Abstract
One of the major difficulties in the treatment of epithelial ovarian cancer (EOC) is the high rate of recurrent disease. This is thought to be due to the survival of a population of chemo-resistant cells within the tumor, the ovarian cancer stem cells (OCSCs), that are able to regenerate the tumor following chemotherapy. Therefore, the identification of a compund that can target the OCSCs is one of the main steps in improving overall survival of ovarian cancer patients. The objective of this study was to determine the effect of N-t-boc-Daidzein, a novel daidzain derivative, on OCSCs. The efficacy of this compound was evaluated in OCSC and mature ovarian cancer cell (mOCC) lines isolated from malignant ovarian cancer asicites. Cells were treated with increasing concentrations of N-t-boc-Daidzein (0.003–10 μM) and cell growth was monitored by “real time in vitro micro-imaging” using the IncuCyte system. Cell viability was measured using the CellTiter 96 Assay. Apoptosis was determined by Caspase-Glo 3/7, 8, and 9 assays. The components of the apoptotic cascade were characterized by Western blot analysis. N-t-boc-Daidzein was able to significantly inhibit cell growth and decrease cell viability of OCSC as well as mOCC cells in a dose and time dependent maner. This effect was due to the induction of apoptosis, which is characterized by caspase activation, XIAP and AKT degradation, and mitochondrial depolarization. This study describes a novel compound that can target the OCSCs. These findings may provide vital aide in improving overall survival in patients with EOC.
Keywords: ovarian cancer stem cells, apoptosis, caspases, XIAP, mitochondrial depolarization
Introduction
Epithelial ovarian cancer (EOC) is the fourth-leading cause of cancer-related deaths in women and the leading cause of gynecologic cancer death 1. This high mortality rate is largely due to difficulties with diagnosis in early stages of the disease and a high rate of recurrence 2, 3. The troubles with early detection of the disease are caused by an absence of signs and symptoms in the initial stages of the disease and the lack of a widely available screening test. Additionally, although eighty percent of cancers initially respond to chemotherapy, the majority ultimately recurs with less than fifteen percent remaining in remission 4. This is thought to be due to a small subset of cells within the tumor, the ovarian cancer stem cells (OCSCs), that are resistant to conventional chemotherapy treatments 5.
Current chemotherapy agents are aimed at targeting rapidly dividing cells 6; however, since the OCSCs are slow-dividing, they are not effectively killed by these compounds 5. A number of previous studies have shown that there is indeed a population of cells within EOC tumors that are CD44-positive (CD44+) 7, chemo-resistant, and are able to regenerate a heterogeneous tumor. In contrast, the CD44-negative (CD44−) cell population (referred to here as the mature ovarian cancer cells or mOCCs) are chemo-sensitive, and non-tumorigenic 5. Thus, the CD44+ cells are thought to be the OCSCs, whose survival during chemotherapy is a large factor in the high recurrence rate of ovarian cancer. Therefore, developing new therapies that can target the OCSCs is extremely important for the treatment of patients with ovarian cancer.
Daidzein is an isoflavone found in soy products that has been shown to affect cell cycle progression and apoptosis in human cancer cells 8. 5-{2-[3-(4-Hydroxy-phenyl)-4-oxo-4H-chromen-7-yloxy]-acetylamino}-pentyl)-carbamic acid tert-butyl ester (N-t-boc-Daidzein) is a synthetic derivative of Daidzein that preliminary studies have demonstrated can inhibit cell growth in ovarian and colon cancer cells in vitro 9. The long chain on the daidzein molecule imparts lipophilic character facilitating endocytosis and the N-t-Boc group at the end of the side chain provides metabolic stability, which may increase its efficacy in vivo 9. The objective of this study was to evaluate the effect of N-t-boc-Daidzein on EOC cells, especially the OCSCs.
We report here the molecular characterization of this novel compound that has the ability to induce apoptosis in OCSCs in vitro.
Results
N-t-boc-Daidzein significantly inhibits growth of EOC cells in vitro
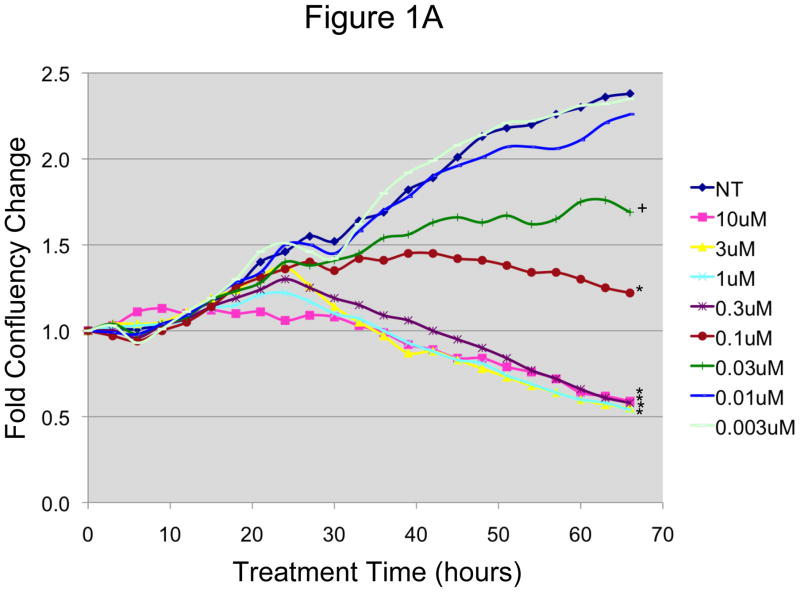
Our first objective was to characterize the in vitro response of EOC cells to N-t-boc-Daidzein treatment. Two CD44+ OCSC cultures (OCSC1, OCSC2) and their corresponding CD44- mOCCs (TARA R182, TARA R127) were incubated in the presence of increasing concentrations of N-t-boc-Daidzein (0.003–10 μM) for an average of 72 (± 11) hours, and cell growth was monitored by “real time in vitro micro- imaging” using the IncuCyte system (Essen Instruments, Ann Arbor, MI). The IncuCyte system allows for the hourly monitoring of cell growth by determining the confluence of the cells and displaying the morphologic changes associated with treatment. Figures 1A–B show the growth curves of representative EOC cell lines. For the OCSCs (figure 1A), the control group follows a time-dependent growth curve characterized by a small increase in growth in the first 10 hours, a logarithmic growth over the next 35 hours, and the beginning of a plateau after 45 hours. During the first 15 hours, no differences were found in growth between the treated and non-treated groups. After this time, the effect of the treatment was clearly delineated according to dose and time. After 18 hours of N-t-boc-Daidzein treatment, we began to observe a decrease in cell growth for cells treated with the highest dose of N-t-boc-Daidzein (10 μM), as represented by changes in the growth curves. These changes further increased following 30 hours of treatment. Significantly decreased cell growth was also observed with treatment doses of 0.3, 1, and 3 μM, although the changes in growth curves with these doses were observed at later times (24–30 hours of treatment). Lower doses such as 0.1 and 0.03 μM affected cell growth curves even later, after 32 hours of treatment, and the effect on cell growth was significantly less than with the higher doses. N-t-boc-Daidzein treatment at doses of 0.01 and 0.003 μM were found to have no effect on cell growth.
