Abstract
Mitochondria are the power engine generating biochemical energy in the cell. Mitochondrial dysfunction and bioenergy deficiency is closely linked to the pathogenesis of neurodegenerative disorders. Mitochondria play a variety of roles by integrating extracellular signals and executing important intracellular events in neuronal survival and death. In this context, the regulation of mitochondrial function via therapeutic approaches may exert some salutary and neuroprotective mechanisms. Understanding the relationship of mitochondria-dependent pathogenesis may provide important pharmacological utility in the treatment of neurodegenerative conditions such as Alzheimer’s disease, amyotrophic lateral sclerosis, Huntington’s disease and Parkinson’s disease. Indeed, the modulation of mitochondrial pathways is rapidly emerging as a novel therapeutic target. This review focuses on how mitochondria are involved in neurodegeneration and what therapeutics are available to target mitochondrial pathways.
Keywords: Neuroprotection, Alzheimer’s disease, Amyotrophic lateral sclerosis, Huntington’s disease, Parkinson’s disease, Therapeutics
1. Introduction
Mitochondria are the power plants of cells generating biochemical energy in the form of adenosine triphosphate (ATP) by passing electrons derived from the oxidation of nutrients down the respiratory chain to react with oxygen, using redox energy to translocate protons across the mitochondrial inner membrane [1]. The electrochemical potential gradient of protons is generated across the inner membrane consisting of a membrane potential (negative inside) and a pH gradient (basic inside) that drives ATP synthesis through F0F1-ATP synthase [2]. Mitochondria are the source of 80% or more of the reactive oxygen species generated in neurons. Neuronal toxins and stress blocking mitochondrial functions cause excessive neuronal damage and cell death by the dysregulation of oxyradicals. Rotenone and the neurotoxin 1-methyl 4- phenyl 1,2,3,6 tetrahydropyridine (MPTP/MPP+) also induce mitochondrial dysfunction that is relevant to Parkinson’s disease (PD). Thus, while the role of mitochondrial dysfunction has been proposed in neurodegenerative diseases, the exact mechanism of mitochondrial pathogenesis is unclear. Mitochondrial function in terms of energy deficiency and oxidative stress is important in characterizing the pathogenesis of neurodegenerative disorders [3-5]. Mitochondrial DNA defects or mutations are also closely linked to neurological disorders. Excitotoxicity is a well-established mechanism of neuronal cell death in neurodegenerative disorders. N-Methyl D-Aspartate (NMDA) stimulation can decrease the mitochondrial membrane potential associated with neuronal excitotoxicity. Mitochondria therefore play an indirect role as executioner in the excitotoxic pathway. Identification of specific molecules and signaling cascades, which may ultimately lead to neuronal dysfunction and/or cell death, may provide important clues in understanding the pathogenesis of neurological disorders. Recent findings have suggested that mitochondria-dependent cellular events are emerging as potential therapeutic targets. In this review, we address how mitochondria dysfuntion contributes to neurodegeneration and discuss which drugs may improve mitochondria-dependent neuroprotective signaling pathways.
2. Mitochondrial Dysfunction in Neurodegenerative Disorders
2-1. Amyotrophic lateral sclerosis (ALS) and mitochondrial dysfunction
Amyotrophic lateral sclerosis (ALS) is a clinically severe, fatal neurodegenerative disorder characterized by a loss of upper and lower motor neurons, resulting in progressive muscle wasting and paralysis [6]. The vast majority of ALS cases occur sporadically, but about 5-10% of ALS cases are familial. The genetic linkage of several mutations in the gene for Cu/Zn superoxide dismutase (SOD 1) with some cases of familial ALS provided the first indication of a potential causal factor in the disease process [7]. The similarity in the course and pathological features of familial (FALS) and sporadic ALS (SALS) has led a number of investigators to search for genetic mutations associated with FALS as a strategy for elucidating disease pathogenesis and defining novel treatments in both sporadic and inherited forms of the disease. Mitochondria dysfunction and oxidative stress is closely linked to oxidative phosphorylation dysfunction and is further implicated in the pathogenesis of FALS. Metabolic processes involving the mitochondrial electron transport chain are known to contribute to the formation of harmful reactive oxygen species (ROS) (Figure 1). In ALS, motor neurons are particularly vulnerable to oxidative stress, a phenomena attributed to a low level of antioxidant enzymes, a high content of easily oxidized substrates (e.g. polyunsaturated membrane lipids), and an inherently high flux of reactive oxygen species generated during energy metabolism. Therefore, based on the well characterized and essential function of SOD1 in limiting free radical accumulation, research has examined the association between SOD1 mutations and the generation of pathological oxidative damage, which results in subsequent motor neuron degeneration [7]. Oxidative damage to spinal cord proteins has been shown to occur in both human SALS and FALS [8]. In addition, previous studies have shown that transgenic mice expressing mSOD1 develop a progressive accumulation of 8-hydroxy-2-deoxyguanosine, a marker of oxidative DNA damage, and have elevated levels of mitochondrial oxidative damage. A proteomics approach has recently showed that SOD1, translationally controlled tumor protein, ubiquitin carboxyl-terminal hydrolase-L1, and alphaB-crystallin are highly carbonylated in the spinal cord of G93A ALS mice [9]. Other oxidative modifications, such as nitrosylation of proteins, could also be important pathogenic mechanisms of ALS. Either excessive or deficient levels of protein S-nitrosylation may contribute to onset of ALS. Recently, deficient S-nitrosylation has been found in the mitochondria of cells expressing SOD1 mutants [10]. In this paradigm, S-nitrosothiol donor compounds rescue cells from mutant SOD1-induced cell death and suggest that this protective mechanism may provide a novel therapeutic strategy in ALS. Recently, it has been shown that mutation of Omi/HtrA2 (MND2), a mitochondrial serine protease, causes motor neuron degeneration [11]. Loss of Omi protease activity increases the susceptibility of mitochondrial permeability transition pore (mtPTP), and increases the sensitivity of mouse embryonic fibroblasts to stress-induced cell death. Thus, mitochondria are especially critical to the motor neurons that die in ALS, as these cells must meet extraordinary demands for cellular energy. A common means to solving many neurodegenerative problems in ALS may come to light by understanding more about the role of mitochondria.
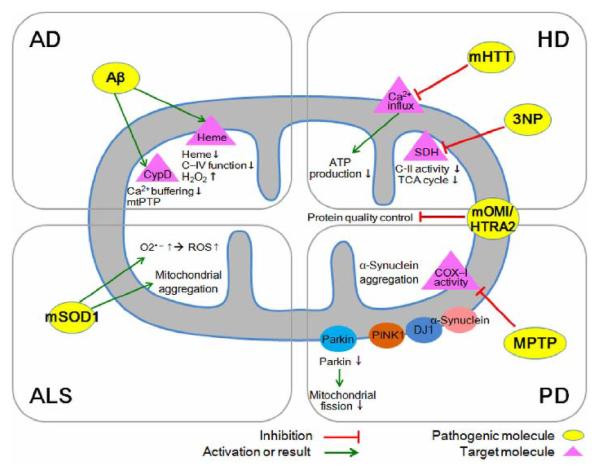
Figure 1. Mitochondria-dependent mechanisms of neurodegeneration and potential therapeutic targets.
A growing body of evidence from in vitro and in vivo studies has implicated Aβ, mutant SOD1 (mSOD1), mutant huntingtin (mHTT), mutant a-synuclein and their ability to induce mitochondrial dysfunction as being toxic to neurons. Aβ-mediated mitochondrial stress through an interaction with cyclophilin D (CypD). The binding of excessive Aβ to heme causes oxidative damage to macromolecules and leads to mitochondrial dysfunction and neurotoxicity. Mitochondria dysfunction and oxidative stress is closely linked to mutation of SOD1. The dysfunction of mitochondrial oxidative phosphorylation is implicated in the pathogenesis of ALS. The neurotoxins 3-NP and MPTP disrupt mitochondrial function and result in idiopathic HD and PD. The neurodegenerative and multiple pathogenic molecules interact with mitochondrial molecules and lead to mitochondrial dysfunction, oxidative stress, and apoptosis of neurons. In this paradigm, the relative pathogenicity of Aβ, mSOD1, mHTT, and mutant α-synuclein is dependent on their mitochondrial interacting molecules and pathways. Omi/HtrA2 has emerged to play a role in protein quality control in AD, HD and PD and its mutation is linked to motor neuronal degeneration in ALS.
2-2. Alzheimer’s disease (AD) and mitochondrial dysfunction
AD is the most common neurodegenerative disease, characterized by neuronal loss and impairment of cognitive function. Deposits of the protein amyloid beta (Aβ) in the form of extracellular insoluble structures or senile plaques are the most prevalent morphological feature. Mitochondrial dysfunction is an early event observed in AD. Recent studies have provided substantial evidence that mitochondria serve as direct targets for Aβ protein-mediated neuronal toxicity. Observations that Aβ progressively accumulates in cortical mitochondria from AD patients and in brains from transgenic AD-type mouse models suggest the role of mitochondrial Aβ in the pathogenesis or development of the disease.
