Abstract
Aims
We sought to investigate the hypothesis that smoking is accompanied by systemic inflammation.
Methods and Results
We examined the relation of smoking to 11 systemic inflammatory markers in Framingham Study participants (n=2944, mean age 60 years, 55% women, 12% ethnic minorities) examined from 1998–2001. The cohort was divided into never (n=1149), former (n=1424), and current smokers with last cigarette >6 hours (n=134) or ≤6 hours (n=237) prior to phlebotomy. In multivariable-adjusted models there were significant overall between-smoking group differences (defined as p<0.0045 to account for multiple testing) for every inflammatory marker tested, except for serum CD40 ligand (CD40L), myeloperoxidase (MPO) and tumor necrosis factor receptor-2 (TNFR2). With multivariable-adjustment, pair-wise comparisons with never smokers revealed that former smokers had significantly lower concentrations of plasma CD40L (p<0.0001) and higher concentrations of C-reactive protein (p=0.002).
Conclusions
As opposed to never smokers, those with acute cigarette smoke exposure (≤6 hours) had significantly higher concentrations of all markers (p<0.0001) except serum CD40L, MPO, and TNFR2; plasma CD40L were significantly lower. Compared with never smokers, cigarette smokers have significantly elevated concentrations of most circulating inflammatory markers, consistent with the hypothesis that smoking is associated with a systemic inflammatory state.
Keywords: smoking, inflammation, cardiovascular disease, epidemiology
Introduction
Cigarette smoking confers a higher risk for peripheral arterial disease, myocardial infarction, heart failure,1 and stroke,2 as well as for lung, pancreatic, renal, cervical, and gastric cancers.3 Whereas efforts have been made to reduce cigarette smoking in public areas both in Europe and North America, 17.5%– 45% of adults in the European Union and United States continue to smoke.4 Because those in lower socioeconomic strata tend to smoke more, a study of adult men in Europe and the United States revealed that smoking accounted for almost half of the mortality in persons in the lowest socio-economic stratum.5,6 Additionally, a study of adolescents in ten European countries reported up to 20% of boys and 24.7% of girls smoke daily, implying that smoking-related health complications will persist in the next 20–30 years.7 The World Health Organization estimates that of the 1.1 billion smokers worldwide, 800 million (73%) are in developing nations,8 thereby placing a disproportionate burden on those health-care systems. Globally, cigarette smoking remains a major public health concern and a premier modifiable risk factor for cardiovascular disease.
We and others have noted that systemic markers of inflammation, such as C-reactive protein (CRP),9–14 fibrinogen,10,12,14 interleukin-6 (IL-6)9,11 soluble intercellular adhesion molecule-1 (ICAM-1),15 and monocyte chemoattractant protein-1 (MCP-1)16 are elevated in smokers but there is little data on other novel markers of inflammation in smokers such as, myeloperoxidase (MPO) and tumor necrosis factor receptor 2 (TNFR2). Whereas previous studies have examined inflammatory markers in smokers, they have been limited by small sample sizes,9,17 hospital-based samples,17 and examination of ≤3 markers.10,18 Additionally, most previous studies have not examined the relation of exposure acuity on markers.
We sought to study markers that would reflect critical phases in the inflammatory pathway of atherosclerotic plaque development. Up-regulation of serum and plasma CD40L, urinary isoprostanes, and P-selectin are thought to represent the initiation of the atherothrombotic cascade by stimulating cytokines (such as tumor necrosis factor-α, whose effects are mediated in part by TNFR2) in response to damage of the endothelium or vascular smooth muscle.19,20 P-selectin and ICAM-1 are involved in the rolling phase of leukocyte adhesion and early plaque development.15,19 MCP-1, a chemokine found in atherosclerotic plaque, attracts monocytes to developing plaque.21,22 MPO, a leukocyte enzyme, is elevated in culprit lesions of patients with acute coronary syndrome.20 Urinary isoprostanes indexed to urinary creatinine (isoprostanes) are measures of oxidative stress.23 The remainder of the markers in our panel (fibrinogen, CRP, and IL-6) are non-specific inflammatory markers found in multiple sites. The current investigation expands upon previous reports by examining a broad panel of circulating biomarkers that we hypothesized reflects the multiple inflammatory pathways activated by smoking. Further, we hypothesized that inflammatory markers would be elevated in a dose-response relation to smoking, and that acute exposure would result in higher inflammatory markers than less proximate exposure. We analyzed the relation of various aspects of smoking behavior to circulating CD40L, CRP, fibrinogen, ICAM-1, IL-6, urinary isoprostanes, MCP-1, MPO, P-selectin, and TNFR2 in the community-based Framingham Heart Study.
