Abstract
The purpose of this study was to characterize the age-related changes of the mouse meibomian gland. Eyelids from adult C57Bl/6 mice at 2, 6, 12 and 24 months of age were stained with specific antibodies against peroxisome proliferator activated receptor gamma (PPARγ) to identify differentiating meibocytes, Oil Red O(ORO) to identify lipid, Ki67 nuclear antigen to identify cycling cells, B-lymphocyte-induced maturation protein-1 (Blimp1) to identify potential stem cells and CD45 to identify immune cells. Meibomian glands from younger mice (2 and 6 months) showed cytoplasmic and perinuclear staining with anti-PPARγ antibodies with abundant ORO staining of small, intracellular lipid droplets. Meibomian glands from older mice (12 and 24 months) showed only nuclear PPARγ localization with less ORO staining and significantly reduced acinar tissue (p<0.04). Acini of older mice also showed significantly reduced (p<0.004) numbers of Ki67 stained nuclei. While Blimp1 appeared to diffusely stain the superficial ductal epithelium, isolated cells were occasionally stained within the meibomian glandduct and acini of older mice that also stained with CD45 antibodies, suggesting the presence of infiltrating plasmacytoid cells. These findings suggest that there is altered PPARγ receptor signaling in older mice that may underlie changes in cell cycle entry/proliferation, lipid synthesis and gland atrophy during aging. These results are consistent with the hypothesis that mouse meibomian glands undergo age-related changes similar to those identified in humans and may be used as a model for age-related meibomian gland dysfunction.
Keywords: Meibomian gland, PPARγ, aging, eyelid, lymphocytic infiltration
Introduction
Meibomian glands are lipid excreting glands embedded in the tarsal plate of the eyelid. Lipids from the meibomian gland play an important role in the maintenance of the ocular surface tear film and form the most superficial layer that prevents evaporation and protects against excessive dehydration (Driver & Lemp, 1996). In meibomian gland dysfunction (MGD) there is thought to be abnormal lipid excretion either in quantity or quality that results in increased evaporation leading to blepharitis and evaporative dry eye syndrome (DES) (2007). During aging, several changes occur within the meibomian gland including acinar atrophy, basement membrane thickening, ductal hyperkeratinization and lipogranulomatous inflammation leading to a decreased volume and quality of meibomian gland excreta (Obata, 2002). There is also evidence showing an association between MGD and androgen deficiency that occurs in the elderly (Den, et al., 2006, Steagall, et al., 2002, Sullivan, et al., 2002a, Sullivan, et al., 2002b, Sullivan, et al., 2002c, Sullivan, et al., 2002d, Suzuki, et al., 2002, Yamagami, et al., 2002a, Yamagami, et al., 2002b). Despite these findings, MGD is not a well characterized entity and further study is necessary to understand the pathogenesis of this disorder.
While the understanding of meibomian gland function is limited, there has been extensive research into the dynamic growth and differentiation of the sebaceous gland, holocrine lipid excreting gland of the skin comprised of sebocytes. These studies indicate that sebocyte differentiation involves signaling by the peroxisome proliferator activator receptor (PPAR) in response to lipogenic factors that initiates lipogenesis and the accumulation of intracellular lipid droplets (sebum) (Tontonoz & Spiegelman, 2008). PPARs represent a family of transcription factors (α, β, σ,γ) that are activated by fatty acids for which PPARγ is the major isoform expressed in the sebaceous gland. As lipid droplets coalesce and fill the cytoplasm, the sebocyte then undergoes degeneration and releases lipid onto the hair shaft for lubrication and protection of the skin against bacterial infection (Fuchs, 2007). Replacement of disintegrated sebocytes is thought to involve sebocyte progenitor cells located within the ductal epithelium of sebaceous gland that express the transcriptional repressor, B-lymphocyte-induced maturation protein 1 (Blimp1). Studies suggest that Blimp1 suppresses c-myc, and that down-regulation of Blimp1 in progenitor cells allows for proliferation and differentiation of sebocytes.
Since aging has been associated with dysfunction of the meibomian gland and the sebaceous gland (Obata, 2002, Pochi, et al., 1979, Zouboulis & Boschnakow, 2001), this study evaluated the effects of age on meibocyte differentiation and renewal by assessing the localization of PPARγ and Blimp1 in association with neutral lipid synthesis, cell cycling and meibomian gland size in the mouse. We report for the first time that there is a marked change from a cytoplasmic to nuclear localization of PPARγ during aging that is associated with significant decrease in meibocyte cell cycling and meibomian gland size. Furthermore, no isolated Blimp1 positive cells could be identified within the meibomian gland that were not CD45 positive indicating that some glands may be infiltrated by plasmacytoid cells, particularly in older mice.
