Abstract
We describe herein the synthesis and characterization of a new class of histone deacetylase (HDAC) inhibitors derived from conjugation of a suberoylanilide hydroxamic acid-like aliphatic-hydroxamate pharmacophore to a nuclear localizasion signal peptide. We found that these conjugates inhibited the histone deacetylase activities of HDACs 1, 2, 6, and 8 in a manner similar to suberoylanilide hydroxamic acid (SAHA). Notably, compound 7b showed a 3 fold improvement in HDAC 1/2 inhibition, a 3 fold increase in HDAC 6 selectivity and a 2 fold increase in HDAC 8 selectivity when compared to SAHA.
Keywords: HDAC, NLS, histone deacetylase inhibition
Histone deacetylase (HDAC) inhibition has been clinically validated as a therapeutic strategy for cancer treatment with the FDA approval of suberoylanilide hydroxamic acid (SAHA) for the treatment of cutaneous T cell lymphoma.1 HDAC inhibitors (HDACi) have shown the ability to block angiogenesis and cell cycling, initiate differentiation and apoptosis. HDACi presumably derived their biological activities through perturbation of chromatin remodeling and acetylation states of key non-histone proteins.2–7 Most HDACi, including SAHA, non-selectively inhibit the deacetylase activity of class I/II HDAC enzymes.8,9 This broad HDAC inhibition is associated with reduced potency and toxic side effects. Attempts aimed at identifying isoform selective HDACi have been modestly successful, resulting in very few HDACi that are only partially isoform selective.10–12
HDACs 1 and 2, the primary targets for the anticancer activity of HDACi, are exclusively localized in the nucleus.13, 14 Thus, the development of a strategy for nuclear delivery and localization of HDACi could be an alternative approach to isoform selective HDACi. Toward this end, we sought novel peptide-HDACi conjugates that are capable of crossing both the plasma and nuclear membrane.15 Most of the nuclear membrane-penetrating peptides described in the literature are derived from viral sources. Common examples include the Simian virus nuclear localization peptides (NLS),15–17 HIV 1 Tat-protein derived peptides,18 and peptides derived from adenovirus fiber protein. NLS peptides are primarily utilized by viruses to cross the nuclear membrane, making them an ideal candidate for HDACi conjugation. Additionally, we reasoned that NLS, with lysine-enriched sequences, could also act as a substrate-mimetic to HDAC by mimicking the N-terminal tail lysine residues of the core histones. We report here the identification of NLS-peptide derived HDACi with anti-HDAC activity and HDAC isoform selectivies that rival or better that of SAHA.
Our design approach involved conjugation of a SAHA-like aliphatic-hydroxamate HDAC inhibition group directly to the NLS peptide through 1,2,3-triazole moeity.11 This very simple initial design could facilitate a facile, high yielding synthesis of the proposed NLS-HDACi conjugates. Accordingly, we prepared compounds 7a–c having two 1,2,3-triazole rings connecting a NLS-derived peptide to a HDAC surface recognition group and a hydroxamate zinc binding group to the surface recognition group through flexible methylene linkers whose lengths are optimized for HDAC inhibition.11, 12
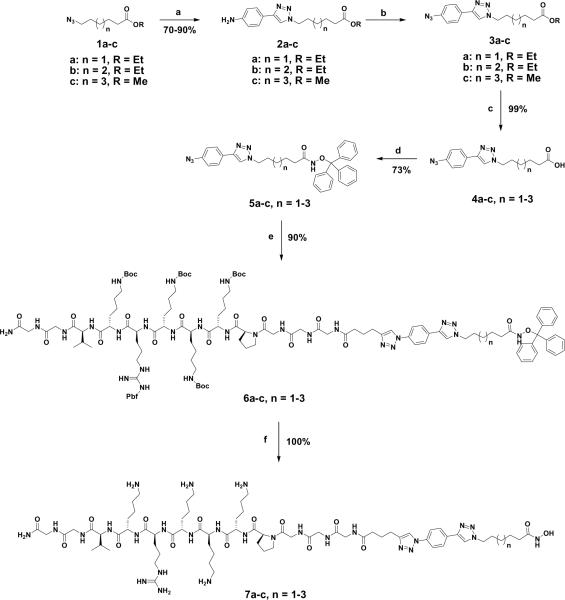
The designed conjugates 7a–c were synthesized through a six-step synthetic route as shown in Scheme 1. Cu(I)-catalyzed reaction of 4-ethynylaniline with azido esters 1a–c gave cycloadducts 2a–c in excellent yields.11, 19 The diazotization of 2a–c by treatment with sodium nitrite followed by exposure of the crude products to sodium azide led to the azido derivatives 3a–c in good yields. However, a portion of azido derivatives 3 was hydrolyzed into carboxylic acid giving a mixture of both ester and carboxylic acid derivatives. To hydrolyze the rest of the ester, lithium hydroxide hydrate was added to the mixture giving a complete conversion to the azido carboxylic acid derivatives 4a–c in excellent yields. The reaction of acid 4a–c with O-trityl hydroxylamine gave the desired O-trityl azido-triazolylhydroxamates 5a–c that were subsequently coupled to the alkyne-terminated protected NLS peptide PCS-37689-PI16 to give cycloadducts 6a–c. Exposure of cycloadducts 6a–c to TFA removed all protecting groups yielding the desired NLS-HDACi conjugates 7a–c in near quantitative yields.
