Abstract
Background
Nonalcoholic fatty liver disease (NAFLD), one of the most common forms of chronic liver disease, is closely associated with obesity and insulin resistance (IR). Recent studies suggest serum retinol-binding protein 4 (RBP4) plays a key role in the pathogenesis of IR. The aims of this study were to determine serum RBP4 levels in patients with biopsy proven NAFLD, and to correlate these levels with the metabolic profile and histological features in this population.
Methods
Our cohort consisted of 51 consecutive patients undergoing liver biopsy for clinical suspicion of NAFLD. Patients were subsequently divided into 3 groups: simple steatosis (n=16), borderline NASH (n=2) and NASH (n=33). The stage of fibrosis was measured using a 4-point scale. RBP4 was measured in triplicates by a specific ELISA assay. The degree of insulin resistance was determined by the homeostatic model assessment (HOMA)
Results
Serum RBP4 levels did not correlate with body mass index, HOMA, fasting glucose, or insulin levels in patients with simple steatosis and NASH. Moreover, RBP4 levels were lower in patients with NASH compared to those with simple steatosis (21.3 mg/L and 26.8 mg/L respectively) although the difference did not reach statistical significance (P = 0.21). A stepwise decrease in RBP4 levels from patients without fibrosis (27.9 mg/L) to patients with cirrhosis (14.1 mg/L) was noted (P = 0.03).
Conclusions
Our study demonstrates that in adult patients with NAFLD, serum RBP4 levels do not correlate with BMI or insulin resistance and identifies a novel association between serum RBP4 levels and hepatocellular injury in these patients.
Keywords: nonalcoholic steatohepatitis, retinol binding protein 4, insulin resistance, steatosis, diabetes
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in Western populations [1] and it has come to be recognized as the hepatic manifestation of the metabolic syndrome [2]. Insulin resistance (IR) is the pathophysiological hallmark of NAFLD. Nonalcoholic steatohepatitis (NASH) is the most severe form of NAFLD with cirrhosis developing in 15% to 25% of patients [3, 4, 5].
Retinol-binding protein 4 (RBP4) is the only specific transport protein for retinol (vitamin A) in the circulation and its main function is thought to be the delivery of retinol to tissues [6]. Recent findings in mice assigned RBP4 a key role in the pathogenesis of IR [7]. However, in humans the link between RBP4 and insulin resistance is less clear. In one study [8], serum RBP4 correlated positively with presence of insulin resistance in individuals with obesity, impaired glucose tolerance, or type 2 diabetes, and was even increased in healthy individuals with a strong family history of type 2 diabetes.
Other reports have found no relationship between circulating RBP4 and IR [9, 10, 11, 12].
Although hepatocytes are regarded the principal source of circulating RBP4 under normal conditions, adipose tissue has the second highest expression level [13]. Changes in adipocyte derived RBP4 can have systemic effects on insulin sensitivity and glucose homeostasis. Recently attention has been addressed to the role of RBP4 in patients with clinical or histological diagnosis of NAFLD [14, 15]. The aim of this study is to evaluate RBP4 levels in a well-characterized cohort of patients with biopsy proven NAFLD.
Methods
Patient selection
The study protocol was approved by the Cleveland Clinic Institutional Review Board, and all patients gave written informed consent prior to participation. Our cohort consisted of 51 patients who underwent liver biopsy for clinical suspicion of NAFLD by their treating hepatologist. Blood was obtained from each patient at the time of liver biopsy. All had persistently elevated liver enzymes in the absence of alternate causes of elevated aminotransferases. Patients were excluded if alcohol consumption was >30 g/day for men, and >20 g/day for women, and if other liver diseases (viral hepatitis, sclerosing cholangitis, primary biliary cirrhosis, autoimmune hepatitis, hemochromatosis, Wilson’s disease, alpha-1-antitrypsin deficiency, and drug-induced liver disease) were detected by serologic testing and imaging studies. Demographic, clinical, and laboratory data were collected. The degree of insulin resistance was determined by the homeostatic model assessment (HOMA) using the formula: insulin resistance = (insulin × glucose)/22.5. Patients were subsequently divided into three groups according to their histological findings: simple steatosis, “borderline NASH” and “definitive NASH”. Only 2 patients were “borderline NASH” and were excluded as no comparisons could be made with only 2 patients. A total of 49 patients with clinically suspected NAFLD were used to perform the final statistical analysis.