Yan and colleagues have found that mtPTP may be central in mitochondrial and neuronal malfunction relevant to AD [12]. Their report provides a plausible mechanism underlying Aβ-mediated mitochondrial stress through an interaction with cyclophilin D (CypD): a protein with prolyl isomerase activity which is linked to synaptic plasticity and learning/memory (Figure 1). CypD is located within the mitochondrial matrix and is an integral part in the formation of the mtPTP leading to cell death. The role of CypD in AD had not been known until Yan and colleagues demonstrated that CypD interacts with Aβ peptide within the mitochondria of AD patients and a transgenic mouse model of AD. Their findings suggest how this mitochondrial process is linked to synaptic failure in AD. Moreover, these findings may help explain the mechanism of action of a medication already in use in clinical trials. The study also provides new insights into the mechanism underlying mitochondrial Aβ-mediated and synaptic stress that links to the mtPTP, an opening that leads to cell death for those with AD. mtPTP causes mitochondrial swelling, outer membrane rupture and release of cell death mediators and enhances production of reactive oxygen species (ROS). The cortical mitochondria isolated from AD mice lacking CypD are resistant to Aβ- and Ca2+-induced mitochondrial swelling; they are also resistant to mitochondrial permeability transition, show increased calcium buffering capacity, and attenuate the generation of mitochondrial ROS. Furthermore, CypD-deficient neurons protect against Aβ- and oxidative stress-induced cell death. Importantly, deficiency of CypD greatly improved the learning, memory, and synaptic function of an AD mouse model and alleviated Aβ-mediated reduction of long term potentiation (LTP). Thus, the CypD/Aβ-mediated mtPTP directly links to the cellular and synaptic perturbation relevant to the pathogenesis of AD.
Aβ also binds to heme to form a peroxidase which catalyzes the oxidation of serotonin and 3,4-dihydroxyphenylalanine by H2O2 (Figure 1) [13]. The binding of Aβ to heme supports a unifying mechanism by which excessive Aβ causes oxidative damage to macromolecules and leads to mitochondrial dysfunction and neurotoxicity and other cytopathologies of AD. Recent studies show that Omi/HtrA2 interacts preferentially with the most toxic oligomeric form of Aβ [14,15]. Omi/HtrA2 inhibits the secretion of Aβ from neurons that decreases the level of toxic Aβ and might reduce Aβ stress in the brain (Figure 1). Thus, Omi/HtrA2-mediated Aβ -detoxification pathway is a promising therapeutic target for AD including other neurodegenerative diseases.
2-3. Huntington’s disease (HD) and mitochondrial dysfunction
Huntington’s disease (HD) is an autosomal dominantly inherited, neurodegenerative disorder caused by a CAG repeat expansion in the gene encoding huntingtin that manifests as a progressive and characteristic increase in chorea and dementia [16]. Although autopsy specimens of HD brains show a generalized cell loss, the earliest and most extensive atrophy is seen in two specific areas: the caudate and putamen [17]. There is evidence to suggest that the cellular degeneration seen in these two areas is responsible for perturbations in the level of neurotransmitters such as glutamate, which in turn cause the involuntary movements [18,19]. Importantly, a local hypometabolism appears prior to the bulk of tissue loss in these specific areas. This finding raises the possibility that impaired glucose utilization and decreased aerobic energy metabolism are the causes of the cellular atrophy in the caudate and putamen. Consistent with this finding, mitochondrial electron transport enzymes are altered in HD. Mitochondria in HD lymphoblasts and fibroblasts show an increased susceptibility to depolarization which directly correlates with CAG repeat length [20]. The maximal rate of mitochondrial ATP generation in muscle is significantly reduced in both symptomatic HD patients and in presymptomatic HD gene carriers. There has been some debate regarding the vulnerability of transgenic HD mice to neurotoxins. The mitochondrial toxins malonate and 3-nitropropionic acid produce striatal lesions that mimic HD and are mediated by excitotoxic mechanisms (Figure 1). It has been reported that both R6/1 and R6/2 mice are resistant to excitotoxic lesions [21,22]. In contrast, the other study has shown that R6/2 mice are more susceptible to the mitochondrial toxin, 3-nitropropionic acid [23]. As such, the discrepancy in the findings may be methodological with regards to periodicity and dosage of injections. It is of interest, however, that YAC mice containing full-length huntingtin are more susceptible to excitotoxicity [24]. In addition, the fact that NMDA antagonists prolong survival in R6/2 mice clearly implicates excitotoxicity [25]. Interestingly, Omi/HtrA2 dysfunction is linked to the degeneration of striatal neurons in mutant Omi/HtrA2 (MND2) mice [11]. Indeed, Omi/HtrA2 was decreased under the expression of mutant huntingtin (htt) in striatal neurons but not in cortical or cerebellar neurons [26]. These data suggest that the homeostatic function of Omi/HtrA2 is linked to selective vulnerability of striatal neurons in HD pathology (Figure 1).
2-4. Parkinson’s disease (PD) and mitochondrial dysfunction
PD is the second most common neurodegenerative disease, affecting ~1.8% of people over 65 years old [27]. PD is characterized by a progressive loss of dopaminergic neurons and dopamine in the substantia nigra and striatum. Oxidative stress and free radicals from both mitochondrial impairment and dopamine metabolism are considered to play critical roles in the etiology of PD. A mitochondrial defect in PD was first identified in 1989 in substantia nigra from patients with PD [28,29]. This study has been expanded over the years and the results to date show that there is approximately a 35% deficiency in complex I in PD nigra [30] Additionally, neurodegeneration occurs in PD, at least in part, through the activation of the mitochondria-dependent apoptotic molecular pathway [31]. Inhibition of mitochondria fueling pumps has been implicated in the MPTP chemical model of PD and provides insight into the aetiology and pathogenesis of idiopathic PD. (Figure 1). In addition, there has been an identification of specific gene mutations in mitochondrial proteins that serve to cause PD. Thus, mitochondrial dysfunction has been brought to attention in PD pathogenesis. Interestingly, mutations in the Omi/HtrA2 gene have been identified in PD patients [32]. The G399S mutation results in parkinsonian phenotype that includes rigidity [33].
3. Therapeutic Targeting of Mitochondria
3-1. Mitochondria as a therapeutic target
Mitochondria are thread shape organelles consisting of several compartments each with different compositions and functions [34]. Therefore, mitochondria have been considered as intracellular targets for small compounds [35]. The porous mitochondrial outer membrane is permeable to molecules which are smaller than ~5kDa. The mitochondrial intermembrane space contains many specific proteins, but is continuous with the cytoplasm for small molecules. The mitochondrial inner membrane - a convoluted and invaginated structure - contains oxidative phosphorylation enzymes and a series of metabolic pathways. Mitochondrial membrane damage contributes to the pathogenesis of many neurodegenerative diseases. Neuronal cell fate is dependent on mitochondria that play key roles in apoptotic and necrotic cell death. Necrotic neuronal cell death occurs in response to acute damage and results in rapid, uncontrolled death with subsequent cell lysis and an inflammatory response. Necrotic cell death follows ATP depletion and cellular calcium overloading, with extensive mitochondrial damage leading to necrotic cell death [36]. Otherwise, apoptotic neuronal cell death accompanies the activation of a cell death program that leads to the ordered self-destruction of the cell, ending with phagocytosis without leakage of damaging contents and thus no inflammatory response. The difference between apoptotic and necrotic neuronal death is rather arbitrary as completion of the apoptotic program requires ATP, and if ATP levels drop lower than a critical threshold after initiation of apoptosis, apoptosis is aborted and neurons die by necrosis [37]. The mtPTP and mitochondrial membrane potential (ΔΨm) play an important role in both necrotic and apoptotic neuronal death. Activation of the mtPTP increases the mitochondrial membrane permeability to molecules with a mass of up to 1.5 kDa [38]. It is activated by increases in calcium and free radicals. Cyclosporine A is a well known compound to inhibit the activation of mitochondrial membrane permeability and prevent neuronal damage provoked by neuronal stresses (Figure 2) [39]. For last decade, many compounds have been tested for their effects on mtPTP, ΔΨm, and mitochondria-dependent neuronal survival pathways as shown in the Table 1.
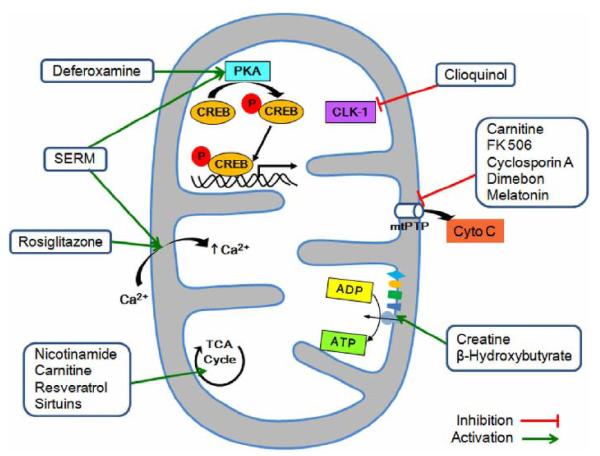
Figure 2. Therapeutic targeting of mitochondria-dependent neuropathogenic mechanisms.
