Abstract
Cell fusion plays an essential role in fertilization, formation of placenta, bone and muscle tissues, immune response, tissue repair and regeneration. Increasing recognition of cell fusion in somatic cell dynamics has revitalized the century-old hypothesis that cell fusion may contribute to the initiation and progression of cancer. In this review, we discuss findings from experimental and clinical studies that suggest a potentially multifaceted involvement of cell fusion in different stages of tumor progression, including aneuploidy and tumor initiation, origin of cancer stem cells, multidrug resistance, and the acquisition and diversification of metastatic abilities.
Keywords: cell fusion, aneuploidy, chromosomal instability, centrosome, metastasis, cancer stem cell, drug resistance
Introduction
Cell fusion is believed to be a relatively rare, and strictly regulated phenomenon that only takes place in specific occasions, such as fertilization (fusion of sperm and egg), the formation of placenta (fusion of trophoblasts) and muscle fibers (fusion of myoblasts), and bone homeostasis (formation of multinuclear osteoclasts for bone resorption) (1). The concept of cell fusion as a mechanism of tumorigenesis dates back to the early 1900s when Otto Aichel postulated that spontaneous fusion between somatic cells may lead to chromosomal abnormalties and cancer (1–4). However, in the modern era of molecular oncology, cell fusion has been considered as an oddity that attracts little attention compared to other fields of cancer research. Recent discoveries of a much broader role of cell fusion in tissue homeostasis and regeneration, particularly during inflammatory conditions, have revitalized the interest in cell fusion as one of the driving forces of cancer progression.
Spontaneous cell fusion in tissue culture or in animal models has been reported for a large variety of tumor cells (1, 5). The frequency of cell fusion can be up to 1% in vivo in experimental tumor models (1). Furthermore, fusion efficiency can be proportional to the malignant level of tumor cells (6). Several reasons may account for the increased fusogenicity of tumor cells. First, enveloped viruses may trigger the fusion of infected tumor cells (7, 8) and may therefore increase the efficiency of fusion in infection-related cancers, such as liver cancer or cervical cancer. Second, deregulation of physiological fusogenic proteins in tumor cells may promote cell fusion (7). For example, syncytin, the Env protein of HERV-W human endogenous retroviruses and putative mediator of trophoblast fusion, was also found to mediate fusion between breast cancer cells and endothelial cells (7). CD44, a cell surface receptor known to be involved in cell fusion during osteoclastogenesis, is frequently overexpressed in cancer cells and has been linked to poor prognosis and cancer stem cell phenotypes (3). Third, chronic inflammation has recently been shown to dramatically increase the frequency of cell fusion between hematopoietic cells and various somatic cells such as cardiomyocytes, skeletal muscle, hepatocytes and Purkinje cells during tissue repair and regeneration (9, 10). Similar as-yet-unknown mechanisms underlying this observation may also stimulate the fusion between bone marrow derived cells (BMDCs) and tumor cells since inflammation is often associated with the tumor microenvironment. Fourth, cell fusion may be the by-product of “cell-eat-cell” processes, such as phagocytosis of tumor cells by macrophages (3) or entosis (11). Finally, while rare accidental somatic cell fusion almost inevitably leads to cell cycle arrest and terminal differentiation in normal tissues, tumor cell fusions may produce proliferating cells capable of clonal expansion. This possibility was supported by a recent study showing that fused cells harboring oncogenes E1A and H-Ras could override the cell cycle arrest and evolve to become tumorigenic cells after massive chromosomal rearrangement (7).
Cell fusion can be easily recognized in cell culture and animal tumor model experiments using lineage-specfic tracking markers. In contrast, fusion events in human cancers are much more difficult to detect due to the lack of applicable genetic tracing tools. Therefore, definitive and direct observation of cell fusion in human cancer is rare and the most striking one was reported in patients who developed renal-cell carcinoma after allogeneic bone-marrow transplantation from donors of the opposite gender (12). Karyotyping of the cancer cells showed that they had adopted chromosomes from bone marrow donors, possibly through fusion of early tumor cells with transplanted bone marrow cells. In multiple myeloma cancer patients, up to 30% of the multinuclear osteoclasts are found to contain malignant nuclei with typical chromosomal translocations of myeloma (13). Premature chromosome condensation, a typical result of heterophasic cell fusion, has been observed in various types of cancer (1). Binuclear and multinuclear cells are also frequently observed in many types of tumors, although cell fusion is only one of several mechanisms to generate such cells. The correlation between increased DNA content and enhanced malignant behavior has been observed in ovarian cancer, prostate cancer, colon carcinoma and breast cancer (1). Despite the paucity of direct evidence of cell fusion in human cancer, accumulating experimental evidence in recent years has suggested a possible broad invovement of cell fusion during the initiation, progression and phenotypic diversification of cancer.
