Abstract
Objective
Detailed characterization of progesterone and ovulation across the menopausal transition provides insight into conception risk and mechanisms of reproductive aging.
Design
Participants (N=108, aged 25–58 years) collected daily urine specimens for six month intervals in each of five consecutive years. Specimens were assayed for pregnanediol-glucuronide (PDG), LH, FSH and estrone-glucuronide (E1G). Reproductive stage was determined using cycle length variance. A hierarchical algorithm was used to identify ovulation. Linear mixed-effects models estimated: 1) the frequency and day of ovulation by age and stage; 2) differences in FSH, LH, and E1G levels between ovulatory (O) and anovulatory (AO) cycles; and 3) total PDG levels and PDG levels in ovulatory cycles by age and stage.
Results
The probability of AO cycles increased across the perimenopause (p<.0001); reproductive stage was a stronger predictor than age of the probability of anovulation. Most cycles in late perimenopause were anovulatory (>60%), but one quarter of cycles longer than 60 days were ovulatory. Average day of ovulation was later in the late perimenopause (mean (SD) cycle day 27 (25)) compared to the premenopause. FSH and LH levels were higher, and E1G levels lower, in AO than O cycles (p<.0001 for each). Total PDG decreased in the late perimenopause, but 95th percentile PDG in ovulatory cycles declined steadily across the transition.
Conclusions
Exposure to the risk of conception in women experiencing cycles long enough to classify them as late perimenopausal is far from negligible. Reproductive stage is more informative than age about PDG levels and the likelihood of anovulation.
Keywords: pregnanediol glucuronide, PDG, urinary hormones, menopause, perimenopause, reproductive aging, reproductive stage, FSH, LH, E1G, anovulation
INTRODUCTION
Luteal inadequacy is linked to reduced fecundity (1, 2), thus a great deal of attention has been paid to luteal function, as assessed by progesterone and its derivatives. Although progesterone levels during the luteal phase of the menstrual cycle appear to decline with advancing age (3, 4), and frequency of ovulatory cycles also declines with advancing age (5, 6), the extent to which fecundity might be affected by these changes is not entirely clear. This lack of information is, in part, a result of the use of limited windows of observation of hormonal changes across the transition to menopause, with regard to both sampling frequency and length of follow-up. Most longitudinal studies of reproductive aging have monitored progesterone levels in serum specimens, thereby limiting sampling frequency and length of follow (e.g. (7–12)). Studies using urine specimens have both honed and broadened the window of observation, by allowing for more frequent sampling (13) and longer periods of prospective monitoring (14). Because the transition to menopause is associated with increasing average cycle length (15–18), it is of particular interest to examine the hormonal and ovulatory status of long cycles. For example, although long cycles are often assumed to be anovulatory (19), few studies have, in fact, been designed to capture and describe the hormonal characteristics of long cycles. The few addressing this have focused on small samples of women who were 40 years of age or older (14, 20, 21). There is thus a need for larger, detailed studies that include substantial numbers of long cycles in order to assess whether they are anovulatory, and how their hormonal characteristics compare with ovulatory cycles.
In addition to providing information on fecundity across the transition to menopause, detailed assessment of progesterone dynamics can provide insight into the underlying mechanisms of reproductive aging. Although there is debate about whether the hypothalamus and pituitary or the gonads are the initial or dominant site contributing to hormonal and cycle changes in the perimenopause (22), it is widely accepted that a declining pool of follicles plays a key role in the onset of menopause and that declining follicle quality is partly responsible for decreased fecundity beginning after age 30 (23). How either of these features of ovarian aging relates to changes in the perimenopause is not well understood, although elevated FSH, decreased inhibin B, and decreased anti-mullerian hormone are believed to reflect a depleting pool of follicles (24). One of our objectives is to interpret progesterone changes across the transition to menopause in light of these features of reproductive aging.
A challenge for studies on reproductive aging is the use of age as a comparative anchor across women. This has confounded studies of reproductive aging because women of similar ages can have very different hormonal levels (25) and because they undergo menopause across a broad range of ages (26). Reproductive stages such as those put forth in the STRAW system have been proposed as an alternative to age for research and clinical purposes (27). In this study we assess hormones by both reproductive stage and chronological age.
This study extends prior work on progesterone and reproductive aging to include longer windows of observation coupled with daily sampling for a relatively large sample of women. Our goal is to examine how progesterone levels and ovulatory status change across the transition to menopause in a five-year prospective study with daily urinary hormone measures. We use a reproductive staging system, based on variance in cycle length (28–30), to monitor the transition to menopause. Our specific aims were to: 1) assess the frequency and day of ovulation by age and reproductive stage; 2) compare FSH, LH, and E1G levels between ovulatory and non-ovulatory cycles, and 3) estimate PDG levels in all cycles and ovulatory cycles by age and reproductive stage.
MATERIALS AND METHODS
Participants
This report used data collected as part of the Biodemographic Models of Reproductive Aging (BIMORA) project (25). The BIMORA participants were recruited from the Tremin Research Program on Women’s Health (TREMIN). BIMORA participants were either from the second cohort of women recruited into TREMIN in 1961–63, or women subsequently recruited into TREMIN after the second cohort but prior to 1997 (25). At the time of recruitment, TREMIN women eligible for BIMORA were between the ages of 25 and 60 years, had at least one intact ovary and were not using any prescription reproductive hormones; pregnant or breastfeeding women, and women receiving cancer treatment, were not eligible. Details on sample recruitment are provided in (25); briefly, of 748 women enrolled in TREMIN in 1997, 225 were eligible, and 156 ultimately participated in BIMORA. For this paper, we excluded all data from participants with no uterus; data were also excluded for ambiguous bleed or cycle day information and for three months following exogenous hormone use, pregnancy, breastfeeding, miscarriage, major surgery, chemotherapy, or use of any medications known to affect reproductive hormone or menstrual bleed patterns. The final anlaysis sample comprised 108 women aged 25–58 years at recruitment and 64,671 woman-days of observation.
Participants provided written informed consent and were paid $150 per year for their participation. Procedures were approved by the institutional review boards of the University of Utah, the Pennsylvania State University, Georgetown University, and the University of Washington.
Data Collection
Daily first-morning urine specimens were collected from January 14 to July 15 of each year from 1998 to 2002. Daily information was collected on menstrual bleeding, major medical conditions and treatments, and all over-the-counter and prescription medication used. During the BIMORA project, most participants continued to record menstrual bleed data on calendar cards for TREMIN. We combined the TREMIN bleed data for July 16 to January 13 with the BIMORA data for January 14 to July 15 for each project year from 1998 to 2002; for 2002, however, we have only BIMORA bleed data. We thus have nearly-complete menstrual cycle data for most participants for the five year period.
Height and weight for body mass index (BMI) come from a 2000 self-administered health survey, at the midpoint of the study. BMI was available for the year 2000 for 90 of the 108 women. For the remaining 18 women all available BMI data from previous years for each woman was used in a linear mixed effects model of BMI by year; we then used the estimated model to impute a value for the year 2000.