Nicotinamide, carnitine, resveratrol, and Sirtuins modulate tricarboxylic acid (TCA) cycle in mitochondria. Desferoxamine may trigger mitochondrial protein kinase A (PKA) activity and mitochondrial CREB-mediated transcription. The antioxidant and bioenergetic compounds creatine, β-hydroxybutyrate, and coenzyme Q10 can improve mitochondrial function by preventing 3-nitropropionic acid (3-NP)-induced cytotoxicity, while cyclosporine A, FK 506, melatonine, and Dimebon influence mitochondrial membrane function and inhibit cytochrome c release and mitochondrial permeability transition pore (mtPTP)-induced cytotoxicity. Rosiglitazone promotes maintenance of mitochondrial Ca2+ activity. Specific estrogen receptor modulators (SERMs) also affect the mitochondrial activity by modulating calcium fluxes and may activate transcription of mitochondrial genes by interacting with mitochondrial ERs. Cloquinol regulates the mitochondrial oxidative phosphorylation pathway via demethoxyubiquinone hydroxylase (CLK-1 gene product) that catalyzes the production of coenzyme Q. The reversible inhibitor of caspase activity reduces pro-apoptotic signaling in the mitochondria. In addition, compounds exhibiting a robust antioxidant effect lead to the improvement of mitochondrial oxidative metabolism, bioenergy production, and neuronal survival.
Table 1.
Therapeutic Treatments for Targeting Neuronal Mitochondria
| Compound | Mitochondria Targeting |
Applicable Disease | Reference | |
|---|---|---|---|---|
| Direct | Indirect | |||
| Calcineurin inhibitor (FK 506, Cyclosporin A) |
Inhibition of mtPTP | AD, PD, HD, ALS | [12,89-91] | |
| Carnitine | TCA cycle, Inhibition of mtPTP |
antioxidant | PD, HD, ALS | [62,65,68,69] |
| Clioquinol | Inhibition of mitochondrial enzyme CLK-1 (CoQ7) |
AD, PD, HD | [92] | |
| Coenzyme Q10 | antioxidant | antioxidant | [42,61,93,94] | |
| Mito Q10 | antioxidant | [95,96] | ||
| Creatine | ATP/ADP regulation | AD, PD, HD, ALS | [47-52, 97-100] | |
| Cystamine | Inhibition of mitochondrial depolarization |
HD | [63,66] | |
| Deferoxamine | Mitochondrial gene regulation |
Metal chelator | [70,101,102] | |
| Dimebon | Inhibition of mtPTP | AD, PD | [103-105] | |
| β-Hydroxybutyrate | mitochondrial respiration and ATP production |
PD | [106,107] | |
| Lipoic acid | antioxidant | AD, PD, HD | [59,60,64] | |
| Melatonin | antioxidant | Inhibition of Cytochrome c release from purified mitochondria |
[53-58, 108] | |
| Minocycline | Inhibition of caspase and cytochrome c release |
PD, HD | [109,110] | |
| Resveratrol, Sirtuins, nicotinamide |
Mitochondrial biogenesis, TCA cycle |
Anti-apoptotic, anti- inflammatory,anti-stress responses |
AD, PD, HD, ALS | [111-118] |
| Rosiglitazone (PPAR-γ agonist) |
Mitochondrial calcium & membrane potential |
Anti-apoptotic, Bcl-2 upregulation |
AD, PD, HD, ALS | [119-123] |
| Szeto-Schiller peptide |
antioxidant | PD, ALS | [124-127] | |
| Triterpenoids (avicins) |
Antioxidant, anti- inflammatory gene regulation |
PD, HD | [128, 12] | |
| Vitamin E | antioxidant | PD, HD | [61,67] | |
| Mito-E | Antioxidant | [130] | ||
3-2. Therapeutics for mitochondria in neurodegenerative conditions
Coenzyme Q
Coenzyme Q10 (CoQ10), a strong antioxidant ubiquinone, is a lipid-soluble benzoquinone that harbors antioxidant properties when reduced to ubiquinol, or through a CoQ10-induced increase in alpha-tocopherol [40]. It is imposed in the inner mitochondrial membrane and is essential for Complex I and II electron transfer activities during oxidative phosphorylation [41], playing a vital role in ATP production. CoQ10 administration has been demonstrated to significantly increase brain mitochondrial CoQ10 concentrations [42]. CoQ10 possesses neuroprotective effects in AD and PD by attenuating mitochondrial dysfunction and nullifying oxidative damage [43]. The safety and tolerability of high doses of coenzyme Q10 (CoQ10) has been assessed in ALS. CoQ10 is safe and well tolerated in ALS patients with high doses. CoQ10 may improve the mitochondrial dysfunction in ALS [44]. CoQ10 provides significant neuroprotection in a dose-dependent manner in a striatal lesion model of HD [45]. CoQ10 treatment significantly delays the typical decline in weight loss and motor performance as assessed on the rotarod and extends survival of R6/2 HD mice [46].
Creatine
The guanidine compound creatine is endogenously produced in neurons but can also be obtained from the diet. It provides an antioxidant capacity and buffers intracellular energy reserves, stabilizes intracellular calcium, and inhibits activation of the mitochondrial pore transition [47]. In neurons, creatine can exist either as a free substrate, or phosphocreatine (PCr). According to the PCr shuttle hypothesis, sites of energy production are connected with sites of energy consumption when a phosphoryl group from PCr is transferred to ADP creating ATP, in a reaction mediated by creatine kinase [48]. Treatment of mutant SOD1 (G93A) ALS transgenic mice with creatine improves motor performance, delays loss of anterior horn motor neurons, and extends survival [49]. In HD, there is a significant shift in the ratio of PCr to phosphate [50]. Therefore, creatine is highly applicable to the restoration of normal metabolic activity in HD. Indeed, several pre-clinical studies have shown the neuroprotective effect of creatine in chemical and animal models of HD [51,52].
Melatonin
Melatonin mediates a neuroprotective role in AD by scavenging oxygen and nitrogen-based reactants generated in mitochondria. One of the mechanisms underlying the neuroprotective effects of melatonin is a counter-action against mitochondrial cell death pathways. For example, melatonin activates the survival signal Bcl-2 dependent-pathway, which stabilizes mitochondrial function. Interestingly, Bcl-2 expression is enhanced by melatonin concomitant with inhibition of Aβ-induced cell death [53]. Chronic high-dose administration of melatonin has been shown to cause a reduction of oxidative damage in patients with sporadic ALS [54]. In addition, melatonin delays the disease progression and extends the survival of mutant SOD1(G93A) ALS transgenic mice. Moreover, melatonin attenuates superoxide-induced cell death and modulates glutamate toxicity in cultured NSC-34 motor neuronal cells [54]. Administration of melatonin significantly delays the development of the signs of AD and prevents cognitive and behavioral deterioration in a human monozygotic twin study [55]; in addition, melatonin improves learning and memory deficits in an APP695 transgenic mouse model of AD [56]. Melatonin inhibits the dissipation of ΔΨm in mutant-htt ST14A striatal cells, a cellular model of HD [57]. Melatonin prevents oxidative stress-induced mitochondrial calcium overload, ΔΨm depolarization, opening of mtPTP, ROS formation, and cytochrome c release in rat astrocytes in a chemical model of PD [58]. Taken together, the large body of evidence suggests that melatonin provides a neuroprotective effective for ALS, AD, HD and PD through its mitochondria-dependent anti-apoptotic activities.
3-3. Antioxidants, specific estrogen receptor modulators (SERMs) and other compounds targeting mitochondria
Antioxidants and small compounds such as lipoic acid, cystamine, vitamin E, and carnitine have shown neuroprotective effects in neurodegenerative conditions (Figure 2) [59-69]. Deferoxamine (DFO), an antioxidant and iron chelator known to inhibit oxidative stress-induced cell death, activates mitochondrial protein kinase A (PKA) and increased mitochondrial CREB phosphorylation (Ser 133) [70]. The catalytic subunit of PKA is found in the mitochondrial matrix to phosphorylate mitochondrial CREB in neurons [70]. The therapeutic approaches of increasing mitochondrial PKA and mitochondrial CREB activity may provide a novel direction in both preclinical and human trials. In this context, DFO increases CREB binding to CRE in the mitochondrial D-loop DNA and D-loop CRE-driven luciferase activity. In contrast, KT5720, a specific inhibitor of PKA, reduces DFO-mediated neuronal survival against oxidative stress induced by glutathione depletion. Neuronal survival by DFO may be, in part, mediated by the mitochondrial PKA-dependent pathway (Figure 2). These results suggest that the regulation of mitochondrial function via the mitochondrial PKA and CREB pathways may underlie some of the salutary effects of DFO in neurons. Taken together, the idea of targeting biologically active molecules to the mitochondria is to modulate selective mitochondrial functions in a specific manner. Therapeutic strategies will allow mitochondria to better cope with oxidative stress, mitochondrial damage by excitotoxicity, and maintain efficient oxidative phosphorylation and respiratory function. This study provides a novel mechanism for preventing mitochondrial transcriptional dysfunction in neurodegenerative conditions and in the design of applicable therapeutic treatments to modulate mitochondrial hormone receptors and transcription factors.
