Abstract
Moraxella catarrhalis is a respiratory tract pathogen causing otitis media in children and respiratory tract infections in adults with chronic obstructive pulmonary disease. This study examined two newly identified proteins as potential vaccine antigens. Antisera raised to recombinant purified proteins Msp22 and Msp75 recognized corresponding native proteins in multiple strains of M. catarrhalis. Vaccine formulations individually administered subcutaneously and intranasally showed enhanced clearance of M. catarrhalis in a mouse pulmonary clearance model by both routes of administration. Msp22 and Msp75 are antigenically conserved proteins that induce potentially protective immune responses and should be examined further as vaccine antigens for M. catarrhalis.
Keywords: Moraxella catarrhalis, vaccine, mouse pulmonary clearance, immunization, otitis media, obstructive lung disease
1. Introduction
Moraxella catarrhalis is an important human respiratory tract pathogen. It is the third most common bacterial pathogen in otitis media in children, causing 15–20% of episodes of acute otitis media based on cultures of middle ear fluid obtained by tympanocentesis [1–3]. Otitis prone children experience recurrent and chronic otitis media, which are associated with delayed speech and language development in children [4, 5]. A vaccine to prevent otitis media would have a large impact in preventing morbidity and reducing healthcare costs associated with otitis media.
M. catarrhalis is also an important cause of lower respiratory tract infections, called exacerbations, in adults with chronic obstructive pulmonary disease (COPD) [1, 6–8]. COPD is the fourth most common cause of death worldwide [9, 10]. The course of COPD is characterized by intermittent exacerbations that result in lost work time, emergency room visits, hospital admissions, respiratory failure and death. M. catarrhalis causes approximately 10% of exacerbations [6]. Thus, adults with COPD are another group that would benefit from a vaccine to prevent M. catarrhalis infection.
Surface proteins of M. catarrhalis are being evaluated as vaccine antigens. An ideal vaccine candidate has several characteristics including surface exposure, conservation among strains, expression during infection, and generation of a protective immune response [2]. A limited number of surface proteins of M. catarrhalis have been examined for their ability to act as vaccine antigens [11–17].
In this study, we examined two highly conserved surface proteins designated Moraxella surface protein (Msp) Msp22 and Msp75, that were identified using a genome mining approach [18]. Msp22 is a ~22kDa lipoprotein of 152 amino acids. Msp22 has homology with cytochrome c and the msp22 gene is part of gene cluster that includes a coproporphyrinogen III and GTP cyclohydrolase II. These two observations suggest that Msp22 may be involved in transport of divalent cations across the outer membrane. Msp75 is a 499 amino acid protein that shares homology (73% identity, 83% similarity) with succinic semialdehyde dehydrogenase of Psychrobacter species and other gram negative bacteria. Msp75 was identified for study as a vaccine antigen based on its high degree of sequence conservation among strains of M. catarrhalis and based on homology with a region of the chromosome of Agrobacterium tumefaciens that is associated with virulence [18].
To assess the immunogenicity of Msp22 and Msp75 and to determine the extent to which antibodies recognized the proteins in multiple strains, rabbits and mice were immunized with purified recombinant protein and antisera were studied. In addition, both proteins were examined using the mouse pulmonary clearance model to assess for the induction of potentially protective immune responses.
2. Materials and methods
2.1. Bacterial strains and culture conditions
M. catarrhalis strain 43617 was obtained from the American Type Culture Collection (Rockville, MD). Strain O35E was provided by Eric Hansen. Middle ear fluid isolates 2951, 7169, and 8184, obtained by tympanocentesis from children with otitis media, were provided by Dr. Howard Faden. Strains 6P29B1, 7P94B1, and 102P19B1 were sputum isolates obtained from adults in our COPD Study Clinic [6, 7]. Chemically competent Escherichia coli strains TOP10 and BL21(DE3) were purchased from Invitrogen.
M. catarrhalis strains were grown on brain heart infusion (BHI) plates at 37°C with 5% CO2 or in BHI broth with shaking at 37°C. E. coli strains were grown on Luria-Bertani (LB) plates, LB broth, or in terrific broth (TB) at 37°C supplemented with the appropriate antibiotics (MoBio Laboratories, Carlsbad, CA).
2.2. Expression and purification of recombinant proteins
Genes were cloned and recombinant proteins were purified as described previously in detail [18]. In brief, genes were amplified by PCR from M. catarrhalis ATCC 43617 genomic DNA and cloned into pRSETB (Invitrogen, San Diego) for msp75, and pCATCH, for msp22 [19] resulting in fusion proteins expressing a 6-histidine tag. Cloning msp22 into the pCATCH plasmid allowed for placement of an N-terminal lipid resulting in expression of a recombinant lipoprotein with the histidine tag on the C-terminus [19, 20]. Msp75 had a histidine tag on its amino terminus. Purification of the recombinant proteins was accomplished by affinity chromatography using TALON Co+2 metal affinity resin (BD Biosciences, Palo Alto, CA) which binds the histidine tags. The purified proteins yielded a single band when subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) [18].
2.3. Development of rabbit antiserum
Antiserum to purified recombinant Msp22 and Msp75 were developed by Proteintech (Chicago IL). Rabbits were immunized individually with each protein subcutaneously with 50 µg of protein emulsified with complete Freund’s adjuvant on day 1. This was followed by boosting with 50 µg protein emulsified with incomplete Freund’s adjuvant on days 14, 28 and 35. Blood was obtained on day 56.
2.4. Systemic immunization of mice
Groups of five Balb/c mice each were immunized subcutaneously with 25 µg or 50 µg of either purified recombinant Msp22 or Msp75 emulsified in incomplete Freund’s adjuvant. Additional groups of five mice each were immunized with either adjuvant alone (negative control) or formalin killed M. catarrhalis O35E emulsified in incomplete Freund’s adjuvant (positive control). Injections were repeated at 14 and 28 days after the initial immunization. Mice were challenged as described below on day 35.
2.5. Mucosal immunization of mice
Groups of five Balb/c mice were immunized intranasally with 25 µg or 50 µg of either purified recombinant Msp22 or Msp75. Proteins were administered together with cholera toxin (1 µg) as a mucosal adjuvant. Additional groups of five mice each were immunized with either 1 µg cholera toxin (negative control) or formalin killed M. catarrhalis O35E with 1 µg cholera toxin (positive control). Intranasal immunization was performed by having awake animals sniff liquid from a pipette tip placed at the nostril. A volume of 5 µl per nostril was administered. Immunizations were repeated on days 10 and 20 after the initial immunization. Mice were challenged as described below on day 28.