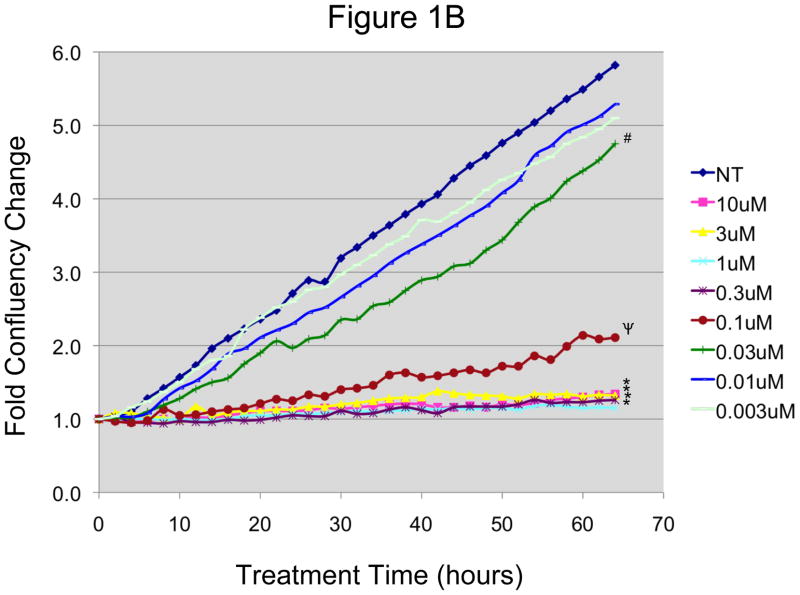
Figure 1. In vitro effect of N-t-boc-Daidzein on EOC cells.
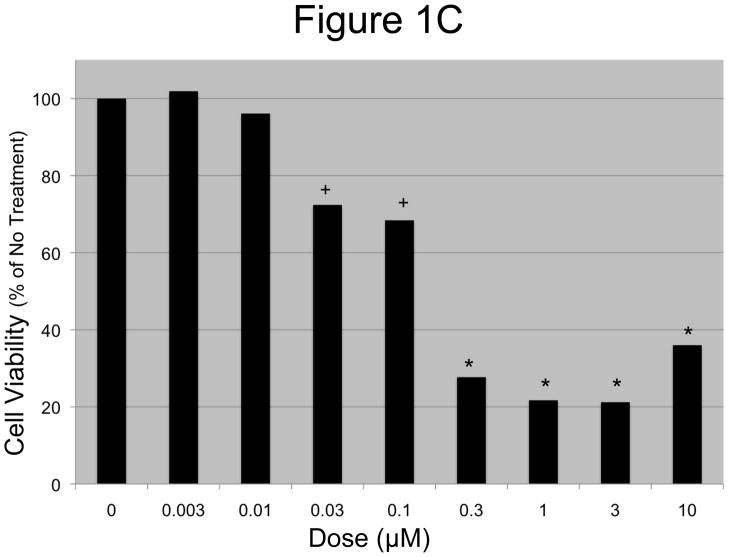
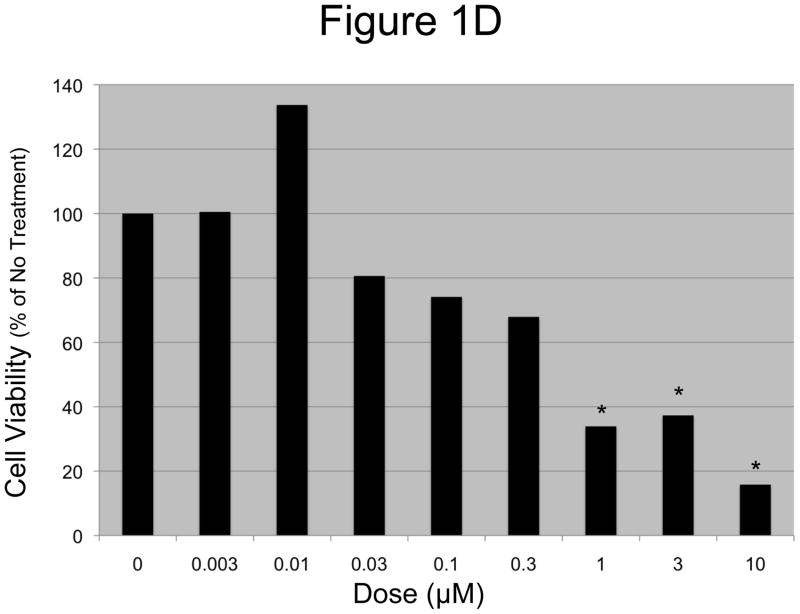
A and B: EOC cells were treated with increasing concentrations of N-t-boc-Daidzein (0–10μM) for an average of 65 hours. Confluence was measured by real time in vitro micro-imaging using the IncuCyte system and fold confluency change over time plotted for (A) OCSC1 and (B) TARA R127. * p <0.001, ψ p <0.01, + p<0.025, # p <0.05, when compared to control. C and D: Percent viable cells after 65h of treatment, (C) OCSC1 and (D) TARA R127. * p<0.001, + p<0.025). Similar results were observed for the other cell lines tested. Experiments were performed in triplicate.