Liver histology
The histological diagnosis was established using H&E and Masson trichrome stains of formalin-fixed paraffin-embedded liver and graded in a blinded fashion according to the NAFLD scoring system proposed by the National Institute of Diabetes and Digestive and Kidney Diseases NASH Clinical Research Network. A NAFLD activity score (NAS) > = 5 corresponded to a diagnosis of “definitive NASH”, a score of 3–4 corresponded to “borderline NASH”, and a score of < 3 corresponded to “simple steatosis”. The stage of fibrosis was similarly measured using a 4-point scale: 0, no fibrosis; 1, perisinusoidal/periportal fibrosis (1a mild zone 3 perisinusoidal, 1b moderate zone 3 perisinusoidal, 1c portal/periportal); 2, perisinusoidal and portal/periportal; 3, bridging fibrosis; 4, cirrhosis).
Measurement of serum RBP4
A blood sample was obtained from each patient at the time of liver biopsy, processed to plasma and stored frozen at − 80° C. Serum RBP4 was measured by an enzyme-linked immunosorbent assay (ELISA) (ALPCO Diagnostics). ELISA samples were run in triplicate, and the absorbance was determined using a microplate reader (Molecular Devices M2, Sunnyvale, CA). The coefficient of variation for interassay replicate samples was less than 10 percent for ELISA.
Statistical analysis
Descriptive statistics were computed for all variables. These included means and standard deviations or medians, as well as 25th and 75th percentiles for continuous factors. For categorical variables, frequencies and percentages were estimated. Spearman’s correlation coefficient was used to estimate the association of serum RBP4 levels with BMI, HOMA, serum glucose, and mean insulin levels. A p value of 0.05 was considered statistically significant.
Results
Patient Characteristics
The main clinical and serological features of the patients are described in table 1. Patient age (49.1 +/− 10.7) and sex (49% males) did not differ significantly between the groups, whereas BMI and HOMA were significantly higher in patients with NASH compared with patients with simple steatosis. AST/ALT ratio was not significantly different between the groups. Thirty four percent of the patients had clinical diabetes, 55.1% had hyperlipidemia, and 77.6% had hypertension. Sixteen patients (32.6 %) had a simple steatosis and 33 patients (67.4%) had definitive NASH. Four patients (8.2%) had cirrhosis (stage 4 fibrosis) and they all had definitive NASH. Of the 33 patients with definitive NASH, ten (31.3%) had stage 3 fibrosis. In addition, a detailed description of the individual components of NAS (steatosis, inflammation, and ballooning) is provided in table 1. The median NAS was 2.0 (1.0, 2.0) for patients with steatosis and 5.5 (5.0, 6.0) for patients with NASH.
Table 1.
Characteristics of the study population
| Factor | All (n=49) |
Steatosis (N=16) |
NASH (N=33) |
p-value |
|---|---|---|---|---|
| Age (yrs) | 49.1(10.7) | 45.1(11) | 51.1(10) | 0.079 |
| BMI (kg/m2) | 32.3(5.0) | 30.4(5.3) | 33.2(4.6) | 0.043 |
| Female | 25 (51.0) | 7 (43.8) | 18 (54.6) | 0.48 |
| Caucasian | 42 (85.7) | 13 (81.3) | 29 (87.9) | 0.67 |
| Diabetes | 15 (30.6) | 3 (18.8) | 12 (36.4) | 0.32 |
| Hyperlipidemia | 25 (51.1) | 5 (31.3) | 20 (60.6) | 0.054 |
