Abstract
We previously have demonstrated that the colonic P-ATPase α subunit cDNA encodes an H,K-ATPase when expressed in Xenopus laevis oocytes. Besides its high level of amino acid homology (75%) with the Na,K-ATPase, the colonic H,K-ATPase also shares a common pharmacological profile with Na,K-ATPase, because both are ouabain-sensitive and Sch 28080-insensitive. These features raise the possibility that an unrecognized property of the colonic H,K-ATPase would be Na+ translocation. To test this hypothesis, ion-selective microelectrodes were used to measure the intracellular Na+ activity of X. laevis oocytes expressing various combinations of P-ATPase subunits. The results show that expression in oocytes of the colonic H,K-ATPase affects intracellular Na+ homeostasis in a way similar to the expression of the Bufo marinus Na,K-ATPase; intracellular Na+ activity is lower in oocytes expressing the colonic H,K-ATPase or the B. marinus Na,K-ATPase than in oocytes expressing the gastric H,K-ATPase or a β subunit alone. In oocytes expressing the colonic H,K-ATPase, the decrease in intracellular Na+ activity persists when diffusive Na+ influx is enhanced by functional expression of the amiloride-sensitive epithelial Na+ channel, suggesting that the decrease is related to increased active Na+ efflux. The Na+ decrease depends on the presence of K+ in the external medium and is inhibited by 2 mM ouabain, a concentration that inhibits the colonic H,K-ATPase. These data are consistent with the hypothesis that the colonic H,K-ATPase may transport Na+, acting as an (Na,H),K-ATPase. Despite its molecular and functional characterization, the physiological role of the colonic (Na,H),K-ATPase in colonic and renal ion homeostasis remains to be elucidated.
Various K-ATPase activities are present along the renal tubule. Recently, Doucet and coworkers (1) proposed that, in addition to the Na,K-ATPase, three other K-ATPases are expressed along the rat nephron. Type I is expressed in the collecting duct, and type II is in the proximal tubule and in the large loop of Henle of animals fed a standard diet; type III expression is induced by a low K+ diet and is restricted to the collecting duct, especially to the medullary collecting duct, similarly to the colonic K-ATPase isoform. By using the Xenopus laevis oocyte as heterologous expression system, we previously have shown that the colonic K-ATPase operates as a ouabain-sensitive and Sch 28080-insensitive H,K-ATPase (2), sharing common features with the pharmacological properties of the Na,K-ATPase. Besides its pharmacological profile, the colonic H,K-ATPase also shares molecular homologies with the members of the Na,K-ATPase gene family (3, 4). These observations raise the hypothesis that the colonic H,K-ATPase may transport other cations besides H+, in particular, Na+, which would result in an exchange of Na+ or H+ for K+. This hypothesis is supported by discrepancies between H+ and K+ fluxes mediated by the human homologue of the rat colonic H,K-ATPase, i.e., the ATP1AL1 H,K-ATPase, when expressed in HEK 293 cells, a nonepithelial human cell line (5).
In this study, our purpose was to determine whether the colonic H,K-ATPase could mediate Na+ transport without imposing drastic experimental conditions. For this purpose, we functionally expressed in X. laevis oocytes the colonic H,K-ATPase (and also the gastric H,K-ATPase, the Bufo marinus bladder H,K-ATPase and the B. marinus Na,K-ATPase), and subsequently measured the intracellular Na+ activity ([Na]i) in oocytes incubated in normal extracellular ionic environment. Our results show that the expression of colonic H,K-ATPase in oocytes decreases [Na]i (measured 2 days after cRNAs injection) under various conditions, but not when the colonic H,K-ATPase is inhibited. The results are consistent with the hypothesis that the colonic H,K-ATPase transports Na+ together with protons, resulting in an (Na,H),K-ATPase.
MATERIALS AND METHODS
cRNAs Synthesis and Expression in X. laevis Oocytes.
cRNAs of (i) the rat colonic (c) H,K-ATPase α subunit (3), (ii) the B. marinus Na,K-ATPase α1 and β1 subunits (10), (iii) the B. marinus bladder (bl) H,K-ATPase α and β subunits (6), (iv) the murine α subunit (7) and the rat β subunit (8) of the gastric (g) H,K-ATPase, and (v) the rat epithelial Na+ channel (rENaC) α, β, and γ subunits (9, 10) were synthesized by using the SP6 RNA polymerase (Promega). Oocytes were injected with either 10 ng of α subunit or 2 ng of β subunit of the various P-ATPase cRNAs, or 2 ng of the rENaC α, β, and γ subunit cRNAs, alone or in combination.