Estrogen attenuates NMDA receptor-mediated excitotoxic neuronal death and oxidative neuronal death [71-76]. Estrogen has a number of neurotrophic effects mediated via different signaling pathways, including activation of PKA, ERK and phosphatidyl inositol 3-kinase (PI3K) cascades and inactivation of glycogen synthase kinase 3beta (GSK3 beta) [77,78]. The important structural motif that elicits estrogenic effects is a phenol ring that is relatively unhindered and attached to a bulky hydrophobic structure [79]. The phenolic A ring is related to its neuroprotective function [80]. Steroids with a hydroxyl group in the C3 position of the A ring provides an antioxidant property. 17 beta-estradiol (E2) can suppress intracellular ROS and prevent neurons from oxidative stress-induced cell death. However, the antioxidant property requires a higher concentration of E2 (10-100 microM). Furthermore, anti-apoptotic neuroprotection may be blocked by ICI 182,720, which has a hydroxyl group at C3 [81,82]. Therefore, it is unlikely that the anti-cell death effect is solely due to the antioxidant property of E2. As neuroprotective molecules, SERMs may act through mitochondria-dependent and —independent signaling pathways (Figure 2). First, they may activate the transcription of mitochondrial genes directly through binding to mitochondrial estrogen receptors (ERs) and subsequently to the estrogen response element (ERE) in mitochondrial genome, which is the mitochondrial transcription-dependent pathway [83]. This classical mode of estrogen action works through the activation of estrogen receptors that target estrogen receptor-responsive areas in the promoter regions of mitochondrial genomes. Therefore, mitochondrial estrogen receptors that are activated by estrogen directly act as mitochondrial transcription factors [84-86]. Second, SERMs directly regulate gene expression through ERs and ERE, as well as indirectly activating gene transcription by performing a crosstalk with various intracellular signaling pathways [87]. In this case, it is predicted that estrogenic compounds bind to estrogen receptors that do not directly bind to the mitochondrial ERE, but rather interact with other signaling cascades in the mitochondrial matrix. Such signaling partners of interaction may include the mitochondrial PKA and the CREB-signaling processes. Third, SERMs may directly affect mitochondrial membranes by modulating Ca2+ fluxes and protect neurons through their antioxidant effects, which promote the transcription-independent pathway (Figure 2) [88].
FK 506, a calcineurin inhibitor, and Dimebon, an antihistamine drug, influence mitochondrial membrane function, inhibit cytochrome c release, and mitochondrial permeability transition pore (mtPTP)-induced cytotoxicity (Table 1 and Figure 2) [62,65,68,69, 89-91, 103-105]. Dimebon has been demonstrated to show efficacy in AD and HD clinical trials [1-3]. Minocycline, a tetracycline antibiotic, inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of HD [109]. Nicotinamide, resveratrol, and Sirtuins modulate tricarboxylic acid (TCA) cycle in mitochondria and provide neuroprotective effects in neurodegenerative conditions [110-118]. Rosiglitazone, as known as a PPAR-gamma agonist and an anti-diabetic drug in the thiazolidinedione class of drugs, shows beneficial effects on neuroprotection to a subset of patients with AD [119-123]. Interestingly, mitochondria-targeted aromatic-cationic peptides (Szeto-Schiller peptides) are neuroprotective in animal models of ALS and PD [124-127]. Avicins are pro-apoptotic and anti-inflammatory triterpene electrophiles that modulate the activity of Nrf2, a redox-regulated transcription factor that controls the expression of a battery of detoxification and antioxidant proteins. Avicins directly target the outer membrane of mitochondria but its neuroprotective effect remains to be determined [128, 129]. Recently, two mitochondrial antioxidants have been developed by conjugating alpha-tocopherol and the ubiquinol moiety of coenzyme Q to the lipophilic triphenylphosphonium cation (TPP+), named MitoE and MitoQ, respectively [130]. Both MitoE and MitoQ treatments result in an increased Ca2+ concentration in the mitochondrial matrix by inhibiting Ca2+ efflux from the organelle; an effect dependent on the TPP+ moiety of these compounds. It will be very interesting to study the effect of MitoE and MitoQ on neurodegenerative conditions.
4. Conclusions
The extraordinary dependence of neurons on the energy provided by mitochondrial oxidative metabolism is directly linked to neurodegenerative conditions. The central role of mitochondria in neurodegeneration has become apparent over the last decade as the molecular mechanisms leading to neuronal cell death have been underscored. Previous findings on the mitochondrial localization and function of nuclear receptors and transcription factors have unraveled interesting mechanisms in the mitochondria. The effectiveness of drug treatments may depend upon targeting bioactive molecules to the appropriate organ, cell type, and subcellular organelle. Therefore, future studies using small compounds to target the mitochondria directly or to modulate nuclear receptors and transcription factors that subsequently convey the signal to the mitochondria, may contribute to improved mitochondrial function in a specific manner. Taken together, small compounds are able to boost the health of mitochondria or tune up the mitochondrial power engine to compensate the damaged or interrupted neuronal power failure in response to neuronal stresses. We expect that novel therapeutic strategies will enable mitochondria to better cope with oxidative stress, excitotoxicity, and other neuronal stresses, as well as maintain efficient respiratory function in neurons.
Acknowledgements
J.L. is an awardee of Les Turner ALS Foundation Grant. NIH P30 AG13846 (J.L.), and NIH NS52724 (H.R.) supported this work. The WCU Neurocytomics Program Grant (800-20080848) through SNU from KOSEF (H.R.) supported this work, in part.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Saraste M. Oxidative phosphorylation at the fin de siecle. Science. 1999;283:1488–1493. doi: 10.1126/science.283.5407.1488. [DOI] [PubMed] [Google Scholar]
- [2].Nicholls DG, Ferguson SJ. Bioenergetics 2. Academic Press; London: 1992. [Google Scholar]
- [3].Albers DS, Beal MF. Mitochondrial dysfunction and oxidative stress in aging and neurodegenerative disease. J. Neural. Transm. Suppl. 2000;59:133–154. doi: 10.1007/978-3-7091-6781-6_16. [DOI] [PubMed] [Google Scholar]
- [4].Beal MF, Brouillet E, Jenkins BG, Ferrante RJ, Kowall NW, Miller JM, Storey E, Srivastava R, Rosen BR, Hyman BT. Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. J. Neurosci. 1993;13:4181–4192. doi: 10.1523/JNEUROSCI.13-10-04181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zamzami N, Kroemer G. The mitochondrion in apoptosis: how Pandora’s box opens. Nat. Rev. Mol. Cell Biol. 2001;2:67–71. doi: 10.1038/35048073. [DOI] [PubMed] [Google Scholar]
- [6].Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- [7].Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. others. [DOI] [PubMed] [Google Scholar]
- [8].Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat. Rev. Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- [9].Poon HF, Hensley K, Thongboonkerd V, Merchant ML, Lynn BC, Pierce WM, Klein JB, Calabrese V, Butterfield DA. Redox proteomics analysis of oxidatively modified proteins in G93A-SOD1 transgenic mice--a model of familial amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2005;39:453–462. doi: 10.1016/j.freeradbiomed.2005.03.030. [DOI] [PubMed] [Google Scholar]