| Hypertension | 38 (77.6) | 10 (62.5) | 28 (84.9) | 0.14 |
| HOMA | 5.0 (2.4, 12.4) | 1.8 (1.0, 2.9) | 6.9 (3.5, 14.2) | <0.001 |
| Glucose (mg/dL) | 111 (100.5, 150) | 103 (93.0, 110.0) | 126 (105.5, 167) | 0.018 |
| Insulin (IU/mL) | 38.2 (15.2, 103.5) | 18.7 (10.7, 96.0) | 49.5 (16.9, 107.0) | 0.17 |
| AST (U/L) | 68.5 (37, 106.5) | 50.0 (28.0, 82.5) | 84.5 (48.0, 113.5) | 0.11 |
| ALT (U/L) | 51.5 (43.5, 82.5) | 41.5 (28.5, 60.5) | 62.5 (46.0, 84.0) | 0.019 |
| RBP4 (mg/L) | 23.2(11.5) | 26.9(13.4) | 21.4(10.3) | 0.16 |
| Fibrosis stage | <0.001 | |||
| 0 | 20 (41.7) | 16 (100) | 5 (15.6) | |
| 1 | 9 (18.8) | 0 (0.0) | 8 (25.0) | |
| 2 | 5 (10.4) | 0 (0.0) | 5 (15.6) | |
| 3 | 10 (20.8) | 0 (0.0) | 10 (31.3) | |
| 4 | 4 (8.3) | 0 (0.0) | 4 (12.5) | |
| Inflammation | <0.001 | |||
| No/minimal | 14 (30.4) | 14 (93.3) | 0 (0.0) | |
| Mild | 14 (30.4) | 1 (6.7) | 13 (41.9) | |
| Moderate | 17 (37) | 0 (0.0) | 17 (54.8) | |
| Severe | 1 (2.2) | 0 (0.0) | 1 (3.2) | |
| Ballooning | <0.001 | |||
| None | 15 (31) | 14 (93.3) | 0 (0.0) | |
| Few | 12 (29) | 1 (6.7) | 12 (40.0) | |
| Many | 18 (40) | 0 (0.0) | 18 (60.0) | |
| Steatosis | <0.001 | |||
| None | 2 (4.4) | 2 (13.3) | 0 (0.0) | |
| 1–33% | 9 (19.6) | 6 (40.0) | 3 (9.7) | |
| 34–66% | 23 (50.0) | 7 (46.7) | 16 (51.6) | |
| >66% | 12 (26.1) | 0 (0.0) | 12 (38.7) | |
| NAS | 5.0 (2.0, 6.0) | 2.0 (1.0, 2.0) | 5.5 (5.0, 6.0) | <0.001 |
Values presented as N (%) for categorical factors, Mean (SD) for age, BMI and RBP4 or Median (P25, P75) otherwise.
P-values correspond to t-tests for age, BMI and RBP4, Pearson's chi-square for gender and hyperlipidemia, Fisher's exact tests for race, diabetes mellitus and hypertension and Wilxocon rank sum tests otherwise.
Serum RBP4 levels and Metabolic Profile in patients with NAFLD
There was no evidence to suggest that serum RBP 4 levels were significantly associated with BMI, or degree of insulin resistance as determined by either HOMA-IR, fasting glucose, or insulin levels (Table 2). Moreover, there was no statistically significant difference in RBP4 levels between patients with clinical diagnosis of diabetes in comparison to those without diabetes (20.2 ± 9.4 mg/ L vs. 24.5 ± 12.3, respectively, p= 0.19). The association between RBP4 levels and HOMA-IR was re-examined after excluding patients with diabetes from the analysis. Again, no correlation was found between HOMA-IR and RBP4 levels [rho= −0.23 (−0.59, 0.14); p= 0.21].
Table 2.
Associations with RBP4 levels: Spearman's correlation coefficients
| Factor | rho (95% CI) | P−value |
|---|---|---|
| BMI | −0.10 (−0.39,0.19) | 0.49 |
| HOMA | −0.27 (−0.57,0.02) | 0.065 |
| Glucose | −0.13 (−0.44,0.17) | 0.37 |
| Mean Insulin | 0.08 (−0.23,0.38) | 0.61 |
| AST | 0.09 (−0.20,0.39) | 0.54 |
| ALT | −0.06 (−0.36,0.23) | 0.66 |
| Fibrosis grade | −0.37 (−0.64,−0.09) | 0.011 |
| Inflammation | −0.27 (−0.56,0.03) | 0.073 |
| Balloon | −0.07 (−0.38,0.24) | 0.65 |
| Steatosis | −0.17 (−0.47,0.13) | 0.26 |
| NAS | −0.17 (−0.47,0.13) | 0.26 |
No evidence to suggest that serum RBP4 is significantly correlated to BMI, HOMA, glucose, insulin, AST, ALT or NAS and its components. Fibrosis grade was found to be correlated to RBP4 levels (rho= −0.37).
