Abstract
Antisense technology holds tremendous potential in the research and clinical settings. However, successful delivery of antisense oligodeoxynucleotides (ODNs) to the intracellular site of action requires the passage of many barriers, including survival against extracellular serum nucleases and escape from endolysosomal degradation. Previous work has shown that the effectiveness of antisense delivery by the cationic liposome, dioleoyl-3-trimethylammonium-propane (DOTAP), is enhanced substantially by the incorporation of a pH-sensitive polymer, poly (propylacrylic acid) (PPAA), in serum-free media. To improve this system for application in serum-containing media conditions, PPAA was modified in this work by grafting onto it either poly(ethylene oxide) (PEO) or a more hydrophobic analog, poly (oxyalkylene amine), known as Jeffamine. The ternary formulation of DOTAP/ODN/PPAA-g-Jeffamine resulted in 8-fold increased uptake of fluorescently-labeled ODNs compared to DOTAP/ODN/PPAA and ∼80% silencing of green fluorescent protein (GFP) expression in CHO-d1EGFP cells treated in the presence of 10% FBS-containing media. In contrast, the carrier systems that contained PPAA or PPAA-g-PEO failed to display any significant antisense activity in the presence of serum, even though all of the delivery systems displayed moderate to high levels of antisense activity in serum-free conditions. The results reveal that the carrier system with the Jeffamine graft copolymer effectively mediates specific gene silencing in the presence of serum, while the system with the PEO graft copolymer fails to do so. While the pH-dependent lytic functionality of PPAA was found to be lost upon grafting with PEO or Jeffamine, the hydrophobicity of the latter was sufficient to mediate cellular internalization and endosomal escape. Thus, the PPAA-g-Jeffamine copolymers hold substantial promise as agents for controlled therapeutic delivery of antisense oligonucleotides.
Keywords: graft copolymers, gene silencing, delivery vector, poly(oxyalkylene amines), endosomal escape
1. Introduction
The ability of exogenously administered oligonucleotides to mediate antisense gene silencing was discovered nearly 30 years ago [1]. Ever since, this technology has been utilized as a research tool to study gene function, and it has been developed for therapeutic applications in cancer, autoimmune and cardiovascular diseases, wound healing, and viral infections [2]. The mechanism of antisense in a cell involves the introduction of a short, single-stranded oligodeoxynucleotide (ODN) molecule that binds to its complementary mRNA in a sequence-specific fashion, thereby causing degradation of the target mRNA strand and silencing of the gene [3]. Although the immense potential of antisense as a therapy is evident by the numerous ongoing clinical trials, relatively few have reached Phase II/III stage [4, 5], and to date only one antisense therapeutic, Vitravene, has been approved by the FDA.
Progress in the field of antisense therapeutics requires improvement in the systemic and cellular delivery of ODNs. Some of the barriers at the systemic level include survival against unfavorable interactions with serum proteins present in the bloodstream, avoidance of accumulation in non-target organs such as the lung, liver and kidney, and targeting of the diseased or infected cells [6]. Once the ODN molecules overcome these barriers, they must maneuver their way into the target cells and finally to the target mRNA within the cell. Some of the antisense delivery challenges at the cellular level include efficient entry into the cell, escape of ODNs from the degradative lysosomes, and finally the release of ODNs into the cytoplasm [7, 8]. While viral vectors are being used for ODN and gene delivery, safety concerns persist. Although iterative design of non-viral vectors has endowed them with attributes for overcoming some of the systemic and cellular barriers in the delivery of nucleic acid therapeutics [9-11], they still suffer from low efficiency and high cytotoxicity.
Non-viral carriers such as cationic polymers, micelles, and liposomes have been studied extensively for their ability to bind with and deliver anionic antisense ODNs and short interfering RNA (siRNA) molecules into cells. Liposomes are composed of phospholipid bilayers that are similar to biological membranes, initiating intimate contact with and entry into cells. Polymers, on the other hand, can be tailored with chemical functionalities for biological tasks. The work presented here combines features from both systems through the use of hydrophobically modified, environmentally protective graft copolymers to enhance liposome-mediated delivery of antisense ODNs.
It has been established that a major barrier to the intracellular delivery of ODNs is their sequestration in endosomes, which eventually fuse with lysosomes, thereby leading to degradation of their contents. To overcome this barrier, the pH-sensitive anionic polyelectrolyte, poly (propylacrylic acid) (PPAA), was used as an adjunct to ODN carrier systems. PPAA has been shown to lyse membranes at endosomal pH (∼5-6), while leaving membranes unperturbed at physiological pH (∼7.2) [12]. This functionality of PPAA improved significantly the in vitro transfection efficiency of liposome-based complexes containing plasmid DNA [13]. Similarly, our research group has demonstrated successful in vitro antisense ODN delivery by utilizing PPAA in complexes of DOTAP/ODN. However, the delivery efficiency of these ternary complexes containing PPAA is reduced in treatment conditions involving serum-containing media [14].
Our strategy to improve the performance of the DOTAP/ODN/PPAA delivery system in the presence of serum involved modifying the chemistry of PPAA by grafting onto it hydrophilic poly(ethylene oxide), PEO, or poly(oxyalkylene amines), Jeffamine. PEO is known for its ability to increase the stability of drug and nucleic acid delivery vectors by providing protection against serum nuclease degradation and removal by the reticuloendothelial system [15-17]. We hypothesized that the introduction of a PPAA-grafted copolymer chain consisting of both EO and hydrophobic propylene oxide (PO) could improve the cell membrane penetration capability of complexes, while retaining the protective properties of the PEO that are required to provide stability in serum. Previous work by Kabanov and coworkers has demonstrated successful ODN delivery using poly(ethylene imine) (PEI) conjugated to Pluronics®, which are triblock copolymers in a PEO-PPO-PEO configuration [18, 19]. In this study we present a comparison of the effects of PEO- and Jeffamine-grafted PPAA copolymers on ODN delivery and antisense activity of ODNs targeted to silence a short half-life green fluorescent protein (d1EGFP) in a stably expressing Chinese hamster ovary (CHO) cell line.
2. Materials and methods
2.1. Materials
A phosphorothioate oligodeoxynucleotide tagged with Cy5 (5′-/5Cy5/TTG TGG CCG TTT ACG TCG CC- 3′) was used for physical and biological studies. The presence of the Cy5 tag did not significantly alter the delivery of ODNs into cells or their ability to bind with mRNA and achieve an antisense effect [20]. This 20-mer oligonucleotide (ODN) (EGFP157), previously selected for down regulation of d1EGFP, was used to assess the degree of silencing or antisense effect. The ODNs were obtained from Integrated DNA Technologies (Coralville, IA) and delivered as HPLC grade. Before use, lyophilized ODNs were resuspended in phosphate buffer saline (PBS) (Invitrogen, Carlsbad, CA) at pH 7.2 to obtain a stock concentration of 100 μM.
