Summary
Administration of stimulant and non-stimulant drugs that inhibit monoamine reuptake is known to improve cognitive and behavioral symptoms of attention deficit hyperactivity disorder (ADHD). Although this may reflect acute actions of these drugs, clinical observations suggest that prolonged treatment with these agents may result in a better therapeutic outcome. In the current study, we compared the effects of acute and repeated treatment with the stimulant drug, methylphenidate (MPH), and the non-stimulant norepinephrine reuptake inhibitor desipramine (DMI) in rats performing a reversal learning task meant to study behavioral flexibility in rats. Furthermore, we tested the effect of an acute challenge administration with these agents or vehicle on reversal performance of rats repeatedly treated with the drug or vehicle. Our results suggest the acute and repeated treatment with DMI improves reversal learning performance in a qualitatively and quantitatively similar manner. Further repeated treatment with DMI seems to produce a reversal learning improvement that persists at least 24 hours after drug administration. Repeated MPH treatment only improved performance in the first within session reversal administered, suggesting that its beneficial effects may depend upon the complexity of the reversal condition tested. The differential outcome produced by stimulant and non-stimulant medications in this study may be explained in light of their distinct actions on brain catecholaminergic systems.
Keywords: ADHD, prefrontal cortex, methylphenidate, norepinephrine reuptake inhibitors, reversal learning, inhibitory control
Introduction
Difficulty with certain facets of executive functioning is a common feature of attention deficit hyperactivity disorder (ADHD). For example, children and adults with ADHD exhibit performance deficits on tasks that require inhibitory control of behavior, including the stop-signal reaction time task, discrimination reversal learning and Go-NoGo tasks (Frank et al., 2007; Itami and Uno, 2002; Schachar et al., 2000; Schachar et al., 1995); other component processes of executive function are also impaired, including working memory (Kempton et al., 1999; Mehta et al., 2000a) and attentional set-shifting (Mehta et al., 2004). Collectively, performance in these tasks depends upon the integrity of brain regions whose functionality appears to be disrupted in ADHD patients, including the striatum and orbital and dorso-lateral divisions of the prefrontal cortex (Hesslinger et al., 2002; Makris et al., 2007; Valera et al., 2007).
Treatment with stimulant or non-stimulant ADHD medications can ameliorate cognitive dysfunction and behavioral symptoms of ADHD (Aron et al., 2003; Chamberlain et al., 2007; Faraone et al., 2005a; Faraone et al., 2005b; Frank et al., 2007; Kempton et al., 1999; Mehta et al., 2000a; Mehta et al., 2000b; Scheres et al., 2003; Spencer et al., 2001; Tannock et al., 1989). Together, agents such as methylphenidate, amphetamine and atomoxetine, likely exert their effects by increasing the extra-cellular levels of the neurotransmitters dopamine and norepinephrine in prefronto-cortical regions. These neurotransmitter responses reflect the acute pharmacodynamic responses elicited by the drugs, either through an increase neurotransmitter output (amphetamine) or blockade of catecholamine reuptake (methylphenidate and atomoxetine).
Multiple clinical reports suggest that repeated dosing with these agents is required before a significant improvement in ADHD behavioral symptoms can be measured (Spencer et al., 2005; Spencer et al., 1998), suggesting that chronic treatment with these drugs may be necessary to relieve behavioral symptoms. However, because physicians often titrate drug doses up over the first weeks of treatment, it is unclear whether the delayed onset of clinical efficacy arises from long-term adaptations secondary to repeated dosing or simply from the fact that an optimal drug dosage is reached only in a later stage of treatment.
Indirect evidence in favor of the latter view is found in some studies of drug effects on executive function deficits in ADHD. For example, acute administration of ADHD medications improves inhibitory control and executive functioning in healthy humans (Chamberlain et al., 2006; Clatworthy et al., 2009; Mehta et al., 2000b) and otherwise normal animal models (Berridge et al., 2006; Eagle et al., 2007; Navarra et al., 2008; Robinson et al., 2007; Seu et al., 2009), indicating that their cognitive-enhancing actions may result from acute dosing, assuming a clinically meaningful dose is used. Very few studies have addressed the issue of whether this is also the case in ADHD patients. In fact, the effect of ADHD medications on cognitive functions in patients has mostly been assessed in individuals who have a long-term history of treatment; in two separate studies, Rhodes et al., found no improvement in executive functions following a single administration of the stimulant methylphenidate in drug-naïve ADHD patients (Rhodes et al., 2004, 2006), while a different group showed an improvement in executive functions and response inhibition after the acute administration of the same drug in a sample consisting mostly of never-medicated ADHD children (Tannock et al., 1995a; Tannock et al., 1995b).
Together these observations seem to indicate that the cognitive-enhancing effect of ADHD medications arises from their acute actions; however, it remains possible that the long-term history of treatment alters the acute effect of these agents resulting in a better and/or longer-lasting outcome. We sought to further explore this topic by comparing the effect of acute and repeated treatment with catecholamine reuptake inhibitors in rats performing an operant reversal learning task designed to study behavioral flexibility and/or response inhibition. In a common reversal learning task, after response-outcome associations are learned by subjects, they are unexpectedly changed by the experimenter, and subjects must modify their behavior in order to find the new predictors of reward. Performance in reversal learning tasks depends upon the integrity of prefronto-cortical regions (Dias et al., 1996) and is impaired in ADHD children (Itami and Uno, 2002). We have recently shown that acute administration of the stimulant methylphenidate (MPH) or the non-stimulant desipramine (DMI) improves reversal learning performance in drug-naïve rats (Seu et al., 2009), and in the current study, we sought to further examine whether the acute administration of these agents in rats with a long-term history of treatment with the same drugs would produce a quantitatively or qualitatively different effect. A previous study has shown that both acute and chronic treatment with DMI improves reversal learning and attentional set shifting in rats (Lapiz et al., 2007); however, because this study used two different doses of the drug for the acute and for the chronic treatment manipulations and the drug was administered chronically using osmotic pumps, it is not possible to clearly disambiguate acute from chronic actions. In contrast, in the current study, the same dose and route of administration were used for the acute and the repeated treatment. Additionally, our experimental design allowed us to establish whether the beneficial effect of repeated dosing with these drugs on reversal performance may extend beyond the range of pharmacodynamic actions of these drugs, by comparing performance of rats repeatedly treated with MPH or DMI at 30 minutes or 24 hours after the last administration of the drug. We hypothesized that the acute administration of DMI and MPH in rats with a previous history of treatment with the same drugs will result in an improvement of reversal learning performance qualitatively similar but quantitatively larger than that obtained with an acute administration of the same drugs, while we did not have any a priori hypothesis relatively to a potential longer lasting effect of repeated treatment.
Material and methods
Subjects
Sixty-eight adult male Long-Evans rats (Harlan, Indianapolis IN) were used in these experiments. The subjects were ~60 days of age at the initiation of training and ranged in weight from 250 to 350 g during the experimental period. All rats were initially food-restricted to 80-85% of their free-feeding weights and subsequently fed ~15 g rat chow per day in their home cage within 1-3 hrs after testing. Water was continuously available, except while in the operant testing chambers. Rats were housed in pairs and were maintained in 14/10 hour light/dark schedule (lights on at 7 am).
