Abstract
Objective
Polycythemia vera (PV) is characterized by erythrocytosis associated with the presence of the activating JAK2V617F mutation in a variable proportion of hematopoietic cells. JAK2V617F is detected in other myeloproliferative neoplasms, does not appear to be the PV-initiating event, and its specific role in deregulated erythropoiesis in PV is incompletely understood. We investigated the pathogenesis of PV to characterize abnormal proliferation and apoptosis responses and aberrant oncogenic pathway activation in primary PV erythroid precursors.
Patients and Methods
Peripheral blood CD34+ cells isolated from PV patients and healthy controls were grown in liquid culture to expand a population of primary erythroblasts for experiments designed to analyze cellular proliferation, apoptosis, JAK2V617F mutation status, cytokine-dependent protein phosphorylation and gene expression profiling using Affymetrix microarrays.
Results
The survival and proliferation of PV erythroblasts were growth factor-dependent under strict serum-free conditions, requiring both erythropoietin (EPO) and stem cell factor. PV erythroblasts exhibited EPO hypersensitivity and enhanced cellular proliferation associated with increased EPO-mediated ERK1/2 phosphorylation. EPO-induced AKT phosphorylation was observed in PV but not normal erythroblasts, an effect associated with apoptosis resistance in PV erythroblasts. Analysis of gene expression and oncogenic pathway activation signatures revealed increased RAS (P<0.01) and PI3-kinase (P<0.05) pathway activation in PV erythroblasts.
Conclusion
Deregulated erythropoiesis in PV involves EPO hypersensitivity and apoptosis resistance of erythroid precursor cells associated with abnormally increased activation of RAS-ERK and PI3-kinase-AKT pathways. These data suggest that investigation of the mechanisms of abnormal RAS and PI3-kinase pathway activation in erythroblasts may contribute to our understanding of the molecular pathogenesis of PV.
Keywords: Polycythemia vera, erythropoietin, Janus kinase 2, MAP-ERK kinase, AKT, apoptosis, signal transduction
Introduction
Polycythemia vera (PV) is characterized by an absolute increase in the red blood cell mass and the development of erythrocytosis due to deregulated red blood cell (RBC) production, the mechanisms of which are not completely understood. Erythrocytosis is the unique clinical feature of PV among related BCR/ABL-negative myeloproliferative neoplasms (MPNs) and is associated with the characteristic biological features of the disease which include the ability of bone marrow and peripheral blood progenitor cells to undergo in vitro erythroid differentiation in the absence of exogenous erythropoietin (EPO) in serum-containing colony formation assays and the hypersensitivity of erythroid progenitors to EPO [1-3]. The overproduction and accumulation of red blood cells in PV occurs at normal oxygen saturations, often with depressed endogenous EPO levels, but without abnormalities in the EPO receptor (EPOR) sequence or expression [4, 5].
The discovery of an acquired, somatic mutation JAK2V617F present within the gene encoding the cytoplasmic tyrosine kinase JAK2 detected in the majority (95%) of PV patients provided insight as to how the molecular and biological characteristics of the disease might relate to its clinical phenotype [6-11]. Believed to play an important role in the pathogenesis of PV and other related MPNs where JAK2V617F is detected in approximately 50% of patients with essential thrombocythemia (ET) or primary myelofibrosis (PMF), the activating JAK2V617F mutation confers a proliferative advantage in EPO-independent PV erythroid colonies and when expressed in hematopoietic cell lines [6-8]. Moreover, in murine bone marrow transplantation models, retroviral expression of JAK2V617F at high levels in hematopoietic cells has been associated with the development of erythrocytosis and a PV-like phenotype [6, 12-15], whereas lower levels of JAK2V617F expression in transgenic murine models has induced variable MPN phenotypes including ET and PMF-like disorders in mice [16, 17]. The findings of other studies involving familial MPNs where JAK2V617F was not found to be a predisposing germ-line factor [18, 19] and the detection of EPO-independent erythroid colonies containing wild-type JAK2 [20] have been consistent with the hypothesis that other primary pathogenic factors may be involved in the development of PV [21]. Thus, the role of the commonly encountered JAK2V617F mutation in the development of specific MPNs with diverse clinicopathologic phenotypes and the mechanism(s) of erythrocytosis as the distinctive clinical feature of PV require further characterization.
The key role of JAK2 protein in red cell development was demonstrated by the observations that JAK2-deficient mice die in-utero due to ineffective erythropoiesis and that fetal liver myeloid progenitors from JAK2-deficient mice fail to respond to exogenous EPO [22]. The binding of EPO to its receptor EPOR leads to the tyrosine phosphorylation of JAK2 at the Y1007/Y1008 residues [23, 24]. JAK2, in turn, catalyzes the phosphorylation of multiple tyrosine residues within the cytoplasmic domain of EPOR, generating docking sites for SH2 domain-containing intracytoplasmic proteins and facilitating the activation of various downstream effectors involved in erythropoiesis, including the signal transducer and activator of transcription 5 (STAT5), phosphatidylinositol 3-kinase (PI3-kinase) and mitogen-activated protein kinase/extracellular regulated kinase (MAPK/ERK) pathways [25].
Enhanced activation of JAK2 and its substrate STAT5 had been previously implicated in the pathogenesis of erythrocytosis in some individuals with primary familial and congenital polycythemia (PFCP) syndromes [26]. Dominant gain-of-function EPOR mutations identified in PFCP patients have been associated with EPO hypersensitivity –but not EPO-independence – of erythroid progenitors and hematopoietic cells, leading to altered kinetics of prolonged EPO-induced JAK2 and STAT5 activation due to failure to efficiently downregulate JAK2 [27-29]. On the other hand, overexpression of the gain-of-function JAK2V617 mutation in hematopoietic cells has been associated with constitutive activation of JAK2 and its substrate STAT5 and enhanced proliferation capacity even in the absence of exogenous EPO [6-8]. The important role in erythropoiesis regulation of STAT5 activation was suggested by the observations that Stat5 knock-out mice exhibit fetal anemia and deficient stress-erythropoiesis response [30, 31]. Furthermore, constitutive STAT5 activity in erythroid progenitors induces EPO-independent differentiation and colony formation [32], while constitutive STAT5 activity within fetal liver cells devoid of JAK2 and EPOR was shown to induce EPO-independent proliferation [33].
The role of signaling effectors other than STAT5 in erythroid cells that may be important in the pathogenesis of PV and deregulation of erythropoiesis requires further investigation. Among other signaling pathways that are involved in erythropoiesis regulation are the MAPK/ERK [34, 35] and PI3-kinase/AKT pathways [36, 37]. In normal primary erythroid precursors, we previously found that activation of STAT5, MAPK-ERK and PI3-kinase-AKT pathways in response to the cooperative action of both EPO and stem cell factor (SCF) is required for the maximal expansion capacity of erythroblasts [38]. In the present study, we investigated the pathogenesis of PV using primary PV erythroid precursors to characterize abnormal proliferation and apoptosis responses associated with deregulation of specific intracellular signaling pathway activation.
