Abstract
Background
ABT-751, an orally bioavailable sulfonamide, binds β-tubulin to inhibit microtubule polymerization. To describe response and event free survival (EFS) in children with neuroblastoma and other solid tumors receiving ABT-751, assess in vitro cytotoxicity of ABT-751 and evaluate the effect of ABT-751 on tubulin polymerization in peripheral blood mononuclear cells (PBMC) and pediatric tumor cell lines.
Procedure
Patients with neuroblastoma (n=50) or other solid tumors (n=26) enrolled on the ABT-751 pediatric phase I and pilot trials were reviewed. The sulforhodamine B (SRB) and ACEA Real-Time Cell Electronic Sensing (RT-CES) assays were used to determine the in vitro cytotoxicity. Pharmacodynamic effects on tubulin polymerization/depolymerization were assessed by western blot and confocal microscopy using antibodies specific for post-translational modifications of polymerized tubulin.
Results
Forty-five patients with neuroblastoma were evaluated for anti-tumor response. No complete or partial responses were documented. The median EFS was 9.3 weeks for children with neuroblastoma and 3.3 weeks for children other solid tumors (P<0.0001). The ABT-751 IC50 was 0.6–2.6 mcM in neuroblastoma and 0.7–4.6 mcM in other solid tumor cell lines. Following drug exposure, polymerized tubulin decreased in a concentration and time dependent manner in cell lines.
Conclusions
In children treated with ABT-751, the EFS is longer in children with neuroblastoma as compared to other diagnoses. In vitro, ABT-751 was cytotoxic at concentrations tolerable in children. Effects of ABT-751 on polymerization and microtubule structure were time and dose-dependent but not dependent on tumor type.
Keywords: neuroblastoma, ABT-751, cytotoxicity, pediatric, tubulin
Introduction
ABT-751 is an orally bioavailable sulfonamide that binds to the colchicine binding site on β-tubulin to inhibit microtubule polymerization. By disrupting the dynamic equilibrium of microtubules, ABT-751 interferes with functions critical to cellular structure, motility, intracellular transport, and chromosome alignment and segregation during mitosis [1,2] resulting in cell cycle arrest in the G2/M phase and apoptosis. [3] Although the mechanism of action of ABT-751 is similar to that of vincristine, ABT-751 binds to a different site on β-tubulin and is not a substrate for multi-drug resistance mediated by P-glycoprotein. ABT-751 is active in vitro against adult human tumor cell lines and in in vivo xenograft models of adult and childhood cancers. [3–7] This agent is currently undergoing clinical evaluation in childhood cancers.
We conducted a pediatric solid tumor phase I clinical trial of ABT-751 and expanded the study to include a pilot study of ABT-751 in children with relapsed or refractory neuroblastoma. In the phase I trial, two schedules of ABT-751 were studied: once daily for 7 days repeated every 21 days (7-day schedule) [8] and once daily for 21 days repeated every 28 days (21-day schedule). [9] The recommended dose and schedule of ABT-751 in children with solid tumors is 200 mg/m2/dose administered orally, daily for 7 days every 21 days. Mean peak (Cmax) and steady state (Css) plasma concentrations at the recommended dose were 46 and 10 mcM, respectively.[10] Patients with neuroblastoma enrolled on the phase I trial appeared to have a prolonged progression free survival and improvement in symptoms such as pain compared to those with other pediatric solid tumors. [11] Based on these preliminary results suggesting a selective effect of ABT-751 in neuroblastoma, the study was expanded to include a pilot study of ABT-751 in patients with neuroblastoma. We analyzed data from all neuroblastoma patients treated on the study and investigated the selective cytotoxicity and effect on tubulin polymerization of ABT-751 in vitro in neuroblastoma cell lines, comparing clinical and pre-clinical activity in neuroblastoma to other solid tumors.
Methods
Patients
After completion of the dose escalation portion of our phase I trial of ABT-751 on the 7-day and 21-day schedules, the study was expanded to include a pilot study of patients with neuroblastoma treated at 200 mg/m2/day on the 7-day schedule. Study design, eligibility criteria, treatment regimen, definitions of dose-limiting toxicity and monitoring parameters have been reported previously [8,9] The clinical trial and all study procedures were approved by the institutional review board at all participating institutions, and all patients and their legal guardians provided informed consent and assent according to institutional guidelines.
Eligibility criteria unique to the neuroblastoma pilot study included a lower threshold for hematologic function [absolute neutrophil count (ANC) >250/mcL, platelet count > 25,000/mcL] and alanine aminotransferase (ALT) ≤5 × the upper limit of normal. Due to clinical benefit observed in patients with neuroblastoma on the phase 1 dose escalation portion of the trial, patients enrolled on the pilot study were not required to have measurable or evaluable disease, but could be enrolled with no evidence of disease (NED). Patients with other solid tumors enrolled on the dose escalation portion of the trial, were required to have measurable or evaluable disease. Hematologic dose-limiting toxicity (H-DLT) was defined as failure to recover an ANC ≥1000/mcL for patients with an ANC ≥1000/mcL at enrollment or to an ANC ≥250/mcL for those with an ANC 250–1000/mcL at enrollment by day 28 of the treatment cycle. Failure to recover the platelet count to ≥50,000/mcL for patients with a platelet count ≥50,000/mcL at enrollment or to≥25,000/mcL for those enrolled with a platelet count 25,000–50,000/mcL by day 28 of the treatment cycle was also considered H-DLT.
Clinical Response Criteria and Survival Analysis
Response was assessed with the CTEP Response Evaluation Criteria in Solid Tumors (RECIST) in those with measurable disease. In patients with neuroblastoma that is evaluable only by MIBG scans, complete response was complete resolution of all lesions, partial response was decrease in the number of sites of disease compared to baseline, progressive disease was any new site of disease, and stable disease was no change in number or size of MIBG positive lesions. Central review of pre-treatment and on study disease evaluations was performed for all patients considered to have disease response by their treating physicians.
