.87 | (0.82, 0.93) | 0.000 |
| African-American | 0.68 | (0.6, 0.77) | 0.000 | 0.99 | (0.9, 1.08) | 0.793 |
| Other Race | 0.74 | (0.62, 0.89) | 0.002 | 0.91 | (0.76, 1.1) | 0.333 |
| Medicaid | 1.09 | (0.99, 1.2) | 0.065 | 1.10 | (1.01, 1.2) | 0.030 |
| HMO member | 1.08 | (0.94, 1.25) | 0.271 | 1.06 | (0.93, 1.21) | 0.359 |
| % in block group Below the Poverty Line |
0.91 | (0.61, 1.36) | 0.644 | 1.05 | (0.77, 1.44) | 0.768 |
| % adults ?25 years in block with College Degree |
0.89 | (0.72, 1.11) | 0.311 | 0.89 | (0.73, 1.08) | 0.234 |
| Prior Medical History | ||||||
| Prior Hospitalization | 1.05 | (0.97, 1.13) | 0.265 | 1.02 | (0.95, 1.09) | 0.669 |
| Prior stroke | 0.83 | (0.72, 0.94) | 0.004 | 0.93 | (0.83, 1.03) | 0.168 |
| Cardiac arrhythmias | 1.48 | (1.38, 1.59) | 0.000 | 1.17 | (1.09, 1.24) | 0.000 |
| Congestive heart failure | 1.43 | (1.33, 1.54) | 0.000 | 1.50 | (1.4, 1.61) | 0.000 |
| Chronic pulmonary disease | 1.05 | (0.96, 1.14) | 0.287 | 1.13 | (1.06, 1.22) | 0.000 |
| diabetes mellitus, uncomplicated | 1.18 | (1.09, 1.28) | 0.000 | 1.38 | (1.29, 1.47) | 0.000 |
| diabetes mellitus, complicated | 1.14 | (1.05, 1.23) | 0.002 | 1.18 | (1.1, 1.27) | 0.000 |
| Hypertension | 1.04 | (0.92, 1.17) | 0.559 | 1.12 | (1, 1.25) | 0.047 |
| Fluid and electrolyte disorders | 0.78 | (0.73, 0.83) | 0.000 | 0.83 | (0.78, 0.89) | 0.000 |
| Valvular disease | 1.41 | (1.31, 1.52) | 0.000 | 1.40 | (1.31, 1.5) | 0.000 |
| Peripheral vascular disorders | 0.84 | (0.77, 0.92) | 0.000 | 0.93 | (0.87, 1.01) | 0.072 |
| Hypothyroidism | 1.55 | (1.31, 1.83) | 0.000 | 1.38 | (1.15, 1.66) | 0.001 |
| Solid tumor without metastasis | 1.12 | (1.02, 1.23) | 0.019 | 1.20 | (1.11, 1.29) | 0.000 |
| Deficiency anemias | 1.02 | (0.94, 1.12) | 0.628 | 0.92 | (0.85, 1.01) | 0.069 |
| Depression | 1.02 | (0.93, 1.13) | 0.631 | 1.04 | (0.95, 1.13) | 0.439 |
| Dementia | 1.01 | (0.92, 1.1) | 0.898 | 1.18 | (1.08, 1.28) | 0.000 |
| Concurrent cardiac event | 0.88 | (0.79, 0.98) | 0.019 | 1.07 | (0.96, 1.18) | 0.213 |
| Other Comorbidity Count | 1.17 | (1.11, 1.23) | 0.000 | 1.25 | (1.2, 1.3) | 0.000 |
In those who survived the initial 30 days, however, ICU admission had no significant impact on 1-year mortality (HR 1.01; 95% CI 0.93–1.09; p=0.869). Mechanical ventilation still resulted in a higher risk of death, although with a decreased hazard ratio compared to the 30-day effect (HR 1.88; 95% CI 1.57–2.25; p<0.000). At this time, patients with a PEG tube had a 2.59 fold greater hazard of death than non-PEG patients (95% CI 2.38–2.82; p<0.000).
Age was associated with a gradually increasing risk of death, with those aged greater than 85 having more than a three-fold increase in mortality hazard, both at 30-days and 1-year, when compared to the baseline group of patients aged 65–70 years. Of the comorbidities examined, the strongest association with mortality was seen with arrhythmias, congestive heart failure (CHF), valvular heart disease and hypothyroidism. Increased risk of death was also seen in those who had concomitant COPD, diabetes mellitus, hypertension, solid tumors and dementia.