Laboratory Methods
Urine specimens were assayed with an enzyme immunoassay (EIA) for pregnanediol-3-glucuronide (PDG), a urinary metabolite of progesterone (31). Inter- and intra-assay CV’s were 9.2% and 10.3%, and the EIA cross-reacted 100% with pregnanediol-3-glucuronide, 119% with 20α hydroxy-4-pregnen-3-one, 8.7% with pregnanediol, 2.7% with 20β hydroxy-4-pregnen-3-one and less than 1% with other progestins. The metabolites and cross-reactants parallel the serum levels of progesterone. Urine specimens were also assayed for intact FSH, beta LH, and estrone conjugates using previously described and validated enzyme immunoassays (32, 33). E1G concentrations were statistically corrected for slight assay non-parallelism, using 1:5 as the dilution standard to which all values were corrected (32). Hormone concentrations were estimated from optical density using a four parameter logistic model (Biolinx 1.0, Dynex Laboratories, Inc., Chantilly, VA). Urine specimens were assayed in duplicate, and adjusted by specimen specific gravity, using a mean specific gravity of 1.020 (34).
Reproductive Stage Determination
A reproductive stage was assigned to each menstrual cycle, using a four-category scale (Table 1) derived from the Staging Reproductive Aging Workshop (STRAW) recommendations (27). Based on the criterion of variability in cycle length employed by other researchers for defining the early and late stages of the transition to menopause (28–30), we developed a unique approach using the coefficient of variation (CV) of menstrual cycle length to assign reproductive stage.
Table 1.
Criteria for assigning reproductive stage.
| Stage Number | Description | Defining Criteria |
|---|---|---|
| Stage-3 | Pre-Menopausal | CV of length of current cycle + previous 5 (or fewer) cycles < 20% |
| Stage-2 | Early Stage of Menopausal Transition | CV of length of current cycle + previous 5 (or fewer) cycles = 20–40% |
| Stage-1 | Late Stage of Menopausal Transition | CV of length of current cycle + previous 5 (or fewer) cycles ≥ 40% OR presence of a cycle length ≥ 60 days. |
| Stage +1 | Post-menopausal | No menstruation for the previous 12 months. |
Cycle length was calculated as the number of days from the first day of a menstrual bleed to the day before the next bleed. A menstrual bleed was defined as an interval with at least 2 days of bleeding in 6 consecutive days; the interval had to be preceded by at least 5 consecutive days of no bleeding.
A rolling cycle-length CV (the standard deviation of cycle length divided by the mean cycle length) was calculated for each cycle using the length of the current cycle and the five previous cycles. When fewer than five previous cycles were available, all cycle lengths observed before the current cycle were used.
Cutoff values for the rolling cycle-length CV were chosen to represent stages similar to the STRAW system (27) (Table 1). Because CV values of less than 20% represent fewer than 7 days of deviation from a 35 day cycle (considered the upper end of normal cycle length (35)), we used it to indicate pre-menopause (stage -3). A twenty to forty percent CV was chosen to represent the STRAW criterion of cycle length variance greater than 7 days (stage -2). A CV greater than forty percent or the presence of a cycle greater than 60 days in length was used for the STRAW criterion of two or more skipped cycles (stage -1). Postmenopause (stage +1) was defined as one year with no menstrual bleeding; this stage was only included in analyses of PDG area under the curve (AUC).
Cycles that were right or left censored at fewer than 60 days within the six-month period of urine collection each year, and for which there was no cycle length information from TREMIN outside of this six-month period, were assigned the stage occurring ≥ 60% of the time within the six-month interval. Cycles censored at 60 or more days in length were assigned either stage -1 or +1; in some censored cycles, it was not possible to reliably differentiate between stages -1 and +1, but this occurred in only 11 of 3303 menstrual segments.
Estimation of Ovulatory Status and Day of Ovulation
All complete cycles were evaluated for presence and timing of ovulation using a hierarchal algorithm, described in detail elsewhere (36). In brief, to identify the presence or absence of ovulation, we used Kassam’s (37) algorithm for menstrual intervals which calculates a ratio of daily PDG to the minimum 5-day moving average PDG in order to identify a sustained PDG rise. This approach avoids absolute threshold levels for defining the presence or absence of ovulation, given potential inter-woman variation in the metabolism and excretion of progesterone (37). Previously we found that the Kassam method had 100% specificity and 100% sensitivity in a sample of 52 menstrual cycles including 25 perimenopausal cycles (36). Censored cycles were inspected visually for the presence or absence of ovulation; evidence of a sustained elevation in PDG (more than 4 consecutive days) was taken as evidence of ovulation, whereas absence of a sustained rise in PDG was coded as having no evidence for ovulation, which was distinct from a cycle coded as anovulatory. To estimate day of ovulation in complete cycles we used a hierarchical algorithm, optimized for precision by prioritizing first, the day of the mid-cycle LH peak, second, a mid-cycle FSH peak, third, a day of luteal transition, and fourth, a modified version of a running PDG average (36). This algorithm estimated day of ovulation within ± 2 days in 93% of a sample of 30 test cycles (36). Censored cycles were kept for analyses if there was clear evidence of ovulation and sufficient hormonal information to estimate day of ovulation.
Statistical Analyses
Hormonal data were log transformed prior to all analyses. In the analyses of ovulatory status, baseline and peak hormone levels, cycle length and day of ovulation, the unit of analysis was the cycle. Cycles with fewer than 32 days and no evidence of ovulation were omitted; censored cycles longer than 32 days with no evidence of ovulation were treated as anovulatory. To account for potential bias in this approach, analyses modeling anovulation were repeated excluding the latter set of cycles. Fifth percentile hormone levels were used to estimate baseline hormone levels in a cycle while the 95th percentile was used to estimate peak hormone levels.
For analysis of total PDG exposure, the unit was the six-month interval; we calculated six-month PDG area under the curve (AUC) for daily PDG over the six-month interval for each study year. If PDG data were missing for fewer than seven consecutive days, AUC was interpolated. When more than seven consecutive days were missing or data were missing at either end, AUC was computed without that segment, and the final result was weighted upward to represent the full 181 day span. If more than 30 days were missing data, the interval was excluded. To assess the relationship between total PDG exposure and stage, the stage occurring ≥ 60% of the time within the six-month segment was assigned for the interval; if the most prevalent stage occurred less than 60% of the time, the interval was coded as a mixed stage; this stage was assigned to 39 of the 359 six-month intervals.
Tables were constructed summarizing the demographic characteristics of the study population, the proportion of ovulatory cycles by reproductive stage and cycle length (greater or less than 60 days), and cycle characteristics by ovulatory status. Generalized linear mixed effects models were used to determine the association between likelihood of an anovulatory (AO) cycle and both age and reproductive stage. Linear mixed effects models were used to compare cycle length and hormonal characteristics between ovulatory and anovulatory cycles, while adjusting for age and stage: the median and 5th and 95th percentiles were compared for E1G, LH and FSH. Day of ovulation, PDG AUC and in ovulatory cycles PDG median, 5th, and 95th percentiles, were each examined for associations with age and stage. Linear and quadratic associations between age and PDG were tested. In order to separate effects due to between-subject differences in age from within-subject aging occurring across the study, age was modeled as two separate terms: age at study entry and years followed. All analyses were repeated with adjustment for BMI.