Cell fusion in aneuploidy and tumor initiation
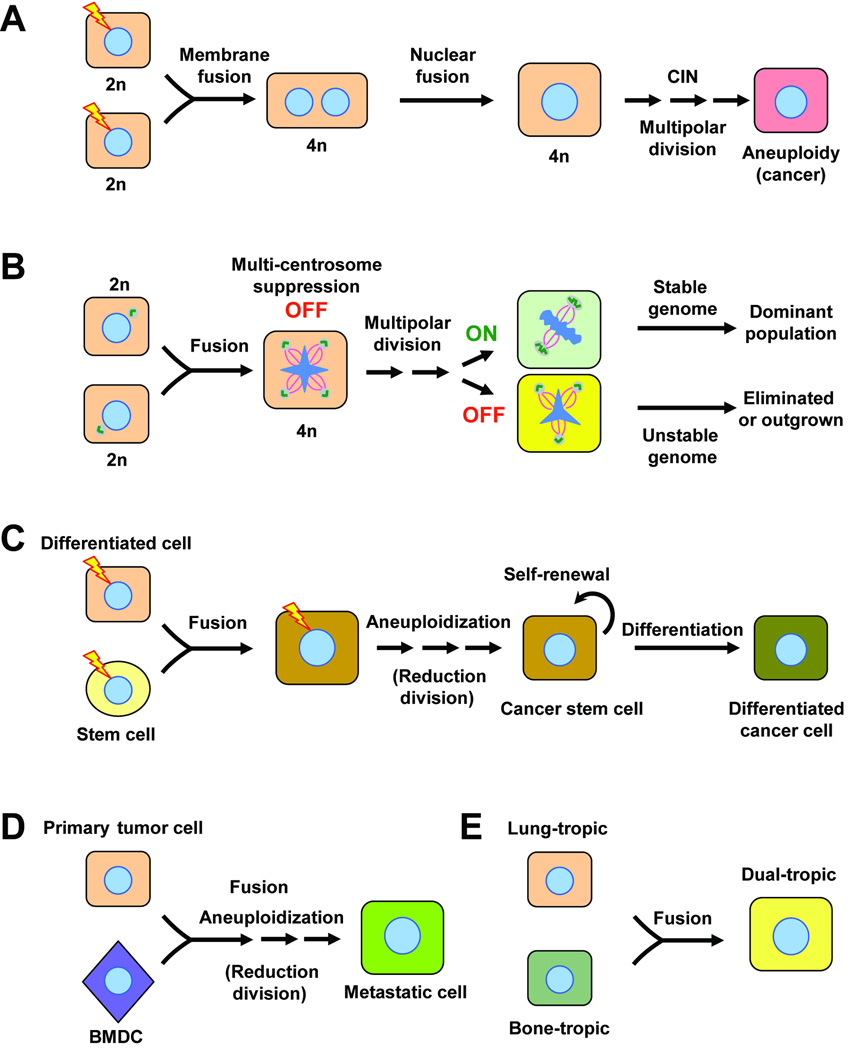
The direct consequence of fusion between two diploid somatic cells is the generation of a tetraploid hybrid. The nuclei of the fusion partners often remain separate, as in the case of cell fusion in placenta, muscle and bone. However, in non-programmed accidental fusion, the two nuclei may fuse and lead to aneuploidy and potentially cancer, given the existence of other genetic alterations, such as loss of p53 function (14). Indeed, tetraploidy frequently precedes aneuploidy in cancer, as was observed in both clinical tumor samples (15) and experimental models (14). A fusion-mediated oncogenesis model based on these observations has been proposed (7, 8, 16, 17) (Fig. 1A). When accidental cell fusion or cytokinesis failure occurs in a cell with inactivated p53-dependent ‘tetraploidy’ checkpoint (17) or ‘fusion’ checkpoint (7), supernumerary centrosome-mediated multipolar division, chromosome missegregation and increased DNA damage rate may then facilitate the progression from tetraploidy to aneuploidy (16). Oncogene activation and loss of tumor suppressor genes caused by aneuploidy in a stochastic manner may select for cell(s) that eventually becomes fully malignant. It should be noted that the prevailing model of aneuploidy focuses on checkpoint mutations and telomere attrition instead of tetraploidy. The exact contribution of cell fusion to aneuploidy still awaits further characterization.