DISCUSSION
Summary of Study Findings
ICU admission was associated with increased 30-day mortality amongst older stroke patients requiring hospitalization in the United States. However, in 30-day survivors, ICU admission had no impact on long-term, 1-year mortality after adjustment for sociodemographic variables and comorbidities. Our data demonstrate that patients who received mechanical ventilation in the setting of acute ischemic stroke had a significantly higher probability of death, both in the short-term and at 1-year following the index hospitalization. By contrast, those who received a PEG did not have a higher 30-day mortality, but demonstrated a markedly increased risk of death at 1 year.
Explanation and relation to previous work
There are a number of indications for the admission of acute stroke patients to the ICU. Most commonly, these are deteriorating neurological status, the need for thrombolytic therapy, brainstem infarcts referable to the basilar artery and large, space-occupying hemispheric infarcts (31, 32). Other indications include fluctuating, hemodynamically induced infarction, multiple or septic emboli, serious cardiac dysrhythmias, and hemodynamic, respiratory or metabolic instabilities (33). These conditions may reflect a greater stroke severity in the acute setting or be the consequence of serious concomitant comorbidities that call for a higher level of care. Hence, the lower survival rate amongst ICU stroke patients, compared to stroke patients not requiring ICU care, is not unexpected. However, our study shows that short-term and 1-year mortalities for ICU stroke patients are both lower than those previously reported, being 21% and 40% respectively. Only two previous investigations have addressed the question of ICU stroke outcomes in the United States. Briggs et al in 2001 reported outcomes of mild to moderate stroke patients admitted to an ICU, but did not report mortality, whilst Rordorf and colleagues reported an in-hospital mortality of 21% in 63 consecutive stroke patients admitted to the ICU of a single tertiary care center (5, 8). A number of other investigations outside of the U.S. have been reported, mostly with very small patient numbers. The largest to date is by Navarrete-Navarro et al., who performed a multicenter, prospective observational study of 132 stroke patients admitted to ICUs in Spain (4). 74% of patients required mechanical ventilation. One-year mortality was 66% in the subgroup of patients (n=27) who had ischemic stroke. A larger study in Taiwan has been completed involving 850 stroke patients admitted to ICUs, 508 of whom suffered ischemic stroke (6). However, this study has very limited comparability to ours, given the much younger patient population and different care practices such as a strikingly longer average length of stay.
Mechanical ventilation is another important reason for ICU admission of stroke patients. Indications for MV in acute ischemic stroke include deteriorating level of consciousness, airway compromise, seizures and respiratory failure due to pneumonia, pulmonary edema (cardiogenic or non-cardiogenic) and pulmonary embolism (10, 15, 16, 21). Our study demonstrates that patients requiring mechanical ventilation experience a significantly higher mortality, both at 30 days and at 1 year, when compared to stroke patients not requiring MV. Most previous studies have focused on ICU or in-hospital mortality. However, short-term (≤ 3 months) mortality has been reported to be in the range 46 – 90% (7, 10, 11, 15, 16, 20, 34–37); this compares to a 30-day mortality in our sample of 65%. Older and smaller studies have generally reported higher mortality rates. The extent to which the changing practice of mechanical ventilation contributes to the observed decreasing trend in mortality remains uncertain. Aggregate 1-year mortality for mechanically ventilated patients in our study population also remained high at 82%. It has been argued that the need for mechanical ventilation in the setting of stroke is a proxy for coma (9). This would certainly be true if the indication for MV was always related to the stroke process. However, other ongoing process such as cardiac or respiratory failure or pulmonary embolism could also be markers for greater illness severity due to comorbidities and thus will be associated with a higher mortality rate. This may partially explain why the effect of MV is much less after 30 days.