2.6. Immunoblot assays
Immunoblot assays for Msp22 were transferred from 15% gels and immunoblots for Msp75 were transferred from 12% gels. Dilutions, blocking conditions, incubation times and developing reagents were optimized for each antiserum. In the case of mouse antiserum to purified recombinant Msp 22, blots were blocked with 5% nonfat dry milk for one hour and were incubated with antiserum at room temperature for 3 hours. In the case of mouse antiserum to Msp75, blots were blocked with 3% bovine serum albumen and incubated overnight with antiserum at room temperature. Blots were probed with peroxidase-conjugated anti rabbit IgG or anti mouse IgG (KPL Gaithersburg, MD). For immunoblots with rabbit antisera, bands were developed with horseradish peroxidase color developer (BioRad, Richmond CA). For immunoblots with mouse antisera, bands were developed with either Super Signal West Pico Chemiluminescence Substrate (Pierce, Rockford IL) or ECL Plus Western Blotting Detection Reagents (Amersham, Piscataway NJ) as per manufacturers’ instructions.
2.7. Construction of mutants
A mutant lacking the msp22 gene and thus deficient in expression of Msp22 was constructed in M. catarrhalis strain O35E. To accomplish this, an ~1000 bp region upstream of msp22 and an ~1000 bp region downstream of msp22 were amplified from O35E genomic DNA using oligonucleotide primers that contained appropriate restriction sites (Table 1). These amplicons were ligated into plasmid pGEM3Zf . The nonpolar kanamycin resistance cassette aphA-3 (850 bp) [21] was amplified from plasmid pUC18K using oligonucleotide primers that contained compatible restriction sites and the amplicon was ligated into the plasmid construct between the fragments upstream and downstream of msp22. Therefore the plasmid contained an insert in which a kanamycin cassette was flanked by sequences corresponding to regions upstream and downstream of msp22 and lacked the msp22 gene itself.
Table 1.
Oligonucleotide primers used for construction of mutants.
| Gene | Primer name and purpose |
Sequencea |
|---|---|---|
| msp22 | 231upstream5′ | ATATGAATTCGTCTTATCAGGCGGGCAAGTC |
| msp22 | 231upstream3′ | ACACGGTACCGGTAATTTTATGAAACATGGC |
| msp22 | 231downstream5′ | GAGAGGATCCCAGCAGTCGTTTAATCTCCC |
| msp22 | 231downstream3′ | ATATCTGCAGGTATGACGCCATTACCAAACC |
| msp22 | kanalphakpn5′ | ATATGGTACCTGACTAACTAGGAGGAATAAA TGGCTAAAATGAGA |
| msp22 | kanalphakpn3′ | GAGAGGATCCTCATTATTCCCTCCAGGTACT AAAACAATTCATC |
| msp75 | 255upstream5′ | ATATGAATTCAATGGACGGGCTAATTTCAGA AC |
| msp75 | 255upstream3′ | GAGAGGATCCAAATCACCCAAAGTGCTTCTC CT |
| msp75 | 255downstream5′ | GAGAGGATCCCCTTAATCATAAAAAAGCAG |
| msp75 | 255 downstream3′ | ACACAAGCTTCCTCAGGCATTTTATTGGTGGC |
| msp75 | kan-bam5′ | TATAGGATCCTCTGCCTCGTGAAGAAGG |
| msp75 | kan-bam3′ | GAGAGGATCCAAAGCCACGTTGTGTCTC |
Restriction enzyme sites are underlined
The entire 2852 bp insert was amplified by PCR, subjected to agarose gel electrophoresis and the band was gel purified. The amplicon was used to transform M. catarrhalis O35E as described by Luke et al [22]. Briefly, a 100µl aliquot of bacterial suspension of strain O35E (OD600 0.2) was plated onto BHI agar and 20 ng of purified DNA was spotted onto the bacterial lawn. After incubation for 5 hours at 37°C in 5% CO2, the area of the bacterial lawn that had been spotted with the amplicon was swabbed onto a BHI plate containing 40 µg/ml kanamycin to select transformants. A mutant was obtained (O35E-Msp22−) and allelic exchange was verified by PCR analysis and sequence determination. The same strategy was used to knock out msp75 and construct a mutant in strain O35E that lacked the entire msp75 gene (O35E-Msp75−).
2.8. Enzyme-linked immunosorbent assay (ELISA)
ELISA was employed to quantitate the antibody responses following both subcutaneous and mucosal immunizations of separate groups of mice with the purified recombinant proteins. The optimal protein concentrations (2 to 10 µg) and starting dilutions were determined in preliminary assays. Wells of Immunolon 4 plates (Thermo Labsystems, Franklin, MA) were coated with purified recombinant protein by overnight incubation at room temperature. Plates were washed 4 times with phosphate buffered saline, 0.5% Tween 20 (PBS-tween) and then blocked using 2% nonfat dry milk in phosphate buffered saline for 1 hour at room temperature. After washing, dilutions of serum or bronchoalveolar lavage fluid in 2% nonfat dry milk in phosphate buffered saline plus 0.02% sodium azide were added to wells and incubated at room temperature overnight. Wells were washed 4 times with PBS-tween and horse radish peroxidase-conjugated anti-mouse IgG (1:3000) or anti-mouse IgA (1:2000) diluted in 2% nonfat dry milk in phosphate buffered saline was added. After incubating at room temperature for 4 to 6 hours, wells were washed and color developing reagent was added to the wells and incubated for 15 minutes in the dark. Color development was stopped using 4N H2SO4. Absorbance at 490nm was read using a BioRad microplate reader.
To calculate antibody concentrations, a standard curve was constructed on each microtiter plate and run simultaneously with experimental samples. Wells were coated with 1 µg/ml of anti-mouse IgG or anti-mouse IgA. Standardized amounts of IgG or IgA were incubated in coated wells overnight. Wells were washed 4 times with PBS-tween and peroxidase-conjugated anti-mouse IgG (1:3000) or anti-mouse IgA (1:2000) was added, followed by color developer as described above. Concentrations of IgG and IgA were determined based on standard curves using a four-parameter logistic method of calculation (Microplate Manager III, BioRad).
IgG and IgA levels in bronchoalveolar lavage fluid were expressed as a percentage of protein-specific antibody level as compared to total IgG or IgA to correct for volume differences obtained in the lavage fluid. To determine this, each sample was examined by ELISA twice, once to assay the amount of antibody that recognized the purified recombinant protein as described above, and a second time to determine the total amount of IgG or IgA in the sample. To measure total IgG and IgA, wells were coated with 1 µg/ml anti-mouse IgG or 1µg/ml anti-mouse IgA instead of recombinant protein. Total immunoglobulin concentrations were calculated from standard curves performed as described in the previous paragraph. To calculate the protein specific antibody level, the concentration of antibody to the recombinant protein was divided by the total antibody concentration individually for IgG and IgA.
