Abstract
Objectives
To date, separate condition-specific instruments have been used to assess symptom severity in men and women with urologic pain conditions. A single instrument for use in both men and women would be helpful for assessing treatment response in clinical trials and cohort studies involving both genders.
Methods
We developed the Genitourinary Pain Index (GUPI) by modifying and adding questions to the NIH Chronic Prostatitis Symptom Index. To assess discriminant validity, concurrent validity, and reliability, we administered the GUPI to 1,653 men and 1,403 women in a large managed care population. To assess responsiveness, we administered the GUPI to 47 men and women who completed an NIH-sponsored trial of pelvic floor physical therapy.
Results
The GUPI discriminated between men with chronic prostatitis or interstitial cystitis, men with other symptomatic conditions (dysuria, frequency, chronic cystitis), and men with none of these diagnoses (p<0.05). It also discriminated between women with interstitial cystitis, women with incontinence, and women with none of these diagnoses (p<0.05). The GUPI demonstrated good internal consistency within subscale domains, and GUPI scores correlated highly with scores on the Interstitial Cystitis Symptom Index and Problem Index. The GUPI was highly responsive to change, and thechange in score was similar in both male and female responders. A reduction of 7 points robustly predicted being a treatment responder (sensitivity 100%, specificity 76%).
Conclusions
The GUPI is a valid, reliable and responsive instrument that can be used to assess the degree of symptoms in both men and women with genitourinary pain complaints.
Keywords: interstitial cystitis, chronic prostatitis, questionnaire, psychometrics
Introduction
Interstitial cystitis/painful bladder syndrome (IC/PBS) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) are enigmatic disorders characterized by pain or discomfort in the pelvic region. Symptoms of these conditions occur in as many as 5–10% of the population, although clinical diagnoses of IC/PBS and CP/CPPS are assigned much less frequently1,2. The two conditions are typically considered separate clinical entities, but they have overlapping symptoms and are treated with similar medications and other therapies (e.g. antibiotics, antidepressants, anti-inflammatory medications, antimuscarinic agents, pelvic floor physical therapy). Recent NIH-sponsored studies of IC/PBS and CP/CPPS utilize inclusive symptom-based criteria to study the epidemiology, response to therapy, natural history, and pathophysiology of these conditions3–5. Generally, assessments of symptoms in men and women with IC/PBS and CP/CPPS have utilized separate instruments. Development of a valid and reliable instrument to assess the degree of pelvic pain symptoms and their impact in both men and women would likely prove useful in epidemiological studies, clinical trials and patient management. We modified the validated and widely-used National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) to permit its use in men and women. We report the validity, reliability, and responsiveness of the new instrument, the Genitourinary Pain Index (GUPI).
Materials and Methods
The male and female Genitourinary Pain Indices (M-GUPI and F-GUPI) (See Appendix) were developed by modifying the NIH Chronic Prostatitis Symptom Index (NIH-CPSI)6. The NIH-CPSI includes subscales for pain symptoms, urinary symptoms and quality-of-life (QOL) impact. To capture IC/PBS symptoms, we added 2 additional pain subscale items to the NIH-CPSI to assess pain/discomfort that worsens as the bladder fills or is relieved by voiding. These complaints have been described as the cardinal symptoms of IC/PBS7. For the female GUPI, we changed the male-specific pain items (perineal pain, testicular pain, penile pain, pain during or after ejaculation) to female-specific items (pain at the entrance to the vagina, pain in the vagina, pain in the urethra, pain during or after sexual intercourse). These symptoms have been commonly reported in women with a clinical diagnosis of IC/PBS8–10. The resulting questionnaire has 10 pain items (total pain subscale score 0–23), 2 urinary symptom items (total urinary subscale score 0–10), and 3 QOL items (total QOL subscale score 0–12). As with the NIH-CPSI, the scores from each item are summed for a total F-GUPI or M-GUPI score that ranges from 0 to 45.