Early reports in the 1990s suggested that stroke patients with dysphagia might have improved outcomes if they received enteral nutrition via a gastrostomy tube (38, 39). A single center cohort study in the U.S. looked at stroke patients with a PEG admitted to a rehabilitation facility (40). Mortality after a mean follow up of two years was 36%. However, a matched case-control study, also focusing on stroke patients at a rehabilitation center, showed that those with a PEG were at increased risk of complications and death (41). More recently, a multicenter randomized trial evaluated feeding policies in hospitalized patients with recent stroke (42). Three separate trials were conducted as part of this study. In one, the benefit of early tube feeding was assessed in 859 dysphagic stroke patients and revealed an absolute reduction of death rate of 5.8% at 6 months follow-up. In another trial, feeding via PEG was compared to a nasogastric tube. 329 patients were recruited; there was no statistically significant difference in the risk of death between the two groups (42). In our study, we have shown that there was no increased risk of death at 30 days in PEG patients (adjusted HR 0.96). If it is assumed that patients requiring a PEG have either a greater severity of dysphagia or a greater severity of stroke itself, then one might postulate that PEG offers a short-term survival advantage, since these patients might otherwise be expected to have a higher death rate. We believe, however, that our findings are the result of a selection effect, whereby the decision to place a PEG by the clinician staff, patients and families is made only in those who are deemed to have a reasonable chance of survival, at least in the short term. Certainly, this advantage is not evident at 12-month follow up, when the adjusted hazard ratio increased to 2.59. Thus in those who survive the initial 30 days, the need for a PEG appears to be a better predictor of long-term mortality.
The principal strength of our study lies in its generalizability to other older patients suffering from ischemic stroke in the United States by virtue of the size and geographical distribution of the sample. We were able to adjust for confounding factors, including comorbidities (43), illness severity (30) and sociodemographic variables. However, we did not have access to stroke-specific severity information such as infarct size and territory, the Glasgow Coma Scale or disability scores that have, in smaller studies, been shown to be predictors of mortality. The impact of specific therapeutics such as the use of thrombolytic agents could not be analyzed owing to lack of information on these interventions. Moreover, we did not have data on the functional status of survivors at 12 months. Such data would have been informative, in particular with regards to assessment of patients who did require ICU care or interventions such as MV and PEG.
CONCLUSIONS
Both short-term and long-term mortality in older patients with acute ischemic stroke admitted to ICUs is lower than previously reported. The same is true for patients who require mechanical ventilation and survive the initial phase of their illness. The need for PEG does appear to be a marker for poor long-term prognosis. Clearly, the care given to stroke patients during the initial hospitalization is related to their long-term outcome. Older age per se should, therefore, not be considered a contraindication to the delivery of intensive care in this setting. The course of events after hospital discharge is less well understood. In patients who require mechanical ventilation or percutaneous gastrostomy tubes, what clinical factors lead to the lower observed survival rate? Is there a greater incidence of aspiration pneumonia or cardiac failure, for example? Understanding the underlying causes will lead to identification of potentially reversible events, ultimately resulting in improved care and reduced death rates. Future research should focus on delineating such clinical factors, on stroke-specific predictors of long-term survival in patients requiring ICU care and on their functional outcomes.
Acknowledgements
This study was supported by a grant (R01-AG19747) from the National Institute of Aging. Additional support was provided by the Health Innovation Program and the Community-Academic Partnerships core of the University of Wisconsin Institute for Clinical and Translational Research (UW ICTR), grant 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health.
Footnotes