2.9. Pulmonary clearance model
On day 35 of the subcutaneous immunization schedule noted above or day 28 of the intranasal immunization schedule, mice were challenged using an inhalational system [23]. All immunizations and procedures on mice were performed in compliance with Veterans Affairs Western New York Healthcare System IACUC guidelines. An overnight culture of M. catarrhalis O35E was diluted in phosphate buffer saline containing gelatin, calcium, and magnesium (PBSG; 137 mM NaCl, 2.7 mM KCl, 4.3 mM NaHPO4, 1.4 mM KH2PO4, 0.12 5mM CaCl2, 0.5 mM MgCl2, 0.1% gelatin, pH 7.3) to an OD600≈0.3–0.4 (~1×108 colony forming units/ml); 10 ml of the diluted culture was placed in the nebulizer of an Inhalational Exposure System model 099C A4212 (Glas-Col, Terre Haute, IN). An aliquot of culture was diluted and plated to determine the starting concentration of bacteria. The equipment settings were as follows: 10 minutes preheat, 40 minutes nebulization, 30 minutes cloud decay, 10 minutes decontamination, vacuum flow meter at 60 cubic feet/hour, compressed air flow meter at 10 cubic feet/hour.
Three hours post-challenge, the mice were anesthetized by inhalation of isoflurane and bled by retro-orbital puncture. Blood from each mouse was allowed to clot on ice. Serum was then isolated, heat inactivated at 56°C for 30 minutes, aliquoted, and frozen at −20°C.
After blood was collected from anesthetized mice, additional isoflurane was administered to ensure death. Bronchoalveolar lavage fluid was then collected by inserting a blunt 22 gauge needle into the trachea. A total of 2ml of PBSG was administered in 3 doses and the lavage fluid was collected by syringe aspiration. The fluid was filter sterilized and stored at −20°C.
Lungs were harvested, placed in 5 ml PBSG and homogenized on ice using a tissue homogenizer. Following homogenization, 250 µl of each lung homogenate was plated and incubated at 35°C with 5% CO2 overnight. Colonies were counted the following day to determine the concentration of bacteria in the lungs. Statistical significance was determined by performing two-tailed t-tests. A p value of ≤0.05 was considered significant.
3. Results
3.1. Characterization of mutants deficient in expression of Msp22 and Msp75
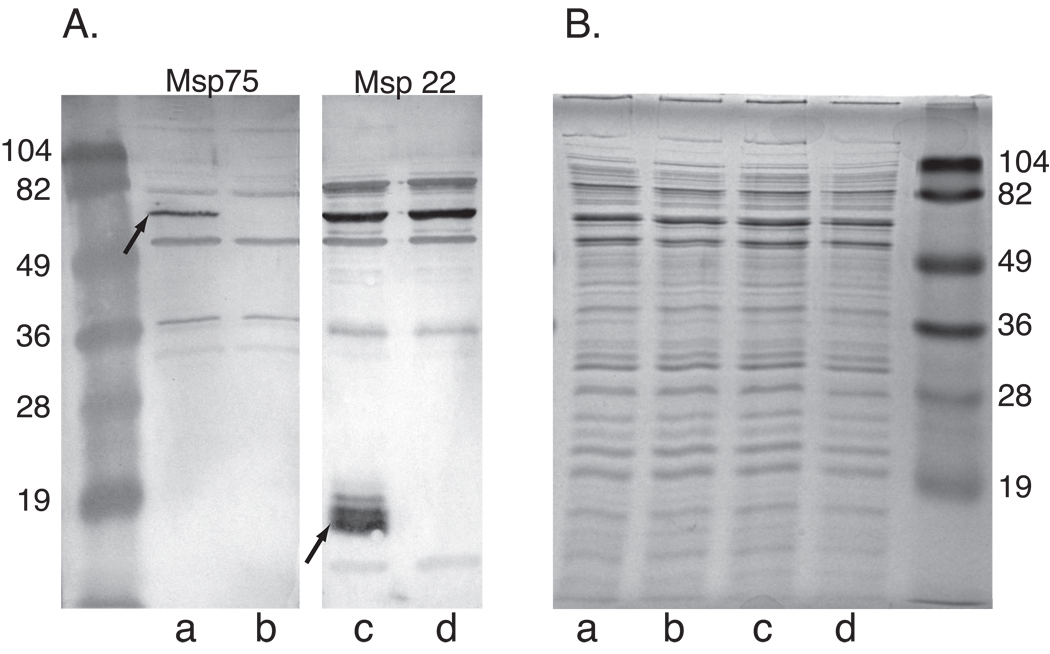
Mutants in which msp22 and msp75 were knocked out were constructed to facilitate the assessment of the specificity of antisera raised to the recombinant proteins. A mutant that completely lacks the gene that encodes Msp22 was created by homologous recombination. Sequencing through the insertion sites confirmed the absence of msp22 and the incorporation of the kanamycin cassette in place of the gene. The same strategy was used to generate a mutant that lacked the entire msp75 gene. Both mutants grew at the same rate as the parent strain in BHI broth. An immunoblot assay with rabbit antiserum to each of the two proteins showed that the corresponding proteins are absent in the mutants but present in the wild type parent strain (Figure 1). An SDS gel of a whole cell lysate of wild type and mutant strains show indistinguishable banding patterns without an obvious absence of bands in the mutant. This results suggests that Msp22 and Msp75 are relatively minor bands among many bands in whole cell lysates prepared under these conditions, or alternatively, that the proteins do not stain well with Coomassie blue in a whole cell lysate (Figure 1).
Figure 1.
Immunoblot assay (A) and Coomassie blue stained SDS gel (B). Lanes contain whole bacterial cell lysates a) O35E, b) O35E-Msp22−, c)O35E, d) O35E-Msp75−. The immunoblots were probed with rabbit antiserum to recombinant Msp75 (Lanes a and b) (1:1000) and rabbit antiserum to recombinant Msp22 (Lanes c and d)(1:1000) as noted. Immunoblots were incubated with peroxidase conjugated anti rabbit IgG and color was developed with horseradish peroxidase color developer. Molecular mass markers are noted in kilodaltons. Arrows denote native Msp22 and Msp75 in strain O35E.
3.2 Immunogenicity of purified recombinant proteins
The immunoblot assay in Figure 1 of rabbit antisera raised individually to the purified recombinant proteins Msp22 and Msp75 shows that antibodies resulting from immunization with recombinant protein recognize bands corresponding to the sizes of Msp22 and Msp75. This observation, in combination with the absence of the corresponding bands in the mutants (Figure 1 lanes b and d), confirms that the antisera contain antibodies that recognize the native proteins. The additional bands in these immunoblots may represent either antibodies to cross reactive proteins or induction of non specific antibodies following immunization with proteins and adjuvant. We conclude that rabbit antibodies raised to recombinant proteins Msp22 and Msp75 recognize the native proteins in M. catarrhalis.