Serum RBP4 levels and liver histolopathological features in NAFLD
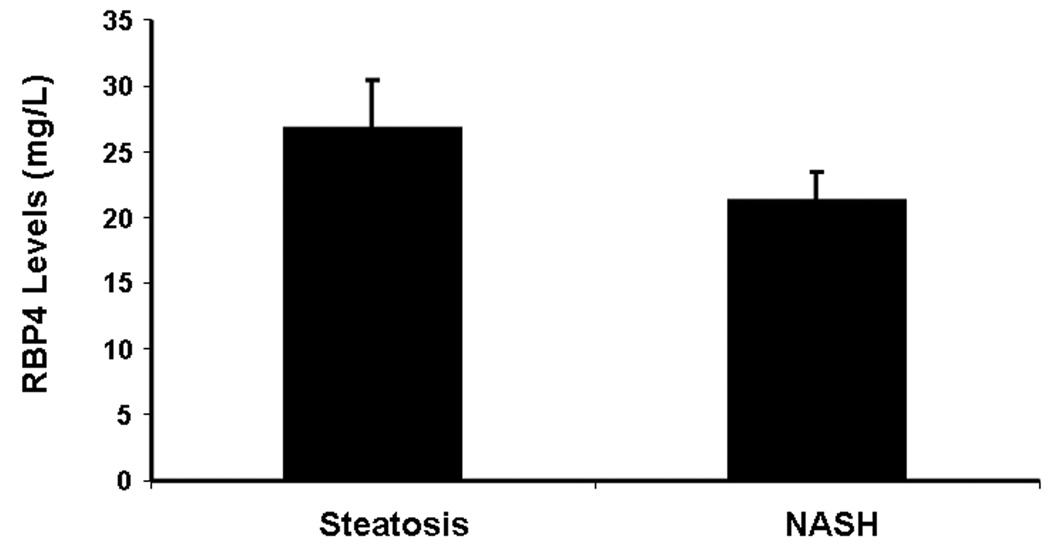
Patients with NASH had lower RBP4 levels compared to those with fatty liver (21.3 ± 2.1 mg/L and 26.8 ± 3.6 mg/L, respectively) although the difference did not reach statistical significance (Figure 1). There was no evidence to suggest that RBP levels were significantly different between the two groups after adjusting for BMI, HOMA and insulin levels (P=0.21) (Table 3). Also, no significant correlation were found between any of the individual histological features of NAS or total NAS and RBP4 levels as demonstrated in table 2.
Figure 1.
Adjusted RBP Means by NAFLD Group (ANCOVA). Although RBP4 levels appears to be slightly higher in patients with steatosis, there was no evidence to suggest that RBP4 levels are significantly different between the two groups even after adjusting for BMI, HOMA and insulin (P=0.21).
*Adjusting for BMI, HOMA and Insulin
Table 3.
