Abstract
Consumption of ω-3 fatty acids from fish oil, specifically eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), decreases risk for heart failure and attenuates pathologic cardiac remodeling in response to pressure overload. Dietary supplementation with EPA+DHA may also impact cardiac mitochondrial function and energetics through alteration of membrane phospholipids. We assessed the role of EPA+DHA supplementation on left ventricular (LV) function, cardiac mitochondrial membrane phospholipid composition, respiration, and sensitivity to mitochondrial permeability transition pore (MPTP) opening in normal and infarcted myocardium. Rats were subjected to sham surgery or myocardial infarction by coronary artery ligation (n=10–14), and fed a standard diet, or supplemented with EPA+DHA (2.3% of energy intake) for 12 weeks. EPA+DHA altered fatty acid composition of total mitochondrial phospholipids and cardiolipin by reducing arachidonic acid content and increasing DHA incorporation. EPA+DHA significantly increased calcium uptake capacity in both subsarcolemmal and intrafibrillar mitochondria from sham rats. This treatment effect persisted with the addition of cyclosporin A, and was not accompanied by changes in mitochondrial respiration or coupling, or cyclophilin D protein expression. Myocardial infarction resulted in heart failure as evidenced by LV dilation and contractile dysfunction. Infarcted LV myocardium had decreased mitochondrial protein yield and activity of mitochondrial marker enzymes, however respiratory function of isolated mitochondria was normal. EPA+DHA had no effect on LV function, mitochondrial respiration, or MPTP opening in rats with heart failure. In conclusion, dietary supplementation with EPA+DHA altered mitochondrial membrane phospholipid fatty acid composition in normal and infarcted hearts, but delayed MPTP opening only in normal hearts.
Keywords: eicosapentaenoic acid, docosahexaenoic acid, myocardial infarction, mitochondrial permeability transition pore
1. Introduction
Clinical studies show that the ω-3 polyunsaturated fatty acids found in fish oil (eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)) exert beneficial effects on the heart, as evidenced by a decreased risk of ischemic heart disease and sudden cardiac death[1;2], and a lower incidence of heart failure[3;4]. Recently, the GISSI heart failure trial found that a low dose of EPA+DHA (0.85 g/day) reduced the risk for mortality and hospitalization in heart failure patients compared with placebo over a 3.9 year period[5]. The mechanisms for the observed beneficial effects of EPA+DHA on the heart are not clear, but a possible mediator is alterations in cardiac phospholipid composition. We recently found that dietary supplementation with EPA+DHA increased DHA and EPA and decreased arachidonic acid (a precursor to inflammatory eicosanoids) in whole tissue cardiac phospholipids[6]. However, changes in whole tissue phospholipids are difficult to interpret, and targeted analysis of specific organelles, particularly mitochondria, have not been reported.
In advanced heart failure, mitochondria exhibit either normal[7–10] or decreased [11–13] respiration and oxidative phosphorylation, and have a greater susceptibility to opening of the mitochondrial permeability transition pore (MPTP)[14;15]. The MPTP is a large diameter, high conductance, voltage-dependent channel that allows passage of water, ions, and large molecules, resulting in mitochondrial swelling and triggering of cardiomyocyte apoptosis[16–18]. High extra-mitochondrial Ca2+ and oxidative stress synergistically trigger MPTP opening, whereas Ca2+ chelation causes it to rapidly close. Previous studies suggest that MPTP opening is affected by phospholipid composition, specifically the release of arachidonic acid and the content of cardiolipin (CL) in mitochondrial membranes[19–21]. CL is an inner membrane tetra-acyl phospholipid that is essential for normal mitochondrial respiration and is decreased in heart failure[22]. Most CL is composed of four linoleic acid side chains (tetralinoleoyl CL or L4CL), which is considered the optimal structure, as substitution with long chain saturated and monounsaturated fatty acids impairs mitochondrial function[22;23]. Dietary supplementation with EPA+DHA can increase CL in cardiac mitochondria[24;25], which could affect MPTP opening. Since MPTP opening is strongly associated with cardiomyocyte death and LV dysfunction in ischemia/reperfusion and heart failure[17;18;26], it is important to determine if EPA+DHA affects MPTP opening in normal and pathological conditions.
Supplementation with EPA+DHA attenuates pathological LV hypertrophy and development of heart failure under conditions of pressure overload in a rat model[6;27–29]. However clinical heart failure is frequently the result of ischemic heart disease and myocardial infarction rather than hypertension-induced LV hypertrophy. Previous studies in the coronary artery ligation infarct-induced heart failure model in the rats found a modest depletion of linoleic acid from total cardiac phospholipids[30], and either no effect on mitochondrial function[7–10] or a significant decrease in respiratory capacity[31]. The effects of infarct-induced heart failure on mitochondrial phospholipid composition have not been reported, nor has the response to dietary supplementation with EPA+DHA. Thus in the present study we assessed the effects of long term treatment with EPA+DHA on phospholipid fatty acid composition and function in cardiac mitochondria from rats subjected to sham surgery or infarct-induced heart failure. We hypothesized that EPA+DHA would: 1) decrease arachidonic acid in mitochondrial phospholipids, 2) maintain total CL and L4CL, 3) delay MPTP opening in response to Ca2+, and 4) prevent LV remodeling in response to myocardial infarction,
2. Methods
2.1. Experimental Design
Investigators were blinded to treatment when measurements were performed. The animal protocol was conducted according to the Guideline for the Care and Use of Laboratory Animals (NIH publication No. 85–23) and was approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee. The animals were maintained on a reverse 12-hour light-dark cycle and all procedures were performed in the fed state between 3 and 6 hours from the start of the dark phase. Male Sprague-Dawley rats weighing approximately 330 g were subjected to myocardial infarction or sham surgery (n=10–14). One week after surgery, the rats were fed a standard chow (STD) or modified standard chow containing fish oil composed primarily of EPA and DHA (EPA+DHA). The rats were maintained on the diet for 12 weeks. LV function was analyzed by echocardiography at 6 and 11 weeks after dietary assignment. Twelve weeks after assignment to dietary treatment, rats were anesthetized with isoflurane, blood and urine were drawn, and the heart was harvested for biochemical analysis.
2.2. Myocardial Infarction
Heart failure was induced by myocardial infarction as previously described[32]. Briefly, rats were anesthetized with 1.5–2.0% isoflurane, intubated and ventilated. An infarct was induced by ligation of the left coronary artery and sham animals were subjected to the same surgical procedure without coronary artery ligation.
2.3. Diets
Both chows were custom-manufactured (Research Diets Inc. New Brunswick, NJ). The STD and EPA+DHA diets both derived 70% of total energy from carbohydrate (40% of total energy from cornstarch, 5% from maltodextrin and 25% from sucrose), 20% protein (casein supplemented with L-cystine) and 10% energy from fat. In the STD diet, fat was made up of 67% lard and 33% soybean oil. The EPA+DHA diet derived 2.3% of the total energy as EPA+DHA (3.3% fish oil that was comprised of 21% EPA and 49% DHA by mass; Ocean Nutrition, Dartmouth, Nova Scotia, Canada), with the balance of fat from lard and soybean oil. The fish oil dose corresponds to a human intake of approximately 5.1 g/d of EPA+DHA (calculated assuming an energy intake of 2000 kcal/d).
2.4. Echocardiography
LV function was assessed using a Vevo 770 High-Resolution Imaging Systems (Visual Sonics) with a 15-MHz linear array transducer (model 716). Anesthetized rats were shaved and placed supine on a warming pad. Two-dimensional cine loops and guided M-mode frames were recorded from the parasternal short and long axis. At the end of the study, all data were analyzed offline with software resident on the ultrasound system, and calculations were made to determine LV volumes as previously described[6]. Ejection fraction was calculated as: (EDV-ESV)/EDV × 100, where EDV is the end diastolic volume and ESV is the end systolic volume. Relative wall thickness is calculated by the equation: (PWTs+AWTs)/EDD, where PWTs is the systolic posterior wall thickness and AWTs is the systolic anterior wall thickness, using measurements made from the long axis parasternal view.