Statistical analyses were done using R 2.6.1 (38).
RESULTS
Demographic data were available for most of the 108 BIMORA participants, and were derived from the 2000 self-administered survey or previous TREMIN surveys. All women in BIMORA are White Caucasians. Over 80% of the 108 women completed a college or postgraduate degree, 62% had no evidence of ever smoking, 6% were current smokers, 33% had unknown parity, 32% had 2 live births, 19% had 3 or 4 live births, and the remainder had 1 live birth. Because of missing data these variables were not used in analyses. Age by reproductive stage is shown in Table 2; note that this was derived using the 6-month stage assigned to women, and thus women can be represented in multiple stages. The mean (SD) and median BMI for the sample of 108 women was 24.2 (5.1) and 23.2 kg/m2 respectively. Seventy percent of the BMIs were below 25 (normal), 18% fell between 25–29 (overweight) and 12% were 30 kg/m2 or greater (obese).
Table 2.
Age by reproductive stage for 108 BIMORA women
| Reproductive | Mean (range) in years |
|---|---|
| Stage-3 | 41 (25–52) |
| Stage-2 | 46 (25–5) |
| Stage-1 | 49 (43–56) |
| Stage +1 | 55 (43–60) |
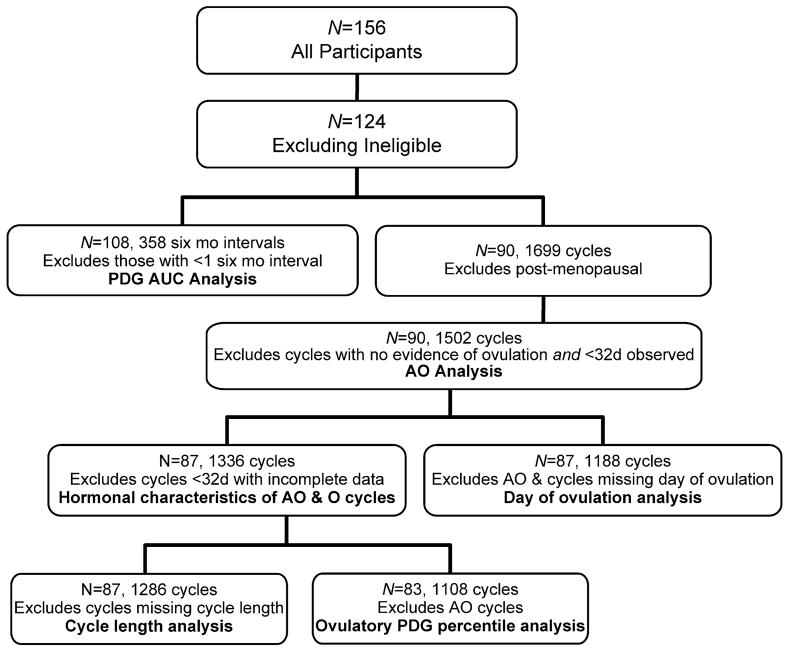
Figure 1 is a flow chart of sample sizes of women and cycles used in the analyses. Cycles that were right- or left-censored at fewer than 32 days were omitted from analyses including hormones, because of insufficient information to estimate hormonal percentiles.
Figure 1.
Flow chart of samples used in analyses. AO = anovulation, O = ovulation.
Table 3 shows the percentage of all ovulatory and all anovulatory cycles that fell within each reproductive stage. The majority of anovulatory cycles (65%) fell into the late perimenopause (−1) stage, whereas the majority of ovulatory cycles (70%) fell into the premenopause (−3) stage. Between-subject age effects and within-subject age effects were similar in their relationship to ovulatory status, so these effects were combined, and age was modeled as a 6 category variable (from 30 to 60 years of age in 5-year intervals). In linear mixed effects models using 1,592 cycles from 90 women, both higher age and more advanced reproductive stage were associated with a greater rate of anovulatory (AO) cycles (p<.0001 for both): of the two covariates, stage was a stronger predictor, with the rate of AO cycles progressively increasing from stage -3 to -2 to -1; the likelihood of AO cycles increased with age only after age 45. Figure 2 illustrates the probability of an AO cycle with age in the left panel and the proportion of AO cycles across age by reproductive stage in the right panel. These results were unchanged when the 99 censored cycles (those longer than 32 days and having no evidence of ovulation) were excluded.
Table 3.
Characteristics of cycles by ovulatory status: means ± SD or % (or median where indicated)
| Ovulatory (N=1108 cycles) |
Anovulatory (N=228 cycles) |
p* | |
|---|---|---|---|
| Age (years) | 44.0 ± 6.9 | 50.2 ± 5.2 | <.0001a |
| Stage | <.0001b | ||
| % Stage -3 | 70 | 11 | |
| % Stage -2 | 16 | 18 | |
| % Stage -1 | 13 | 65 | |
| % censored | 1 | 7 | |
| FSH (per cycle) (ng/mL) | |||
| 5th %-tile | 0.30 ± 0.31 | 0.96 ± 1.39 | <.0001 |
| Median | 1.10 ± 1.20 | 3.99 ± 1.22 | <.0001 |
| 95th %-tile | 4.06 ± 4.00 | 11.9 ± 10.3 | <.0001 |
| LH (per cycle) (ng/mL) | |||
| 5th %-tile | 10 ± 14 | 24 ± 45 | <.0001 |
| Median | 58 ± 85 | 188 ± 378 | <.0001 |
| 95th %-tile | 559 ± 728 | 1141 ± 1414 | <.0001 |
| E1G (per cycle) (pg/mL) | |||
| 5th %-tile | 15600 ± 7200 | 11200 ± 8700 | <.0001 |
| Median | 31900 ± 11900 | 24600 ± 16600 | <.0001 |
| 95th %-tile | 68200 ± 2900 | 60700 ± 38700 | <.0001 |
| Cycle length (days) | |||
| Mean | 29.1 ± 17.1 | 47.6 ± 50.6 | <.0001 |
| (median) | 27 | 32 | |
| % missing cycle length | 1 | 18 | |
Linear mixed effects models on ovulatory status adjusting for age and stage unless otherwise indicated.
Linear mixed effects model of age on ovulatory status
Linear mixed effects model of ovulatory status on stage
Figure 2.
Anovulation by age and stage. Left panel: Probability of anovulation (AO) by age using restricted cubic splines without adjustment for repeated measures, triangles indicate group estimates (N=50) of probability of AO ordered by age. Right panel: Proportion of cycles AO by 5 year age interval by reproductive stage. N=1,592 cycles from 90 women.
Mean BMI for women with AO cycles was lower than for women with O cycles: out of 1,592 cycles, the mean (SD) BMI for women with O cycles was 24.5 (5.9) and the mean BMI for women with AO cycles was 23.6 (5.0). Note that the same women can be in both the O and AO groups for different cycles. This difference in BMI by ovulatory status is borderline significant (p=.05) when adjusted for stage and age. However, adding BMI in the model did not alter the association between AO status and age or stage.