Various combinations of P-ATPase α and/or β subunits were expressed in oocytes: (i) control oocytes were injected with cRNAs coding for β1 or βg, and (ii) experimental oocytes were coinjected with the cRNAs coding for (a) the α1 and the β1 subunits, to express a ouabain-resistant Na,K-ATPase, (b) the αg and βg subunits, to express the ouabain-insensitive, Sch 28080-sensitive gastric H,K-ATPase, (c) the αbl and βbl subunits, to express the B. marinus bladder H,K-ATPase, or (d) the αc and βg or the αc and β1 subunits, to express the colonic H,K-ATPase.
Intracellular Ionic Activity Measurements.
Simultaneous measurements of membrane potential, Vm, and intracellular activity of Na+ ([Na]i) or protons (expressed as intracellular pH, pHi) were performed by using double-barreled (ion-selective and conventional) microelectrodes connected to a high impedance electrometer (WPI Instruments FD 223, Waltham, MA). Manufacture of microelectrodes and composition of their filling solutions have been described in detail elsewhere (11). In the present study, as in our previous work (2, 12), the Na+ ionophore 71176 and the H+ ionophore 95291 (Fluka) were used to measure [Na]i and pHi, respectively. Before use, microelectrodes were beveled on a microgrinder (De Marco Engineering, Geneva, Switzerland). Their slopes, S, were determined before any intracellular measurement by measuring the variation of potential induced by a decade change of concentration of Na+ or H+ in the extracellular (o) fluid; S was checked again after each puncture. Intracellular activity, A, of the ion i, Ai, was calculated from the following relation: Ai = Aref⋅ 10 (Vsel−Vm)/S, where Aref is the ionic activity (in mM) of ion i in the reference solution, and Vsel is the electrochemical potential difference (in mV) for i, measured by using the ion-selective barrel of the microelectrode.
For pH-microelectrodes, S was 55–58 mV (pH of the testing solutions: 7.4–8.4).
For Na+-selective microelectrodes, S was 51–57 mV when Nao was changed from 100 to 10 mM. In the latter solution, 90 mM KCl was added to maintain osmolarity and to take into account the slight interference of the high intracellular K+ activity value on [Na]i measurements (11). S fell to 35–40 mV in the following mixed solutions: 1 mM NaCl + 99 mM KCl vs. 10 mM NaCl + 90 mM KCl. No interference by H+ ions was found in pilot tests of Na+-selective microelectrodes, excluding the possibility that a change in pHi (resulting from H,K-ATPase expression) influences the measured [Na]i value.
Because 48 hr are needed for the functional expression of the colonic H,K-ATPase (2), measurements were performed 2 days after the injection of the various β or of α and β subunit cRNAs (as detailed above), the oocytes being incubated in amphibian Ringer’s solution containing: 85 mM NaCl, 1 mM KCl, 1 mM CaCl2, 1 mM MgCl2, buffered at pH 7.4 with N-tris[hydroxymethyl]methyl-2-aminoethane sulfonic acid (TES)/NaOH. This incubating solution was sometimes supplemented with 10 μM or 2 mM ouabain or 10−5 M 5-(N-ethyl-N-isopropyl) amiloride.
Voltage-Clamp Experiments.
To obtain current/voltage (I/V) curves, two microelectrodes filled with 3 M KCl were inserted into a single oocyte. Increments of 20 mV from holding potential were imposed by using a Gene Clamp amplifier (Axon Instruments, Foster City, CA), using the software program pclamp (Axon Instruments).
Results are given as means ± SEM. The significance of the results was assessed by unpaired t test and were considered significant for a P value < 0.05.
RESULTS AND DISCUSSION
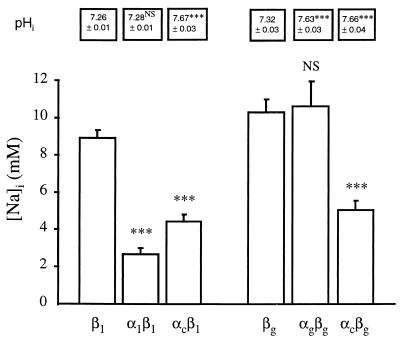
In our first experimental series, [Na]i and pHi were measured in control (β1 or βg) oocytes and in oocytes expressing various K-ATPases. Oocytes coinjected with cRNAs coding for αg and βg, αc and βg or αc and β1 exhibited more alkaline pHi values than control (β1 or βg) oocytes or oocytes coinjected with α1 and β1 cRNAs (see Fig. 1), thus confirming the functional expression of the gastric and colonic H,K-ATPases. As shown in Fig. 1, in α1β1 oocytes, expressing the B. marinus Na,K-ATPase, [Na]i was significantly lower than in the control β1 oocytes (P < 0.001). Similarly, oocytes expressing the colonic H,K-ATPase (either as the αcβ1 or the αcβg heterodimer) exhibited a significant decrease in [Na]i compared with the control (β1 or βg) oocytes (P < 0.001, Fig. 1). It previously was reported (13), then debated (14), that in vesicules Na+ may substitute for H+ in the gastric H,K-ATPase reaction; Fig. 1 indicates that the expression of the gastric H,K-ATPase did not affect [Na]i when compared with control (βg) oocytes. In this first experimental series, a low [Na]i value also was observed in oocytes expressing the B. marinus bladder H,K-ATPase (2.4 ± 0.2 mM, n = 10). Thus, our results show a reduction of oocyte [Na]i not only by expression of B. marinus Na,K-ATPase but also by expression of colonic or bladder H,K-ATPases.