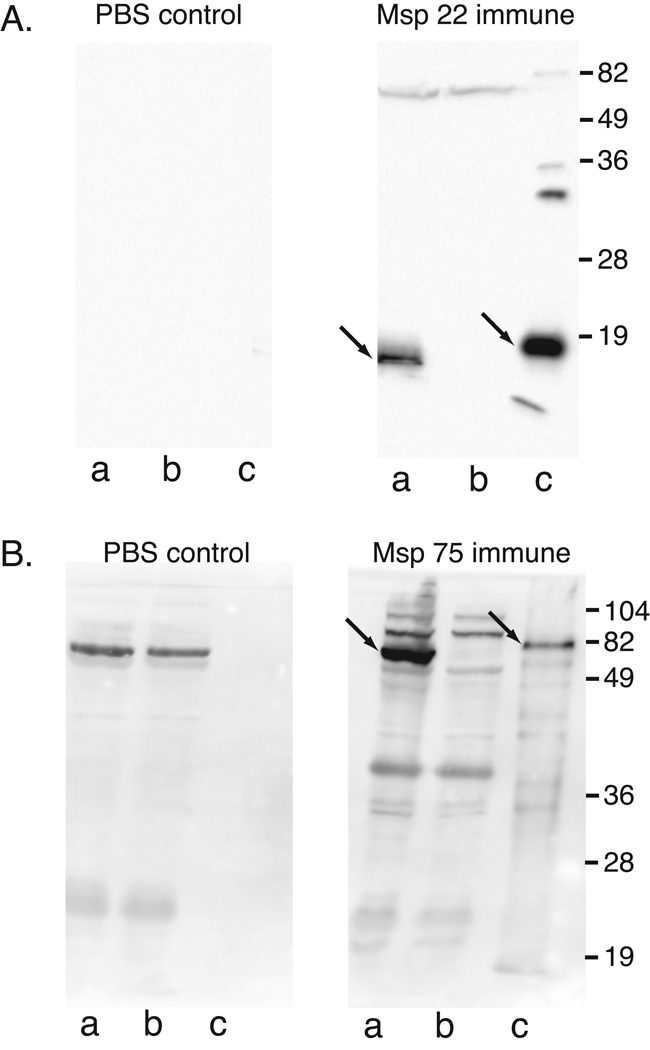
To further assess the immunogenicity of the purified recombinant proteins, antisera raised in mice were subjected to immunoblot assay and ELISA. Figure 2 shows that mouse antibodies raised by immunization with purified recombinant proteins recognize both recombinant (lanes c) and native proteins (lanes a). The presence of bands at the predicted size in the wild type parent strain (lanes a) in combination with the absence of the corresponding bands in the mutants (lanes b) confirms the specificity of the antibodies for the native proteins. The PBS control immunoblot assays which were probed with sera from mice immunized with adjuvant in PBS showed absence of antibodies to both recombinant and native proteins. The presence of background bands in the PBS control panel corresponding to the antiserum to Msp 75 is due to the longer exposure time required to develop the blots compared to the blots for Msp22. The slightly higher apparent molecular mass of the recombinant proteins compared to native proteins is due to the histidine tag on the recombinant proteins. We conclude that mouse antibodies raised individually to purified recombinant Msp22 and Msp75 recognize both recombinant proteins and native proteins in M. catarrhalis.
Figure 2.
Immunoblot assays of 15% SDS gels (A) and 12% SDS gels (B). A: lanes contain a) whole cell lysate of M. catarrhalis O35E, b) whole cell lysate of mutant O35EMsp22−, c) lysate of E. coli expressing recombinant Msp22. Blots were probed with pooled serum (1:10,000) from mice immunized with purified recombinant Msp22 plus adjuvant (Msp 22 immune) and with pooled serum (1:10,000) from mice immunized with PBS plus adjuvant (PBS control). B: Lanes contain a) whole cell lysate of M. catarrhalis O35E, b) whole cell lysate of mutant O35E-Msp75−, c) purified recombinant Msp75. Blots were probed with pooled serum (1:10,000) from mice immunized with purified recombinant Msp75 plus adjuvant (Msp 75 immune) and with pooled serum (1:10,000) from mice immunized with PBS plus adjuvant (PBS control). All immunoblots were probed with peroxidase conjugated anti mouse IgG and developed with chemiluminescence. Molecular mass markers are noted on the right in kilodaltons. Arrows denote native and recombinant Msp22 and Msp 75.
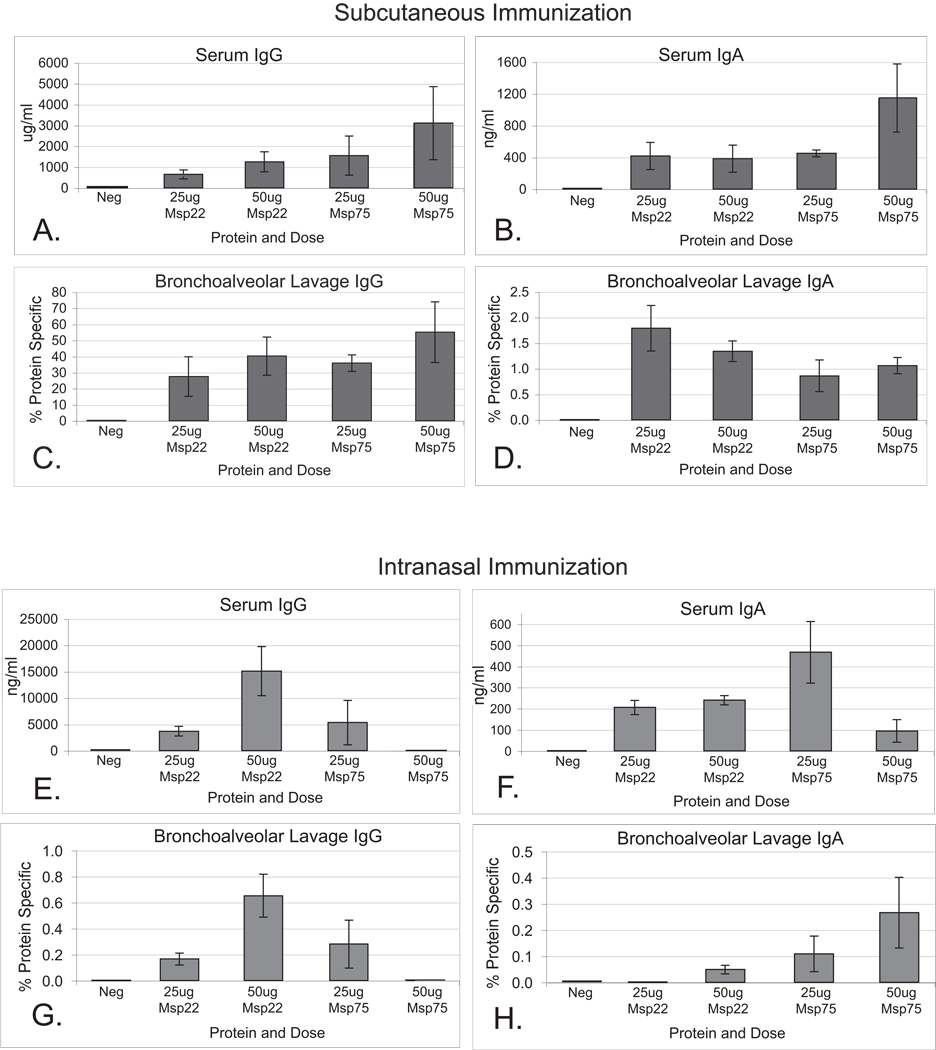
ELISAs were employed to further assess antibody responses in mice to the recombinant proteins following subcutaneous and intranasal (Figure 3) immunization. To correct for volume differences obtained in the lavage fluid, IgG and IgA levels in bronchoalveolar lavage fluids were expressed as a percentage of protein-specific antibody level by dividing the protein specific antibody concentration by total antibody concentration for both IgG and IgA.