The experimental protocols employed were consistent with the NIH “Guide for the Care and Use of Laboratory Animals” and were approved by the Chancellor’s Animal Research Committee at UCLA.
Drugs
Desipramine hydrochloride (5.0 mg/kg; Sigma-Aldrich; St Louis MO) and methylphenidate hydrochloride (0.33 mg/kg; Sigma-Aldrich; St Louis MO) were dissolved in sterile saline (0.9%) and were administered intra-peritoneally (i.p.) in a volume of 1.0 ml/kg. Doses of both drugs were chosen based upon our previous work showing an acute effect of these drugs on reversal learning tasks (Seu and Jentsch, 2006; Seu et al., 2009).
Rats were injected with DMI, MPH or saline (SAL) daily for a total of 19-20 days; on behavioral testing days, drugs or vehicle were administered 30 min before the beginning of behavioral testing.
Rat behavioral testing apparatus
Standard extra-tall aluminum and Plexiglas operant conditioning chambers with a photocell-equipped pellet delivery magazine on one side and a curved panel with five photocell-equipped apertures on the opposite side (Med Associates, Mount Vernon, Vt., USA) were used. The boxes were housed inside of a sound-attenuating cubicle; background white noise was broadcasted, and the environment was illuminated with a house light (a light diffuser that was located outside of the operant chamber but within the cubicle).
Pre-treatment training
The procedure for the initial training was similar to that used for a lateralized reaction time task (Jentsch, 2003). Rats were first trained in a single session in which the house light was continuously illuminated and single pellets (45 mg Dustless Precision Pellets; Bio-serv, Inc., Frenchtown, N.J., USA) were delivered into an illuminated magazine on a fixed-time 20-s schedule over a 45-min period. Across three subsequent daily sessions, the rats were then trained to make a sustained, variable duration nose poke (200, 500, 700 or 1000 ms) in an illuminated center nose poke aperture to receive a pellet. This response (called the observing response) was used in the subsequent sessions to begin a new trial in order to demonstrate task engagement and to avoid random responding. All rats were trained until they earned at least 70-80 pellets in each of these three sessions
Rats were then tested for the acquisition of a 2-position discrimination. Briefly, rats were tested in a session in which the initiation of individual trials was signaled by the illumination of the central aperture. A variable-duration observing response at that location resulted in the immediate switching off of the central light and illumination of the far left and right apertures; a nose poke in only one of the two apertures (correct response) resulted in the illumination of the food magazine and a pellet released; the correct position (left [L] or right [R]) was chosen randomly and balanced across experimental groups.
If the rat responded at a location that was not the established target, all lights in the box were extinguished, and the rat was given a 3-s time-out period in complete darkness (incorrect response). If no response was made within 15 s, the rat received a 3-s time-out in darkness (omission). The inter-trial interval that followed a completed trial or omission was 3 s. On occasion, rats responded into one of the lateral apertures before completing the sustained nose poke (and before the target presentation); in this case, a 3-s time-out was delivered (as above), and a pre-mature response was scored.
Sessions were terminated when rats reached a criterion of 18 correct responses in 20 consecutive trials, after 1 hr or when 200 trials were completed, whichever came first. If rats failed to achieve criterion performance in 1 hr or 200 trials, the discrimination was repeated on subsequent days until criterion was met.
Once performance criterion on the 2-position discrimination task was achieved, rats were given an additional session with the same position-reward contingency as in the discrimination, i.e. retention (Figure 1); the performance criterion was always set to 18 correct responses in 20 consecutive trials.
Figure1.
Schematic representation of the experimental design employed, including acquisition (ACQ), retention (RET) and between- or within- session reversal tests (BET REV and WITH REV, respectively).
Post-treatment testing and experimental design
Rats were assigned to three experimental groups (DMI, MPH or SAL). The three groups were balanced according their performance of the task prior to initiation of the pharmacological study; they did not differ on total trials required to achieve behavioral criterion on the acquisition of the 2-position discrimination (SAL: 40.2 ± 3.3; DMI: 41.7 ± 4.5; MPH: 41.7 ± 5.4) or on retention of the discrimination (SAL: 23.4 ± 0.9; DSI: 23.9 ± 1.5; MPH: 24.8 ± 1.8).
Daily injections with DMI, MPH or SAL were initiated after baseline training; for the first 6 days, the rats were injected and then returned to their home cages. On the 7th day of dosing, the rats were injected, and 30-min later, they were tested for the retention of the position discrimination learned before beginning pharmacological treatment (Figure 1).
On the 8th day of dosing, subjects were tested for reversal of the learned discrimination (Figure 1); here, the position-reward associations were switched, such that previously correct position became the incorrect one and vice versa. Sessions were terminated when rats reached a criterion of 18 correct responses in 20 consecutive trials, after 90 min or when 200 trials were completed, whichever came first. If a rat failed to reach these criteria, the session was repeated the following day. Three rats from the MPH group needed two sessions to complete this phase: one reached the maximum number of trials with passing, while the other two timed out.
On day 9, they were tested using a within session reversal (Figure 1), wherein the contingencies were initially the same as on day 8, but once the rats achieved performance criterion (18 correct in 20 consecutive trials), the reversal phase, in which the contingencies previously retained were reversed, was automatically initiated; we utilized a within session reversal in order to make the reversal more challenging and unpredictable. This session was terminated when at least 18 correct responses in 20 consecutive trials were made on the reversal phase, after 90 min or when 250 trials were completed.
At this point, the rats received two days with no behavioral testing, though pharmacological treatments were administered as usual. Subsequently, the rats were tested in two blocks of three days separated by two days off-testing (see Figure 1 for the experimental design). On the first day of each block, rats performed a session that we refer to as “acquisition”: the correct aperture for this session was chosen pseudorandomly so that for half of the subjects in each group it was the same as in the preceding session (i.e. in the preceding within session reversal). The second day of each block, the rats were tested on the “retention” of the position-reward associations learned the day before. Finally, on the third day of each block, rats were exposed to a “within session reversal” (retention followed by reversal). As stated earlier, drugs or vehicle were always administered 30 min before behavioral testing during these sessions. For the DMI group, they received pre-testing acute injection with desipramine (dmi, 5.0 mg/kg) on one block and saline (sal, 1ml/kg) on one block; for the MPH group, they received pre-testing injection with methylphenidate (mph, 0.33 mg/kg) on one block and saline (sal) on one block. Finally, the SAL group was divided in two subgroups: they received pre-testing injection with “sal” on one block and “dmi” or “mph” on the other block. [For presentational purposes, chronic treatment groups are designated by capital letters (e.g., SAL vs. DMI) while acute challenge injections are designated with small letters (e.g., sal vs. dmi)]. For all groups, the order of the treatments on the two blocks was counterbalanced.
