Abstract
This review will focus on neural plasticity and recovery of respiratory function after spinal cord injury and feature the “crossed phrenic phenomenon” (CPP) as a model for demonstrating such plasticity and recovery. A very brief summary of the earlier literature on the CPP will be followed by a more detailed review of the more recent studies. Two aspects of plasticity associated with the CPP that have been introduced in the literature recently have been spontaneous recovery of ipsilateral hemidiaphragmatic function following chronic spinal cord injury and drug-induced persistent recovery of the ipsilateral hemidiaphragm lasting long after animals have been weaned from drug treatment. The underlying mechanisms for this plasticity and resultant recovery will be discussed in this review. Moreover, two new models involving the CPP have been introduced: a mouse model which now provides for an opportunity to study CPP plasticity at a molecular level using a genetic approach and light-stimulated induction of the CPP accomplished by transfecting mammalian cells with channelrhodopsin. Both models provide an opportunity to sort out the intracellular signaling cascades that may be involved in motor recovery in the respiratory system after spinal cord injury. Finally, the review will examine developmental plasticity of the CPP and discuss how the expression of the CPP changes in neonatal rats as they mature to adults. Understanding the underlying mechanisms behind the spontaneous expression of the crossed phrenic pathway either in the developing animal or after chronic spinal cord injury in the adult animal may provide clues to initiating respiratory recovery sooner to alleviate human suffering and eventually eliminate the leading cause of death in human cases of spinal cord injury.
1. Introduction
According to the National Spinal Cord Injury Statistical Center, the most frequent category of spinal cord injury (SCI) in man since 2000 has been incomplete tetraplegia in which one or more of the eight cervical spinal cord segments is injured (National Spinal Cord Injury Statistical Center,2008). By definition, an “incomplete” spinal cord injury involves sensory and motor sparing of the most caudal segments, S4-S5, whereas in a complete injury there is no sensory or motor function detected at these levels. Cervical spinal cord injury, particularly when it occurs rostral to the phrenic nucleus, and whether it is complete or incomplete, may interrupt the descending bulbospinal respiratory pathways and cause life-threatening weakness of respiratory muscle function. In fact, the leading causes of death among persons with spinal cord injury since 1973, according to the National SCI Database, are from pneumonia, pulmonary emboli and septicemia (National Spinal Cord Statistical Center, 2008). These mortality factors may be caused directly by the weakening of the respiratory muscles after SCI or indirectly by the need to place patients on long-term mechanical ventilator support. Because of the life-threatening nature of respiratory dysfunction, it is imperative to understand the underlying mechanisms related to plasticity and recovery of respiratory function after SCI. This information may ultimately lead to the resolution of the problem of post-SCI respiratory insufficiency in man.
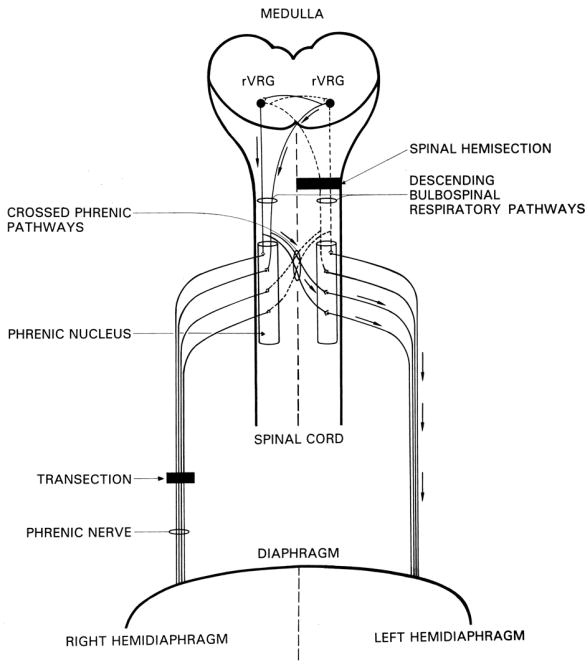
This review will focus on neural plasticity and recovery of respiratory function after spinal cord injury and feature the “crossed phrenic phenomenon” (CPP) as a model for demonstrating such plasticity and recovery. A very brief summary of the earlier literature on the CPP will be followed by a more detailed review of the more recent studies. Briefly, the CPP may be explained as follows: spinal cord hemisection (Goshgarian, 1979) or a lateralized contusion injury (el-Bohy et al., 1998; Baussart et al., 2006;) rostral to the level of the phrenic nucleus in the cervical spinal cord may interrupt both crossed and uncrossed descending bulbospinal respiratory pathways extending from the rostral ventral respiratory group (rVRG) of neurons in the medulla to the ipsilateral phrenic motoneurons. The interruption of these pathways results in an immediate paralysis of the ipsilateral hemidiaphragm (Fig. 1). Hemidiaphragmatic paralysis results in a significant reduction in tidal volume and increase in respiratory frequency. This has been demonstrated both in un-anesthetized awake rats (Goshgarian et al, 1986; Fuller et al, 2006) and in anesthetized spontaneously breathing rats (Golder et al, 2001). Vagal feedback, most likely arising from changes in lung and chest wall compliance after hemisection, is the primary cause for the change in breathing pattern (Golder et al, 2001). Although the breathing pattern is significantly altered in response to C2 hemisection, total ventilation (i.e., breathing frequency X tidal volume) (Golder et al, 2001; Fuller et al, 2006) and blood gases (Goshgarian et al, 1986) remain unchanged compared to non-injured controls. Thus, animals are able to compensate for altered respiratory function due to hemidiaphragmatic paralysis.
Fig. 1.
Surgical Procedures and pathways involved in the crossed phrenic phenomenon (CPP). Inspiratory drive to phrenic motoneurons is mediated by medullary neurons in the rostral division of the medullary respiratory group (rVRG). These neurons project bilaterally to the phrenic nuclei. Moreno et al. (1992) showed that both crossed and uncrossed descending respiratory pathways have spinal decussating collaterals that project to both phrenic nuclei (i.e., the crossed phrenic pathways). Hemisection rostral to the phrenic nucleus interrupts (dotted lines) the major bulbospinal drive to the ipsilateral phrenic nucleus, which results in paralysis of the left hemidiaphragm. Transection of the right phrenic nerve immediately after hemisection paralyzes the right hemidiaphragm and induces the CPP in most mammalian species. Arrows indicate the pathways followed by respiratory impulses during the CPP to restore function to the hemidiaphragm paralyzed by spinal cord injury. Reprinted from Castro-Moure and Goshgarian (1997) with Permission by Academic Press.
2. Brief review of the CPP literature
In his seminal studies conducted in 1895, Porter showed in both dogs and rabbits that following C2 (second cervical segment) spinal cord hemisection, transection of the contralateral phrenic nerve immediately restored function to the hemidiaphragm paralyzed by the hemisection. This example of functional recovery after SCI was later termed, the “crossed phrenic phenomenon” (Rosenblueth and Ortiz, 1936) and over the years was confirmed by several different laboratories in a variety of mammalian species (for a complete review, see Goshgarian, 2003). Although Porter suggested that the anatomical substrate for the CPP may consist of phrenic motoneuron dendrites that cross the midline of the spinal cord to receive descending respiratory bulbospinal input from the spinal cord contralateral to hemisection, Moreno et al (1992) showed that axon collaterals from these crossed and uncrossed descending respiratory pathways reach phrenic motoneurons ipsilateral to hemisection by crossing the midline of the spinal cord at the level of the phrenic nuclei (Fig. 1). They referred to these crossing collaterals as the crossed phrenic pathway. It is possible, however, that crossed phrenic dendrites may still, in some instances convey crossed phrenic activity (Lindsay et al., 1991; Prakash et al., 2000). Moreover, a recent study has suggested that propriospinal neurons may also help mediate the CPP (Lane et al., 2008).