Scheme 1.
Synthesis of NLS-HDACi conjugates. Conditions: (a) 4-ethynylaniline, CuI, DIPEA, THF, (b) NaNO2, NaN3, 17.2% HCl(aq), (c) LiOH•H2O, THF/H2O, (d) o-tritylhydroxylamine, EDCI, HOBT, NMM, DMF, (e) alkyne-terminated NLS peptides, CuI, TBTA, DIPEA, THF/DMF, (f) 90:5:5 TFA/TIPS/Phenol
The HDAC inhibition activity of 7a–c was tested using a cell free assay (Fluor de Lys).20 We found that the NLS-HDACi conjugates display potent HDAC inhibition activities, similar to SAHA, that is somewhat linker-length dependent against HDAC 1 and 2 from HeLa cell nuclear extract (Table 1). An increase in the linker length from C6 to C7 conferred a better anti-HDAC activity. The NLS-HDACi conjugates also presented similar isoform selectivity to that of SAHA with respect to HDAC 6 and HDAC 8 (Table 1). Compound 7b, with a C7 linker, stood out as it showed not only a 3-fold improvement in HDAC 1/2 activity when compared to SAHA, but also a 2-fold increase in HDAC 8 selectivity and a 3-fold increase in HDAC 6 selectivity.
Table 1.
HDAC inhibitory activity and isoform selectivity of SAHA and NLS-linked compounds.
| HDAC 1/2 IC50 (nM) | HDAC 8 IC50 (nM) | HDAC 8 Selectivity* | HDAC 6 IC50 (nM) | HDAC 6 Selectivity* | |
|---|---|---|---|---|---|
| SAHA | 49 | 2185 | 45 | 800 | 16 |
| 7a | 36 | 3503 | 96 | 575 | 16 |
| 7b | 14 | 1528 | 108 | 716 | 51 |
| 7c | 25 | 1243 | 50 | 714 | 29 |
Selectivity is the activity of the HDAC isoform (6 or 8) divided by the activity of HDAC 1/2.
Encouraged by the potent anti-HDAC activities of these NLS-HDACi conjugates, we then evaluated their whole cell anti-proliferative activities using trypan blue exclusion and MTT assay. Sadly, none of the conjugates exhibited any appreciable, anti-proliferative activity in DU-145 or HSC3 cell lines at drug concentrations up to 25μM in both assays (Data not shown). In an attempt to shed light on the lack of whole cell activity of these conjugates, we prepared a BODIPY tagged analog of the NLS (Supplementary Data).21 The syntheses of alkyne BODIPY and NLS-BODIPY are described in the supplementary data (schemes S1 and S2 respectively). The intracellular localization of the dye labeled NLS was then monitored using fluorescence microscopy at a variety of incubation times (Supplementary Data). It was found that the dye labeled NLS was not taken up into the nucleus; instead it was sequestered in the cytosol. This preliminary result may provide an explanation for the poor whole cell activity of the compounds.
We have described a class of novel NLS-peptide derived HDACi. All of the compounds demonstrated HDAC inhibition and isoform selectivity similar to, and in the case of 7b better than, SAHA. Despite these promising HDAC inhibitory activities, none of the compounds exhibited whole cell activity at concentrations up to 25μM. The lack of appreciable whole cell activity of these NLS-HDACi conjugates may be partly due to their cytosolic entrapment. Efforts are currently underway in our laboratory to enhance the nuclear penetration of these conjugates by optimizing their NLS sequence linker, and HDAC inhibition moiety.