- [10].Schonhoff CM, Matsuoka M, Tummala H, Johnson MA, Estevez AG, Wu R, Kamaid A, Ricart KC, Hashimoto Y, Gaston B, Macdonald TL, Xu Z, Mannick JB. S-nitrosothiol depletion in amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. U. S. A. 2006;103:2404–2409. doi: 10.1073/pnas.0507243103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jones JM, Datta P, Srinivasula SM, Ji W, Gupta S, Zhang Z, Davies E, Hajnoczky G, Saunders TL, Van Keuren ML, Fernandes-Alnemri T, Meisler MH, Alnemri ES. Loss of Omi mitochondrial protease activity causes the neuromuscular disorder of mnd2 mutant mice. Nature. 2003;425:721–727. doi: 10.1038/nature02052. [DOI] [PubMed] [Google Scholar]
- [12].Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat. Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Atamna H, Boyle K. Amyloid-beta peptide binds with heme to form a peroxidase: relationship to the cytopathologies of Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3381–3386. doi: 10.1073/pnas.0600134103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Huttunen HJ, Guenette SY, Peach C, Greco C, Xia W, Kim DY, Barren C, Tanzi RE, Kovacs DM. HtrA2 regulates beta-amyloid precursor protein (APP) metabolism through endoplasmic reticulum-associated degradation. J. Biol. Chem. 2007;282:28285–28295. doi: 10.1074/jbc.M702951200. [DOI] [PubMed] [Google Scholar]
- [15].Liu ML, Liu MJ, Shen YF, Ryu H, Kim HJ, Klupsch K, Downward J, Hong ST. Omi is a mammalian heat-shock protein that selectively binds and detoxifies oligomeric amyloid-{beta} J. Cell Sci. 2009;122:1917–1926. doi: 10.1242/jcs.042226. [DOI] [PubMed] [Google Scholar]
- [16].A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- [17].Bruyn GW. Huntington’s chorea. Tijdschr. Ziekenverpl. 1979;32:101–105. [PubMed] [Google Scholar]
- [18].Spokes EG, Garrett NJ, Rossor MN, Iversen LL. Distribution of GABA in post-mortem brain tissue from control, psychotic and Huntington’s chorea subjects. J. Neurol. Sci. 1980;48:303–313. doi: 10.1016/0022-510x(80)90103-3. [DOI] [PubMed] [Google Scholar]
- [19].Spokes EG. Neurochemical alterations in Huntington’s chorea: a study of post-mortem brain tissue. Brain. 1980;103:179–210. doi: 10.1093/brain/103.1.179. [DOI] [PubMed] [Google Scholar]
- [20].Sawa A, Wiegand GW, Cooper J, Margolis RL, Sharp AH, Lawler JF, Jr., Greenamyre JT, Snyder SH, Ross CA. Increased apoptosis of Huntington disease lymphoblasts associated with repeat length-dependent mitochondrial depolarization. Nat. Med. 1999;5:1194–1198. doi: 10.1038/13518. [DOI] [PubMed] [Google Scholar]
- [21].Hansson O, Petersen A, Leist M, Nicotera P, Castilho RF, Brundin P. Transgenic mice expressing a Huntington’s disease mutation are resistant to quinolinic acid-induced striatal excitotoxicity. Proc. Natl. Acad. Sci. U. S. A. 1999;96:8727–8732. doi: 10.1073/pnas.96.15.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hansson O, Guatteo E, Mercuri NB, Bernardi G, Li XJ, Castilho RF, Brundin P. Resistance to NMDA toxicity correlates with appearance of nuclear inclusions, behavioural deficits and changes in calcium homeostasis in mice transgenic for exon 1 of the huntington gene. Eur. J. Neurosci. 2001;14:1492–1504. doi: 10.1046/j.0953-816x.2001.01767.x. [DOI] [PubMed] [Google Scholar]
- [23].Bogdanov MB, Ferrante RJ, Kuemmerle S, Klivenyi P, Beal MF. Increased vulnerability to 3-nitropropionic acid in an animal model of Huntington’s disease. J. Neurochem. 1998;71:2642–2644. doi: 10.1046/j.1471-4159.1998.71062642.x. [DOI] [PubMed] [Google Scholar]
- [24].Zeron MM, Chen N, Moshaver A, Lee AT, Wellington CL, Hayden MR, Raymond LA. Mutant huntingtin enhances excitotoxic cell death. Mol. Cell. Neurosci. 2001;17:41–53. doi: 10.1006/mcne.2000.0909. [DOI] [PubMed] [Google Scholar]
- [25].Schiefer J, Landwehrmeyer GB, Luesse HG, Sprunken A, Puls C, Milkereit A, Milkereit E, Kosinski CM. Riluzole prolongs survival time and alters nuclear inclusion formation in a transgenic mouse model of Huntington’s disease. Mov. Disord. 2002;17:748–757. doi: 10.1002/mds.10229. [DOI] [PubMed] [Google Scholar]
- [26].Inagaki R, Tagawa K, Qi ML, Enokido Y, Ito H, Tamura T, Shimizu S, Oyanagi K, Arai N, Kanazawa I, Wanker EE, Okazawa H. Omi / HtrA2 is relevant to the selective vulnerability of striatal neurons in Huntington’s disease. Eur. J. Neurosci. 2008;28:30–40. doi: 10.1111/j.1460-9568.2008.06323.x. [DOI] [PubMed] [Google Scholar]
- [27].Foley P, Riederer P. Pathogenesis and preclinical course of Parkinson’s disease. J. Neural Transm. Suppl. 1999;56:31–74. doi: 10.1007/978-3-7091-6360-3_2. [DOI] [PubMed] [Google Scholar]
- [28].Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- [29].Gu M, Cooper JM, Taanman JW, Schapira AH. Mitochondrial DNA transmission of the mitochondrial defect in Parkinson’s disease. Ann. Neurol. 1998;44:177–186. doi: 10.1002/ana.410440207. [DOI] [PubMed] [Google Scholar]
- [30].Mann VM, Cooper JM, Daniel SE, Srai K, Jenner P, Marsden CD, Schapira AH. Complex I, iron, and ferritin in Parkinson’s disease substantia nigra. Ann. Neurol. 1994;36:876–881. doi: 10.1002/ana.410360612. [DOI] [PubMed] [Google Scholar]
- [31].Vila M, Przedborski S. Targeting programmed cell death in neurodegenerative diseases. Nat. Rev. Neurosci. 2003;4:365–375. doi: 10.1038/nrn1100. [DOI] [PubMed] [Google Scholar]
- [32].Strauss KM, Martins LM, Plun-Favreau H, Marx FP, Kautzmann S, Berg D, Gasser T, Wszolek Z, Muller T, Bornemann A, Wolburg H, Downward J, Riess O, Schulz JB, Krüger R. Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson’s disease. Hum. Mol. Genet. 2005;14:2099–2111. doi: 10.1093/hmg/ddi215. [DOI] [PubMed] [Google Scholar]
- [33].Martins LM, Morrison A, Klupsch K, Fedele V, Moisoi N, Teismann P, Abuin A, Grau E, Geppert M, Livi GP, Creasy CL, Martin A, Hargreaves I, Heales SJ, Okada H, Brandner S, Schulz JB, Mak T, Downward J. Neuroprotective role of the Reaper-related serine protease HtrA2/Omi revealed by targeted deletion in mice. Mol. Cell. Biol. 2004;24:9848–9862. doi: 10.1128/MCB.24.22.9848-9862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Murphy MP, Smith RA. Drug delivery to mitochondria: the key to mitochondrial medicine. Adv. Drug Deliv. Rev. 2000;41:235–250. doi: 10.1016/s0169-409x(99)00069-1. [DOI] [PubMed] [Google Scholar]
- [35].Murphy MP. Selective targeting of bioactive compounds to mitochondria. Trends Biotechnol. 1997;15:326–330. doi: 10.1016/S0167-7799(97)01068-8. [DOI] [PubMed] [Google Scholar]
- [36].Nicotera P, Leist M, Ferrando-May E. Intracellular ATP, a switch in the decision between apoptosis and necrosis. Toxicol. Lett. 1998;102-103:139–142. doi: 10.1016/s0378-4274(98)00298-7. [DOI] [PubMed] [Google Scholar]
- [37].Leist M, Nicotera P. The shape of cell death. Biochem. Biophys. Res. Commun. 1997;236:1–9. doi: 10.1006/bbrc.1997.6890. [DOI] [PubMed] [Google Scholar]
- [38].Bernardi P. Mitochondria in muscle cell death. Ital. J. Neurol. Sci. 1999;20:395–400. doi: 10.1007/s100720050057. [DOI] [PubMed] [Google Scholar]
- [39].Friberg H, Ferrand-Drake M, Bengtsson F, Halestrap AP, Wieloch T. Cyclosporin A, but not FK 506, protects mitochondria and neurons against hypoglycemic damage and implicates the mitochondrial permeability transition in cell death. J. Neurosci. 1998;18:5151–5159. doi: 10.1523/JNEUROSCI.18-14-05151.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Beal MF, Ferrante RJ. Experimental therapeutics in transgenic mouse models of Huntington’s disease. Nat. Rev. Neurosci. 2004;5:373–384. doi: 10.1038/nrn1386. [DOI] [PubMed] [Google Scholar]
- [41].Chan TS, Wilson JX, O’Brien PJ. Coenzyme Q cytoprotective mechanisms. Methods Enzymol. 2004;382:89–104. doi: 10.1016/S0076-6879(04)82006-8. [DOI] [PubMed] [Google Scholar]
- [42].Matthews RT, Yang L, Browne S, Baik M, Beal MF. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc. Natl. Acad. Sci. U. S. A. 1998;95:8892–8897. doi: 10.1073/pnas.95.15.8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Beal MF. Mitochondrial dysfunction and oxidative damage in Alzheimer’s and Parkinson’s diseases and coenzyme Q10 as a potential treatment. J. Bioenerg. Biomembr. 2004;36:381–386. doi: 10.1023/B:JOBB.0000041772.74810.92. [DOI] [PubMed] [Google Scholar]