Methods
Animals
A total of 49 C57Bl/6 mice were used in this study. Fifteen animals at 2 months old, 13 animals at 6 months old, 15 animals at 12 months old and 6 animals at 24 months of age were humanely sacrificed by cervical neck dislocation after anesthesia using ketamine HCl (100 mg/Kg body weight, Bioniche Pharma, Lake Forest, IL) and xylazine (20 mg/Kg body weight, Lloyd Laboratories, Shenandoah, IO). All procedures were approved by the UCI IACUC and conducted in accordance with ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Inmunohistochemistry
Eyelids were fixed overnight in 2% paraformaldehyde in phosphate buffered saline (PBS, pH 7.4). Eyelids were then trimmed of fur and excess tissue, placed in15% sucrose for 4 hours and then transferred to 30% sucrose overnight. Lids were then embedded in OCT, frozen in liquid nitrogen and then sectioned using a Leica CM1850 Crytotome (Leica, Wetzlar, Germany).
Cryostat sections (8 μm) were placed on glass slides and allowed to dry before staining. Sections were then re-hydrated with PBS and placed in freezing acetone (−20C) for 3 minutes. Tissue sections were then blocked with 1% BSA in PBS for 30 minutes at 37°C, excess BSA removed and then incubated with primary antibodies including rabbit polyclonal anti-PPARγ (1:50, Abcam, Cambridge, MA), goat polyclonal anti- PRDM1/BLIMP-1 (1:50, Abcam), rabbit polyclonal anti Ki67 (Abcam) and FITC conjugated anti-mouse common leukocyte antigen (CD45, clone 30F11, 1:50, R&D Systems, Minneapolis, MN). Sections were then incubated for 1 hour at 37°C, washed with PBS and reacted with FITC conjugated secondary antibodies against mouse, rabbit or goat IgG (Invitrogen, Carlsbad, CA) for 1 hour at 37°C. All samples were counterstained with DAPI (300 nM) in PBS for 15 minutes. For negative controls, sections were left either un-stained or incubated with irrelevant IgG or secondary antibodies alone. Sections were then evaluated and imaged using a Nikon Eclipse E600 fluorescence microscope (Melville, NY, U.S.A).
For detection of neutral lipids, fresh frozen sections were re-hydrated in PBS and then stained with 0.5% Oil Red O (ORO, Sigma Chemical Co., St Louis, MO) for 10 minutes. Samples were then rinsed with PBS and counterstained with hematoxylin.
Measurement of Cell Cycling Labeling Index
Digital images were taken of stained tissue sections and then analyzed using Metamorph Image analysis software (Molecular Devices, Downingtown, PA). Regions of the gland containing acini were first identified and the number of nuclei positively stained with Ki67 marked and recorded using the cell count subroutine. The total number of nuclei within the basal cell layer was then counted using the same subroutine and the number recorded. Data was then downloaded to an Excel spreadsheet and the labeling index (LI) calculated by dividing the number of positive Ki67 cell nuclei by the total number of acinar basal cells and multiplying by 100. All acini were counted in 3 different sections from each eyelid and the average and standard deviation determined. A total of 6 eyelids from mice ages 2, 6 and 12 months and 2 eyelids from mice 24 months of age were measured.
Measurement of Meibomian Gland Acinar Area
Eyelids were embedded in LR White plastic embedding media and then 3 μm thick serial sections cut using Leica Ultramicrome ((Leica, Wetzlar, Germany). Each eyelid was sectioned in the coronal plane starting at the distal meibomian gland orifice and extending proximal completely through the gland for 125 to 285 sections. Images of every 5th tissue section were then photographed and digitized. The area of 3–4 glands in each section was then measured using Metamorph and the average over the 25–57 images calculated. The average gland size for each eyelid was determined by averaging the areas for the 3–4 glands per each eyelid. Two eyelids from mice aged 2 months and 1 year were measured.
Statistics
Results are reported as the mean ± the standard deviation. Differences between groups were calculated by Sigma Stat (Systat Software, Inc., Point Richmond, CA) using All Pairwise Multiple Comparison Procedures, Holm-Sidak method.