Figure 1.
[Na]i and pHi in X. laevis oocytes expressing various P-ATPase subunits. Oocytes were injected with various P-ATPase subunit cRNAs, as indicated below the bars. After a 2-day incubation of the oocytes in amphibian Ringer’s solution, [Na]i was measured by using intracellular Na+-selective microelectrodes: results are expressed as mean ± SE; n = 6–25 oocytes, from 2–6 independent experiments. Under the same experimental conditions, pHi was measured by using intracellular pH-selective microelectrodes: results are expressed as mean ± SE; n = 4–7 oocytes, from 2–3 independent experiments. Statistics were performed compared with control (β1 or βg expressing-oocytes) [Na]i value or pHi value. Statistical significance: NS, not significant; ∗∗∗, P < 0.001.
[Na]i results from a balance between Na+ efflux and Na+ influx. The increase of active Na+ efflux induced by the expression of the B. marinus Na,K-ATPase reduces [Na]i (Fig. 1). For the other oocytes presenting a reduced [Na]i as compared with control (β1 or βg) oocytes, in particular for oocytes expressing the colonic H,K-ATPase (on which we will focus the remainder of this study), it is not possible from this first experimental series to decide whether the low [Na]i value reflects an increase of Na+ efflux or a decrease of Na+ influx.
A tentative explanation of the low [Na]i value observed in oocytes expressing the colonic H,K-ATPase would be that in these oocytes the intracellular alkalinization (consecutive to H,K-ATPase expression) tends to reverse the direction of net ionic fluxes mediated by the inherent Na-H exchanger, resulting in Na+ efflux, thus lowering [Na]i. This possibility is contradicted by our observation that the intracellular alkalinization of oocytes expressing the gastric H,K-ATPase was not accompanied by any decrease in [Na]i compared with that of oocytes expressing βg alone (see Fig. 1). Moreover, in experiments in which oocytes expressing the colonic H,K-ATPase were incubated in a Ringer’s solution supplemented with 10−5 M 5-(N-ethyl-N-isopropyl) amiloride (to inhibit the Na-H exchanger), [Na]i was 4.4 ± 0.3 mM (n = 9), not different from the previously measured [Na]i value (4.9 ± 0.3 mM, n = 25, P > 0.4). Also, we calculated from measurements of [Na]i and pHi in colonic H,K-ATPase-expressing oocytes (4.9 ± 0.3 mM, n = 25, and 7.66 ± 0.04, n = 4, respectively) that the chemical gradients for Na+ and H+ were +65.9 ± 1.6 mV and −15.1 ± 2.6 mV, respectively, thus favoring ionic fluxes in the usual direction for Na-H exchange. We conclude that the low [Na]i value observed in oocytes expressing the colonic H,K-ATPase is not related to a putative Na+ efflux via the Na-H antiport.
Next, we considered the possibility that diffusive Na+ entry might be reduced in oocytes expressing the colonic H,K-ATPase, resulting in the observed decrease in [Na]i. Diffusional Na+ influx depends on two parameters: (i) the Na+ electromotive force (EMF), and (ii) the membrane partial conductance for Na+. A decrease of either one in oocytes expressing the colonic H,K-ATPase would result in reduced diffusive Na+ influx. Calculation of Na+ EMF from paired Vm and [Na]i measurements (first experimental series) showed that it was significantly higher (P < 0.001) in oocytes expressing colonic H,K-ATPase (−80.3 ± 3.4 mV, n = 25) than in control (βg) oocytes (−67.5 ± 4.2 mV, n = 11). Because the membrane partial Na+ conductance could not be evaluated from the previous results, and consequently its possible decrease could not be excluded, we decided to solve the problem by expressing the rENaC in oocytes.
To overwhelm the oocyte membrane Na+ conductance, we injected oocytes with cRNAs coding for the three subunits (α, β, and γ) of rENaC. The expression of rENaC was verified in voltage-clamp experiments: an amiloride-sensitive current was found only in the oocytes injected with the cRNAs coding for rENaC subunits. In oocytes expressing rENaC, [Na]i was greatly increased: from the reversal potential (13.3 ± 2.5 mV, n = 7) of the amiloride-sensitive current induced by rENaC expression, [Na]i was calculated to be 38.8 ± 3.9 mM, n = 7. As a check, [Na]i also was measured by using Na+-selective microelectrodes and found to be 38.6 ± 1.9 mM, n = 10. These results indicate that the expression of rENaC results in higher [Na]i because of increased diffusional Na+ influx. This increased Na+ influx is caused by increased membrane conductance for Na+ and not by an increased Na+ EMF: from 10 paired Vm and [Na]i measurements in rENaC-expressing oocytes, Na+ EMF was calculated to be −50.6 ± 2.0 mV, i.e., lower than in control βg oocytes (−67.5 ± 4.2 mV, n = 11). Finally, from I/V curves, we verified that oocytes coexpressing both the colonic H,K-ATPase and rENaC exhibited similar amiloride-sensitive Na+ currents over the range of imposed voltages, and thus had a Na+ conductance similar to that of oocytes expressing only rENaC (data not shown). At this point, it was appropriate to test whether the reduced [Na]i measured in oocytes expressing the colonic H,K-ATPase was caused by a reduction in diffusive Na+ entry into the cell.