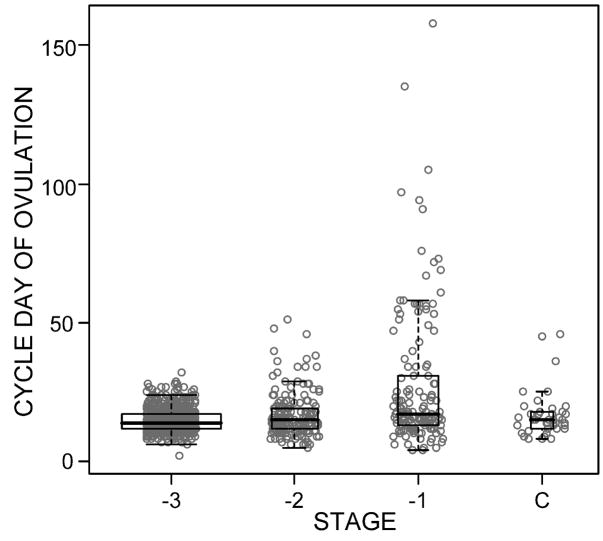
Mean and median day of ovulation were progressively later, with larger standard deviations, for each successive reproductive stage (Figure 3). Mean (SD) and median cycle days of ovulation were 15(4) and 14 for stage -3, 17(8) and 15 for stage -2, and 27(25) and 17 for stage -1. Day of ovulation was significantly later in stage -1 than the other stages (p<.0001). Day of ovulation was not associated with within-subject aging (p=.076). Adjustment for BMI did not change these results.
Figure 3.
Boxplot of day of ovulation by age and reproductive stage. “C” refers to censored cycles. Box width is proportional to the number of intervals in a stage. N= 1,144 cycles from 87 women.
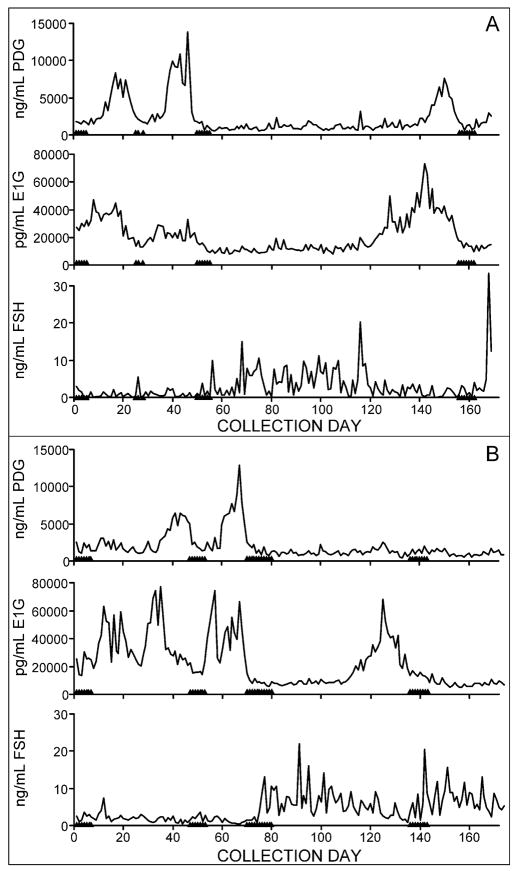
Cycle length was significantly longer for AO than O cycles (Table 3) irrespective of adjustment for BMI. Although AO cycles were longer than O cycles, there were still numerous long O cycles. We examined this in more detail by tabulating the frequency of ovulation in cycles that were longer than 60 days. Out of a total of 1,699 cycles (complete and censored) assessed for ovulatory status, 115 (7%) were greater than 60 days. Of these 115 segments, 29 (25%) were ovulatory, whereas 29 (25%) were anovulatory. The remaining 57 (50%) were indeterminate; that is, no evidence for ovulation was observed, but hormone data were not available for a full segment from menses to menses. Thus, at least one quarter of all cycles longer than 60 days were ultimately ovulatory. Figure 4 shows illustrative examples of long ovulatory and anovulatory cycles in stage -1 women aged 46 and 55 years.
Figure 4.
Illustrative examples of six months of PDG, E1G, FSH and menses for two participants in reproductive stage -1. Panel A; 46 yrs old, shows a long ovulatory cycle; Panel B; 55 years old, shows long ovulatory and anovulatory cycles. Triangles on x-axis represent days of menstrual bleeding.
Comparison of hormonal and cycle length characteristics between AO and O cycles used 1,336 cycles. The results are shown in Table 3. All indicators (median and 5th and 95th percentiles) show that FSH and LH levels were higher and E1G levels lower, in AO vs. O cycles. These results remained unchanged after adjustment for BMI.
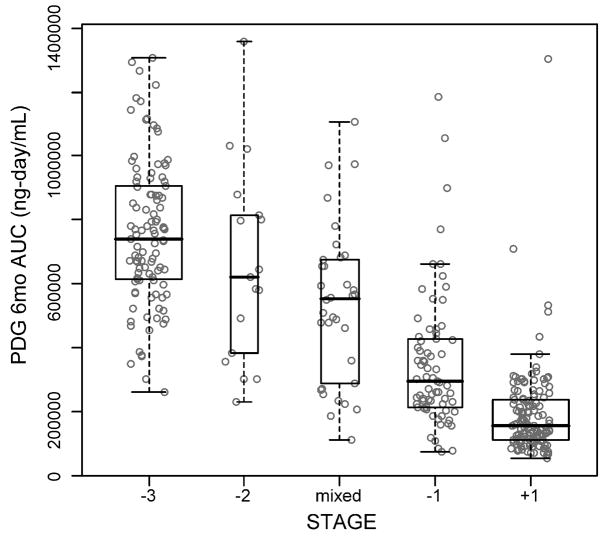
Based on 358 six-month intervals from 108 women (103 stage -3, 17 stage -2, 70 stage -1, 134 stage 1 and 34 mixed stage), there were significant differences in mean PDG AUC by stage (p<.0001) while adjusting for both between-subject and within-subject age. Mean PDG AUC significantly decreased from stages –3 and –2 to stage –1, then to stage +1 (p<.0001 for each difference) with no significant differences among stages –3, -2 and a mixed stage (Figure 5). The relationship between PDG AUC and age-at-study entry was mainly linear, with PDG AUC decreasing across subject age. There was a significant interaction between stage and within-subject age with mean PDG AUC decreasing as subjects aged in stages –1 and –2. There was no consistent association between within-subject age and PDG AUC for stage -3. These results remained unchanged with adjustment for BMI.
Figure 5.
Boxplot of 6 month PDG AUC by reproductive stage. Box width is proportional to the number of intervals in a stage. N= 108 women, 358 six month-intervals.