This design was employed in order to compare the effect of acute (SAL group treated acutely with drugs) and repeated treatment (e.g., DMI group treated with dmi) with these drugs. Additionally by comparing reversal performance of rats repeatedly treated with DMI or MPH after an acute administration of saline (essentially 24 hours after the last administration of the drug), we are able to explore whether the potential beneficial effect of repeated treatment is independent from acute actions these drugs.
The measures collected during daily sessions included total number of trials (number of trials required to reach criterion), the mean trial initiation latency (the average interval between illumination of the central aperture and the initiation of the observing response), the mean pellet retrieval time (the average interval between pellet delivery and head entry into the magazine), the number of pre-mature responses and omissions (calculated as a fraction of completed trials).
Statistical analysis
The dependent measures collected on retention and between session reversal sessions were subjected to one-way ANOVA with repeated treatment (DMI vs. MPH vs. SAL) as a between subjects factor. For within session reversal sessions, total trials to criteria and latencies to initiate trials were subjected to repeated measures ANOVA with phase (retention vs. reversal) as the repeated measure, while pellet retrieval times and the proportion of pre-mature responses and omissions were subjected to simple ANOVA. Our hypothesis that repeated and acute treatment with MPH and DMI would reduce total trials to criteria on reversal conditions was tested using 1-tailed unpaired t-tests, while for all the other post hoc comparisons, 2-tailed t-tests were used.
Finally the effect of acute challenge administration of drug or saline on rats repeatedly treated with saline or drugs was analyzed with repeated treatment as between subject factor and acute treatment (dmi vs. mph vs. sal) and phase as repeated measures.
Results
Effect of the repeated treatment with DMI or MPH on the retention and reversal of a 2-position discrimination task
Two rats in the DMI group, one in the MPH group and one in the saline group consistently failed to initiate trials when returned to behavioral testing after the initial 6 days of treatment and were excluded from the study, resulting in a total sample of 64 rats. Performance of the retention of the discrimination acquired prior to the initiation of drug administration was not affected by group; as shown in Figure 2A, after 7 days of drug administration, there was no effect of group for total trials to criterion (F(2,61)= 0.03, p=0.97) or other measures, such as pre-mature responses per total trials completed (F(2,61)= 2.0, p=0.14), average latency to initiate a trial (F(2,61)= 0.7, p=0.48) or the time required to retrieve the reward (F(2,61)= 1.7, p=0.18).
Figure 2.
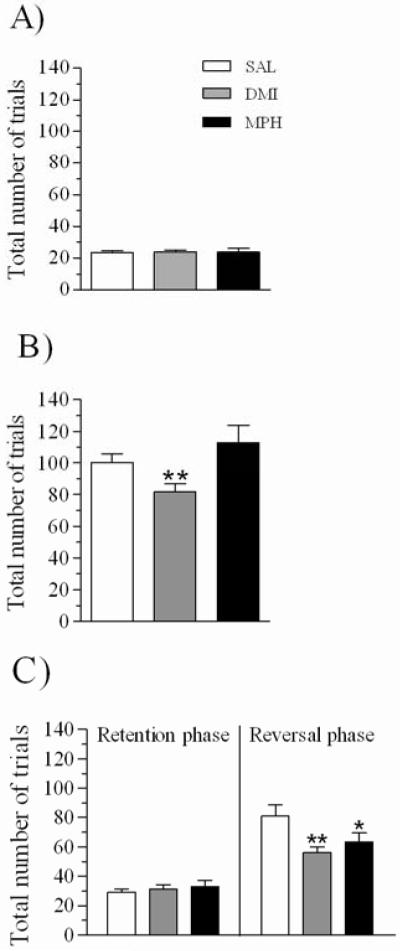
Total trials required to complete the retention session (A) and the between session (B) and within session reversals (C); data is shown for rats repeatedly treated with SAL (1ml/kg; n=27), DMI (5 mg/kg; n=20) and MPH (0.33 mg/kg; n=17). Data are expressed as mean ± sem. * p≤0.05, significantly different from SAL group; ** p≤0.01.
Rats were then tested the following day (day 8; Figure 1) on the reversal of the learned contingency. ANOVA detected a significant effect of repeated treatment for total trials required to complete this session (F(2,61)= 4.4, p≤ 0.05); as shown in Figure 2B, rats repeatedly treated with DMI, but not with MPH, required significantly fewer trials to reach criterion than did rats treated with SAL (DMI: t=-2.3, df=45, p≤0.01; MPH: t=-1.1, df=42, p=0.86; all 1-tailed t-tests). There was no main effect of group for pre-mature responses (F(2,61)= 2.0, p= 0.13) or mean trial initiation latencies (F(2,61)= 0.6, p= 0.50), but there was a significant effect of group for pellet retrieval times (F(2,61)= 5.4, p≤0.01) due to the fact that DMI (t=2.6, df=45, p≤0.01), but not MPH (t=-0.5, df=45, p=0.61), rats were slower in retrieving the reward than were SAL rats (Table 1).
Table 1.
Mean trial initiation latency, mean pellet retrieval time and the number of premature responses per trial in the retention (RET) between- and within session reversals (BET REV and WITH REV, respectively) performed after the first week of treatment by rats repeatedly treated with SAL (1ml/kg; n=27), DMI (5 mg/kg; n=20) or MPH (0.33 mg/kg; n=17). Data are expressed as mean ± sem
| Stage | Group | Mean Trial Initiation Latency (s) | Mean Pellet Retrieval Time (s) | Tot Premature / Tot Trials | |
|---|---|---|---|---|---|
| Retention | Reversal | ||||
| RET (day 7) | SAL | 7.09 ±0.56 | n/a | 1.40 ±0.04 | 1.55 ±0.15 |
| DMI | 6.40 ±0.56 | n/a | 1.34 ±0.05 | 1.11 ±0.16 | |
| MPH | 7.88 ±1.33 | n/a | 1.48 ±0.05 | 1.36 ±0.13 | |
| BET REV (day8) | SAL | n/a | 9.72 ±2.08 | 1.54 ±0.06 | 1.09 ±0.13 |
| DMI | n/a | 13.0 ±1.60 | 1.88 ±0.12** | 0.79 ±0.10 | |
| MPH | n/a | 10.1 ±2.66 | 1.50 ±0.07 | 0.83 ±0.08 | |
| WITH REV (day 9) | SAL | 7.13 ±1.01 | 6.43 ±0.88 | 1.41 ±0.03 | 0.90 ±0.07 |
| DMI | 10.8 ±1.98 | 10.3 ±1.96* | 1.55 ±0.05* | 0.85 ±0.07 | |
| MPH | 5.57 ±0.55 | 5.85 ±0.55 | 1.34 ±0.05 | 0.69 ±0.05 | |
p≤0.05
p≤0.01: significantly different from rats repeatedly treated with SAL
Rats were subsequently tested on a within session reversal (day 9; Figure 1). One rat treated with MPH and one treated with DMI failed to reach behavioral criterion in the within session reversal, and they were excluded from the analysis of this session. Considering phase (retention vs. reversal) as a within subject factor and group as the between subjects factor, ANOVA detected a significant phase x group interaction for total trials to criteria (F(2,59)= 4.1, p≤0.05); as shown in Figure 2C, both, rats repeatedly treated with DMI and MPH, took significantly fewer trials to complete the reversal phase than did SAL rats (DMI: t=-2.5, df=44, p≤0.01; MPH: t=-1.6, df=41, p≤0.05; all 1-tailed t-tests), while neither group differed in terms of the number of trials required to complete the retention phase (DMI: t=-0.5, df=44, p=0.57; MPH: t=-0.8, df=41, p=0.41; all 2-tailed t-tests). A significant effect of group on the latency to initiate a trial was also found (repeated treatment: F(2,59)= 4.2, p≤0.05; phase x treatment interaction: F<1, ns); post hoc comparisons revealed that rats treated with DMI, but not with MPH, tended to be slower in initiating trials than SAL rats in the reversal (DMI: t=2.0, df=44, p≤0.05; MPH: t=-0.4, df=41, p=0.64; Table 1) and retention phases (DMI: t=1.8, df=44, p=0.07; MPH: t=-1.1, df=41, p=0.27; Table 1). In addition, the ANOVA detected a significant effect of group for pellet retrieval time (F(2,59)= 4.8, p≤0.01); rats treated with DMI, but not with MPH, were slower than SAL rats in retrieving the reward (DMI: t=2.2, df=44, p≤0.05; MPH: t=-1.1, df=41, p=0.24; Table 1). Finally, while none of the drugs affected pre-mature responses (F(2,59)= 2.1, p=0.12; Table 1), there was a main effect of group on omissions (F(2,59)= 4.9, p≤0.01; data not shown), due to an increase in the fraction of omissions in the DMI (t=2.4, df=44, p≤0.01) but not in the MPH treated rats (t=-0.3, df=41, p=0.76).