Figure 1.
The potential multi-faceted role of cell fusion in cancer progression. A, fusion-induced tetraploidy as an intermediate step before aneuploidy (7, 8, 16, 17). Mutations of certain oncogenes (e.g. H-Ras) or tumor suppressor genes (e.g. p53), represented by lightning bolts, in the fusion partners may be required to generate a synkaryonic proliferating cell. CIN, chromosomal instability; n denotes haploidy. B, a biphasic role of supernumerary centrosomes in tumor progression (18). Fusion-induced centrosome amplification causes multipolar division and aneuploidy in nascent tumor cells, when the mechanism(s) for suppressing spindle multipolarity is not activated (“OFF” state). Once the tumor cells acquire the ability to suppress supernumerary centrosomes (“ON” state, e.g. centrosome clustering or orphan centrioles), this mechanism allows the maintenance of a relatively stable chromosomal composition and the clonal expansion of the dominant population. C, generation of a cancer stem cell by having an adult stem cell as a fusion partner (2). Oncogenic mutations could occur in the stem cell, the differentiated cell or the hybrid. D, fusion between a tumor cell and a bone marrow derived cell (BMDC) generates a metastatic cell (3). In C and D, reduction division could occur in the hybrid so that the transient increased of ploidy can not be detected. E. fusion between tumor cells with different metastatic organotropism leads to rapid acquisition of dual-tropisms and may increase dissemination and promote metastatic recurrence after therapy (18). The original single organotropism could be derived from a previous fusion of a tumor cell with an organotropic leukocyte.
The doubling of centrosomes after cell fusion does not always lead to chromosome instability. For example, stable chromosome numbers were reported in hybrids resulting from fusion between Hela cells and fibroblasts, between mouse mammary tumor cells, or between ES cells and fibroblasts (18). In a recent study from our laboratory, spontaneous fusion hybrids between two sublines of the MDA-MB-231 breast cancer cell line were found to have robust chromosomal stability over long-term proliferation in vitro or in vivo (18), despite the fact that more than 50% of hybrid tumor cells have an abnormally high number of centrioles. Further analysis revealed that extra centrioles can be discarded, coalesced or orphaned, so that tumor cells were able to maintain bipolar division with two functional centrosomes. The ability to control supernumerary centrosomes through such mechanisms is not specific to breast cancer cells, as similar phenomenon was also observed in neuroblastomas (19). The stark contrast between highly malignant tumor cells and pre-cancerous or early stage tumor cells (14) in terms of chromosomal stability after cell fusion suggested a possible biphasic role of centrosome duplication in tumor progression (Fig.1B). In the early stage of tumorigenesis, centrosome amplification caused by cell fusion, abortive division or abnormal centrosome duplication leads to multipolar division and chromosome instability, resulting in a heterogeneous pool of aneuploid cells. A random activation of certain intrinsic mechanisms for suppressing supernumerary centrosomes in some tumor cells allows the clonal outgrowth of the cells to become the dominated population with a relative stable chromosomal composition. Indeed, many previous studies have repeatedly shown that the chromosome aberration spectrum stabilizes in advanced cancers (20). The ability to avoid mitotic catastrophe in the face of supernumerary centrosomes may be an important tumor survival mechanism that can be exploited for therapeutic intervention. For example, several proteins have been implicated in the suppression of spindle multipolarity caused by supernumerary centrosomes, including NuMA and dynein (21). Targeting these proteins may lead to catastrophic chromosome missegregation and cell death. Importantly, the ability for fusion hybrids to sustain a relatively stable genomic composition will allow tumor cells to acquire and stably maintain additional malignant properties, such as stemness, drug resistance and metastatic abilities, through cell fusion.