Figure 3.
Panels A –D. Results of quantitative ELISA measuring IgG and IgA levels to their respective recombinant proteins in serum (A and B) and bronchoalveolar lavage fluids (C and D) from mice immunized subcutaneously with purified recombinant proteins Msp22 and Msp75. For bronchoalveolar lavage fluids, y-axes represent protein-specific IgG or IgA divided by the total amount of IgG or IgA in the sample. Error bars represent standard error of the mean (n=5).
Panels E –H. Results of quantitative ELISA measuring IgG and IgA levels to their respective recombinant proteins in serum (E and F) and bronchoalveolar lavage fluids (G and H) from mice immunized intranasally with purified recombinant proteins Msp22 and Msp75. For bronchoalveolar lavage fluids, y-axes represent protein-specific IgG or IgA divided by the total amount of IgG or IgA in the sample. Error bars represent standard error of the mean (n=5).
Immunization with purified recombinant Msp22 and Msp75 induced an antibody response with measurable levels of IgG and IgA antibodies in the serum and bronchoalveolar lavage fluids as compared to the adjuvant control mice, which had undetectable levels (Figure 3). Subcutaneous immunizations induced approximately one log greater serum IgG level as compared to intranasal immunization, as expected. A trend towards dose dependence was seen in the serum IgG levels for both proteins with subcutaneous immunization. Both routes of immunization generated IgG and IgA antibodies in bronchoalveolar lavage fluid. Subcutaneous immunization induced a greater percentage of antigen specific IgG in the bronchoalveolar lavage fluids compared to intranasal immunization. Intranasal immunization resulted in protein specific IgA antibodies in the bronchoalveolar lavage fluids that were all less than 2% of the total IgA, a lower proportion than expected. Overall we conclude that purified recombinant Msp22 and Msp75 were both immunogenic in mice when administered by systemic and mucosal routes.
3.4. Expression of Msp22 and Msp75 in heterologous strains
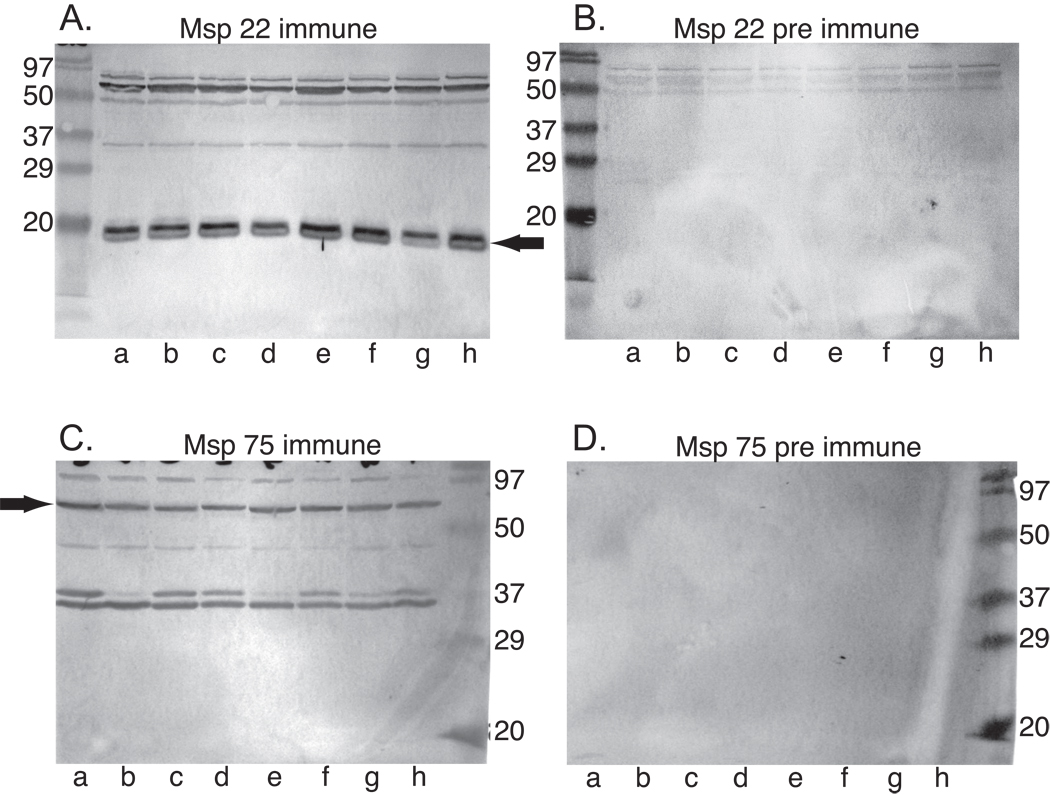
We investigated whether Msp22 and Msp75 are expressed by heterologous strains of M. catarrhalis and the degree to which antibodies raised to the purified recombinant proteins recognized the native proteins in heterologous strains. Immunoblot assays were performed by probing whole bacterial cell lysates of heterologous isolates, including strains isolated from middle ear fluid of children with otitis media and from the sputum of adults experiencing exacerbations of COPD. Figure 4 shows that rabbit antiserum raised to the purified recombinant proteins contain antibodies that recognize the corresponding native proteins in 8 of 8 strains of M. catarrhalis. The bands are of similar intensity among all strains. The immunoblot assays of immune sera show the presence bands in addition to those corresponding to Msp22 and Msp75. These likely represent either cross reacting proteins or induction of non specific antibodies following immunization with proteins and adjuvant. We conclude that Msp22 and Msp75 are expressed by multiple strains of M. catarrhalis and that antibodies raised to the purified recombinant proteins recognize the corresponding native proteins in multiple strains.
Figure 4.
Immunoblot assays probed with rabbit antisera (1:4000) to recombinant Msp22 (A) and Msp75 (C). Panels B and D were probed with corresponding pre immune sera (1:4000). Lanes contain whole bacterial cell lysates of M. catarrhalis strains a) O35E, b) 43617, c) 6P29B1, d) 102P19B1, e) 7P94B1, f) 2951, g) 8184, h) 7169. Immunoblots were probed with peroxidase conjugated anti rabbit IgG and color was developed with horseradish peroxidase color developer. Molecular mass markers are noted in kilodaltons. Arrows denote native Msp22 and Msp75.