Effect of the acute drug challenge on the retention and reversal performance of DMI or SAL rats
Total trials to criteria on retention and reversal phases for rats repeatedly treated with SAL or DMI after an acute administration of the same drug or vehicle is shown in Figure 3. An ANOVA considering group (SAL vs. DMI) as a between subjects factor and phase (retention vs. reversal) and acute challenge (sal vs. dmi) as within subjects factors was conducted. There was no main effect of group (F(1,33)= 1.5, p=0.21), nor was there a phase x group interaction for this measure (F(1,33)= 1.0, p=0.31). The ANOVA did, however, detect a significant phase x acute challenge interaction (F(1,33)= 5.6, p≤0.05), without any acute challenge x group interaction (F(1,33)= 2.0, p=0.15) nor any higher order interactions (phase x acute challenge x group: F(1,33)= 2.4, p=0.12). As shown in Figure 3, the acute administration of dmi (30 min prior to testing) reduced the number of trial to complete reversal phase (compared to SAL-sal controls) irrespective of repeated treatment. Post-hoc comparisons revealed that, as opposed to SAL group under acute challenge with vehicle, both SAL- and DMI-treated rats took significantly fewer trials to complete reversal sessions when they were given an acute administration of dmi 30′ prior to testing (SAL group: t=2.7, df=14, p≤0.01; DMI group: t=-2.4, df=33, p≤0.01; all 1-tailed t-tests; Figure 3), while there was no difference on trials to complete retention phase (SAL group: t=0.3, df=14, p=0.71; DMI group: t=-0.7, df=33, p=0.47; all 2-tailed t-tests; Figure 3). Considering only the DMI rats, performance on retention or reversal did not differ when a pre-testing injection of vehicle or dmi were given, likely due to a floor effect (reversal: t=0.8, df=19, p=0.20, 1-tailed t-test; retention: t=0.6, df=19, p=0.50, 2-tailed t-test). As shown in Figure 3, performance of the reversal phase after an acute administration of vehicle, tended to be better in DMI, as compared with SAL, rats (reversal: t=-1.5, df=33, p=0.12; retention: t=-0.0, df=33, p=0.93; all 2-tailed t-tests.
Figure 3.
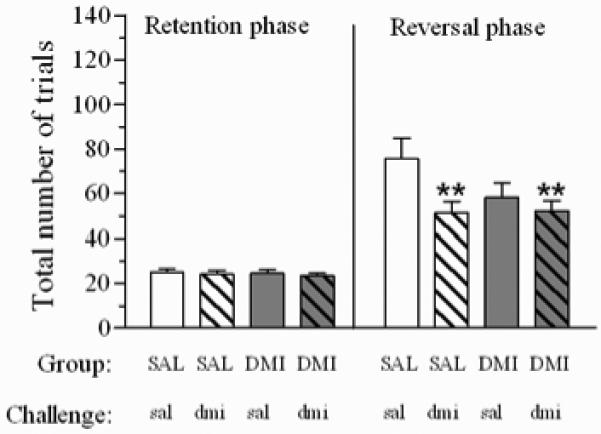
Total trials to criteria on retention and reversal phases after an acute challenge administration of dmi (5 mg/kg) or sal (1ml/kg) in rats repeatedly treated with DMI (n=20) or SAL (n=16). Acute treatments were delivered 30 min prior to testing. Data are expressed as mean ± sem. ** p≤0.01, significantly different from SAL group after sal challenge.
Because this was a within subjects design with the potential for an order effect, we separately considered only the data collected on the first day of the challenge study (day 14) and evaluated the data using a between subjects analysis. As with the counterbalanced, within subject analyses, there was a main effect of acute challenge (F(1,31)= 7.2, p≤0.01), but no main effect of group (F(1,31)= 1.9, p=0.17) nor a significant group x acute challenge interaction (F(1,31)= 1.1, p=0.28). Rats in the SAL and DMI groups that received an acute challenge with dmi took significantly fewer trials to reach criterion than did rats in the SAL group that were treated with sal (SAL group: t=-2.7, df=9.3, p≤0.01; DMI group: t=-2.6, df=12.8, p≤0.01; all 1-tailed t-tests). Additionally, there was no difference in number of trials to criteria between the rats in the DMI group that received an acute challenge with saline and those that were treated with the drug (t=-1.1, df=17.2, p=0.12; 1-tailed t-test); furthermore, following a challenge administration of vehicle, the DMI group tended to perform better than SAL group (t=-1.5, df=14.6, p=0.14).
In regard to latency to initiate trials, there was main effect of acute challenge (F(1,33)= 14.7, p≤0.001) and phase (F(1,33)= 9.8, p≤0.01), but no main effect of group (F<1, ns) nor any significant higher level interactions involving treatment (acute challenge x phase: F(1,33)= 2.5, p=0.11; all other Fs<1, ns). Further comparisons revealed that during the reversal phase, all rats were slower in initiating trials than they were in retention phase, irrespective of whether they were treated with vehicle (SAL group: t=-2.4, df=14, p≤0.05; DMI group: t=-2.2, df=19, p≤0.05; Table 2) or dmi (SAL group: t=-2.1, df=14, p≤0.05; DMI group: t=-2.4, df=19, p≤0.05; Table 2). Additionally, as shown in Table 2A, when they were given an acute challenge with dmi, both SAL and DMI group were slower in initiation of trials than when they received an acute challenge with vehicle; this effect was consistent across retention (Saline group: t=-2.2, df=14, p≤0.05; DMI group: t=-2.3, df=19, p≤0.05) and reversal (Saline group: t=-2.5, df=14, p≤0.05; DMI group: t=-3.1, df=19, p≤0.01) phases.