- [44].Ferrante KL, Shefner J, Zhang H, Betensky R, O’Brien M, Yu H, Fantasia M, Taft J, Beal MF, Traynor B, Newhall K, Donofrio P, Caress J, Ashburn C, Freiberg B, O’Neill C, Paladenech C, Walker T, Pestronk A, Abrams B, Florence J, Renna R, Schierbecker J, Malkus B, Cudkowicz M. Tolerance of high-dose (3,000 mg/day) coenzyme Q10 in ALS. Neurology. 2005;65:1834–1836. doi: 10.1212/01.wnl.0000187070.35365.d7. [DOI] [PubMed] [Google Scholar]
- [45].Beal MF, Henshaw DR, Jenkins BG, Rosen BR, Schulz JB. Coenzyme Q10 and nicotinamide block striatal lesions produced by the mitochondrial toxin malonate. Ann. Neurol. 1994;36:882–888. doi: 10.1002/ana.410360613. [DOI] [PubMed] [Google Scholar]
- [46].Ferrante RJ, Andreassen OA, Dedeoglu A, Ferrante KL, Jenkins BG, Hersch SM, Beal MF. Therapeutic effects of coenzyme Q10 and remacemide in transgenic mouse models of Huntington’s disease. J. Neurosci. 2002;22:1592–1599. doi: 10.1523/JNEUROSCI.22-05-01592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].O’Gorman E, Beutner G, Dolder M, Koretsky AP, Brdiczka D, Wallimann T. The role of creatine kinase in inhibition of mitochondrial permeability transition. FEBS Lett. 1997;414:253–257. doi: 10.1016/s0014-5793(97)01045-4. [DOI] [PubMed] [Google Scholar]
- [48].Bessman SP, Geiger PJ. Transport of energy in muscle: the phosphorylcreatine shuttle. Science. 1981;211:448–452. doi: 10.1126/science.6450446. [DOI] [PubMed] [Google Scholar]
- [49].Klivenyi P, Ferrante RJ, Matthews RT, Bogdanov MB, Klein AM, Andreassen OA, Mueller G, Wermer M, Kaddurah-Daouk R, Beal MF. Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nat. Med. 1999;5:347–350. doi: 10.1038/6568. [DOI] [PubMed] [Google Scholar]
- [50].Koroshetz WJ, Jenkins BG, Rosen BR, Beal MF. Energy metabolism defects in Huntington’s disease and effects of coenzyme Q10. Ann. Neurol. 1997;41:160–165. doi: 10.1002/ana.410410206. [DOI] [PubMed] [Google Scholar]
- [51].Andreassen OA, Dedeoglu A, Ferrante RJ, Jenkins BG, Ferrante KL, Thomas M, Friedlich A, Browne SE, Schilling G, Borchelt DR, Hersch SM, Ross CA, Beal MF. Creatine increase survival and delays motor symptoms in a transgenic animal model of Huntington’s disease. Neurobiol. Dis. 2001;8:479–491. doi: 10.1006/nbdi.2001.0406. [DOI] [PubMed] [Google Scholar]
- [52].Ferrante RJ, Andreassen OA, Jenkins BG, Dedeoglu A, Kuemmerle S, Kubilus JK, Kaddurah-Daouk R, Hersch SM, Beal MF. Neuroprotective effects of creatine in a transgenic mouse model of Huntington’s disease. J. Neurosci. 2000;20:4389–4397. doi: 10.1523/JNEUROSCI.20-12-04389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Jang MH, Jung SB, Lee MH, Kim CJ, Oh YT, Kang I, Kim J, Kim EH. Melatonin attenuates amyloid beta25-35-induced apoptosis in mouse microglial BV2 cells. Neurosci. Lett. 2005;380:26–31. doi: 10.1016/j.neulet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- [54].Weishaupt JH, Bartels C, Polking E, Dietrich J, Rohde G, Poeggeler B, Mertens N, Sperling S, Bohn M, Huther G, Schneider A, Bach A, Sirén AL, Hardeland R, Bähr M, Nave KA, Ehrenreich H. Reduced oxidative damage in ALS by high-dose enteral melatonin treatment. J. Pineal Res. 2006;41:313–323. doi: 10.1111/j.1600-079X.2006.00377.x. [DOI] [PubMed] [Google Scholar]
- [55].Brusco LI, Marquez M, Cardinali DP. Monozygotic twins with Alzheimer’s disease treated with melatonin: Case report. J. Pineal Res. 1998;25:260–263. doi: 10.1111/j.1600-079x.1998.tb00396.x. [DOI] [PubMed] [Google Scholar]
- [56].Feng Z, Zhang JT. Melatonin reduces amyloid beta-induced apoptosis in pheochromocytoma (PC12) cells. J. Pineal Res. 2004;37:257–266. doi: 10.1111/j.1600-079X.2004.00164.x. [DOI] [PubMed] [Google Scholar]
- [57].Trettel F, Rigamonti D, Hilditch-Maguire P, Wheeler VC, Sharp AH, Persichetti F, Cattaneo E, MacDonald ME. Dominant phenotypes produced by the HD mutation in STHdh(Q111) striatal cells. Hum. Mol. Genet. 2000;9:2799–2809. doi: 10.1093/hmg/9.19.2799. [DOI] [PubMed] [Google Scholar]
- [58].Jou MJ, Peng TI, Reiter RJ, Jou SB, Wu HY, Wen ST. Visualization of the antioxidative effects of melatonin at the mitochondrial level during oxidative stress-induced apoptosis of rat brain astrocytes. J. Pineal Res. 2004;37:55–70. doi: 10.1111/j.1600-079X.2004.00140.x. [DOI] [PubMed] [Google Scholar]
- [59].Andreassen OA, Ferrante RJ, Dedeoglu A, Beal MF. Lipoic acid improves survival in transgenic mouse models of Huntington’s disease. Neuroreport. 2001;12:3371–3373. doi: 10.1097/00001756-200110290-00044. [DOI] [PubMed] [Google Scholar]
- [60].Karunakaran S, Diwakar L, Saeed U, Agarwal V, Ramakrishnan S, Iyengar S, Ravindranath V. Activation of apoptosis signal regulating kinase 1 (ASK1) and translocation of death-associated protein, Daxx, in substantia nigra pars compacta in a mouse model of Parkinson’s disease: protection by alpha-lipoic acid. FASEB J. 2007;21:2226–2236. doi: 10.1096/fj.06-7580com. [DOI] [PubMed] [Google Scholar]
- [61].Kasparova S, Sumbalova Z, Bystricky P, Kucharska J, Liptaj T, Mlynarik V, Gvozdjakova A. Effect of coenzyme Q10 and vitamin E on brain energy metabolism in the animal model of Huntington’s disease. Neurochem. Int. 2006;48:93–99. doi: 10.1016/j.neuint.2005.09.002. [DOI] [PubMed] [Google Scholar]
- [62].Kira Y, Nishikawa M, Ochi A, Sato E, Inoue M. L-carnitine suppresses the onset of neuromuscular degeneration and increases the life span of mice with familial amyotrophic lateral sclerosis. Brain Res. 2006;1070:206–214. doi: 10.1016/j.brainres.2005.11.052. [DOI] [PubMed] [Google Scholar]
- [63].Lesort M, Lee M, Tucholski J, Johnson GV. Cystamine inhibits caspase activity. Implications for the treatment of polyglutamine disorders. J. Biol. Chem. 2003;278:3825–3830. doi: 10.1074/jbc.M205812200. [DOI] [PubMed] [Google Scholar]
- [64].Maczurek A, Hager K, Kenklies M, Sharman M, Martins R, Engel J, Carlson DA, Munch G. Lipoic acid as an anti-inflammatory and neuroprotective treatment for Alzheimer’s disease. Adv. Drug Deliv. Rev. 2008;60:1463–1470. doi: 10.1016/j.addr.2008.04.015. [DOI] [PubMed] [Google Scholar]
- [65].Nishimura M, Okimura Y, Fujita H, Yano H, Lee J, Suzaki E, Inoue M, Utsumi K, Sasaki J. Mechanism of 3-nitropropionic acid-induced membrane permeability transition of isolated mitochondria and its suppression by L-carnitine. Cell Biochem. Funct. 2008;26:881–891. doi: 10.1002/cbf.1521. [DOI] [PubMed] [Google Scholar]
- [66].Mao Z, Choo YS, Lesort M. Cystamine and cysteamine prevent 3-NP-induced mitochondrial depolarization of Huntington’s disease knock-in striatal cells. Eur. J. Neurosci. 2006;23:1701–1710. doi: 10.1111/j.1460-9568.2006.04686.x. [DOI] [PubMed] [Google Scholar]
- [67].Testa CM, Sherer TB, Greenamyre JT. Rotenone induces oxidative stress and dopaminergic neuron damage in organotypic substantia nigra cultures. Brain Res. Mol. Brain Res. 2005;134:109–118. doi: 10.1016/j.molbrainres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- [68].Wang C, Sadovova N, Ali HK, Duhart HM, Fu X, Zou X, Patterson TA, Binienda ZK, Virmani A, Paule MG, Slikker W, Jr, Ali SF. L-carnitine protects neurons from 1-methyl-4-phenylpyridinium-induced neuronal apoptosis in rat forebrain culture. Neuroscience. 2007;144:46–55. doi: 10.1016/j.neuroscience.2006.08.083. [DOI] [PubMed] [Google Scholar]
- [69].Binienda Z, Przybyla-Zawislak B, Virmani A, Schmued L. L-carnitine and neuroprotection in the animal model of mitochondrial dysfunction. Ann. N. Y. Acad. Sci. 2005;1053:174–182. doi: 10.1196/annals.1344.015. [DOI] [PubMed] [Google Scholar]
- [70].Ryu H, Lee J, Impey S, Ratan RR, Ferrante RJ. Antioxidants modulate mitochondrial PKA and increase CREB binding to D-loop DNA of the mitochondrial genome in neurons. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13915–13920. doi: 10.1073/pnas.0502878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Weaver CE, Jr., Park-Chung M, Gibbs TT, Farb DH. 17beta-Estradiol protects against NMDA-induced excitotoxicity by direct inhibition of NMDA receptors. Brain Res. 1997;761:338–341. doi: 10.1016/s0006-8993(97)00449-6. [DOI] [PubMed] [Google Scholar]