Results
Lipogenesis
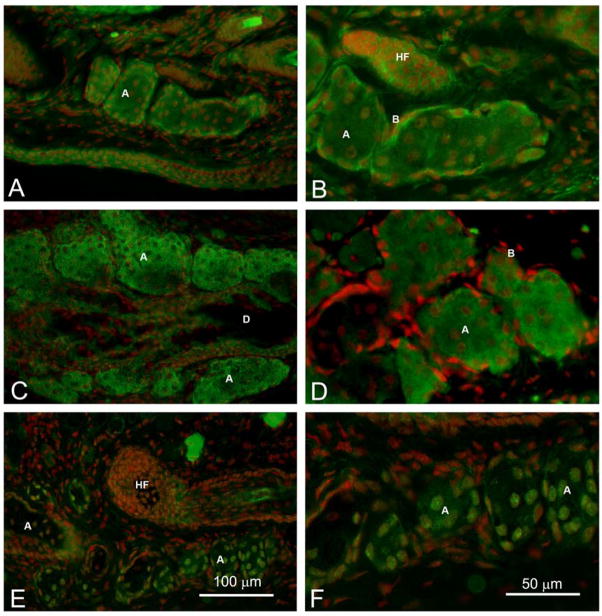
The effect of age on meibocyte differentiation was first evaluated by identifying the location and expression of PPARγ within the meibomian gland acinar cells (Figure 1). Immunofluorescent staining of eyelids from 2 month old and 6 month old mice showed perinuclear and cytoplasmic staining of meibocytes within the acini, which appeared more intense in basal cells of 2 month old mice and more vesicular in 6 month old mice (Figure 1, A to D). This pattern of staining was noticeably changed in older mice with a complete shift from cytoplasmic to nuclear staining at 12 months (not shown) and 24 months (Figure 1E and F). Slides stained with secondary antibody alone or irrelevant primary antibodies showed no staining (not shown). The cytoplasmic PPARγ localization in younger mice was associated with the presence of multiple small lipid droplets stained by ORO within the cytoplasm of differentiating meibocytes from 2 month and 6 month old mice (Figure 2, A and B). By contrast, ORO staining of meibomian glands from older mice appeared generally more intense (Figure 2C and D). Sections through the gland also showed less overall ORO staining in older mice compared to younger mice suggesting a decrease in lipid producing acinar tissue.
Figure 1.
PPARγ localization (Green) in adult mice. We observed cytoplasmic and nuclear anti-PPARγ staining in 2 month (A and B) and 6 month old glands (C and D) that change to a nuclear staining in 2 year old mice (E and F). (Red=Dapi) (A=Acini, D=Duct, HF=Hair follicle, C=Conjunctiva).
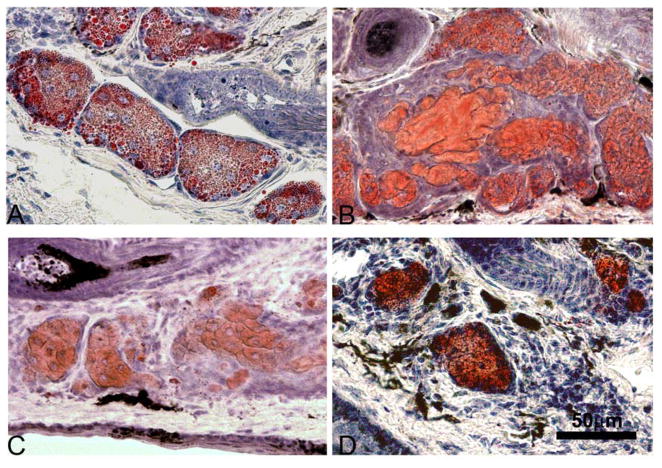
Figure 2.
ORO staining for lipid in adult mice. We observed abundant lipid droplets in 2 months (A) and 6 months old glands (B) while in 1 year and 2 year old mice we observed fewer and more condensed ORO stained lipid dropplets (C and D respectively) (A=Acini, D=Duct, HF=Hair follicle).