Supplementary Material
Acknowledgments
This work was financially supported by Georgia Institute of Technology, by the Blanchard fellowship and by NIH Grant R01CA131217. J.C.C. and P.C.C. are recipients of the GAANN predoctoral fellowship from the Georgia Tech Center for Drug Design, Development, and Delivery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(a) Marks PA. Oncogene. 2007;26:1351–1356. doi: 10.1038/sj.onc.1210204. [DOI] [PubMed] [Google Scholar]; (b) FDA approves vorinostat (Zolinza) for the treatment of cutaneous manifestations of cutaneous T-cell lymphoma (CTCL) http://www.fda.gov/AboutFDA/CentersOffices/CDER/ucm094952.htm
- 2.Marson CM, Mahadevan T, Dines J, Segmany S, Morrell JM, Alao JP, Joel SP, Vigushin DM, Coombes RC. Bioorg. & Med. Chem. Lett. 2007;17:136–141. doi: 10.1016/j.bmcl.2006.09.085. [DOI] [PubMed] [Google Scholar]
- 3.Moradei O, Maroun CR, Paquin I, Vaisburg A. Curr. Med. Chem.: Anti-Cancer Agents. 2005;5:529–560. doi: 10.2174/1568011054866946. [DOI] [PubMed] [Google Scholar]
- 4.Hildmann C, Riester D. Appl. Microbiol. Biotechnol. 2007;75:487–497. doi: 10.1007/s00253-007-0911-2. [DOI] [PubMed] [Google Scholar]
- 5.Marks P, Rifkind R, Richon V, Breslow R, Miller T, Kelly W. Nat. Rev. Cancer. 2001;1:194. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 6.Johnstone RW, Licht JD. Cancer Cell. 2003;4:13. doi: 10.1016/s1535-6108(03)00165-x. [DOI] [PubMed] [Google Scholar]
- 7.Carey N, Thangue N. Curr. Opin. Pharmacol. 2006;6:369–375. doi: 10.1016/j.coph.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Estiu G, Greenberg E, Harrison C, Kwiatkowski N, Mazitschek R, Bradner J, Wiest O. J. Med. Chem. 2008;51:2898–2906. doi: 10.1021/jm7015254. [DOI] [PubMed] [Google Scholar]
- 9.Hkan N, Jeffers M, Kumar S, Hackett C, Boldog F, Khramtsov N, Qian X, Mills E, Berghs S, Carey N, Finn P, Collins N, Tumber A, Ritchie J, Jensen P, Lichenstein H, Sehested M. Biochem. J. 2008;409:581–589. doi: 10.1042/BJ20070779. [DOI] [PubMed] [Google Scholar]
- 10.(a) Miller TA, Witter DJ, Belvedere S. Histone Deacetylase Inhibitors. J. Med. Chem. 2003;46:5097–5116. doi: 10.1021/jm0303094. [DOI] [PubMed] [Google Scholar]; (b) Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc. Natl. Acad. Sci. USA. 2003;100:4389–4394. doi: 10.1073/pnas.0430973100. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Carew JS, Francis JG, Nawrocki ST. Cancer Lett. 2008;269:7–17. doi: 10.1016/j.canlet.2008.03.037. [DOI] [PubMed] [Google Scholar]; (d) Bieliauskas AV, Pflum MKH. Isoform-selective histone deacetylase inhibitors. Chem Soc Rev. 2008;37:1402–1413. doi: 10.1039/b703830p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen P, Patil V, Guerrant W, Green P, Oyelere A. Bioorg. Med. Chem. 2008;16:4839–4853. doi: 10.1016/j.bmc.2008.03.050. [DOI] [PubMed] [Google Scholar]
- 12.Oyelere A, Chen P, Guerrant W, Mwakwari S, Hood R, Zhnag Y, Fan Y. J. Med. Chem. 2009;52:456–468. doi: 10.1021/jm801128g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolden JE, Part MJ, Johnstone RW. Nat. Rev. Drug Discovery. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 14.Johnstone RW. Nat. Rev. Drug Discovery. 2002;1:287–299. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 15.Tkachenko AG, Xie H, Coleman D, Glomm W, Ryan J, Anderson MF, Franzen S, Feldheim DL. J. Am. Chem. Soc. 2003;125:4700–4701. doi: 10.1021/ja0296935. [DOI] [PubMed] [Google Scholar]
- 16.Oyelere AK, Chen PC, Huang X, El-sayed IH, El-sayed MA. Bioconjugate Chem. 2007;18:1490–1497. doi: 10.1021/bc070132i. [DOI] [PubMed] [Google Scholar]
- 17.Kalderon D, Richardson WD, Markham AF, Smith AE. Nature (London) 1984;311:33–38. doi: 10.1038/311033a0. [DOI] [PubMed] [Google Scholar]
- 18.De la Furente JM, Berry CC. Bioconjugate Chem. 2005;16:1176–1180. doi: 10.1021/bc050033+. [DOI] [PubMed] [Google Scholar]
- 19.(a) Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew. Chem. Int. Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]; (b) Tornoe CW, Christensen C, Meldal MJ. Org. Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 20.HDAC Fluorimetric Assay/Drug Discovery Kit - AK-500 Manual. Fluorescent Assay System; BIOMOL® International, Plymouth Meeting; PA. 2005. [Google Scholar]
- 21.Loudet A, Burgess K. Chem. Rev. 2007;107:4891–4932. doi: 10.1021/cr078381n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.