Methods
Study sample
We examined participants of the Framingham Heart Study Offspring cohort (largely white of European descent) and the Omni study of ethnic/racial minorities (African Americans, Hispanic Americans and Asian Americans), who attended Exams 7 and 2, respectively (1998 –2001). Design and inclusion criteria of the Framingham Heart Study and the Omni Cohort have been described elsewhere.24–26 The Framingham Study is reviewed by the Boston University Medical Center Institutional Review Board and all participants gave written informed consent.
Baseline measurements and definitions
Smoking status information was obtained by self-report. Current smokers were defined as those who smoked ≥1 cigarette per day regularly during the year preceding the exam. Current smokers were subdivided into 2 categories: those with acute smoke exposure, defined as having smoked ≤6 hours prior to phlebotomy, and those having smoked >6 hours prior to phlebotomy at the index examination. Former smokers were defined as those who denied having smoked regularly for the year preceding the exam, but reported regular smoking more than one year prior to the exam. In both the current and former smokers, assessment of cumulative smoke exposure was measured by calculating pack-years, defined as the number of packs per day multiplied by the number of years smoked. Never smokers were defined as those who reported no regular smoking at or prior to any examination. Chewing tobacco, pipe, cigar smoking, and secondhand smoke exposure were not collected at Offspring examination 7 and therefore were not considered in our analysis.
Laboratory analyses
For analysis, serum, plasma and urine samples stored at −80° C were thawed at room temperature. Samples were assayed between 2001 and 2005 (3 years [isoprostanes] to 8 years [TNFR2] after collection). Measurement reproducibility was good, with intra-assay coefficients of variation (CV) as follows: serum and plasma CD40L 4.7±4.4% and 4.4±3.4%, respectively, serum ICAM-1 3.7±2.4%, serum IL-6 3.1±2.1%, serum MCP-1 3.8±3.3%, serum MPO 3.0±2.5%, plasma P-selectin 3.0±2.2%, and plasma TNFR2 2.2±1.6% were measured using the Molecular Devices VersaMax microplate reader and commercially-available quantitative ELISA kits [Bender MedSystems (CD40L), R&D Systems (ICAM-1, MCP-1, P-selectin, TNFR2), OXIS (MPO), and Cayman (isoprostanes)]. Plasma fibrinogen was measured using the Clauss method (clot time), with Diagnostica Stago Reagents; the intra-assay CV was 2.1%. Serum and plasma CD40L, fibrinogen, ICAM-1, IL-6, isoprostanes, MCP-1, MPO, P-selectin, and TNFR2 were run in duplicate and averaged. Serum CRP was measured on a Dade Behring BN100 nephelometer intra-assay CV 3.2%. Urinary isoprostane concentrations, 8-Epi-PGF2α, were indexed to creatinine and had an intra-assay CV 9.1±5.8% ng/mL. Urinary creatinine was measured on an Abbott Spectrum CCX using a technique previously reported.23
Statistical analysis
Since the biomarker concentrations were skewed, we logarithmically transformed the markers. In order to have comparable interpretations, we further standardized all biomarkers to have mean 0 and standard deviation 1. Multivariable analysis of covariance was performed for each inflammatory marker with smoking status as the primary exposure of interest and adjusting for 16 covariates: age, sex, cohort (Offspring vs. Omni), body mass index (BMI), waist circumference, systolic blood pressure, diastolic blood pressure, total/HDL cholesterol ratio, triglycerides, fasting glucose, diabetes mellitus, medications (hypertensive, lipid-lowering, aspirin [>3 days per week], hormone replacement), and prevalent cardiovascular disease. Least-squares means and standard errors were generated for markers according to smoking status (Table 2). A 2-sided p<0.0045 (α=0.05/11) was considered statistically significant for all analyses to account for multiple testing (11= number of markers analyzed). In secondary analyses, we checked for effect modification by age (>60 vs. ≤60 years old), sex, obesity (BMI <30 vs. ≥30kg/m2), and cohort (Offspring vs. Omni). We also looked for smoking dose-response relations with inflammatory markers by 2 additional analyses. First, we examined relations between markers and cigarettes per day in current smokers (grouped as 1–9, 10–19, 20–29, ≥30). Second, in ever smokers, we analyzed packs years (grouped as 0–19, 20–39, ≥40) adjusted for former versus current smoking status, and in current smokers for acute versus >6 hours smoking category. SAS version 8.1 was used for statistical analyses.27
Table 2.