Proliferative Potential and Gland Size
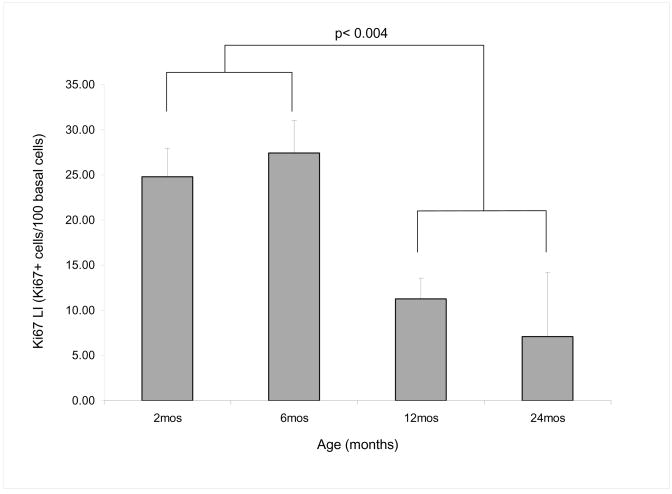
Since the loss of cytoplasmic staining by PPARγ suggested a decrease in the nuclear to cytoplasmic signaling important for lipogenesis and cell proliferation, we next evaluated the effect of age on the number of actively cycling cells in the meibomian glands. Ki67 is a nuclear antigen that is expressed during cell cycling and is present at stages G1 through mitosis but absent in resting cells and therefore can be used as a marker of the number of cells that are undergoing cell division. Staining of sections with antibodies to Ki67 labeled many of the basal nuclei present in the acini of meibomian glands from 2 month and 6 month old mice (Figure 3, A and B, respectively). By comparison, Ki67 labeling of basal nuclei from 12 month and 24 month old mice appeared greatly reduced (Figure 3, C and D, respectively). In order to confirm this observation, the number of stained nuclei within the acini of the meibomian gland were counted and a labeling index (LI) calculated based on the number of Ki67 stained nuclei per 100 total nuclei in the basal cell layer. Results showed that younger meibomian glands had an average LI of 24.79 ± 7.77 and 27.43 ± 8.82 in the acini of eyelids from 2 month and 6 month old mice respectively. This was significantly higher (p<0.004) than the LI in older mice which averaged 11.28 ± 5.62 and 7.1 ± 10.04 for eyelids from 12 month and 24 month old mice respectively, indicating that indeed the cell growth and differentiation rate of meibocytes was reduced in older mice (Figure 4)
Figure 3.
Ki67 (Green) staining of mouse meibomian glands at 2 months (A), 6 months (B), 1 year (C) and 2 years (D) of age. Note the loss of Ki67 staining of acinar cells at 12 and 24 months of age (Red= Dapi) (A=Acini, B= basal cells, D=Duct, HF=Hair follicle, C=Conjunctiva, M=Levator palpebrae muscle).
Figure 4.
Labeling Index (LI) calculated by the number of positive stained nuclei fo Ki67 per 100 total basal cells. We observed a significant decrease of Ki67 positive stained cells at 12 and 24 months of age compared to 2 and 6 months old mice (p=0.004). (2 month and 6 month old p=not significant (NS), 2 month and 12 month old p < 0.01, 2 month and 24 month old p < 0.05, 6 month and 12 month old p < 0.01, 6 month and 24 month old p <0.05, 12 month and 24 month old p =NS).
This marked decrease in cell cycling also suggested that the mouse meibomian glands may atrophy with age, consistent with the marked decrease ORO staining seen in the older eyelids. In order to assess this possibility we measured the average gland area cut in cross section for meibomian glands in younger (2 month) and older (12 month) mice. In 2 month old mice the meibomian glands had an average cross-sectional area of 25,748 ± 4,573 μm2. By contrast, meibomian glands from 12 month old mice were significantly reduced (p<0.04) in area to 7,782 ± 2,956 μm2 or by almost 70%.