PDG indicators from ovulatory cycles are shown by reproductive stage in Table 4. Ninety-fifth percentile averages declined linearly from stage -3 to stage -1 (p=.023). In contrast, the median and 5th percentile averages increased from stage -3 to -2 (p=.2, p<.01 respectively). Median, but not 5th percentile, levels were ultimately lower in stage -1 than stage -3 (p=.011).
Table 4.
PDG median and 5thand 95th percentiles from ovulatory cycles by stage: mean ± SD (ng/mL); N = number of cycles.
| Stage-3 (N=776) |
Stage-2 (N=174) |
Stage -1 (N=140) |
Censored (N=18) | p | |
|---|---|---|---|---|---|
| 5th percentile | 872 ± 571 | 1033 ± 834 | 820 ± 475 | 849 ± 482 | .015 |
| Median | 2751 ± 1424 | 3054 ± 1843 | 2365 ± 1435 | 2133 ± 1308 | .0051 |
| 95th percentile | 11571 ± 5002 | 10490 ± 4987 | 8729 ± 5524 | 10540 ± 6487 | .023 |
DISCUSSION
Using prospectively-collected daily hormone data from over one thousand cycles we found that significant increases in anovulation, and decreases in PDG, did not occur until women were in late perimenopause (stage -1). Most cycles (>60%) in the late perimenopause were anovulatory but 25% of cycles longer than 60 days were ovulatory. Thus, exposure to the risk of conception, theoretically, is far from negligible in women experiencing cycles long enough to classify them as late perimenopausal. Moreover, we found that day of ovulation increased, on average, as women progressed through the transition to menopause. Together, these findings support that women and clinicians should not rely on cycle length in the perimenopause as a benchmark for identifying whether or not a cycle is ovulatory or for pinpointing a fertile period of the menstrual cycle (13, 39).
As expected, anovulation increased as women progressed through the transition to menopause. Although the probability of anovulation increased with age after the age of 45, reproductive stage was a stronger predictor than age of the probability of anovulation (see Figure 2). However, there were very few anovulatory cycles in women under age 40: out of 1,592 cycles only 16 of the AO cycles were in women less than 40 years of age. The few studies which have examined changes in ovulatory frequency in perimenopausal women did not use a formal staging system. The two most consistent findings in these studies, however, are first, that anovulation increases as women progress through the perimenopause (13, 40, 41) and second, that anovulation tends to be more common in longer cycles (13, 42). Our results concur with these findings.
PDG AUC and ovulatory median and 95th percentile PDG declined by the late perimenopause (stage -1). Previously, we showed that average PDG slowly declined with age (25); here we show that this is only the case for women classified as being in the menopausal transition (stages -2 and -1); women classified as pre-transitional (stage -3) did not experience declining PDG AUC with age. Reproductive stage was a more useful predictor than chronological age of PDG AUC; in recent work we similarly found that stage was more useful than age as a predictor of E1G AUC levels. Our finding that PDG declined late in the transition to menopause is concordant with the many studies that have reported declining progesterone with increasing age or advancement of perimenopause (7, 8, 13, 20, 21, 25, 40, 43–45). However, our study disentangled the effects of reproductive stage and age. Only one other study has examined progesterone changes with well-defined reproductive stages: Hale and colleagues (11) found that mean luteal phase serum progesterone was not significantly different across stages of the transition until the late menopausal transition.
Given that progesterone and progesterone metabolite levels are implicated in the etiology of breast cancer (46), which increases through the perimenopausal years up to at least 60 years of age (47), our observation that total progesterone exposure (PDG AUC) does not decline until late in the transition to menopause (stage -1; Fig 5) may be informative for research on the prevention and control of breast cancer. It is likely that declining PDG AUC in the late perimenopause is largely a function of increased anovulation. While this provides useful information on total progesterone exposure, it does not address whether luteal function is also declining across the transition. We therefore examined PDG levels in ovulatory cycles.
We found that median and 5th percentile levels of PDG in ovulatory cycles did not decline until late in the transition, but 95th percentile averages declined linearly across the transition to menopause. These results indicate that peak PDG in ovulatory cycles declines first (stage -2) and is followed in the next stage by declining PDG AUC, declining ovulatory median and 5th percentile PDG, and increasing anovulation. Our results are generally consistent with other studies examining luteal function across the transition to menopause. Miro and colleagues (14) prospectively examined PDG levels in a sample of 34 perimenopausal women and found a progressive decline in PDG associated with cycles showing evidence of delayed follicular development; they suggested that this was indicative of luteal phase defect. Santoro and colleagues reported a significant decrease in PDG ovulatory cycles across three years in perimenopausal women, and attributed this to declining follicle quality (13). Thus, declining progesterone across the menopausal transition is likely a function of both increasing anovulation and declining luteal secretion, which together support a model of declining follicle competency (23). Evidence from studies of fetal loss and in-vitro fertilization support that oocyte quality monotonically declines with age after the late 20’s (23, 48). We suggest that decreased PDG in ovulatory cycles and increasing anovulation with reproductive aging could be later stage manifestations of this process.
The average day of ovulation increased progressively from stage -3 to -1 (Figure 3). We also found that at least one quarter of cycles 60 days or longer were ultimately ovulatory. We chose to examine more closely only cycles longer than 60 days because that was the cut-off used in the original STRAW staging system as well as the one we used here for late perimenopause. Metcalf and colleagues (21) similarly reported that long cycles were frequently ovulatory: in a study of 31 perimenopausal women they found that 32% of cycles greater than 50 days in length were ovulatory. We are not aware of other studies that have examined the timing of ovulation with respect to age or reproductive stage. Increasingly delayed ovulation is consistent with a model of follicular depletion across the lifespan that predicts increasing delays in follicular development across the transition to menopause (49). This model predicts that a natural outcome of a negative exponential process, which characterizes follicular depletion across the lifespan, is stochastically occurring periods of ovarian inactivity (i.e. delayed follicular development and therefore delayed ovulation) as the follicle pool approaches exhaustion (49). In contrast to our focus on follicular depletion as an explanatory mechanism for delayed follicular development and ovulation in the perimenopause, Miro and colleagues (14) favor a ‘refractory follicle’ model to explain delayed follicular development, placing primacy on follicle quality. In support of their model, they found reduced PDG in cycles with delayed follicular development; however, E1G was higher in these cycles, suggesting that this aspect of follicle health was not compromised. Thus, currently, it is not clear whether increasingly delayed ovulation reflects changing follicle quality and/or follicle quantity in models of reproductive aging.