Cell fusion as an origin of cancer stem cells
Increasing evidence suggests that tumor growth and recurrence relies on the existence of cancer stem cells, also known as tumor initiating cells, that are capable of self-renewal and differentiation into diverse tumor cell populations(2). Inspired by the recent findings that hematopoietic stem cells are frequently found to be fused with several resident tissue cells during tissue repair and regeneration, Bjerkvig et al. proposed that fusion of an adult stem cell (local or hematopoietic) with a differentiated cell may leads to the formation of a cancer stem cell if either one of the cells harbors appropriate mutations to sustain a proliferative fusion (2) (Fig. 1C). Several lines of evidence support the feasibility of this hypothesis. Fusion of BMDCs with differentiated adult tissue cells was dramatically increased by chronic inflammation, which is a major risk factor for tumorigenesis (9, 10). In a mouse model of gastric cancer produced through chronic inflammation from Helicobacter pylori infection, BMDCs were found to replace the damaged epithelial lining of gastric mucosa and eventually gave rise to gastric cancer (22). Although no direct evidence of cell fusion was found in this study, additional experiments using fusion tracing techniques, such as Cre-mediated activation of a LacZ reporter preceded by a lox-franked stop signal (Cre and LacZ constructs are separately integrated in the donor cells and host cells), should be used to definitely investigate the potential role of cell fusion in tumor initiation using this experimental model.
Acquisition of drug resistance through cell fusion
Cell fusion may allow the fused cells to become more resistant to chemotherapeutic drugs in two ways: acquisition of drug resistance from both parental cells and emergence of new drug resistance. For example, when a 5-fluorouracil-resistant and a methotrexate-resistant mammary tumor cell lines were fused, the resulted hybrids became resistant to both drugs as well as another drug, melphalan (1). In a separate study, fusion of a drug-sensitive E1A-transformed cell with a primary fibroblast generated a hybrid that is resistant to drug-induced apoptosis (7). Acquisition of new drug resistance may occur at a low frequency and in a transient manner from a vast variety of the aneuploid progenies resulting from cell fusion. However, even a temporal advantage over other tumor cells in drug resistance could allow the escape of rare cells which may become the source of tumor relapse (1). As drug treatment of cancer is often accompanied by increased inflammation, which is known to promote cell fusion, the potential contribution of cell fusion to drug resistance should not be underestimated.
Cell fusion and metastasis
Metastasis is a multi-step process requiring the tumor cells to be equipped with properties they rarely need in the primary site, including higher motility, survival in circulation, invasion through tissue layers and growth in an unfamiliar microenvironment (23). Many of these are intrinsic properties of migratory BMDCs, such as macrophages. Thus, fusion between tumor cells and BMDCs may be an efficient way for rapid acquisition of metastatic phenotypes (Fig. 1D) (3). Indeed, in vitro and in vivo fusion between tumor cells and macrophages has been shown to produce hybrids with increased metastatic abilities (3). To test the contribution of cell fusion to the natural development of metastasis, fusion of transplanted bone marrow cells with resident tumor cells should be distinguished from unfused tumor cells in mouse tumor models using reporter genes (e.g. Cre-activated LacZ expression). Examination of nascent distant metastases may reveal whether fusion between tumor cells with BMDCs may play a significant role in promoting metastasis.
The organ that tumor cells prefer to colonize, or organotropism, can also be influenced by cell fusion. For example, fusion of myeloma cells with B lymphocytes resulted in a hybrid cell metastasizing to the spleen and liver, yet fusion with macrophages led to metastasis to lung (24). Importantly, fusion of tumor cells with resident cells in a secondary organ may allow disseminated tumor cells to survive in a hostile microenvironment as minimal residual diseases and emerge as overt metastasis after accumulation of additional oncogenic alternations. In a recent study, untransformed mammary cells were found to persist in the lung as small clusters until inducible activation of oncogenes stimulated the formation of pulmonary metastases (25). It is conceivable that fusion between the disseminated mammary cells and the local resident cells in lung, including lung epithelial cells and recruited BMDCs, may allow the survival of disseminated non-transformed mammary epithelial cells and contribute to the formation of metastases when additional oncogenes are activated. Again, such a hypothesis could be tested definitively by using fusion-dependent activation of reporters in transgenic animal models.