In spite of numerous studies on the CPP appearing in the literature subsequent to Porter’s 1895 work, the physiological basis for the CPP was not elucidated until more than 50 years later. In 1951, Lewis and Brookhart concluded that the expression of the CPP was related to the intensity of central respiratory discharge. Any condition that enhances central respiratory discharge will enhance the expression of the CPP. When central respiratory discharge is decreased, there will be an attenuation of the intensity of CPP expression (Lewis and Brookhart, 1951).
2.1 Plasticity associated with the CPP
The initial demonstrations of the CPP published over the first 66 years following Porter’s work did not involve plasticity in the respiratory circuitry per se (Goshgarian, 2003). Since, induction of the CPP occurs within seconds to minutes following hemisection and contralateral phrenicotomy in many different mammalian species (see Goshgarian, 2003 for a complete literature listing), the CPP was classically thought of as a simple respiratory reflex in which changes in descending respiratory discharge had a temporary affect on modulating respiratory output. Once the modulatory stimulus was removed, the affect on enhancing respiratory output was reversed (Aserinsky, 1961; Goshgarian, 1981). There was no long-term or permanent change on respiratory output performance (i.e., “plasticity”, as defined by Mitchell and Johnson, 2003) that would persist after the inducting stimulus was removed regardless of how long the inducing stimulus was applied (several hours to weeks). The first demonstrations of plasticity associated with the CPP were not published until more than 90 years after Porter’s work.
In 1976, Guth noted that although the CPP could be readily induced in many different mammalian species, it was not readily elicited in guinea pigs (see also Rosenblueth and Ortiz, 1936). When spinal hemisection is followed within minutes by contralateral phrenictomy in anesthetized, spontaneously breathing guinea pigs, both sides of the diaphragm remain paralyzed and these animals always die of asphyxia (Guth, 1976; Rosenblueth and Ortiz, 1936) Guth (1976) found that the CPP could be induced in guinea pigs if an interoperative interval of several months elapsed between the spinal hemisection and contralateral phrenicotomy. In a subsequent study, Goshgarian and Guth (1977) showed the CPP could be induced when the interoperative interval was as short as 3.5 hours in guinea pigs. The above studies suggested that the spinal cord pathway that mediated the CPP in guinea pigs was normally present in these animals (as it is in all other mammalian species), but in this case the synaptic connections of the pathway were initially “functionally ineffective”. The authors reasoned that some change (e.g. plasticity) occurs in the respiratory circuitry within hours after spinal hemisection that converted the ineffective synaptic connections to “latent connections”, termed as such because they did not restore function to the ipsilateral hemidiaphragm until the animal was subjected to asphyxia by contralateral phrenicotomy several hours after the spinal cord injury. Thus, there were specific plasticity-related alterations that occurred in the respiratory neural circuitry within hours after injury that permanently changed the system response to the modulatory stimulus (i.e. asphyxia).
Over the next 20 years, (mid 1970’s to mid 1990’s) Goshgarian and colleagues showed that functionally ineffective synapses were also found in the crossed phrenic pathway of rats and through a series of correlative quantitative physiological and anatomical studies at both EM and TM levels (Goshgarian, 1979; Goshgarian and Rafols, 1981, 1984; Goshgarian et. al., 1989; O’Hara and Goshgarian, 1991; Sperry and Goshgarian, 1993) these authors suggested how specific spinal cord hemisection-induced morphological changes in the phrenic nucleus could be related to the conversion of functionally ineffective synapses in the crossed phrenic pathway. Furthermore, by employing a reversible cold conduction block of the spinal cord at C2 instead of a hemisection, they showed that similar morphological changes could be induced in the phrenic nucleus and thus, argued that the morphological alterations are phrenic nucleus “activity-dependent” rather than spinal cord “injury-induced”. (Castro-Moure and Goshgarian, 1996, 1997). Specifically, both hemisection and cold block induced the following ultrastructural changes in the cytoarchitecture of the phrenic nucleus as determined by quantitative morphometric analysis: 1) an increase in both the length and number of dendrodendritic appositions attributed to an active retraction of astroglial processes that normally intervene between adjacent phrenic dendrites, 2) an increase in the number of double, triple and quadruple synapses in the phrenic nucleus defined as single synaptic terminals contacting either two, three or four post-synaptic profiles in the same plane of section respectively, and 3) an increase in the length of synaptic contacts on phrenic motoneurons.
In was hypothesized that functionally ineffective synaptic terminations of the crossed phrenic pathway may be quantitatively insufficient to fully depolarize phrenic motoneurons in spinal hemisected adult rats. Thus, when the animals are subjected to contralateral phrenicotomy within minutes after hemisection, the summated action potentials of the crossed phrenic input is insufficient to fire the cells and the animals usually die of asphyxia from a completely paralyzed diaphragm. Within hours after spinal cord injury or cold block, however, astroglial process retraction and increases in phrenic membrane apposition could result in an enhanced excitability of phrenic motoneurons. We reasoned that if astroglial processes are no longer present to absorb extracellular potassium, the resulting increase in the concentration of extracellular potassium could increase phrenic neuronal membrane excitability by partial depolarization (Goshgarian et al, 1989; Sperry and Goshgarian, 1993; Goshgarian, 2003). The above postsynaptic changes coupled with the rapid presynaptic augmentation of crossed phrenic input mediated both by an increase in the length of synaptic contacts and the associated double, triple and quadruple synapse formation, could be the mechanism enabling previously ineffective synapses of the crossed phrenic pathway to depolarize phrenic motoneurons and bring about functional recovery (Goshgarian et al. 1989; Sperry and Goshgarian, 1993; Castro-Moure and Goshgarian, 1997). Most of the above studies were reviewed in detail in the first CPP review (Goshgarian, 2003). The following are some of the more recent studies demonstrating plasticity associated with the CPP.
3. Expression of the CPP in mice
Although the CPP was demonstrated in many mammalian species (Goshgarian, 2003), it was never demonstrated in mice. Recently, Minor et al. (2006) not only demonstrated the CPP in mice but also showed that there may be functionally ineffective synapses in the crossed phrenic pathway of mice since the CPP is either not expressed at all or it is only weakly expressed when spinal hemisection is followed within minutes by contralateral phrenicotomy in this species. However, the percentage of mice showing the CPP increased significantly when the interoperative interval between C2 hemisection and contralateral phrenicotomy increased. When the interoperative interval was 1-2 hours, 77% of the mice expressed the CPP. This percentage increased to 86% when the interoperative period was increased to 4-8 hours and 95% of all mice tested expressed the CPP when the interoperative delay between the two surgical procedures was overnight (Minor et al., 2006). The demonstration of the CPP in mice is a notable achievement because this species is amenable to studying respiratory plasticity from a molecular genetic perspective (e.g. with transgenic and knock-out strains). In fact, the same group of investigators have recently suggested that plasminogen activators (PA) may partly mediate the synaptic plasticity that underlies the enhanced expression of the CPP within hours after hemisection (Minor and Seeds, 2008). They showed that both PA mRNAs and protein levels are enhanced in phrenic motoneurons within the first two hours after C2 hemisection in wild-type mice and that uPA knock-out mice do not demonstrate a strong CPP response as late as 6 hours after hemisection (Minor and Seeds, 2008). It will be interesting to see what additional information will be acquired as the above and other laboratories further employ the mouse model to study injury-induced plasticity in the respiratory circuitry at the molecular level.
In the last 5 years several different investigators have studied the CPP in their laboratories and at least five reviews have been written on the subject since 2003 (Goshgarian, 2003; Nantwi and Goshgarian, 2005; Zimmer et al. 2007, 2008; Lane et al., 2008). Although many of the recent studies have already been summarized in the later reviews, a significant body of work has appeared in the literature since the last review and these studies will be the focus of the remainder of the present article.