Similar results were observed during the treatment of mOCCs with N-t-boc-Daidzein (figure 1B), with doses of 0.3 μM and higher significantly inhibiting cell growth, doses of 0.1 and 0.03 μM causing some, but less, of an effect, and doses of 0.01 and 0.003 μM having no significant effect on growth. Cell growth of the mOCCs was inhibited by treatment at earlier time points than in the OCSCs, with changes in the mOCC growth curves after 10 hours of treatment for all effective concentrations of N-t-boc-Daidzein (0.03–10 μM).
In order to determine whether the changes in the cell growth curves observed following treatment with N-t-boc-Daidzein were due to a decrease in cell viability, we performed the conventional cell viability assay on the cultures at the end of the treatment (figures 1C–D) using the CellTiter 96 assay. These results correlated with those observed using the IncuCyte system, with no changes in cell viability for the OCSCs treated with doses 0.003 and 0.01 μM when compared to the control, small decreases in cell viability (P<0.025) with doses 0.03 and 0.1 μM, and significantly decreased cell viability (P<0.001) for doses of 0.3 μM or greater. The GI50 for OCSCs treated with N-t-boc-Daidzein was approximately 0.2 μM. Cell viability results for the mOCCs also correlated with the data obtained from the Incucyte sytem with the GI50 for mOCCs at approximately 0.6 μM.
Decrease in cell growth with N-t-boc-Daidzein treatment is associated with apoptosis
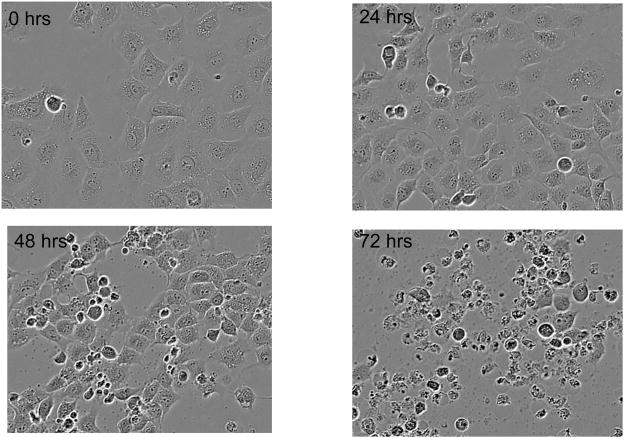
Our next objective was to determine the morphologic changes associated with the decrease in cell growth and viability observed with N-t-boc-Daidzein treatment. As shown in figure 2 and Movies 1–4 (supplementary material), OCSCs and mOCCs treated with N-t-boc-Daidzein (10 μM) undergo morphologic changes characteristic of apoptosis, including cell shrinkage, chromatin condensation, membrane blebbing, formation of apoptotic bodies, and lastly, DNA fragmentation 10.
Figure 2. Induction of apoptosis in OCSCs with N-t-boc-Daidzein.
OCSCs were treated with 10μM N-t-boc-Daidzein and their morphologic changes were followed using real time in vitro micro-imaging. Results shown are for OCSC1. Similar results were observed with the other cell lines tested.
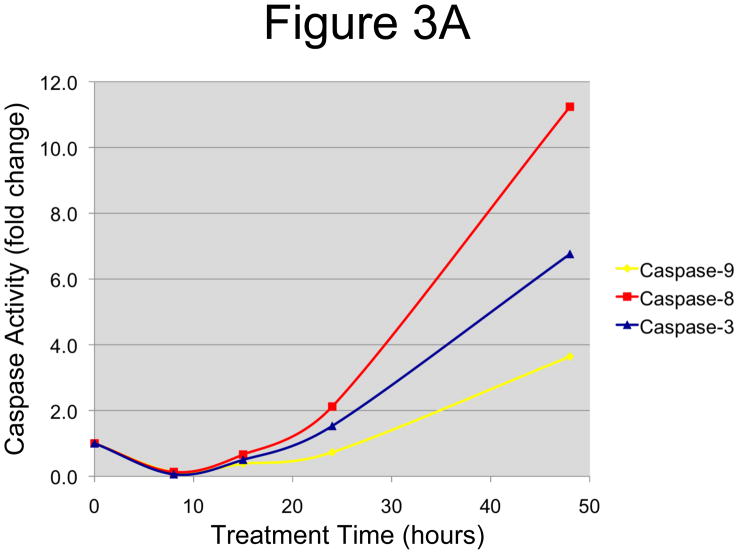
In order to determine whether these morphological changes were indeed induced by apoptosis, we evaluated the activation levels of the caspases, the main mediators of apoptotic cell death. OCSCs were treated with 10 μM N-t-boc-Daidzein for different time points (0–48 hours) and the activity of initiator caspases-8 and -9 and the effector caspase-3 was measured using the Caspase-Glo 8, 9, and 3/7 Assays, respectively. As shown in figure 3A, a significant increase in caspase activation was observed in a time-dependent manner for all three caspases tested. Activity of caspases-3 and -8 doubled after 24h, while caspase-9 activity doubled after 48h, suggesting that N-t-boc-Daidzein is able to induce apoptosis in OCSCs.