Table 2.
Mean trial initiation latency, mean pellet retrieval time and the number of premature responses per trial in rats repeatedly treated with DMI (n=20) or SAL (n=16) following an acute challenge administration of dmi (5 mg/kg) or sal (1ml/kg). Acute treatments were delivered 30 min prior to testing. Data are expressed as mean ± sem.
| Group | Acute challenge | Mean Trial Initiation Latency (s) | Mean Pellet Retrieval Time (s) | Tot Premature/Tot Trials | |
|---|---|---|---|---|---|
| Retention | Reversal | ||||
| SAL | sal | 3.37 ±0.49 | 4.15 ±0.62 | 1.40 ±0.03 | 0.62 ±0.06 |
| dmi | 5.68 ±1.26* | 7.09 ±1.40* | 1.49 ±0.04** | 0.50 ±0.09 | |
| DMI | sal | 3.48 ±0.41 | 5.11 ±0.86 | 1.39 ±0.05 | 0.94 ±0.12# |
| dmi | 5.27 ±0.76* | 7.58 ±1.31** | 1.61 ±0.07*** | 0.65 ±0.08** | |
significant difference between sal and dmi treatment: p<0.05
p≤0.01
p≤0.001
p≤0.05, significant difference between SAL and DMI groups.
A similar effect was also found for pellet retrieval times (acute challenge: F(1,33)= 21.4, p≤0.0001; group: F<1, ns; acute challenge x group: F(1,33)= 3.0, p=0.08), due to the fact that both SAL (t=-3.5, df=14, p≤0.01) and DMI (t=-3.9, df=19, p≤0.001) groups were slower in retrieving the reward when they were given an acute injection of dmi, as opposed to a vehicle injection (Table 2). Additionally, ANOVA detected a main effect of acute challenge and a strong trend for a main effect of group without any higher level interactions for the fraction of pre-mature responses per total trials completed (acute challenge: F(1,33)= 7.5, p≤0.01; group: F(1,33)= 3.7, p=0.06; acute treatment x group: F(1,33)= 1.2, p=0.27); post hoc comparisons revealed that the DMI (t=2.6, df=19, p≤0.01), but not the SAL (t=1.2, df=14, p=0.22), group made fewer pre-mature responses per trials when they were given an acute challenge injection of dmi (Table 2). Further analysis, however, indicates that, when received they vehicle, the DMI group made more premature responses than did rats in the SAL group (t=2.0, df=33, p≤0.05; Table 2). Omissive trials during these sessions were rare and were not affected by any treatment (data not shown).
Effect of a challenge injection with mph or sal on the retention and reversal performance of rats treated repeatedly with MPH or SAL
Figure 4 shows the effect of the acute challenge administration of mph on total trials to criteria in rats repeatedly treated with MPH or SAL; there were no main effects, nor any significant interactions, for this measure (all Fs<1, ns). However, the ANOVA detected a main effect of acute challenge on trial initiation latencies (F(1,27)= 33.3, p≤0.0001), without a main effect of group or phase, nor any significant higher level interactions (all Fs≤1, ns). This was due to the fact that all rats were slower in initiating trials when they were given a challenge injection of the drug, either in retention (SAL: t=-2.9, df=11, p≤0.01; MPH: t=-2.4, df=16, p≤0.05) or reversal (SAL: t=-3.4, df=11, p≤0.01; MPH: t=-2.8, df=16, p≤0.01) phases (Table 3).
Figure 4.
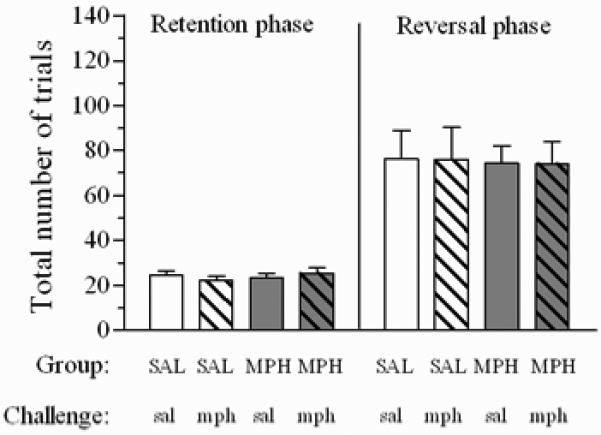
Total trials to criteria on retention and reversal phases after acute challenge administration of mph (0.33 mg/kg) or sal (1ml/kg) in rats repeatedly treated with MPH (n=17) or SAL (n=12). Acute treatments were delivered 30 min prior to testing. Data are expressed as mean ± sem.
Table 3.
Mean trial initiation latency, mean pellet retrieval time and the number of premature responses per trial in rats repeatedly treated with MPH (n=17) or SAL (n=12) following an acute challenge administration of mph (0.33 mg/kg) or sal (1ml/kg). Acute treatments were delivered 30 min prior to testing .Data are expressed as mean ± sem
| Group | Acute challenge | Mean Trial Initiation Latency (s) | Mean Pellet Retrieval Time (s) | Tot Premature/Tot Trials | |
|---|---|---|---|---|---|
| Retention | Reversal | ||||
| SAL | sal | 3.39 ±0.36 | 3.15 ±0.37 | 1.27 ±0.04 | 0.85 ±0.19 |
| mph | 4.10 ±0.39** | 4.58 ±0.33** | 1.30 ±0.04 | 0.81 ±0.16 | |
| MPH | sal | 2.99 ±0.35 | 3.11 ±0.38 | 1.26 ±0.03 | 0.79 ±0.09 |
| mph | 3.91 ±0.42* | 4.20 ±0.43** | 1.30 ±0.04** | 0.66 ±0.09 | |
p≤0.05
p≤0.01: significant difference between sal and mph.
In regards to pellet retrieval times, there was a main effect of acute challenge (F(1,27)= 9.8,p≤0.01), but no effect of group, nor any significant higher level interactions (all Fs<1, ns); this was due to the fact that, when given an acute administration of mph, the MPH group (t=-2.7, df=16, p≤0.01) but not the SAL group (t=-1.8, df=11, p=0.09) was significantly slower in retrieving the reward (Table 3).
Finally, there was no main effects of group or acute challenge, nor any higher level interactions, for the proportion of pre-mature responses (all Fs<1, ns; Table 3). Omissive trials during these sessions were rare and were not affected by any treatment (data not shown).