RBP4 levels and NAFLD
| NAFLD | Unadjusted Mean RBP4 (95% CI) |
P- value |
Adjusted Mean RBP4* (95% CI) |
P- value |
|---|---|---|---|---|
| Steatosis | 26.9 (21.2, 32.7) | 0.12 | 26.8 (19.6, 34.1) | 0.21 |
| NASH | 21.4 (17.4, 25.4) | 21.3 (16.9, 25.6) |
Adjusted for BMI, HOMA, and insulin levels
Results correspond to ANOVA and ANCOVA
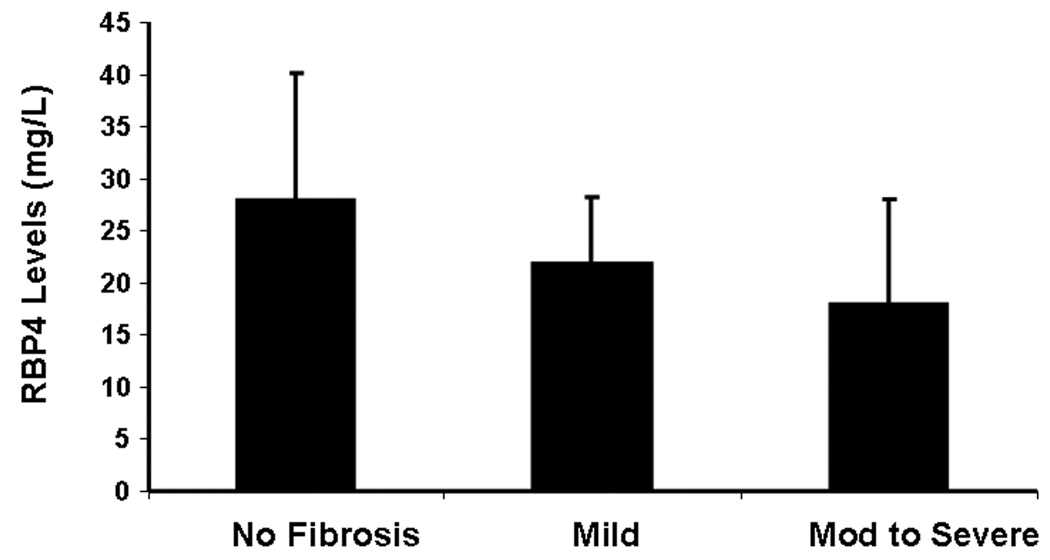
More importantly, a stepwise decrease in RBP4 levels from patients without fibrosis (27.9 mg/L) to patients with cirrhosis (14.1 mg/L) was noted (p = 0.03) as shown in table 4. A significant inverse correlation was found between the stage of fibrosis and serum RBP4 levels (Figure 2). After adjusting for factors associated with fibrosis (age, HOMA and ALT), fibrosis remained significantly associated with RBP 4 levels (p = 0.025) in a multivariable linear regression analysis. For every one stage increase in fibrosis stage the mean RBP 4 levels decreased by 3.06 mg/ L. These results demonstrated that the lowest RBP4 levels were seen in patients with advanced fibrosis and cirrhosis, indicating that serum RBP4 is closely linked to liver function.
Table 4.
Fibrosis grade and RBP4 levels
| Fibrosis grade |
N | Mean (SD) |
|---|---|---|
| 0 | 20 | 27.9 (13.6) |
| 1 | 9 | 21.7 (6.2) |
| 2 | 5 | 18.6 (10.2) |
| 3 | 10 | 20.8 (9.7) |
| 4 | 4 | 14.1 (11.1) |
A stepwise decrease in RBP4 levels from patients without fibrosis (27.9q mg/L) to patients with cirrhosis (14.1 mg/L) was noted (p value=0.03).
Figure 2.
A stepwise decrease in RBP4 levels was noted from patients without fibrosis (28 mg/L) to patients with mild fibrosis (22 mg/L) to patients with moderate to severe fibrosis (18 mg/L) (p value < 0.05).
Discussion
The recently reported association between RBP4 and insulin resistant states prompted us to evaluate RBP4 levels in patients with NAFLD as a potential marker for disease severity and the development of NASH in these patients. Our study demonstrated a lack of association between serum RBP4 levels and features of the metabolic syndrome including obesity and insulin resistance in NAFLD patients. RBP4 levels were lower in NASH patients compared to fatty liver patients. The most important finding of our study was the inverse correlation between the stage of fibrosis and RBP4 levels with the lowest levels in patients with advanced fibrosis or cirrhosis. The results identify RBP4 as a potential novel marker to assess fibrosis progression in patients with NAFLD.
In their original paper on RBP4 and IR, Yang et al [7] showed that RBP4 expression was elevated in adipose tissue of insulin resistant mice with elevated serum RBP4 levels. Furthermore, transgenic overexpression of RBP4 or injection of RBP4 caused IR, whereas genetic deletion enhanced insulin sensitivity. A great deal of interest followed this initial report and multiple studies tried to evaluate serum RBP4 levels in humans with insulin resistant states with contrasting results. Initial studies demonstrated increased serum RBP4 levels in obesity, impaired glucose tolerance and type 2 diabetes (all considered insulin resistant states and are associated with NAFLD) [16, 17, 18, 19].