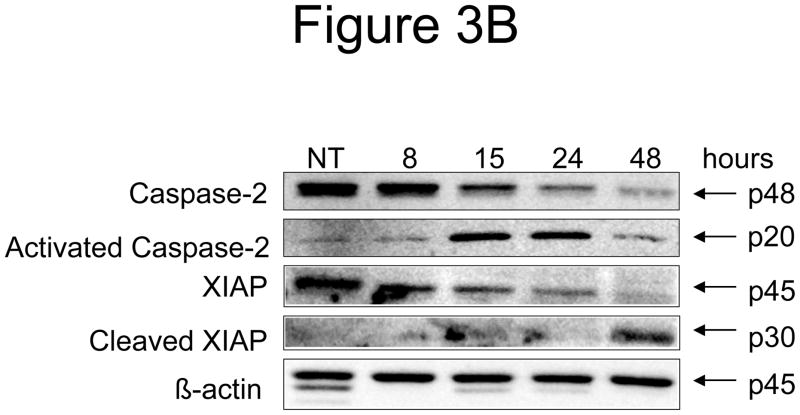
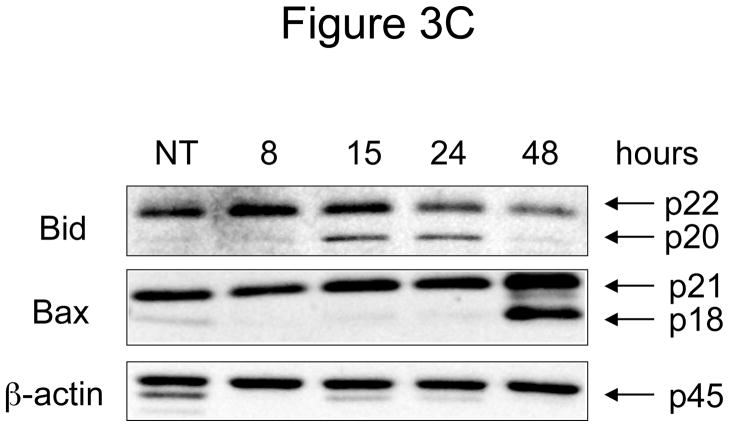
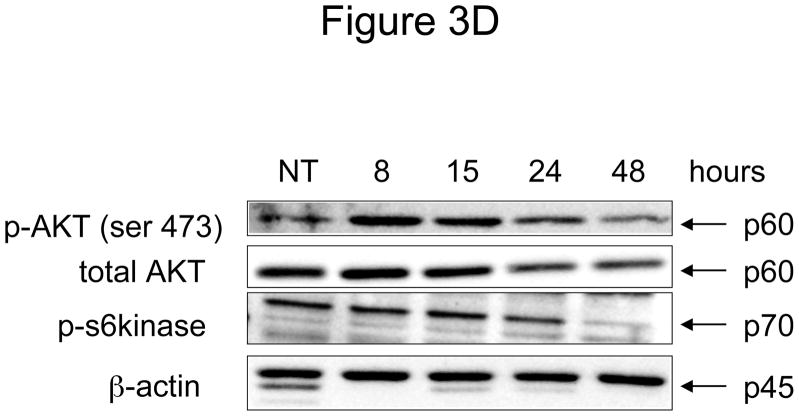
Figure 3. Effect of N-t-boc-Daidzein treatment on components of the apoptotic cascade.
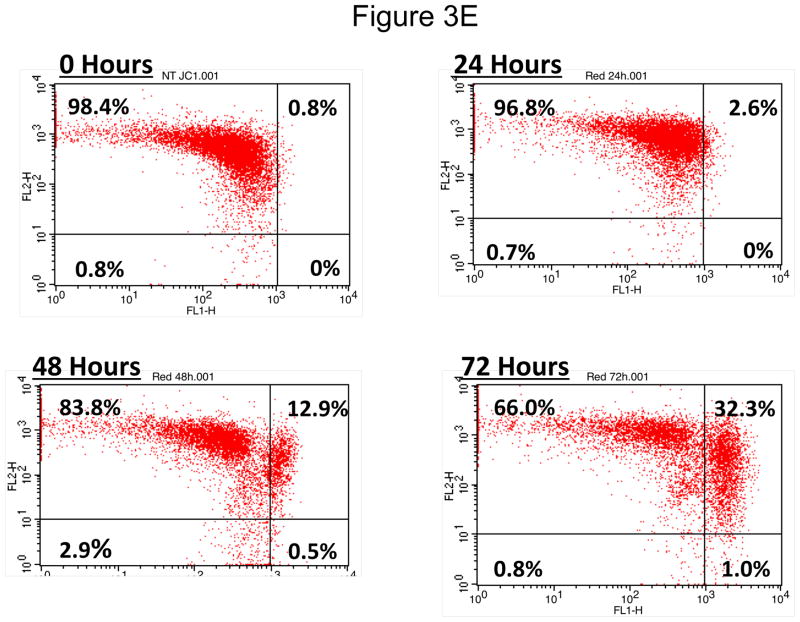
OCSCs were treated with 10μM N-t-boc-Daidzein for 8, 15, 24, or 48h. (A) Activity of caspases-3/7, -8, and -9 was measured using Caspase-Glo assays. (B–D) Effect on pro- and anti-apoptotic proteins, and pro-survival proteins was determined using Western blot analyses. (E) Effect on mitochondrial membrane stability was determined using the JC-1 dye. Results shown are for OCSC1. Similar results were observed with the other cell lines tested.
Early changes in the apoptotic pathway induced by N-t-boc-Daidzein treatment
There are two main pathways that lead to caspase activation and the initiation of apoptosis. One is the intrinsic, or mitochondrial, pathway that involves disruption of the mitochondrial membrane and caspase-9 activation. The second is the extrinsic pathway, which is activated by binding of ligands to transmembrane death receptors on the surface of the cells, leading to caspase-8 activation and initiation of the apoptotic cascade. These two pathways are interconnected and can activate each other. Both the intrinsic and extrinsic apoptosis pathways are controlled by a delicate balance between pro and anti-apoptotic proteins 11, 12. Our next objective was to characterize the activation status of some of these key regulatory proteins in OCSCs following N-t-boc-Daidzein treatment.
The earliest changes observed after N-t-boc-Daidzein treatment were caspase-2 activation and XIAP degradation. As shown in figure 3B, following 8 hours of N-t-boc-Daidzein treatment we observed a decrease in the inactive form of caspase-2 (p48), representing capase-2 activation, and appearance of the activated caspase-2 fragment at 15 hours (p20). Additionally, after 8 hours of treatment we observed a decrease in the anti-apoptotic protein, X-linked inhibitor of apoptosis protein (XIAP) and later observed the appearance of its p30 cleavage product, thought to be formed when XIAP is cleaved by caspases.
Late involvement of the intrinsic apoptosis pathway in N-t-boc-Daidzein-induced apoptosis
The increase in caspase-9 activity that we observed suggested involvement of the mitochondrial pathway in N-t-boc-Daidzein-induced apoptosis. To further study mitochondrial pathway involvement, we looked at several proteins in the Bcl2 family of proteins, which are the main regulators of mitochondrial stability and the intrinsic apoptosis pathway. These proteins can be divided into three groups: 1) pro-survival proteins, such as Bcl2, 2) pro-apoptotic proteins, including Bid, and 3) the Bax subfamily. In normal, healthy cells, the pro-survival proteins outweigh the pro-apoptotic proteins, resulting in cell survival. However, when a cell is given a stress signal, the activated pro-apoptotic proteins bind and neutralize the pro-survival molecules, allowing pro-apoptotic family members like Bak and Bax to oligomerize and create an open channel in the mitochondrial membrane, resulting in mitochondrial depolarization 13. These events may lead to the activation of caspase-9, which we observed after 48 hours of N-t-boc-Daidzein treatment.