2.5. Metabolic and Biochemical Parameters
Free fatty acids, triglycerides, and glucose (Wako, Richmond, VA)) were assessed in the plasma and creatinine was assessed in the urine (Cayman Chemical, Ann Arbor, MI) using enzymatic spectrophotometric methods. Enzyme-linked immunosorbent assays were used to measure TNFα (Alpco Diagnostics, Salem, NH) and adiponectin (Millipore, St. Charles, MO) levels in the serum, and thromboxane B2 in the urine (Cayman Chemical, Ann Arbor, MI). The accumulation of the lipid peroxidation products malondialdehyde (MDA) and 4-hydroxyalkenals (HAE) was measured using a colorimetric microplate assay (Oxford Biomedical Research, Oxford, MI). Powdered LV tissue was assessed based on the manufacturer’s protocol, and the results were normalized to total protein content. Activities of the mitochondrial marker enzymes citrate synthase, medium chain acyl-CoA dehydrogenase (MCAD), and succinate dehydrogenase (SDH) were measured spectrophotometrically in homogenates of the LV, and citrate synthase and SDH were measured in isolated cardiac mitochondria. Protein was extracted from frozen LV mitochondria, separated by electrophoresis in 4–12% NuPage gels, transferred onto a nitrocellulose membrane, and incubated with specific antibodies to cyclophilin D and voltage-dependent anion channel (VDAC) (1:10,000 and 1:5,000, respectively, both from Mitosciences, Eugene, OR). Fluorescence-conjugated secondary antibodies (IRDye 800, 1:10,000; LI-COR Bioscience) were used for incubation before the membranes were scanned with Odyssey® infrared imaging system (LI-COR Bioscience). The digitized image was analyzed with Odyssey® software.
2.6. Mitochondrial Preparation
Mitochondria were isolated based on the method of Palmer[33]. Non-infarcted LV tissue (400–500 mg) was minced and homogenized in 1:10 cold modified Chappel-Perry buffer, and the homogenates were centrifuged at 500 × g and then treated to isolate subsarcolemmal (SSM) and interfibrillar (IFM) mitochondria. The IFM were obtained after treatment of supernatant with 5 mg/g wet weight trypsin for 10 min at 4°C. The concentration of mitochondrial protein was measured by the Lowry method using bovine serum albumin as a standard.
2.7. Mitochondrial Respiration
Oxygen consumption in SSM and IFM was measured using a Clark-type oxygen electrode (Qubit Systems, Ontario, Canada). Mitochondria (0.25 mg protein) were maintained in 0.5 ml solution consisting of 100 mM KCl, 50 mM MOPS, 1 mM EGTA, and 0.5 mg fatty acid-free albumin, at pH 7.0 and 37°C. State 3 (ADP-stimulated) and State 4 (ADP-limited) respiration were measured with glutamate + malate (10 mM and 5 mM, respectively), pyruvate (10 mM), palmitoyl-CoA + carnitine (10 mM and 25 mM, respectively), and palmitoylcarnitine (10 mM), and succinate plus rotenone (10 mM and 7.5 µM, respectively), was used to assess respiration through complex II of the ETC exclusively. State 4 respiration was measured ± oligomycin. Possible defects in the F0/F1 ATPase were assessed with the uncoupler carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP).
2.8. Mitochondrial Permeability Transition Pore (MPTP) Opening Probability
Isolated IFM and SSM (0.75 mg protein) were resuspended in respiration buffer with 10 mM glutamate and 5 mM malate. A 5 mM calcium solution was continuously infused at a rate of 2 µl/min for 20 minutes, and free Ca2+ was monitored by use of 1 µl Fura-6-F (0.1 mM). Fluorescence was recorded continuously in a water-jacketed cuvette holder at 37°C using a Hitachi F2500 fluorescence spectrometer with excitation wavelengths for the free and calcium-bound forms of 340 and 380 nm, respectively and emission wavelength of 550 nm. At the end of each experiment, calibrations were performed to establish (a) a zero (by adding 30 µl of 0.1 M EGTA) and (b) a saturated Ca2+ level (by adding 30 µl of 0.1 M CaCl2). Free Ca2+ concentration was calculated from the following equation: where F is the fluorescence of the indicator at experimental Ca2+ levels, Fmin is the fluorescence in the absence of Ca2+ (after adding EGTA), and Fmax is the fluorescence of the Ca2+-saturated probe (1 M CaCl2 added). A KD for Fura-6-F and Ca2+ of 5.3 µM was used[34]. MPTP opening was defined as the cumulative Ca2+ load at which the extra-mitochondrial [Ca2+] equaled twice the baseline extra-mitochondrial [Ca2+].
In a follow-up study a separate group of rats were fed the same STD and EPA+DHA diets for 8 weeks, and SSM were isolated from the LV as described above. The protocol for the MPTP Ca2+ sensitivity assay was modified so that MPTP would be triggered in all samples by using 0.50 mg mitochondrial protein, and infusing 5 mM calcium solution at 5 µL/min. The assay was also performed with the addition of cyclosporin A (CsA; 100 nM)), an inhibitor of MPTP.
2.9. Reactive Oxygen Species Production
To determine whether EPA+DHA supplementation affected mitochondrial generation of reactive oxygen species, hydrogen peroxide production was measured in respiring mitochondria as previously described[35;36]. Briefly, 5 U/ml horseradish peroxidase, 40 U/ml Cu-Zn superoxide dismutase, and 1 µM amplex red were added to respiration buffer containing 0.75 mg mitochondria and malate+glutamate. Superoxide generation was measured with sequential additions of ADP (0.5 mM), oligomycin (1.25µg/ml), and rotenone (1 µM). H2O2 production was measured as an increase in fluorescence of amplex red. The experiment was terminated by the addition of 1 nmol H2O2 to calibrate the dye response.
2.10. Membrane Lipid Composition
Cardiac phospholipid fatty acid composition was assessed in a subset of animals (n=7–8/group) on isolated cardiac IFM and SSM homogenates by gas chromatography with a flame ionization detector according to a modification of the transesterification method as previously described [37]. Cardiolipin composition was assessed on isolated cardiac mitochondria by electrospray ionization mass spectrometry using 1,1’,2,2’-tetramyristoyl CL as an internal standard as previously described (n=6/group)[38;39].
2.11. Statistical Analyses
A two-way analysis of variance (ANOVA) was used to assess the differences among groups based on diet or surgical intervention. Post hoc comparisons were made using the Bonferroni t-test for multiple comparisons. Mean values are presented ± SEM, and the level of significance was set at p < 0.05.
3. Results
3.1. Body and Heart Mass
All sham rats survived the 12 weeks of treatment. Survival at 12 weeks in the infarct groups was not significantly affected by dietary treatment (68% for STD and 82% for EPA+DHA). Body mass at baseline was not different among groups, and was similar at the termination of the study (Table 1). Infarction yielded a similar scar size in both STD and EPA+DHA-fed animals, and had no effect on total LV mass. Infarction resulted in significant increases in right ventricular and biventricular mass in both the STD and EPA+DHA groups (Table 1). Atrial mass was increased by infarction in the STD chow group (38% compared to sham), but was not significantly increased in the EPA+DHA-fed animals (Table 1).
Table 1.