For event-free survival analysis, events were defined as discontinuation of protocol therapy for tumor progression, toxicity or withdrawal of consent; whereas the progression-free survival analysis only included tumor progression as an event. Patients were followed until death or date of last documented follow up visit. Event-free survival, progression-free survival, and overall survival (OS) from the date of the first dose of ABT-751 were estimated using the Kaplan-Meier method, with a two-tailed log rank test used to compare EFS in patients with neuroblastoma to patients who were enrolled on the phase I trial of ABT-751 with other solid tumors.
Research evaluation for patients with neuroblastoma in the pilot study included serial assessments of heath related quality of life using the PedsQL Generic Core Scale version 4.0. This 23 item self-administered questionnaire has been validated in children with cancer.[12] Prior to each cycle, the patient’s performance status was assigned by a health care provider using the Lansky Scale for children 10 years of age or the Karnofsky scale for children >10 years of age.
Peripheral Blood Mononuclear Cells (PBMC)
Whole blood (6 mL) was obtained from patients prior to, 5 hours and 24 hours after the first dose and then prior to the dose of ABT-751 on day 5 of cycle 1. Peripheral blood mononuclear cells (PBMCs) were harvested by density centrifugation using Ficoll Hypaque. PBMCs were washed in PBS and stored at −70°C until analysis. Analysis of tubulin polymerization was performed using a polyclonal anti-detyrosinated α-tubulin antibody (Chemicon International, Temecula, CA) and monoclonal anti-acetylatedα-tubulin antibody (Sigma-Aldrich, St. Louis, MO) as described below.
Pediatric Tumor Cells Lines
Neuroblastoma (SK-N-AS, SK-N-DZ), rhabdomyosarcoma (RD), Ewing sarcoma (TC-71), medulloblastoma (HTB-186 Daoy) and osteosarcoma (HOS) pediatric tumor cell lines were obtained from American Type Culture Collection (ATCC, Manassas, Virginia). A second Ewing sarcoma line (LD) was established at the NCI from a patient with a scapular lesion. The neuroblastoma cell line, KCNR, was obtained from C.P. Reynolds. Cell lines were maintained in 1640 RPMI media with 10% fetal bovine serum (FBS) or 15% FBS for the KCNR line at 37°C/5% CO2 for optimal cell growth.
Drug Preparation
Vincristine (VCR), 1 mg/ml solution (Vincasar PFS, Sicor Pharmaceuticals, Inc, Irvine, California), was diluted in sterile H2O to a 100 mcM solution. ABT-751, which was supplied as a powdered compound by Abbott Laboratories (Abbott Park, Illinois), was dissolved in 1.25 % DMSO/sterile H2O to a final concentration of 135 mcM. Combretastatin, from a 10 mM DMSO solution, was diluted in sterile H2O to a 10 mcM solution. Aliquots of stock drug solutions were stored at −20°C. Prior to each assay, stock drug solutions were further diluted in sterile H2O to concentrations ranging from 0.1 nM to 1000 nM for VCR, from 0.1 nM to 100 mcM for ABT-751 and from 0.1 nM to 1000 nM for combretastatin.
Sulforhodamine B (SRB) Cytotoxicity Assay
Cells, in 1640 RPMI media with FBS, were plated in triplicate onto 96 well tissue culture plates in numbers determined optimal for confluent monolayer growth (5,000 cells/well for HOS, HTB-186 Daoy; 10,000 cells/well for TC-71, RD, SK-N-AS, SK-N-DZ, LD; 30,000 cells/well for KCNR), with an automated, multichannel pipette system (SerialMate, Matrix Technologies, Hudson, New Hampshire). Cells were incubated for 24 hours at 37°C/5% CO2 then exposed to vehicle control (1.25% DMSO/H2O), VCR (0.1–1000 nM), ABT-751 (0.1 nM–100 mcM), and in 4 cell lines (SK-N-AS, KCNR, RD, TC-71) combretastatin (0.1–1000 nM) for 72 hours. Cells were fixed with trichloroacetic acid (final concentration 10%) at 4°C, washed, then dried at room temperature, stained with SRB in 1% acetic acid and dye was then solubilized with Tris base. Optical density measurements were performed at 540 and 405 nm dual wavelengths in a Bio-Tek EL 340 UV plate reader (Bio-Tek Instruments, Inc., Winooski, Vermont). [13–16]
ACEA RT-CES Assay
The ACEA RT-CES device (ACEA Biosciences, San Diego, California) utilizes microelectrodes imbedded in the well base of a multi-well tissue culture plate to continuously measure changes in electrical impedance of plated cells as a determinant of cell surface area. The unit of measure, Cell Index (CI), relates the impedance of a media blank at time zero to impedance following cell plating and drug exposure. [17–20]
Each cell line was initially plated in duplicate onto 16 well ACEA E-plates (ACEA Biosciences, San Diego, California) at concentrations ranging from 2,500–40,000 cells/well, to characterize growth curves in drug-free media and determine optimal cell number for confluent monolayer growth. Number of cells plated, drug concentrations and exposure were performed as described for the SRB assay. Hourly measurements of electrical impedance were performed for 96 hours (time of cell plating through the end of the 72 hour drug exposure).
Dose Response Curves and IC50 Determination
Endpoint cytotoxicity results from the SRB assay were compared to the continuous data obtained from the ACEA RT-CES assay following the 72 hour drug exposure. Growth curves initially performed with the ACEA system confirmed all tumor lines remained in the log phase of cell growth 72 hours after plating; therefore, this time point was evaluated. Cell survival for the SRB and ACEA assays was calculated as a percentage of vehicle control.
IC50 concentrations and the slope (h) of the sigmoidal dose-response curves for VCR and ABT-751 in each of the 8 pediatric tumor cell lines were estimated by fitting the mathematical model below to the combined drug concentration-survival data from all experiments using MLAB (Civilized Software Inc, Silver Spring, Maryland).
Where C(d) represents the percent survival at concentration d, ME is the maximum effect, IC50 is the concentration that produces half of the maximum effect and h is the slope of the dose response curve.