Discriminant validity
The GUPI was administered to 1,403 male and 1,653 female randomly selected enrollees in the Kaiser Permanente Northwest health maintenance organization population. Details about the accrual of this cohort have been published previously11,12. Male participants were stratified into four groups based on diagnoses coded in their medical record as follows: Interstitial Cystitis (ICD-9 code 595.1; n=14), Prostatitis (601.1 or 601.9; n=593), Other Symptoms (chronic cystitis – 595.2, unspecified cystitis – 595.5, urinary frequency – 788.41, or dysuria – 788.1; n=244), and Controls (none of the above codes; n=552). Female participants were similarly classified as follows: Interstitial Cystitis (595.1; n=82), Incontinence (unspecified urinary incontinence – 788.30, urge incontinence – 788.31, mixed incontinence – 788.33, incontinence without sensory awareness – 788.34, continuous leakage – 788.37, overflow incontinence – 788.38, female stress incontinence – 625.6, or intrinsic sphincter deficiency – 599.82; n=427), Other Symptoms (chronic cystitis – 595.2, unspecified cystitis – 595.5, urinary frequency – 788.41, or dysuria – 788.1; n=268), and Controls (none of the above codes; n=876). The category of ‘Other Symptoms’ was chosen to reflect the presence of irritative or painful urologic symptoms that may not have reached the threshold to yield a diagnosis of IC or prostatitis. One-way ANOVA was used to compare mean GUPI scores across each of these 4 groups in each gender in order to assess discriminant validity. When this analysis indicated significant differences across these mean values, comparisons between individual groups were conducted using the Bonferroni adjustment for multiple comparisons.
Concurrent validity
All individuals in the cohort also completed the Interstitial Cystitis Symptom Index (ICSI) and Problem Index (ICPI)13. GUPI scores in individuals diagnosed with IC (82 women and 14 men) were correlated with ICSI and ICPI scores in order to assess concurrent validity.
Reliability
Internal consistency reliability was assessed by measuring the correlation (Cronbach’s alpha) between the items in each GUPI subscale (pain, urinary and QOL), with separate analyses performed for men and women in the Kaiser Permanente dataset. This analysis was limited to the 82 women with a diagnosis of IC and the 607 men with a diagnosis of IC or prostatitis. Analysis of the pain subscale was limited to the 8 items with dichotomous responses (GUPI items 1 and 2), as the other 2 items (frequency and severity of pain symptoms) are contingent upon a positive response to one of the dichotomous items.
Responsiveness
To assess responsiveness, we administered the GUPI to 47 men and women who completed an NIH-sponsored pilot clinical trial of pelvic floor physical therapy14. Participants in this study were adults with clinical diagnoses of either IC/PBS or CP/CPPS, with symptoms of less than 3 years duration who had previously failed at least one course of treatment. These individuals were randomized to receive 10 hour-long weekly treatments with either myofascial physical therapy or global therapeutic massage. Questionnaires were administered before and after completion of the treatment program, and the change in NIH-GUPI scores were correlated with the response to therapy as measured by a Global Response Assessment (GRA) item. The GRA asks trial participants to rate their treatment response on a 7-point scale (markedly worsened, moderately worsened, slightly worsened, no change, slightly improved, moderately improved, markedly improved). Participants who indicated that they were moderately or markedly improved were considered responders, and GUPI scores were compared between responders and non-responders. Changes in mean GUPI values before and after the treatment program were assessed using the signed-rank test.
Results
Discriminant validity (Table 1)
Table 1.