Subsequently, we coinjected cRNAs coding for the rENaC α, β, and γ subunits and cRNAs coding for (i) βg, (ii) β1, (iii) α1 and β1 (to express the B. marinus Na,K-ATPase), (iv) αc and βg (to express the colonic H,K-ATPase), or (v) αg and βg (to express the gastric H,K-ATPase). Table 1 shows that all [Na]i values were higher in these oocytes expressing rENaC than in oocytes of the first set of experiments (see Fig. 1), reflecting the increase in the passive Na+ influx. Coexpression of β1, βg, or αgβg with rENaC did not affect [Na]i, as compared with the expression of rENaC alone (Table 1), but oocytes coexpressing the colonic H,K-ATPase (αcβg) and rENaC, like oocytes expressing the B. marinus Na,K-ATPase and rENaC, exhibited a significantly lower [Na]i value (P < 0.001, Table 1). From the Na+ EMF values (calculated from paired Vm and [Na]i measurements), we see that in oocytes coexpressing the colonic H,K-ATPase and rENaC the low [Na]i value could not simply reflect a decreased Na+ diffusive influx, because (i) Na+ EMF is not reduced but on the contrary is significantly increased compared with that of oocytes expressing rENaC alone or coexpressing rENaC and the βg subunit (Table 1), and (ii) the membrane Na+ conductance previously was shown to be similar in oocytes coexpressing rENaC and the colonic H,K-ATPase than in oocytes expressing rENaC alone (see above). We conclude that oocytes expressing the colonic H,K-ATPase exhibit a lower [Na]i value because of increased Na+ efflux. This efflux is necessarily active, because it occurs against the Na+ EMF.
Table 1.
Intracellular Na+ activities and electromotive force for Na+ across the cell membrane in X. laevis oocytes expressing the rENaC and various α and/or β P-ATPase subunits
| Injected cRNAs | [Na]i, mM | Na+ electromotive force, mV | n |
|---|---|---|---|
| rENaC | 38.6 ± 1.9 | −50.6 ± 2.0 | 10 |
| rENaC + βg | 34.7 ± 3.7NS | −48.7 ± 4.7NS | 5 |
| rENaC + β1 | 33.9 ± 1.7NS | −45.1 ± 1.9NS | 9 |
| rENaC + αgβg | 35.5 ± 1.9NS | −49.4 ± 1.6NS | 7 |
| rENaC + α1β1 | 9.4 ± 0.7*** | −57.6 ± 0.7*** | 14 |
| rENaC + αcβg | 17.8 ± 1.3*** | −57.0 ± 1.1*** | 11 |
Oocytes were coinjected with rENaC α, β, and γ subunits cRNAs alone or with βg, β1, αgβg, α1β1, and αcβg subunit cRNAs. After a 2-day incubation in amphibian Ringer’s solution, [Na]i and Vm were measured by using intracellular Na+-selective microelectrodes. The electrochemical potential for Na+ across the oocyte membrane was calculated as Vm − ENa, where Vm is the measured membrane potential difference, and ENa is the equilibrium potential for Na+, calculated from extracellular Na+ concentration (using an activity coefficient of 0.75) and from measured [Na]i. Results are expressed as mean ± SE. n, number of oocytes from 2-3 independent experiments. Statistics were performed by unpaired t-test referenced to values in rENaC-expressing oocytes. Statistical significance: NS, not significant; ∗∗∗, P < 0.001.