Tubulin Polymerization Assay
Acetylation and detyrosination are reversible post-translational modifications made to α-tubulin. Polymerized tubulin has been shown to be the preferred substrate for these enzymatic reactions as compared to tubulin heterodimers, [21,22] and smaller amounts of acetylated and detyrosinated α-tubulin have been found in dynamic microtubules. Therefore, these modifications represent markers of microtubule stability. [23,24]
Cells in 1640 RPMI media with FBS were plated onto 100 mm diameter tissue culture dishes (500,000 cells for HOS, HTB-186 Daoy; 1,000,000 cells for TC-71, RD, SK-N-AS, SK-N-DZ, LD; 3,000,000 cells for KCNR), incubated for 24 hours at 37°C/5% CO2 then exposed to vehicle control (1.25% DMSO/H2O), VCR, ABT-751, and in selected cell lines (SK-N-AS, KCNR, RD, TC-71) combretastatin for 72 hours at the IC10, IC50 and IC90 concentrations as estimated from dose response curves for each cell line. Scraped cells were pelleted and then resuspended in lysis buffer (0.1 M PIPES, 1 mcM EGTA, 1 mM MgSO4, 30% glycerol, 5% DMSO, 0.125% IGEPAL, Roche Diagnostics complete mini protease inhibitor; 1 tablet/10 mL, aprotinin; 9,500 units/mL, GTP; 5 mM), vortexed, centrifuged and supernatant samples were standardized for protein concentration with the Bradford Reagent assay and BSA standards by measuring single reference wavelength absorbance (Hewlett Packard 8452A Diode Array Spectrophotometer).
Samples containing 15 mcg total protein were separated by SDS-PAGE gel electrophoresis, transferred to PVDF membrane and then exposed to a polyclonal anti-detyrosinated α-tubulin antibody and monoclonal anti-acetylated α-tubulin antibody, which are antibodies specific for post-translational modifications made to α-tubulin. A monoclonal anti-tyrosinated α-tubulin antibody (Sigma-Aldrich, St. Louis, MO) was used as a measure of dynamic tubulin and a monoclonal anti-α tubulin antibody (Sigma-Aldrich, St. Louis, MO) as a measured control of total tubulin.
Following the protocol described above for cell plating and drug exposure, four cell lines (SK-N-AS, KCNR, RD, TC-71) were lysed with buffer containing 5 mcM paclitaxel (Sigma-Aldrich, St. Louis, MO), immediately vortexed and centrifuged. Pellet samples were resuspended in lysis buffer and supernatant samples were standardized for total protein concentration. Samples of 3 mcg total protein were separated by gel electrophoresis, transferred to a PVDF membrane and exposed to the anti-acetylated and anti-tyrosinated α-tubulin antibodies followed by GAPDH and C-21 monoclonal antibodies as a gel loading control. Membranes were developed by a chemiluminescent technique (Supersignal Femto West Kit, Pierce Biotechnology, Inc, Rockford, IL). [25–27]
Confocal Microscopy
SK-N-AS, KCNR, RD, and TC-71 cell lines were plated onto 12 mm diameter glass cover slips, incubated for 24 hours and then exposed to vehicle control (1.25% DMSO/H2O), VCR, ABT-751, or combretastatin for 72 hours at the IC10, IC50 and IC90 concentrations as estimated from the dose response curves specific for each cell line. Cells were fixed with cold methanol (−20°C), blocked with 5% bovine serum albumin in PBS then exposed to primary antibodies; anti-detyrosinated and anti-acetylatedα-tubulin antibodies specific for post-translationally modified tubulin, anti-tyrosinatedα-tubulin antibody as a measure of dynamic tubulin and anti-α-tubulin as a control for total tubulin, followed by fluorescein-5-isothiocyanate (FITC) labeled secondary antibodies. Cover slips were mounted onto glass slides using Fluor-Gel (Electron Microscopy Sciences). Intracellular microtubule structure was observed on an Axiovert 100M fluorescent microscope (Carl Zeiss, Inc., Oberkochen, Germany) equipped with a PlanApochromat 100x/1.4 oil objective and confocal images were generated on a Zeiss LSM510. [28–30]
Results
Pediatric Phase I Clinical Trial
Patients
Between June 2002 and January 2008, 50 patients with neuroblastoma were enrolled on the pediatric ABT-751 phase I trial. Seventeen were treated on the phase 1 portion of the trial (8 on the 7-day schedule at doses ranging 100–250 mg/m2 and 9 on 21 day schedule at doses ranging 100–165 mg/m2) and 33 were treated on the neuroblastoma pilot study with 200 mg/m2 on the 7-day schedule (Table I).
Table I.
Neuroblastoma Patient Characteristics
| Enrolled/Evaluable: | 50/45 | |
| Median (range) Age [yrs]: | 8 (2.5–17) | |
| Male/Female: | 27/23 | |
| Diagnosis: | Neuroblastoma | 46 |
| Ganglioneuroblastoma | 4 | |
| Prior Therapy: | ≥ chemotherapy regimens | 25 |
| <3 chemotherapy regimens | 25 | |
| 131I MIBG | 26 | |
| Biologic Therapy | 44 | |
| HSCT | 39 | |
| Radiation Therapy | 41 | |
| Disease Status at Enrollment: | Measurable | 9 |
| Evaluable | 33 | |
| No Evidence of Disease | 8 | |
| ABT-751 Dosing Schedule: | Daily × 7d every 21d | 8 |
| Daily × 21d every 28d | 9 | |
| Expansion cohort | 33 | |
| ABT-751 cycles: median (range) | 4 (1–53) | |
Five patients were not fully evaluable for disease response because they discontinued protocol therapy due to toxicity (n=4) or withdrawal of consent unrelated to clinical progression or toxicity (n=1) prior to the first scheduled radiographic evaluation of response. Patients were heavily pre-treated (Table I), 78% had received high-dose chemotherapy or radiotherapy requiring autologous hematopoietic stem cell transplant (HSCT). Eighty-eight percent had received biologic therapy, defined as retinoids or antibody therapy. At the time of enrollment, 9 patients (18%) had measurable disease on CT scan or MRI, 33 patients (66%) had evaluable disease [on MIBG scan (n=18), both on MIBG scan and positive bone marrow aspirate/biopsy (n=8), positive bone marrow aspirate/biopsy (n=6) or on bone scan (n=1)], and 8 (16%) had no evidence of disease on imaging and histologic studies. At the time of enrollment, patients with no evidence of disease were a median of 77 days (range 20–196 days) from completion of their last therapy regimen.