Fusion between tumor cells in a heterogeneous population, such as pleural effusions, may contribute to the rapid acquisition of multiple metastasis organotropisms. In vivo selection of the MDA-MB-231 breast cancer cell line in mouse models successfully derived bone-and lung-tropic sublines with distinct organ-specific metastasis gene signatures (23). In our recent study, spontaneous fusion in vitro or in vivo between the bone-tropic and the lung-tropic subpopulations resulted in stable hybrids with dual tropisms to both organs (18) (Fig. 1E). Expression analysis showed that the hybrids co-expressed both organ-specific metastasis gene signatures. Hybrid cells with multiple metastasis organotropisms may increase the efficiency of metastatic dissemination since the hybrids are able to colonize several different organs when they are in circulation, while tumor cells with only a single metastasis organotropism can only colonize one organ and will be lost when they are lodged into an inhospitable microenvironment. Furthermore, since metastases in different organs often have different responses to therapeutic treatments, tumor cells with multiple metastasis organotropisms can survive in a different organ and then quickly re-colonize the organ that has positively responded to therapies.
Overall, accumulating evidence has suggested a plausible involvement of cell fusion in several aspects of cancer progression. Rigorous genetic studies in animal tumor models will be needed to definitely demonstrate the extent of cell fusion to tumor initiation, drug resistance and metastasis. Such experiments, together with more extensive investigations of cell fusion in human cancer patients, may lead to novel therapeutics for cancer.
Acknowledgment
We thank members of our laboratory for helpful discussions. We also apologize to the many investigators whose important studies could not be cited directly here owing to space limitations. Y.K. is a Champalimaud Investigator and a Department of Defense Era of Hope Scholar Award recipient. Research in the authors’ laboratory is additionally supported by a research scholar grant from the American Cancer Society, the National Institute of Health and the New Jersey Commission on Cancer Research. X.L. is a recipient of a Harold W. Dodds Fellowship from Princeton University.
References
- 1.Duelli D, Lazebnik Y. Cell fusion: A hidden enemy? Cancer Cell. 2003;3(5):445–448. doi: 10.1016/s1535-6108(03)00114-4. [DOI] [PubMed] [Google Scholar]
- 2.Bjerkvig R, Tysnes BB, Aboody KS, Najbauer J, Terzis AJ. Opinion: the origin of the cancer stem cell: current controversies and new insights. Nature Rev Cancer. 2005;5:899–904. doi: 10.1038/nrc1740. [DOI] [PubMed] [Google Scholar]
- 3.Pawelek JM, Chakraborty AK. Fusion of tumour cells with bone marrow-derived cells: a unifying explanation for metastasis. Nat Rev Cancer. 2008;8(5):377–386. doi: 10.1038/nrc2371. [DOI] [PubMed] [Google Scholar]
- 4.Pawelek JM, Chakraborty AK, George FVWaGK. Chapter 10 The Cancer Cell-Leukocyte Fusion Theory of Metastasis. Advances in Cancer Research: Academic Press; 2008. pp. 397–444. [DOI] [PubMed] [Google Scholar]
- 5.Rachkovsky M, Sodi S, Chakraborty A, et al. Melanoma x macrophage hybrids with enhanced metastatic potential. Clinical and Experimental Metastasis. 1998;16(4):299–312. doi: 10.1023/a:1006557228604. [DOI] [PubMed] [Google Scholar]
- 6.Miller FR, McInerney D, Rogers C, Miller BE. Spontaneous fusion between metastatic mammary tumor subpopulations. Journal Of Cellular Biochemistry. 1988;36(2):129–136. doi: 10.1002/jcb.240360204. [DOI] [PubMed] [Google Scholar]
- 7.Duelli D, Lazebnik Y. Cell-to-cell fusion as a link between viruses and cancer. Nat Rev Cancer. 2007;7(12):968–976. doi: 10.1038/nrc2272. [DOI] [PubMed] [Google Scholar]