All work was performed at the University of Wisconsin School of Medicine and Public Health.
No reprints will be ordered.
The authors have no potential conflicts of interest to disclose.
REFERENCES
- 1.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics--2007 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Poalillo FE, Jimenez EJ, Falk J. Critical care in the United States of America. Crit Care Clin. 2006;22:447–455. doi: 10.1016/j.ccc.2006.03.013. ix. [DOI] [PubMed] [Google Scholar]
- 3.Fanshawe M, Venkatesh B, Boots RJ. Outcome of stroke patients admitted to intensive care: Experience from an Australian teaching hospital. Anaesth Intensive Care. 2002;30:628–632. doi: 10.1177/0310057X0203000515. [DOI] [PubMed] [Google Scholar]
- 4.Navarrete-Navarro P, Rivera-Fernandez R, Lopez-Mutuberria MT, et al. Outcome prediction in terms of functional disability and mortality at 1 year among ICU-admitted severe stroke patients: A prospective epidemiological study in the south of the European Union (Evascan Project, Andalusia, Spain) Intensive Care Med. 2003;29:1237–1244. doi: 10.1007/s00134-003-1755-6. [DOI] [PubMed] [Google Scholar]
- 5.Rordorf G, Koroshetz W, Efird JT, et al. Predictors of mortality in stroke patients admitted to an intensive care unit. Crit Care Med. 2000;28:1301–1305. doi: 10.1097/00003246-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Jeng J-S, Huang S-J, Tang S-C, et al. Predictors of survival and functional outcome in acute stroke patients admitted to the stroke intensive care unit. J Neurol Sci. doi: 10.1016/j.jns.2008.01.015. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 7.Handschu R, Haslbeck M, Hartmann A, et al. Mortality prediction in critical care for acute stroke: Severity of illness-score or coma-scale? J Neurol. 2005;252:1249–1254. doi: 10.1007/s00415-005-0853-5. [DOI] [PubMed] [Google Scholar]
- 8.Briggs DE, Felberg RA, Malkoff MD, et al. Should mild or moderate stroke patients be admitted to an intensive care unit? Stroke. 2001;32:871–876. doi: 10.1161/01.str.32.4.871. [DOI] [PubMed] [Google Scholar]
- 9.Horner RD, Sloane RJ, Kahn KL. Is use of mechanical ventilation a reasonable proxy indicator for coma among Medicare patients hospitalized for acute stroke? Health Serv Res. 1998;32:841–859. [PMC free article] [PubMed] [Google Scholar]
- 10.Berrouschot J, Rossler A, Koster J, et al. Mechanical ventilation in patients with hemispheric ischemic stroke. Crit Care Med. 2000;28:2956–2961. doi: 10.1097/00003246-200008000-00045. [DOI] [PubMed] [Google Scholar]
- 11.Bushnell CD, Phillips-Bute BG, Laskowitz DT, et al. Survival and outcome after endotracheal intubation for acute stroke. Neurology. 1999;52:1374–1381. doi: 10.1212/wnl.52.7.1374. [DOI] [PubMed] [Google Scholar]
- 12.Ely EW, Evans GW, Haponik EF. Mechanical ventilation in a cohort of elderly patients admitted to an intensive care unit. Ann Intern Med. 1999;131:96–104. doi: 10.7326/0003-4819-131-2-199907200-00004. [DOI] [PubMed] [Google Scholar]
- 13.Foerch C, Kessler KR, Steckel DA, et al. Survival and quality of life outcome after mechanical ventilation in elderly stroke patients. J Neurol Neurosurg Psychiatry. 2004;75:988–993. doi: 10.1136/jnnp.2003.021014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holloway RG, Benesch CG, Burgin WS, et al. Prognosis and decision making in severe stroke. JAMA. 2005;294:725–733. doi: 10.1001/jama.294.6.725. [DOI] [PubMed] [Google Scholar]
- 15.Leker RR, Ben-Hur T. Prognostic factors in artificially ventilated stroke patients. J Neurol Sci. 2000;176:83–87. doi: 10.1016/s0022-510x(00)00316-6. [DOI] [PubMed] [Google Scholar]
- 16.Mayer SA, Copeland D, Bernardini GL, et al. Cost and outcome of mechanical ventilation for life-threatening stroke. Stroke. 2000;31:2346–2353. doi: 10.1161/01.str.31.10.2346. [DOI] [PubMed] [Google Scholar]
- 17.Santoli F, De Jonghe B, Hayon J, et al. Mechanical ventilation in patients with acute ischemic stroke: Survival and outcome at one year. Intensive Care Med. 2001;27:1141–1146. doi: 10.1007/s001340100998. [DOI] [PubMed] [Google Scholar]
- 18.Schielke E, Busch MA, Hildenhagen T, et al. Functional, cognitive and emotional long-term outcome of patients with ischemic stroke requiring mechanical ventilation. J Neurol. 2005;252:648–654. doi: 10.1007/s00415-005-0711-5. [DOI] [PubMed] [Google Scholar]
- 19.Steiner T, Mendoza G, De Georgia M, et al. Prognosis of stroke patients requiring mechanical ventilation in a neurological critical care unit. Stroke. 1997;28:711–715. doi: 10.1161/01.str.28.4.711. [DOI] [PubMed] [Google Scholar]