Response and Survival
Forty-five patients with neuroblastoma were evaluated for response. The median (range) number of cycles administered was 4 (1–53). No complete or partial responses were documented. In patients who remained progression free a minimum of 6 weeks (n=26, 58%), the median (range) duration of treatment was 25 weeks (9–151 weeks). Of these patients, 16 (62%) had evaluable disease (MIBG n=11, histological evidence of disease in bone marrow n=4, bone scan n=1), 4 (15%) had measurable disease and 6 (23%) had no evidence of disease at study enrollment. Six patients (13%) completed study or continue ABT-751 without progression of disease. Of these six patients, three (1 with no evidence of disease, 1 with measurable disease and 1 with bone marrow involvement at enrollment) completed 50 cycles (150 weeks) of ABT-751 and treatment was stopped at the discretion of the treating physician. As of January 1, 2009, the other three patients (1 with no evidence of disease, 1 with measurable disease and 1 with bone marrow disease at enrollment) remain on therapy with no evidence of disease progression for 31, 35, and 53 cycles (93, 105, and 159 weeks). The median event-free survival (Figure 1) was 3.3 weeks for children with all other types of solid tumors (n=26), all who had disease present on initial CT or MRI imaging, and 9.3 weeks for children with neuroblastoma (n=50) (P<0.0001). The median progression free and overall survival for patients with neuroblastoma was 11.7 weeks and 62.5 weeks, respectively. There was no statistically significantly difference in median PFS when the extent of disease at study entry was considered; 24 weeks for NED, 9.6 weeks for evaluable disease, 5.9 weeks for measurable disease.
Figure 1.
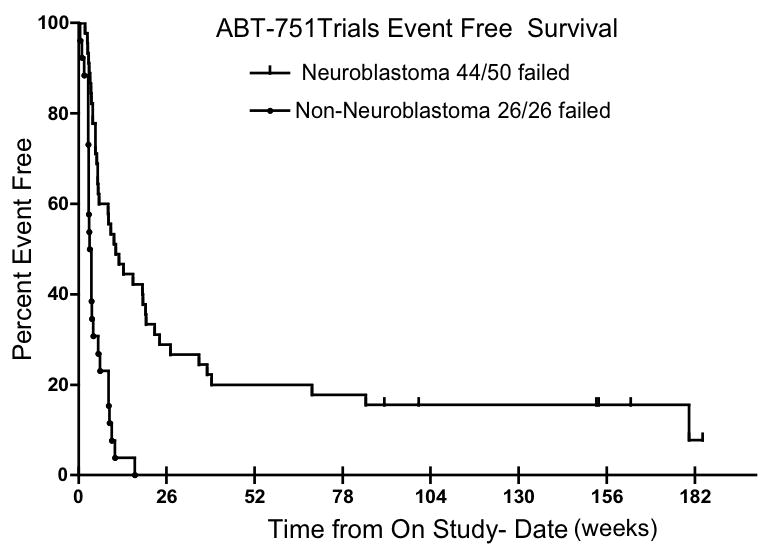
Event Free Survival (EFS) of patients treated with ABT-751. The median EFS was 9.3 weeks in children with neuroblastoma (NBL) and 3.3 weeks in children with other solid tumors (P<0.0001)
Twenty patients in the neuroblastoma pilot study completed the baseline PedsQL Generic Core Scales version 4.0 to assess health related quality of life. The median (range) baseline score was 70.6 (34.8–100). The median (range) change in the quality of life score from baseline to best reported score was 0 (−27.2–22.1). The median (range) clinician assigned performance status at baseline was 100 (70–100) and was only weakly to moderately correlated with the baseline PedsQL score (r=0.40; p=0.08).
Toxicity
ABT-751 related toxicities observed in the neuroblastoma patients (phase 1 and pilot study) were similar to those previously described on the two dose escalation schedules of the phase I trial. [8,9] Dose-limiting toxicities seen in patients with neuroblastoma included grade 2 decrease in left ventricular function (asymptomatic decrease in left ventricular shortening fraction); grade 3 thrombocytopenia, neutropenia, neuropathic pain, dehydration and fatigue and grade 4 elevated ALT and weight loss. A severe adverse event, encephalopathy, was reported in one patient with neuroblastoma. This patient did not have central nervous system disease, but had received TBI and neck irradiation 4 years prior to enrollment.
Tubulin Polymerization in PBMCs
The effect of ABT-751 on tubulin polymerization was evaluated in 10 patients with neuroblastoma, each with PBMC samples collected at 3 time points; pre-dose and 5 h and 24 h after ABT-751 (n=4) or pre-dose and 24 h and 5 days after ABT-751 (n=6). In some patients (n=6) there was a decrease in polymerized tubulin following ABT-751 administration as compared to baseline when measured by the anti-detyrosinated α-tubulin antibody. However, a consistent pattern was not observed across all patient samples. Anti-acetylated α-tubulin could not be detected in these patient samples perhaps related to degradation of the acetylated component over time.
Cytotoxicity Assays
We evaluated the in vitro cytotoxicity of three agents that inhibit tubulin polymerization; ABT-751 and combretastatin bind the colchicine site on tubulin, vincristine binds the vinca site, distinct from the colchicines binding site.
The cytotoxicity profiles of VCR and ABT-751 over a range of concentrations were evaluated in each pediatric tumor cell line by ACEA RT-CES with hourly cell index measurements. Performed in parallel as a comparison, cell survival was measured at the end of the 72 h drug exposure using the SRB assay. Both agents were cytotoxic in all pediatric tumor cell lines, although VCR was more potent than ABT-751 (Figure 2).