Blimp-1 and CD45 staining
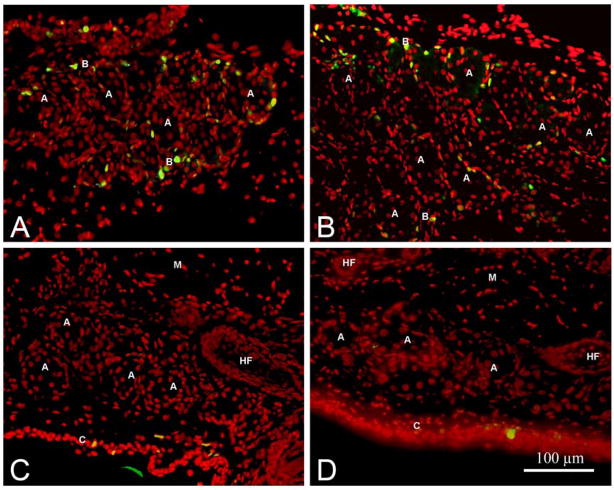
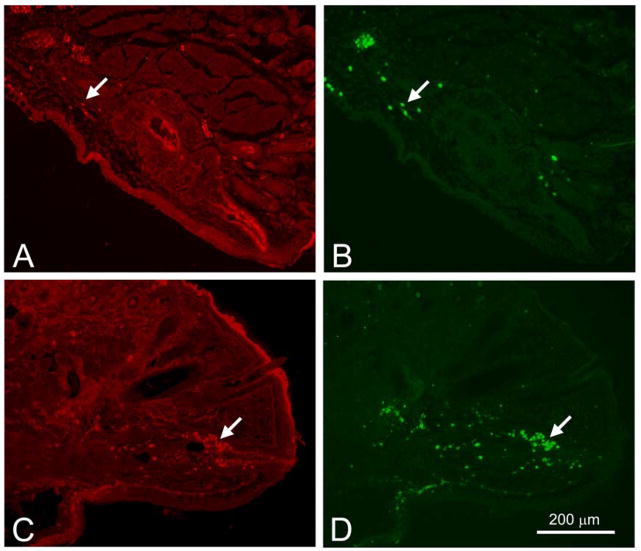
Recent studies have suggested that sebaceous and meibomian gland progenitor cells may express Blimp1 and reside within the distal ductal epithelium of the gland. Since aging appears to result in meibomian gland atrophy with possible reduction or loss of progenitor cells, we stained mouse meibomian glands for Blimp1. When young mice were evaluated, Blimp1 showed intense staining of the suprabasal ductal epithelium, and occasional cells in the interstitial tissues (Figure 5A, arrow). Counterstaining sections with antibodies to CD45 also stained isolated cells in the interstitial tissue indicating that these cells were plasmacytoid cells (Figure 5B, arrow). In meibomian glands from older mice, clusters of cells within the distal ductal epithelium showed staining with Blimp1 (Figure 5C, arrow) that stained with antibodies to CD45 (Figure 5D, arrow) indicating that these cells were also plasmacytoid cells and not meibomian gland progenitor cells.
Figure 5.
Blimp1 (Red, A and C) and CD45 (Green, B and D) localization in young (2 month-old, A and B) and old (2 year-old, C and D) mice. (A) and (B) shows Blimp1 staining the duct and a few cells in the interstitial tissue that stained with CD45. (C) shows intraductal Blimp1 positive cells (arrow) that colocalize with CD45 (arrow) in(D) (A=Acini, D=Duct, HF=Hair follicle, C=Conjunctiva, M=Levator palpebrae muscle).
Discussion
We have identified distinct age-related changes in the mouse meibomian gland that appear to model changes in aging human meibomian glands (Obata, 2002) thought to underlie the development of age-related evaporative dry eye and meibomian gland dysfunction. In comparing young (2 to 6 month) to old (12 to 24 month) meibomian glands there was a distinct change from a cytoplasmic to a nuclear localization of the nuclear receptor PPARγ that plays an important role in controlling differentiation of the sebaceous gland. This change was also associated with a significant decrease in Ki67 labeling of basal acinar cells suggesting decreased cell proliferation and growth. Meibomian glands from older mice were also significantly smaller in area compared to meibomian glands from younger mice, and showed distinctly less ORO staining. Furthermore, aging appeared to be associated with immune cell infiltration of the meibomian gland, similar to that observed in human lids. Together, these changes suggest that older meibomian glands in the mouse undergo atrophic changes that include reduced meibocyte differentiation and lipid production combined with increased immune cell infiltration that together may lead to MGD. Further studies identifying the role of PPARγ in regulating meibocyte differentiation during aging may provide insight into the mechanism of these age related changes.