Estimated geometric means (95% CI) of inflammatory markers by smoking category
| Never smoker | Former smoker | >6 hours | Acute(≤6 hours) | p-value | |
|---|---|---|---|---|---|
| CD40L, plasma, ng/mL | 1.83 (1.70, 1.96) | 1.43 (1.34, 1.52)* | 0.96 (0.78, 1.18)* | 1.05 (0.90, 1.23)* | <0.0001 |
| CD40L, serum, ng/mL | 2.28 (2.09, 2.48) | 2.24 (2.07, 2.42) | 2.45 (1.90, 3.14) | 2.67 (2.21, 3.23) | 0.36 |
| CRP, mg/L | 1.99 (1.88, 2.10) | 2.25 (2.14, 2.36)* | 2.60 (2.21, 3.06)* | 3.17 (2.80, 3.58)* | <0.0001 |
| Fibrinogen, mg/dL | 370 (367, 374) | 368 (365, 372) | 374 (363, 384) | 411 (402, 420)* | <0.0001 |
| ICAM-1, ng/mL | 234 (230, 238) | 239 (235, 243) | 266 (253, 280)* | 309 (298, 321)* | <0.0001 |
| IL-6, pg/mL | 2.72 (2.62, 2.82) | 2.79 (2.70, 2.88) | 3.23 (2.89, 3.60)* | 4.21 (3.88, 4.57)* | <0.0001 |
| Isoprostanes, ng/mmol | 120 (116, 124) | 126 (122, 129)† | 177 (161, 194)* | 223 (208, 240)* | <0.0001 |
| MCP-1, pg/mL | 295 (289, 301) | 298 (292, 303) | 317 (299, 336) † | 331 (317, 346)* | <0.0001 |
| MPO, ng/mL | 39.4 (38.1, 40.7) | 39.3 (38.1, 40.4) | 40.3 (36.7, 44.4) | 44.1 (41.1, 47.4)† | 0.03 |
| P-selectin, ng/mL | 35.0 (34.3, 35.7) | 36.0 (35.3, 36.6) | 37.0 (34.9, 39.3) | 42.2 (40.3, 44.1)* | <0.0001 |
| TNFR2, pg/mL | 2022 (1991, 2055) | 1969 (1941, 1997)† | 2040 (1948, 2135) | 2048 (1978, 2120) | 0.03 |
P-value from ANCOVA test of equality among all 4 group means adjusted for covariates listed in statistical methods. P-value from test of specific smoking category versus never smokers:
significant p<0.0045;
borderline 0.0045 < p < 0.05
Results
Participant characteristics and inflammatory markers
We studied the relation of smoking to inflammatory markers in the Framingham Heart Study (n=2944, mean age 60 years, 55% women, 12% ethnic minorities) participants examined from 1998–2001. Clinical characteristics of the cohort stratified by smoking status at examinations 7 and 2 for Offspring and Omni participants, respectively, are shown in Table 1.