3.5. Enhancement of pulmonary clearance
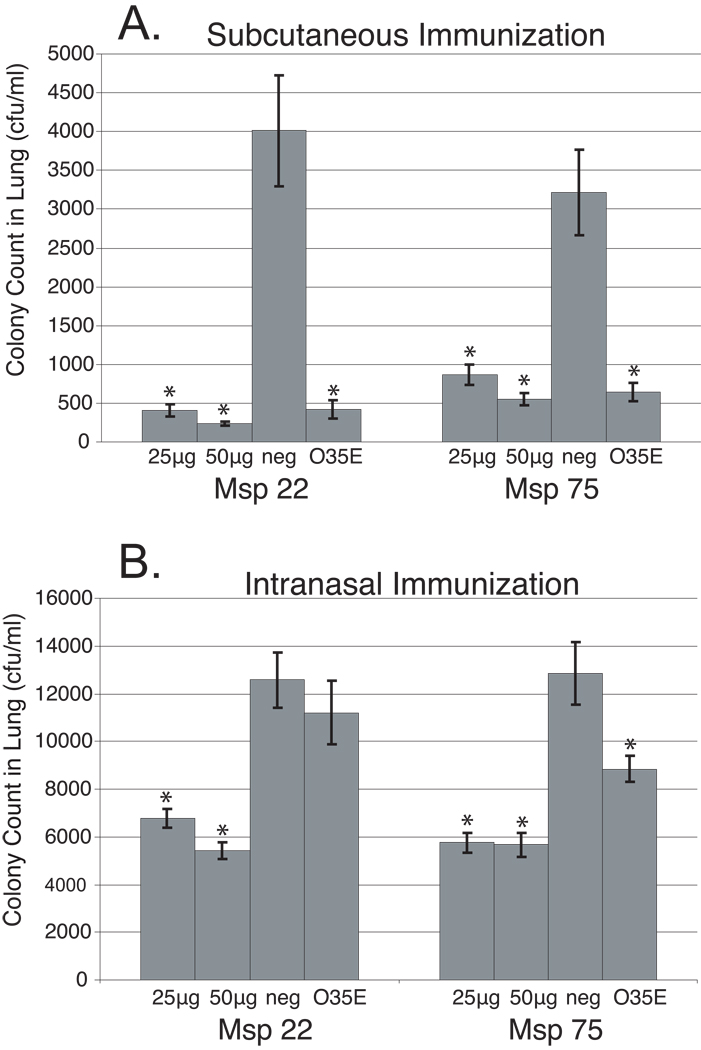
To determine whether immunization with purified recombinant Msp22 and/or Msp75 would enhance pulmonary clearance of M. catarrhalis in mice, groups of mice were immunized three times each either subcutaneously or intranasally. Positive control mice were immunized with formalin-killed M. catarrhalis O35E, because immunization with whole organism is known to induce enhanced clearance in this model [16, 24]. Mice were challenged with live bacteria one week after their last boost. Following challenge with M. catarrhalis, the quantity of bacteria recovered from the lungs of mice that received the recombinant proteins was compared with control mice immunized with adjuvant alone. Two-tailed t-tests were performed to assess statistical significance of clearance of bacteria from the lungs between protein immunized groups compared to negative control groups (Figure 5).
Figure 5.
Results of pulmonary clearance after aerosol challenge with M. catarrhalis following subcutaneous immunization (A) and intranasal immunization (B) of mice with purified recombinant proteins as noted in the x-axes. Error bars represent the standard error of the mean (n=5). * p-value < 0.05 as compared to the negative control (neg).
Groups of mice immunized with purified recombinant Msp22 showed significantly greater clearance of bacteria from lungs compared to sham (adjuvant) immunized mice for both the 25 µg and 50 µg doses via the subcutaneous route (Figure 5, Panel A). In addition, subcutaneous immunization with recombinant purified Msp75 showed significantly greater clearance compared to sham (adjuvant) immunized mice for both the 25 µg and 50 µg doses (Figure 5, panel A).
Mice immunized intranasally with recombinant purified Msp22 showed enhanced clearance of bacteria from the lungs compared to sham (adjuvant) immunized mice with both the 25 µg and 50 µg doses (Figure 5, Panel B). Intranasal immunization with purified recombinant Msp75 also induced enhanced clearance of bacteria from the lungs compared to sham (adjuvant) immunized mice for both the 25 µg and 50 µg doses (Figure 5, Panel B).
The enhanced clearance observed was one half to one log of bacteria, a level consistent with previous studies utilizing this model [15–17, 25]. Enhanced clearance was observed in separate groups of animals with both 25 µg and 50 µg doses for both Msp22 and Msp 75. Furthermore, assessment of subcutaneous and intranasal immunization on pulmonary clearance were performed as independent experiments and each induced statistically significant enhanced clearance at two doses. Finally the experiments depicted in Figure 5 representing 4 separate groups of animals for each of the proteins (2 doses, 2 routes of immunization) were repeated and a similar level of enhanced clearance was observed in all groups (data not shown). Therefore we conclude that immunization individually with purified recombinant Msp22 and Msp75 induced enhanced pulmonary clearance following immunization both by a systemic (subcutaneous) and a mucosal (intranasal) route.
4. Discussion
Msp22 and Msp75 are two novel proteins of M. catarrhalis that were identified in previous work using a genome mining approach based on sequence analyses to predict proteins that are likely to be on the bacterial surface. Additional characterization showed that the sequences of these proteins were highly conserved among strains (99% and 97% amino acid identity, respectively). Finally, both proteins are expressed by M. catarrhalis in the human respiratory tract [18].
The present study assessed these two proteins as potential vaccine antigens. The work shows 1) Purified recombinant Msp22 and Msp75 were immunogenic in rabbits with systemic immunization and in mice with both systemic and mucosal immunization. 2) Immunization with purified recombinant Msp22 and Msp75 induced antibodies that recognize the corresponding native proteins. 3) Antibodies raised to purified recombinant Msp22 and Msp75 recognize the native proteins in multiple strains of M. catarrhalis. And 4) systemic and mucosal immunization of mice with purified recombinant Msp22 and Msp75 induced enhanced pulmonary clearance in a mouse model.
M. catarrhalis is an exclusively human pathogen. Adults with COPD make systemic and mucosal antibody responses to M. catarrhalis and apparently develop strain specific protection following clearance from the respiratory tract [6]. However, little is known about the elements of a protective immune response to M. catarrhalis. For example, no good correlate of protection such as bactericidal antibody or osponizing antibody has been identified for M. catarrhalis. A further obstacle to determining protective immune responses to M. catarrhalis is the lack of an animal model that simulates human infection [1, 2].
Currently, the mouse pulmonary clearance model used in the present study is the most widely used model to assess the ability of antigens to generate a potentially protective response [2, 16, 23, 26–28]. In this model, mice are challenged with bacteria, and after a period of 3 hours, lungs are harvested and clearance of bacteria is determined. A limitation of the model is that mice do not develop pneumonia. Rather, the endpoint is clearance of bacteria from the respiratory tract. This model is simple, reproducible, and allows for examination of a functional response. The degree of enhancement of pulmonary clearance observed in this study was consistently between one half-log to one log difference between the adjuvant control and recombinant protein immunized groups of mice. This level is quite similar to the level of clearance demonstrated by other groups who have studied putative vaccine antigens of M. catarrhalis in the mouse pulmonary clearance model [15–17, 25].