PPARs are nuclear receptors implicated in cell proliferation, differentiation and apoptosis (Keller, et al., 2000). PPARγ, the most studied of these receptors, is a key regulator of adipogenesis, sebum production and adipocyte differentiation (Mao-Qiang, et al., 2004, Sandouk, et al., 1993). Our finding that PPARγ staining changed in older mice is consistent with age-related changes in the human sebaceous gland that has been observed by others (Trivedi, et al., 2006). The function of PPARs has been studied using PPAR agonists in different models and have been implicated in the fetal development of the epidermis, including keratinocyte differentiation and the development of skin appendages such as hair follicles and sebaceous glands (Rosenfield, et al., 1999). The activation of PPARγ using agonists like thiazolidinediones, linolenic acid and fibrates have been shown to increase the formation of intracellular lipid droplets in rat preputial sebocytes (Rosenfield, et al., 1999). In agreement with this animal study, increased sebum production has been noted in hyperlipidemia patients following treatment with fibrates and diabetics following treatment with thiazolidinediones (Trivedi et al., 2006). There is also evidence that PPARγ agonists, ciglitazone and troglitazone, reduce epithelial hyperplasia in both mouse models (Demerjian, et al., 2006) and patients with psoriasis (Ellis, et al., 2000).
A role for PPARγ in meibomian gland function is suggested by our finding of age-related changes in PPARγ localization in association with changes in the lipid production and meibomian gland size. Specifically, we observed abundant small lipid droplets in young meibomian glands (2 and 6 months) that changed in older mice to fewer and larger droplets with more intense staining. Furthermore, the size of the meibomian glands showed a significant decrease with aging indicating that these glands become atrophic in older mice. These findings are consistent with previous studies of human meibomian glands, which show reduced and viscous excretions, reduced cellular turnover, and acinar cell atrophy in older people (Hykin & Bron, 1992, Norn, 1987, Obata, 2002, Obata, et al., 1994). Furthermore, Sullivan et al (Sullivan, et al., 2006) demonstrated changes in the polar and neutral lipid profiles in human meibomian glands associated with aging, with a significant increase in the levels of neutral lipid that correlated with increased opacity of meibomian gland excretion. Finally, Hykin et al (Hykin & Bron, 1992) has shown human eyelid changes with age, includin hyperkeratinization, lid margin vascularity, decrease gland expressibility and inspissation of excreta.
Interestingly, endogenous ligands for the PPARs include free fatty acids and eicosanoids and include the essential fatty acids omega-3 (i.e. α-linolenic acid, n-3) and omega-6 (i.e. linolenic acid, n-6). Both omega-3 and omega-6 fatty acids are also considered natural modulators of inflammation and shown to be beneficial agents in many inflammatory diseases. Since inflammation has an important role in the development of DES there are some reports showing the benefits of n-3 dietary intake in reducing the prevalence of DES (Miljanovic, et al., 2005) and the effects of topical α-linolenic acid in the treatment inflammatory changes in DES (Rashid, et al., 2008).
In this regard it should be noted that the changes in PPARγ expression identified in older mice were also associated with increased lymphocytic infiltration. While attempting to identify putative meibocyte progenitor cells, we identified Blimp1/CD45 positive cells that infiltrated the meibomian gland of older mice. Lymphocytic infiltrates have been noted in human meibomian glands by Obata, et al (Obata, 2002, Obata et al., 1994). Furthermore, proteomic studies of meibomian gland excreta have identified IgA as a major constituent, suggesting that plasma cells may be present within human meibomian glands (Tsai, et al., 2006). Our findings are therefore consistent with these human observations and suggest that in older individuals these glands may become infiltrated with lymphocytic cells. In this context, PPAR ligands such as n-3 fatty acids could play an important therapeutic role in DES by not only stimulating meibocyte differentiation, but also in reducing inflammation and meibomian gland infiltration. However, further studies are needed to identify the potential role of lymphocytic infiltration in the development of MGD and DES. Specifically, studies identifying the inflammatory cell type and presence of pro inflammatory cytokines are needed.
It is not clear why we were unable to identify Blimp1 positive/CD45 negative progenitor meibocytes, although differences in antibody specificity between those used in this study and the ones used by others may be an explanation. Recently, Horshley et al (Horsley, et al., 2006) showed that Blimp1, a transcriptional repressor protein, plays a key role as a marker of progenitor cells located at the entrance of the sebaceous gland. These Blimp1 positive cells are capable of regenerating the entire sebaceous gland and when targeted for ablation in transgenic mice result in sebaceous gland hyperplasia mediated by the loss of c-myc repression (Fuchs, 2008, Horsley et al., 2006). In addition, Blimp1 is a regulator of terminal differentiation of myeloid cells and B lymphocytes (Angelin-Duclos, et al., 2000, Cattoretti, et al., 2005, Chang, et al., 2002).