Body Mass and Cardiac Parameters
| Standard Chow | EPA+DHA | P Value | |||
|---|---|---|---|---|---|
| Sham | Infarct | Sham | Infarct | Infarct vs. Sham | |
| Terminal body mass (g) | 474±6 | 464±8 | 468±8 | 473±10 | NS |
| Tibia length (cm) | 4.50±0.04 | 4.41±0.03 | 4.40±0.03 | 4.41±0.03 | NS |
| LV mass/tibia length (mg•cm−1) |
232.8±5.8 | 249.6±7.0 | 228.7±6.2 | 253.3±7.4# | 0.003 |
| RV mass/tibia length (mg•cm−1) |
58.1±3.1 | 78.2±7.5# | 57.9±2.2 | 72.3±5.2# | 0.009 |
| Atrial mass/tibia length (mg•cm−1) |
20.7±2.1 | 29.2±3.3# | 21.7±1.8 | 26.8±2.2 | 0.002 |
| Infarct size (% of LV) | 1.1±0.6 | 13.0±2.7* | 0.8±0.5 | 13.4±2.2* | <0.001 |
| Infarct size (mg) | 12±6 | 146±32* | 8±5 | 155±28* | <0.001 |
| Relative Wall Thickness (cm) | 0.69±0.04 | 0.54±0.03# | 0.67±0.03 | 0.51±0.03* | <0.001 |
Data are the mean±SEM; n=10–14.
p<0.05 vs sham;
p<0.001 vs. sham. The Infarct vs. Sham column represents significant main effects of surgery. NS, not significant; RV, right ventricle; LV, left ventricle.
3.2. Echocardiographic Data
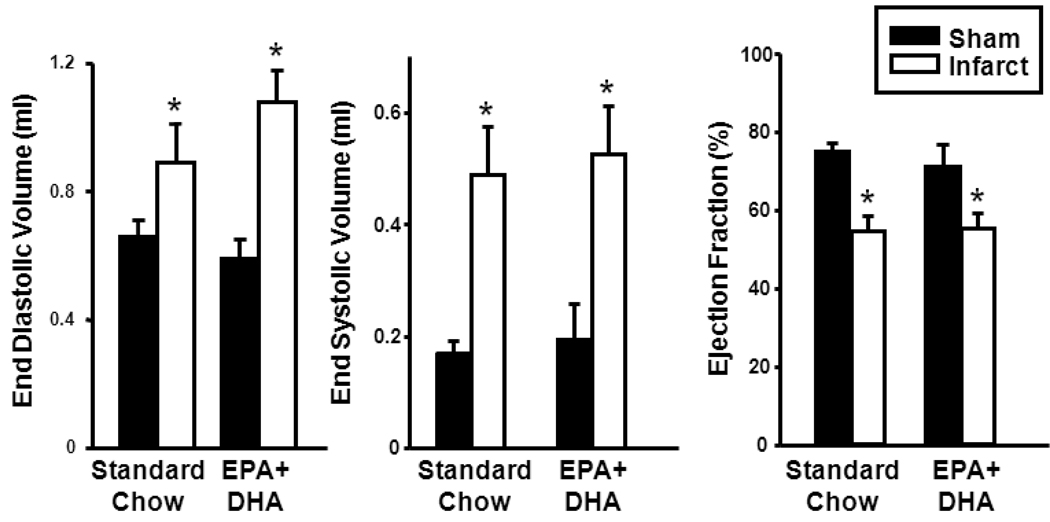
After 12 wk of treatment, LV EDV and ESV were increased and ejection fraction was decreased by infarction in the STD and EPA+DHA diet groups compared with their respective shams (Figure 1). There were no differences in function between the STD and EPA+DHA groups. Relative wall thickness was decreased with infarction in both STD and EPA+DHA-fed rats reflecting LV remodeling, with no effects of EPA+DHA supplementation (Table 1).
Figure 1.
Echocardiographic assessment of LV function. End diastolic volume (left panel); end systolic volume (middle panel); and ejection fraction (right panel); n=10–14/group. *P < 0.001 vs. respective sham.
3.3. Mitochondrial Phospholipid Analysis
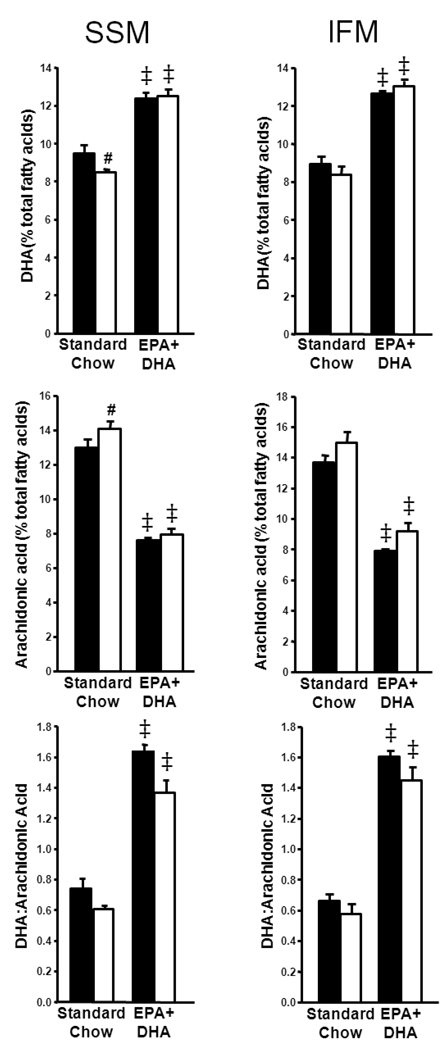
EPA+DHA significantly altered phospholipid composition in cardiac SSM and IFM. Notably, EPA+DHA increased the content of DHA in SSM and IFM membranes compared to STD diet, and in SSM, infarction decreased DHA in the STD chow-fed rats only (Figure 2). The content of EPA was not detectable in all but one out of 7–8 samples in IFM and SSM from STD-fed rats (limit of detection=0.41% of total phospholipid fatty acids) but was detectable in all EPA+DHA-fed rats (Table 2). EPA+DHA also decreased the content of arachidonic acid in SSM and IFM membranes, and arachidonic acid was significantly elevated in STD-fed rats with infarction in SSM only (Figure 2). The ratio for DHA to arachidonic acid was significantly increased in EPA+DHA-fed rats in both SSM and IFM (Figure 2). Palmitic acid was modestly but significantly reduced by EPA+DHA as a main effect in SSM only, but was increased by infarct in IFM (Table 2). Stearic acid was slightly but significantly reduced by EPA+DHA in both SSM and IFM, and oleic acid was decreased with EPA+DHA in sham rats in SSM and with EPA+DHA as a main effect in IFM (Table 2). Linoleic acid was modestly decreased in the EPA+DHA infracted rats in SSM only (Table 2).
Figure 2.
Cardiac mitochondrial fatty acid content expressed as percentage of total fatty acids in SSM and IFM phospholipids. Docosahexaenoic acid content in SSM (top left panel) and IFM (top right panel); arachidonic acid content in SSM (middle left panel) and IFM (middle right panel); and the ratio of docosahexaenoic acid content to arachidonic acid content in SSM (lower left panel) and IFM (lower right panel). n=7–8/group. *P < 0.001 vs. standard chow sham; ‡P < 0.001 vs. standard chow.
Table 2.