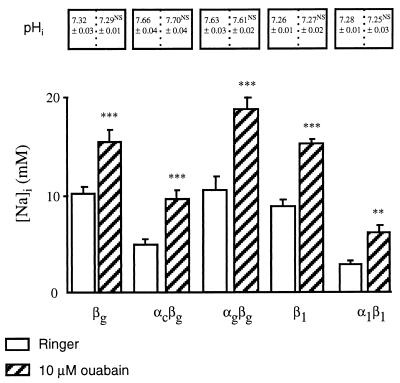
It remained to establish whether the low [Na]i value observed in colonic H,K-ATPase-expressing oocytes was caused by increased Na+ efflux via the endogenous Na,K-ATPase or via the exogenous colonic pump. The possible activation of the endogenous Na,K-ATPase thanks to the functional association of the exogenous β subunit with the endogenous oocyte Na,K-ATPase α subunit (15) was considered first. This Na,K-ATPase heterodimer is inhibited by 10 μM ouabain (16), a concentration that does not inhibit the colonic H,K-ATPase or the B. marinus Na,K-ATPase (2, 15). We did not observe any acute effect of ouabain on [Na]i (results not shown), possibly because of the large volume of the oocyte (about 1 μl) that would “buffer” the effect of acute changes in Na+ fluxes on [Na]i, as suggested in ref. 17. Thus, we measured [Na]i and pHi in oocytes after their incubation in the presence of 10 μM ouabain for 48 hr. From results (shown in Fig. 2) it can be seen that the presence of 10 μM ouabain did not modify pHi. In this experimental condition, [Na]i was higher than in the absence of ouabain, consistent with inhibition of the endogenous Na,K-ATPase. The observed [Na]i increase is less than that reported in other studies in which oocytes were incubated in a K+-free, Ca2+-free, and ouabain-free medium, which allowed [Na]i increase to about 35 mM (17) or 80 mM (18). These discrepancies may be because the different experimental conditions. When incubated in 10 μM ouabain, oocytes expressing either the B. marinus Na,K-ATPase or the rat colonic H,K-ATPase still exhibited significantly lower [Na]i values (6.1 ± 0.4 mM, n = 8, and 9.9 ± 0.9 mM, n = 10, respectively) than those of the control (βg) oocytes or the oocytes expressing the gastric H,K-ATPase (15.6 ± 1.2 mM, n = 11 and 18.9 ± 1.2 mM, n = 11, respectively). The persistence of a lower [Na]i in oocytes expressing the colonic H,K-ATPase than in oocytes expressing βg or the gastric H,K-ATPase rules out the hypothesis that activation of the endogenous Na,K-ATPase could account for the [Na]i decrease observed in oocytes expressing the colonic H,K-ATPase.
Figure 2.
Effect of 10 μM ouabain on [Na]i and pHi in X. laevis oocytes expressing various P-ATPase subunits. Oocytes were injected with various P-ATPase subunit cRNAs, as indicated below the bars. [Na]i was measured by using intracellular Na+-selective microelectrodes after a 2-day incubation of oocytes in a Ringer’s solution (empty bars), or in a Ringer’s solution supplemented with 10 μM ouabain (hatched bars). Results are expressed as mean ± SE; n = 6–25 oocytes, from 2–6 independent experiments. Under the same experimental conditions, pHi was measured by using intracellular pH-selective microelectrodes: results are expressed as mean ± SE; n = 4–7 oocytes, from 2–3 independent experiments. Statistics were performed compared with [Na]i or pHi values measured in oocytes incubated in the Ringer’s solution. Statistical significance: ∗∗, P < 0.01; ∗∗∗, P < 0.001.
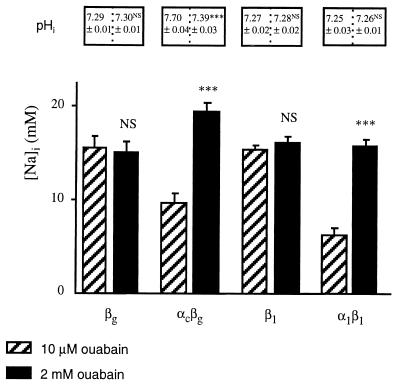
Finally, we tested our initial hypothesis that Na+ is actively transported by the colonic H,K-ATPase, as it is by the B. marinus Na,K-ATPase. In this case, addition to the incubation medium of 2 mM ouabain, a concentration high enough to block the colonic H,K-ATPase (2) and the B. marinus Na,K-ATPase (15), would raise [Na]i in oocytes expressing these pumps and would acidify pHi only in oocytes expressing the colonic H,K-ATPase. We performed an experimental series in which control (βg or β1) oocytes or oocytes expressing the colonic H,K-ATPase or the B. marinus Na,K-ATPase were incubated in the presence of 2 mM ouabain. Results are summarized in Fig. 3. Compared with results after 10 μM ouabain incubation, incubation in a solution containing 2 mM ouabain did not further affect [Na]i in control (βg or β1) oocytes. However, incubation in the presence of 2 mM ouabain significantly increased [Na]i in oocytes expressing the colonic H,K-ATPase or the B. marinus Na,K-ATPase, compared with their respective [Na]i values observed under a 10-μM ouabain incubation. The increase in [Na]i measured in colonic H,K-ATPase-expressing oocytes (and in B. marinus Na,K-ATPase-expressing oocytes) after incubating them with 2 mM ouabain is consistent with the hypothesis that, like the Na,K-ATPase, the colonic H,K-ATPase has a direct role in Na+ extrusion.
Figure 3.