Discriminant Validity of the Genitourinary Pain Index (GUPI)
| Men (n=1653) | Women (n=1403) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GUPI Score | IC n=14 | Prostatitis n=593 | Other Symptoms n=244 | Controls n=552 | p-valuea | IC n=82 | Incontinence n=427 | Other Symptoms n=268 | Controls n=876 | p-valuea |
| Total | ||||||||||
| Mean | 16.3 | 8.9 | 11.2 | 4.5 | <0.0001c | 20.7 | 11.6 | 11.1 | 5.9 | <0.0001b |
| s.d. | 12.8 | 7.1 | 8.4 | 4.8 | 9.5 | 6.9 | 8.5 | 5.2 | ||
| Median | 15 | 8 | 9 | 3 | 24 | 11 | 9 | 5 | ||
| Pain | ||||||||||
| Mean | 5.6 | 3.3 | 3.7 | 1.4 | <0.0001c | 7.4 | 3.4 | 3.9 | 1.8 | <0.0001b |
| s.d. | 7.4 | 3.8 | 4.4 | 2.2 | 5.1 | 3.4 | 4.0 | 2.4 | ||
| Median | 2.5 | 2 | 2 | 0 | 8 | 2 | 2 | 1 | ||
| Urinary | ||||||||||
| Mean | 4.6 | 2.6 | 3.5 | 1.4 | <0.0001c | 5.8 | 3.5 | 3.0 | 1.7 | <0.0001b |
| s.d. | 2.8 | 2.4 | 2.7 | 1.8 | 2.8 | 2.7 | 2.6 | 1.9 | ||
| Median | 6 | 2 | 3 | 1 | 6 | 3 | 2 | 1 | ||
| QOL | ||||||||||
| Mean | 6.2 | 3.3 | 4.5 | 1.7 | <0.0001c | 7.2 | 5.1 | 4.4 | 2.4 | <0.0001b |
| s.d. | 4.0 | 2.6 | 3.0 | 2.1 | 3.1 | 3.0 | 3.3 | 2.4 | ||
| Median | 6 | 3 | 4 | 1 | 8 | 5 | 4 | 2 | ||
ANOVA indicates that the four patient groups differ for total GUPI score as well as subscales in males and females. After adjustment for multiple comparisons, mean GUPI scores (total score and subscales) were significantly lower in Controls than in any of the other groups.
In women, mean total scores for the GUPI as well as all subscales were significantly greater in the IC group than in the Other Symptoms group and the Incontinence groups. Mean total and subscale scores were not significantly different between the Other Symptoms group and Incontinence group.
In men, mean scores in the IC group and in the Other Symptoms group were significantly greater than those in the Prostatitis group for total GUPI scores and all subscales except the pain subscale. Mean total and subscale scores were not significantly different between the IC group and the Other Symptoms group.
In both men and women in the Kaiser Permanente population, mean GUPI total scores as well as all subscale scores were significantly lower (better) in the Control group than in the other groups. In women, mean scores for the GUPI as well as all subscales were significantly greater (worse) in the IC group than in the Other Symptoms group and the Incontinence groups. Mean scores were not significantly different between the Other Symptoms group and Incontinence group. In men, mean scores in the IC group and in the Other Symptoms group were significantly greater than those in the Prostatitis group for all GUPI scores except the pain subscale. Mean scores in men were not significantly different between the IC group and the Other Symptoms group.
Concurrent validity
In women diagnosed with IC, the Spearman correlation coefficients between GUPI scores and ICSI and ICPI scores were 0.68 and 0.74, respectively. In men with IC, the corresponding values were 0.99 and 0.97, respectively. All correlations were highly statistically significant (p<0.005).
Reliability
In men, the Cronbach’s alpha values for the pain, urinary and QOL subscales were 0.80, 0.73 and 0.74, respectively. Corresponding values in women were 0.88, 0.60 and 0.78, respectively. These values indicate excellent internal consistency for all subscales except the urinary subscale in women (Cronbach’s alpha value 0.60). The urinary subscale consists of only 2 items which assess symptoms of urinary frequency and sense of incomplete emptying, respectively. Frequency distributions of responses for these 2 items demonstrate that many women reported urinary frequency, while fewer endorsed the symptom of incomplete bladder emptying (data not shown).