Bid functions as a link between the extrinsic apoptosis pathway, involving caspase-8 activation, and mitochondrial integrity. Indeed, it has been shown that Bid is cleaved and activated by caspase-8, and the truncated form translocates to the mitochondria initiating the process of mitochondrial depolarization 14, 15. After 15 hours of N-t-boc-Daidzein treatment, Bid was activated, as shown by a decrease in the amount of full-length, inactive Bid (p22) and the appearance of its truncated, active form (p20) (figure 3C). The other pro-apoptotic Bcl2 family member, which was activated after N-t-boc-Daidzein treatment was Bax. We detected the cleaved form of Bax (p18) after 48 hours of treatment, which is indicative of Bax activation and mitochondrial translocation.
To further confirm the involvement of the mitochondrial pathway, we measured mitochondrial depolarization using JC1 dye, and found that there is a late, slow mitochondrial depolarization, beginning after 48 hours of treatment with N-t-boc-Daidzein (figure 3E). This time point for mitochondrial depolarization correlated more with Bax activation, which occurred at 48h, than with Bid activation, which occurred earlier at 15h. Furthermore, a significant increase in caspase-9 activity was observed after 48h of treatment. Taken together, our results suggest that N-t-boc-Daidzein primarily initiates apoptosis via the extrinsic pathway and later engages the intrinsic pathway to amplify the cascade.
Effect of N-t-boc-Daidzein on AKT survival pathway
As indicated above, apoptosis is the result of changes in the balance of pro- and anti-apoptotic pathways. Our next objective was to characterize the effect of N-t-boc-Daidzein on one of the main pro-survival pathways, the AKT survival pathway.
AKT promotes cell survival by controlling the activities of caspase-9, Bax, and the mTOR pathway and protecting XIAP from degradation. AKT has been shown to protect ovarian cancer cells against pro-apoptotic signals 16. Interestingly, we observed a time-dependent decrease in activated, phosphorylated AKT (p-AKT) following N-t-boc-Daidzein treatment. Furthermore, we also observed a decrease in total AKT levels, suggesting that N-t-boc-Daidzein not only affects AKT phosphorylation and activation, but also triggers its degradation. This had downstream effects on other pro-survival pathways regulated by AKT, including the mTOR pathway. We measured the activity of p-p70 S6 kinase (p-p70S6K), one of the main effectors of the mTOR pathway, using Western blot analysis and found that the level of p-p70S6K began to decrease after 24 hours of treatment, with only a faint band remaining at 48 hours (figure 3D).
EOCs fail to recover from 12-Hours of N-t-boc-Daidzein treatment
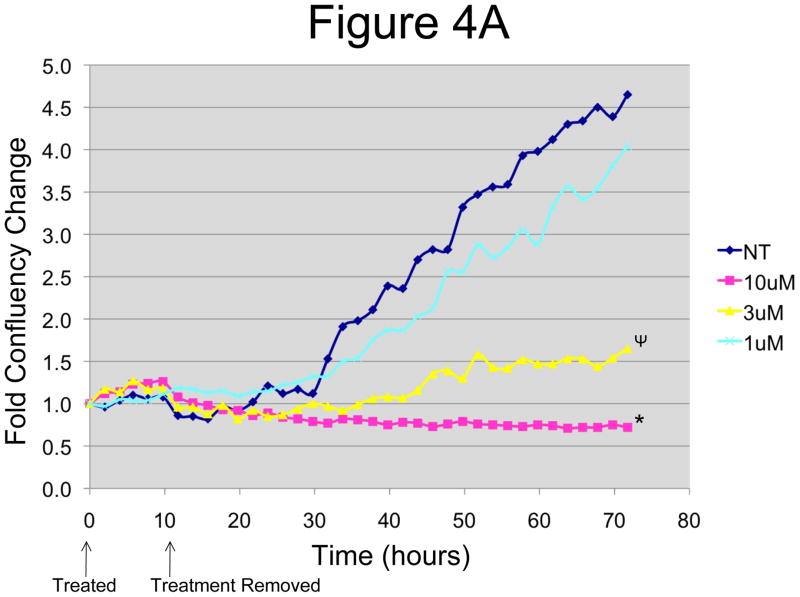
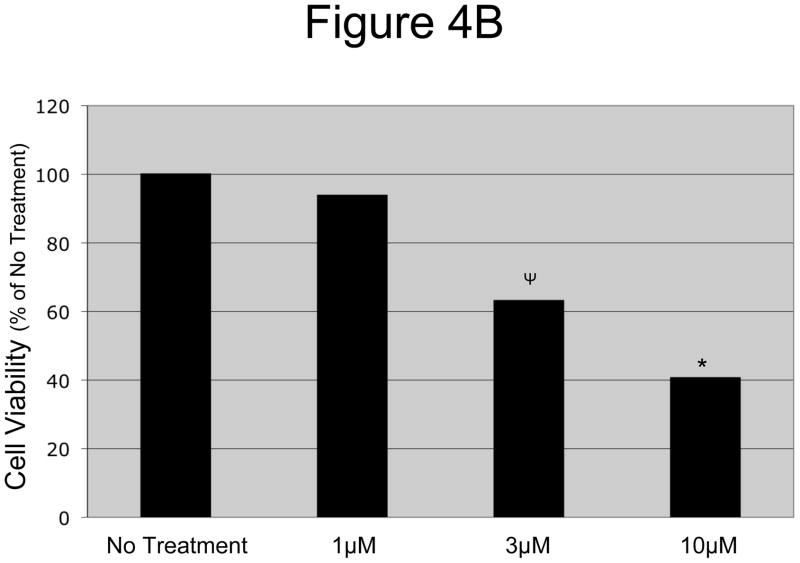
In order to get a better idea of how effective the drug would be in vivo where it is cleared from the blood, we designed an in vitro wash out system. OCSCs and mOCCs were treated with increasing concentrations (1–10μM) of N-t-boc-Daidzein for 12-hours, after which we removed and washed out the compounds, replaced the cultures with regular growth media, and followed the cell confluence using the IncuCyte system. As shown in figure 4A, OCSCs treated for 12-hours with 3 μM or greater N-t-boc-Daidzein still exhibited significantly decreased cell growth, even after 60 hours of recovery. This gives promising results that the compound will be able to induce cell death prior to its clearance from the blood in vivo. These results were confirmed by cell viability testing after the 60 hour recovery period. As shown in figure 4B, there is a significant decrease in OCSC viability following 12 hours of N-t-boc-Daidzein treatment and 60 hours of recovery.