Figure 2.
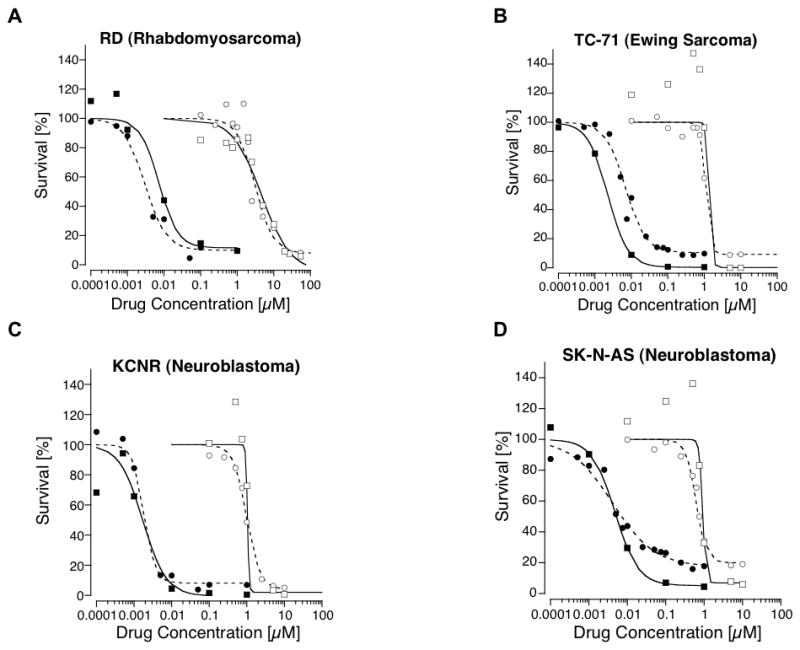
Cell survival curves for 2 non-neuroblastoma (A. RD and B. TC-71) and 2 neuroblastoma (C. KCNR and D. SK-N-AS) cell lines exposed to a range of concentrations of ABT-751 or vincristine (VCR). Cell survival was measured using from the Sulforhodamine B (SRB) and ACEA RT-CES cytotoxicity assays. Open circles represent the mean survival from multiple replicates and multiple experiments for cell lines exposed to ABT-751 with survival measured with the SRB assay; open squares represent the mean survival for cell lines exposed to ABT-751 with survival measured with the ACEA RT-CES assay; closed circles represent the mean survival for cell lines exposed to VCR with survival measured with the SRB assay; and closed squares represent the mean survival for cell lines exposed to VCR with survival measured with the ACEA RT-CES assay. Lines represent the mathematical model fit to the concentration-survival data - the dashed line for the SRB assay and solid line for the ACEA RT-CES assay.
IC50 concentrations were derived by fitting the mathematical model to the drug concentration-cell survival curves from the SRB and ACEA assays. The IC50 for ABT-751 ranged from 0.6 to 4.6 mcM and for VCR the IC50 ranged from 1.2 to 8.8 nM (Table II). In 4 select tumor cell lines combretastatin was cytotoxic with estimated IC50 concentrations ranging from 2.9 to 9.1 nM utilizing the SRB assay. The plasma steady state concentration of ABT-751 at the recommended dose from our phase I trial exceeded 10 mcM, a concentration greater than the IC50 of ABT-751 in all 8 pediatric tumor cell lines.
Table II.
Cytotoxicity of ABT-751 and Vincristine in Pediatric Solid Tumor Cell Lines
| Cell Line | Lineage | Assay | Vincristine |
ABT-751 |
||
|---|---|---|---|---|---|---|
| IC50 (mcM) | h | IC50 (mcM) | h | |||
| RD | Rhabdomyosarcoma | SRB | 0.0030 | 1.5 | 3.1 | 1.8 |
| ACEA | 0.0073 | 1.7 | 4.6 | 1.1 | ||
| TC-71 | Ewing sarcoma | SRB | 0.0069 | 1.6 | 1.1 | 6.0 |
| ACEA | 0.0023 | 1.6 | 1.4 | 10.9 | ||
| LD | Ewing sarcoma | SRB | 0.0019 | 2.0 | 0.68 | 3.5 |
| ACEA | 0.0012 | 7.6 | 0.85 | 6.1 | ||
| HTB-186 | Medulloblastoma | SRB | 0.0025 | 2.0 | 2.6 | 0.66 |
| ACEA | 0.0039 | 2.7 | 1.2 | 4.0 | ||
| HOS | Osteosarcoma | SRB | 0.0072 | 1.6 | 1.4 | 4.3 |
| ACEA | 0.0088 | 4.0 | 3.8 | 4.2 | ||
| SK-N-AS | Neuroblastoma | SRB | 0.0041 | 0.80 | 0.61 | 3.3 |
| ACEA | 0.0048 | 1.4 | 0.91 | 10.5 | ||
| SK-N-DZ | Neuroblastoma | SRB | 0.0029 | 0.78 | 1.2 | 2.7 |
| ACEA | 0.0013 | 5.8 | 2.6 | 2.9 | ||
| KCNR | Neuroblastoma | SRB | 0.0018 | 2.8 | 0.98 | 2.5 |
| ACEA | 0.0017 | 1.4 | 1.0 | 20.7 | ||
The IC50s estimated from the ACEA RT-CES cytotoxicity assay for the various cell lines were similar to the IC50s from the standard SRB assay for ABT-751 and VCR (Table II). However, the shape of the drug concentration-survival curves differed for a number of cell lines for the two methods with ABT-751 exposure (Figure 2). At low, non-cytotoxic concentrations of ABT-751, the cell index increased by up to 50% in some lines (e.g., SK-N-AS, TC-71) compared to the vehicle treated control, presumably due to a drug-related alteration in cell size or morphology. These survival values in excess of 100% at the low concentrations resulted in a steeper downward slope (higher value for h, Table II) at the higher cytotoxic concentrations on the survival curve.