Effect of 2 mM ouabain on [Na]i and pHi in X. laevis oocytes expressing various P-ATPase subunits. Oocytes were injected with various P-ATPase subunit cRNAs, as indicated below the bars. [Na]i was measured by using intracellular Na+-selective microelectrodes after a 2-day incubation of oocytes in a Ringer’s solution supplemented with 10 μM ouabain (hatched bars, from results presented in Fig. 2), or a Ringer’s solution supplemented with 2 mM ouabain (filled bars). Results are expressed as mean ± SE; n = 6–25 oocytes, from 2–6 independent experiments. Under the same experimental conditions, pHi was measured by using intracellular pH-selective microelectrodes: results are expressed as mean ± SE; n = 6–7 oocytes, from two independent experiments. Statistics were performed compared with [Na]i or pHi values measured in oocytes incubated in the Ringer’s solution supplemented with 10 μM ouabain. Statistical significance: NS, not significant; ∗∗∗, P < 0.001.
To further support this conclusion, we looked for a relation between the decrease in [Na]i and the extracellular K+ concentration in oocytes expressing the colonic H,K-ATPase. To this end, in a separate experimental series, we measured [Na]i in the presence of 10 μM ouabain (to inhibit the endogenous Na,K-ATPase) and in the presence of various concentrations of extracellular K+, i.e., 0, 1, or 5 mM of K+. In this experimental series, incubating the oocytes in a K+-free medium containing 10 μM ouabain would prevent ionic exchange by the endogenous Na,K-ATPase or by the colonic H,K-ATPase, whereas in the presence of 5 mM K+ and 10 μM ouabain the colonic H,K-ATPase would be almost fully activated [K1/2 for K+ is 730 μM, and Ki for ouabain is 970 μM in the presence of 5 mM K+ (2)], whereas the endogenous Na,K-ATPase would be inhibited. [Na]i values obtained on oocytes incubated in K+-free solution are in good agreement with values measured in control (βg) oocytes in the presence of ouabain 10 μM (see Fig. 2). Regarding the external K+ dependency of [Na]i, our results clearly show that in the presence of 10 μM ouabain: (i) In control (βg) oocytes, [Na]i is not dependent on the presence of external K+, because measured [Na]i were (in mM), 19.4 ± 1.1, n = 6; 15.6 ± 1.2, n = 11; 18.9 ± 1.9, n = 6, in the absence of external K+ and in the presence of 1 and 5 mM K+, respectively; and (ii) in αcβg oocytes, [Na]i decreases as external K+ concentration is raised; measured [Na]i were 15.8 ± 0.7 mM (n = 11) in the nominal absence of K+, 8.7 ± 0.7 mM (n = 6) in the presence of 1 mM K+ (P < 0.001), and 4.1 + 0.2 mM (n = 13) in the presence of 5 mM K+ (P < 0.001). Despite this strong relationship between [Na]i and extracellular K+, an increase in [Na]i (induced by coexpression of rENaC) did not affect the 86Rb transport mediated by the colonic H,K-ATPase as tested in a separate experimental series (data not shown). Such lack of effect also was reported for the human ATP1AL1 H,K-ATPase, when expressed in HEK cells and could be caused by a very high affinity of the pump for Na+ (5). Analysis of the Na+ stimulation kinetics of the colonic H,K pump would require measurement of a Na+-dependent and K+-dependent ATPase activity in an appropriate experimental system.
In conclusion, our data are consistent with the exchange of Na+ for K+ by the colonic H,K-ATPase, resulting in an (Na,H),K-ATPase. This property may be common to the three currently characterized H,K-ATPases of the nongastric H,K-ATPase α subunit gene subfamily, because a low [Na]i value also was observed in oocytes expressing the B. marinus bladder H,K-ATPase (present study), and transport of Na+ was suggested for the human ATP1AL1 H,K-ATPase (5). It is known that under suitable conditions of intracellular and extracellular ionic concentrations, the Na,K-ATPase activity may sustain not only forward or backward Na+/K+ exchanges, but also Na+/Na+ or K+/K+ exchanges (19), and it should be noticed that the experiments performed in the present study were performed in standard conditions. In very special experimental conditions, the Na,K-ATPase and the gastric H,K-ATPase have been reported to be activated by analogs different from the substrates usually translocated during the pump cycle. In the presence of a K+-free and low Na+ external medium, the Na,K-ATPase was reported to carry H+ in its E2 conformation (20). Concerning the gastric H,K-ATPase, Na+ recently was reported to behave as K+, but not as H+ (14), whereas a “H+-like” effect of Na+ previously was suggested when [H+] is < 10−8 M (13). Our data suggest, however, that Na+ transport through the colonic pump occurs in standard conditions that may be relevant for studying its physiological role. The K+-dependence of [Na]i values in oocytes expressing the rat colonic H,K-ATPase and the discrepancies between proton and 86Rb fluxes mediated by the human ATP1AL1 H,K-ATPase (with 10-fold more K+ to be transported than H+) (5) suggest that Na+ transport by these P-ATPases is coupled to K+. Therefore, we propose to name the nongastric H,K-ATPases (Na,H),K-ATPases rather than H,K-ATPases.