The cationic liposomal formulation, N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium methyl sulphate (DOTAP), was purchased from Roche Applied Science (Indianapolis, IN). Lipofectamine2000 was purchased from Invitrogen and used as directed. Poly(α-propylacrylic acid) (PPAA) (Mn = 27kDa) was purchased from Polymer Source (Montreal, Canada). PEO monomethyl ether (MW=5kDa) was purchased from Fluka. 1-(3-dimethylamnopropyl)-3-ethyl-carbodiimide (EDCI) was purchased from Kawaguchi Chemical Industry Co., Ltd. (Tokyo, Japan). 4-(dimethylamino) pyridinium 4-toluenesulfonate (DPTS) was synthesized following a published procedure [21]. Jeffamine M-2070 (MW=2kDa, EO/PO=31/10) was a gift from Huntsman International, LLC (Woodlands, TX).
PPAA polymer, which was received as a dried powder, was solubilized in 0.1 N NaOH in PBS (pH 13). The Jeffamine M-2070 grafted PPAA copolymer (PPAA-g-Jeffamine) was solubilized in a NaOH-PBS formulation (pH 12.5) and further diluted in PBS. The PEO grafted PPAA copolymer (PPAA-g-PEO) was solubilized directly in PBS (pH 7.4). Solutions of DOTAP, ODN and polymers were stored at 4°C and vortexed prior to use. All other reagents and solvents were purchased from Sigma-Aldrich (St. Louis, MO), unless noted otherwise. All buffers were prepared in MilliQ ultrapure water and filtered (0.22 μm) prior to use.
2.2. Synthesis of graft copolymers
The synthesis procedure of the graft copolymers was similar to previously described methods for the synthesis of similar polyacrylate-grafted PEO and Jeffamine comb copolymers [22, 23]. For the PPAA-g-PEO copolymer, 200 mg PPAA was added to 5 mL DMSO along with 11 mg 4-(dimethylamino) pyridinium 4-toluenesulfonate (DPTS), and a slight molar excess of PEO monomethyl ether required to achieve the target graft ratio. The mixture was stirred for 30 min at room temperature, after which 41 mg 1-(3-dimethylamnopropyl)-3-ethyl-carbodiimide (EDCI) was added. The reaction was allowed to proceed at room temperature and driven to completion with subsequent 100 mg aliquots of EDCI added on days 4, 9, and 11. The reaction mixture was then transferred to a Slide-A-Lyzer cassette with 10 kDa MW cut off and dialyzed exhaustively against deionized water. The dialyzed solution was then lyophilized.
For the PPAA-g-Jeffamine copolymer, the same synthesis, dialysis and lyophilization protocols that were used for the PPAA-g-PEO copolymer were followed, with the exception that Jeffamine M-2070 replaced the PEO monomethyl ether and 1-hydroxy-1H-benzotriazole (HOBt) was used as the catalyst in place of DPTS. The extent of PEO and Jeffamine M-2070 grafting onto PPAA backbones was determined by NMR spectroscopy, which was performed on a Varian 400 MHz spectrometer. The percentage of grafting was calculated by the ratio of the integrated peak areas of the methylene protons in the grafted chains to the methyl protons in PPAA. For the PPAA-g-PEO: 1H NMR (DMSO-d6) δ 0.80 (s, CH3), 0.95-2.0 (m, br, CH2(PPAA), 2.10(br), 2.17 (br), 2.29 (br), 2.35, 2.41, 2.64, 2.77, 2.9-3.3 (br), 3.2 (OCH3), 3.29 (t), 3.47 (s, -OCH2CH2O-), 3.65 (t), 4.10 (br, C(O)OCH2CH2OPEO), 4.6-4.8 (br), 4.9-5.2 (br), 7.05 (br), 8.45 (br). For the PPAA-g-Jeffamine: 1H NMR (DMSO-d6) δ 0.82 (s, CH3), 1.0-2.0 (br, CH2), 2.10, 2.17, 2.2-3.0 (br), 2.9-3.0 (br), 3.2 (OCH3), 3.24-3.42 (m, CH+CH2 (PPO), 3.47 (s, -OCH2CH2O-), 3.50, 3.51, 3.52, 3.65 (t), 4.6-4.8 (br), 5.0-5.3 (br), 5.50 (s), 7.08 (d), 7.45-7.60 (br), 7.66 (C(O)NH).
Conventional gel permeation chromatography was used to monitor the progress of the reactions and determine the final molecular weight and polydispersity of the graft copolymers. This was performed with the Waters 510 HPLC unit equipped with a Waters 410 Differential Refractometer, a 5 μm PL gel precolumn, and two PL gel columns (pore size 103-105 Angstroms) that have been calibrated with polystyrene standards by using DMF containing 0.1% trifluoroacetic acid as the mobile phase at a flow rate of 0.8 mL min-1.
2.3. Vector preparation
The delivery vectors were self-assembled from their components by electrostatic interactions, first between the cationic DOTAP liposomes and anionic ODN, and then between the DOTAP/ODN complexes and the anionic polyelectrolytes (PPAA, PPAA-g-PEO or PPAA-g-Jeffamine). Complexes were prepared using a DOTAP/ODN weight ratio of 10:1, which corresponded to a charge ratio of 4.7 (+/-). The net charge ratio is defined as the ratio of the moles of DOTAP amine groups to the sum of the moles of ODN phosphate groups and PPAA carboxylic acid groups. The DOTAP working concentration was 20 μg/ml (as recommended by Roche). All DOTAP/ODN/polyelectrolyte complexes were formed by mixing equal volumes of DOTAP and ODN, followed by incubation for 30 minutes at room temperature. Polyelectrolyte was then added to the DOTAP/ODN solution to produce the desired net charge ratio, and incubated for an additional 30 minutes at room temperature. DOTAP and ODN were assumed to be completely ionized (100%), while the carboxylic acids of PPAA were assumed to be 33% ionized at pH 7.4 based on its pKa value [24]. This assumption was also used to determine the ionization degrees of the carboxylic acid groups in PPAA-g-PEO and PPAA-g-Jeffamine copolymers. LipofectAMINE 2000 (Invitrogen, Carlsbad, CA), the control delivery agent, was complexed to ODN in a weight ratio of 2:1. The ratio of complex volume to buffer/media volume was maintained constant at 1:4.