PATIENTS AND METHODS
Cytokines and antibodies
Recombinant human stem cell factor (SCF) and insulin-like growth factor-I (IGF-I) were purchased from Sigma (St. Louis, MO). Human recombinant EPO (Procrit) was purchased from the outpatient pharmacy at Duke University Medical Center (Ortho-Biotech, Bridgewater, NJ). The primary antibodies against phospho-STAT5 (Tyr694), phospho-AKT (Ser473), total AKT, phospho-p44/42 ERK1/2 (Thr202/Tyr204), and total ERK1/2 were purchased from Cell Signaling Technologies (Danvers, MA). Antibody against total STAT5 was from Santa Cruz Biotechnology (Santa Cruz, CA).
Patients and primary hematopoietic cell cultures
Peripheral blood mononuclear cells were collected from patients diagnosed with PV (14 patients, 9 men, 5 women, median age 66) and from healthy normal volunteers (10 total, 7 men, 3 women, median age 36) in accordance with a research protocol approved by the Institutional Review Board at Duke University. All patients with PV had erythrocytosis and peripheral blood granulocytes were positive for the JAK2V617F mutation. Peripheral blood samples collected in tubes containing acid-citrate-dextrose (ACD) anticoagulant were diluted in 50mL phosphate-buffered saline containing 0.6% ACD and 0.8% bovine serum albumin (Stem Cell Technologies, Vancouver, BC, Canada) and then subjected to Ficoll 1.077 density gradient separation (Amersham-Pharmacia, Upsala, Sweden) prior to liquid cultures performed as described previously [38, 39]. Briefly, CD34+ cells were isolated from peripheral blood mononuclear cells by positive selection using anti-CD34 antibody (QBEND/10) and magnetic cell sorting on Mini-MACS columns (Miltenyi Biotech, Auburn, CA). CD34+ cells were placed in erythroblast culture medium containing Dulbeco’s Modified Eagle’s Medium (Cat no. 11965-092; Gibco, Carlsbad, CA), 15% fetal bovine serum (Hyclone, Logan, UT), 1.9 mmol sodium bicarbonate (Cat no. 25080-094, Gibco), 0.1 mmol β-mercaptoethanol (Cat. no. M7522, Sigma), 0.125mg/mL iron-saturated transferrin (Cat. No. T0665, Sigma), 1% bovine serum albumin (Cat. no. A8806, Sigma), 10-6 mol/L dexamethasone (Sigma), 10-6 mol/L β-estradiol (Sigma) supplemented with 100 ng/mL recombinant SCF, 1 unit/mL EPO, 40 ng/mL IGF-I. The presence of a homogeneous population of proerythroblasts at days 12-14 of culture was confirmed as described [38].
JAK2V617F mutation detection and allele burden determination in expanded erythroblasts
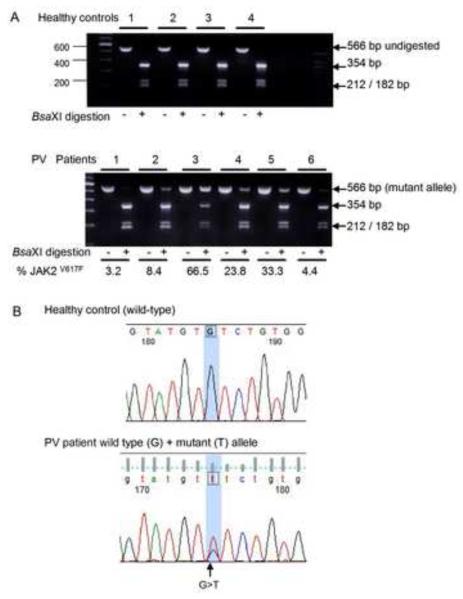
Total RNA from primary erythroblasts was extracted using Qiagen RNAeasy Mini Kit (Qiagen, Studio City, California) and 1.5 μg RNA was used in cDNA synthesis reaction by reverse transcription performed using oligo-dT priming and superscript II (Life Technologies). PCR amplification for JAK2 was performed using expanded high fidelity PCR system in a final reaction mixture of 50μL, containing 300 nmol/L of each primer, 0.5U of the enzyme solution, 200μmol/L each of dNTP, 1.5mmol/L magnesium chloride, and 2.0μL cDNA. The reaction mixture was preheated at 95°C for 2 minutes, followed by 40 cycles at 94°C, 57°C, and 72°C each for 60 seconds. Primers for JAK2 were forward 5′-AGCCTTGGCCAAGGCACTTTT-3′, and JAK2 reverse 5′-CTCCATTTGTCTGTTGCCAAAT-3′. The resulting amplification product was 566-bp (Figure 1A) and the presence of the G>T mutation at position 1849 in JAK2V617F (GenBank sequence AF001362) was confirmed in all cases by purifying the DNA from 1% agarose gels and direct sequencing at the Duke University Medical Center DNA sequencing facility using an ABI PRISM(™) 377 DNA sequencer and BigDye Terminator Cycle Sequencing system (Perkin-Elmer). To determine JAK2V617F allele ratio, the amplified PCR products were subjected to restriction enzyme digestion with BsaXI (New England Biolabs, Beverly, MA) for 19 hours at 37°C. The BsaXI enzyme recognizes a site that is abolished by the mutant G>T nucleotide in JAK2V617F [9]. PCR products subjected to BsaXI digestion were visualized in 2.5% agarose gels and the JAK2V617F allele ratio was expressed as the percentage of undigested DNA (as a result of G>T mutation) of the total DNA amount determined by densitometry analysis of the bands. RT-PCR experiments, direct sequencing and BsaXI digestions using RNA from normal cultured erythroblasts from healthy individuals served as controls (Figure 1B).
Figure 1. Detection of JAK2V617F mutation in primary erythroid precursors.
Total RNA isolated from primary erythroblasts of healthy controls and PV patients was reverse transcribed followed by PCR amplification for JAK2 yielding a 566 bp product. (A) Detection of JAK2V617F by restriction enzyme BsaXI digestion. The wild-type allele is digested into 354, 212, and 182 bp fragments whereas the mutant allele, detected only in PV patients, remains undigested. The allele-burden of JAK2V617F in PV patients was quantified by densitometry and expressed as percentage. (B) Sequence traces illustrating wild-type sequence (a healthy control) and confirmation of mixed G>T sequences in a representative patient with PV. The presence of the mutant JAK2V617F allele was confirmed by direct sequencing in all patient samples.