In contrast to the animal data, Janke et al. [9] found that RBP4 mRNA was downregulated in subcutaneous adipose tissue of obese postmenopausal women and circulating RBP4 levels were similar in normal weight, overweight and obese women. In addition, Promintzer et al. [12] found no association between serum RBP4 and IR in two groups of nondiabetic humans who were markedly different regarding insulin sensitivity. A new longitudinal study by Lewis et al. [11] demonstrated that nondiabetic overweight patients who developed significant IR (during the 3 year follow up period) showed no change in serum RBP4 levels. An intriguing study in obese girls showed a negative correlation of RBP4 levels with BMI and no association with IR measured by HOMA index [20] suggesting a different role of RBP4 in pediatric patients. These studies challenged the concept that circulating RBP4 correlates with IR and highlighted the fact that adipose tissue may be a less important source of circulating RBP4 in humans.
The liver remains the primary source of RBP4 synthesis and liver function has a tremendous impact on RBP4 levels. In a study of 93 patients with Child A–C cirrhosis [21], no correlation was found for RBP4 with fasting glucose, C-peptide, or HOMA index. A recent study assessed RBP4 levels in NAFLD patients [15]. The study suggested that serum RBP4 levels were significantly associated with ultrasonographic evidence of fatty liver and transaminases in these patients. However, no liver biopsy was available and thus the presence of NASH, severity of disease and stage of fibrosis could not be determined. To date, the only study that evaluated serum RBP4 levels in biopsy proven NAFLD patients (n=37) have found higher serum levels of RBP4 in these patients compared to controls (35.2 vs. 28.9 mg/L, respectively) [14]. However, most of these patients had no fibrosis or mild fibrosis (stage 0–1 in 83.8%) with only 2 patients with stage 3–4 fibrosis. Furthermore, there was no association between RBP4 levels and AST or gamma-glutamyl transferase.
In our study, more than half of NAFLD patients (53.5%) had stage 2–4 fibrosis which may explain the lower serum RBP4 levels. We also found a trend toward having lower RBP4 levels in NASH patients compared to fatty liver patients. Limitations to our study include the lack of a matched control group of patients with high BMI and IR without liver disease to compare their RBP4 levels to our patient population. Another limitation is the lack of data about RBP4 expression in the liver and adipose tissues to determine if RBP4 mRNA was downregulated in either of these sites. However, these limitations do not lessen the significance of our results as there is an urgent need to develop accurate and noninvasive tests to help distinguishing NASH from simple steatosis and assess histological severity in patients with suspected NAFLD [22, 23, 24].
In the future, larger studies are needed to further establish the role of measuring serum RBP4 levels in patients with NAFLD. Our findings suggest that liver injury due to NASH may result in decreased levels of RBP4. Our study was not designed to clarify the mechanism by which the liver down regulated the production of RBP4 in this condition, although it is unrelated to the overall synthetic function of the liver as all our patients had well compensated state of the disease. The inverse relationship between RBP4 levels and fibrosis could be hypothetically explained by the fact that retinoic acid is a suppressor of type I collagen expression by hepatic stellate cells, which have an essential role in fibrogenesis [25]. Lower RBP4 levels may be involved in activating stellate cells to overexpress and deposit type I collagen in the liver. Lower RBP4 levels in patients with NAFLD could potentially be used as a screening tool to identify patients at higher risk for developing NASH and advanced fibrosis.
Acknowledgments
This work was supported by NIH grant (DK076852) and the AGA Research Scholar Award (RSA) to AEF
Footnotes
No conflicts of interest exist for any of the authors of this manuscript.