2.4. Particle sizing and zeta potential
Complexes were prepared by first mixing DOTAP and ODN solutions (final ODN concentration 300nM, DOTAP/ODN charge ratio 4.7), followed by the addition of polyelectrolyte (PPAA, PPAA-g-PEO or PPAA-g-Jeffamine) to yield a net charge ratio within the range of 3 to 0.25. All solutions of DOTAP, ODN and polymers were diluted in PBS. The ratio of complex volume to buffer (PBS, Opti-MEM, or MEM) volume was maintained at 1:4. Complexes were analyzed using a Malvern Instruments Zetasizer Nano ZS-90 instrument (Southboro, MA) with reproducibility being verified by collection of sequential measurements. DLS measurements were performed at a 90° scattering angle at 25°C. DLS and z-average sizes were collected and analyzed immediately after the formation of DOTAP/ODN/Polyelectrolyte complexes (total incubation time of 60 minutes).
2.5. Hemolysis
The pH-dependant membrane-disruptive functionality of PPAA and the grafted polymers was assessed using a hemolysis assay [12]. Stock solutions of polymers were vortexed thoroughly to ensure complete solubility, and dilutions of the polymer were prepared fresh. Phosphate buffers, in the pH range 5.5–7.0, and citrate buffer of pH 5.0, were prepared by titration of 100 mM sodium mono and diphosphate and 100 mM sodium citrate, respectively, to achieve the appropriate pH values. Solutions of PPAA, PPAA-g-PEO and PPAA-g-Jeffamine were added to pH buffers 5.0, 5.5, 6.0, 6.5 and 7.0 at 40, 240 and 400 μg/ml and vortexed thoroughly. These various amounts of polymer corresponded to equivalent moles of carboxylic acid groups. To these polymer solutions, fresh RBCs that had been washed three times with 100 mM NaCl were added at a concentration of 108 cells/ml, incubated in a waterbath at 37°C for 1 hr, and then centrifuged for 4 min at 400 g to pellet the intact RBCs. The absorbance of the supernatant (541 nm) was determined on a UV spectrophotometer (Thermo Spectronic). Experimental controls included RBCs in pH buffers in the absence of polymer (negative control) and RBCs in distilled water (positive control). The percentage of hemolysis was determined using the formula below. Each test was performed in triplicate.
| (1) |
2.6. Pyrene assay
Pyrene has been used as a hydrophobic fluorescent probe in polymeric solutions to assess environmental polarity [25, 26]. Stock solutions of all tested polymers were prepared in either a basic solution of NaOH or PBS, depending on their solubility. The polymer stock solutions were diluted with phosphate buffer solution of various pHs from 5.0 to 7.0 to yield a final polymer concentration of 1 mg/ml. Pyrene was added to each sample at a final concentration of 10-7 M. Emission spectra of pyrene was recorded on a spectrofluorometer (excitation at 335 nm, Shimadzu corporation, NJ, USA) at room temperature and the intensities of emission peaks at 382 and 392 nm were recorded.
2.7. Cell culture
The preparation of Chinese hamster ovary (CHO-K1) cells stably integrated with destabilized EGFP (d1EGFP) transgene has been described previously [14, 20]. The CHO-d1EGFP cell line was maintained in F-12K medium (Kaighn's modification of Ham's F-12; ATCC, Manassas, VA) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA), 100 U/mL penicillin (Invitrogen, Carlsbad, CA) and 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA). The cell type was maintained under constant selective pressure by using G418 antibiotic (500 μg/ml). Cells were cultivated in a humidified atmosphere at 5% CO2 and 37°C.
2.8. Oligonucleotide delivery/gene silencing
Cells were split at approximately 70% confluence and seeded onto 12-well plates (Fisher, Suwanee, GA) at 105cells/ml (with 1 ml volume per well) ∼18 h prior to ODN treatment. For cellular uptake and antisense studies, cells were treated with Cy5-labeled antisense ODN (targeting d1EGFP) at a final ODN concentration of 300 nM per well. In the case of treatment, 200 μl of complexes were prepared as described in the ‘Vector preparation’ section, mixed with either Opti-MEM reduced serum (Invitrogen, Carlsbad, CA) or 10% FBS-containing medium and added to each well. For control samples, complexes were substituted with 200 μl of PBS. After 4 hours of cell exposure to treatment, medium containing complexes was aspirated and replaced by fresh medium. Cells were assayed for Cy5- ODN uptake and GFP activity 24 hrs post-ODN treatment using fluorescence activated cell sorting (FACS).
Cells were prepared for FACS analysis first by washing with PBS buffer, followed by the addition of trypsin-EDTA (Invitrogen, Carlsbad, CA) to remove cells from the plate surface. Immediately after cell detachment, cell culture medium was added to neutralize the trypsin. Next, cells were collected in pellet form by centrifugation for 3.5 min at 200 g, resuspended in 150 μl of PBS and maintained on ice until the time of analysis. Cells were analyzed for size (side scatter), granularity (forward scatter), intensity of Cy5 fluorescence (FL4 channel) and intensity of GFP (FL1 channel). Geometric mean fluorescence intensities for 10,000 cells were determined on the FACS Calibur three-laser flow cytometer (BD Biosciences). CellQuest software was used to acquire and analyze results. The degree of silencing was calculated from the GFP fluorescence of treatment samples normalized to control (untreated cells). The background GFP fluorescence from cells lacking d1EGFP plasmid was negligible.
2.9. Cytotoxicity assay
CHO-d1EGFP cells were seeded onto a 96-well plate and treated with 20 μL of complexes, prepared according to methods described above, and mixed with 80 μL of 10% FBS-containing media. After 4 hours of exposure to treatment, complexes were aspirated from wells and cells were replenished with fresh media. After 24 hours, 20 μL of MTS reagent (Promega, Madison, WI) was added to 100 μL of media, per the manufacturer's protocol, and cells were incubated for a period of two hours under humidified conditions. The reduction of MTS tetrazolium into a colored formazan product by the cells was quantified colorimetrically to represent cell metabolic activity. Metabolic activity was calculated from recording the absorbance (at 490 nm) of treatment samples normalized to control (untreated cells).
2.10. Statistics
Groups from gene silencing and cell viability experiments were compared using the one-way ANOVA test, and results were represented as mean ± standard deviation. Pair-wise comparisons between the various polymer-containing delivery systems were made using a Tukey HSD post-hoc test; a p-value less than 0.05 indicated significant difference compared with the control, as indicated by asterisks on the figures.