Figure 4. OCSCs failed to recover after a 12h treatment with N-t-boc-Daidzein.
OCSCs were treated with increasing concentrations of N-t-boc-Daidzein (0–10μM) for 12 hours, after which media containing the compound was removed and replaced with regular growth media. (A) Fold confluency change over time was measured for the succeeding 60h using real time in vitro micro-imaging. * p<0.001, ψ p<0.01. (B) Cell viability was measured at the end of the 60h recovery period using the CellTiter Assay. * p <0.001, ψ p<0.01). Experiments were performed in triplicate and the averages were graphed. The results shown are for OCSC2. Similar results were observed with other lines tested.
Discussion
We demonstrate in this study that the novel compound N-t-boc-Daidzein is capable of inducing apoptosis in EOC cells, including the OCSCs. The drug exerts its effect through the activation of pro-apoptotic proteins, such as Caspases-2, 3, 8, and 9, Bid and Bax, and the degradation of anti-apoptotic proteins XIAP and AKT. Additionally, the failure of OCSCs to recover following 12 hours of treatment gives promising results for the compound’s effectiveness in vivo.
Cancer recurrence is a major obstacle in the treatment of EOC, with over 80% of initially responsive tumors resulting in recurrence. This high rate of recurrence is thought to be due to the OCSCs, which are resistant to conventional treatments 17. Cancer stem cells are defined as cells within the tumor that have the ability to self-renew and to differentiate into the heterogeneous cell types that make up the bulk of the tumor 18. The initial response to chemotherapy that is seen in the majority of ovarian cancers is due to death of the bulk of the tumor cells, resulting in a decrease in tumor size, as evidenced by radiography tests and a downward trend in CA-125 levels. However, most of the CSCs survive the treatment and over time, they are able to regenerate the bulk of the tumor resulting in recurrence. Therefore, finding a compound that is capable of killing the OCSCs is extremely important to increasing overall survival and improving rates of tumor remission.
N-t-boc-Daidzein is a derivative of the isoflavone Daidzein. Isoflavones are plant hormones that regulate normal cell cycle kinetics in plants. Isoflavone derivatives have been shown to be effective in inducing cell death in many forms of cancers, including ovarian cancer 19. However, many of these compounds have a very short half-life, and therefore, loose their efficacy once tested in humans 20. The long alkyl chain on the Daidzein molecule in N-t-Boc-Daidzein imparts lipophilic character facilitating endocytosis and the N-t-Boc group at the end of the side chain provides metabolic stability, which we hypothesize will increase its efficacy in vivo 9.
Our in vitro testing revealed that concentrations of 0.03 μM and higher of N-t-boc-Daidzein lead to significant inhibition of OCSC growth, along with a significant decrease in cell viability. Morphologic changes typical of apoptosis, as well as the activation of a number of important caspases, including initiator caspases-2, -8, and -9 and the main effector caspase, caspase-3, proved that N-t-boc-Daidzein treatment is, in fact, activating the apoptotic cascade, resulting in cell death. These results confirmed those found in a preliminary study done by Kohen et al. which showed that N-t-boc-Daidzein was successful in inhibiting the growth of ovarian and colon cancer cells in vitro, as measured by l[3H]-labeled thymidine incorporation into DNA 9. They also showed that the action of the drug was inhibited by the addition of the pan-caspase inhibitor Z-VADFK, supporting our findings that N-t-boc-Daidzein induces a caspase-dependent cell death 9.
The earliest change in the regulatory proteins of the apoptotic cascade that we observed following in vitro treatment of OCSCs with N-t-boc-Daidzein was a decrease in XIAP levels, beginning after 8 hours of treatment. XIAP has been shown to bind directly to and block the activity of caspases-3, 7, and 9, thus inhibiting apoptosis 21. There is substantial evidence that suggests that XIAP plays a role in chemo-resistance of ovarian cancer cells, with increased ovarian cancer cell death both in vitro and in vivo following downregulation of XIAP expression using antisense oligonucleotides 22, 23. Therefore, this early decrease in XIAP levels that we observed following N-t-boc-Daidzein treatment may be an important step in the induction of death in OCSCs.
We also observed a decrease in the amount of anti-apoptotic phosphorylated AKT and total AKT protein levels. It has been shown that AKT levels can decrease due to cleavage by caspases, degradation by the proteasome, or translational down-regulation as a result of decreasing XIAP levels 24. Our results show a reduction in both p-AKT and total AKT levels after 15 hours of treatment with N-t-boc-Daidzein. This occurs prior to caspase activation, but following the down-regulation of XIAP, suggesting that AKT is degraded by XIAP-related mechanisms or by proteasomal degradation. Along with supporting cell growth and survival, AKT has been found to play a role in promoting angiogenesis, increasing telomerase activity, improving cell motility, and altering cell-cell adhesion, all of which are important in tumor metastasis. Hence, N-t-boc-Daidzein’s ability to promote the degradation of AKT may have significant implications in reducing tumor growth and spread.
In addition to these important changes observed in the apoptotic pathway in response to N-t-boc-Daidzein treatment, alterations in the mTOR survival pathway were also observed. Levels of p-p70S6K, an important effector kinase in the mTOR pathway, decreased significantly after 24 hours of treatment. Previous studies have found that increased nuclear expression of p-p70S6K was associated with malignant ovarian tumors, when compared to benign or borderline tumors 25. Thus, the ability of N-t-boc-Daidzein to decrease p-p70S6K levels may be important in diminishing tumor aggressiveness and promoting cancer cell death.
In addition to these promising in vitro results, preliminary animal studies done by Kohen et al. showed a reduction in tumor size with N-t-boc-Daidzein treatment in an in vivo model of ovarian cancer in CD1 nude mice 9. Furthermore, they found that there was no difference in weight reduction or death of mice between the treatment and control groups, suggesting that the drug was not toxic to normal cells and was well tolerated in vivo 9. Our next step is to perform additional animal studies to assess the effectiveness of N-t-boc-Daidzein in killing the OCSCs in vivo and also to provide additional toxicology studies.