Cardiac Mitochondrial Phospholipid Composition in SSM and IFM.
| Standard Chow | EPA+DHA | P Value | ||||
|---|---|---|---|---|---|---|
| Fatty Acid | Sham | Infarct | Sham | Infarct |
Infarct vs. Sham |
EPA+DHA vs. STD |
| SSM | ||||||
| EPA (C20:5n3) | BQL | BQL | 4.2±0.3‡ | 4.7±0.2‡ | NS | <0.001 |
| Palmitic Acid (C16:0) |
12.5±0.8 | 11.3±0.4 | 11.1±0.4† | 10.7±0.1 | NS | 0.035 |
| Stearic Acid (C18:0) |
18.6±0.2 | 18.1±0.1 | 17.0±0.3‡ | 17.2±0.1† | NS | <0.001 |
| Oleic Acid (C18:1n9) |
15.2±1.1 | 13.0±0.5# | 12.5±0.2† | 12.9±0.3 | NS | NS |
| Linoleic Acid (C18:2n6) |
21.6±0.3 | 20.9±0.7 | 21.5±0.4 | 18.4±0.5† | 0.005 | NS |
| IFM | ||||||
| EPA (C20:5n3) | BQL | BQL | 4.2±0.2‡ | 4.0±0.2‡ | NS | <0.001 |
| Palmitic Acid (C16:0) |
10.9±0.5 | 12.0±0.5 | 11.1±0.3 | 12.3±0.3# | 0.007 | NS |
| Stearic Acid (C18:0) |
18.2±0.2 | 18.6±0.2 | 17.5±0.1† | 18.0±0.1† | 0.031 | <0.001 |
| Oleic Acid (C18:1n9) |
13.7±0.9 | 12.5±1.0 | 11.8±0.2 | 11.2±0.7 | NS | 0.039 |
| Linoleic Acid (C18:2n6) |
21.8±0.4 | 21.9±0.8 | 21.8±0.4 | 20.6±0.4 | NS | NS |
Data are expressed as percent of total mitochondrial membrane phospholipid content. Data are the mean±SEM. n=7–8/group.
p<0.05 vs EPA+DHA sham, # p<0.05 vs sham,
p<0.001 vs sham,
p<0.05 vs standard chow,
p<0.001 vs. standard chow sham. The Infarct vs. Sham column represents significant main effects of surgery. The EPA+DHA vs. STD column represents significant main effects of diet. BQL, Below Quantifiable Limits; NS, not significant.
Previous studies have reported a decrease in total CL and L4CL with heart failure[39;40], and an increase in total CL with high intake of EPA+DHA[24;41], therefore we assessed CL in SSM and IFM. Total CL was unchanged in SSM, but decreased in IFM with EPA+DHA in infarcted rats (Table 3). The percent of CL that was L4CL was significantly reduced by infarction in both SSM and IFM from almost 80% to <70% (Table 3). EPA+DHA altered CL composition, as seen in an increase in CL containing one DHA and three linoleic acid side chains (DHA1L3CL), in both SSM and IFM regardless of infarction. Infarction also increased DHA1L3CL in both mitochondrial populations (Table 3). The CL species containing one arachidonic acid and three linoleic acid side chains, AA1L3CL, was increased with infarction on STD diet in SSM and IFM, but EPA+DHA prevented this increase, and further decreased AA1L3CL in sham rats (Table 3).
Table 3.
Mitochondrial Cardiolipin Composition.
| CL Species | Standard Chow | EPA+DHA | P Value | |||
|---|---|---|---|---|---|---|
| SSM | Sham | Infarct | Sham | Infarct |
EPA+DHA vs. STD |
Infarct vs. Sham |
| L4CL (%) | 77.7±1.3 | 66.9±4.8# | 79.3±1.1 | 69.8±2.1# | NS | 0.003 |
| AA1L3CL (%) | 2.9±0.3 | 5.2±0.9# | 1.6±0.2 | 2.7±0.5† | 0.004 | 0.009 |
| DHA1L3CL (%) | 1.9±0.2 | 3.5±0.8 | 4.0±0.5† | 6.0±0.8† | 0.003 | 0.015 |
| Total CL (nmol• mg mito protein1−) |
10.7±1.1 | 10.3±0.8 | 11.3±1.1 | 10.5±1.1 | NS | NS |
| IFM | ||||||
| L4CL (%) | 77.9±0.7 | 65.9±4.8# | 80.6±0.8 | 68.7±2.6# | NS | <0.001 |
| AA1L3CL (%) | 2.7±0.2 | 5.6±1.1# | 1.9±0.2 | 2.9±0.4† | 0.015 | 0.010 |
| DHA1L3CL (%) | 2.5±0.5 | 3.7±0.8 | 3.1±0.5 | 5.8±0.4#† | 0.031 | 0.004 |
| Total CL (nmol• mg mito protein1−) |
11.6±1.0 | 9.2±1.2 | 11.1±0.8 | 7.5±1.3# | NS | 0.021 |
Data are expressed as percentage of total cardiolipin content. Data are the mean±SEM. n=6/group.
p<0.05 vs. sham.
p<0.05 vs standard chow. The EPA+DHA vs. STD column represents the significant main effects of diet and the Infarct vs. Sham represents the main effects of surgery. NS, not significant; CL, cardiolipin; SSM, subsarcolemmal mitochondria; IFM, interfibrillar mitochondria; L4CL, tetralinoleoyl cardiolipin; AA1L3CL, cardiolipin containing one arachidonic acid and three linoleic acid chains; DHA1L3CL, cardiolipin containing one docosahexaenoic acid and three linoleic acid chains.
3.4. Metabolic and Biochemical Parameters
As expected, plasma levels of TG were significantly reduced by EPA+DHA in both sham and infarct groups, and serum adiponectin was significantly elevated by EPA+DHA supplementation (Table 4). Plasma FFA (Table 4) and glucose (data not shown) were unaffected by either diet or surgical treatment. In the past, we observed a reduction in inflammatory markers with EPA+DHA feeding in a pressure overload model[6], therefore we measured serum tumor necrosis factor (TNF) α and urine thromboxane B2 concentrations in the present study. Serum TNF and thromboxane B2 were not altered by diet or surgery (Table 4).
Table 4.
Metabolites
| Standard Chow | EPA+DHA | P Value | |||
|---|---|---|---|---|---|
| Sham | Infarct | Sham | Sham | EPA+DHA vs. STD | |
| Plasma Triglycerides (mg•dl−1) |
113.0±14.0 | 106.3±9.7 | 70.3±6.5† | 72.2±8.3† | <0.001 |
| Serum Adiponectin (µg•ml−1) |
19.3±2.4 | 21.7±1.6 | 24.1±1.7 | 25.0±1.9 | 0.032 |
| Plasma Free Fatty Acids (mmol•L−1) |
0.16±0.02 | 0.20±0.01 | 0.16±0.01 | 0.15±0.02 | NS |
| Serum TNFα (pg•ml−1) | 5.80±1.80 | 5.10±0.45 | 5.62±0.60 | 4.37±0.43 | NS |
| Urine Thromboxane B2 (pg•mol−1 creatinine) |
24.4±2.5 | 35.6±6.4 | 36.7±8.9 | 49.8±14.0 | NS |
Data are the mean±SEM; n=10–14.
p<0.05 vs standard chow. The Infarct vs. Sham column represents significant main effects of surgery. The EPA+DHA vs. STD column represents significant main effects of diet. NS, not significant; TNFα, tumor necrosis factor α.
3.5. Enzyme Activities
The activities of citrate synthase and SDH were measured in whole cardiac tissue homogenates as an index of myocardial mitochondrial content (Table 5). Both citrate synthase and SDH activities were significantly reduced by ~10–15% with infarction in both STD and EPA+DHA groups, suggesting a modest decrease in mitochondrial content.
Table 5.
Mitochondrial Enzyme Activities and ROS production.
| Standard Chow | EPA+DHA | P Value | |||
|---|---|---|---|---|---|
| LV Whole Tissue | Sham | Infarct | Sham | Infarct | Infarct vs. Sham |
| Citrate Synthase (µmol•gww−1•min−1) |
115±3 | 103±5 | 117±5 | 107±5 | 0.033 |
| MCAD (µmol•gww−1•min−1) | 14.7±0.3 | 12.8±0.6 | 14.3±0.5 | 13.7±0.7 | 0.068 |
| SDH(µmol•gww−1•min−1) | 3.9±0.2 | 3.5±0.2 | 4.3±0.1 | 3.6±0.2# | 0.004 |
|
Mitochondrial Protein Yield |
|||||
| SSM (mg•gww−1) | 23.9±1.9 | 19.8±2.2 | 23.3±2.9 | 20.2±1.8 | NS |
| IFM (mg•gww−1) | 23.1±1.1 | 17.0±1.2* | 21.6±0.9 | 17.3±0.6* | <0.001 |
|
Mitochondrial ROS Production (+ rotenone) |
|||||
| SSM (nmol H202•min−1) | 2.18±0.26 | 3.02±0.53 | 2.03±0.23 | 2.83±0.33 | 0.024 |
| IFM (nmol H202•min−1) | 2.04±0.19 | 3.48±0.50# | 2.13±0.16 | 2.85±0.34 | 0.003 |
Data are the mean±SEM. n=10–14.
p<0.05 vs sham,
p<0.001 vs EPA+DHA sham. The Infarct vs. Sham column represents significant main effects of surgery. NS, not significant; LV, left ventricle; MCAD, medium chain acyl-CoA dehydrogenase; SDH, succinate dehydrogenase; SSM, subsarcolemmal mitochondria; IFM, interfibrillar mitochondria.