3. Results
3.1. Synthesis and characterization of graft copolymers
The set of copolymers was synthesized by grafting either poly (ethylene oxide) monomethyl ether (PEO, MW=5 kDa) or Jeffamine M-2070 monomethyl ether (Jeffamine, MW=2 kDa) onto the backbone of poly(propylacrylic acid) (PPAA, MW=27 kDa). The reactions were performed using carbodiimide coupling to yield graft copolymers with target graft densities from 1 to 25 mole % (Scheme 1). The grafting reactions were confirmed by 1H NMR spectroscopy and GPC. The NMR spectra of PPAA-g-PEO copolymer contained chemical shifts expected for methylene protons of the PEO ester moiety formed by reaction of the PPAA carboxylic acids with the primary alcohol of PEO (4.1 ppm). In the case of PPAA-g-Jeffamine, the spectra contained the amide proton (NH) that is formed by reaction of PPAA carboxylic acid groups with the primary amine of Jeffamine (7.6 ppm). The GPC chromatograms of the isolated products also revealed the disappearance of the starting materials and the appearance of new multimodal peaks at high molecular weight.
Scheme 1.
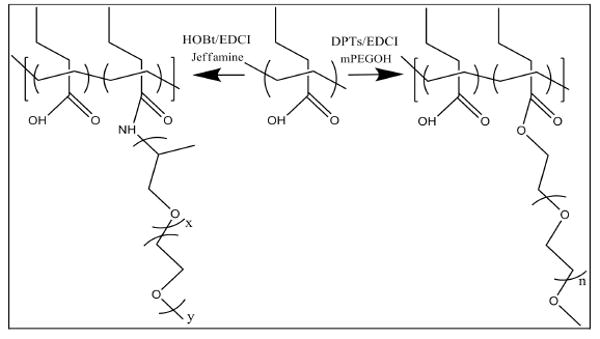
Synthesis of graft copolymers: PPAA-g-PEO and PPAA-g-Jeffamine. A fraction of the carboxylic acid groups on the PPAA backbone were reacted with the end group of PEO/Jeffamine chains to form ester/amide bonds, respectively.
Only a few of the graft copolymers from the initial set synthesized were soluble in aqueous buffer, even when NaOH was added to enhance solubility. Hence, the physical and biological studies presented here are limited to a comparison of two similar graft copolymers that were soluble in aqueous buffer: 1) PPAA-g-PEO containing 21 mole% grafting of the 5 kDa PEO monomethyl ether, total number-average molecular weight of 57 kDa, and polydispersity index of 1.4; and, 2) PPAA-g-Jeffamine containing 25 mole% grafting of the 2 kDa Jeffamine M-2070, total number-average molecular weight of 58 kDa, and polydispersity index of 1.9. These two graft copolymers provided us with sufficient structural similarities to evaluate the relative effects of PEO and Jeffamine chains on the delivery of ODNs to cells in the presence of serum-containing treatment conditions.
3.2. Physical characteristics of DOTAP/Polymer/ODN complexes
At the DOTAP/ODN charge ratio of 4.7 used in this work, over 80% of ODN is encapsulated, and this degree of association is not diminished significantly by addition of PPAA, PPAA-g-PEO or PPAA-g-Jeffamine (Supplementary Data, Fig. S1). Furthermore, the ternary polyelectrolyte complexes formed stable particles in PBS, Opti-MEM or serum-containing MEM with sizes ranging from 215-300 nm, and no statistically significant effect of the added polymers was observed (Supplementary Data, Fig. S2). Since PPAA and its graft copolymers are charged species, their incorporation into DOTAP/ODN complexes was monitored by measuring changes in their zeta potential. A progressive decrease was observed in the zeta potential of DOTAP/ODN complexes upon addition of increasing amounts of anionic polymer or graft copolymer (Fig. 1). Furthermore, as might be expected, the zeta potential of these complexes is close to 0 mV when the net charge of the polyelectrolyte mixture was neutral (N/P = 1). In the cases of PPAA and PPAA-g-Jeffamine, the decrease in zeta potential was marked compared to PPAA-g-PEO. Overall, this result confirms the integration of these polymers into DOTAP/ODN complexes.
Figure 1.
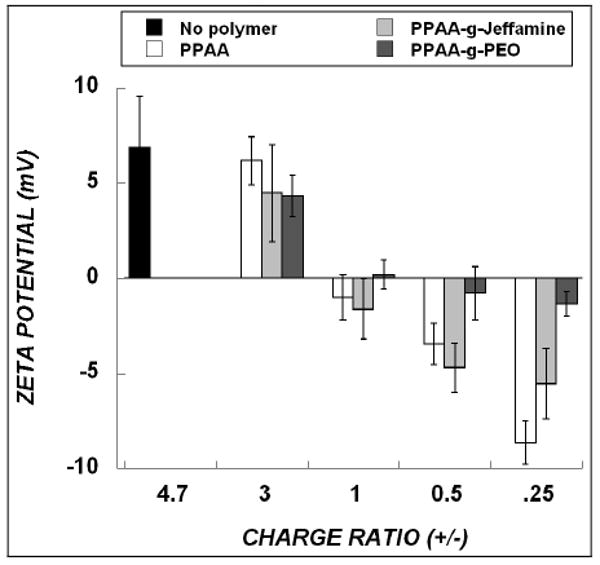
Zeta potential of DOTAP/ODN complexes in the absence of (“No polymer”) or presence of the indicated polyelectrolyte. The theoretical charge ratio of DOTAP/ODN was constant at 4.7. Addition of increasing amount of polymer to DOTAP/ODN complexes is indicated by charge ratios from 3 (lowest polymer concentration) to 0.25 (highest polymer concentration). Data represent mean ± s.d. (n=3).
3.3. pH-dependent physical characteristics of polymers
Previous work has correlated the ability of poly(alkylacrylic) acids to mediate intracellular endosomal escape with their pH-dependent hemolytic properties [12, 13]. To understand the effect of alkylene oxide grafting on the lytic properties of PPAA, the ability of PPAA, PPAA-g-PEO and PPAA-g-Jeffamine to disrupt erythrocyte (RBC) membranes was evaluated by quantifying the release of hemoglobin from RBCs spectroscopically. Solutions of PPAA, PPAA-g-PEO and PPAA-g-Jeffamine were prepared in pH buffers ranging from 5.0 to 7.0 at polymer concentrations of 40, 240 and 400 μg/ml, respectively, which corresponded to equivalent moles of carboxylic acid groups in the polymers. PPAA produced significant hemolytic activity between the pH of 5.5 and 6.5, with maximum effect at pH 6.0 (which is the pH of endosomes) and a minimum hemolytic effect at pH 5.0 and 7.0 (Fig. 2), consistent with previous work by Murthy et al. [12]. In comparison, the graft copolymers, PPAA-g-PEO and PPAA-g-Jeffamine, displayed low levels of hemolytic activity throughout the pH range of 5.0 to 7.0.
Figure 2.