The physiological relevance of Na+ transport by the colonic (Na,H),K-ATPase remains to be established. Because of its apical localization in the renal medullary collecting duct as well as in the distal colon (21), the colonic (Na,H),K-ATPase would allow Na+ extrusion into the renal tubular lumen and into the digestive tract, but one should expect that the colocalization of a Na+ entry pathway(s) in the apical membrane would prevent net Na+ secretion and result in net transepithelial K+ absorption only. Thus, the role of the colonic (Na,H),K-ATPase in Na+ homeostasis is not obvious at present. In the kidney, the colonic (Na,H),K-ATPase recently has been reported to be localized in the apical membrane of the principal cells in the outer medullary collecting duct of K+-restricted rats (21). The expression of an apical ouabain-sensitive pump in the distal part of the nephron postulated more than 25 years ago by Giebisch and coworkers (22, 23) might represent the colonic (Na,H),K-ATPase. Translocation of Na+ in exchange for K+ by the colonic (Na,H),K-ATPase may account for the apical Na,K-ATPase activity reported by Hayashi and Katz (24) in the medullary collecting duct of K+-depleted rats. The colonic (Na,H),K-ATPase, however, may be different from the renal type III K-ATPase (1), because type III K-ATPase is highly sensitive to both ouabain and Sch 28080, whereas the colonic (Na,H),K-ATPase is moderately ouabain-sensitive but Sch 28080-insensitive, at least when expressed in X. laevis oocytes (2). Moreover, Buffin-Meyer et al. (1) showed that Na+ is as potent as K+ for type III-K-ATPase activation, suggesting that Na+ can compete with K+ for its binding site. These discrepancies might be explained by the physiological association of a different β subunit to αc in the native cells. However, as have others (25), we did not observe any effect of the β subunit isoform on the colonic (Na,H),K-ATPase functional properties (present data and data not shown). The colonic (Na,H),K-ATPase could not account for the ouabain-resistant H,K-ATPase activity that has been shown to be modulated by metabolic acidosis (26), nor could it be implicated in intercalated cell proton transport, because the colonic (Na,H),K-ATPase is not expressed in intercalated cells (21) and has different pharmacological properties from those of the H,K-ATPase described in intercalated cells (27). Therefore, the role of the colonic (Na,H),K-ATPase in acid-base balance also appears to be minor.
In summary, based on its functional properties, its cellular and subcellular localization, and the metabolic regulation of its renal expression, the physiological role of the colonic (Na,H),K-ATPase appears to be devoted to K+ homeostasis. Further studies especially in genetically modified animals (28) and in polarized cells expressing in an inducible way the colonic (Na,H),K-ATPase are needed to discover whether this is its unique role. The results presented here on Na+ transport by the colonic (Na,H),K-ATPase are a basis for initiating studies on the potential physiological role of this pump.
Acknowledgments
We thank M.S. Crowson and G.E. Shull for the kind gift of the full-length cDNA coding for the α subunit of the colonic P-ATPase. We are indebted to B.C. Rossier for providing us with the rENaC α, β, γ, and bladder Na,K-ATPase α and β subunits cDNAs, and to J.B. Lingrell for providing the rat Na,K-ATPase β1 subunit cDNA. We also thank J.D. Horisberger for reading a previous version of this manuscript, A. Edelman for his critical suggestions, S.R. Thomas for helpful comments, and T. Anagnostopoulos for his continuous encouragement during this study. M.C. is supported by a fellowship from the Société Française de Néphrologie, and P.B. is supported by a fellowship from the Association pour la Recherche contre le Cancer.