3.6. Mitochondrial Respiration and Superoxide Production
The yield of mitochondrial protein was significantly decreased in the IFM of infarcted animals in both the STD and EPA+DHA groups (Table 5). There was a trend for a decrease in the SSM, but this difference did not reach statistical significance (p=0.095). State 3 and state 4 mitochondrial respiration rates were not affected by diet or surgery with glutamate+malate, pyruvate, palmitoyl-CoA, palmitoylcarnitine, or succinate+rotenone as a substrate in both SSM and IFM (Table S1). State 4 respiration measured in the presence of oligomycin was not different between groups, indicating the absence of uncoupling.
In both the SSM and IFM, ROS production with succinate+rotenone as a substrate was increased with infarction (Table 5). The production of ROS with glutamate, ADP, and oligomycin were not different with dietary or surgical intervention (Table S2).
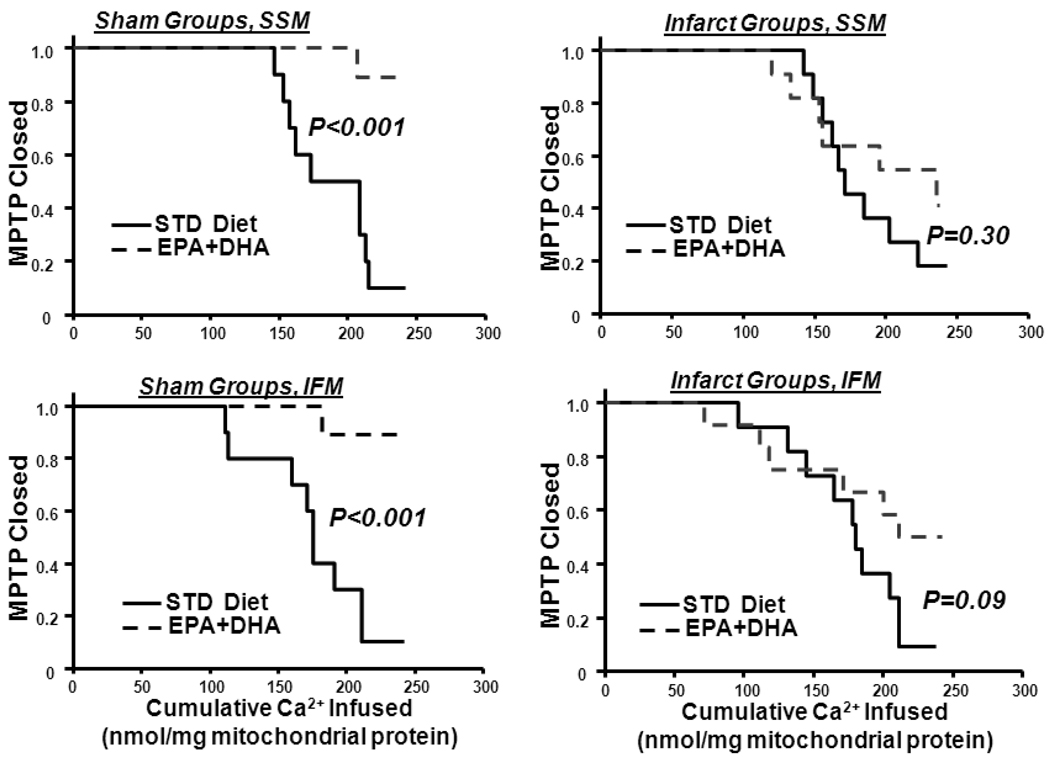
3.7. Ca2+-Induced MPTP Opening
EPA+DHA feeding decreased susceptibility to Ca2+-induced MPTP compared to the STD diet in sham animals in both SSM and IFM, indicating delayed MPTP opening (Figure 3). Infarction had no effect on Ca2+-induced MPTP opening in both mitochondria populations, and EPA+DHA had no effect on MPTP in rats with infarction (Figure 3). Cyclophilin-D is considered to be a key regulatory component of the MPTP, however western blot analysis found no effect of EPA+DHA on cyclophilin-D protein expression in either SSM or IFM (Figure S1 in Supplemental Data). The voltage-dependent anion channel (VDAC) is thought to play a role in regulation of MPTP, however protein expression of VDAC1 was significantly decreased by infarction regardless of diet, while VDAC2 was significantly reduced with infarction in the STD group, but not in the EPA+DHA-fed rats (Figure S1). Thus the delay in Ca2+-induced MPTP we observed does not appear to be due to changes in the mitochondrial content of cyclophilin-D or VDAC.
Figure 3.
Effect of diet on MPTP. The fraction of preparations with the MPTP open plotted as a function of the cumulative amount of Ca2+ added to the cuvette containing isolated mitochondria. Upper panels: SSM from sham rats (left) and infarcted rats (right). Lower panels: IFM from sham rats (left) and infarct rats (right). n=10–14/group.
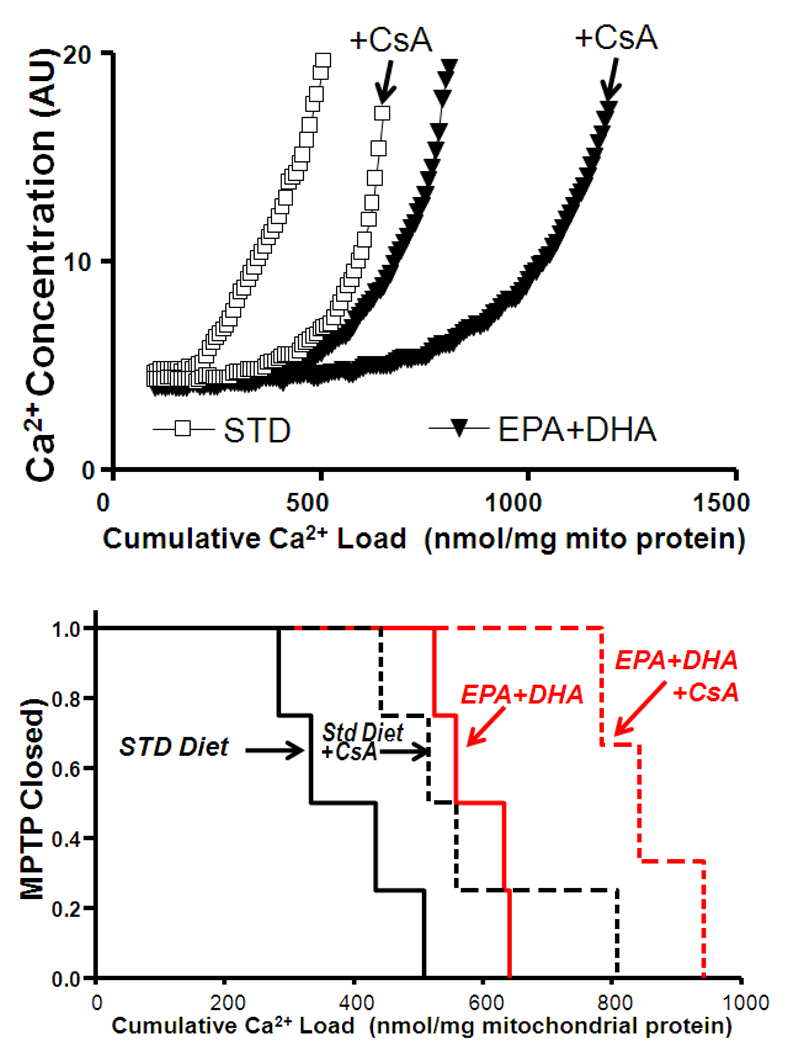
Since the duration of Ca2+ infusion was not sufficient to trigger MPTP opening in all mitochondrial preparations, we subsequently measured MPTP opening using less mitochondria and a higher rate of Ca2+ infusion. A separate group of normal healthy rats were fed EPA+DHA or STD diet for 8 weeks, and Ca2+ induced MPTP opening was measured in SSM. In this group, the duration of Ca2+ infusion was extended to allow all samples to undergo MPTP. EPA+DHA resulted in MPTP opening at a higher cumulative Ca2+ load compared to mitochondria from rats fed the STD diet (Figure 4). Cyclosporin A (CsA; 100 nM) significantly shifted MPTP opening to the right in both STD and EPA+DHA treatment groups (p<0.01) (Figure 4).