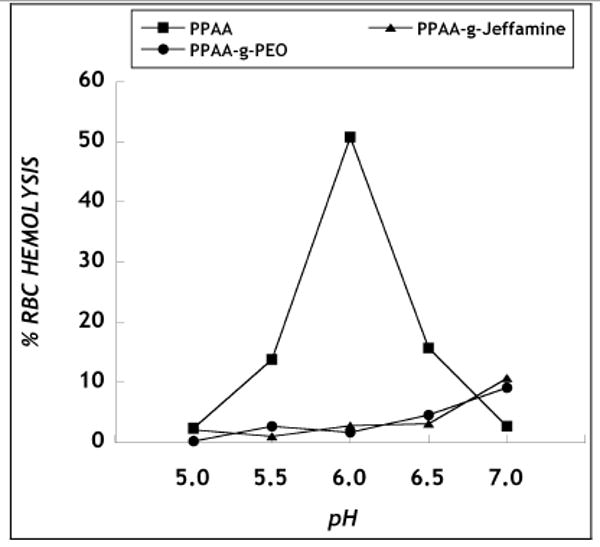
Hemolysis induced by PPAA and the graft copolymers. The concentration of PPAA, PPAA-g-PEO and PPAA-g-Jeffamine present in the pH buffers was 40, 240 and 400 μg/ml, respectively, corresponding to equivalent moles of carboxylic acid groups. RBC hemolysis induced by polymer was normalized to control (distilled water). Data represent mean ± s.d. (n=3).
The environmental polarity of the polymers used in this work was monitored by measuring the steady-state fluorescence of pyrene. The emission spectrum of pyrene displays peaks at 371 and 392 nm, and an additional peak at 382 nm that appears only in the presence of a hydrophobic environment. The ratio of pyrene emission intensity at 382 nm to that at 392 nm was used to quantify the degree of polymer hydrophobicity in the various pH environments. The peak at 382 nm was absent for pyrene solution in the absence of polymer (control) and for pyrene solutions in the presence of PEO (ungrafted) or Jeffamine (ungrafted), which each failed to display any hydrophobicity throughout the pH range. PPAA binding of pyrene is consistent with a conformational shift from an expanded, hydrophilic coiled polymer at neutral pH to a more compact, globular structure at acidic pH [25]. The pH value at which the ratio of I382/I392 is a maximum was 5.5 (Fig. 3). This pH-dependent hydrophobic effect of PPAA is responsible for its hemolytic activity at acidic pH (Fig. 2). The graft copolymers, PPAA-g-PEO and PPAA-g-Jeffamine, also resulted in pyrene emission enhancement, however, independent of the pH environment. Throughout the pH range, PPAA-g-Jeffamine demonstrated greater hydrophobicity compared to PPAA-g-PEO, consistent with the presence of hydrophobic propylene oxide groups in PPAA-g-Jeffamine.
Figure 3.
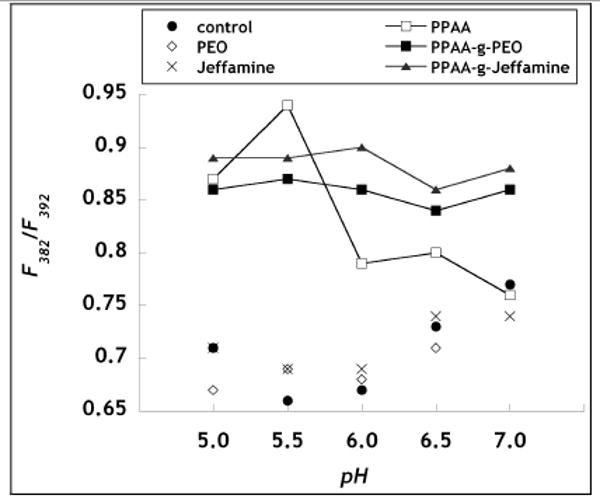
Pyrene assay of hydrophobicity. The ratio of pyrene fluorescence intensity at 382 nm to that at 392 nm was measured for polymer solutions incubated in the various pH buffers. Polymer concentration was 1 mg/ml. Control refers to buffer only. Standard deviations are within 10%.
3.4. Intracellular ODN delivery and gene silencing
The biological activity of the ODN complexes was assessed in CHO cells that stably express the gene encoding d1EGFP. The short intracellular half-life of the protein encoded by pd1EGFP gene provides a tight temporal coupling between pd1EGFP gene silencing at the mRNA level and fluorescence of GFP protein [14]. The intracellular delivery of ODN molecules into cells and the gene silencing effects were quantitatively determined from the fluorescence of Cy5-labeled ODN and d1EGFP expression, respectively, using flow cytometry. Under serum-free media conditions, DOTAP mediated sufficient ODN delivery that produced moderate silencing of d1EGFP, but the inclusion of PPAA mediated greater silencing of GFP expression (Fig. 4). Substitution of PPAA with PPAA-g-Jeffamine produced somewhat less gene silencing and with PPAA-g-PEO significantly less (Fig. 4) (p-value < 0.05 for PPAA-g-PEO vs. PPAA). Nonetheless, in serum-free medium, all of the delivery systems resulted in at least moderate levels of gene silencing.
Figure 4.
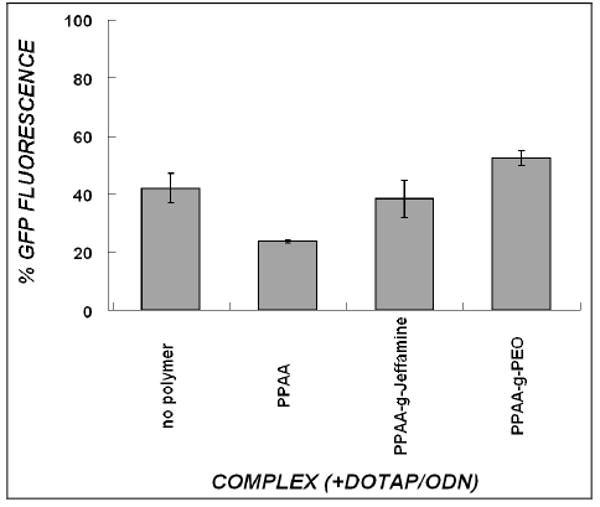
Silencing of target d1EGFP expression by various DOTAP/ODN formulations in serum-free medium. CHO-d1EGFP cells received 300nM ODN treatment for a period of 4hr in the absence of serum, and were assayed 24 hours post-treatment. The “no polymer” treatment refers to DOTAP/ODN (charge ratio 4.7) in the absence of polymer, and all other groups refer to the polymer that is added to this combination (to yield a net charge ratio of 1). GFP Fluorescence is normalized to untreated cells (100%). Data represent mean ± s.d. (n=3).