- 8.Parris GE. The role of viruses in cell fusion and its importance to evolution, invasion and metastasis of cancer clones. Med Hypotheses. 2005;64:1011–1014. doi: 10.1016/j.mehy.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Johansson CB, Youssef S, Koleckar K, et al. Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation. Nat Cell Biol. 2008;10(5):575–583. doi: 10.1038/ncb1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nygren JM, Liuba K, Breitbach M, et al. Myeloid and lymphoid contribution to non-haematopoietic lineages through irradiation-induced heterotypic cell fusion. Nat Cell Biol. 2008;10(5):584–592. doi: 10.1038/ncb1721. [DOI] [PubMed] [Google Scholar]
- 11.Overholtzer M, Mailleux AA, Mouneimne G, et al. A Nonapoptotic Cell Death Process, Entosis, that Occurs by Cell-in-Cell Invasion. Cell. 2007;131(5):966–979. doi: 10.1016/j.cell.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 12.Yilmaz Y, Lazova R, Qumsiyeh M, Cooper D, Pawelek J. Donor Y chromosome in renal carcinoma cells of a female BMT recipient: visualization of putative BMT-tumor hybrids by FISH. Bone Marrow Transplant. 2005;35(10):1021–1024. doi: 10.1038/sj.bmt.1704939. [DOI] [PubMed] [Google Scholar]
- 13.Andersen TL, Boissy P, Sondergaard TE, et al. Osteoclast nuclei of myeloma patients show chromosome translocations specific for the myeloma cell clone: a new type of cancer-host partnership? The Journal of pathology. 2007;211(1):10–17. doi: 10.1002/path.2078. [DOI] [PubMed] [Google Scholar]
- 14.Fujiwara T, Bandi M, Nitta M, Ivanova EV, Bronson RT, Pellman D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437(7061):1043–1047. doi: 10.1038/nature04217. [DOI] [PubMed] [Google Scholar]
- 15.Heselmeyer K. Gain of chromosome 3q defines the transition from severe dysplasia to invasive carcinoma of the uterine cervix. Proc Natl Acad Sci USA. 1996;93:479–484. doi: 10.1073/pnas.93.1.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganem NJ, Storchova Z, Pellman D. Tetraploidy, aneuploidy and cancer. Curr Opin Genet Dev. 2007 doi: 10.1016/j.gde.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Margolis RL. Tetraploidy and tumor development. Cancer Cell. 2005;8(5):353–354. doi: 10.1016/j.ccr.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Lu X, Kang Y. Efficient acquisition of dual metastasis organotropism to bone and lung through stable spontaneous fusion between MDA-MB-231 variants. Proceedings of the National Academy of Sciences. 2009 doi: 10.1073/pnas.0900108106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brinkley BR. Managing the centrosome numbers game: from chaos to stability in cancer cell division. Trends in Cell Biology. 2001;11(1):18–21. doi: 10.1016/s0962-8924(00)01872-9. [DOI] [PubMed] [Google Scholar]
- 20.Albertson DG, Collins C, McCormick F, Gray JW. Chromosome aberrations in solid tumors. Nat Genet. 2003;34(4):369–376. doi: 10.1038/ng1215. [DOI] [PubMed] [Google Scholar]
- 21.Quintyne NJ, Reing JE, Hoffelder DR, Gollin SM, Saunders WS. Spindle Multipolarity Is Prevented by Centrosomal Clustering. Science. 2005;307(5706):127–129. doi: 10.1126/science.1104905. [DOI] [PubMed] [Google Scholar]
- 22.Houghton J, Stoicov C, Nomura S, et al. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306(5701):1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 23.Lu X, Kang Y. Organotropism of Breast Cancer Metastasis. Journal of Mammary Gland Biology and Neoplasia. 2007;12(2):153–162. doi: 10.1007/s10911-007-9047-3. [DOI] [PubMed] [Google Scholar]
- 24.De Baetselier P, Roos E, Brys L, et al. Nonmetastatic tumor cells acquire metastatic properties following somatic hybridization with normal cells. Cancer Metastasis Reviews. 1984;3(1):5–24. doi: 10.1007/BF00047690. [DOI] [PubMed] [Google Scholar]
- 25.Podsypanina K, Du Y-CN, Jechlinger M, Beverly LJ, Hambardzumyan D, Varmus H. Seeding and Propagation of Untransformed Mouse Mammary Cells in the Lung. Science. 2008;321(5897):1841–1844. doi: 10.1126/science.1161621. [DOI] [PMC free article] [PubMed] [Google Scholar]