Cell proliferation assays
Expanded erythroblasts were washed free of serum and growth factors using Roswell Park Memorial Institute (RPMI) 1640 medium (Cat. no. 11835-030; Gibco) and suspended in serum-free medium consisting of RPMI supplemented with 2% bovine serum albumin, 300μg/mL iron-saturated transferrin, 40μg/mL low-density lipoprotein (Cat. no. I2139; Sigma), and 5×10-5 mol/L β-mercaptoethanol. To generate growth curves, cells (1×105/well) were seeded in triplicates in 35mm tissue cultures plates in a volume of 2 mL in the presence of EPO (1 unit/mL), SCF (100 ng/mL), or both cytokines as described [38]. Cells were counted daily using the trypan blue exclusion technique and the total cell numbers were plotted. Erythroblast proliferation in response to EPO, SCF, or both growth factors was also assessed in an assay using the vital dye 3-(4,5-dimethylthiazol-2-yl)-2,4-diphenyltetrazolium bromide (MTT, Sigma). Cells were washed free of serum and growth factors and placed in 96-well plates at a density of 1×104/well in 50μL of serum free medium. Another 50μL of serum-free medium was added with either no growth factor as negative control, EPO (final concentration 1 unit/mL), SCF (final concentration 100 ng/mL), or both cytokines. In assays of EPO sensitivity, cells were treated with SCF plus variable EPO concentrations ranging from 0.01 to 1 unit/mL. Cells were cultured for 4 days, MTT reagent was added to the medium at final concentration of 1 mg/mL, incubated for 4 hours at 37°C, and dissolved in isopropyl alcohol containing 0.04N hydrochloric acid. The optical density in the wells was assessed at wavelength of 562 nm (562-650 nm) using a microtitre plate reader. In some experiments, the kinase inhibitor PD98059 targeting MAPK/ERK kinase (MEK1) was added to the culture medium.
Apoptosis assays
In vitro expanded primary erythroblasts were deprived of growth factors to induce apoptosis. Cells (1 106 cells/condition) were cultured for 16 hours in erythroblast culture medium either in the presence or absence of growth factors (SCF, EPO and IGF-I), with or without kinase inhibitors. Cells were then analyzed for the degree of apoptosis induction upon growth factor withdrawal using an Annexin-V and propidium iodide staining kit (Roche, Indianapolis, IN) followed by flow cytometry. To determine the effect of signaling pathway inhibition on apoptosis, the kinase inhibitors PD98059 (MEK inhibitor) or LY294002 (PI3-kinase inhibitor) were added during the 16-hour incubation at final concentrations of 50μm. Incubations with the kinase inhibitor solvent DMSO served as negative controls.
In a second apoptosis assay, PV or normal erythroblasts (5×105 cells/condition) were plated in a 48-well plate containing erythroblast culture medium either in the presence or absence of growth factors for 16 hours with or without kinase inhibitors. Following the 16 hour incubation, cells were fixed in solution containing 4% paraformaldehyde, permeabilized in solution containing 0.1% Triton X-100 in 0.1% sodium citrate, and then treated with nucleotide label mixture using an In-Situ Cell Death Detection Kit, TMR Red (Roche). Cells were incubated in a solution containing 14.3mM DAPI for 30 minutes, then analyzed by fluorescence microscopy for counting apoptotic nuclei expressed as a percentage of total nuclei (approximately 1000 cells).
Cell treatments, protein assays, and immunoblotting
Proerythroblasts were washed free of serum and growth factors and placed in serum-free starvation medium at a density of 0.5-1×107/mL in 12-well plates. After 4 hours of culture at 37°C, cells were either left untreated or treated with EPO (10 U/mL) for 5, 10, and 30 minutes. In some experiments, kinase inhibitors or DMSO vehicle (negative control) were added to the cultures for 30 minutes prior to stimulation with EPO. Cells were then washed with ice-cold phosphate-buffered saline, lysed in a buffer containing 20 mmol/L Tris-HCL (pH 7.4), 150 mmol/L NaCl, 1% Triton X-100, 10% glycerol, 5 mmol/L EDTA supplemented freshly with 10 mmol/L NaF, 1 mmol/L Na3VO4, 1 mmol/L phenylmethylsulphonyl fluoride, 1 μg/μL aprotinin, and 1 μg/μL leupeptin. The whole cell lysates were cleared by centrifugation at 16,000 g for 15 minutes, supernatants were transferred to fresh tubes, and the concentration of soluble proteins was determined using the Bio-Rad protein assay (Bio-Rad, Hercules, CA). The proteins were heated to 95°C for 4 minutes in 2X Laemmli sample buffer (Bio-Rad), separated in 4-12% Tris-glycine gels (Novex, Carlsbad, CA) by SDS-polyacrylamide gel electrophoresis and transferred to Immobilon polyvinylidenedifluoride membranes (Millipore, Billeric, MA). The membranes were blocked with 5% non-fat milk for 1 h in TBST buffer (20 mmol/L Tris-HCL (pH 7.4); 137 mmol/L NaCl; 0.1% (v/v) Tween-20) and incubated with the primary antibodies at recommended dilutions overnight at 4°C. Antigen-antibody complexes were visualized by incubation with horseradish peroxidase-conjugated secondary antibodies (Pierce, Rockford, IL) and proteins were detected using SuperSignal Enhancer (Pierce) and visualized by autoradiography. In some experiments, after hybridization to phospho-specific antibodies, membranes were stripped and reprobed with the appropriate antibodies. Signal intensity was quantified using NIH ImageJ software for densitometry measurements.
Gene expression microarrays and analysis of RAS and PI3-K pathway activation signatures
Total RNA was extracted from expanded erythroblasts using Trizol reagent (Invitrogen) followed by a clean-up of the RNA sample using RNeasy kit (Qiagen). The quality of the RNA was verified on the Agilent Bioanalyzer at the Duke Microarray Core Facility. Human Affymetrix U133 plus 2.0 gene expression microarrays were used for gene expression analysis. Total RNA samples from independent primary erythroblast cultures from 6 PV patients and 5 healthy controls were analyzed. Biologically validated gene expression signatures representative of PI3-kinase and RAS pathway activation were applied and the probability of PI3-kinase and RAS pathway activation was determined using regression models to assign the relative probability of pathway deregulation in PV or normal erythroblast control samples, as previously described [40-42].
Statistical Analysis
The data are presented as mean±SEM. Data were analyzed using GraphPad InStat software version 3.0 (San Diego, CA, USA). Comparisons between two groups was performed using t-tests and multiple groups were analyzed by using one-way analysis of variance (ANOVA) and Bonferroni’s multiple comparisons post-hoc test. A two-tailed P-value of P<0.05 was considered statistically significant.