Our observations of the hormonal characteristics of ovulatory and anovulatory cycles are also consistent with both models of reproductive aging. We found higher LH and FSH, and lower E1G in anovulatory cycles when compared with ovulatory cycles. Higher LH and FSH in anovulatory cycles have been observed in other studies (11, 13, 40). Elevated gonadotropins in anovulatory cycles is consistent with loss of negative feedback from delayed ovarian activity (49); this occurs early in a cycle, and would thus be observable across the different sampling designs in these studies. The studies differ, however, with respect to estrogen: Santoro and colleagues found no difference in E1C between ovulatory and anovulatory cycles in a sample of 848 women followed for up to 50 days per year for three years; similarly, Landgren and colleagues found no differences in ovulatory and anovulatory cycles in early follicular phase estradiol in a sample of 13 women monitored for up to 4 weeks per year across 4–9 years; Hale and colleagues (11) found lower estradiol in anovulatory cycles. Differences in estrogen findings may be partly a function of differences across studies in the reproductive stages included, the different forms of estrogen measured, and variation in the length of the observation period, which ranged from 4 weeks to 50 days; in contrast, our sample included observation periods up to 182 consecutive days per year for up to 5 years. Hale’s intriguing finding of low estradiol in anovulatory cycles, though similar, is not strictly comparable to our finding, as their observation period was one menstrual cycle. The lower estrogen we observed in anovulatory cycles may reflect the long periods of delayed follicular development. Further data are needed to address whether estrogen dynamics in ovulatory cycles show evidence of reduced follicular competence.
Our study has several limitations. The BIMORA sample is biased toward women who chose not to use either hormone replacement therapy as they went through the menopausal transition or oral contraceptives to prevent pregnancy. Additionally, the sample consists primarily of white, middle-class, college educated, and non-obese women (25). Our results may therefore not be applicable to all women.
The BIMORA sample is likely to be biased toward women with later ages at menopause: TREMIN cohort II women with early ages at menopause completed the transition to menopause before the start of BIMORA in 1998. In a study of an earlier cohort of TREMIN women Lisabeth and colleagues (28) found that although the pattern of perimenopausal change in cycle length mean and standard deviation did not differ by age at menopause, women with later menopause had longer, more variable cycles during the transition than those menopausal before 52 years of age.
We confined urinary hormone data collection to the same months of each year for all women in order to avoid confounding by potential seasonal variation in reproductive function; however, this design also obscures any potential circum-annual variation in patterns of reproductive aging (50).
Another limitation of our study is our single mid-study measure of BMI, derived from participant reports of weight and height. The pattern of bias commonly found for self-reports of height and weight is slight, but can result in overestimates of lower BMI and underestimates of higher BMI (51, 52). Most studies find that obesity has a dampening effect on ovulation, reproductive hormones, and fecundity (13, 53–57). In contrast, we found that anovulatory cycles were weakly associated with slightly lower, not higher BMI, while controlling for age and stage. Progesterone and PDG have been found to be lower in overweight and obese women (BMI > 25kg/m2) compared to women of normal weight (13, 55, 58). Although adding BMI did not alter the results of any of our analyses, our midpoint estimate may not be an accurate representation across the study period, particularly given that BMI has been found in other studies to increase across the transition to menopause (59, 60). Our results are nevertheless consistent with other studies that found a decrease in ovulatory cycles and progesterone levels with progression through the menopausal transition, while controlling for clinically assessed annual BMI (13, 41).
The variance in cycle length we chose as cutoffs for each reproductive stage could have been determined in a different manner. Our goal was to operationalize the STRAW system for use with a large collection of cycle data. Like the methods tested by Harlow and colleagues (28–30), our approach replicated the spirit of the original STRAW system, designed to be used by women, clinicians, and researchers alike. We note that our stage -3, premenopause, was based solely on variation in cycle length, but in the STRAW staging system FSH levels are used to subdivide women without cycle length variation into premenopausal and perimenopausal stages (27). Thus, our -3 stage likely includes cycles that would be classified as perimenopausal based on FSH levels. Our aim here was to use only non-hormonal criteria to classify cycles into reproductive stages, so that we could assess the hormonal characteristics of those stages.
CONCLUSIONS
Anovulation increased, and PDG AUC decreased, in the late perimenopause (stage -1), when cycles were quite variable and frequently long. Day of ovulation also increased across the transition, and was significantly later in the late perimenopause (stage -1) than in the premenopausal stage (stage -3). The long cycles characteristic of the late transition were anovulatory more often than not (65% of all anovulatory cycles occurred in stage -1), but up to 25% of stage -1 cycles 60 days or longer were ultimately ovulatory. These findings reiterate previous work suggesting that women and clinicians should not rely on cycle length in the perimenopause as a benchmark for identifying whether a cycle is ovulatory or for pinpointing a fertile period of the menstrual cycle (13, 39). Reproductive stage is more informative than chronological age with respect to the likelihood of anovulation and changing PDG levels across the transition to menopause. Delayed ovulation in long cycles as the menopausal transition progresses is consistent with models of both declining follicle quantity and quality. Increasing anovulation and decreasing ovulatory PDG across the transition are consistent with declining follicle quality, and may reflect late-stage manifestations of declining follicle competence beginning decades earlier.
Acknowledgments
This research was partly supported by the Population Research Institute of Pennsylvania State University, the Center for Studies in Demography and Ecology of the University of Washington, and the Center for Population and Health, Georgetown University. Thanks to S Barsom, PK Mansfield, RC Miller and JW Wood for their contributions to this work. We extend our deep gratitude to the BIMORA participants for their extraordinary participation.
Sources of Financial Support: NIA RO1 AG15141, NIA 5 T32 AG00208, NICHD HD34159, NICHD 2 P30 HD28263, NICHD F32 HD 07994-02, and NICHD 5 T32 HD007543-07.