Responsiveness
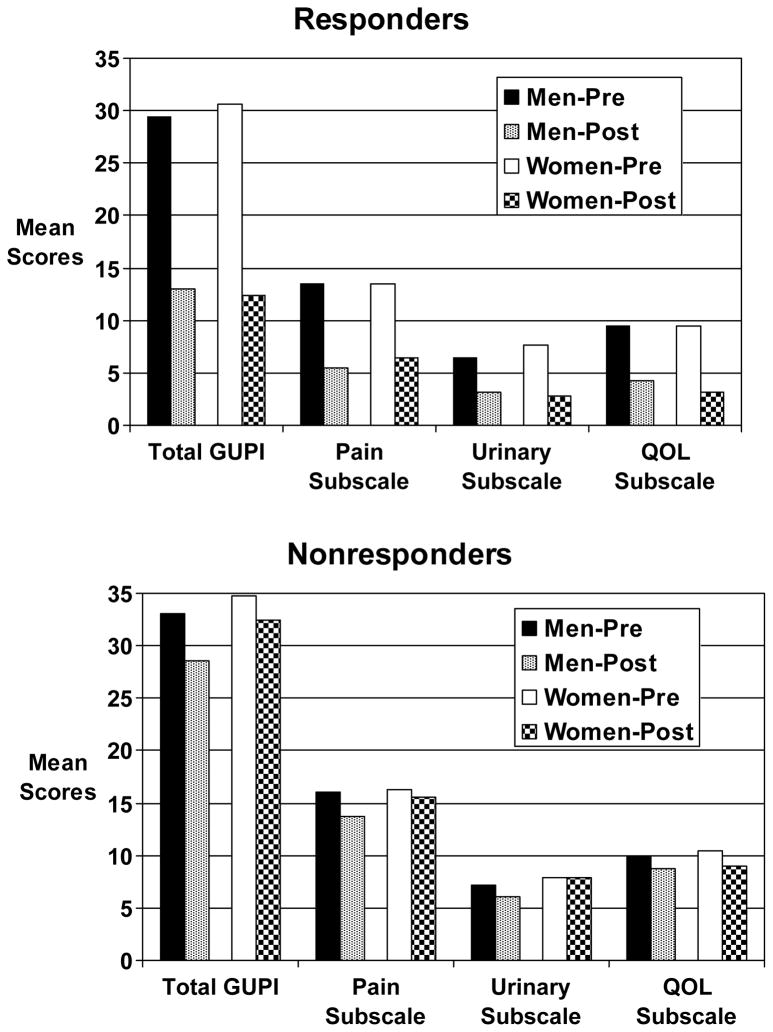
Baseline GUPI total and subscale scores were not significantly different between men and women participating in the NIH-sponsored physical therapy trial (Table 3). Table 4 and Figure 1 present the change in the GUPI total score and its subscales from baseline to end of study in male and female responders and non-responders in this trial. We observed a significant decrease for the GUPI total and subscale scores among both male and female clinical trial responders. A decrease of 7 points in the GUPI total score robustly predicted being a treatment responder (sensitivity 100%, specificity 76%). A reduction of 4 points in the GUPI total score predicted a clinically perceptible difference in global response (slightly improved, moderately improved, or markedly improved on the GRA) (sensitivity 79%, specificity 90%).
Table 3.