References
- 1.Bloomgarden ZT. Second World Congress on the Insulin Resistance Syndrome: hypertension, cardiovascular disease, and treatment approaches. Diabetes Care. 2005;28:2073–2080. doi: 10.2337/diacare.28.8.2073. [DOI] [PubMed] [Google Scholar]
- 2.Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42:987–1000. doi: 10.1002/hep.20920. [DOI] [PubMed] [Google Scholar]
- 3.Bacon BR, Farahvash MJ, Janney CG, et al. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994;107:1103–1109. doi: 10.1016/0016-5085(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 4.Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 5.McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006;40 Suppl 1:S17–S29. doi: 10.1097/01.mcg.0000168645.86658.22. [DOI] [PubMed] [Google Scholar]
- 6.Quadro L, Blaner WS, Salchow DJ, et al. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. Embo J. 1999;18:4633–4644. doi: 10.1093/emboj/18.17.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 8.Graham TE, Yang Q, Bluher M, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 9.Janke J, Engeli S, Boschmann M, et al. Retinol-binding protein 4 in human obesity. Diabetes. 2006;55:2805–2810. doi: 10.2337/db06-0616. [DOI] [PubMed] [Google Scholar]
- 10.Lewis JG, Shand BI, Frampton CM, et al. An ELISA for plasma retinol-binding protein using monoclonal and polyclonal antibodies: plasma variation in normal and insulin resistant subjects. Clin Biochem. 2007;40:828–834. doi: 10.1016/j.clinbiochem.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Lewis JG, Shand BI, Frampton CM, et al. Plasma retinol-binding protein is not a marker of insulin resistance in overweight subjects: A three year longitudinal study. Clin Biochem. 2008 doi: 10.1016/j.clinbiochem.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Promintzer M, Krebs M, Todoric J, et al. Insulin resistance is unrelated to circulating retinol binding protein and protein C inhibitor. J Clin Endocrinol Metab. 2007;92:4306–4312. doi: 10.1210/jc.2006-2522. [DOI] [PubMed] [Google Scholar]
- 13.Tsutsumi C, Okuno M, Tannous L, et al. Retinoids and retinoid-binding protein expression in rat adipocytes. J Biol Chem. 1992;267:1805–1810. [PubMed] [Google Scholar]
- 14.Petta S, Camma C, Di Marco V, et al. Retinol-binding protein 4: a new marker of virus-induced steatosis in patients infected with hepatitis c virus genotype 1. Hepatology (Baltimore, Md. 2008;48:28–37. doi: 10.1002/hep.22316. [DOI] [PubMed] [Google Scholar]
- 15.Seo JA, Kim NH, Park SY, et al. Serum retinol-binding protein 4 levels are elevated in non-alcoholic fatty liver disease. Clinical endocrinology. 2008;68:555–560. doi: 10.1111/j.1365-2265.2007.03072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho YM, Youn BS, Lee H, et al. Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care. 2006;29:2457–2461. doi: 10.2337/dc06-0360. [DOI] [PubMed] [Google Scholar]
- 17.Gavi S, Stuart LM, Kelly P, et al. Retinol-binding protein 4 is associated with insulin resistance and body fat distribution in nonobese subjects without type 2 diabetes. J Clin Endocrinol Metab. 2007;92:1886–1890. doi: 10.1210/jc.2006-1815. [DOI] [PubMed] [Google Scholar]
- 18.Lee DC, Lee JW, Im JA. Association of serum retinol binding protein 4 and insulin resistance in apparently healthy adolescents. Metabolism. 2007;56:327–331. doi: 10.1016/j.metabol.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Yao-Borengasser A, Varma V, Bodles AM, et al. Retinol binding protein 4 expression in humans: relationship to insulin resistance, inflammation, and response to pioglitazone. J Clin Endocrinol Metab. 2007;92:2590–2597. doi: 10.1210/jc.2006-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanaka-Gantenbein C, Margeli A, Pervanidou P, et al. Retinol-binding protein 4 and lipocalin-2 in childhood and adolescent obesity: when children are not just "small adults". Clin Chem. 2008;54:1176–1182. doi: 10.1373/clinchem.2007.099002. [DOI] [PubMed] [Google Scholar]
- 21.Yagmur E, Weiskirchen R, Gressner AM, et al. Insulin resistance in liver cirrhosis is not associated with circulating retinol-binding protein 4. Diabetes Care. 2007;30:1168–1172. doi: 10.2337/dc06-2323. [DOI] [PubMed] [Google Scholar]
- 22.Cortez-Pinto H, de Moura MC, Day CP. Non-alcoholic steatohepatitis: from cell biology to clinical practice. J Hepatol. 2006;44:197–208. doi: 10.1016/j.jhep.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 24.Wieckowska A, Zein NN, Yerian LM, et al. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Attard FA, Tankersley LR, et al. Effect of retinoic acid on the enhancing effect of acetaldehyde on mouse type I collagen expression. Archives of biochemistry and biophysics. 2000;376:191–198. doi: 10.1006/abbi.2000.1723. [DOI] [PubMed] [Google Scholar]