Results
In strict serum-free conditions, PV erythroblasts are dependent on the cooperative effect of EPO and SCF for growth and exhibit hypersensitivity to EPO
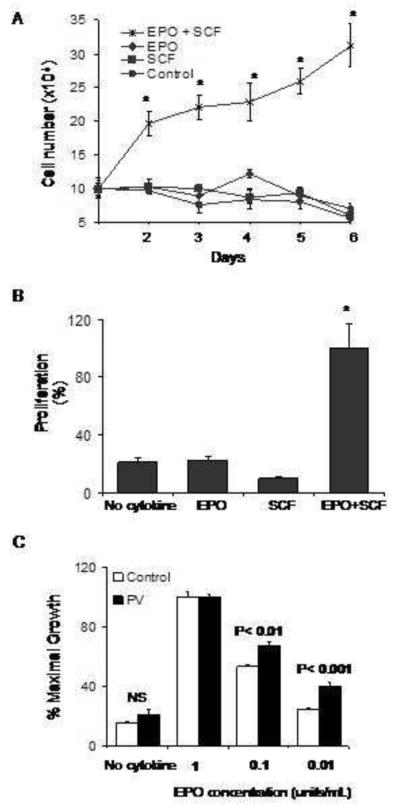
In order to assess the growth characteristics of primary PV erythroblasts, we isolated CD34+ cells from peripheral blood and established liquid cultures containing SCF, EPO, and IGF–I. The cells were expanded over a 12 to 14 day period, yielding a homogenous population of erythroblasts as described previously [38, 39]. Under serum-free conditions, the growth of normal cultured erythroblasts derived from healthy individuals is dependent upon the co-stimulatory effect of EPO and SCF [38]. To determine whether the proliferation of erythroblasts derived from individuals with PV may exhibit growth factor independence, PV erythroblasts were cultured in serum-free medium containing no added growth factors, EPO alone, SCF alone, or both EPO and SCF. PV erythroblasts did not survive and proliferate when cultured in serum-free medium containing no growth factor or either EPO or SCF alone, but required both EPO and SCF to exhibit maximal growth (Figure 2A). As illustrated in Figure 2B, these results were corroborated by the MTT proliferation assay, which similarly demonstrated the requirement of PV erythroblasts for the presence of both EPO and SCF.
Figure 2. Proliferation of PV erythroid precursors in response to cytokines under serum-free conditions.
(A) Growth curves of PV erythroblasts plated in serum-free medium in the presence of EPO, SCF or both growth factors in a representative experiment. Cells were counted daily in a hemocytometer using trypan blue exclusion technique and total cell counts were plotted. The data represent mean ± SEM values of triplicate cultures. *P<0.001 (B) Short-term expansion of erythroblasts in serum-free cultures (four days) in response to cooperative effect of EPO and SCF determined by MTT assay. Control group contained no cytokines. Data represent mean ± SEM values (n=6 replicates in each group). *P<0.001. (C) EPO hypersensitivity of PV erythroblasts in serum-free conditions containing decreasing concentrations of EPO ranging as indicated with assessment of proliferation in an MTT assay. Data are represented as % of maximal growth. NS: not significant.
As demonstrated by previous experiments that examined erythroid colony formation in vitro in semi-solid medium cultures, erythroid progenitors in polycythemia vera are hypersensitive to EPO [3, 43, 44]. We next investigated whether EPO-hypersensitivity characterizes the growth of erythroid precursor cells under serum-free conditions. Proerythroblasts from individuals with PV and healthy controls were washed and transferred to serum-free medium containing either no growth factor or SCF with varying concentrations of EPO (range from 0.01unit/mL to 1 unit/mL). After 4 days of culture, proliferation was assessed by the MTT assay. In both PV and control erythroblasts, maximum growth was achieved with SCF and the highest concentration of EPO (Figure 2C). There was no significant difference in the in % maximal proliferation between PV and control erythroblasts in the absence of cytokines. A significantly higher level of proliferation was observed in PV erythroblasts compared to normal erythroblasts at EPO concentrations of 0.1 unit/mL (68±2% versus 53±1%, P<0.01) and at the physiologic EPO concentration of 0.01 unit/ml (40±3 versus 24±1%, P<0.001).
RAS/ERK and PI3-kinase /AKT pathway activation is increased in PV erythroblasts
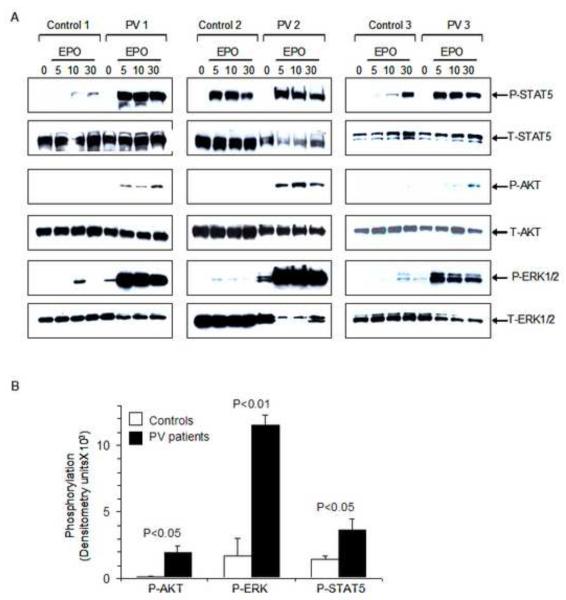
We investigated the mechanism of the enhanced EPO sensitivity of PV erythroblasts by examining EPO-dependent activation of JAK2/STAT5, RAS/ERK and PI3-kinase/AKT pathways. PV erythroblasts were placed in starvation medium for 4 hours and then either left untreated or treated with EPO for 5, 10, and 30 minutes and cell lysates were subjected to immunoblotting. EPO-induced STAT5 phosphorylation was increased in PV erythroblasts compared to normal erythroblasts (Figure 3A). We observed constitutive phosphorylation of p44/42 mitogen-activated protein kinases ERK1/2 in the absence of EPO stimulation in PV but not in control erythroblasts (Figure 3A). Furthermore, EPO stimulation resulted in marked increase in ERK1/2 phosphorylation in PV erythroblasts relative to that observed in control erythroblasts at all three time points. In addition, EPO stimulation resulted in weak but detectable AKT phosphorylation in PV erythroblasts, but no AKT phosphorylation was observed in control erythroblasts consistent with the results of our previous studies involving normal primary erythroblasts in which AKT phosphorylation was dependent primarily on SCF-KIT signaling [38]. Quantification of the phosphorylation was performed by densitometry analysis of untreated and EPO-stimulated samples (10 minutes) demonstrating significant increase in EPO-induced phosphorylation of ERK1/2, AKT and STAT5 in PV erythroblasts compared to normal erythroblasts (Figure 3B). The degree of increase in ERK phosphorylation was prominent and did not appear to be related the percentage of JAK2V617F allele burden, although some patient variability in EPO-induced phosphorylation (Figure 3A) did not allow a conclusive assessment of the relationship between JAK2V617F allele burden and EPO-induced phosphorylation.
Figure 3. Phosphorylation of STAT5, AKT and ERK1/2, in primary erythroblasts in response to EPO.
(A) Proerythroblasts were washed free of serum and growth factors, incubated in serum-free starvation medium and then either left unstimulated (0) or stimulated with EPO (10 units/mL) for the indicated time period (5, 10, 30 minutes). Whole cell lysates were analyzed by immunoblotting. Increased EPO-induced phosphorylation of STAT5, AKT and ERK1/2 are noted. Comparable protein loading in each lane and protein integrity were demonstrated by stripping the blots and hybridizing to an antibody that detects total protein levels. (B) Quantification and comparison of levels of phosphorylated STAT5, AKT, and ERK1/2 in PV versus control erythroblasts stimulated with EPO at 10 minutes. Densitometry measurements were performed and represented as mean ± SEM from three PV patients and three healthy controls.