To date, a limited number of vaccine antigens have been demonstrated to induce a potentially protective response in this model, including UspA1, UspA2, Hag, OMP CD, TbpB, CopB, and dLOS [12, 14–17, 25, 29–32]. All of these antigens, except OMP CD, show substantial sequence heterogeneity among strains of M. catarrhalis. Therefore the high degree of sequence conservation of Msp22 and Msp75 makes them attractive vaccine antigens for further testing.
Alternatives to the mouse pulmonary clearance model include chinchilla and SCID mouse models. The chinchilla model of otitis media has been quite valuable in studies of Streptococcus pneumoniae and Haemophilus influenzae. However, M. catarrhalis is cleared readily following instillation into the chinchilla middle ear, precluding the use of the model to study otitis media due to M. catarrhalis. [33, 34]. Chinchillas have been used to assess nasopharyngeal colonization with M. catarrhalis so may be useful for examining inhibition or disruption of colonization, an important initial step in the development of otitis media in humans [33]. The systemic SCID mouse model of infection is even less favored as the mice often succumb to infection with postmortem findings that are not consistent with human respiratory tract infections [35].
Both Msp22 and Msp75 were immunogenic when administered systemically and mucosally. In both rabbits and mice, immunization with recombinant proteins induced antibodies that recognized the native proteins. Not surprisingly, systemic immunization produced a substantially greater level of serum IgG antibodies. IgG detected in bronchoalveolar fluid was most likely due to leakage across the mucosal surface from the serum.
The group of mice immunized intranasally with the 50 µg dose of Msp75 did not generate a serum IgG antibody response whereas the 25 µg group of immunized mice did (Figure 3). We do not have a good explanation for this observation; it may represent an aberrant response by this group of mice. All other groups of mice and both rabbits immunized systemically with purified recombinant Msp75 developed serum IgG to the protein. In spite of undetectable levels of serum IgG in this single group immunized intranasally, the animals demonstrated enhanced pulmonary clearance in the mouse. The observation that IgG antibodies are not present in bronchoalveolar lavage fluid in this group supports the hypothesis that the IgG antibodies in bronchoalveolar lavage fluid are due to transudation of IgG from the serum.
The relatively low IgA levels observed when Msp22 and Msp75 were administered intranasally was somewhat unexpected and apparently indicate that the proteins did not stimulate robust IgA production when presented at mucosal inductive sites under the conditions used in this study. To overcome this, the proteins could be coupled to a carrier that targets M cells [36]. Alternatively, antigen-adjuvant formulations other than cholera toxin administered intranasally could also be adjusted to induce a greater IgA response. In spite of modest mucosal IgA responses, intranasal immunization induced enhanced pulmonary clearance.
In summary, we characterized the immunogenicity of two highly conserved putative vaccine antigens of M. catarrhalis, Msp22 and Msp75. Both purified recombinant proteins were immunogenic in rabbits and mice with subcutaneous immunization and in mice with intranasal immunization. Both proteins are expressed in multiple strains and antibodies raised to purified recombinant proteins bound to native proteins that are present in multiple strains of M. catarrhalis. Finally, in vivo pulmonary clearance studies demonstrated that immunization with both purified recombinant Msp22 and Msp75 individually enhanced pulmonary clearance in a mouse model. This observation suggests that immunization with either of these two proteins generates an immune response that mediates bacterial clearance from the lungs. The conservation among strains, immunogenicity, and the induction of protective responses in the mouse pulmonary clearance model collectively indicate that Msp22 and Msp75 may make good vaccine antigens. These proteins should be studied further as promising vaccine antigens of M. catarrhalis.
Acknowledgements
We thank Rebecca Benz for her expert assistance in the animal facility and Karen Ostberg for her expertise and assistance in collecting bronchoalveolar lavage fluids.
This work was supported by NIH grant AI28304 (TFM) from the National Institutes of Allergy and Infectious Diseases and by the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murphy TF, Paramswaran GI. Moraxella catarrhalis, a human respiratory tract pathogen. Clin Infect Dis. 2009;49:124–131. doi: 10.1086/599375. [DOI] [PubMed] [Google Scholar]
- 2.Murphy TF. Vaccine development for non-typeable Haemophilus influenzae and Moraxella catarrhalis: progress and challenges. Expert Rev Vaccines. 2005;4(6):843–853. doi: 10.1586/14760584.4.6.843. [DOI] [PubMed] [Google Scholar]
- 3.Klein JO. Otitis media. Clin Infect Dis. 1994;19:823–833. doi: 10.1093/clinids/19.5.823. [DOI] [PubMed] [Google Scholar]
- 4.American Academy of Pediatrics. Diagnosis and management of acute otitis media. Pediatrics. 2004;113(5):1451–1465. doi: 10.1542/peds.113.5.1451. [DOI] [PubMed] [Google Scholar]
- 5.Teele DW, Klein JO, Chase C, Menyuk P, Rosner BA Greater Boston Otitis Media Study Group. Otitis media in infancy and intellectual ability, school achievement, speech, and language at age 7 years. J Infect Dis. 1990;162:685–694. doi: 10.1093/infdis/162.3.685. [DOI] [PubMed] [Google Scholar]
- 6.Murphy TF, Brauer AL, Grant BJ, Sethi S. Moraxella catarrhalis in chronic obstructive pulmonary disease. Burden of disease and immune response. Am J Respir Crit Care Med. 2005;172:195–199. doi: 10.1164/rccm.200412-1747OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sethi S, Evans N, Grant BJB, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347:465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 8.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359(22):2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 9.Mannino DM. COPD: epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest. 2002;121 5 Suppl:121S–126S. doi: 10.1378/chest.121.5_suppl.121s. [DOI] [PubMed] [Google Scholar]