The mean IC50 for ABT-751 in the three neuroblastoma cell lines based on the SRB assay was 0.93 mcM compared to 1.8 mcM in the other five non-neuroblastoma pediatric solid tumor cell lines. The mean IC50 for VCR was lower in the neuroblastoma cell lines (2.9 nM) than in the non-neuroblastoma cell lines (4.3 nM). However, these differences were not statistically significant.
Tubulin Polymerization Assay
The effect of ABT-751 and VCR on tubulin polymerization was studied in the 8 cell lines by Western blot at the IC50 and IC90 concentrations determined from the model fit to the drug concentration-survival curves. In all cell lines, a concentration and time dependent decrease in the amount of stable polymerized tubulin was detected by Western blot utilizing two antibodies specific for post-translational modifications made to polymerized tubulin as compared to a vehicle control. A representative Western blot is shown (Figure 3). A similar dose- and time-dependent decrease in stable, polymerized tubulin was documented in 4 select cell lines after exposure to combretastatin and on evaluation of pellet and supernatant samples. Of note, the degree of decrease in stable polymerized tubulin at equipotent drug concentrations was greater with VCR and combretastatin than ABT-751, particularly at the IC90.
Figure 3.
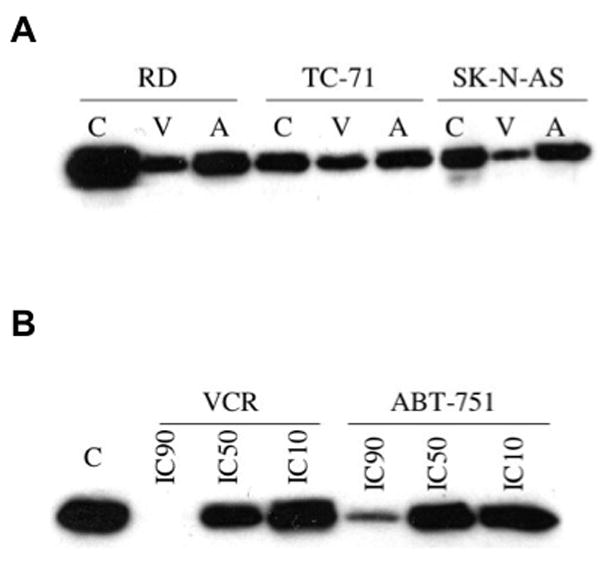
A. Western blot of polymerized tubulin at the IC50 of vincristine (V) or ABT-751 (A) compared to vehicle control (C) utilizing an anti-detyrosinated ! tubulin antibody in three of the pediatric tumor cell lines. B. Western blot of polymerized tubulin in KCNR cells at the IC90, IC50. and IC10 of vincristine (VCR), ABT-751 or vehicle control (C) utilizing an anti-detyrosinated ! tubulin antibody.
Confocal Microscopy
A concentration-dependent decrease in stable polymerized tubulin was demonstrated in 4 tumor cell lines following VCR, ABT-751 and combretastatin exposure through fluorescence labeling with antibodies specific for post-translational modifications, anti-detyrosinated and anti-acetylated, of polymerized tubulin (Figure 4). The IC90 concentrations of VCR and combretastatin resulted in near complete loss of stable polymerized tubulin compared to the vehicle control. In contrast, following exposure to ABT-751 a significant proportion of acetylated and detyrosinated α-tubulin positive microtubules remained. A distinctive microtubule pattern was observed at the IC90 concentration of ABT-751 with course, thick, curled microtubules.
Figure 4.
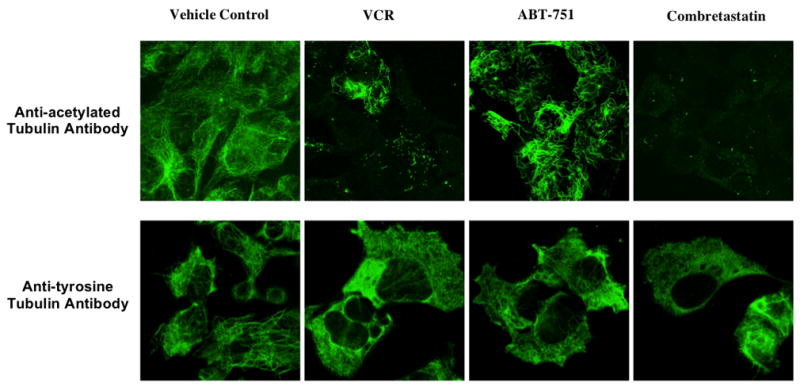
Confocal microscopy of polymerized tubulin utilizing fluorescence labeled anti-acetylated ! tubulin antibody and of dynamic tubulin using anti-tyrosinated ! tubulin antibody at the IC90 concentrations of VCR, ABT-751, combretastatin and a vehicle control in KCNR cells.
The anti-tyrosinated α-tubulin antibody, which was used to assess the affect of the three tubulin binding agents on dynamic microtubules, demonstrated that the tubulin developed a granular appearance with increasing concentration of VCR, ABT-751 and combretastatin, consistent with depolymerization, as opposed to a complete loss or significant decrease in the fluorescence signal.
Discussion
The tubulin binding chemotherapeutic drugs, most of which are natural products (e.g., the vinca alkaloids and taxanes), have a broad spectrum of anti-tumor activity in cancers occurring in children and adults. ABT-751 is a synthetic tubulin binding agent that has a number of favorable pharmacological properties. The drug is orally bioavailable, it is not significantly myelosuppressive at the recommended dose of 200 mg/m2/day for 7 days in children, it binds to a site on β-tubulin that is distinct from the vinca alkaloid and taxane binding sites, and it is not a substrate for multi-drug resistance mediated by P-glycoprotein.