Change in total GUPI and its subscales in Male and Female Physical Therapy Trial Responders and Non-Responders
| Responders (n= 18) | Nonresponders (n=26) | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean GUPI Scores | Pre-Tx | Post-Tx | Change (%) | p-value* | Pre-Tx | Post-Tx | Change (%) | p-value* |
| Men (n =23) | ||||||||
| Total | 29.4 ± 6.6 | 13.0 ± 6.1 | −16.4 (−55.8) | 0.0002 | 33.0 ± 1.6 | 28.6 ± 7.7 | −4.4 (−13.3) | 0.09 |
| Pain | 13.5 ± 2.6 | 5.5 ± 3.6 | −8.0 (−59.3) | 0.0002 | 16.0 ± 3.1 | 13.7 ± 4.4 | −2.5 (−15.6) | 0.19 |
| Urinary | 6.4 ± 3.4 | 3.2 ± 1.9 | −3.2 (−50.0) | 0.003 | 7.2 ± 2.3 | 6.1 ± 2.5 | −0.9 (−12.5) | 0.30 |
| QOL | 9.5 ± 2.0 | 4.3 ± 2.6 | −5.2 (−54.7) | 0.0002 | 9.9 ± 2.3 | 8.8 ± 2.6 | −0.9 (−9.1) | 0.13 |
| Women (n=21) | ||||||||
| Total | 30.6 ± 5.8 | 12.4 ± 7.8 | −18.2 (−59.5) | 0.06 | 34.8 ± 4.4 | 32.5 ± 6.5 | −2.3 (−6.6) | 0.25 |
| Pain | 13.5 ± 2.6 | 6.4 ± 4.6 | −7.1 (−52.6) | 0.06 | 16.3 ± 2.7 | 15.6 ± 3.8 | −0.7 (−4.3) | 0.67 |
| Urinary | 7.6 ± 2.8 | 2.8 ± 1.8 | −4.8 (−63.2) | 0.06 | 7.9 ± 2.4 | 7.9 ± 2.5 | −0.0 (0.0) | 0.57 |
| QOL | 9.5 ± 1.6 | 3.2 ± 2.8 | −6.3 (−66.3) | 0.06 | 10.5 ± 1.2 | 9.0 ± 2.3 | −1.5 (−14.3) | 0.002 |
| All (n=44) | ||||||||
| Total | 29.7 ± 6.2 | 12.8 ± 6.3 | −16.9 (−56.9) | <.0001 | 34.1 ± 5.0 | 31.1 ± 7.1 | −3.1 (−9.1) | 0.03 |
| Pain | 13.5 ± 2.6 | 5.8 ± 3.8 | −7.8 (−57.8) | <.0001 | 16.2 ± 2.8 | 14.9 ± 4.0 | −1.4 (−8.6) | 0.24 |
| Urinary | 6.7 ± 3.2 | 3.1 ± 1.8 | −3.7 (−55.2) | <.0001 | 7.6 ± 2.3 | 7.2 ± 2.6 | −0.4 (−5.3) | 0.75 |
| QOL | 9.5 ± 1.9 | 4.0 ± 2.6 | −5.5 (−57.9) | 0.0001 | 10.3 ± 1.7 | 8.9 ± 2.4 | −1.3 (−12.6) | 0.0001 |
Signed rank test
Figure 1.
Magnitude of change in GUPI total score and subscales among responders and nonresponders in trial of pelvic floor physical therapy.
Comment
The Genitourinary Pain Index (GUPI) is a valid, reliable and responsive condition-specific instrument that can be used to quantify symptoms in men and women with urologic pain conditions. The index could be an important tool for clinical use as well as for research purposes to assess baseline symptom severity and response to therapy. Such an index will be especially useful in urologic pain trials that include both men and women.
Our discriminant analysis was based on administrative claims data diagnoses. Using these methods, the GUPI discriminated well between controls and patients with pain symptoms in both genders. The female GUPI also discriminated between those diagnosed with IC and those with other diagnoses (e.g. dysuria, frequency, incontinence). In men, male GUPI scores were higher (worse) in men diagnosed with IC or other conditions (dysuria, frequency) than in those with prostatitis. This is likely because the prostatitis group in this sample represents a heterogeneous group of men, many of whom have mild or self-limited symptoms12,15. The patients were identified based on the presence of specific ICD-9 diagnostic codes in the electronic medical record. Since IC is a relatively uncommon diagnosis, it was encountered less frequently than prostatitis, which was diagnosed more frequently in this population. The specific diagnostic criteria that were used to make these diagnoses reflect the general criteria that are used in practice rather than specific criteria typically utilized as part of a trial or cohort study. Better discrimination would be expected in a more specifically defined clinic cohort. Since the male GUPI is comprised almost entirely of the NIH-CPSI, we would expect it to have discriminant validity as robust as the NIH-CPSI in a clinic cohort6.