4. Drug-induced plasticity in the crossed phrenic pathway: the effects of cAMP
The recent broad interest in the CPP as a model to study recovery of respiratory function after SCI is based primarily on the discovery that various drugs can be used to activate the crossed phrenic pathway and restore function to the hemidiaphragm paralyzed by SCI without having to carry out a contralateral phrenicotomy and thus sacrifice function in the intact hemidiaphragm. The compounds that have been used to induce recovery are specific serotonin receptor agonists and antagonists, NMDA receptor antagonists and the methylxanthines, such as theophylline (Alilain and Goshgarian, 2007, 2008; Ling et al, 1994; Zhou et al, 2001; Basura et al, 2002; Fuller et al, 2005; Nantwi et al, 1996). The effects of serotonin have already been summarized in detail in an earlier review (Zimmer et al, 2008) and the role of the glutamate receptor compounds will be discussed in the next section of this review.
Interestingly, when theophylline is administered acutely (a single systemic administration) in rats, it activates the crossed phrenic pathway transiently and its effects on inducing recovery only lasts for about 3 hours (Nantwi et al, 1996). However, when theophylline is administered chronically (3 times/day orally for as little as three consecutive days) it induces plasticity in the respiratory pathways which results in recovery that persists weeks after the animal has been weaned from the drug (Nantwi et al, 2003). Specifically, Nantwi et al showed that three days of chronically administered theophylline could induce recovery that persisted for 3, 7, 12 and 30 days after the last drug administration in separate groups of rats. This above drug-induced plasticity study is in marked contrast to earlier studies demonstrating that the modulatory actions of asphyxia induced by placing a topical anesthetic on the phrenic nerve contralateral to hemisection (Aserinsky, 1961; Goshgarian, 1981) or crushing the phrenic nerve (Goshgarian, 1981) are not permanent. Regardless of how long the modulatory effects are induced (i.e., hours to weeks), when the stimulus is removed, the expression of the CPP is attenuated (Aserinsky, 1961; and Goshgarian, 1981). However, weaning a rat from theophylline after as little as a 3-day exposure to the drug will not attenuate activity transmitted over the crossed phrenic pathway (Nantwi et al, 2003). From the above studies, one can conclude that a long duration of CPP activation alone will not lead to its persistent expression. Rather, theophylline causes a specific change in the respiratory circuitry that allows for a persistent expression of the CPP after the animal is weaned from the drug. The underlying mechanisms for this drug-induced plasticity in the respiratory circuitry have been the subject of recent investigations in the Goshgarian laboratory (Kajana and Goshgarian, 2008a, 2008b, 2009).
Theophylline is both a non-selective phosphodiesterase inhibitor and an adenosine receptor antagonist that has an equal affinity for blocking both the A1 and A2 adenosine receptors. Both actions of this drug can lead to an increase in 31 - 51 – cyclic adenosine monophosphate (cAMP) levels by blocking phosphodiesterase enzymes normally responsible for the cellular breakdown of cAMP (Horn and McAfee, 1977) or by inhibiting adenosine A1 receptors which normally prevent the synthesis of cAMP (Marks et al, 2005). Elevated levels of cAMP can be a powerful mediator of synaptic plasticity in the CNS. Following spinal cord injury, administration of cAMP analogs as well as drugs that elevate cAMP levels in neurons have been shown to promote regeneration and functional recovery (Pearse et al, 2004; Nikulina et al, 2004; Qiu et al, 2002a). Furthermore, increased cAMP levels have been associated with improvements in respiratory neural function (Ruangkittisakul and Ballanyi, 2006). We therefore hypothesized that theophylline-induced respiratory plasticity in C2 hemisected rats leading to the persistent recovery of the ipsilateral hemidiaphragm may be mediated by the drug’s non-selective inhibition of phosphodiesterase enzymes and/or by the blockade of adenosine A1 receptors, both of which can increase cAMP levels. We tested this hypothesis in three studies.
In the first study (Kajana and Goshgarian, 2008a), we set out to determine which of the two actions of chronically administered theophylline (phosphodiesterase inhibition or adenosine receptor antagonism) is responsible for mediating the persistent recovery. In separate groups of C2 hemisected animals, we administered 1) a non-selective phosphodiesterase inhibitor (pentoxifylline, 20mg/kg orally, 3 times/day for 3 days), 2) a selective phosphodiesterase 4-specific inhibitor (rolipram, 2mg/kg, intraperitoneal (i.p.) injection 2 times/day for 3 days) or 3) a selective adenosine A1 receptor antagonist (DPCPX, 0.1mg/kg, i.p. 2 times/day for 3 days. Half of the rats in each group were assessed for respiratory recovery 5 days after the last drug administration while the other half were not assessed for recovery until 10 days after the last drug administration. Recovery was assessed by recording from the phrenic nerve ipsilateral to the C2 hemisection under standardized physiological recording conditions. In every case functional confirmation of a complete hemisection was made prior to drug administration. There was no respiratory-related activity detected ipsilateral to hemisection in any animal prior to drug administration. The results indicated that there was recovery at both time points in all 3 drug treatment groups. Thus, both actions of theophylline are capable of inducing persistent recovery in the CPP spinal cord injury model (Kajana and Goshgarian, 2008a).
In the second study (Kajana and Goshgarian, 2009) we set out to determine whether cAMP levels could be modulated after a single administration of rolipram (2mg/kg, IV) and whether a single dose of rolipram could induce recovery in our spinal cord injury model as we have demonstrated in our early theophylline studies (Nantwi et al., 1996). The results indicated that rolipram restored respiratory-related activity to the left phrenic nerve made quiescent by hemisection. In addition, the single dose of rolipram increased cAMP levels at the level of the phrenic nucleus in the spinal cord and in the medulla at the level of the rVRG as measured by ELISA. In spite of the elevation of cAMP levels and induction of recovery, it is important to note that a single administration of rolipram (or theophylline) will not induce the persistent recovery that we have demonstrated following chronic drug administration. Moreover, it is important to stress that an elevation of cAMP in the medulla at the level of the rVRG and in the spinal cord at the level of the phrenic nucleus does not necessarily mean that cAMP levels are increasing in rVRG and phrenic motoneurons specifically. The above studies do not establish a cause-effect relationship and should be interpreted with caution. Thus, a simple correlation of elevated cAMP levels with functional recovery is not sufficient to understand the underlying mechanisms leading to persistent recovery. There is a need to further explore the intracellular mechanisms by which an increase in cAMP could mediate its effects.
To gather more evidence for the intracellular involvement of cAMP in the activation of the latent crossed phrenic pathway in a third study (Kajana and Goshgarian, 2008b), a cAMP analog, 8-Br-cAMP, was administered intrathecally into the spinal cord at the level of the phrenic nucleus. The cAMP analog induced recovery in C2 spinal cord hemisected rats. Furthermore, the cAMP-PKA intracellular signaling cascade was implicated by pre-treating other animals with a PKA inhibitor, Rp-8-Br-cAMP, before the cAMP analog was administered. Pre-treatment with the PKA inhibitor totally abolished the cAMP analog-induced recovery. Thus, PKA activation appears to be necessary for the cAMP-mediated recovery in the CPP spinal cord injury model (Kajana and Goshgarian, 2008b).
The PKA pathway can modulate both short and long-term synaptic plasticity (Huang and Kandel, 1998; Lee et al, 2000; Yamamoto et al, 2005). PKA may regulate short term effects on synaptic function by modulating ion channels. The cAMP-PKA system can increase excitatory glutamate neurotransmission by increasing cationic currents through the NMDA and AMPA receptors (Shao et al, 2003). PKA has also been shown to phosphorylate AMPA receptor subunits and increase the open probability of AMPA receptors (Banke et al, 2000). In addition to modulating short-term synaptic efficacy, PKA activation can also mediate long-lasting changes in synaptic transmission by regulating new protein synthesis. For instance, activation of the cAMP-PKA pathway can lead to the induction of Brain Derived Neurotrophic Factor (BDNF) and recently Baker-Herman et al. (2004) showed that BDNF is both necessary and sufficient for enhanced phrenic motor output (i.e., phrenic long term facilitation, pLTF) following chronic intermittent hypoxia. The association of the cAMP-PKA pathway-induced up regulation of BDNF resulting in an enhancement of phrenic motor output over the long term is an extremely important issue which we are currently pursuing in the laboratory to completely understand the underlying mechanisms associated with drug-induced plasticity and persistent recovery in the CPP spinal cord injury model.