In conclusion, this study establishes that the mouse meibomian gland undergoes distinct age-related changes similar to those already established for the human meibomian gland and may be used as a model to study age-related MGD. Our findings that meibomian glands show altered PPARγ localization in conjunction with reduced growth and differentiation point to the intriguing possibility that PPAR ligands may play an important role in the development of these age-related changes. The findings would also suggest that PPAR agonists and antagonist may be efficacious in the treatment of age-related MGD.
Acknowledgments
Supported in part by NIH Infrastructure Grant EY016663; Support Grant from Research to Prevent Blindness, Inc, The Skirball Program in Molecular Ophthalmology, and a Research Gift from Alcon.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- International Dry Eye WorkShop. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- Angelin-Duclos C, et al. Commitment of B lymphocytes to a plasma cell fate is associated with Blimp-1 expression in vivo. J Immunol. 2000;165(10):5462–5471. doi: 10.4049/jimmunol.165.10.5462. [DOI] [PubMed] [Google Scholar]
- Cattoretti G, et al. PRDM1/Blimp-1 is expressed in human B-lymphocytes committed to the plasma cell lineage. J Pathol. 2005;206(1):76–86. doi: 10.1002/path.1752. [DOI] [PubMed] [Google Scholar]
- Chang DH, et al. The dynamic expression pattern of B lymphocyte induced maturation protein-1 (Blimp-1) during mouse embryonic development. Mech Dev. 2002;117(1–2):305–309. doi: 10.1016/s0925-4773(02)00189-2. [DOI] [PubMed] [Google Scholar]
- Demerjian M, et al. Topical treatment with thiazolidinediones, activators of peroxisome proliferator-activated receptor-gamma, normalizes epidermal homeostasis in a murine hyperproliferative disease model. Exp Dermatol. 2006;15(3):154–160. doi: 10.1111/j.1600-0625.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- Den S, et al. Association between meibomian gland changes and aging, sex, or tear function. Cornea. 2006;25(6):651–655. doi: 10.1097/01.ico.0000227889.11500.6f. [DOI] [PubMed] [Google Scholar]
- Driver PJ, Lemp MA. Meibomian gland dysfunction. Surv Ophthalmol. 1996;40(5):343–367. doi: 10.1016/s0039-6257(96)80064-6. [DOI] [PubMed] [Google Scholar]
- Ellis CN, et al. Troglitazone improves psoriasis and normalizes models of proliferative skin disease: ligands for peroxisome proliferator-activated receptor-gamma inhibit keratinocyte proliferation. Arch Dermatol. 2000;136(5):609–616. doi: 10.1001/archderm.136.5.609. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. Skin stem cells: rising to the surface. J Cell Biol. 2008;180(2):273–284. doi: 10.1083/jcb.200708185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V, et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126(3):597–609. doi: 10.1016/j.cell.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hykin PG, Bron AJ. Age-related morphological changes in lid margin and meibomian gland anatomy. Cornea. 1992;11(4):334–342. doi: 10.1097/00003226-199207000-00012. [DOI] [PubMed] [Google Scholar]
- Keller JM, et al. Implications of peroxisome proliferator-activated receptors (PPARS) in development, cell life status and disease. Int J Dev Biol. 2000;44(5):429–442. [PubMed] [Google Scholar]
- Mao-Qiang M, et al. Peroxisome-proliferator-activated receptor (PPAR)-gamma activation stimulates keratinocyte differentiation. J Invest Dermatol. 2004;123(2):305–312. doi: 10.1111/j.0022-202X.2004.23235.x. [DOI] [PubMed] [Google Scholar]
- Miljanovic B, et al. Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am J Clin Nutr. 2005;82(4):887–893. doi: 10.1093/ajcn/82.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norn M. Expressibility of meibomian secretion. Relation to age, lipid precorneal film, scales, foam, hair and pigmentation. Acta Ophthalmol (Copenh) 1987;65(2):137–142. doi: 10.1111/j.1755-3768.1987.tb06991.x. [DOI] [PubMed] [Google Scholar]