For treatments in the presence of 10% FBS, however, relatively low levels of Cy5-ODN uptake and minimal antisense activity were induced by the DOTAP/ODN and DOTAP/ODN/PPAA systems over the range of charge ratios from 4 to 0.25, corresponding to increasing amounts of PPAA (Fig. 5a, b). Taken together with the serum-free data, this result suggests that the presence of serum in treatment media hinders the intracellular uptake of ODN in complexes containing PPAA. Under similar treatment conditions with 10% FBS, the incorporation of PPAA-g-PEO copolymer into DOTAP/ODN complexes failed to increase intracellular ODN levels, which resulted in insignificant antisense activity throughout the range of charge ratios tested (Fig. 5a, b). In marked contrast, DOTAP/ODN complexes containing PPAA-g-Jeffamine copolymer produced an 8-fold increase in intracellular levels of ODN compared to DOTAP/ODN/PPAA in the presence of serum (Fig. 5a). This enhanced uptake with PPAA-g-Jeffamine correlated with a gene silencing effect of ∼ 80% (Fig. 5b). These results imply that PPAA-g-Jeffamine is able to mediate both serum avoidance and membrane penetration. Moreover, complexes with PPAA-g-Jeffamine produced a greater antisense effect than those utilizing the commercial standard, Lipofectamine 2000. PPAA-g-Jeffamine-containing complexes were active over a range of net charge ratios from 2.0 to 0.25 and yielded maximum ODN delivery and gene silencing effects at the net charge ratio of 0.5.
Figure 5.
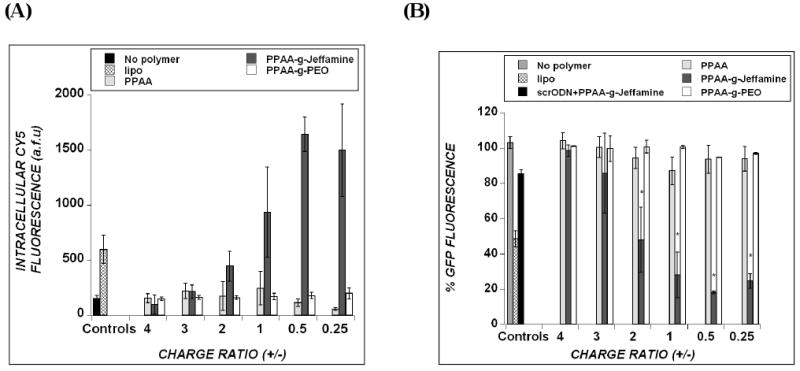
Cellular uptake of ODNs (A) and inhibition of target d1EGFP expression (B) in treatments with serum-containing medium. CHO-d1EGFP cells received 300nM ODN treatment in the presence of 10% FBS and were assayed 24 hours post-treatment. “No polymer” refers to DOTAP/ODN in the absence of polymer and all other groups refer to the polymer that is added to this combination. “Lipo” refers to delivery of ODN using Lipofectamine2000. In panel B, the % GFP fluorescence is normalized to untreated cells (100%). Asterisk in (B) represents statistical significance (p<0.05) between the polymer-containing delivery systems, as indicated at the charge ratios of 2, 1, 0.5 and 0.25. Data represent mean ± s.d. (n=3).
The non-specific antisense silencing effects were tested by the use of a scrambled ODN sequence and found to be minimal (10-20%) for DOTAP/ODN/PPAA-g-Jeffamine (Fig. 5b) or any of the other delivery formulations (data not shown). In a separate experiment, non-grafted Jeffamine polymer was added to DOTAP/ODN/PPAA complexes. The resulting degree of ODN delivery and antisense effect are no different than those for DOTAP/ODN/PPAA (data not shown). Thus, it appears that chemical grafting of Jeffamine onto PPAA is necessary for the increase in cellular internalization and antisense effects that are observed.
3.5. Cytotoxicity
The use of polymeric carrier systems to enhance nucleic acid delivery has generally been accompanied by significant levels of cell death and/or impairment of cellular function [27, 28]. In order to test the cytotoxicity induced by these novel delivery systems, cell metabolic activity was quantified 24 hrs post-treatment using a standard MTS assay. Increasing amounts of PPAA polymer to DOTAP/ODN complexes increased toxicity, with lowest cell metabolic activity (∼55%) at the charge ratio of 0.25. In general, comparable levels of toxicity are observed when PPAA is replaced by PPAA-g-Jeffamine (Fig.6). In comparison, the least effective polymer, PPAA-g-PEO, induced negligible toxicity throughout the range of charge ratios tested, consistent with the idea that the presence of PEO resists cellular interactions.
Figure 6.
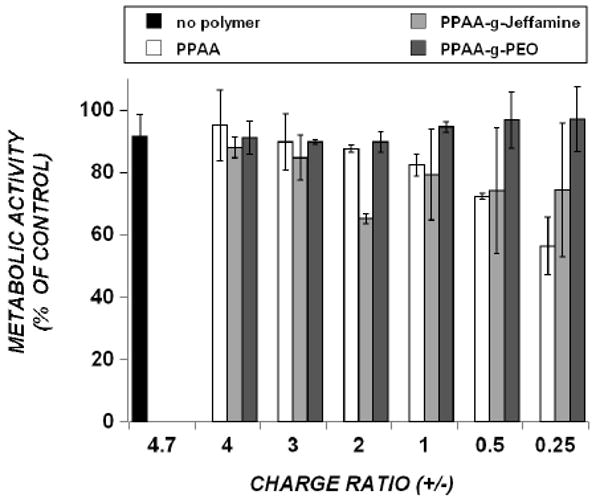
Effect of polyelectrolyte-containing complexes on CHO-d1EGFP cell metabolic activity, measured by the MTS assay. “No polymer” treatment refers to DOTAP/ODN (charge ratio 4.7) in the absence of polymer, and all other groups refer to the polymer that is added to this combination. Addition of increasing amounts of polymer to DOTAP/ODN complexes is indicated by charge ratios from 4 (lowest polymer concentration) to 0.25 (highest polymer concentration). Cells were treated with 300 nM ODN for 4 hr, in 10% FBS-containing cell culture media, and assayed 24 h post-treatment. Data represent mean ± s.d. (n=3).