5. Spontaneous Recovery of hemidiaphragmatic function after ipsilateral spinal cord hemisection: role of glutamate receptor subunit plasticity
Another aspect of plasticity associated with the CPP spinal cord injury model was discovered in 1940 by Pitts and reconfirmed by Nantwi and Colleagues in 1999. These authors showed that recovery of the hemidiaphragm paralyzed by C2 spinal hemisection can occur spontaneously without any additional intervention provided a sufficient time is allowed to elapse after the hemisection. Specifically, in the Nantwi et al. study (1999), spontaneous recovery did not occur in any of the animals (i.e., female Sprague-Dawley rats) tested 4 weeks after C2 hemisection. However, spontaneous recovery was detected qualitatively in the ipsilateral phrenic nerve and hemidiaphragm in 2 of 5 spontaneously breathing rats at 6 weeks post injury, 2 of 4 animals at 8 weeks post injury, 4 of 5 animals at 10 weeks after injury and in all animals at 12 (6 of 6) and 16 (4 of 4) weeks post hemisection. Moreover, the extent of spontaneous recovery of respiratory-related function in the ipsilateral phrenic nerve was assessed quantitatively under standardized recording conditions (i.e., bilateral vagotomy, paralysis with pancuronium bromide, artificial ventilation, constant body temperature, and constant PCO2 maintained at 25-28mm Hg) in a separate group of animals. The quantitative analysis of recovery was assessed at the latest post-hemisection interval tested (16 weeks). The mean area under the rectified and integrated phrenic burst was expressed as 1) a percent of activity in the contralateral phrenic nerve in the same hemisected animal (56.76 ± 5.10 percent) and 2) a percent of activity in the homolateral nerve of non-injured control rats (28.75 ± 3.10 percent). The hemisected animals had a mean respiratory frequency (55.7 ± 1.22 bursts/min) higher than the control rats (41.08 ± 1.18 bursts/min).
Two recent studies have been published (Alilain and Goshgarian, 2007, 2008) which have begun to investigate the underlying mechanisms associated with this spontaneous recovery of hemidiaphragm function in chronically injured animals.
Since the descending bulbospinal respiratory pathways including the crossed phrenic pathway are glutamatergic (McCrimmon et al., 1989), it seemed reasonable to begin an investigation of glutamate receptor plasticity on phrenic motoneurons which may help to explain the spontaneous expression of the CPP. Both N-methyl-D-aspartate (NMDA) and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptors and their subunits are found on phrenic motoneurons (Robinson and Ellenber, 1997). The NMDA receptor has been thought of as a “coincidence detector” of simultaneous pre- and post–synaptic activity since NMDA receptor activation requires pre-synaptic glutamate release and post-synaptic membrane depolarization. This makes the NMDA receptor an ideal candidate for mediating long term potentiation (LTP) (Seeburg et al, 1975; Teng et al,2006).
The NMDA receptor complex is formed by distinct subunits: the NRI, NR2A,B,C,D and NR3 subunits which confer distinctive properties to the NMDA receptor (Monyer et al, 1992). Following contusive spinal cord injury at T8, the mRNA levels of the NR2A subunit are upregulated caudal to the site of injury, and the upregulation has been correlated with improved hindlimb function suggesting that the upregulation strengthens excitatory synaptic connections on hindlimb motoneurons (Grossman et al, 2000). Later studies demonstrated that the NR2A subunit is a possible mediator of synaptic strengthening during LTP (Liu et al, 2004; Massey et al. 2004).
AMPA receptor complexes are composed of four subunits, GluR1,2,3 and 4 which confer distinct properties to the AMPA receptor. For instance, the GluR3/GluR4 heteromer receptor complex is continuously delivered to the synapse, regardless of synaptic activity. However, the GluR1/GluR2 heteromer complex is delivered to the synapse on the basis of synaptic activity, particularly through NMDA receptor activation (Esteban, 2003). Furthermore, the GluR1 subunit is delivered to the post-synaptic membrane during LTP (Hayashi et al, 2000) and the GluR2 subunit is downregulated following traumatic CNS injury, including T8 spinal cord injury (Grossman et al, 1999; Gorter et al, 1997).
Based on the above discussion, we hypothesized that following C2 spinal cord hemisection there may be an increase of the NR2A subunit and a downregulation of GluR2 at the level of the phrenic nucleus to initiate synaptic strengthening in the crossed phrenic pathway (Alilain and Goshgarian, 2008). Subsequently, an upregulation of GluR1 could result in enhanced glutamatergic drive to phrenic motoneurons bringing about spontaneous recovery of the hemidiaphragm ipsilateral to C2 hemisection in chronically injured animals.
To test this hypothesis, different groups of female rats were hemisected at C2 and allowed to recover for 4, 6, 12 and 16 weeks. Following the assessment of recovery, protein levels of NR2A, GluR1 and GluR2 subunits were assessed by Western blot analysis and immunohistochemistry on labeled phrenic neurons (Alilain and Goshgarian, 2008).
The physiological results indicated that there was no spontaneous recovery in any of the 4 week animals, but some of the animals showed recovery at 6 weeks and the majority of the rats displayed recovery by 12 and 16 weeks post hemisection. These results were similar to those obtained by Nantwi et al (1999). Western blot analysis indicated that at 4 weeks the level of NR2A was not significantly higher than controls, but at six and twelve weeks there were statistically significant increases of NR2A in the spinal cord at the level of the phrenic nucleus compared to controls. However, at 16 weeks post hemisection, there was a drop in the level of NR2A subunit to a level not significantly different from control. Western blot analysis of GluR1 and GluR2 AMPA receptor subunits at the same time points indicated that there was no significant change from control levels at 4 weeks. However, at 6 weeks there was a significant increase in the GluR1 AMPA receptor subunit in the spinal cord at the level of the phrenic nucleus ipsilateral to hemisection as compared to control. Furthermore, this increase persisted out to the latest time point studied (16 weeks). For the GluR2 subunit, there was no significant changes four, six and twelve weeks after injury. However, by the 16 week time-point, there was a significant decrease of GluR2 levels compared to controls (Alilain and Goshgarian, 2008). The above Western blot quantitative analysis was confirmed qualitatively by immunochemistry suggesting that similar changes occur specifically on phrenic motoneurons (Alilain and Goshgarian, 2008). Furthermore, double immunofluorescence showed that activity-regulated cytoskeletal associated protein (Arc) was co-localized in phrenic motoneurons along with the NR2A subunit. Since others have shown that Arc is immediately transcribed following the onset of synaptic stimulation and LTP induction protocols, and is localized to newly activated excitatory post-synaptic membranes through NMDA receptor activation (Steward et al, 1998; Steward and Worley, 2001), it was suggested that Arc could regulate AMPA receptor subunit synthesis and its delivery to the crossed phrenic synaptic pathway after chronic spinal cord hemisection in a similar manner (Alilain and Goshgarian, 2008). This would strengthen synaptic connections of the crossed phrenic pathway and allow for the spontaneous deplorization of phrenic motoneurons ipsilateral to hemisection in the chronic spinal cord injury model.
As pointed out in the Alilain and Goshgarian (2008) study, glutamate receptor subunit plasticity may only be part of the underlying mechanism leading to spontaneous recovery of hemidiaphragm function after chronic high cervical spinal cord injury. Other neurotransmitter systems such as serotonin may also be involved as explained in detail in this study.