Discussion
We have previously shown that acute administration of catecholamine reuptake inhibitors, including the stimulant drug MPH and the non-stimulant norepinephrine reuptake inhibitors DMI and atomoxetine, improve reversal learning performance in rats (Seu et al., 2009). Accordingly, other groups have reported a beneficial effect of these drugs on rat performance in tasks that measure different aspects of inhibitory control (Eagle et al., 2007; Navarra et al., 2008; Robinson et al., 2007). Moreover, a recent report has demonstrated that acute and chronic treatment with the selective norepinephrine reuptake inhibitor, DMI, improves reversal learning and attentional set shifting in rats (Lapiz et al., 2007); because this study used two different doses of the drug for the acute and for the chronic treatment manipulations, as well as the fact that the drug was administered chronically using osmotic pumps, it is not possible to clearly disambiguate acute from chronic actions. In the current study, we tested the effect of the acute administration of DMI or MPH, in rats repeatedly treated with the drug or vehicle. The same doses of these agents were used for acute and repeated treatments; further, the performances of rats repeatedly treated with DMI and MPH were also assessed 24 hours after the last drug injection (when rats repeatedly treated with DMI or MPH were injected with saline), enabling us to detect any potential long lasting effects caused by repeated drug administration.
Acute and repeated treatment with DMI similarly affects reversal learning performance
As our results show, rats treated daily with DMI took fewer trials to complete the first between- and within-session reversals administered after one week of drug exposure, while there was never any effects of the drug on retention of a previously learned discrimination. Additionally, DMI tended to increase latency measures and sometimes increased the proportion of omissions; similar effects, as well as reductions in pre-mature responding, have been shown to be elicited by acute administration of selective norepinephrine reuptake inhibitors (Jentsch et al., 2009; Navarra et al., 2008; Robinson et al., 2007). In our study, we did not find a consistent reduction in pre-mature responses elicited by DMI, perhaps due to the fact that the number of pre-mature responses emitted in our task is very low, resulting in a floor effect.
Interestingly, the acute administration of DMI in the later stage of repeated treatment produced a similar outcome in drug naïve rats and rats repeatedly treated with the drug, including a reduction in trials to criteria and an increase in latency measures, further suggesting that the acute effects of this agent are qualitatively similar in drug-treated and drug-naïve rats. In fact both, SAL and DMI groups, when given an acute challenge of the drug completed the reversal phase with significantly fewer trials than the SAL group after acute administration of vehicle. On the other hand, the reversal performance of rats repeatedly treated with DMI did not differ whether they were given an injection of vehicle (no drug) rather than their usual DMI treatment. The half life of DMI in rats is approximately 4.6 hours (Kozisek et al., 2007), so it is unlikely that the superior reversal performance of the DMI group after vehicle injection was due to persistent drug levels from the previous day’s treatment; however we cannot rule out the possibility that physiologically-relevant levels of the active metabolite of DMI, desmethyldesipramine, were present up to 24 hours after the injection, resulting in the observed long-lasting action of the drug. Alternatively, the persistency of behavioral effects observed in this study after a 24 wash-out period may be explained by the neuroadaptations induced by repeated treatment with DMI. For instance, although a previous study has shown maintenance of alpha-2 adrenergic autoreceptor function following chronic treatment with DMI (Garcia et al., 2004), down-regulation of beta-1 adrenergic receptors and norepinephrine transporters have been reported to persist even after discontinuation of treatment (Zhao et al., 2008).
Similarly, a recent clinical study reported that behavioral symptoms of ADHD worsened but did not return to pretreatment levels after discontinuation of treatment with the non-stimulant, atomoxetine (Wernicke et al., 2004) perhaps indicating that repeated administration of norepinephrine reuptake inhibitors produces long lasting changes in brain circuitry that is affected in ADHD. Further evidence in support of this possibility stems from the observation that, when they were treated with vehicle, rats with a history of repeated DMI treatment tended to make more pre-mature responses than when treated with DMI, as well as more pre-mature responses than drug-naïve animals made; such outcome may also reflect a long-lasting adaptation due to the repeated treatment with the drug.
MPH effects on reversal learning: effect of task complexity?
Our results show that the acute administration of MPH to rats treated with the same drug, did not affect performance on the between session reversal performed after 1 week of treatment, while it reduced the number of trials to criteria in the within session reversal administered after 9 days of drug exposure. Using a more difficult 4-position discrimination task, we previously showed that the acute administration of MPH at the same dose as is used here improved reversal learning (Seu et al., 2009). Similar doses of MPH were shown to improve inhibitory control and others executive functions in rats (Berridge et al., 2006; Eagle et al., 2007; Navarra et al., 2008), suggesting that the absence of a consistent effect of MPH in the current study was not due to the employment of a sub-threshold dose. The fact that we previously found a beneficial effect of MPH on a 4-choice task and that, in the current study, MPH only improved the first within session reversal may suggest that the effect of this drug depends upon the complexity of the condition under which subjects are tested. A multiple choice task would be more cognitively demanding than a 2-choice task, and the first within session reversal is generally considered to be more challenging and to require greater frontal lobe contributions (Boulougouris et al., 2007).
Although MPH failed to affect latency measures in the first retention and reversals tested after beginning the repeated treatment, it increased latencies to initiate trials and pellet retrieval times in both drug-naïve and drug-treated rats during the two blocks of within session reversal performed at a later stage of treatment. This paradoxical slowing effect induced by MPH is reportedly associated with the acute administration of low doses of stimulants and may contribute to their efficacy in treating behavioral and cognitive symptoms of ADHD (Arnsten, 2006). The same slowing action was also shown by DMI in the current study; however, the increase in latencies induced by DMI was associated with improved reversal performance. These observations suggest that this “slowing effects” are not causal for the reversal learning improvement.
Mechanism of action of stimulants and non-stimulants medications: relevance to their acute and chronic effects
For decades, stimulant drugs such as MPH have been the first choice treatment for children and adults with ADHD. More recently, clinical trials have shown the efficacy of non-stimulant selective norepinephrine reuptake inhibitors, such as DMI and atomoxetine, in the treatment of the disorder (Faraone et al., 2005a; Maidment, 2003; Spencer et al., 2002). The stimulant and non-stimulant drugs used in this study are known to increase the extracellular levels of dopamine and norepinephrine in different brain regions through blockade of their respective reuptake proteins. Differently from MPH that binds to both the dopamine and the norepinephrine transporter, DMI acts selectively on norepinephrine reuptake (Bymaster et al., 2002). As a result, while MPH increases dopamine levels in the ventral and dorsal striatum (Carboni et al., 2006; Kuczenski and Segal, 1997), selective norepinephrine reuptake inhibitors do not affect dopamine levels in this brain region (Bymaster et al., 2002). However, similarly to MPH, atomoxetine and DMI increase both dopamine and norepinephrine levels in prefrontal regions (Berridge et al., 2006; Bymaster et al., 2002; Swanson et al., 2006) because a significant portion of extracellular dopamine is cleared by the norepinephrine transporter in cortical regions (Carboni et al., 2006; Mazei et al., 2002; Moron et al., 2002). These actions reflect the acute response to these agents and they are likely to be responsible for the cognitive and behavioral effects induced by ADHD medications. However, clinical observations indicate that weeks of treatment with these agents are needed to observe a significant improvement in rated symptoms of ADHD (Spencer et al., 2005; Spencer et al., 1998), suggesting that chronic treatment may result in a better therapeutic outcome; these effects may depend upon long-term adaptations induced by chronic treatment that, in turn, enhance or facilitate their acute effects. For example, repeated treatment with stimulant or non stimulants medications can result in long term adaptations, including changes in receptors and reuptake proteins (Izenwasser et al., 1999; Thanos et al., 2007; Zhao et al., 2008), that may impact the acute neurochemical responses to these drugs (Vitiello, 2001).