Figure 4.
Effects of CsA on MPTP. Upper panel: Representative results for animals treated with the STD diet or EPA+DHA in the presence and absence of cyclosporin A (CsA; 100nM). Lower panel: The fraction of preparations with the MPTP open plotted as a function of the cumulative amount of Ca2+ added to the cuvette containing isolated cardiac SSM. n=3–4/group. CsA, cyclosporin A.
4. Discussion
The goal of this investigation was to determine the effects of EPA+DHA supplementation on cardiac mitochondrial membrane composition, mitochondrial respiration, and MPTP opening in normal hearts and following myocardial infarction. We found that EPA+DHA altered fatty acid composition of mitochondrial phospholipids, specifically decreasing arachidonic acid and increasing DHA. EPA+DHA delayed Ca2+-induced MPTP opening in both SSM and IFM in sham rats. This effect was enhanced by the addition of cyclosporin A, and was not accompanied by changes in mitochondrial respiration, coupling, or cyclophilin D protein expression. On the other hand, supplementation with EPA+DHA did not improve LV function nor delay MPTP opening in failure myocardium. Nevertheless our findings suggest the novel concept that dietary intake of ω-3 polyunsaturated fatty acids can alter MPTP opening in normal hearts.
A delay in MPTP opening with EPA+DHA has not been previously reported, and could be clinically useful if it translates into less myocardial injury in response to acute and chronic cardiac stress (e.g. ischemia/reperfusion or hypertension). The EPA+DHA-induced delay in MPTP opening was enhanced by cyclosporin A, an inhibitor of the isomerase activity of cyclophilin D, which plays a regulatory role in MPTP opening. Cyclophilin D protein expression did not change with diet or surgery in this study (Supplementary Figure 1), suggesting that changes in cyclophilin D do not mediate the effects of EPA+DHA. More definitive studies with EPA+DHA supplementation in cycolphilin D knockout mice are warranted [42]. VDAC is another protein that has been implicated in the regulation of MPTP opening, however the infarct-induced reduction in VDAC1 and 2 that we observed were not related to MPTP opening. At present the molecular components and regulation of the MPTP are unclear[17;18;43], which makes it difficult to draw conclusions regarding how EPA+DHA affect MPTP opening at a molecular level. One limitation of the method used to measure MPTP opening in the present study is that the assay conditions minimized the potential contribution of oxidative stress. Future studies should assess whether diet and heart failure affect MPTP opening at different mitochondrial redox states and in response to acute oxidative stress, e.g., that induced by the metabolism of exogenous peroxides. In addition, the lack of beneficial effect of EPA+DHA on LV remodeling and function in infarcted animals may be due to the delayed initiation of treatment 7 days after coronary ligation. Since MPTP opening is associated with apoptosis and myocardial damage during ischemia and chronic stress [17;18;44], EPA+DHA treatment prior to infarction may lead to less MPTP opening and thus prevent myocardial damage and initiation of LV remodeling in the week following infarction.
Previous studies show a decrease in arachidonic acid in total myocardial phospholipids with EPA+DHA[6;45], and in the present study we extend this observation to show a similar decrease in arachidonic acid in total mitochondrial phospholipids and in cardiolipin. A number of studies found that arachidonic acid plays an important role in Ca2+ mediated MPTP opening and apoptosis. Arachidonic release by Ca2+-independent phospholipase A2γ mediates MPTP opening in renal cortex mitochondria[46]. The release of cytochrome c from the mitochondria, which leads to downstream apoptosis, may also be regulated by arachidonic acid release from the membrane in mouse embryonic fibroblasts[47] and in rat liver mitochondria[20;48]. The role of arachidonic acid may differ in cardiac mitochondria compared to mitochondria from other tissues, nonetheless there is clear evidence that arachidonic acid can contribute to MPTP opening[49]. In the present study there may have been a decrease in arachidonic acid release from the membrane with EPA+DHA treatment, which would delay or inhibit MPTP opening. In addition, alterations in cardiolipin composition (i.e. decreased arachidonic acid and increased DHA incorporation) may also play a role in MPTP opening. Further studies are needed to fully assess the role of mitochondrial arachidonic acid on MPTP opening.
Supplementation with EPA+DHA was ineffective in delaying Ca2+-induced MPTP opening in mitochondria from infarcted hearts despite increasing DHA and decreasing in arachidonic acid in cardiac phospholipids. This suggests that changes in phospholipid composition are not responsible for the delay in MPTP opening observed in sham rats. Myocardial infarction resulted in myocardial pathology, as evidenced by LV dilation and contractile dysfunction, decreased activity of mitochondrial marker enzymes in LV tissue, and a lower IFM yield. Consistent with previous studies there was no impairment in mitochondrial respiration in either SSM or IFM[7–10]. At present it is unclear why the beneficial effects of EPA+DHA supplementation on MPTP opening are absent in mitochondria from infarcted rats.
Our recent studies in rats with pressure overload found that supplementation with EPA+DHA attenuated pathological LV hypertrophy and development of heart failure[6;27;29], however no such protective effect was observed in the present investigation in rats with infarct-induced heart failure. The reasons for the lack of benefit in the present investigation may be due to the timing and duration of treatment with EPA+DHA. In our previous study with abdominal aortic banding, EPA+DHA treatment was initiated either one week prior to aortic banding [6;27] or immediately after banding [29], and resulted in significant cardioprotection. Since the purpose of the present study was to determine if EPA+DHA exerts a beneficial effect in a model of irreversible myocardial injury, we initiated treatment 7 days after coronary ligation to insure that the myocardial infarct was established. Our previous results from the aortic constriction model suggests that if supplementation with EPA+DHA had been initiated prior to coronary ligation a protective effect may have been observed. Another possibility is that more prolonged treatment is needed to fully elicit and maintain the effects of EPA+DHA. Previous work in patients undergoing elective cardiac surgery established that supplementation at a dose equivalent to ours show changes in membrane DHA and arachidonic acid by 7 to 10 days of treatment, and a plateau by ~30 days. Additional studies are needed to assess the effects of earlier and more prolonged treatment with EPA+DHA.
In the present investigation myocardial infarction resulted in heart failure as evidenced by LV dilation and contractile dysfunction, decreased activity of mitochondrial marker enzymes in LV tissue, and a decrease in the yield of IFM. Consistent with previous studies there was no impairment in mitochondrial respiration in either SSM or IFM[7–10]. On the other hand, infarct-induced alterations in cardiac mitochondria in this model prevented the beneficial effects of EPA+DHA supplementation on MPTP opening. Future studies should clarify the relationship among heart failure, mitochondrial dyfunction and EPA+DHA supplementation by assessing the effects of more advanced heart failure, and initiating treatment after mitochondrial dysfunction is established.