The ICSI and ICPI are commonly used instruments to assess IC symptom severity. They have been used as outcome variables in NIH-sponsored clinical trials of IC/PBS therapies16,17. In our sample of men and women diagnosed with IC, GUPI scores correlated highly with ICSI and ICPI scores, with correlation coefficients ranging from 0.59 to 0.99. In the same sample, the correlation between the ICSI and the ICPI were 0.84 in women and 0.92 in men. These findings suggest that the GUPI, the ICSI and the ICPI are measuring similar constructs, and support the use of the GUPI in IC/PBS patients.
The internal consistency of the GUPI was excellent for all subscales except for the urinary subscale results in women. Given that the urinary subscale includes only 2 items, it is perhaps not surprising that this subscale demonstrated the lowest Cronbach’s alpha values. Although the symptoms of urinary frequency and incomplete bladder emptying are common complaints in women with IC/PBS, they did not track together to large degree in our managed care cohort.
The data obtained from the NIH physical therapy trial include subjects recruited from high-volume tertiary care centers and therefore may be more representative of refractory IC/PBS and CP/CPPS patients seen in urology clinics. In these individuals, baseline scores for the GUPI and subscales were similar in men and women. Furthermore, treatment responders had remarkably similar changes in GUPI scores, regardless of gender (−55.8% in men, −59.5% in women). This suggests that the GUPI is equally appropriate for use in men and women with urologic pain syndromes, and indicates that the index is equally responsive to treatment effects in both genders.
Given the advantages of using the GUPI in clinical studies, it is important to understand how much change in the GUPI is significant in order to determine an appropriate effect size for power analyses. Receiver operator curves identified a 7-point decline in the GUPI total score as the optimal threshold to predict treatment response. In addition, a 4-point decline in total GUPI score was the optimal threshold to detect a clinically perceptible difference (slightly improved or greater).
Conclusions
The Genitourinary Pain Index is a single instrument that can be used to assess symptom severity and impact in both men and women with genitourinary pain complaints. It demonstrates good discriminant validity, reliability, and responsiveness to change.
Male Genitourinary Pain Index
Female Genitourinary Pain Index
Table 2.
Baseline Genitourinary Pain Index scores by gender in participants in NIH Physical Therapy trial
| Baseline GUPI Score | Women n=24 | Men* n=23 | Total n=47 | p-value** | |
|---|---|---|---|---|---|
| Total Score | Mean +/− s.d. | 33.9 +/− 4.9 | 31.0 +/− 6.5 | 32.4 +/− 5.9 | 0.15 |
| Median | 34.8 | 31.5 | 33.0 | ||
| Range | 21 to 41 | 20 to 42 | 20 to 42 | ||
| Pain Subscale | Mean +/− s.d. | 15.8 +/− 2.9 | 14.6 +/− 3.0 | 15.2 +/− 3.0 | 0.21 |
| Median | 15.8 | 15.5 | 15.5 | ||
| Range | 10 to 21 | 9 to 20 | 9 to 21 | ||
| Urinary Subscale | Mean +/− s.d. | 7.6 +/− 2.4 | 6.7 +/− 2.9 | 7.2 +/− 2.7 | 0.34 |
| Median | 8.5 | 7.0 | 8.0 | ||
| Range | 2 to 10 | 1 to 10 | 1 to 10 | ||
| QOL Subscale | Mean +/− s.d. | 10.4 +/− 1.3 | 9.6 +/− 2.1 | 10.0 +/− 1.8 | 0.37 |
| Median | 10.8 | 10.0 | 10.5 | ||
| Range | 8 to 12 | 6 to 12 | 6 to 12 | ||
Includes 21 men diagnosed with CP/CPPS and 2 men diagnosed with IC/PBS
Wilcoxon rank sum test
Acknowledgments
NIDDK 5U01DK065257