ABBREVIATIONS
- [Na]i
intracellular Na+ activity
- rENaC
rat epithelial Na+ channel
- pHi
intracellular pH
- EMF
electromotive force
References
- 1.Buffin-Meyer B, Younes-Ibrahim M, Barlet-Bas C, Cheval L, Marsy S, Doucet A. Am J Physiol. 1997;272:F124–F131. doi: 10.1152/ajprenal.1997.272.1.F124. [DOI] [PubMed] [Google Scholar]
- 2.Cougnon M, Planelles G, Crowson M S, Shull G E, Rossier B C, Jaisser F. J Biol Chem. 1996;271:7277–7280. doi: 10.1074/jbc.271.13.7277. [DOI] [PubMed] [Google Scholar]
- 3.Crowson M S, Shull G E. J Biol Chem. 1992;267:13740–13748. [PubMed] [Google Scholar]
- 4.Jaisser F, Coutry N, Farman N, Binder H J, Rossier B C. Am J Physiol. 1993;265:C1080–C1089. doi: 10.1152/ajpcell.1993.265.4.C1080. [DOI] [PubMed] [Google Scholar]
- 5.Grishin A V, Bevensee O M, Modyanov N N, Rajendran V, Boron W F, Caplan M J. Am J Physiol. 1996;271:F539–F551. doi: 10.1152/ajprenal.1996.271.3.F539. [DOI] [PubMed] [Google Scholar]
- 6.Jaisser F, Horisberger J D, Geering K, Rossier B C. J Cell Biol. 1993;123:1421–1429. doi: 10.1083/jcb.123.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathews P M, Claeys D, Jaisser F, Geering K, Horisberger J D, Krahenbuhl J M, Rossier B C. Am J Physiol. 1995;268:C1207–C1214. doi: 10.1152/ajpcell.1995.268.5.C1207. [DOI] [PubMed] [Google Scholar]
- 8.Shull G E. J Biol Chem. 1990;265:12123–12126. [PubMed] [Google Scholar]
- 9.Canessa C M, Horisberger J D, Rossier B C. Nature (London) 1993;361:467–470. doi: 10.1038/361467a0. [DOI] [PubMed] [Google Scholar]
- 10.Canessa C M, Schild L, Buell G, Thorens B, Gautschi I, Horisberger J D, Rossier B C. Nature (London) 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 11.Anagnostopoulos T, Planelles G. J Physiol (London) 1987;393:73–89. doi: 10.1113/jphysiol.1987.sp016811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cougnon M, Bouyer P, Hulin P, Anagnostopoulos T, Planelles G. Pflügers Arch. 1996;431:658–667. doi: 10.1007/BF02191917. [DOI] [PubMed] [Google Scholar]
- 13.Polvani C, Sachs G, Blostein R. J Biol Chem. 1989;264:17854–17859. [PubMed] [Google Scholar]
- 14.Swarts H G P, Klaasen C H, Schuurmans Stekhoven F M A H, De Pont J J H H M. J Biol Chem. 1995;270:7890–7895. doi: 10.1074/jbc.270.14.7890. [DOI] [PubMed] [Google Scholar]
- 15.Jaisser F, Canessa C M, Horisberger J D, Rossier B C. J Biol Chem. 1992;267:16895–16903. [PubMed] [Google Scholar]
- 16.Canessa C M, Horisberger J D, Louvard D, Rossier B C. EMBO J. 1992;11:1681–1697. doi: 10.1002/j.1460-2075.1992.tb05218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lafaire A V, Schwarz W. J Membr Biol. 1986;91:43–51. doi: 10.1007/BF01870213. [DOI] [PubMed] [Google Scholar]
- 18.Vasilets L A, Ohta T, Noguchi S, Kawamura M, Schwarz W. Eur Biophys J. 1993;21:433–443. doi: 10.1007/BF00185871. [DOI] [PubMed] [Google Scholar]
- 19.Bahinski A, Nakao M, Gadsby D C. Proc Natl Acad Sci USA. 1988;85:3412–3416. doi: 10.1073/pnas.85.10.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Horisberger J D. Amer J Physiol. 1995;268:C590–C595. doi: 10.1152/ajpcell.1995.268.3.C590. [DOI] [PubMed] [Google Scholar]
- 21.Sangan P, Rajendran V M, Mann A S, Kashgarian M, Binder H J. Am J Physiol. 1997;272:C685–C696. doi: 10.1152/ajpcell.1997.272.2.C685. [DOI] [PubMed] [Google Scholar]
- 22.Wiederholt M, Sullivan W J, Giebisch G. J Gen Physiol. 1971;57:495–525. doi: 10.1085/jgp.57.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strieder N, Khuri P, Wiederholt M, Giebisch G. Pflügers Arch. 1974;349:91–107. doi: 10.1007/BF00586621. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi M, Katz A. Am J Physiol. 1987;252:F437–F446. doi: 10.1152/ajprenal.1987.252.3.F437. [DOI] [PubMed] [Google Scholar]
- 25.Codina J, Kone B C, Delmasmata J T, Dubose T D. J Biol Chem. 1997;271:29759–29763. doi: 10.1074/jbc.271.47.29759. [DOI] [PubMed] [Google Scholar]
- 26.Eiam-Ong S, Kurtzman N A, Sabatini S. J Clin Invest. 1993;91:2385–2392. doi: 10.1172/JCI116471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silver R B, Mennitt P A, Saltin L M. Am J Physiol. 1996;270:F539–F547. doi: 10.1152/ajprenal.1996.270.3.F539. [DOI] [PubMed] [Google Scholar]
- 28.Meneton P, Schulthels P J, Greeb J, Nieman M L, Liu L H, Clarke L L, Duffy J J, Doetschman T, Lorenz J N, Shull G E. J Clin Invest. 1998;101:536–542. doi: 10.1172/JCI1720. [DOI] [PMC free article] [PubMed] [Google Scholar]