Discussion
The delivery of antisense oligonucleotides (ODNs) into cells is impeded severely by a variety of extracellular and intracellular barriers. An efficient carrier system must associate with ODN molecules to form stable particles, resist attack from serum proteins present in the bloodstream/media, enter the target cell by means of an efficient uptake route, and finally be able to escape the endosomes in order to avoid being trafficked to the degradative lysosomes. Previous work has shown that the inclusion of poly(propylacrylic acid) (PPAA), an anionic polymer with lytic abilities exclusively at endosomal pH, has improved the cellular delivery of ODN mediated by DOTAP liposome [14]. However, this system failed to display any significant antisense activity in CHO (Chinese Hamster Ovary) cells that were treated in the presence of 10% FBS. We have, therefore, modified PPAA to create graft copolymers containing hydrophilic or amphipathic moieties, based on the hypothesis that these structures would resist serum attack and possibly aid in delivery across cell membranes. Specifically, the backbone structure of PPAA was modified by grafting poly(ethylene oxide) (PEO) or Jeffamine (10:31 mole ratio of ethylene oxide to propylene oxide groups) at 21 and 25 mol% graft densities, respectively.
The results raise the interesting question of why there exist differences among the PPAA, PPAA-g-Jeffamine and PPAA-g-PEO-containing complexes in their ability to mediate cellular delivery of ODNs and initiate an antisense effect. The formation of the ternary delivery system from its individual components is driven by electrostatic interactions between: (1) the cationic liposome, DOTAP, and anionic ODN molecules and, (2) the resulting DOTAP/ODN complex and anionic polymers: PPAA, PPAA-g-PEO or PPAA-g-Jeffamine. A fluorescence quenching assay demonstrated that the addition of PPAA, PPAA-g-PEO, or PPAA-g-Jeffamine to the DOTAP/ODN complexes did not affect the ability of DOTAP to maintain association with anionic ODN molecules, even when the net charge ratio was neutral (Fig. S1). Moreover, it is noteworthy that the sizes of DOTAP/ODN complexes in the presence of any of the polymers are quite stable in a variety of buffer solutions ranging from PBS, Opti-MEM (reduced serum medium) and MEM containing 10% FBS (Fig. S2). Furthermore, micellization of the free polymers was not observed by light scattering. Thus, it appears that the inhibitory effects of serum occur by modulating carrier-cell interactions, perhaps by competitive adsorption to cell membrane proteins, rather than by “attacking” the delivery vector directly.
PPAA and its graft copolymers have been designed towards the goal of creating molecules that can penetrate membranes at endosomal pH to deliver their ODN cargo into the cytoplasm. The characterization of the polymers by the pyrene and hemolysis assays provides some insight regarding how the grafting chemistry influences these key properties of PPAA. The results from these assays indicate that the parent polymer, PPAA, undergoes a pH-dependent conformational change that imparts significantly greater hydrophobicity at pH 5.0 to 5.5 as compared to pH 6.0 and above. The hemolytic activity likewise exhibits a maximum at acidic pH, that of endosomes. This physical property of PPAA suggests that the incorporation of this polymer into DOTAP/ODN complexes can mediate timely destabilization of endosomes and release of the contents into the cytoplasm, thereby allowing the antisense ODN to bind with complementary mRNA. Previous fluorescence microscopy has indicated that addition of PPAA to DOTAP/ODN complexes allows for the release of ODNs from endosomes, and that doing so results in an enhanced antisense effect [14].
In the case of the graft copolymers, we did not observe any significant red blood cell hemolysis throughout the pH range of 5.0-7.0, suggesting that the intracellular mechanism that these polymers employ to initiate carrier escape from endosomes is different from that of PPAA. Interestingly, the pyrene assay demonstrated that the graft copolymers, PPAA-g-PEO and PPAA-g-Jeffamine, displayed significant degrees of hydrophobicity over the entire pH range of 5.0 to 7.0, suggesting favorable interactions with the hydrophobic membranes of the cell and endosome. These results suggest that the graft copolymers behave differently in terms of their association with the cell and intracellular membranes. It is likely that the pH-insensitive hydrophobic components of the graft copolymers facilitate entry into cells and escape from endosomes by a fusion-mediated process as opposed to the pH-dependent lysis effects mediated by PPAA. Similar interactions with cell membranes were found with Pluronics, triblock copolymers of PEO-b-PPO-b-PEO architecture [19]. Previous work by Kabanov et al. demonstrated successful drug and gene delivery with Pluronic-PEI conjugates [18]. The efficacy of the Pluronics has been attributed to 1) favorable interactions between propylene oxide blocks of the polymer and the cell lipid bilayer membranes, leading to enhanced translocation into cells and 2) the formation of micelles that aid in the release of block copolymers into the cell [18, 29]. However, the organization of hydrophobic groups on the polymer also seems to play a role. While PPAA-g-PEO possesses hydrophobic domains from the PPAA backbone that can bind a small dye such as pyrene, the highly grafted PEO chains inhibit interactions of the PPAA-g-PEO copolymer with cells.
Thus, the PPAA-g-Jeffamine graft copolymer has been developed as part of a formulation designed to satisfy the following important prerequisites for cellular ODN delivery in biological milieu: (1) serum-stability, (2) uptake of ODNs into cells, and (3) release of ODNs into the cytoplasm before lysosomal degradation. The DOTAP/PPAA-g-Jeffamine formulation is also effective for delivery of siRNA (Peddada et al., unpublished data) and thus has broad application in technologies for gene silencing. While PPAA is effective at mediating intracellular delivery, it inhibits cellular uptake in the presence of serum. While PPAA-g-PEO may resist the attack of serum, this polymer appears to resist cellular uptake. On the other hand, PPAA-g-Jeffamine appears to strike an appropriate balance of hydrophilic/lipophilic character to allow serum resistance, cellular uptake, and endosomal escape. This graft copolymer can be tuned to further improve delivery or tailor the delivery system for a particular application. Thus, the PPAA-g-Jeffamine copolymers hold substantial promise as excipients for oligonucleotide delivery.