More severe and prolonged heart failure may derive benefit with EPA+DHA supplementation, as suggested by the results of the recent GISSI-HF trial[5]. For example, EPA+DHA could improve long term survival in infarct-induced heart failure. Fiaccavento et al. showed that high intake of α-linolenic acid, a ω-3 polyunsaturated fatty acid found in vegetable oils, prolonged survival and prevented myocardial pathology in cardiomyopathic Syrian hamsters compared to those fed standard chow[50]. It is important to note that the dose of EPA+DHA used in this study corresponds to an equivalent dose of in humans of approximately 5 g/day (assuming an energy intake of 2000 kcal/day), which is in the range of safe and effective doses recommended for the treatment of hypertriglyceridemia ( 3 to 4 g/day). In the GISSI-HF trial, a much lower dose of 0.85 g/day was used. Additional studies are needed to more fully assess the effects of various ω-3 polyunsaturated fatty acids at higher doses on outcomes in heart failure.
In summary, the results of the present investigation demonstrate that MPTP opening is delayed by dietary EPA+DHA supplementation in sham rat hearts. This effect was associated with alterations in membrane phospholipids, specifically a decrease in arachidonic acid and elevated DHA. In contrast, LV function and MPTP opening were not affected by EPA+DHA supplementation in infarcted myocardium. Nevertheless, inhibition of MPTP opening in cardiac mitochondria should be cardioprotective, and thus the novel effect observed here may contribute to the lower incidence of heart failure observed in people consuming high amount of EPA+DHA in population based studies[3;4].
Supplementary Material
Acknowledgments
Funding.
WCS was supported by NIH grants HL074237 and HL091307.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2003 Feb 1;23:e20–e30. doi: 10.1161/01.atv.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 2.Leaf A, Kang JX, Xiao YF, Billman GE. Clinical prevention of sudden cardiac death by n−3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n−3 fish oils. Circulation. 2003 Jun 3;107:2646–2652. doi: 10.1161/01.CIR.0000069566.78305.33. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Bryson CL, Lemaitre RN, Burke GL, Siscovick DS. Fish intake and risk of incident heart failure. J Am Coll Cardiol. 2005 Jun 21;45:2015–2021. doi: 10.1016/j.jacc.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 4.Yamagishi K, Iso H, Date C, Fukui M, Wakai K, Kikuchi S, Inaba Y, Tanabe N, Tamakoshi A. Fish, omega-3 Polyunsaturated Fatty Acids, and Mortality From Cardiovascular Diseases in a Nationwide Community-Based Cohort of Japanese Men and Women The JACC (Japan Collaborative Cohort Study for Evaluation of Cancer Risk) Study. J Am Coll Cardiol. 2008 Sep 16;52:988–996. doi: 10.1016/j.jacc.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G. Effect of n−3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008 Oct 4;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 6.Duda MK, O'shea KM, Tintinu A, Xu W, Khairallah RJ, Barrows BR, Chess DJ, Azimzadeh AM, Harris WS, Sharov VG, Sabbah HN, Stanley WC. Fish oil, but not flaxseed oil, decreases inflammation and prevents pressure overload-induced cardiac dysfunction. Cardiovasc Res. 2009 Feb 1;81:319–327. doi: 10.1093/cvr/cvn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray AJ, Cole MA, Lygate CA, Carr CA, Stuckey DJ, Little SE, Neubauer S, Clarke K. Increased mitochondrial uncoupling proteins, respiratory uncoupling and decreased efficiency in the chronically infarcted rat heart. J Mol Cell Cardiol. 2008;44:694–700. doi: 10.1016/j.yjmcc.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Rennison JH, McElfresh TA, Okere IC, Vazquez EJ, Patel HV, Foster AB, Patel KK, Chen Q, Hoit BD, Tserng KY, Hassan MO, Hoppel CL, Chandler MP. High-fat diet postinfarction enhances mitochondrial function and does not exacerbate left ventricular dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1498–H1506. doi: 10.1152/ajpheart.01021.2006. [DOI] [PubMed] [Google Scholar]
- 9.Rennison JH, McElfresh TA, Okere IC, Patel HV, Foster AB, Patel KK, Stoll MS, Minkler PE, Fujioka H, Hoit BD, Young ME, Hoppel CL, Chandler MP. Enhanced Acyl-CoA Dehydrogenase Activity is Associated with Improved Mitochondrial and Contractile Function in Heart Failure. Cardiovasc Res. 2008 Mar 13; doi: 10.1093/cvr/cvn066. [DOI] [PubMed] [Google Scholar]
- 10.Rennison JH, McElfresh TA, Chen X, Anand VR, Hoit BD, Hoppel CL, Chandler MP. Prolonged exposure to high dietary lipids is not associated with lipotoxicity in heart failure. J Mol Cell Cardiol. 2009 Mar 3; doi: 10.1016/j.yjmcc.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharov VG, Todor AV, Silverman N, Goldstein S, Sabbah HN. Abnormal mitochondrial respiration in failed human myocardium. J Mol Cell Cardiol. 2000;32:2361–2367. doi: 10.1006/jmcc.2000.1266. [DOI] [PubMed] [Google Scholar]
- 12.Rosca MG, Vazquez EJ, Kerner J, Parland W, Chandler MP, Stanley W, Sabbah HN, Hoppel CL. Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation. Cardiovasc Res. 2008 Oct 1;80:30–39. doi: 10.1093/cvr/cvn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Javadov S, Huang C, Kirshenbaum L, Karmazyn M. NHE-1 inhibition improves impaired mitochondrial permeability transition and respiratory function during postinfarction remodelling in the rat. J Mol Cell Cardiol. 2005;38:135–143. doi: 10.1016/j.yjmcc.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Matas J, Young NT, Bourcier-Lucas C, Ascah A, Marcil M, Deschepper CF, Burelle Y. Increased expression and intramitochondrial translocation of cyclophilin-D associates with increased vulnerability of the permeability transition pore to stress-induced opening during compensated ventricular hypertrophy. J Mol Cell Cardiol. 2009;46:420–430. doi: 10.1016/j.yjmcc.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Marcil M, Ascah A, Matas J, Belanger S, Deschepper CF, Burelle Y. Compensated volume overload increases the vulnerability of heart mitochondria without affecting their functions in the absence of stress. J Mol Cell Cardiol. 2006;41:998–1009. doi: 10.1016/j.yjmcc.2006.08.117. [DOI] [PubMed] [Google Scholar]
- 16.Baines CP. The molecular composition of the mitochondrial permeability transition pore. J Mol Cell Cardiol. 2009;46:850–857. doi: 10.1016/j.yjmcc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honda HM, Ping P. Mitochondrial permeability transition in cardiac cell injury and death. Cardiovasc Drugs Ther. 2006;20:425–432. doi: 10.1007/s10557-006-0642-0. [DOI] [PubMed] [Google Scholar]
- 18.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol. 2009;46:821–831. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Penzo D, Petronilli V, Angelin A, Cusan C, Colonna R, Scorrano L, Pagano F, Prato M, Di LF, Bernardi P. Arachidonic acid released by phospholipase A(2) activation triggers Ca(2+)-dependent apoptosis through the mitochondrial pathway. J Biol Chem. 2004 Jun 11;279:25219–25225. doi: 10.1074/jbc.M310381200. [DOI] [PubMed] [Google Scholar]