Table 1.
Clinical characteristics of study sample by smoking status*
| Never smokers n=1149 | Former smokers n=1424 | Current smokers, >6 hours n= 134 | Acute Exposure ≤6 hours n=237 | |
|---|---|---|---|---|
| Age, years | 60±10 | 62±9 | 57±9 | 57±9 |
| Sex, % female | 60 | 50 | 56 | 54 |
| Omni cohort, % | 20 | 7 | 11 | 6 |
| Systolic blood pressure, mmHg | 128±20 | 128±18 | 124±17 | 123±17 |
| Diastolic blood pressure, mmHg | 76±10 | 74±10 | 74±10 | 74±10 |
| Waist circumference, cm | 39.1±5.7 | 39.7±5.4 | 39.1±6.2 | 38.2±5.9 |
| Body mass index, kg/m2 | 28.1±5.5 | 28.5±5.3 | 27.8±5.5 | 27.2±5.3 |
| Total cholesterol/HDL ratio | 4.0±1.4 | 4.0±1.3 | 4.0±1.3 | 4.2±1.5 |
| Triglycerides, mg/dl | 135±88 | 137±85 | 149±117 | 139±99 |
| Diabetes, % | 12 | 15 | 7 | 11 |
| Fasting glucose, mg/dl | 104±30 | 105±30 | 103±27 | 104±29 |
| Hypertension treatment, % | 31 | 37 | 20 | 24 |
| Lipid-lowering treatment, % | 16 | 24 | 15 | 13 |
| ASA treatment, % | 26 | 35 | 22 | 25 |
| Prevalent CVD, % | 9 | 15 | 11 | 13 |
| Hormone replacement therapy, % | 16 | 17 | 19 | 14 |
| Mean pack-years of cigarettes | - | 23±21 | 32±22 | 48±23 |
| Current mean # of cigarettes/day | - | - | 12±10 | 20±10 |
Characteristics expressed as % (dichotomous) or mean±SD (continuous)
Inflammatory markers and smoking status
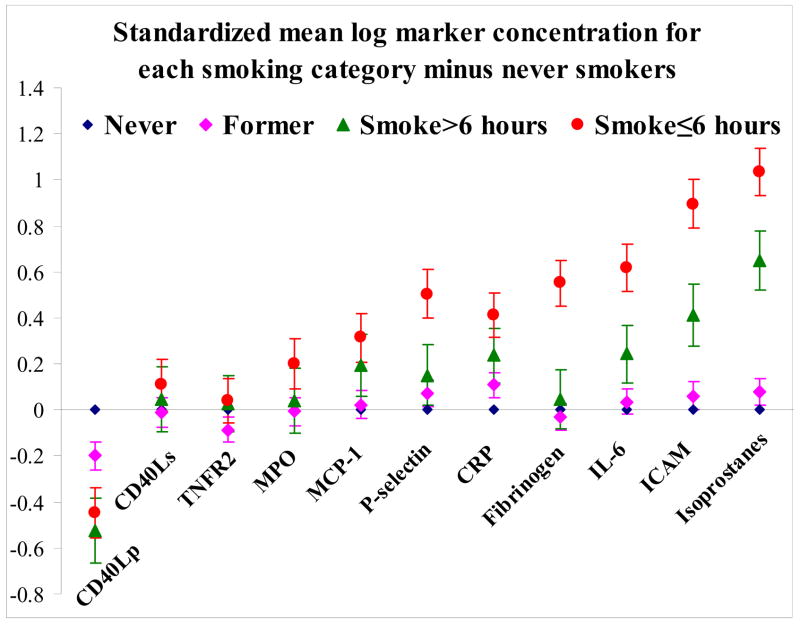
Adjusting for 16 potential clinical confounders, we observed that marker concentrations differed significantly (p<0.0045) across smoking groups (never, former, >6 hours smoking, and acute smoking (≤6 hours from phlebotomy), except for serum CD40L, MPO, and TNFR2 (Figure 1; Table 2). MPO and TNFR2 showed borderline significant differences by smoking category (p<0.05 but >0.0045). Former smokers had significantly lower concentrations of plasma CD40L (p<0.0001), and higher concentrations of CRP (p=0.002), as compared with never smokers. Compared with never smokers, those with exposure >6 hours prior to marker assessment had significantly lower concentrations of plasma CD40L, and higher concentrations CRP, ICAM-1, IL-6, and isoprostanes. Similarly, compared with never smokers, smokers with acute exposure (≤6 hours) had significantly higher concentrations of all markers except serum CD40L, MPO, and TNFR2 compared with never smokers (p<0.0001); plasma CD40L concentrations were lower.