Supplementary Material
Acknowledgments
We are grateful for funding support from the NIH (R01GM65913), the Charles and Johanna Busch Foundation and an NSF IGERT Fellowship (DGE-0333196) to LP. We acknowledge Professor Kathryn Uhrich for access to the Zetasizer, Professor Ed Castner for access to the Shimadzu Spectrofluorimeter, and Professor Joachim Kohn for access to the polymer synthesis laboratory of the NJ Center for Biomaterials under the auspices of NIH grant P41 EB001046 (RESBIO biomedical technology resource). We thank Sumati Sundaram, Carolyn Waite, Ronn Friendlander and Ariell Joiner for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zamecnik PC, Stephenson ML. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci U S A. 1978;75:280–4. doi: 10.1073/pnas.75.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dove A. Antisense and sensibility. Nat Biotechnol. 2002;20:121–4. doi: 10.1038/nbt0202-121. [DOI] [PubMed] [Google Scholar]
- 3.Opalinska JB, Gewirtz AM. Nucleic-acid therapeutics: basic principles and recent applications. Nat Rev Drug Discov. 2002;1:503–14. doi: 10.1038/nrd837. [DOI] [PubMed] [Google Scholar]
- 4.Gleave ME, Monia BP. Antisense therapy for cancer. Nat Rev Cancer. 2005;5:468–79. doi: 10.1038/nrc1631. [DOI] [PubMed] [Google Scholar]
- 5.Potera C. Antisense--down, but not out. Nat Biotechnol. 2007;25:497–9. doi: 10.1038/nbt0507-497. [DOI] [PubMed] [Google Scholar]
- 6.Shi F, Hoekstra D. Effective intracellular delivery of oligonucleotides in order to make sense of antisense. J Control Release. 2004;97:189–209. doi: 10.1016/j.jconrel.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Roth CM, Sundaram S. Engineering synthetic vectors for improved DNA delivery: insights from intracellular pathways. Annu Rev Biomed Eng. 2004;6:397–426. doi: 10.1146/annurev.bioeng.6.040803.140203. [DOI] [PubMed] [Google Scholar]
- 8.Khalil IA, Kogure K, Akita H, Harashima H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol Rev. 2006;58:32–45. doi: 10.1124/pr.58.1.8. [DOI] [PubMed] [Google Scholar]
- 9.Park TG, Jeong JH, Kim SW. Current status of polymeric gene delivery systems. Adv Drug Deliv Rev. 2006;58:467–86. doi: 10.1016/j.addr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Fenske DB, Cullis PR. Liposomal nanomedicines. Expert Opin Drug Deliv. 2008;5:25–44. doi: 10.1517/17425247.5.1.25. [DOI] [PubMed] [Google Scholar]
- 11.Kabanov A, Zhu J, Alakhov V. Pluronic Block Copolymers for Gene Delivery. Adv Genet. 2005;53PA:231–261. doi: 10.1016/S0065-2660(05)53009-8. [DOI] [PubMed] [Google Scholar]
- 12.Murthy N, Robichaud JR, Tirrell DA, Stayton PS, Hoffman AS. The design and synthesis of polymers for eukaryotic membrane disruption. J Control Release. 1999;61:137–43. doi: 10.1016/s0168-3659(99)00114-5. [DOI] [PubMed] [Google Scholar]
- 13.Cheung CY, Murthy N, Stayton PS, Hoffman AS. A pH-sensitive polymer that enhances cationic lipid-mediated gene transfer. Bioconjug Chem. 2001;12:906–10. doi: 10.1021/bc0100408. [DOI] [PubMed] [Google Scholar]
- 14.Lee LK, Williams CL, Devore D, Roth CM. Poly(propylacrylic acid) enhances cationic lipid-mediated delivery of antisense oligonucleotides. Biomacromolecules. 2006;7:1502–8. doi: 10.1021/bm060114o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roux E, Passirani C, Scheffold S, Benoit JP, Leroux JC. Serum-stable and long-circulating, PEGylated, pH-sensitive liposomes. J Control Release. 2004;94:447–51. doi: 10.1016/j.jconrel.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 16.Lee M, Kim SW. Polyethylene glycol-conjugated copolymers for plasmid DNA delivery. Pharm Res. 2005;22:1–10. doi: 10.1007/s11095-004-9003-5. [DOI] [PubMed] [Google Scholar]
- 17.Kim JK, Choi SH, Kim CO, Park JS, Ahn WS, Kim CK. Enhancement of polyethylene glycol (PEG)-modified cationic liposome-mediated gene deliveries: effects on serum stability and transfection efficiency. J Pharm Pharmacol. 2003;55:453–60. doi: 10.1211/002235702928. [DOI] [PubMed] [Google Scholar]
- 18.Kabanov AV, Batrakova EV, Sriadibhatla S, Yang Z, Kelly DL, Alakov VY. Polymer genomics: shifting the gene and drug delivery paradigms. J Control Release. 2005;101:259–71. doi: 10.1016/j.jconrel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Kabanov A, Zhu J, Alakhov V. Pluronic block copolymers for gene delivery. Adv Genet. 2005;53:231–61. [PubMed] [Google Scholar]
- 20.Sundaram S, Lee LK, Roth CM. Interplay of polyethyleneimine molecular weight and oligonucleotide backbone chemistry in the dynamics of antisense activity. Nucleic Acids Res. 2007;35:4396–408. doi: 10.1093/nar/gkm450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.S SI, Moore JS. Cleavage of Aldehyde Hydrazonium Iodides under Mild Conditions – a Convenient Route to Chiral Nitriles of High Enantiomeric Purity. Macromolecules. 1990;23 [Google Scholar]
- 22.Moore J, Stupp SI. Cleavage of Aldehyde Hydrazonium Iodides Under Mild Conditions. A Convenient Route to Chiral Nitriles of High Enantiomeric Purity. Journal of Organic Chemistry. 1990;55:3374. [Google Scholar]
- 23.LA F, Hourdet D, Audebert R. Synthesis of thermoassociative copolymers. Polymer. 1997;38:2535–2547. [Google Scholar]
- 24.Kyriakides TR, Cheung CY, Murthy N, Bornstein P, Stayton PS, Hoffman AS. pH-sensitive polymers that enhance intracellular drug delivery in vivo. J Control Release. 2002;78:295–303. doi: 10.1016/s0168-3659(01)00504-1. [DOI] [PubMed] [Google Scholar]
- 25.Kusonwiriyawong C, van de Wetering P, Hubbell JA, Merkle HP, Walter E. Evaluation of pH-dependent membrane-disruptive properties of poly(acrylic acid) derived polymers. Eur J Pharm Biopharm. 2003;56:237–46. doi: 10.1016/s0939-6411(03)00093-6. [DOI] [PubMed] [Google Scholar]
- 26.Sakaguchi N, Kojima C, Harada A, Kono K. Preparation of pH-sensitive poly(glycidol) derivatives with varying hydrophobicities: their ability to sensitize stable liposomes to pH. Bioconjug Chem. 2008;19:1040–8. doi: 10.1021/bc7004736. [DOI] [PubMed] [Google Scholar]
- 27.Chirila TV, Rakoczy PE, Garrett KL, Lou X, Constable IJ. The use of synthetic polymers for delivery of therapeutic antisense oligodeoxynucleotides. Biomaterials. 2002;23:321–42. doi: 10.1016/S0142-9612(01)00125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duncan R, Izzo L. Dendrimer biocompatibility and toxicity. Adv Drug Deliv Rev. 2005;57:2215–37. doi: 10.1016/j.addr.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen HK, Lemieux P, Vinogradov SV, Gebhart CL, Guerin N, Paradis G, Bronich TK, Alakhov VY, Kabanov AV. Evaluation of polyether-polyethyleneimine graft copolymers as gene transfer agents. Gene Ther. 2000;7:126–38. doi: 10.1038/sj.gt.3301052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