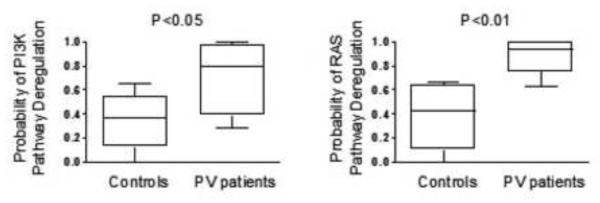
In the next set of experiments, the activation status of RAS/ERK and PI3-kinase/AKT pathways in PV erythroid precursor cells was further evaluated using genome-wide gene expression analysis and utilization of oncogenic pathway signatures designed to detect abnormal signaling [40, 41]. In these experiments, total cellular RNA was extracted directly from expanded erythroblasts during exponential growth phase in erythroblast culture medium supplemented with EPO, SCF and IGF without a starvation period. The probability of RAS and PI3-kinase pathway activation was assessed by applying previously validated signatures of pathway activity to RNA expression data from 6 PV and 5 healthy control erythroblast samples. Importantly, gene expression analysis using pathway activation signatures revealed significantly increased RAS (P<0.01) and PI3-kinase (P<0.05) pathway deregulation probability in PV erythroblasts (Figure 4).
Figure 4. Analysis of RAS and PI3-Kinase pathway activation signatures.
Gene expression signatures of oncogenic signaling pathways to characterize the activation status of RAS and PI3-kinase pathways were applied to data from patients with PV and healthy controls. The probability of PI3-kinase (panels on left) and RAS pathway (panels on right) deregulation in PV erythroblasts are significantly increased compared to normal control erythroblasts.
PV erythroblasts exhibit reduced apoptosis following growth factor withdrawal
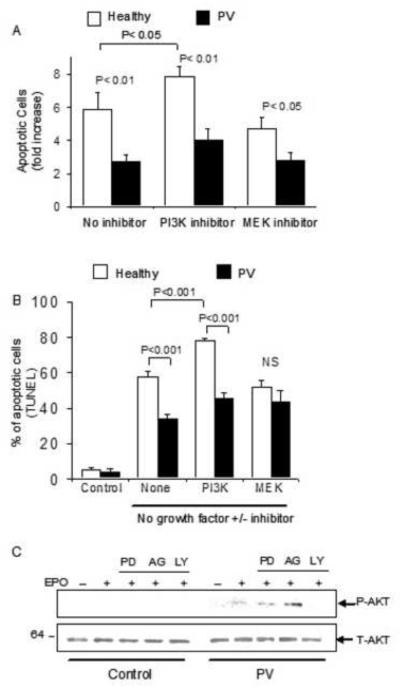
Growth factor withdrawal from primary human erythroid progenitors leads to induction of apoptosis [45]. To compare the apoptosis response of PV and control erythroblasts following growth factor withdrawal, expanded erythroblasts were washed and cultured in erythroblast culture medium with or without added growth factors (EPO, SCF, and IGF). The degree of apoptosis induction was assessed at 16 hours, in the presence or absence of growth factor, using Annexin-V/propidium iodide staining followed by flow cytometry, as well as in TUNEL assays. The basal amount of apoptosis in cultured erythroblasts from PV patients and healthy controls was similar as reported previously [46]. Following growth factor withdrawal, both normal and PV erythroblasts exhibited increased apoptosis (5.8±1-fold compared to 2.7±0.3-fold, respectively) with significantly decreased apoptosis in PV erythroblasts (P<0.01, Figure 5A). Similar results were obtained using TUNEL assays where growth factor withdrawal was associated with significantly lower degree of apoptosis induction in PV erythroblasts compared to normal erythroblasts (34±3% versus 58±3%, respectively, P<0.001, Figure 5B). To investigate the role of RAS/ERK and PI3-kinase/AKT pathways in erythroblast apoptosis, cells were treated with kinase inhibitors during growth factor deprivation. Treatment with MEK inhibitor PD98059 did not have a significant effect on erythroblast apoptosis in normal or PV erythroblasts (Figure 5A and B). When primary erythroblasts were treated with LY294002– a kinase inhibitor targeting PI3-kinase – during growth factor withdrawal, normal erythroblasts exhibited a further significant increase in apoptosis as determined by Annexin-V assays from 5.8 to 7.8 fold (P<0.05, Figure 5A), or in TUNEL assays from 57±3% to 77±2% (P<0.001, Figure 5B), whereas growth factor-deprived PV erythroblasts also exhibited an increase in apoptosis when exposed to PI3-kinase inhibitor from 33±2% to 45±3% in TUNEL assays that was not statistically significant. Treatment of PV erythroblasts with PI3-kinase inhibitor abolished the EPO-induced phosphorylation of AKT whereas treatment with MEK inhibitor PD98059 or JAK2 inhibitor AG490 had no effect on AKT phosphorylation in PV erythroblasts (Figure 5C).
Figure 5. PV erythroid precursors exhibit reduced apoptosis following growth factor withdrawal.
Proerythroblasts from PV patients and healthy individuals were induced to undergo apoptosis by growth factor (EPO, SCF, IGF-I) withdrawal in the presence or absence of kinase inhibitors for 16 hours. Apoptosis was assessed using (A) Annexin V assays, expressed as fold-increase in apoptotic cells compared to erythroblasts cultured in complete erythroblast medium supplemented with EPO, SCF and IGF-I as control, and (B) TUNEL assays. Control cultures containing complete medium with growth factor exhibit low basal apoptotic cell percentage that is significantly increased following growth factor withdrawal for 16 hours. (C) EPO-induced AKT phosphorylation was observed only in PV erythroblasts and not in healthy control cells. AKT phosphorylation is inhibited by treatment with PI3-kinase inhibitor LY but not after treatment with kinase inhibitors targeting MEK (PD) or JAK2 (AG).