Morton et al. [7] evaluated ABT-751 in childhood cancer xenograft murine models. ABT-751 100 mg/kg (approximately 250 mg/m2 in children), which was administered daily for 5 days followed by a 5 day rest period and then another 5 day course of drug every 21 days for 2 cycles, induced objective responses in 4 of 25 of models including embryonal rhabdomyosarcoma, Wilms tumor, and 2 neuroblastoma models. ABT-751 extended event free survival in 10 of 25 models. For pediatric non-neuroblastoma solid tumor xenograft models (n=21), the median (range) EFS in untreated cohorts was 11.4 days (7.4–25.2) compared to 19.4 days (11.3–64.4) in cohorts receiving ABT-751. In neuroblastoma models (n=4) the median EFS in untreated cohorts was 12.0 days (9.3–15.1) compared to 37.5 days (16.2–59.7) in ABT-751 treated cohorts. The percent increase in life span was greater in the neuroblastoma xenografts.
Our analysis of the pediatric phase I trial and pilot study of ABT-751 suggested patients with neuroblastoma have a longer event-free interval than patients with other solid tumors. Longer event-free survival in neuroblastoma patients could be due to a drug effect, a difference in the natural history of neuroblastoma compared to other solid tumors, or a selection bias resulting from inclusion of children with neuroblastoma who had a high risk of relapse of disease but had no evidence of disease at the time of study enrollment. The preclinical component of this study compared the effects of ABT-751 in neuroblastoma cell lines to cell lines from other common types of childhood cancers to determine if there was a selective effect of ABT-751 in neuroblastoma.
ABT-751 was cytotoxic in all 8 pediatric solid tumor cell lines, and IC50 values were below the steady state concentration shown to be achievable in plasma at recommended doses in children. The in vitro cytotoxicity evaluation revealed a trend towards greater potency of ABT-751 and VCR in neuroblastoma cell lines with mean IC50 concentrations less than those in non-neuroblastoma tumor lines. The clinical outcomes observed in the phase I trial and pilot study and have led to the development of a pediatric phase II clinical trial evaluating ABT-751 in a neuroblastoma patient population.
Although VCR is 300 times more potent than ABT-751, the clinically tolerated dose of ABT-751 (200 mg/m2/day for 7 consecutive days every 21 days) is approximately 250 times greater than that of VCR (2.0 mg/m2/week). Inhibition of tubulin polymerization was more extensive and more rapid with VCR and combretastatin than with ABT-751 at equipotent (IC50 and IC90) drug concentrations. This difference was observed in the context of increased overall cell death following exposure to VCR and combretastatin, particularly at the IC90 concentrations. In an attempt to better understand this difference in the relative extent of the inhibition of tubulin polymerization at equipotent doses of the three agents, we studied the effect of drug exposure on microtubule structure using confocal microscopy. The microtubule pattern seen with high concentrations of VCR and combretastatin was similar and showed complete loss of polymerized tubulin (absence of stained microtubules). After ABT-751 exposure microtubules remained but their appearance compared to vehicle treated controls suggests that ABT-751 has a selective effect on dynamic microtubules and spares stable microtubules, accounting for the persistence of acetylated and detyrosinated α-tubulin positive polymerized tubules at the IC90 concentration of ABT-751.
Prolonged disease stabilization and event free survival were observed in a large, heavily pretreated population of patients with neuroblastoma who received ABT-751 therapy. Toxicity associated with ABT-751 was similar to that previously reported and did not appear increased in patients in the pilot study for whom less stringent hematologic eligibility criteria were utilized. In a subpopulation of patients with neuroblastoma, health related quality of life during treatment with ABT-751 was not significantly changed from baseline and clinician assigned performance status was not correlated with patient-reported health related QOL scores. However, a larger sample size, including more complete longitudinal data, is necessary to confirm these observations. Currently, a Children’s Oncology Group phase 2 study of ABT-751 in children with relapse or refractory neuroblastoma is evaluating the progression free survival and health related quality of life of children with neuroblastoma.
Acknowledgments
This research was supported, in part, by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and by intramural funds of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Conflict of Interest Statement
Andrew Krivoshik, MD, PhD is employed by and has ownership interest in Abbott Laboratories. The other authors of this manuscript do not have conflicts of interest to disclose.
References
- 1.Iwamoto Y, Nisho K, Fukumoto H, Yoshimatsu K, Yamakido M, Saijo N. Preferential binding of E7010 to murine beta 3-tubulin and decreased beta 3-tubulin in E7010 resistant cell lines. Japanese J Cancer Research. 1998;89(9):954–962. doi: 10.1111/j.1349-7006.1998.tb00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nogales E. A structural view of microtubule dynamics. Cell Mol Life Sci. 1999;56:133–142. doi: 10.1007/s000180050012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshimatsu K, Yamaguchi A, Yoshino H, Koyanagi N, Kitoh K. Mechanism of action of E7010, an orally active sulfonamide antitumor agent: inhibition of mitosis by binding to the colchicine site of tubulin. Cancer Research. 1997;57(15):3208–3213. [PubMed] [Google Scholar]
- 4.Funahashi Y, Koyanagi N, Kitoh K. Effect of E7010 on liver metastasis and life span of syngeneic C57BL/6 mice bearing orthotopically transplanted murine Colon 38 tumor. Cancer Chemother Pharmacol. 2001;47(2):179–184. doi: 10.1007/s002800000199. [DOI] [PubMed] [Google Scholar]