In summary, our results show that N-t-boc-Daidzein is able to successfully induce apoptotic cell death in OCSCs, which are extremely resistant to conventional chemotherapy agents. These results have two major implications. First, despite previous reports, which show that cancer stem cells in general are resistant to apoptosis, our data show that OCSCs can be induced to undergo apoptosis. Second, we show that a naturally occurring compound is potent enough to activate the caspases in OCSCs in vitro. The increased stability that the N-t-boc hexylene diamine side chain confers may be vital to improving the effectiveness of this compound on OCSCs in vivo. The failure of cells to recover following 12 hours of treatment with N-t-boc-Daidzein provides promising evidence for its ability to initiate the apoptotic cascade in OCSCs in vivo, prior to its clearance from the blood. As mentioned above, our next objective is to demonstrate if the potency of this compound against OCSCs is reflected in vivo. The ability to kill OCSCs in vivo is vital to decreasing rates of recurrence and improving overall survival in patients with EOC.
Materials and Methods
Cell lines and culture conditions
Primary EOC cell lines were isolated from malignant ovarian ascites and cultured as previously described 26. Cell lines were propagated in RPMI plus 10% fetal bovine serum (Gemini Bio-Products, West Sacramento, CA).
Growth curve and cell viability assay
Cells (4500 cells/well for OCSCs and 8000 cells/well for mOCCs) were plated in a 96-well plate. After 24h, the medium was replaced with OptiMem (Gibco, Invitrogen, Carlsbad, CA) for 4 hours followed by treatment. Growth curves were determined by placing cells in the Incucyte system (Essen Instruments, Ann Arbor, MI), which allows an automated and non-invasive method of monitoring live cells in culture. Growth curve is constructed automatically from data points acquired during round-the-clock kinetic imaging. Parallel plates were prepared for cell viability assays where 20 μL CellTiter Assay (Promega, Madison, WI) was added to each well and optical density acquired after 1 h at 490nm. Cell viability was normalized as a percentage of the untreated cells. Each experiment was done in triplicate and the results were averaged.
Caspase Activity Assay
The activity of caspases-3, -8, and -9 was measured using Caspase-Glo™ 3/7, 8, and 9 (Promega, Madison, Wisconsin), respectively, as previously described 11 using 5 μg protein in a 50 μL total volume. Fold-increase in activity was calculated based on the activity measured in the untreated cells.
SDS-PAGE and Western blots
SDS-PAGE and western blots were performed as previously described 11 using the following antibodies: mouse anti-caspase-2 (BD Biosciences, San Jose, CA 1:1,000), rabbit anti-Bid (Cell Signaling, Beverly, MA, 1:1,000), mouse anti-XIAP (BD Biosciences, 1:1,000), rabbit anti-phosphorylated Akt (Cell Signaling, 1:1,000), rabbit anti-Akt (Cell Signaling, 1:1,000), mouse anti-Bax (BD Biosciences, 1:250), anti-phosphorylated p70 S6 Kinase (Millipore, Billerica, MA, 1:2,000), and rabbit anti-actin (Sigma, St Louis, MO 1:10,000).
Mitochondrial Depolarization Assay
After treatment, cells were trypsinized and stained with JC-1 dye using the Mitocapture™ mitochondrial apoptosis detection kit (BioVision Research Products Mountain View, CA) according to the manufacturer’s instructions. Data was gathered using the FACS Calibur System and analyzed using CellQuest software (BD Biosciences, Rockville, MD).
Statistical Analysis
The two-sided t test was used to determine statistical differences between treatment and control groups at the end of treatment. p<0.05 was considered statistically significant.
Supplementary Material
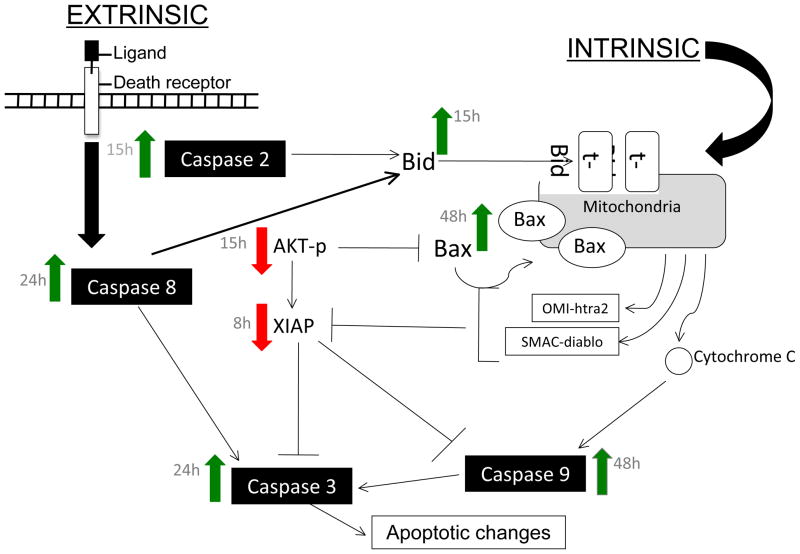
Figure 5. Proposed mechanism for the induction of apoptosis in EOC cells by N-t-boc-Daidzein.
N-t-boc-Daidzein induced apoptosis is characterized by activation of caspases- 2, 3/7, 8, and 9, down-regulation of XIAP, dephosphorylation of Akt and S6 kinase, activation of Bid and Bax, and mitochondrial depolarization.
Acknowledgments
This study was supported in part by grants from NCI/NIH RO 1CA127913 and RO1 CA118678; The Janet Burros Memorial Foundation, the Sands Foundation, and the Discovery To Cure Research Program. JMG is the recipient of the Doris Duke Clinical Research Fellowship from the Doris Duke Charitable Foundation.