Footnotes
Publisher's Disclaimer: Disclaimers: None
Reprints: None will be available
REFERENCES CITED
- 1.Bukulmez O, Arici A. Luteal phase defect: myth or reality. Obstet Gynecol Clin North Am. 2004;31(4):727–744. doi: 10.1016/j.ogc.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Cramer DW, Wise LA. The epidemiology of recurrent pregnancy loss. Semin Reprod Med. 2000;18(4):331–9. doi: 10.1055/s-2000-13722. [DOI] [PubMed] [Google Scholar]
- 3.Prior JC. Perimenopause: the complex endocrinology of the menopausal transition. Endocr Rev. 1998;19(4):397–428. doi: 10.1210/edrv.19.4.0341. [DOI] [PubMed] [Google Scholar]
- 4.Burger HG, Dudley EC, Robertson DM, Dennerstein L. Hormonal changes in the menopause transition. Recent Prog Horm Res. 2002;57:257–75. doi: 10.1210/rp.57.1.257. [DOI] [PubMed] [Google Scholar]
- 5.Prior JC. The ageing female reproductive axis II: ovulatory changes with perimenopause. Novartis Found Symp. 2002;242:172–86. discussion 186–92. [PubMed] [Google Scholar]
- 6.Burger HG, Hale GE, Dennerstein L, Robertson DM. Cycle and hormone changes during perimenopause: the key role of ovarian function. Menopause. 2008;15(4 Pt 1):603–12. doi: 10.1097/gme.0b013e318174ea4d. [DOI] [PubMed] [Google Scholar]
- 7.Longcope C, Franz C, Morello C, Baker R, Johnston CCJ. Steroid and gonadotrophin levels in women during hte peri-menopausal years. Maturitas. 1986;8:189–196. doi: 10.1016/0378-5122(86)90025-3. [DOI] [PubMed] [Google Scholar]
- 8.Sherman BM, West JH, Korenman SG. The menopausal transition: analysis of LH, FSH, estradiol, and progesterone concentrations during menstrual cycles of older women. J Clin Endocrinol Metab. 1976;42(4):629–36. doi: 10.1210/jcem-42-4-629. [DOI] [PubMed] [Google Scholar]
- 9.Burger HG, Robertson DM, Baksheev L, Collins A, Csemiczky G, Landgren BM. The relationship between the endocrine characteristics and the regularity of menstrual cycles in the approach to menopause. Menopause. 2005;12(3):267–74. doi: 10.1097/01.gme.0000147172.21183.86. [DOI] [PubMed] [Google Scholar]
- 10.Santoro N, Isaac B, Neal-Perry G, Adel T, Weingart L, Nussbaum A, et al. Impaired folliculogenesis and ovulation in older reproductive aged women. J Clin Endocrinol Metab. 2003;88(11):5502–5509. doi: 10.1210/jc.2002-021839. [DOI] [PubMed] [Google Scholar]
- 11.Hale GE, Zhao X, Hughes CL, Burger HG, Robertson DM, Fraser IS. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the Staging of Reproductive Aging Workshop (STRAW) staging system. J Clin Endocrinol Metab. 2007;92(8):3060–7. doi: 10.1210/jc.2007-0066. [DOI] [PubMed] [Google Scholar]
- 12.Klein NA, Battaglia DE, Fujimoto VY, Davis GS, Bremner WJ, Soules MR. Reproductive aging: accelerated ovarian follicular development associated with a monotropic follicle-stimulating hormone rise in normal older women. J Clin Endocrinol Metab. 1996;81(3):1038–45. doi: 10.1210/jcem.81.3.8772573. [DOI] [PubMed] [Google Scholar]
- 13.Santoro N, Crawford SL, Lasley WL, Luborsky JL, Matthews KA, McConnell D, et al. Factors related to declining luteal function in women during the menopausal transition. J Clin Endocrinol Metab. 2008;93(5):1711–21. doi: 10.1210/jc.2007-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miro F, Parker SW, Aspinall LJ, Coley J, Perry PW, Ellis JE. Origins and consequences of the elongation of the human menstrual cycle during the menopausal transition: The FREEDOM study. J Clin Endocrinol Metab. 2004;89:4910–4915. doi: 10.1210/jc.2003-031731. [DOI] [PubMed] [Google Scholar]
- 15.Gorrindo T, Lu Y, Pincus S, Riley A, Simon JA, Singer BH, et al. Lifelong menstrual histories are typically erratic and trending: a taxonomy. Menopause. 2007;14(1):74–88. doi: 10.1097/01.gme.0000227853.19979.7f. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein M, Gorrindo T, Riley A, Mormino J, Niedfeldt J, Singer B, et al. Timing of menopause and patterns of menstrual bleeding. Am J Epidemiol. 2003;158(8):782–91. doi: 10.1093/aje/kwg223. [DOI] [PubMed] [Google Scholar]
- 17.Treloar A, Boynton R, Behn B, Brown B. Variation of the human menstrual cycle through reproductive life. Int J Fertil. 1967;12:77–126. [PubMed] [Google Scholar]
- 18.Taffe JR, Dennerstein L. Menstrual patterns leading to the final menstrual period. Menopause. 2002;9(1):32–40. doi: 10.1097/00042192-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Terry KL, Willett WC, Rich-Edwards JW, Hunter DJ, Michels KB. Menstrual cycle characteristics and incidence of premenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(6):1509–13. doi: 10.1158/1055-9965.EPI-05-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shideler SE, DeVane GW, Kaira PS, Benirschke K, Lasley BL. Ovarian-pituitary hormone interactions during the perimenopause. Maturitas. 1989;11:331–339. doi: 10.1016/0378-5122(89)90029-7. [DOI] [PubMed] [Google Scholar]
- 21.Metcalf MG, Donald RA, Livesey JH. Pituitary-ovarian function before, during, and after the menopause: a longitudinal study. Clinical Endocrinology. 1981;17:489–494. doi: 10.1111/j.1365-2265.1982.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 22.Wu JM, Zelinski MB, Ingram DK, Ottinger MA. Ovarian aging and menopause: current theories, hypotheses, and research models. Exp Biol Med. 2005;230(11):818–28. doi: 10.1177/153537020523001106. [DOI] [PubMed] [Google Scholar]
- 23.te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. 2002;8(2):141–54. doi: 10.1093/humupd/8.2.141. [DOI] [PubMed] [Google Scholar]
- 24.Hall JE. Neuroendocrine changes with reproductive aging in women. Semin Reprod Med. 2007;25(5):344–51. doi: 10.1055/s-2007-984740. [DOI] [PubMed] [Google Scholar]
- 25.Ferrell RJ, O’Connor KA, Rodriguez G, Gorrindo T, Holman DJ, Brindle E, et al. Monitoring Reproductive Aging in a Five Year Prospective Study: Aggregate and Individual Changes in Steroid Hormones and Menstrual Cycle Lengths with Age. Menopause. 2005;12(5):567–577. doi: 10.1097/01.gme.0000172265.40196.86. [DOI] [PubMed] [Google Scholar]
- 26.Wood JW. Dynamics of Human Reproduction: Biology, Biometry, Demography. New York: Aldine de Gruyter; 1994. [Google Scholar]
- 27.Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, et al. Executive Summary: Stages of Reproductive Aging Workshop (STRAW) Fertil Steril. 2001;76:874–878. doi: 10.1016/s0015-0282(01)02909-0. [DOI] [PubMed] [Google Scholar]
- 28.Lisabeth L, Harlow S, Qaqish B. A new statistical approach demonstrated menstrual patterns during the menopausal transition did not vary by age at menopause. J Clin Epidemiol. 2004;57:484–96. doi: 10.1016/j.jclinepi.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Lisabeth LD, Harlow SD, Gillespie B, Lin X, Sowers MF. Staging reproductive aging: a comparison of proposed bleeding criteria for the menopausal transition. Menopause. 2004;11(2):186–197. doi: 10.1097/01.gme.0000082146.01218.86. [DOI] [PubMed] [Google Scholar]