- 20.Wijdicks EF, Scottx JP. Outcome in patients with acute basilar artery occlusion requiring mechanical ventilation. Stroke. 1996;27:1301–1303. doi: 10.1161/01.str.27.8.1301. [DOI] [PubMed] [Google Scholar]
- 21.Wijdicks EF, Scott JP. Causes and outcome of mechanical ventilation in patients with hemispheric ischemic stroke. Mayo Clin Proc. 1997;72:210–213. doi: 10.4065/72.3.210. [DOI] [PubMed] [Google Scholar]
- 22.Benesch C, Witter DM, Jr, Wilder AL, et al. Inaccuracy of the International Classification of Diseases (ICD-9-CM) in identifying the diagnosis of ischemic cerebrovascular disease. Neurology. 1997;49:660–664. doi: 10.1212/wnl.49.3.660. [DOI] [PubMed] [Google Scholar]
- 23.Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care. 2004;42:801–809. doi: 10.1097/01.mlr.0000132391.59713.0d. [DOI] [PubMed] [Google Scholar]
- 24.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341–378. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- 25.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 27.Pippenger M, Holloway RG, Vickrey BG. Neurologists' use of ICD-9CM codes for dementia. Neurology. 2001;56:1206–1209. doi: 10.1212/wnl.56.9.1206. [DOI] [PubMed] [Google Scholar]
- 28.Samsa GP, Bian J, Lipscomb J, et al. Epidemiology of recurrent cerebral infarction: A Medicare claims-based comparison of first and recurrent strokes on 2-year survival and cost. Stroke. 1999;30:338–349. doi: 10.1161/01.str.30.2.338. [DOI] [PubMed] [Google Scholar]
- 29.Fisher ES, Whaley FS, Krushat WM, et al. The accuracy of Medicare's hospital claims data: Progress has been made, but problems remain. Am J Public Health. 1992;82:243–248. doi: 10.2105/ajph.82.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pope GC, Kautter J, Ellis RP, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25:119–141. [PMC free article] [PubMed] [Google Scholar]
- 31.Andrews PJ. Prospects for acute stroke-- what can intensive care medicine offer? Intensive Care Med. 2003;29:1214–1217. doi: 10.1007/s00134-003-1840-x. [DOI] [PubMed] [Google Scholar]
- 32.Becker K. Intensive care unit management of the stroke patient. Neurol Clin. 2000;18:439–454. doi: 10.1016/s0733-8619(05)70201-2. [DOI] [PubMed] [Google Scholar]
- 33.Hacke W, Stingele R, Steiner T, et al. Critical care of acute ischemic stroke. Intensive Care Med. 1995;21:856–862. doi: 10.1007/BF01700973. [DOI] [PubMed] [Google Scholar]
- 34.Gujjar AR, Deibert E, Manno EM, et al. Mechanical ventilation for ischemic stroke and intracerebral hemorrhage: Indications, timing, and outcome. Neurology. 1998;51:447–451. doi: 10.1212/wnl.51.2.447. [DOI] [PubMed] [Google Scholar]
- 35.Burtin P, Bollaert PE, Feldmann L, et al. Prognosis of stroke patients undergoing mechanical ventilation. Intensive Care Med. 1994;20:32–36. doi: 10.1007/BF02425052. [DOI] [PubMed] [Google Scholar]
- 36.el-Ad B, Bornstein NM, Fuchs P, et al. Mechanical ventilation in stroke patients: Is it worthwhile? Neurology. 1996;47:657–659. doi: 10.1212/wnl.47.3.657. [DOI] [PubMed] [Google Scholar]
- 37.Grotta J, Pasteur W, Khwaja G, et al. Elective intubation for neurologic deterioration after stroke. Neurology. 1995;45:640–644. doi: 10.1212/wnl.45.4.640. [DOI] [PubMed] [Google Scholar]
- 38.Allison MC, Morris AJ, Park RH, et al. Percutaneous endoscopic gastrostomy tube feeding may improve outcome of late rehabilitation following stroke. J R Soc Med. 1992;85:147–149. [PMC free article] [PubMed] [Google Scholar]
- 39.Norton B, Homer-Ward M, Donnelly MT, et al. A randomized prospective comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding after acute dysphagic stroke. BMJ. 1996;312:13–16. doi: 10.1136/bmj.312.7022.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ickenstein GW, Stein J, Ambrosi D, et al. Predictors of survival after severe dysphagic stroke. J Neurol. 2005;252:1510–1516. doi: 10.1007/s00415-005-0906-9. [DOI] [PubMed] [Google Scholar]
- 41.Iizuka M, Reding M. Use of percutaneous endoscopic gastrostomy feeding tubes and functional recovery in stroke rehabilitation: A case-matched controlled study. Arch Phys Med Rehabil. 2005;86:1049–1052. doi: 10.1016/j.apmr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 42.Dennis M, Lewis S, Cranswick G, et al. FOOD: A multicentre randomized trial evaluating feeding policies in patients admitted to hospital with a recent stroke. Health Technol Assess. 2006;10:iii–iv. ix–x, 1–120. doi: 10.3310/hta10020. [DOI] [PubMed] [Google Scholar]
- 43.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]