- [72].Montal M. Mitochondria, glutamate neurotoxicity and the death cascade. Biochim. Biophys. Acta. 1998;1366:113–126. doi: 10.1016/s0005-2728(98)00124-8. [DOI] [PubMed] [Google Scholar]
- [73].Kajta M, Lason W, Bien E, Marszal M. Neuroprotective effects of estrone on NMDA-induced toxicity in primary cultures of rat cortical neurons are independent of estrogen receptors. Pol. J. Pharmacol. 2002;54:727–729. [PubMed] [Google Scholar]
- [74].Linford NJ, Dorsa DM. 17beta-Estradiol and the phytoestrogen genistein attenuate neuronal apoptosis induced by the endoplasmic reticulum calcium-ATPase inhibitor thapsigargin. Steroids. 2002;67:1029–1040. doi: 10.1016/s0039-128x(02)00062-4. [DOI] [PubMed] [Google Scholar]
- [75].Dribben W, Nemmers B, Nardi A, Taylor G, Olney J, Farber N. Chronic but not acute estradiol treatment protects against the neurodegenerative effects of N-methyl-D-aspartate receptor antagonists. Endocrine. 2003;21:53–58. doi: 10.1385/endo:21:1:53. [DOI] [PubMed] [Google Scholar]
- [76].Xue B, Hay M. 17beta-estradiol inhibits excitatory amino acid-induced activity of neurons of the nucleus tractus solitarius. Brain Res. 2003;976:41–52. doi: 10.1016/s0006-8993(03)02629-5. [DOI] [PubMed] [Google Scholar]
- [77].Mendez P, Azcoitia I, Garcia-Segura LM. Estrogen receptor alpha forms estrogen-dependent multimolecular complexes with insulin-like growth factor receptor and phosphatidylinositol 3-kinase in the adult rat brain. Brain Res. Mol. Brain Res. 2003;112:170–176. doi: 10.1016/s0169-328x(03)00088-3. [DOI] [PubMed] [Google Scholar]
- [78].Yu X, Rajala RV, McGinnis JF, Li F, Anderson RE, Yan X, Li S, Elias RV, Knapp RR, Zhou X, Cao W. Involvement of insulin/phosphoinositide 3-kinase/Akt signal pathway in 17 beta-estradiol-mediated neuroprotection. J. Biol. Chem. 2004;279:13086–13094. doi: 10.1074/jbc.M313283200. [DOI] [PubMed] [Google Scholar]
- [79].Schultz TW, Sinks GD, Cronin MT. Structure-activity relationships for gene activation oestrogenicity: evaluation of a diverse set of aromatic chemicals. Environ. Toxicol. 2002;17:14–23. doi: 10.1002/tox.10027. [DOI] [PubMed] [Google Scholar]
- [80].Green PS, Gordon K, Simpkins JW. Phenolic A ring requirement for the neuroprotective effects of steroids. J. Steroid Biochem. Mol. Biol. 1997;63:229–235. doi: 10.1016/s0960-0760(97)00124-6. [DOI] [PubMed] [Google Scholar]
- [81].Murashov AK, Islamov RR, McMurray RJ, Pak ES, Weidner DA. Estrogen increases retrograde labeling of motoneurons: evidence of a nongenomic mechanism. Am. J. Physiol. Cell Physiol. 2004;287:C320–326. doi: 10.1152/ajpcell.00542.2003. [DOI] [PubMed] [Google Scholar]
- [82].Stroppolo A, Tian C, Guinea B, Olm V, Sheffield R, Sommer J, Ehrlich ME. 17beta-Estradiol promotes striatal medium size spiny neuronal maturation in vitro. Neuroendocrinology. 2004;79:259–267. doi: 10.1159/000079320. [DOI] [PubMed] [Google Scholar]
- [83].Yager JD, Chen JQ. Mitochondrial estrogen receptors--new insights into specific functions. Trends Endocrinol. Metab. 2007;18:89–91. doi: 10.1016/j.tem.2007.02.006. [DOI] [PubMed] [Google Scholar]
- [84].Chen JQ, Yager JD. Estrogen’s effects on mitochondrial gene expression: mechanisms and potential contributions to estrogen carcinogenesis. Ann. N. Y. Acad. Sci. 2004;1028:258–272. doi: 10.1196/annals.1322.030. [DOI] [PubMed] [Google Scholar]
- [85].Chen OS, Crisp RJ, Valachovic M, Bard M, Winge DR, Kaplan J. Transcription of the yeast iron regulon does not respond directly to iron but rather to iron-sulfur cluster biosynthesis. J. Biol. Chem. 2004;279:29513–29518. doi: 10.1074/jbc.M403209200. [DOI] [PubMed] [Google Scholar]
- [86].Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM, Jr., Valencia T, Brun-Zinkernagel AM, Prokai L, Will Y, Dykens J, Koulen P, Simpkins JW. Mitochondrial localization of estrogen receptor beta. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4130–4135. doi: 10.1073/pnas.0306948101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Leong H, Riby JE, Firestone GL, Bjeldanes LF. Potent ligand-independent estrogen receptor activation by 3,3′-diindolylmethane is mediated by cross talk between the protein kinase A and mitogen-activated protein kinase signaling pathways. Mol. Endocrinol. 2004;18:291–302. doi: 10.1210/me.2003-0196. [DOI] [PubMed] [Google Scholar]
- [88].Farhat MY, Lavigne MC, Ramwell PW. The vascular protective effects of estrogen. FASEB J. 1996;10:615–624. [PubMed] [Google Scholar]
- [89].Almeida S, Domingues A.i., Rodrigues L, Oliveira CR, Rego AC. FK506 prevents mitochondrial-dependent apoptotic cell death induced by 3-nitropropionic acid in rat primary cortical cultures. Neurobiol. Dis. 2004;17:435–444. doi: 10.1016/j.nbd.2004.07.002. [DOI] [PubMed] [Google Scholar]
- [90].Keep M, Elm E, Fong KSK, Csiszar K. Intrathecal cyclosporin prolongs survival of late-stage ALS mice. Brain Res. 2001;894:327–331. doi: 10.1016/s0006-8993(01)02012-1. [DOI] [PubMed] [Google Scholar]
- [91].Tanaka Y, Engelender S, Igarashi S, Rao RK, Wanner T, Tanzi RE, Sawa A, Dawson VL, Dawson TM, Ross CA. Inducible expression of mutant alpha-synuclein decreases proteasome activity and increases sensitivity to mitochondria-dependent apoptosis. Hum. Mol. Genet. 2001;10:919–926. doi: 10.1093/hmg/10.9.919. [DOI] [PubMed] [Google Scholar]
- [92].Wang Y, Branicky R, Stepanyan Z, Carroll M, Guimond MP, Hihi A, Hayes S, McBride K, Hekimi S. The anti-neurodegeneration drug clioquinol inhibits the aging-associated protein CLK-1. J. Biol. Chem. 2009;284:314–323. doi: 10.1074/jbc.M807579200. [DOI] [PubMed] [Google Scholar]
- [93].Moon Y, Lee KH, Park JH, Geum D, Kim K. Mitochondrial membrane depolarization and the selective death of dopaminergic neurons by rotenone: protective effect of coenzyme Q10. J. Neurochem. 2005;93:1199–1208. doi: 10.1111/j.1471-4159.2005.03112.x. [DOI] [PubMed] [Google Scholar]
- [94].Yang X, Yang Y, Li G, Wang J, Yang ES. Coenzyme Q10 attenuates beta-amyloid pathology in the aged transgenic mice with Alzheimer presenilin 1 mutation. J. Mol. Neurosci. 2008;34:165–171. doi: 10.1007/s12031-007-9033-7. [DOI] [PubMed] [Google Scholar]
- [95].Dhanasekaran A, Kotamraju S, Kalivendi SV, Matsunaga T, Shang T, Keszler A, Joseph J, Kalyanaraman B. Supplementation of endothelial cells with mitochondria-targeted antioxidants inhibit peroxide-induced mitochondrial iron uptake, oxidative damage, and apoptosis. J. Biol. Chem. 2004;279:37575–37587. doi: 10.1074/jbc.M404003200. [DOI] [PubMed] [Google Scholar]
- [96].Lu C, Zhang D, Whiteman M, Armstrong JS. Is antioxidant potential of the mitochondrial targeted ubiquinone derivative MitoQ conserved in cells lacking mtDNA? Antioxid. Redox Signal. 2008;10:651–660. doi: 10.1089/ars.2007.1865. [DOI] [PubMed] [Google Scholar]
- [97].Dedeoglu J.K.K. Alpaslan, Yang Lichuan, Ferrante Kimberly L., Hersch Steven M., Beal M. Flint, Ferrante Robert J. Creatine therapy provides neuroprotection after onset of clinical symptoms in Huntington’s disease transgenic mice. J. Neurochem. 2003;85:1359–1367. doi: 10.1046/j.1471-4159.2003.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Brewer GJ, Wallimann TW. Protective effect of the energy precursor creatine against toxicity of glutamate and beta-amyloid in rat hippocampal neurons. J. Neurochem. 2000;74:1968–1978. doi: 10.1046/j.1471-4159.2000.0741968.x. [DOI] [PubMed] [Google Scholar]
- [99].Dupuis L, Oudart H, Rene F, de Aguilar J.L. Gonzalez, Loeffler JP. Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: Benefit of a high-energy diet in a transgenic mouse model. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11159–11164. doi: 10.1073/pnas.0402026101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Matthews RT, Ferrante RJ, Klivenyi P, Yang L, Klein AM, Mueller G, Kaddurah-Daouk R, Beal MF. Creatine and Cyclocreatine attenuate MPTP neurotoxicity. Exp. Neurol. 1999;157:142–149. doi: 10.1006/exnr.1999.7049. [DOI] [PubMed] [Google Scholar]