To further associate the functional expression of the crossed phrenic pathway with glutamate receptor subunit plasticity, MK-801, an antagonist of the NMDA receptor was used in another study in acutely injured C2 hemisected adult rats to determine if the drug would induce an upregulation of the NR2A subunit and also recovery of the paralyzed hemidiaphragm (Alilain and Goshgarian, 2007). Recall that the NR2A subunit is a possible mediator of synaptic strengthening during LTP (Liu et al., 2004; Massey et al., 2004), and we hypothesized that its upregulation several weeks after hemisection mediates synaptic strengthening in the crossed phrenic pathway (Alilain and Goshgarian, 2008). We did not detect an upregulation of NR2A until six weeks after hemisection (Alilain and Goshgarian, 2008). If we could induce NR2A sooner with a drug, recovery could perhaps be accelerated. This was the focus of our acute study (Alilain and Goshgarian, 2007). Previously, MK-801 was shown to induce an upregulation of the NR2A subunit in neonatal rats (Wilson et al., 1998), but this action was never before demonstrated in adults. To develop a dose-response curve, adult non-injured rats were treated with varying doses of MK-801 and their spinal cords harvested and assessed for NR2A as well as AMPA GluR1 and GluR2 subunit protein levels. In the second part of the study, one week after injury, C2-hemisected animals received MK-801. Following treatment the animals were assessed for drug-induced recovery of the hemidiaphragm through electromyographic recordings and their spinal cords assessed for NR2A, GluR1 and GluR2.
Dose-response results indicated that there was a significant upregulation of the NR2A subunit following 0.25 or 0.5 mg/kg MK-801 injected intraperitoneally once per day for 2 days. The other doses (0.125 and 1.0 mg/kg) did not induce an upregulation of NR2A compared to saline-injected controls (Alilain and Goshgarian, 2007). The AMPA receptor subunits, GluR1 and GluR2 were not affected by MK-801 treatment at any dosage in non-injured animals. In C2-hemisected rats, treatment with either 0.125 or 0.5 mg/kg MK-801 for 2 consecutive days starting 1 week after hemisection resulted in a significant increase of NR2A compared to saline-treated animals. Treatment with either dose of MK-801 resulted in no change of the AMPA GluR1 subunit compared to saline-treated animals. However, administration of 0.5 mg/kg MK-801 to hemisected rats resulted in a significant decrease of the GluR2 subunit. Hemisected rats that received the 0.125 mg/kg MK-801 dosage did not have a significant change in the expression of GluR2. Ten days following hemisection and treatment with vehicle there were no significant changes of NR2A, GluR1 and GluR2 compared to non-injured animals. Immunocytochemical analysis qualitatively supported all of the above Western blot findings (Alilain and Goshgarian, 2007) and suggested that similar changes were occurring specifically in phrenic motoneurons.
Animals treated with 0.5 mg/kg MK-801 for 2 days starting 7 days after hemisection showed a return of hemidiaphragm motor function by EMG analysis in 8 of 11 animals tested. At the lesser dose, 0.125 mg/kg, recovery was present in only 1 out of 6 animals tested. Treatment with vehicle resulted in no recovery in any animal.
From the above results, it can be concluded that administration of MK-801 leads to an increase in the NR2A subunit and this, in turn restores recovery to the hemidiaphragm paralyzed by C2 spinal cord hemisection. Buck et al (2006) showed that treatment of adult rats with MK-801 led to an enhancement of LTP at the CAl-subiculum synapse. Since it has been demonstrated that the NR2A subunit is the primary mediator of LTP (Liu et al. 2004), it is plausible that upregulation of NR2A through MK-801 treatment mediates a form of LTP in the C2-spinal cord hemisection injury model. Finally, in both the chronic spinal hemisection model (Alilain and Goshgarian, 2008) as well as the study involving MK-801 treatment of acutely injured rats (Alilain and Goshgarian, 2007), there was a reduction of the AMPA GluR2 subunit along with the upregulation of the NMDA NR2A subunit. The GluR2 subunit is the calcium gate of the AMPA receptor. It has been demonstrated that reductions of the GluR2 subunit lead to increases of calcium ion influx and an enhanced expression of LTP (Jia et al., 1996). Thus, it is possible that the above changes in glutamate receptor subunits could lead to the induction of changes associated with synaptic strengthening in phrenic motor neurons and the enhancement of LTP-like mechanisms. The resulting increase in synaptic strength and efficacy in turn would lead to recovery of hemidiaphragmatic activity through strengthening of the crossed phrenic synapse.
6. Light-induced Rescue of Breathing after Spinal Cord Injury
Alilain et al. (2008) have recently introduced a new and exciting approach to studying plasticity and recovery of respiratory function after spinal cord injury. Using the C2 spinal hemisection model in rats, they tranduced spinal neurons in and around the phrenic nucleus to express channelrhodopsin-2 (ChR2) ipsilateral and caudal to the hemisection. ChR2 is an algal protein light-activated cation channel which can be infected in mammalian neurons by viral gene delivery protocols. The above authors tested the hypothesis that after C2 hemisection and infection of spinal neurons at the level of the ipsilateral phrenic nucleus to express ChR2, patterned photostimulation would lead to recovery of motor function and a return of hemidiaphragmatic activity through direct or indirect stimulation of phrenic motor neurons or potentiation of the phrenic nucleus to spared inputs (Alilain et al. 2008). The authors clearly show that 4 days after injection of the viral vector, light activation of ChR2- expressing rats was sufficient to bring about respiratory-related diaphragmatic activity. The pattern and frequency of light stimulation dictated the extent and duration of recovery after the stimulation was ceased. The evidence presented also suggested that this new form of respiratory plasticity is NMDA receptor dependent since the NMDA antagonist, MK-801 applied to the spinal cord shortly before light stimulation totally blocked the recovery response in infected animals.
Briefly, the initial protocol for light stimulation included sustained exposure of light (1 min) on the exposed dorsal surface of the C3-C6 spinal cord. In addition, the authors tried intermittent exposure to light at about once per second for 1 minute. The authors found, however, that recovery was more extensive and long-lasting (greater than 1 hr) when the following light stimulation protocol was used: alternating 5 min rest/5 min intermittent light stimulation for 3-4 cycles (30-40 min total) (Alilain et al., 2008). Interestingly, this patterned intermittent light stimulus protocol is similar to the intermittent hypoxia protocol which induces phrenic long-term facilitation (pLTF) (Fuller et al, 2003). The intermittent light stimulation was at 0.5 Hz with each flash of light lasting about 1 sec long. Another interesting aspect of this study was discovered when this pattern of light stimulation was employed. A small amount of EMG activity would appear in the ipsilateral (paralyzed) hemidiaphragm about 30 to 90 sec from the start of the stimulation. The EMGs waxed and waned in intensity, but gradually increased in intensity over time. Moreover, EMG recordings from both sides of the diaphragm showed an unique alternating interaction. As intense activity on the lesioned side would decrease, EMG activity on the opposite side would increase (see Fig. 3B, C in Alilain et al, 2008). The waxing and waning of activity slowly disappeared until recovery in the paralyzed hemidiaphragm closely resembled activity in the contralateral hemidiaphragm. The near-normal bilateral EMG activity then lasted at least 2 hours. Bilateral EMG activity was present the next day, but at a much lower level (Alilain et al., 2008).