- 5.Koyanagi N, Nagasu T, Fujita F, Watanabe T, Tsukahara K, Funahashi Y, Fujita M, Taguchi T, Yoshino H, Kitoh K. In vivo tumor growth inhibition produced by a novel sulfonamide, E7010, against rodent and human tumors. Cancer Research. 1994;54(7):1702–1706. [PubMed] [Google Scholar]
- 6.Owa T, Yoshino H, Okauchi T, et al. Discovery of novel antitumor sulfonamides targeting G1 phase of the cell cycle. J Med Chem. 1999;42(19):3789–3799. doi: 10.1021/jm9902638. [DOI] [PubMed] [Google Scholar]
- 7.Morton CL, Favours EG, Mercer KS, et al. Evaluation of ABT-751 against childhood cancer models in vivo. Invest New Drugs. 2007;25(4):285–295. doi: 10.1007/s10637-007-9042-y. [DOI] [PubMed] [Google Scholar]
- 8.Fox E, Maris JM, Widemann BC, et al. A phase 1 study of ABT-751, an orally bioavailable tubulin inhibitor, administered daily for 7 days every 21 days in pediatric patients with solid tumors. Clin Cancer Res. 2006;12(16):4882–4887. doi: 10.1158/1078-0432.CCR-06-0534. [DOI] [PubMed] [Google Scholar]
- 9.Fox E, Maris JM, Widemann BC, et al. A phase I study of ABT-751, an orally bioavailable tubulin inhibitor, administered daily for 21 days every 28 days in pediatric patients with solid tumors. Clin Cancer Res. 2008;14(4):1111–1115. doi: 10.1158/1078-0432.CCR-07-4097. [DOI] [PubMed] [Google Scholar]
- 10.Fox E, Widemann BC, Maris JM, et al. The pharmacokinetics (PK) and pharmacodynamics (PD) of ABT-751 in children with recurrent neuroblastoma or other solid tumors. Proceedings ASCO; 2007. Abstract #9557. [Google Scholar]
- 11.Fox E, Adamson PC, Hagey A, Widemann BC, Maris JM, Cohn SL, Cai Y, Medina D, Meek KA, Balis FM. Phase 1 trial of oral ABT-751 in pediatric patients: Preliminary evidence of activity in neuroblastoma. Proceedings ASCO; 2005. p. 806s. Abstract #8527. [Google Scholar]
- 12.Varni JW, Burwinkle TM, Katz ER, et al. The PedsQL in pediatric cancer: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer. 2002;94(7):2090–2106. doi: 10.1002/cncr.10428. [DOI] [PubMed] [Google Scholar]
- 13.Monks A, Scudiero D, Skehan P, et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst. 1991;83(11):757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 14.Perez RP, Godwin AK, Handel LM, et al. A comparison of clonogenic, microtetrazolium and sulforhodamine B assays for determination of cisplatin cytotoxicity in human ovarian carcinoma cell lines. Eur J Cancer. 1993;29A(3):395–399. doi: 10.1016/0959-8049(93)90394-u. [DOI] [PubMed] [Google Scholar]
- 15.Rubinstein LV, Shoemaker RH, Paull KD, et al. Comparison of in vitro anticancer-drug-screening data generated with a tetrazolium assay versus a protein assay against a diverse panel of human tumor cell lines. J Natl Cancer Inst. 1990;82(13):1113–1118. doi: 10.1093/jnci/82.13.1113. [DOI] [PubMed] [Google Scholar]
- 16.Skehan P, Storeng R, Scudiero D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82(13):1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 17.Atienza JM, Zhu J, Wang X, et al. Dynamic monitoring of cell adhesion and spreading on microelectronic sensor arrays. J Biomol Screen. 2005;10(8):795–805. doi: 10.1177/1087057105279635. [DOI] [PubMed] [Google Scholar]
- 18.Solly K, Wang X, Xu X, et al. Application of real-time cell electronic sensing (RT-CES) technology to cell-based assays. Assay Drug Dev Technol. 2004;2(4):363–372. doi: 10.1089/adt.2004.2.363. [DOI] [PubMed] [Google Scholar]
- 19.Xing JZ, Zhu L, Jackson JA, et al. Dynamic monitoring of cytotoxicity on microelectronic sensors. Chem Res Toxicol. 2005;18(2):154–161. doi: 10.1021/tx049721s. [DOI] [PubMed] [Google Scholar]
- 20.Xiao C, Lachance B, Sunahara G, et al. Assessment of cytotoxicity using electric cell-substrate impedance sensing: concentration and time response function approach. Anal Chem. 2002;74(22):5748–5753. doi: 10.1021/ac025848f. [DOI] [PubMed] [Google Scholar]
- 21.Barra HS, Arce CA, Argarana CE. Posttranslational tyrosination/detyrosination of tubulin. Mol Neurobiol. 1988;2(2):133–153. doi: 10.1007/BF02935343. [DOI] [PubMed] [Google Scholar]
- 22.Maruta H, Greer K, Rosenbaum JL. The acetylation of alpha-tubulin and its relationship to the assembly and disassembly of microtubules. J Cell Biol. 1986;103(2):571–579. doi: 10.1083/jcb.103.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreis TE. Microtubules containing detyrosinated tubulin are less dynamic. Embo J. 1987;6(9):2597–2606. doi: 10.1002/j.1460-2075.1987.tb02550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulze E, Asai DJ, Bulinski JC, et al. Posttranslational modification and microtubule stability. J Cell Biol. 1987;105(5):2167–2177. doi: 10.1083/jcb.105.5.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gershoni JM, Palade GE. Protein blotting: principles and applications. Anal Biochem. 1983;131(1):1–15. doi: 10.1016/0003-2697(83)90128-8. [DOI] [PubMed] [Google Scholar]
- 26.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vachereau A. Luminescent immunodetection of western-blotted proteins from coomassie-stained polyacrylamide gel. Anal Biochem. 1989;179(1):206–208. doi: 10.1016/0003-2697(89)90227-3. [DOI] [PubMed] [Google Scholar]
- 28.Baschong W, Duerrenberger M, Mandinova A, et al. Three-dimensional visualization of cytoskeleton by confocal laser scanning microscopy. Methods Enzymol. 1999;307:173–189. doi: 10.1016/s0076-6879(99)07013-5. [DOI] [PubMed] [Google Scholar]
- 29.Carlsson K. Three-dimensional specimen reconstruction by confocal microscopy and digital image processing. Bull Assoc Anat (Nancy) 1991;75(229):105–108. [PubMed] [Google Scholar]
- 30.Wright SJ, Schatten G. Confocal fluorescence microscopy and three-dimensional reconstruction. J Electron Microsc Tech. 1991;18(1):2–10. doi: 10.1002/jemt.1060180103. [DOI] [PubMed] [Google Scholar]