- 10.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370(9589):765–773. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 11.Adlowitz DG, Hiltke T, Lesse AJ, Murphy TF. Identification and characterization of outer membrane proteins G1a and G1b of Moraxella catarrhalis. Vaccine. 2004;22(20):2533–2540. doi: 10.1016/j.vaccine.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Aebi C, Maciver I, Latimer JL, Cope LD, Stevens MK, Thomas SE, et al. A protective epitope of Moraxella catarrhalis is encoded by two different genes. Infect Immun. 1997;65:4367–4377. doi: 10.1128/iai.65.11.4367-4377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen D, McMichael JC, van der Meid KR, Hahn D, Mininni T, Cowell J, et al. Evaluation of purified UspA from Moraxella catarrhalis as a vaccine in a murine model after active immunization. Infect Immun. 1996;64:1900–1905. doi: 10.1128/iai.64.6.1900-1905.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen D, McMichael JC, van der Meid KR, Masi AW, Bortell E, Caplan JD, et al. Evaluation of a 74-kDa transferrin-binding protein from Moraxella (Branhamella) catarrhalis as a vaccine candidate. Vaccine. 1999;18(1–2):109–118. doi: 10.1016/s0264-410x(99)00188-7. [DOI] [PubMed] [Google Scholar]
- 15.Forsgren A, Brant M, Riesbeck K. Immunization with the truncated adhesion Moraxella catarrhalis immunoglobulin D-binding protein (MID764-913) is protective against M. catarrhalis in a mouse model of pulmonary clearance. J Infect Dis. 2004;190(2):352–355. doi: 10.1086/422155. [DOI] [PubMed] [Google Scholar]
- 16.Murphy TF, Kyd JM, John A, Kirkham C, Cripps AW. Enhancement of pulmonary clearance of Moraxella (Branhamella) catarrhalis following immunization with outer membrane protein CD in a mouse model. J Infect Dis. 1998;178:1667–1675. doi: 10.1086/314501. [DOI] [PubMed] [Google Scholar]
- 17.Liu DF, McMichael JC, Baker SM. Moraxella catarrhalis outer membrane protein CD elicits antibodies that inhibit CD binding to human mucin and enhance pulmonary clearance of M. catarrhalis in a mouse model. Infect Immun. 2007;75(6):2818–2825. doi: 10.1128/IAI.00074-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruckdeschel EA, Kirkham C, Lesse AJ, Hu Z, Murphy TF. Mining the Moraxella catarrhalis genome: identification of potential vaccine antigens expressed during human infection. Infect Immun. 2008;76(4):1599–1607. doi: 10.1128/IAI.01253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cullen PA, Lo M, Bulach DM, Cordwell SJ, Adler B. Construction and evaluation of a plasmid vector for the expression of recombinant lipoproteins in Escherichia coli. Plasmid. 2003;49(1):18–29. doi: 10.1016/s0147-619x(02)00150-6. [DOI] [PubMed] [Google Scholar]
- 20.Adlowitz DG, Sethi S, Cullen P, Adler B, Murphy TF. Human antibody response to outer membrane protein G1a, a lipoprotein of Moraxella catarrhalis. Infect Immun. 2005;73(10):6601–6607. doi: 10.1128/IAI.73.10.6601-6607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menard R, Sansonetti PJ, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993;175(18):5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luke NR, Howlett AJ, Shao J, Campagnari AA. Expression of type IV pili by Moraxella catarrhalis is essential for natural competence and is affected by iron limitation. Infect Immun. 2004;72(11):6262–6270. doi: 10.1128/IAI.72.11.6262-6270.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu W-G, Chen J, Collins FM, Gu X-X. An aerosol challenge mouse model for Moraxella catarrhalis. Vaccine. 2000;18:799–804. doi: 10.1016/s0264-410x(99)00335-7. [DOI] [PubMed] [Google Scholar]
- 24.Kyd J, John A, Cripps A, Murphy TF. Investigation of mucosal immunisation in pulmonary clearance of Moraxella (Branhamella) catarrhalis. Vaccine. 2000;18:398–406. doi: 10.1016/s0264-410x(99)00262-5. [DOI] [PubMed] [Google Scholar]
- 25.Becker PD, Bertot GM, Souss D, Ebensen T, Guzman CA, Grinstein S. Intranasal vaccination with recombinant outer membrane protein CD and adamantylamide dipeptide as the mucosal adjuvant enhances pulmonary clearance of Moraxella catarrhalis in an experimental murine model. Infect Immun. 2007;75(4):1778–1784. doi: 10.1128/IAI.01081-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verghese A, Berro E, Berro J, Franzus BW. Pulmonary clearance and phagocytic cell response in a murine model of Branhamella catarrhalis infection. J Infect Dis. 1990;162:1189–1192. doi: 10.1093/infdis/162.5.1189. [DOI] [PubMed] [Google Scholar]
- 27.Maciver I, Unhanand M, McCracken GH, Jr., Hansen EJ. Effect of immunization on pulmonary clearance of Moraxella catarrhalis in an animal model. J Infect Dis. 1993;168:469–472. doi: 10.1093/infdis/168.2.469. [DOI] [PubMed] [Google Scholar]
- 28.Unhanand M, Maciver I, Ramilo O, Arencibia-Mireles O, Argyle JC, McCracken GH, Jr., et al. Pulmonary clearance of Moraxella catarrhalis in an animal model. J Infect Dis. 1992;165:644–650. doi: 10.1093/infdis/165.4.644. [DOI] [PubMed] [Google Scholar]
- 29.Helminen ME, Maciver I, Latimer JL, Klesney-Tait J, Cope LD, Paris M, et al. A large, antigenically conserved protein on the surface of Moraxella catarrhalis is a target for protective antibodies. J Infect Dis. 1994;170:867–872. doi: 10.1093/infdis/170.4.867. [DOI] [PubMed] [Google Scholar]
- 30.Helminen ME, Maciver I, Latimer JL, Cope LD, McCracken GH, Jr., Hansen EJ. A major outer membrane protein of Moraxella catarrhalis is a target for antibodies that enhance pulmonary clearance of the pathogen in an animal model. Infect Immun. 1993;61:2003–2010. doi: 10.1128/iai.61.5.2003-2010.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiao X, Hirano T, Hou Y, Gu XX. Specific immune responses and enhancement of murine pulmonary clearance of Moraxella catarrhalis by intranasal immunization with a detoxified lipooligosaccharide conjugate vaccine. Infect Immun. 2002;70(11):5982–5989. doi: 10.1128/IAI.70.11.5982-5989.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu WG, Berry J, Chen J, Gu XX. Exploration of Moraxella catarrhalis outer membrane proteins, CD and UspA, as new carriers for lipooligosaccharide-based conjugates. FEMS Immunol Med Microbiol. 2004;41(2):109–115. doi: 10.1016/j.femsim.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Bakaletz LO, Murwin DM, Billy JM. Adenovirus serotype 1 does not act synergistically with Moraxella (Branhamella) catarrhalis to induce otitis media in the chinchilla. Infect Immun. 1995;63:4188–4190. doi: 10.1128/iai.63.10.4188-4190.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung M-H, Enrique R, Lim DJ, DeMaria TF. Moraxella (Branhamella) catarrhalis-induced experimental otitis media in the chinchilla. Acta Otolaryngol. 1994;114:415–422. doi: 10.3109/00016489409126080. [DOI] [PubMed] [Google Scholar]
- 35.Harkness RE, Guimond M-J, McBey B-A, Klein MH, Percy DH, Croy BA. Branhamella catarrhalis pathogenesis in SCID and SCID/beige mice. APMIS. 1993;101:805–810. [PubMed] [Google Scholar]
- 36.van Ginkel FW, Nguyen HH, McGhee JR. Vaccines for mucosal immunity to combat emerging infectious diseases. Emerg Infect Dis. 2000;6(2):123–132. doi: 10.3201/eid0602.000204. [DOI] [PMC free article] [PubMed] [Google Scholar]