- [101].Gonzalez-Polo RA, Soler G, Rodriguezmartin A, Moran JM, Fuentes JM. Protection against MPP+ neurotoxicity in cerebellar granule cells by antioxidants. Cell Biol. Int. 2004;28:373–380. doi: 10.1016/j.cellbi.2004.03.005. [DOI] [PubMed] [Google Scholar]
- [102].Sangchot P, Sharma S, Chetsawang B, Porter J, Govitrapong P, Ebadi M. Deferoxamine attenuates iron-induced oxidative stress and prevents mitochondrial aggregation and alpha-synuclein translocation in SK-N-SH cells in culture. Dev. Neurosci. 2002;24:143–153. doi: 10.1159/000065700. [DOI] [PubMed] [Google Scholar]
- [103].Bachurin S, Bukatina E, Lermontova N, Tkachenko S, Afanasiev A, Grigoriev V, Grigorieva I, Ivanov Y, Sablin S, Zefirov N. Antihistamine agent Dimebon as a novel neuroprotector and a cognition enhancer. Ann. N. Y. Acad. Sci. 2001;939:425–435. doi: 10.1111/j.1749-6632.2001.tb03654.x. [DOI] [PubMed] [Google Scholar]
- [104].Bachurin SO, Shevtsova EP, Kireeva EG, Oxenkrug GF, Sablin SO. Mitochondria as a target for neurotoxins and neuroprotective agents. Ann. N. Y. Acad. Sci. 2003;993:334–344. doi: 10.1111/j.1749-6632.2003.tb07541.x. discussion 345-349. [DOI] [PubMed] [Google Scholar]
- [105].Burns A, Jacoby R. Dimebon in Alzheimer’s disease: old drug for new indication. Lancet. 2008;372:179–180. doi: 10.1016/S0140-6736(08)61046-6. [DOI] [PubMed] [Google Scholar]
- [106].Imamura T.T. Keiko, Kashiwaya Yoshihiro, Nakaso Kazuhiro, Nakashima Kenji. D-beta-hydroxybutyrate protects dopaminergic SH-SY5Y cells in a rotenone model of Parkinson’s disease. J. Neurosci. Res. 2006;84:1376–1384. doi: 10.1002/jnr.21021. [DOI] [PubMed] [Google Scholar]
- [107].Tieu K, Perier C, Caspersen C, Teismann P, Wu DC, Yan SD, Naini A, Vila M, Jackson-Lewis V, Ramasamy R, Przedborski S. D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J. Clin. Invest. 2003;112:892–901. doi: 10.1172/JCI18797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wang X, Figueroa BE, Stavrovskaya IG, Zhang Y, Sirianni AC, Zhu S, Day AL, Kristal BS, Friedlander RM. Methazolamide and melatonin inhibit mitochondrial cytochrome C release and are neuroprotective in experimental models of ischemic injury. Stroke. 2009;40:1877–1885. doi: 10.1161/STROKEAHA.108.540765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wang X, Zhu S, Drozda M, Zhang W, Stavrovskaya IG, Cattaneo E, Ferrante RJ, Kristal BS, Friedlander RM. Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington’s disease. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10483–10487. doi: 10.1073/pnas.1832501100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, Sarang S, Liu AS, Hartley DM, Wu DC, Gullans S, Ferrante RJ, Przedborski S, Kristal BS, Friedlander RM. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- [111].Hallows WC, Lee S, Denu JM. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc. Natl. Acad. Sci. U. S. A. 2006;103:10230–10235. doi: 10.1073/pnas.0604392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Okawara M, Katsuki H, Kurimoto E, Shibata H, Kume T, Akaike A. Resveratrol protects dopaminergic neurons in midbrain slice culture from multiple insults. Biochem. Pharmacol. 2007;73:550–560. doi: 10.1016/j.bcp.2006.11.003. [DOI] [PubMed] [Google Scholar]
- [114].Outeiro TF, Marques O, Kazantsev A. Therapeutic role of sirtuins in neurodegenerative disease. Biochim. Biophy. Acta. 2008;1782:363–369. doi: 10.1016/j.bbadis.2008.02.010. [DOI] [PubMed] [Google Scholar]
- [115].Parker JA, Arango M, Abderrahmane S, Lambert E, Tourette C, Catoire H, Neri C. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat. Genet. 2005;37:349–350. doi: 10.1038/ng1534. [DOI] [PubMed] [Google Scholar]
- [116].Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, Puigserver P, Sadoshima J, Deng H, Pedrini S, Gandy S, Sauve AA, Pasinetti GM. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J. Biol. Chem. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- [117].Schlicker C, Gertz M, Papatheodorou P, Kachholz B, Becker CFW, Steegborn C. Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5. J. Mol. Biol. 2008;382:790–801. doi: 10.1016/j.jmb.2008.07.048. [DOI] [PubMed] [Google Scholar]
- [118].Westphal CH, Dipp MA, Guarente L. A therapeutic role for sirtuins in diseases of aging? Trends Biochem. Sci. 2007;32:555–560. doi: 10.1016/j.tibs.2007.09.008. [DOI] [PubMed] [Google Scholar]
- [119].Fuenzalida K, Quintanilla R, Ramos P, Piderit D, Fuentealba RA, Martinez G, Inestrosa NC, Bronfman M. Peroxisome proliferator-activated receptor gamma up-regulates the Bcl-2 anti-apoptotic protein in neurons and induces mitochondrial stabilization and protection against oxidative stress and apoptosis. J. Biol. Chem. 2007;282:37006–37015. doi: 10.1074/jbc.M700447200. [DOI] [PubMed] [Google Scholar]
- [120].Jung TW, Lee JY, Shim WS, Kang ES, Kim SK, Ahn CW, Lee HC, Cha BS. Rosiglitazone protects human neuroblastoma SH-SY5Y cells against MPP+ induced cytotoxicity via inhibition of mitochondrial dysfunction and ROS production. J. Neurol. Sci. 2007;253:53–60. doi: 10.1016/j.jns.2006.11.020. [DOI] [PubMed] [Google Scholar]
- [121].Kiaei M, Kipiani K, Chen J, Calingasan NY, Beal MF. Peroxisome proliferator-activated receptor-gamma agonist extends survival in transgenic mouse model of amyotrophic lateral sclerosis. Exp. Neurol. 2005;191:331–336. doi: 10.1016/j.expneurol.2004.10.007. [DOI] [PubMed] [Google Scholar]
- [122].Quintanilla RA, Jin YN, Fuenzalida K, Bronfman M, Johnson GVW. Rosiglitazone treatment prevents mitochondrial dysfunction in mutant huntingtin-expressing cells: possible role of peroxisome proliferator-activated receptor-gamma (PPARgamma) in the pathogenesis of Huntington disease. J. Biol. Chem. 2008;283:25628–25637. doi: 10.1074/jbc.M804291200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Santos MJ, Quintanilla RA, Toro A, Grandy R, Dinamarca MC, Godoy JA, Inestrosa NC. Peroxisomal proliferation protects from beta-amyloid neurodegeneration. J. Biol. Chem. 2005;280:41057–41068. doi: 10.1074/jbc.M505160200. [DOI] [PubMed] [Google Scholar]
- [124].Szeto HH. Cell-permeable, mitochondrial-targeted, peptide antioxidants. AAPS J. 2006;8:E277–283. doi: 10.1007/BF02854898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Szeto HH. Mitochondria-targeted peptide antioxidants: novel neuroprotective agents. AAPS J. 2006;8:E521–531. doi: 10.1208/aapsj080362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Szeto HH. Development of mitochondria-targeted aromatic-cationic peptides for neurodegenerative diseases. Ann. N. Y. Acad. Sci. 2008;1147:112–121. doi: 10.1196/annals.1427.013. [DOI] [PubMed] [Google Scholar]
- [127].Yang L, Zhao K, Calingasan NY, Luo G, Szeto HH, Beal F. Mitochondria targeted peptides protect against 1-Methyl-4-Phenyl-1, 2, 3, 6-Tetrahydropyridine neurotoxicity. Antioxid. Redox Signal. 2009;11:1–10. doi: 10.1089/ars.2009.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Haridas V, Hanausek M, Nishimura G, Soehnge H, Gaikwad A, Narog M, Spears E, Zoltaszek R, Walaszek Z, Gutterman JU. Triterpenoid electrophiles (avicins) activate the innate stress response by redox regulation of a gene battery. J. Clin. Invest. 2004;113:65–73. doi: 10.1172/JCI18699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Haridas V, Li X, Mizumachi T, Higuchi M, Lemeshko VV, Colombini M, Gutterman JU. Avicins, a novel plant-derived metabolite lowers energy metabolism in tumor cells by targeting the outer mitochondrial membrane. Mitochondrion. 2007;7:234–240. doi: 10.1016/j.mito.2006.12.005. [DOI] [PubMed] [Google Scholar]
- [130].Leo S, Szabadkai G, Rizzuto R. The mitochondrial antioxidants MitoE2 and MitoQ10 increase mitochondrial Ca2+ load upon cell stimulation by inhibiting Ca2+ efflux from the organelle. Ann. N. Y. Acad. Sci. 2008;1147:264–274. doi: 10.1196/annals.1427.019. [DOI] [PMC free article] [PubMed] [Google Scholar]