- 30.Harlow SD, Mitchell ES, Crawford S, Nan B, Little R, Taffe J. The ReSTAGE Collaboration: defining optimal bleeding criteria for onset of early menopausal transition. Fertil Steril. 2008;89(1):129–40. doi: 10.1016/j.fertnstert.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Connor KA, Brindle E, Holman DJ, Klein NA, Soules MR, Campbell KL, et al. Urinary estrone conjugate and pregnanediol-3-glucuronide enzyme immunoassays for population research. Clin Chem. 2003;49(7):1139–48. doi: 10.1373/49.7.1139. [DOI] [PubMed] [Google Scholar]
- 32.O’Connor KA, Brindle E, Shofer JB, Miller RC, Klein NA, Soules MR, et al. Statistical correction for non-parallelism in a urinary enzyme immunoassay. J Immunoassay Immunochem. 2004;25(3):259–278. doi: 10.1081/ias-200028078. [DOI] [PubMed] [Google Scholar]
- 33.Brindle E, Miller RC, Shofer JB, Klein NA, Soules MR, O’Connor KA. Urinary beta-luteinizing hormone and beta-follicle stimulating hormone immunoenzymometric assays for population research. Clin Biochem. 2006;39(11):1071–9. doi: 10.1016/j.clinbiochem.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller RC, Brindle E, Holman DJ, Shofer JB, Klein NA, Soules MR, et al. Comparison of specific gravity and creatinine methods for normalizing urinary reproductive hormone concentrations. Clin Chem. 2004;50(5):924–932. doi: 10.1373/clinchem.2004.032292. [DOI] [PubMed] [Google Scholar]
- 35.Speroff L, Fritz MA. Clinical Gynecologic Endocrinology and Infertility. 7. Philadelphia, PA: Lippincott Williams and Wilkins; 2005. [Google Scholar]
- 36.O’Connor KA, Brindle E, Miller RC, Shofer JB, Ferrell RJ, Klein NA, et al. Ovulation detection methods for urinary hormones: precision, daily and intermittent sampling and a combined hierarchical method. Hum Reprod. 2006;21(6):1442–52. doi: 10.1093/humrep/dei497. [DOI] [PubMed] [Google Scholar]
- 37.Kassam A, Overstreet JW, Snow-Harter C, De Souza MJ, Gold EB, Lasley BL. Identification of Anovulation and transient luteal function using a urinary pregnanediol-3-glucuronide ratio algorithm. Environ Health Perspect. 1996;104(4):408–413. doi: 10.1289/ehp.96104408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R DCT. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2007. [Google Scholar]
- 39.Kaunitz AM. Oral contraceptive use in perimenopause. Am J Obstet Gynecol. 2001;185(2 Suppl):S32–7. doi: 10.1067/mob.2001.116525. [DOI] [PubMed] [Google Scholar]
- 40.Landgren B, Collins A, Csemiczyk G, Burger HG, Baksheev L, Robertson DM. Menopause transition: annual changes in serum hormonal patterns over the menstrual cycle in women during a nine-years period prior to menopause. J Clin Endocrinol Metab. 2004;89:2763–2769. doi: 10.1210/jc.2003-030824. [DOI] [PubMed] [Google Scholar]
- 41.Van Voorhis BJ, Santoro N, Harlow S, Crawford SL, Randolph J. The relationship of bleeding patterns to daily reproductive hormones in women approaching menopause. Obstet Gynecol. 2008;112(1):101–8. doi: 10.1097/AOG.0b013e31817d452b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Metcalf MG. Incidence of ovulatory cycles in women approaching the menopause. J Biosoc Sci. 1979;11:39–48. doi: 10.1017/s0021932000012037. [DOI] [PubMed] [Google Scholar]
- 43.O’Connor KA, Ferrell RJ, Brindle E, Shofer JB, Holman DJ, Miller RC, et al. Total and unopposed estrogen exposure across stages of the transition to menopause. Cancer Epidemiol Biomarkers Prev. 2009;18(3):828–36. doi: 10.1158/1055-9965.EPI-08-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santoro N, Brown JR, Adel T. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81(4):1495–1501. doi: 10.1210/jcem.81.4.8636357. [DOI] [PubMed] [Google Scholar]
- 45.Metcalf MG, Livesey DR. Pregnadiol excretion in fertile women: age-related changes. J Endocrinol. 1988;119:153–157. doi: 10.1677/joe.0.1190153. [DOI] [PubMed] [Google Scholar]
- 46.Wiebe JP. Progesterone metabolites in breast cancer. Endocr Relat Cancer. 2006;13(3):717–738. doi: 10.1677/erc.1.01010. [DOI] [PubMed] [Google Scholar]
- 47.Surveillance EaERSPwscg. SEER*Stat Database: Incidence- SEER 17 Regs Limited-Use, Nov 2006 Sub (2000–2004), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released 2007, based on the November 2006 submission.
- 48.Holman DJ, Wood JW. Pregnancy loss and fecundability in women. In: Ellison PL, editor. Reproductive Ecology and Human Evolution. New York: Aldine de Gruyter; 2001. pp. 15–38. [Google Scholar]
- 49.O’Connor KA, Holman DJ, Wood JW. Menstrual cycle variability and the perimenopause. Am J Hum Biol. 2001;13(4):465–478. doi: 10.1002/ajhb.1078. [DOI] [PubMed] [Google Scholar]
- 50.Garai J, Vilagi S, Repasy I, Koppan M, Bodis J. Short communication: seasonal onset of menopause? Hum Reprod. 2004;19(7):1666–1667. doi: 10.1093/humrep/deh260. [DOI] [PubMed] [Google Scholar]
- 51.Spencer EA, Appleby PN, Davey GK, Key TJ. Validity of self-reported height and weight in 4808 EPIC-Oxford participants. Public Health Nutr. 2002;5(4):561–5. doi: 10.1079/PHN2001322. [DOI] [PubMed] [Google Scholar]
- 52.Palta M, Prineas RJ, Berman R, Hannan P. Comparison of self-reported and measured height and weight. Am J Epidemiol. 1982;115(2):223–30. doi: 10.1093/oxfordjournals.aje.a113294. [DOI] [PubMed] [Google Scholar]
- 53.Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22(2):414–20. doi: 10.1093/humrep/del400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jensen TK, Scheike T, Keiding N, Schaumburg I, Grandjean P. Fecundability in relation to body mass and menstrual cycle patterns. Epidemiology. 1999;10(4):422–8. doi: 10.1097/00001648-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 55.Jain A, Polotsky AJ, Rochester D, Berga SL, Loucks T, Zeitlian G, et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab. 2007;92(7):2468–73. doi: 10.1210/jc.2006-2274. [DOI] [PubMed] [Google Scholar]
- 56.Randolph J, John F, Sowers M, Gold EB, Mohr BA, Luborsky J, Santoro N, et al. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab. 2003;88(4):1516–1522. doi: 10.1210/jc.2002-020777. [DOI] [PubMed] [Google Scholar]
- 57.van der Steeg JW, Steures P, Eijkemans MJ, Habbema JD, Hompes PG, Burggraaff JM, et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod. 2008;23(2):324–8. doi: 10.1093/humrep/dem371. [DOI] [PubMed] [Google Scholar]
- 58.Santoro N, Lasley BL, McConnell D, Allsworth J, Crawford SL, Gold EB, et al. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: The study of women’s health across the nation (SWAN) daily hormone study. J Clin Endocrinol Metab. 2004;89(6):2622–2631. doi: 10.1210/jc.2003-031578. [DOI] [PubMed] [Google Scholar]
- 59.Sowers M, Zheng H, Tomey K, Karvonen-Gutierrez C, Jannausch M, Li X, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metab. 2007;92(3):895–901. doi: 10.1210/jc.2006-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wing RR, Matthews KA, Kuller LH, Meilahn EN, Plantinga PL. Weight gain at the time of menopause. Arch Intern Med. 1991;151(1):97–102. [PubMed] [Google Scholar]