The method of injecting the virus into the cervical cord may have had an effect on the present results. The authors made 3 injections of Sandbis virus (250 nl per injection) containing either the dual ChR2-GFP vector or the green fluorescent protein (control) vector alone into the dorsal surface of the spinal cord at the C3-C6 levels. The injections were made 0.11 cm from the midline and 0.16 cm ventral from the dorsal surface of the spinal cord. There was no indication of how far apart the injections were spaced. The virus injection infected about 650 cells/animal identified as ventral horn motor neurons including phrenic neurons as well as propriospinal neurons. Many of the infected cells had processes that approached the spinal cord midline and the authors were able to follow some labeled propriospinal neuron dendrites across the midline into the contralateral cord. Although some phrenic motor neurons were unequivocally labeled by double immunofluorescence, attempts to refine the virus delivery method (e.g. by using a double-barrel recording electrode and recording respiratory-related activity from phrenic motor cells before injecting) may improve the extent of recovery in future studies. It could also alter the pattern of recovery described above.
Alilain has considerable experience working on the plasticity of glutamate receptors and their subunits in the respiratory system after spinal cord injury (Alilain and Goshgarian 2007, 2008, see also the previous section in this review). The observation that MK-801, an NMDA receptor antagonist blocked the cycling of increasing diaphragmatic activity after photostimulation suggests that the NMDA receptor is involved in the mechanism underlying this newly described aspect of respiratory plasticity and recovery. It is possible that when the ChR2 induces cell depolarization by photostimulation, magnesium ions blocking the NMDA channel may be released. The result would be an influx of calcium ions through the NMDA receptor which could initiate a wide-array of intracellular signaling cascades (see Fig. 5 in Alilain et al, 2008) leading to synaptic strengthing. What the precise down-stream mechanisms are is still unknown, but undoubtedly, this new model of plasticity in the respiratory system will be useful in determining the specific components of the signaling cascade in many useful future studies to come.
7. Developmental Plasticity of the Crossed Phrenic Pathway
Although the crossed phrenic pathway is latent and non-functional in adult rats subjected to C2 spinal hemisection, it was demonstrated that the neural pathway in neonatal brainstem-spinal cord preparations is active functionally (Zimmer and Goshgarian, 2005). The in vitro model was used to demonstrate spontaneous crossed phrenic activity following a complete C2 hemisection from postnatal days 0-4 (PO-P4, Zimmer and Goshgarian, 2005). The study also showed that the spontaneous crossed phrenic activity is age-dependent because younger preparations (P0-P2) exhibited a significant higher incidence of crossed phrenic activity than older preparations (P3-P4) (Zimmer and Goshgarian, 2005). Since the brainstem-spinal cord preparation has been criticized as not reflecting true respiratory-related activity (Li and Duffin, 2004), there is a possibility that what was observed in the in vitro study (Zimmer and Goshgarian, 2005) may not be observed in vivo. However, numerous studies have suggested that the organization of the central respiratory network in perinatal rats and neonatal rats is different from adult rats and some non-functional neural projections are eliminated during development (Greer et al, 1999; Cameron and Nunez-Abades, 2000;). Since the latent crossed phrenic pathway still exists and has not been eliminated in adult rats (Moreno et al, 1992) we hypothesized that the crossed phrenic pathway may be spontaneously functional in neonatal rats, otherwise it would have been eliminated. Furthermore, we hypothesized that activity mediated over the crossed phrenic pathway (i.e., “crossed phrenic activity”) may be converted from a spontaneously active state to a latent and non-functional state during postnatal development. These ideas prompted a time course study designed to analyze crossed phrenic activity in rat pups at different ages (Huang and Goshgarian, 2009). The functional status of the ipsilateral and contralateral hemidiaphragm was tested by EMG analysis following C2 hemisection in spontaneously breathing rat pups. Crossed phrenic activity was expressed in ventral (sternal), lateral and dorsal (crural) parts of the ipsilateral hemidiaphragm in all P2 and some P3 and P4 neonatal rats tested. The crossed phrenic activity was spontaneously expressed and did not need to be induced by any intervention (e.g. hypoxia, contralateral phrenicotomy, etc.). During further postnatal development, the activity was observed only in the ventral area of the ipsilateral hemidiaphragm in P7, P14, P21 and P28 animals. There was no activity detected in either the lateral or dorsal areas of the hemidiaphragm ipsilateral to hemisection in any of the animals from P7-P28 (Huang and Goshgarian, 2009). Semi-quantitative analysis of EMG activity demonstrated that there was a significant reduction in the extent of crossed phrenic activity from P2 to P28. The pathway generating this activity becomes completely latent (i.e., the adult status) by postnatal day 35 (Huang and Goshgarian, 2009). Additional studies are underway to analyze differences in the anatomical components of the pathway during development as well as changes in glutamate receptors on phrenic motor neurons that may help to explain the above differences in the expression of the crossed phrenic pathway during postnatal development.
8. Summary
Over the last 5 years, there has been a considerable increase in using the crossed phrenic phenomenon as a means of exploring plasticity and recovery of respiratory function after spinal cord injury. This review has summarized only a small portion of the total work that has recently emerged in this area. Since many of the investigators who have utilized the CPP in their recent studies will also be contributing reviews in this special issue of Respiratory Physiology and Neurobiology, there was a conscious effort not to include some of that work in this review to avoid redundancy. Some of this work, however, is directly translatable to the clinical level suggesting possible therapeutic strategies that are now ready for testing on human subjects while other work has given us new insight into the intracellular signaling cascades involved in synaptic strengthening that may be targeted for future therapeutic interventions (Baker-Herman et al., 2004; Alilain et al. 2008). With the mouse CPP model, we have a new tool to study plasticity and recovery of respiratory function at the molecular level using a genetic approach (Minor and Seeds, 2008). The similarities in recovery following chronic intermittent hypoxia (Fuller et al.,. 2003) and chronic photostimulation (Alilain et al. 2008) are remarkable and suggest several common pathways that investigators may focus on to induce recovery not only in the respiratory motor system, but in other motor systems following spinal cord injury as well (Goshgarian, 2003; Alilain et al. 2008). Finally, understanding the underlying mechanisms behind the spontaneous expression of the crossed phrenic pathway either in the developing animal (Huang and Goshgarian, 2009) or after chronic spinal cord injury in the adult animal (Alilain and Goshgarian, 2007, 2008) may provide clues to initiating respiratory recovery sooner to alleviate suffering and eventually eliminate the leading cause of death in human cases of spinal cord injury.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aerinsky E. Effect of usage of a dormant respiratory nerve pathway upon its subsequent activity. Exp Neurol. 1961;3:467–475. doi: 10.1016/0014-4886(61)90022-x. [DOI] [PubMed] [Google Scholar]