PV erythroblasts are less sensitive to the growth-inhibitory effect of MEK kinase inhibitor compared to normal erythroblasts
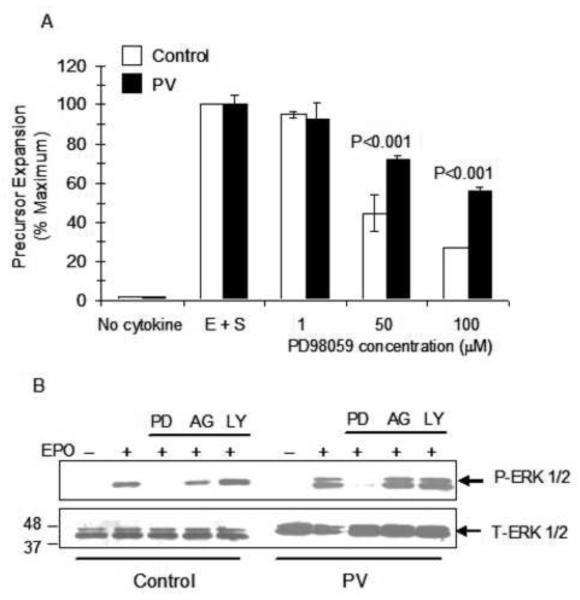
We investigated the significance of deregulated RAS/ERK pathway activation in PV erythroid precursors during cellular proliferation since this pathway did not appear to be involved in apoptosis signaling during growth factor deprivation (Figure 5). Proliferation assays were performed in which PV and control erythroblasts were cultured in the presence of increasing concentrations of MEK inhibitor PD98059 in serum-free medium supplemented with both EPO and SCF that are required for maximal growth. PD98059 treatment of erythroblasts at concentrations of 50 and 100μM significantly decreased the proliferation of normal erythroblasts consistent with the results of our previous studies [38]. At the same concentrations of the kinase inhibitor, the growth inhibitory effect was significantly lower in PV erythroblasts compared to normal (control) erythroblasts (Figure 6A). At the 50μM concentration proliferation of PV erythroblasts was 72±2% compared to 45±3% in normal erythroblasts (P<0.001) and in the presence of 100 μM the proliferation of PV erythroblasts was 56±2% compared to 27±2% in normal erythroblasts (P<0.001). Treatment of PV erythroblasts with MEK inhibitor abolished EPO-induced phosphorylation of ERK1/2 whereas treatment with PI3-kinase inhibitor or JAK2 inhibitor had no effect on ERK1/2 phosphorylation in PV erythroblasts, as well as in normal (control) erythroblasts (Figure 6B).
Figure 6. Decreased sensitivity of PV erythroblasts to the growth-inhibitory effect of MEK kinase inhibitor compared to normal erythroblasts.
(A) PV or healthy control erythroblasts were placed in serum-free medium for short-term expansion (four days) in the presence of EPO and SCF (E+S) and the indicated concentrations of MEK inhibitor PD98059. Proliferation was measured by MTT assay. Control group contained no growth factors. E+S group contained DMSO vehicle. (B) EPO-induced ERK phosphorylation was inhibited by treatment of erythroblasts with MEK inhibitor PD98059 (PD) but not after treatment with kinase inhibitors targeting PI3-kinase (LY) or JAK2 (AG).
Discussion
The excessive production and accumulation of RBCs in PV that result in the distinctive clinical feature of erythrocytosis is characterized by EPO-independent growth of early erythroid progenitors associated with cytokine hypersensitivity and the major JAK2V617 molecular abnormality detected in the majority of patients. In the present studies, we focused on characterization of EPO responses and signaling in primary PV erythroid precursor cells using an in vitro culture system to expand a homogenous population of proerythroblasts. The data indicates that PV erythroblasts are indistinguishable from normal erythroid cells with respect to the dependence on the cooperative effect of both EPO and SCF to survive and proliferate under strict serum-free conditions. EPO alone is not sufficient to maintain the viability of primary PV erythroblasts, but in the presence of SCF and limiting physiologic concentrations of EPO, PV erythroid precursors exhibit EPO hypersensitivity as has been described for erythroid progenitor cells [44]. The EPO hypersensitivity we observed in PV erythroid precursor cells was associated with increased activation of the RAS-MAPK/ERK pathway as determined by markedly increased phosphorylation of ERK1/2, consistent with the findings of a previous study involving cultured PV erythroid precursors [47]. In our studies, we also investigated oncogenic pathway activation signatures in PV using gene expression profiling and found significantly increased RAS pathway activation probability in PV erythroblasts.
The mechanisms of increased RAS pathway and ERK activation in PV cells that we observed in our studies require further exploration. A previous study has implicated a PI3-kinase-dependent pathway in EPO-induced ERK activation in primary human erythroid progenitors [48], although other studies including experiments from our laboratory have not found that PI3-kinase inhibition modulates ERK activation in erythroid cells [38, 49]. We found that treatment of PV or normal erythroblasts with MEK inhibitor abolished EPO-induced increase in ERK phosphorylation, whereas treatment with PI3-kinase inhibitor or JAK inhibitor had no effect, suggesting a PI3-kinase- or JAK2-independent mechanism of ERK activation (Figure 6B). Regardless of the mechanism, that ERK activation in PV erythroblasts may contribute to increased cellular proliferation and the development of erythrocytosis in PV, is consistent with the findings of previous studies in other experimental models. For instance, the activation of ERK in mouse erythroid progenitors was found to be the primary effector mediating EPO-independent growth as a result of oncogenic H-ras-induced transformation [34]. Furthermore, expression of constitutively active MEK in conjunction with constitutively active AKT synergized to result in EPO-independent proliferation of erythroid progenitors. In another study, ERK hyperactivation observed in primary mouse erythroblasts harboring a mutant EPOR was associated with increased numbers of immature erythroblasts and a differentiation defect that was reversible by treatment with a MEK kinase inhibitor [35].
The role of the JAK2V617F mutation in the induction of downstream signaling abnormalities in hematopoietic cells has been investigated in several cell line models, as well as in transgenic mice. In human erythroid leukemia HEL cells which express endogenous JAK2V617F, treatment of the cells with WP1066, a novel JAK2 inhibitor, was associated with reduced ERK1/2 but no change in AKT phosphorylation [50]. In stably transfected IL-3-dependent hematopoietic or non-hematopoietic cell lines expressing high levels of mutant JAK2V617F protein, increased constitutive and EPO-induced phosphorylation of ERK and AKT was reported [6, 11]. In studies involving transplanted or transgenic mice engineered to overexpress JAK2V617F in bone marrow cells, increased constitutive and EPO-induced phosphorylation of ERK and AKT was observed [14, 17]. In our studies using expanded primary erythroblasts, we observed markedly increased RAS pathway activation by gene expression profiling. Importantly, this was true despite a variable level of JAK2V617F expression (Figure 1) observed in the cultured erythroid cells. Whether the hyperactivation of RAS-ERK pathway in PV erythroblasts involves, at least in part, JAK2V617F-independent mechanisms will require further studies.
EPO-induced AKT phosphorylation was observed in PV erythroblasts and not in normal erythroblasts. This finding is consistent with the results of previous studies from our laboratory and others demonstrating that AKT phosphorylation in normal erythroblasts requires treatment of the cells with SCF [38, 51]. Given this ability of EPO to phosphorylate AKT in PV erythroblasts, we further investigated PI3-kinase pathway activation using gene expression profiling. These analyses revealed significantly increased probability of PI3-kinase pathway activation in PV erythroblasts. The observed increase in PI3-kinase pathway activation and AKT phosphorylation in PV erythroblasts were associated with a significant reduction in the degree of apoptosis upon growth factor withdrawal compared to normal erythroblasts. Treatment of erythroblasts with the PI3-kinase inhibitor LY294002 that abolishes AKT phosphorylation resulted in increased apoptosis in both normal and PV erythroblasts – but the increase in apoptosis was relatively minor in PV erythroblasts compared to normal erythroblasts –suggesting resistance to apoptosis in PV cells. Interestingly, treatment of erythroblasts with MEK inhibitor did not have any effect on apoptosis of the cells. We did not observe constitutive phosphorylation of AKT in PV erythroblasts, a finding which is different than the reported results of a study by Zeuner and colleagues demonstrating constitutive AKT phosphorylation in PV erythroid precursors associated with increased resistance to death receptor-induced proliferation arrest and apoptosis [47]. Our findings of apoptosis resistance upon growth factor withdrawal in erythroid precursors are consistent with the results of a prior study that found reduced apoptosis of PV erythroid progenitors upon EPO withdrawal associated with overexpression of the anti-apoptotic bcl-xL protein [52].