In the current study, we show that previous experience with the non-stimulant, DMI, did not alter its acute cognitive effects. In fact, acute administration of this drug produced the same effect as long-term treatment, including a reduction in total trial to reversal criteria and increase in latencies measures; these actions did not appear to be quantitatively different, although it is possible we could not detect any additional improvement on reversal performance because of scaling effects. On the other hand, our results suggest that repeated treatment with DMI results in enhanced behavioral flexibility that persists at least 24 hours after drug injection; this effect may arise from neuronal adaptations induced by repeated treatment.
Finally, the differential effect produced in our study by DMI and MPH may arise from the distinct neurochemical responses elicited by acute administration of these agents; these neurochemical changes may impact differently reversal performance. For instance, while the increase in catecholamine contents in the prefrontal cortex produced by both drugs seems to be crucial for their cognitive enhancing effects, the increase in dopamine in subcortical regions induced by MPH may be detrimental to some of the executive processes involved in reversal learning paradigm. Further studies will be required to precisely define the receptor mechanisms in cortical and subcortical regions that mediate these diverse actions of ADHD medications on cognitive control.
Acknowledgments
Funding: These experiments were funded, in part, by PHS grants P50-MH77248 and P20-DA22539 to JDJ, as well as a grant from the Tenenbaum Creativity Initiative at UCLA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnsten AF. Stimulants: Therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31:2376–2383. doi: 10.1038/sj.npp.1301164. [DOI] [PubMed] [Google Scholar]
- Aron AR, Dowson JH, Sahakian BJ, Robbins TW. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2003;54:1465–1468. doi: 10.1016/s0006-3223(03)00609-7. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav Brain Res. 2007;179:219–228. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Carboni E, Silvagni A, Vacca C, Di Chiara G. Cumulative effect of norepinephrine and dopamine carrier blockade on extracellular dopamine increase in the nucleus accumbens shell, bed nucleus of stria terminalis and prefrontal cortex. J Neurochem. 2006;96:473–481. doi: 10.1111/j.1471-4159.2005.03556.x. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Del Campo N, Dowson J, Muller U, Clark L, Robbins TW, Sahakian BJ. Atomoxetine Improved Response Inhibition in Adults with Attention Deficit/Hyperactivity Disorder. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Muller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science. 2006;311:861–863. doi: 10.1126/science.1121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatworthy PL, Lewis SJ, Brichard L, Hong YT, Izquierdo D, Clark L, Cools R, Aigbirhio FI, Baron JC, Fryer TD, Robbins TW. Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J Neurosci. 2009;29:4690–4696. doi: 10.1523/JNEUROSCI.3266-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Tufft MR, Goodchild HL, Robbins TW. Differential effects of modafinil and methylphenidate on stop-signal reaction time task performance in the rat, and interactions with the dopamine receptor antagonist cis-flupenthixol. Psychopharmacology (Berl) 2007;192:193–206. doi: 10.1007/s00213-007-0701-7. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Spencer T, Michelson D, Adler L, Reimherr F, Glatt SJ. Efficacy of atomoxetine in adult attention-deficit/hyperactivity disorder: a drug-placebo response curve analysis. Behav Brain Funct. 2005a;1:16. doi: 10.1186/1744-9081-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Spencer T, Michelson D, Adler L, Reimherr F, Seidman L. Atomoxetine and stroop task performance in adult attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2005b;15:664–670. doi: 10.1089/cap.2005.15.664. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Santamaria A, O’Reilly RC, Willcutt E. Testing computational models of dopamine and noradrenaline dysfunction in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2007;32:1583–1599. doi: 10.1038/sj.npp.1301278. [DOI] [PubMed] [Google Scholar]
- Garcia AS, Barrera G, Burke TF, Ma S, Hensler JG, Morilak DA. Autoreceptor-mediated inhibition of norepinephrine release in rat medial prefrontal cortex is maintained after chronic desipramine treatment. J Neurochem. 2004;91:683–693. doi: 10.1111/j.1471-4159.2004.02748.x. [DOI] [PubMed] [Google Scholar]
- Hesslinger B, Tebartz van Elst L, Thiel T, Haegele K, Hennig J, Ebert D. Frontoorbital volume reductions in adult patients with attention deficit hyperactivity disorder. Neurosci Lett. 2002;328:319–321. doi: 10.1016/s0304-3940(02)00554-2. [DOI] [PubMed] [Google Scholar]
- Itami S, Uno H. Orbitofrontal cortex dysfunction in attention-deficit hyperactivity disorder revealed by reversal and extinction tasks. Neuroreport. 2002;13:2453–2457. doi: 10.1097/00001756-200212200-00016. [DOI] [PubMed] [Google Scholar]
- Izenwasser S, Coy AE, Ladenheim B, Loeloff RJ, Cadet JL, French D. Chronic methylphenidate alters locomotor activity and dopamine transporters differently from cocaine. Eur J Pharmacol. 1999;373:187–193. doi: 10.1016/s0014-2999(99)00274-5. [DOI] [PubMed] [Google Scholar]
- Jentsch JD. Genetic vasopressin deficiency facilitates performance of a lateralized reaction-time task: altered attention and motor processes. J Neurosci. 2003;23:1066–1071. doi: 10.1523/JNEUROSCI.23-03-01066.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Aarde SM, Seu E. Effects of atomoxetine and methylphenidate on performance of a lateralized reaction time task in rats. Psychopharmacology (Berl) 2009;202:497–504. doi: 10.1007/s00213-008-1181-0. [DOI] [PubMed] [Google Scholar]
- Kempton S, Vance A, Maruff P, Luk E, Costin J, Pantelis C. Executive function and attention deficit hyperactivity disorder: stimulant medication and better executive function performance in children. Psychol Med. 1999;29:527–538. doi: 10.1017/s0033291799008338. [DOI] [PubMed] [Google Scholar]
- Kozisek ME, Deupree JD, Burke WJ, Bylund DB. Appropriate dosing regimens for treating juvenile rats with desipramine for neuropharmacological and behavioral studies. J Neurosci Methods. 2007;163:83–91. doi: 10.1016/j.jneumeth.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J Neurochem. 1997;68:2032–2037. doi: 10.1046/j.1471-4159.1997.68052032.x. [DOI] [PubMed] [Google Scholar]
- Lapiz MD, Bondi CO, Morilak DA. Chronic treatment with desipramine improves cognitive performance of rats in an attentional set-shifting test. Neuropsychopharmacology. 2007;32:1000–1010. doi: 10.1038/sj.npp.1301235. [DOI] [PubMed] [Google Scholar]