- Alilain WJ, Goshgarian HG. MK-801 upregulates NR2A protein levels and induces functional recovery of the ipsilateral hemidiaphragm following acute C2 hemisection in adult rats. J Spinal Cord Med. 2007;30:346–354. doi: 10.1080/10790268.2007.11753950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alilain WJ, Goshgarian HG. Glutamate receptor plasticity and activity-regulated cytoskeleton associated protein regulation in the phrenic motor nucleus may mediate spontaneous recovery of the hemidiaphragm following chronic cervical spinal cord injury. Exp Neurol. 2008;212:348–357. doi: 10.1016/j.expneurol.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alilain WJ, Li X, Horn KP, Dhingra R, Dick TE, Herlitze S, Silver J. Light-induced rescue of breathing after spinal cord injury. J Neurosci. 2008;28:11862–11870. doi: 10.1523/JNEUROSCI.3378-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Banke TG, Bowie D, Lee H, Huganir RL, Schousboe A, Traynelis SF. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci. 2000;20:89–102. doi: 10.1523/JNEUROSCI.20-01-00089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basura GJ, Nantwi KD, Goshgarian HG. Theophylline-induced respiratory recovery following cervical spinal cord hemisection is augmented by serotonin 2 receptor stimulation. Brain Res. 2002;956:1–13. doi: 10.1016/s0006-8993(02)03097-4. [DOI] [PubMed] [Google Scholar]
- Baussart B, Stamegna JC, Polentes J, Tadié M, Gauthier P. A new model of upper cervical spinal contusion inducing a persistent unilateral diaphragmatic deficit in the adult rat. Neurobiol Dis. 2006;22:562–574. doi: 10.1016/j.nbd.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Buck N, Cali S, Behr J. Enhancement of long-term potentiation at CAI-subiculum synapses in MK-801-treated rats. Neurosci Lett. 2006;392:5–9. doi: 10.1016/j.neulet.2005.08.054. [DOI] [PubMed] [Google Scholar]
- Cameron WE, Nunez-Abades PA. Physiological changes accompanying anatomical remodeling of mammalian motoneurons during postnatal development. Brain Res Bull. 2000;53:523–527. doi: 10.1016/s0361-9230(00)00385-3. [DOI] [PubMed] [Google Scholar]
- Castro-Moure F, Goshgarian HG. Reversible cervical hemispinalization of the rat spinal cord by a cooling device. Exp Neurol. 1996;141:102–112. doi: 10.1006/exnr.1996.0143. [DOI] [PubMed] [Google Scholar]
- Castro-Moure F, Goshgarian HG. Morphological plasticity induced in the phrenic nucleus following cervical cold block of descending respiratory drive. Exp Neurol. 1997;147:299–310. doi: 10.1006/exnr.1997.6615. [DOI] [PubMed] [Google Scholar]
- El-Bohy AA, Schrimsher GW, Reier PJ, Goshgarian HG. Quantitative assessment of respiratory function following contusion injury of the cervical spinal cord. Exp Neurol. 1998;150:143–152. doi: 10.1006/exnr.1997.6757. [DOI] [PubMed] [Google Scholar]
- Esteban JA. AMPA receptor trafficking: a road map for synaptic plasticity. Mol Interv. 2003;3:375–385. doi: 10.1124/mi.3.7.375. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Baker-Herman TL, Golder FJ, Doperalski NJ, Watters JJ, Mitchell GS. Cervical spinal cord injury upregulates ventral spinal 5-HT2A receptors. J Neurotrauma. 2005;22:203–213. doi: 10.1089/neu.2005.22.203. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Golder FJ, Olson EB, Jr, Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol. 2006;100:800–806. doi: 10.1152/japplphysiol.00960.2005. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Johnson SM, Olson EB, Jr, Mitchell GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci. 2003;23:2993–3000. doi: 10.1523/JNEUROSCI.23-07-02993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Reier PJ, Davenport DW, Bolser DC. Cervical spinal cord injury alters the pattern of breathing in anesthetized rats. J Appl Physiol. 2001;91:2451–2458. doi: 10.1152/jappl.2001.91.6.2451. [DOI] [PubMed] [Google Scholar]
- Gorter JA, Petrozzino JJ, Aronica EM, Rosenbaum DM, Opitz T, Bennett MV, Connor JA, Zukin RS. Global ischemia induces downregulation of GluR2 mRNA and increases AMPA receptor-mediated Ca2+ influx in hippocampal CAl neurons of gerbil. J Neurosci. 1997;17:1779–1788. doi: 10.1523/JNEUROSCI.17-16-06179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG. Developmental plasticity in the respiratory pathway of the adult rat. Exp Neurol. 1979;66:547–555. doi: 10.1016/0014-4886(79)90201-2. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. The role of cervical afferent nerve fiber inhibition of the crossed phrenic phenomenon. Exp Neurol. 1981;72:211–225. doi: 10.1016/0014-4886(81)90139-4. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. Plasticity in respiratory motor control. Invited review: The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol. 2003;94:795–810. doi: 10.1152/japplphysiol.00847.2002. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Guth L. Demonstration of functionally ineffective synapses in the guinea pig spinal cord. Exp Neurol. 1977;57:613–621. doi: 10.1016/0014-4886(77)90093-0. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Moran MF, Prcevski P. Effect of cervical spinal cord hemisection and hemidiaphragm paralysis on arterial blood gases, pH and respiratory rate in the adult rat. Exp Neurol. 1986;93:440–445. doi: 10.1016/0014-4886(86)90206-2. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Rafols JA. The phrenic nucleus of the albino rat: a correlative HRP and Golgi study. J Comp Neurol. 1981;201:441–456. doi: 10.1002/cne.902010309. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Rafols JA. The ultrastructure and synaptic architecture of phrenic motor neurons in the spinal cord of the adult rat. J Neurocytol. 1984;13:85–109. doi: 10.1007/BF01148320. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Yu XJ, Rafols JA. Neuronal and glial changes in the rat phrenic nucleus occurring within hours after spinal cord injury. J Comp Neurol. 1989;284:519–533. doi: 10.1002/cne.902840404. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Allan DW, Martin-Caraballo M, Lemke RP. An overview of phrenic nerve and diaphragm muscle development in the perinatal rat. J Appl Physiol. 1999;86:779–786. doi: 10.1152/jappl.1999.86.3.779. [DOI] [PubMed] [Google Scholar]
- Grossman SD, Wolfe BB, Yasuda RP, Wrathall JR. Changes in NMDA receptor subunit expression in response to contusive spinal cord injury. J Neurochem. 2000;75:174–184. doi: 10.1046/j.1471-4159.2000.0750174.x. [DOI] [PubMed] [Google Scholar]
- Guth L. Functional plasticity in the respiratory pathway of the mammalian spinal cord. Exp Neurol. 1976;51:414–420. doi: 10.1016/0014-4886(76)90265-x. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Horn JP, McAfee DA. Modulation of cyclic nucleotide levels in peripheral nerve without effect on resting or compound action potentials. J Physiol. 1977;269:753–766. doi: 10.1113/jphysiol.1977.sp011927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Goshgarian HG. Postnatal conversion of crossed phrenic activity from an active to latent state. Exp Neurol. 2009 Feb 9; doi: 10.1016/j.expneurol.2009.01.024. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Postsynaptic induction and PKA-dependent expression of LTP in the lateral amygdale. Neuron. 1998;21:169–178. doi: 10.1016/s0896-6273(00)80524-3. [DOI] [PubMed] [Google Scholar]
- Jia Z, Agopyan N, Miu P, Xiong Z, Henderson J, Gerial R, Taverna FA, Velumian A, MacDonald J, Carlen P, Abramov-Newerly W, Roder J. Enhanced LTP in mice deficient in the AMPA receptor GluR2. Neuron. 1996;17:945–956. doi: 10.1016/s0896-6273(00)80225-1. [DOI] [PubMed] [Google Scholar]
- Kajana S, Goshgarian HG. Administration of phosphodiesterase inhibitors and an adenosine A1 receptor antagonist induces phrenic nerve recovery in high cervical spinal cord injured rats. Exp Neurol. 2008a;210:671–680. doi: 10.1016/j.expneurol.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajana S, Goshgarian HG. Spinal activation of the cAMP-PKA pathway induces respiratory motor recovery following high cervical spinal cord injury. Brain Res. 2008b;1232:206–213. doi: 10.1016/j.brainres.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajana S, Goshgarian HG. Systemic administration of rolipram increases medullary and spinal cord cAMP and activates a latent respiratory motor pathway after high cervical spinal cord injury. J Spinal Cord Med. 2009;32(2):175–182. doi: 10.1080/10790268.2009.11760769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Fuller DD, White TE, Reier PJ. Respiratory neuroplasticity and cervical spinal cord injury: translation a perspectives. Trends Neurosci. 2008;31:538–547. doi: 10.1016/j.tins.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Yates BJ, Fuller DD, Reier PJ. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol. 2008;511:692–709. doi: 10.1002/cne.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- Lewis LJ, Brookhart JM. Significance of the crossed phrenic phenomenon. Am J Physiol. 1951;166:241–254. doi: 10.1152/ajplegacy.1951.166.2.241. [DOI] [PubMed] [Google Scholar]