Taken together, the findings of the present studies suggest that disruption of erythropoiesis in PV involves 1)- increased EPO sensitivity and proliferation responses associated with RAS/ERK pathway hyperactivation and 2)- decreased sensitivity to pro-apoptotic stimuli such as growth factor withdrawal associated with increased PI3-kinase/AKT pathway activation in PV erythroid precursors compared to normal erythroblasts. The elucidation of the mechanisms involved in abnormal RAS/ERK and PI3-kinase/AKT pathway activation in PV erythroblasts may contribute to our understanding of the molecular pathogenesis of PV.
Acknowledgements
This work was supported in part by a grant from the National Institutes of Health NIDDK K08 DK02566. The authors thank all the referring physicians and the patients who participated in this study.
Footnotes
Conflict of Interest Disclosure No financial interest/relationships with financial interest relating to the topic of this article have been declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prchal JF, Axelrad AA. Letter: Bone-marrow responses in polycythemia vera. N Engl J Med. 1974;290:1382. doi: 10.1056/nejm197406132902419. [DOI] [PubMed] [Google Scholar]
- 2.Eaves CJ, Eaves AC. Erythropoietin (Ep) dose-response curves for three classes of erythroid progenitors in normal human marrow and in patients with polycythemia vera. Blood. 1978;52:1196–1210. [PubMed] [Google Scholar]
- 3.Casadevall N, Vainchenker W, Lacombe C, et al. Erythroid progenitors in polycythemia vera: demonstration of their hypersensitivity to erythropoietin using serum free cultures. Blood. 1982;59:447–451. [PubMed] [Google Scholar]
- 4.Means RT, Jr., Krantz SB, Sawyer ST, Gilbert HS. Erythropoietin receptors in polycythemia vera. J Clin Invest. 1989;84:1340–1344. doi: 10.1172/JCI114303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hess G, Rose P, Gamm H, Papadileris S, Huber C, Seliger B. Molecular analysis of the erythropoietin receptor system in patients with polycythaemia vera. Br J Haematol. 1994;88:794–802. doi: 10.1111/j.1365-2141.1994.tb05119.x. [DOI] [PubMed] [Google Scholar]
- 6.James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 7.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 8.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 9.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 10.Jones AV, Kreil S, Zoi K, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005;106:2162–2168. doi: 10.1182/blood-2005-03-1320. [DOI] [PubMed] [Google Scholar]
- 11.Zhao R, Xing S, Li Z, et al. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem. 2005;280:22788–22792. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wernig G, Mercher T, Okabe R, Levine RL, Lee BH, Gilliland DG. Expression of Jak2V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood. 2006;107:4274–4281. doi: 10.1182/blood-2005-12-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bumm TG, Elsea C, Corbin AS, et al. Characterization of murine JAK2V617F-positive myeloproliferative disease. Cancer Res. 2006;66:11156–11165. doi: 10.1158/0008-5472.CAN-06-2210. [DOI] [PubMed] [Google Scholar]
- 14.Lacout C, Pisani DF, Tulliez M, Gachelin FM, Vainchenker W, Villeval JL. JAK2V617F expression in murine hematopoietic cells leads to MPD mimicking human PV with secondary myelofibrosis. Blood. 2006;108:1652–1660. doi: 10.1182/blood-2006-02-002030. [DOI] [PubMed] [Google Scholar]
- 15.Zaleskas VM, Krause DS, Lazarides K, et al. Molecular pathogenesis and therapy of polycythemia induced in mice by JAK2 V617F. PLoS ONE. 2006;1:e18. doi: 10.1371/journal.pone.0000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiedt R, Hao-Shen H, Sobas MA, et al. Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood. 2008;111:3931–3940. doi: 10.1182/blood-2007-08-107748. [DOI] [PubMed] [Google Scholar]
- 17.Shide K, Shimoda HK, Kumano T, et al. Development of ET, primary myelofibrosis and PV in mice expressing JAK2 V617F. Leukemia. 2008;22:87–95. doi: 10.1038/sj.leu.2405043. [DOI] [PubMed] [Google Scholar]
- 18.Rumi E, Passamonti F, Pietra D, et al. JAK2 (V617F) as an acquired somatic mutation and a secondary genetic event associated with disease progression in familial myeloproliferative disorders. Cancer. 2006;107:2206–2211. doi: 10.1002/cncr.22240. [DOI] [PubMed] [Google Scholar]
- 19.Bellanne-Chantelot C, Chaumarel I, Labopin M, et al. Genetic and clinical implications of the Val617Phe JAK2 mutation in 72 families with myeloproliferative disorders. Blood. 2006;108:346–352. doi: 10.1182/blood-2005-12-4852. [DOI] [PubMed] [Google Scholar]
- 20.Nussenzveig RH, Swierczek SI, Jelinek J, et al. Polycythemia vera is not initiated by JAK2V617F mutation. Exp Hematol. 2007;35:32–38. doi: 10.1016/j.exphem.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Kralovics R, Teo SS, Li S, et al. Acquisition of the V617F mutation of JAK2 is a late genetic event in a subset of patients with myeloproliferative disorders. Blood. 2006;108:1377–1380. doi: 10.1182/blood-2005-11-009605. [DOI] [PubMed] [Google Scholar]
- 22.Parganas E, Wang D, Stravopodis D, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 23.Witthuhn BA, Quelle FW, Silvennoinen O, et al. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993;74:227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- 24.Feng J, Witthuhn BA, Matsuda T, Kohlhuber F, Kerr IM, Ihle JN. Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol Cell Biol. 1997;17:2497–2501. doi: 10.1128/mcb.17.5.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richmond TD, Chohan M, Barber DL. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol. 2005;15:146–155. doi: 10.1016/j.tcb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Klingmuller U, Lorenz U, Cantley LC, Neel BG, Lodish HF. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell. 1995;80:729–738. doi: 10.1016/0092-8674(95)90351-8. [DOI] [PubMed] [Google Scholar]
- 27.Sokol L, Luhovy M, Guan Y, Prchal JF, Semenza GL, Prchal JT. Primary familial polycythemia: a frameshift mutation in the erythropoietin receptor gene and increased sensitivity of erythroid progenitors to erythropoietin. Blood. 1995;86:15–22. [PubMed] [Google Scholar]