Abbreviations Used
- EOC
epithelial ovarian cancer
- OCSC
ovarian cancer stem cell
- mOCC
mature ovarian cancer cell
- N-t-boc-Daidzein
5-{2-[3-(4-Hydroxy-phenyl)-4-oxo-4H-chromen-7-yloxy]-acetylamino}-pentyl)-carbamic acid tert-butyl ester
- CD44+
CD44-positive
- CD44−
CD44-negative
- XIAP
X-linked inhibitor of apoptosis protein
- p-p70S6K
p-p70 S6 kinase
References
- 1.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Berchuck A, Elbendary A, Havrilesky L, Rodriguez GC, Bast RC., Jr Pathogenesis of ovarian cancers. J Soc Gynecol Investig. 1994;1:181–90. doi: 10.1177/107155769400100302. [DOI] [PubMed] [Google Scholar]
- 3.Lu KH, Patterson AP, Wang L, Marquez RT, Atkinson EN, Baggerly KA, et al. Selection of potential markers for epithelial ovarian cancer with gene expression arrays and recursive descent partition analysis. Clin Cancer Res. 2004;10:3291–300. doi: 10.1158/1078-0432.CCR-03-0409. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz PE, Chambers JT, Taylor KJ. Early detection and screening for ovarian cancer. J Cell Biochem Suppl. 1995;23:233–7. doi: 10.1002/jcb.240590932. [DOI] [PubMed] [Google Scholar]
- 5.Alvero AB, Chen R, Fu H, Montagna M, Schwartz PE, Rutherford T, Silasi D, Steffensen KD, Visintin I, Mor G. Molecular phenotyping of human ovarian cancer stem cells unravel the mechanisms for repair and chemo-resistance. Cell Cycle. 2008 doi: 10.4161/cc.8.1.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser M, Leung B, Jahani-Asl A, Yan X, Thompson WE, Tsang BK. Chemoresistance in human ovarian cancer: the role of apoptotic regulators. Reprod Biol Endocrinol. 2003;1:66. doi: 10.1186/1477-7827-1-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–20. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Somjen D, Katzburg S, Kohen F, Gayer B, Livne E. Daidzein but not other phytoestrogens preserves bone architecture in ovariectomized female rats in vivo. J Cell Biochem. 2008;103:1826–32. doi: 10.1002/jcb.21565. [DOI] [PubMed] [Google Scholar]
- 9.Kohen F, Gayer B, Kulik T, Frydman V, Nevo N, Katzburg S, et al. Synthesis and evaluation of the antiproliferative activities of derivatives of carboxyalkyl isoflavones linked to N-t-Boc-hexylenediamine. J Med Chem. 2007;50:6405–10. doi: 10.1021/jm070727z. [DOI] [PubMed] [Google Scholar]
- 10.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–8. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 11.Alvero AB, O’Malley D, Brown D, Kelly G, Garg M, Chen W, et al. Molecular mechanism of phenoxodiol-induced apoptosis in ovarian carcinoma cells. Cancer. 2006;106:599–608. doi: 10.1002/cncr.21633. [DOI] [PubMed] [Google Scholar]
- 12.Kamsteeg M, Rutherford T, Sapi E, Hanczaruk B, Shahabi S, Flick M, et al. Phenoxodiol--an isoflavone analog--induces apoptosis in chemoresistant ovarian cancer cells. Oncogene. 2003;22:2611–20. doi: 10.1038/sj.onc.1206422. [DOI] [PubMed] [Google Scholar]
- 13.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–9. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 14.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–90. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 15.Wagner KW, Engels IH, Deveraux QL. Caspase-2 can function upstream of bid cleavage in the TRAIL apoptosis pathway. J Biol Chem. 2004;279:35047–52. doi: 10.1074/jbc.M400708200. [DOI] [PubMed] [Google Scholar]
- 16.Dan HC, Sun M, Kaneko S, Feldman RI, Nicosia SV, Wang HG, et al. Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP) J Biol Chem. 2004;279:5405–12. doi: 10.1074/jbc.M312044200. [DOI] [PubMed] [Google Scholar]
- 17.Chen R, Alvero AB, Silasi DA, Mor G. Inflammation, cancer and chemoresistance: taking advantage of the toll-like receptor signaling pathway. Am J Reprod Immunol. 2007;57:93–107. doi: 10.1111/j.1600-0897.2006.00441.x. [DOI] [PubMed] [Google Scholar]
- 18.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–84. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 19.Kelly GE, Husband AJ. Flavonoid compounds in the prevention and treatment of prostate cancer. Methods Mol Med. 2003;81:377–94. doi: 10.1385/1-59259-372-0:377. [DOI] [PubMed] [Google Scholar]
- 20.Constantinou AI, Mehta R, Husband A. Phenoxodiol, a novel isoflavone derivative, inhibits dimethylbenz[a]anthracene (DMBA)-induced mammary carcinogenesis in female Sprague-Dawley rats. Eur J Cancer. 2003;39:1012–8. doi: 10.1016/s0959-8049(03)00124-2. [DOI] [PubMed] [Google Scholar]
- 21.Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM, et al. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. Embo J. 1998;17:2215–23. doi: 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Feng Q, Kim JM, Schneiderman D, Liston P, Li M, et al. Human ovarian cancer and cisplatin resistance: possible role of inhibitor of apoptosis proteins. Endocrinology. 2001;142:370–80. doi: 10.1210/endo.142.1.7897. [DOI] [PubMed] [Google Scholar]
- 23.Sapi E, Alvero AB, Chen W, O’Malley D, Hao XY, Dwipoyono B, et al. Resistance of ovarian carcinoma cells to docetaxel is XIAP dependent and reversible by phenoxodiol. Oncol Res. 2004;14:567–78. doi: 10.3727/0965040042707943. [DOI] [PubMed] [Google Scholar]
- 24.Asselin E, Mills GB, Tsang BK. XIAP regulates Akt activity and caspase-3-dependent cleavage during cisplatin-induced apoptosis in human ovarian epithelial cancer cells. Cancer Res. 2001;61:1862–8. [PubMed] [Google Scholar]
- 25.Castellvi J, Garcia A, Rojo F, Ruiz-Marcellan C, Gil A, Baselga J, et al. Phosphorylated 4E binding protein 1: a hallmark of cell signaling that correlates with survival in ovarian cancer. Cancer. 2006;107:1801–11. doi: 10.1002/cncr.22195. [DOI] [PubMed] [Google Scholar]
- 26.Flick MB, O’Malley D, Rutherford T, Rodov S, Kamsteeg M, Hao XY, et al. Apoptosis-based evaluation of chemosensitivity in ovarian cancer patients. J Soc Gynecol Investig. 2004;11:252–9. doi: 10.1016/j.jsgi.2003.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.