- Obata H. Anatomy and histopathology of human meibomian gland. Cornea. 2002;21(7 Suppl):S70–74. doi: 10.1097/01.ico.0000263122.45898.09. [DOI] [PubMed] [Google Scholar]
- Obata H, et al. [Histopathological study of the meibomian glands in 72 autopsy cases] Nippon Ganka Gakkai Zasshi. 1994;98(8):765–771. [PubMed] [Google Scholar]
- Pochi PE, et al. Age-related changes in sebaceous gland activity. J Invest Dermatol. 1979;73(1):108–111. doi: 10.1111/1523-1747.ep12532792. [DOI] [PubMed] [Google Scholar]
- Rashid S, et al. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol. 2008;126(2):219–225. doi: 10.1001/archophthalmol.2007.61. [DOI] [PubMed] [Google Scholar]
- Rosenfield RL, et al. Rat preputial sebocyte differentiation involves peroxisome proliferator-activated receptors. J Invest Dermatol. 1999;112(2):226–232. doi: 10.1046/j.1523-1747.1999.00487.x. [DOI] [PubMed] [Google Scholar]
- Sandouk T, et al. The antidiabetic agent pioglitazone increases expression of glucose transporters in 3T3-F442A cells by increasing messenger ribonucleic acid transcript stability. Endocrinology. 1993;133(1):352–359. doi: 10.1210/endo.133.1.8319581. [DOI] [PubMed] [Google Scholar]
- Steagall RJ, et al. Androgen control of gene expression in the rabbit meibomian gland. Adv Exp Med Biol. 2002;506(Pt A):465–476. doi: 10.1007/978-1-4615-0717-8_65. [DOI] [PubMed] [Google Scholar]
- Sullivan BD, et al. Complete androgen insensitivity syndrome: effect on human meibomian gland secretions. Arch Ophthalmol. 2002a;120(12):1689–1699. doi: 10.1001/archopht.120.12.1689. [DOI] [PubMed] [Google Scholar]
- Sullivan BD, et al. Impact of androgen deficiency on the lipid profiles in human meibomian gland secretions. Adv Exp Med Biol. 2002b;506(Pt A):449–458. doi: 10.1007/978-1-4615-0717-8_63. [DOI] [PubMed] [Google Scholar]
- Sullivan BD, et al. Influence of aging on the polar and neutral lipid profiles in human meibomian gland secretions. Arch Ophthalmol. 2006;124(9):1286–1292. doi: 10.1001/archopht.124.9.1286. [DOI] [PubMed] [Google Scholar]
- Sullivan DA, et al. Androgen deficiency, Meibomian gland dysfunction, and evaporative dry eye. Ann N Y Acad Sci. 2002c;966:211–222. doi: 10.1111/j.1749-6632.2002.tb04217.x. [DOI] [PubMed] [Google Scholar]
- Sullivan DA, et al. Sex steroids, the meibomian gland and evaporative dry eye. Adv Exp Med Biol. 2002d;506(Pt A):389–399. doi: 10.1007/978-1-4615-0717-8_56. [DOI] [PubMed] [Google Scholar]
- Suzuki T, et al. Estrogen and progesterone effects on the morphology of the mouse meibomian gland. Adv Exp Med Biol. 2002;506(Pt A):483–488. doi: 10.1007/978-1-4615-0717-8_67. [DOI] [PubMed] [Google Scholar]
- Tontonoz T, Spiegelman BM. Fat and beyond: The diverse biology of PPARgamma. Ann Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- Trivedi NR, et al. Peroxisome proliferator-activated receptors increase human sebum production. J Invest Dermatol. 2006;126(9):2002–2009. doi: 10.1038/sj.jid.5700336. [DOI] [PubMed] [Google Scholar]
- Tsai PS, et al. Proteomic analysis of human meibomian gland secretions. Br J Ophthalmol. 2006;90(3):372–377. doi: 10.1136/bjo.2005.080846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagami H, et al. Gender-associated differences in gene expression of the meibomian gland. Adv Exp Med Biol. 2002a;506(Pt A):459–463. doi: 10.1007/978-1-4615-0717-8_64. [DOI] [PubMed] [Google Scholar]
- Yamagami H, et al. Androgen influence on gene expression in the meibomian gland. Adv Exp Med Biol. 2002b;506(Pt A):477–481. doi: 10.1007/978-1-4615-0717-8_66. [DOI] [PubMed] [Google Scholar]
- Zouboulis CC, Boschnakow A. Chronological ageing and photoageing of the human sebaceous gland. Clin Exp Dermatol. 2001;26(7):600–607. doi: 10.1046/j.1365-2230.2001.00894.x. [DOI] [PubMed] [Google Scholar]