- 28.Arcasoy MO, Harris KW, Forget BG. A human erythropoietin receptor gene mutant causing familial erythrocytosis is associated with deregulation of the rates of Jak2 and Stat5 inactivation. Exp Hematol. 1999;27:63–74. doi: 10.1016/s0301-472x(98)00003-4. [DOI] [PubMed] [Google Scholar]
- 29.Watowich SS, Xie X, Klingmuller U, et al. Erythropoietin receptor mutations associated with familial erythrocytosis cause hypersensitivity to erythropoietin in the heterozygous state. Blood. 1999;94:2530–2532. [PubMed] [Google Scholar]
- 30.Socolovsky M, Fallon AE, Wang S, Brugnara C, Lodish HF. Fetal anemia and apoptosis of red cell progenitors in Stat5a-/-5b-/- mice: a direct role for Stat5 in Bcl-X(L) induction. Cell. 1999;98:181–191. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 31.Socolovsky M, Nam H, Fleming MD, Haase VH, Brugnara C, Lodish HF. Ineffective erythropoiesis in Stat5a(-/-)5b(-/-) mice due to decreased survival of early erythroblasts. Blood. 2001;98:3261–3273. doi: 10.1182/blood.v98.12.3261. [DOI] [PubMed] [Google Scholar]
- 32.Garcon L, Rivat C, James C, et al. Constitutive activation of STAT5 and Bcl-xL overexpression can induce endogenous erythroid colony formation in human primary cells. Blood. 2006;108:1551–1554. doi: 10.1182/blood-2005-10-009514. [DOI] [PubMed] [Google Scholar]
- 33.Grebien F, Kerenyi MA, Kovacic B, et al. Stat5 activation enables erythropoiesis in the absence of EPOR and Jak2. Blood. 2008;111:4511–4522. doi: 10.1182/blood-2007-07-102848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Lodish HF. Constitutive activation of the MEK/ERK pathway mediates all effects of oncogenic H-ras expression in primary erythroid progenitors. Blood. 2004;104:1679–1687. doi: 10.1182/blood-2004-04-1362. [DOI] [PubMed] [Google Scholar]
- 35.Menon MP, Fang J, Wojchowski DM. Core erythropoietin receptor signals for late erythroblast development. Blood. 2006;107:2662–2672. doi: 10.1182/blood-2005-02-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klingmuller U, Wu H, Hsiao JG, et al. Identification of a novel pathway important for proliferation and differentiation of primary erythroid progenitors. Proc Natl Acad Sci U S A. 1997;94:3016–3021. doi: 10.1073/pnas.94.7.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghaffari S, Kitidis C, Zhao W, et al. AKT induces erythroid-cell maturation of JAK2-deficient fetal liver progenitor cells and is required for Epo regulation of erythroid-cell differentiation. Blood. 2006;107:1888–1891. doi: 10.1182/blood-2005-06-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arcasoy MO, Jiang X. Co-operative signalling mechanisms required for erythroid precursor expansion in response to erythropoietin and stem cell factor. Br J Haematol. 2005;130:121–129. doi: 10.1111/j.1365-2141.2005.05580.x. [DOI] [PubMed] [Google Scholar]
- 39.Panzenbock B, Bartunek P, Mapara MY, Zenke M. Growth and differentiation of human stem cell factor/erythropoietin-dependent erythroid progenitor cells in vitro. Blood. 1998;92:3658–3668. [PubMed] [Google Scholar]
- 40.Bild AH, Yao G, Chang JT, et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 2006;439:353–357. doi: 10.1038/nature04296. [DOI] [PubMed] [Google Scholar]
- 41.Potti A, Dressman HK, Bild A, et al. Genomic signatures to guide the use of chemotherapeutics. Nat Med. 2006;12:1294–1300. doi: 10.1038/nm1491. [DOI] [PubMed] [Google Scholar]
- 42.Anders CK, Acharya CR, Hsu DS, et al. Age-specific differences in oncogenic pathway deregulation seen in human breast tumors. PLoS ONE. 2008;3:e1373. doi: 10.1371/journal.pone.0001373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montagna C, Massaro P, Morali F, Foa P, Maiolo AT, Eridani S. In vitro sensitivity of human erythroid progenitors to hemopoietic growth factors: studies on primary and secondary polycythemia. Haematologica. 1994;79:311–318. [PubMed] [Google Scholar]
- 44.Dupont S, Masse A, James C, et al. The JAK2 617V>F mutation triggers erythropoietin hypersensitivity and terminal erythroid amplification in primary cells from patients with polycythemia vera. Blood. 2007;110:1013–1021. doi: 10.1182/blood-2006-10-054940. [DOI] [PubMed] [Google Scholar]
- 45.Somervaille TC, Linch DC, Khwaja A. Growth factor withdrawal from primary human erythroid progenitors induces apoptosis through a pathway involving glycogen synthase kinase-3 and Bax. Blood. 2001;98:1374–1381. doi: 10.1182/blood.v98.5.1374. [DOI] [PubMed] [Google Scholar]
- 46.Gaikwad A, Nussenzveig R, Liu E, Gottshalk S, Chang K, Prchal JT. In vitro expansion of erythroid progenitors from polycythemia vera patients leads to decrease in JAK2 V617F allele. Exp Hematol. 2007;35:587–595. doi: 10.1016/j.exphem.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeuner A, Pedini F, Signore M, et al. Increased death receptor resistance and FLIPshort expression in polycythemia vera erythroid precursor cells. Blood. 2006;107:3495–3502. doi: 10.1182/blood-2005-07-3037. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt EK, Fichelson S, Feller SM. PI3 kinase is important for Ras, MEK and Erk activation of Epo-stimulated human erythroid progenitors. BMC Biol. 2004;2:7. doi: 10.1186/1741-7007-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halupa A, Chohan M, Stickle NH, Beattie BK, Miller BA, Barber DL. Erythropoietin receptor Y479 couples to ERK1/2 activation via recruitment of phospholipase Cgamma. Exp Cell Res. 2005;309:1–11. doi: 10.1016/j.yexcr.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 50.Verstovsek S, Manshouri T, Quintas-Cardama A, et al. WP1066, a novel JAK2 inhibitor, suppresses proliferation and induces apoptosis in erythroid human cells carrying the JAK2 V617F mutation. Clin Cancer Res. 2008;14:788–796. doi: 10.1158/1078-0432.CCR-07-0524. [DOI] [PubMed] [Google Scholar]
- 51.Dai C, Chung IJ, Krantz SB. Increased erythropoiesis in polycythemia vera is associated with increased erythroid progenitor proliferation and increased phosphorylation of Akt/PKB. Exp Hematol. 2005;33:152–158. doi: 10.1016/j.exphem.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 52.Silva M, Richard C, Benito A, Sanz C, Olalla I, Fernandez-Luna JL. Expression of Bcl-x in erythroid precursors from patients with polycythemia vera. N Engl J Med. 1998;338:564–571. doi: 10.1056/NEJM199802263380902. [DOI] [PubMed] [Google Scholar]