- 20.Gadd ME, Broekemeier KM, Crouser ED, Kumar J, Graff G, Pfeiffer DR. Mitochondrial iPLA2 activity modulates the release of cytochrome c from mitochondria and influences the permeability transition. J Biol Chem. 2006 Mar 17;281:6931–6939. doi: 10.1074/jbc.M510845200. [DOI] [PubMed] [Google Scholar]
- 21.Ott M, Zhivotovsky B, Orrenius S. Role of cardiolipin in cytochrome c release from mitochondria. Cell Death Differ. 2007;14:1243–1247. doi: 10.1038/sj.cdd.4402135. [DOI] [PubMed] [Google Scholar]
- 22.Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2007;292:C33–C44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- 23.Schlame M, Rua D, Greenberg ML. The biosynthesis and functional role of cardiolipin. Prog Lipid Res. 2000;39:257–288. doi: 10.1016/s0163-7827(00)00005-9. [DOI] [PubMed] [Google Scholar]
- 24.Pepe S, Tsuchiya N, Lakatta EG, Hansford RG. PUFA and aging modulate cardiac mitochondrial membrane lipid composition and Ca2+ activation of PDH. Am J Physiol. 1999;276:H149–H158. doi: 10.1152/ajpheart.1999.276.1.H149. [DOI] [PubMed] [Google Scholar]
- 25.McMillin JB, Bick RJ, Benedict CR. Influence of dietary fish oil on mitochondrial function and response to ischemia. Am J Physiol. 1992;263:H1479–H1485. doi: 10.1152/ajpheart.1992.263.5.H1479. [DOI] [PubMed] [Google Scholar]
- 26.Baines CP. The molecular composition of the mitochondrial permeability transition pore. J Mol Cell Cardiol. 2009;46:850–857. doi: 10.1016/j.yjmcc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duda MK, O'shea KM, Lei B, Barrows BR, Azimzadeh AM, McElfresh TE, Hoit BD, Kop WJ, Stanley WC. Dietary supplementation with omega-3 PUFA increases adiponectin and attenuates ventricular remodeling and dysfunction with pressure overload. Cardiovasc Res. 2007 Jul 20;76:303–310. doi: 10.1016/j.cardiores.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duda MK, O'shea KM, Stanley WC. {omega}-3 polyunsaturated fatty acid supplementation for the treatment of heart failure: mechanisms and clinical potential. Cardiovasc Res. 2009 doi: 10.1093/cvr/cvp169. %20.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah KB, Duda MK, O'shea KM, Sparagna GC, Chess DJ, Khairallah RJ, Robillard-Frayne I, Xu W, Murphy RC, des RC, Stanley WC. The Cardioprotective Effects of Fish Oil During Pressure Overload Are Blocked by High Fat Intake. Role Of Cardiac Phospholipid Remodeling. Hypertension. 2009 Jul 13; doi: 10.1161/HYPERTENSIONAHA.109.135806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nasa Y, Sakamoto Y, Sanbe A, Sasaki H, Yamaguchi F, Takeo S. Changes in fatty acid compositions of myocardial lipids in rats with heart failure following myocardial infarction. Mol Cell Biochem. 1997;176:179–189. [PubMed] [Google Scholar]
- 31.Javadov S, Karmazyn M, Escobales N. Mitochondrial permeability transition pore opening as a promising therapeutic target in cardiac diseases. J Pharmacol Exp Ther. 2009 Jun 9; doi: 10.1124/jpet.109.153213. [DOI] [PubMed] [Google Scholar]
- 32.Morgan EE, Chandler MP, Young ME, McElfresh TA, Kung TA, Rennison JH, serng KY, Hoit BD, Stanley WC. Dissociation between gene and protein expression of metabolic enzymes in a rodent model of heart failure. Eur J Heart Fail. 2006;8:687–693. doi: 10.1016/j.ejheart.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem. 1977 Dec 10;252:8731–8739. [PubMed] [Google Scholar]
- 34.Rajdev S, Reynolds IJ. Calcium green-5N, a novel fluorescent probe for monitoring high intracellular free Ca2+ concentrations associated with glutamate excitotoxicity in cultured rat brain neurons. Neurosci Lett. 1993 Nov 12;162:149–152. doi: 10.1016/0304-3940(93)90582-6. [DOI] [PubMed] [Google Scholar]
- 35.Schuh RA, Kristian T, Gupta RK, Flaws JA, Fiskum G. Methoxychlor inhibits brain mitochondrial respiration and increases hydrogen peroxide production and CREB phosphorylation. Toxicol Sci. 2005;88:495–504. doi: 10.1093/toxsci/kfi334. [DOI] [PubMed] [Google Scholar]
- 36.Starkov AA, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem. 2003;86:1101–1107. doi: 10.1046/j.1471-4159.2003.01908.x. [DOI] [PubMed] [Google Scholar]
- 37.Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res. 1986;27:114–120. [PubMed] [Google Scholar]
- 38.Sparagna GC, Johnson CA, McCune SA, Moore RL, Murphy RC. Quantitation of cardiolipin molecular species in spontaneously hypertensive heart failure rats using electrospray ionization mass spectrometry. J Lipid Res. 2005;46:1196–1204. doi: 10.1194/jlr.M500031-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Sparagna GC, Chicco AJ, Murphy RC, Bristow MR, Johnson CA, Rees ML, Maxey ML, McCune SA, Moore RL. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J Lipid Res. 2007;48:1559–1570. doi: 10.1194/jlr.M600551-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Sparagna GC, Lesnefsky EJ. Cardiolipin remodeling in the heart. J Cardiovasc Pharmacol. 2009;53:290–301. doi: 10.1097/FJC.0b013e31819b5461. [DOI] [PubMed] [Google Scholar]
- 41.McMillin JB, Bick RJ, Benedict CR. Influence of dietary fish oil on mitochondrial function and response to ischemia. Am J Physiol. 1992;263:H1479–H1485. doi: 10.1152/ajpheart.1992.263.5.H1479. [DOI] [PubMed] [Google Scholar]
- 42.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005 Mar 31;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 43.Baines CP. The molecular composition of the mitochondrial permeability transition pore. J Mol Cell Cardiol. 2009;46:850–857. doi: 10.1016/j.yjmcc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baines CP. The molecular composition of the mitochondrial permeability transition pore. J Mol Cell Cardiol. 2009;46:850–857. doi: 10.1016/j.yjmcc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Metcalf RG, James MJ, Gibson RA, Edwards JR, Stubberfield J, Stuklis R, Roberts-Thomson K, Young GD, Cleland LG. Effects of fish-oil supplementation on myocardial fatty acids in humans. Am J Clin Nutr. 2007;85:1222–1228. doi: 10.1093/ajcn/85.5.1222. [DOI] [PubMed] [Google Scholar]
- 46.Kinsey GR, McHowat J, Patrick KS, Schnellmann RG. Role of Ca2+-independent phospholipase A2gamma in Ca2+-induced mitochondrial permeability transition. J Pharmacol Exp Ther. 2007;321:707–715. doi: 10.1124/jpet.107.119545. [DOI] [PubMed] [Google Scholar]
- 47.Mizuta T, Shimizu S, Matsuoka Y, Nakagawa T, Tsujimoto Y. A Bax/Bak-independent mechanism of cytochrome c release. J Biol Chem. 2007 Jun 1;282:16623–16630. doi: 10.1074/jbc.M611060200. [DOI] [PubMed] [Google Scholar]
- 48.Di PM, Zaccagnino P, Oliveros-Celis C, Lorusso M. Arachidonic acid induces specific membrane permeability increase in heart mitochondria. FEBS Lett. 2006 Feb 6;580:775–781. doi: 10.1016/j.febslet.2005.12.090. [DOI] [PubMed] [Google Scholar]
- 49.Di PM, Lorusso M. Interaction of free fatty acids with mitochondria: coupling, -uncoupling and permeability transition. Biochim Biophys Acta. 2006;1757:1330–1337. doi: 10.1016/j.bbabio.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 50.Fiaccavento R, Carotenuto F, Minieri M, Masuelli L, Vecchini A, Bei R, Modesti A, Binaglia L, Fusco A, Bertoli A, Forte G, Carosella L, Di Nardo P. Alpha-linolenic acid-enriched diet prevents myocardial damage and expands longevity in cardiomyopathic hamsters. Am J Pathol. 2006;169:1913–1924. doi: 10.2353/ajpath.2006.051320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.