- Li YM, Duffin J. Developmental changes in transmission of respiratory rhythm in the rat. Resp Physiol Neurobiol. 2004;142:153–163. doi: 10.1016/j.resp.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Lindsay AD, Greer JJ, Feldman JL. Phrenic motoneuron morphology in the neonatal rat. J Comp Neurol. 1991;308:169–179. doi: 10.1002/cne.903080204. [DOI] [PubMed] [Google Scholar]
- Ling L, Bach KB, Mitchell GS. Serotonin reveals ineffective spinal pathways to contralateral phrenic motoneurons in spinally hemisected rats. Exp Brain Res. 1994;101:35–43. doi: 10.1007/BF00243214. [DOI] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Marks GA, Birabil CG, Speciale SG. Adenosine A1 receptors mediate inhibition of cAMP formation in vitro in the pontine, REM sleep induction zone. Brain Res. 2005;1061:124–127. doi: 10.1016/j.brainres.2005.08.038. [DOI] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon DR, Smith JC, Feldman JL. Involvement of excitatory amino acids in neurotransmission of inspiratory drive to spinal respiratory motoneurons. J Neurosci. 1989;9:1910–1921. doi: 10.1523/JNEUROSCI.09-06-01910.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor KH, Akison LK, Goshgarian HG, Seeds NW. Spinal cord injury-induced plasticity in the mouse-the crossed phrenic phenomenon. Exp Neurol. 2006;200:486–495. doi: 10.1016/j.expneurol.2006.02.125. [DOI] [PubMed] [Google Scholar]
- Minor KH, Seeds NW. Plasminogen activator induction facilitates recovery of respiratory function following spinal cord injury. Mol Cell Neurosci. 2008;37:143–152. doi: 10.1016/j.mcn.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Invited Review: Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Monyer H, Spengel R, Schoepfer R, Herb A, Higuchi M, Lorneli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Moreno DE, Yu XJ, Goshgarian HG. Identification of the axon pathways which mediate functional recovery of the paralyzed hemidiaphragm following spinal cord hemisection in the adult rat. Exp Neurol. 1992;116:219–228. doi: 10.1016/0014-4886(92)90001-7. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, Basura GJ, Goshgarian HG. Effects of long-term theophylline exposure on recovery of respiratory function and expression of adenosine A1 mRNA in cervical spinal cord hemisected rats. Exp Neurol. 2003;182:232–239. doi: 10.1016/s0014-4886(03)00109-2. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, El-Bohy A, Goshgarian HG. Actions of systemic theophylline on hemidiaphragmatic recovery in rats following cervical spinal cord hemisection. Exp Neurol. 1996;140:53–59. doi: 10.1006/exnr.1996.0114. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, El-Bohy A, Schrimsher GW, Reier PJ, Goshgarian HG. Spontaneous functional recovery in a paralyzed hemidiaphragm following upper cervical spinal cord injury in adult rats. Neurorehab Neural Repair. 1999;13:225–234. [Google Scholar]
- Nantwi KD, Goshgarian HG. Adenosinergic mechanisms underlying recovery of diaphragm motor function following upper cervical spinal cord injury: potential therapeutic implications. Neurol Res. 2005;27:195–205. doi: 10.1179/016164105X21977. [DOI] [PubMed] [Google Scholar]
- National Spinal Cord Injury Statistical Center. Spinal Cord Injury: facts and figures at a glance. J Spinal Cord Med. 2006;29:89–90. [Google Scholar]
- Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phophodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Nat Acad Sci. 2004;101:8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara TE, Jr, Goshgarian HG. Quantitative assessment of phrenic nerve functional recovery mediated by the crossed phrenic reflex at various time intervals after spinal cord injury. Exp Neurol. 1991;111:244–250. doi: 10.1016/0014-4886(91)90012-2. [DOI] [PubMed] [Google Scholar]
- Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- Pitts RF. The respiratory center and its descending pathways. J Comp Neurol. 1940;73:605–625. [Google Scholar]
- Porter WT. The path of the respiratory impulse from the bulb to the phrenic nuclei. J Physiol. 1985;17:455–485. doi: 10.1113/jphysiol.1895.sp000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash YS, Mantilla CB, Zhan WZ, Smithson KG, Sieck GC. Phrenic motoneuron morphology during rapid diaphragm muscle growth. J Appl Physiol. 2000;89:563–572. doi: 10.1152/jappl.2000.89.2.563. [DOI] [PubMed] [Google Scholar]
- Qiu J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS, Filbin MT. Spinal axon regeneration induced by elevation of cAMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- Robinson D, Ellenber H. Distribution of N-methyl-D-aspartate glutamate receptor subunits on respiratory motor and premotor neurons in the rat. J Comp Neurol. 1997;389:94–116. doi: 10.1002/(sici)1096-9861(19971208)389:1<94::aid-cne7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Rosenbleuth A, Ortiz T. The crossed respiratory impulses to the phrenic. Am J Physiol. 1936;117:495–513. [Google Scholar]
- Ruangkittisakul A, Ballanyi K. Reversal by phosphodiesterase-4 blockers of in vitro apnea in the isolated brainstem-spinal cord preparation from newborn rats. Neurosci Lett. 2006;401:194–198. doi: 10.1016/j.neulet.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Seeburg PH, Burnashev N, Köht G, Kuner T, Sprengel R, Monyer H. The NMDA receptor channel: molecular design of a coincidence detector. Recent Prog Horm Res. 1995;50:19–34. doi: 10.1016/b978-0-12-571150-0.50006-8. [DOI] [PubMed] [Google Scholar]
- Shao XM, Ge Q, Feldman JL. Modulation of AMPA receptors by cAMP-dependent protein kinase in pre Bötzinger complex inspiratory neurons regulates respiratory rhythm in the rat. J Physiol. 2003;547:543–553. doi: 10.1113/jphysiol.2002.031005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry MA, Goshgarian HG. Ultrastructural changes in the rat phrenic nucleus developing within 2 h after cervical spinal cord hemisection. Exp Neurol. 1993;120:233–244. doi: 10.1006/exnr.1993.1058. [DOI] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley P. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron. 2001;30:227–240. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- Teng YD, Liao WL, Choi H, Konya D, Sabbarwal S, Langer R, Sidman RL, Snyder EY, Frontera WR. Physical activity-mediated functional recovery after spinal cord injury: potential roles of neural stem cells. Regen Med. 2006;1:763–776. doi: 10.2217/17460751.1.6.763. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Kinsman SL, Johnson MV. Expression of NMDA receptor subunit mRNA after MK-801 treatment in neonatal rats. Brain Res Dev Brain Res. 1998;109:211–220. doi: 10.1016/s0165-3806(98)00084-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Urakubo T, Tominaga-Yoshino K, Ogura A. Long-lasting synapse formation in cultured rat hippocampal neurons after repeated PKA activation. Brain Res. 2005;1042:6–16. doi: 10.1016/j.brainres.2005.01.102. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Basura GJ, Goshgarian HG. Serotonin (2) receptors mediate respiratory recovery after cervical spinal cord hemisection in adult rats. J Appl Physiol. 2001;91:2665–2673. doi: 10.1152/jappl.2001.91.6.2665. [DOI] [PubMed] [Google Scholar]
- Zimmer MB, Goshgarian HG. Spontaneous crossed phrenic activity in the neonatal respiratory network. Exp Neurol. 2005;194:530–540. doi: 10.1016/j.expneurol.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Zimmer MB, Nantwi K, Goshgarian HG. Effect of spinal cord injury on the respiratory system: basic research and current clinical treatment options. J Spinal Cord Med. 2007;30:319–330. doi: 10.1080/10790268.2007.11753947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer MB, Nantwi K, Goshgarian HG. Effect of spinal cord injury on the neural regulation of respiratory function. Exp Neurol. 2008;209:399–406. doi: 10.1016/j.expneurol.2007.05.015. [DOI] [PubMed] [Google Scholar]