- Maidment ID. The use of antidepressants to treat attention deficit hyperactivity disorder in adults. J Psychopharmacol. 2003;17:332–336. doi: 10.1177/02698811030173016. [DOI] [PubMed] [Google Scholar]
- Makris N, Biederman J, Valera EM, Bush G, Kaiser J, Kennedy DN, Caviness VS, Faraone SV, Seidman LJ. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb Cortex. 2007;17:1364–1375. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- Mazei MS, Pluto CP, Kirkbride B, Pehek EA. Effects of catecholamine uptake blockers in the caudate-putamen and subregions of the medial prefrontal cortex of the rat. Brain Res. 2002;936:58–67. doi: 10.1016/s0006-8993(02)02542-8. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Calloway P, Sahakian BJ. Amelioration of specific working memory deficits by methylphenidate in a case of adult attention deficit/hyperactivity disorder. J Psychopharmacol. 2000a;14:299–302. doi: 10.1177/026988110001400314. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Goodyer IM, Sahakian BJ. Methylphenidate improves working memory and set-shifting in AD/HD: relationships to baseline memory capacity. J Child Psychol Psychiatry. 2004;45:293–305. doi: 10.1111/j.1469-7610.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000b;20:RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci. 2002;22:389–395. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarra R, Graf R, Huang Y, Logue S, Comery T, Hughes Z, Day M. Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:34–41. doi: 10.1016/j.pnpbp.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Rhodes SM, Coghill DR, Matthews K. Methylphenidate restores visual memory, but not working memory function in attention deficit-hyperkinetic disorder. Psychopharmacology (Berl) 2004;175:319–330. doi: 10.1007/s00213-004-1833-7. [DOI] [PubMed] [Google Scholar]
- Rhodes SM, Coghill DR, Matthews K. Acute neuropsychological effects of methylphenidate in stimulant drug-naive boys with ADHD II--broader executive and non-executive domains. J Child Psychol Psychiatry. 2006;47:1184–1194. doi: 10.1111/j.1469-7610.2006.01633.x. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, Dalley JW, Robbins TW. Similar Effects of the Selective Noradrenaline Reuptake Inhibitor Atomoxetine on Three Distinct Forms of Impulsivity in the Rat. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301487. [DOI] [PubMed] [Google Scholar]
- Schachar R, Mota VL, Logan GD, Tannock R, Klim P. Confirmation of an inhibitory control deficit in attention-deficit/hyperactivity disorder. J Abnorm Child Psychol. 2000;28:227–235. doi: 10.1023/a:1005140103162. [DOI] [PubMed] [Google Scholar]
- Schachar R, Tannock R, Marriott M, Logan G. Deficient inhibitory control in attention deficit hyperactivity disorder. J Abnorm Child Psychol. 1995;23:411–437. doi: 10.1007/BF01447206. [DOI] [PubMed] [Google Scholar]
- Scheres A, Oosterlaan J, Swanson J, Morein-Zamir S, Meiran N, Schut H, Vlasveld L, Sergeant JA. The effect of methylphenidate on three forms of response inhibition in boys with AD/HD. J Abnorm Child Psychol. 2003;31:105–120. doi: 10.1023/a:1021729501230. [DOI] [PubMed] [Google Scholar]
- Seu E, Jentsch JD. Alpha-2 noradrenergic mechanisms modulate the acquisition and reversal of a position discrimination task in rats. Soc. Neurosci. Abstr., 571.517. 2006 [Google Scholar]
- Seu E, Lang A, Rivera RJ, Jentsch JD. Inhibition of the norepinephrine transporter improves behavioral flexibility in rats and monkeys. Psychopharmacology (Berl) 2009;202:505–519. doi: 10.1007/s00213-008-1250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Coffey B, Geller D, Crawford M, Bearman SK, Tarazi R, Faraone SV. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2002;59:649–656. doi: 10.1001/archpsyc.59.7.649. [DOI] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Heiligenstein J, Wilens T, Faries D, Prince J, Faraone SV, Rea J, Witcher J, Zervas S. An open-label, dose-ranging study of atomoxetine in children with attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol. 2001;11:251–265. doi: 10.1089/10445460152595577. [DOI] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Wilens T, Doyle R, Surman C, Prince J, Mick E, Aleardi M, Herzig K, Faraone S. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:456–463. doi: 10.1016/j.biopsych.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Wilens T, Prince J, Hatch M, Jones J, Harding M, Faraone SV, Seidman L. Effectiveness and tolerability of tomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry. 1998;155:693–695. doi: 10.1176/ajp.155.5.693. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Perry KW, Koch-Krueger S, Katner J, Svensson KA, Bymaster FP. Effect of the attention deficit/hyperactivity disorder drug atomoxetine on extracellular concentrations of norepinephrine and dopamine in several brain regions of the rat. Neuropharmacology. 2006;50:755–760. doi: 10.1016/j.neuropharm.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Tannock R, Ickowicz A, Schachar R. Differential effects of methylphenidate on working memory in ADHD children with and without comorbid anxiety. J Am Acad Child Adolesc Psychiatry. 1995a;34:886–896. doi: 10.1097/00004583-199507000-00012. [DOI] [PubMed] [Google Scholar]
- Tannock R, Schachar R, Logan G. Methylphenidate and cognitive flexibility: dissociated dose effects in hyperactive children. J Abnorm Child Psychol. 1995b;23:235–266. doi: 10.1007/BF01447091. [DOI] [PubMed] [Google Scholar]
- Tannock R, Schachar RJ, Carr RP, Chajczyk D, Logan GD. Effects of methylphenidate on inhibitory control in hyperactive children. J Abnorm Child Psychol. 1989;17:473–491. doi: 10.1007/BF00916508. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Benveniste H, Wang GJ, Volkow ND. Effects of chronic oral methylphenidate on cocaine self-administration and striatal dopamine D2 receptors in rodents. Pharmacol Biochem Behav. 2007;87:426–433. doi: 10.1016/j.pbb.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Vitiello B. Long-term effects of stimulant medications on the brain: possible relevance to the treatment of attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol. 2001;11:25–34. doi: 10.1089/104454601750143384. [DOI] [PubMed] [Google Scholar]
- Wernicke JF, Adler L, Spencer T, West SA, Allen AJ, Heiligenstein J, Milton D, Ruff D, Brown WJ, Kelsey D, Michelson D. Changes in symptoms and adverse events after discontinuation of atomoxetine in children and adults with attention deficit/hyperactivity disorder: a prospective, placebo-controlled assessment. J Clin Psychopharmacol. 2004;24:30–35. doi: 10.1097/01.jcp.0000104907.75206.c2. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Baros AM, Zhang HT, Lapiz MD, Bondi CO, Morilak DA, O’Donnell JM. Norepinephrine transporter regulation mediates the long-term behavioral effects of the antidepressant desipramine. Neuropsychopharmacology. 2008;33:3190–3200. doi: 10.1038/npp.2008.45. [DOI] [PMC free article] [PubMed] [Google Scholar]