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McNaughton-Collins M, Joyce GF, Wise M, Pontari MA. Prostatitis: Urologic Diseases in America . In: Litwin MS, Saigal CS, editors. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Washington, DC: US Government Publishing Office; 2007. pp. 9–42. NIH Publication No. 07-5512. [Google Scholar]
- 2.Clemens JQ, Joyce GF, Wise M, Payne CK. Interstitial Cystitis/Painful Bladder Syndrome: Urologic Diseases in America. In: Litwin MS, Saigal CS, editors. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Washington, DC: US Government Publishing Office; 2007. pp. 123–156. NIH Publication No. 07-5512. [Google Scholar]
- 3.Clemens JQ, Link CL, Eggers PW, et al. Prevalence of painful bladder symptoms and effect on quality of life in black, Hispanic and white men and women. J Urol. 2007;177:1390–1394. doi: 10.1016/j.juro.2006.11.084. [DOI] [PubMed] [Google Scholar]
- 4.www.cceb.med.upenn.edu/uppcrn
- 5.www.mappnetwork.org
- 6.Litwin MS, McNaughton-Collins M, Fowler FJ, Jr, et al. The National Institutes of Health chronic prostatitis symptom index: development and validation of a new outcome measure. J Urol. 1999;162:369–75. doi: 10.1016/s0022-5347(05)68562-x. [DOI] [PubMed] [Google Scholar]
- 7.Hanno PM, Landis JR, Matthews-Cook Y, et al. The diagnosis of interstitial cystitis revisited: lessons learned from the National Institutes of Health Interstitial Cystitis Database study. J Urol. 1999;161:553–557. doi: 10.1016/s0022-5347(01)61948-7. [DOI] [PubMed] [Google Scholar]
- 8.FitzGerald MP, Kenton KS, Brubaker L. Localization of the urge to void in patients with painful bladder syndrome. Neurourol Urodyn. 2005;24:633–637. doi: 10.1002/nau.20177. [DOI] [PubMed] [Google Scholar]
- 9.Peters KM, Killinger KA, Carrico DJ, et al. Sexual function and sexual distress in women with interstitial cystitis: a case-control study. Urology. 2007;70:543–547. doi: 10.1016/j.urology.2007.04.037. [DOI] [PubMed] [Google Scholar]
- 10.Peters KM, Carrico DJ, Ibrahim IA, et al. Characterization of a clinical cohort og 87 women with interstitial cystitis/painful bladder syndrome. Urology. 2008;71:634–640. doi: 10.1016/j.urology.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Clemens JQ, Meenan R, O’Keeffe Rosetti MC, et al. Prevalence of interstitial cystitis symptoms in a managed care population. J Urol. 2005;174:576–580. doi: 10.1097/01.ju.0000165170.43617.be. [DOI] [PubMed] [Google Scholar]
- 12.Clemens JQ, Meenan RT, O’Keeffe Rosetti MC, et al. Prevalence of prostatitis-like symptoms in a managed care population. J Urol. 2006;176:593–596. doi: 10.1016/j.juro.2006.03.089. [DOI] [PubMed] [Google Scholar]
- 13.O’Leary MP, Sant GR, Fowler FJ, Jr, et al. The interstitial cystitis symptom index and problem index. Urology. 1997;49:58–63. doi: 10.1016/s0090-4295(99)80333-1. [DOI] [PubMed] [Google Scholar]
- 14.FitzGerald MP, Anderson RU, Potts J, et al. Randomized multicenter feasibility trial of myofascial physical therapy for treatment of urologic chronic pelvic pain syndrome. J Urol. 2009;182:1–11. doi: 10.1016/j.juro.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clemens JQ, Meenan RT, O’Keeffe Rosetti MC, et al. Incidence and clinical characteristics of NIH type III prostatitis in the community. J Urol. 2005;174:2319–2322. doi: 10.1097/01.ju.0000182152.28519.e7. [DOI] [PubMed] [Google Scholar]
- 16.Mayer R, Propert KJ, Peters KM, et al. A randomized controlled trial of intravesical bacillus calmette-guerin for treatment refractory interstitial cystitis. J Urol. 2005;173:1186–91. doi: 10.1097/01.ju.0000152337.82806.e8. [DOI] [PubMed] [Google Scholar]
- 17.Sant GR, Propert KJ, Hanno PM, et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J Urol. 2003;170:810–5. doi: 10.1097/01.ju.0000083020.06212.3d. [DOI] [PubMed] [Google Scholar]