Figure 1.
Estimated mean differences (with standard error bars) for smoking categories versus referent never smokers for standardized log marker concentrations Zero represents no difference between smoking category and never smokers.
Secondary analyses
A subgroup analysis restricted to current smokers was performed to determine if there was a dose-response relation between marker concentrations and number of cigarettes smoked daily. Current smokers were parsed into 4 groups by number of cigarettes smoked per day (mean±SE): <10 (4.4±0. 2) cigarettes per day, 10–19 (12.3±0.3) cigarettes per day, 20–29 (20.5±0.1) cigarettes per day, and 30 or more cigarettes per day (36.0±0.9). There was a significant trend (p=0.001) for increased isoprostanes concentration across increased category of cigarettes smoked per day: the mean±SE isoprostanes concentration (ng/mmol) was 191±12 for <10; 232±11 for 10–19; 252±15 for 20–29 and 269±18 for ≥30 cigarettes per day. The trend was not significant for the remaining markers in the panel.
We also examined ever smokers by pack-years of exposure to determine whether there existed a dose-response relation between marker concentrations and cumulative cigarette smoking exposure, adjusting for smoking status (former vs. current) and acuity of exposure (>6 hours vs. acute exposure). Ever smokers were parsed into the following groups according to pack-years of exposure (mean +/− SE): <20 (8.3±0.2), 20–39 (29.7±0.3), and 40 or more pack-years exposure (60.7±0.8). Compared with smokers who had fewer than 20 pack-years of exposure, there was a significant trend towards elevation of CRP, ICAM-1, IL-6, isoprostanes, P-selectin, and fibrinogen concentrations (Table 3) and lower concentrations of plasma CD40L with increasing pack-years of exposure.
Table 3.
Estimated geometric means (95% CI) of marker concentrations by pack year category in ever smokers
| Pack-years
|
p-value for trend across pack year categories | |||
|---|---|---|---|---|
| <20 | 20–39 | ≥40 | ||
| CD40L plasma, ng/mL | 1.40 (1.22, 1.60) | 1.05 (0.91, 1.22) | 0.88 (0.76, 1.01) | <0.0001 |
| CD40L serum, ng/mL | 2.43 (2.06, 2.86) | 2.66 (2.24, 3.16) | 2.38 (2.00, 2.82) | 0.98 |
| CRP, mg/L | 2.22 (2.00, 2.47) | 2.83 (2.53, 3.16) | 2.76 (2.48, 3.09) | <0.0001 |
| Fibrinogen, mg/dL | 383 (376, 390) | 395 (387, 403) | 394 (387, 402) | 0.003 |
| ICAM-1, ng/mL | 246 (238, 254) | 266 (257, 275) | 263 (254, 271) | <0.0001 |
| IL-6, pg/mL | 3.05 (2.84, 3.27) | 3.51 (3.26, 3.79) | 3.65 (3.39, 3.93) | <0.0001 |
| Isoprostanes, ng/mL | 139 (131, 148) | 162 (152, 172) | 181 (170, 193) | <0.0001 |
| MCP-1, pg/mL | 279 (268, 290) | 293 (281, 305) | 292 (281, 304) | 0.02 |
| MPO, ng/mL | 37.8 (35.5, 40.2) | 36.8 (34.5, 39.4) | 38.5 (36.0, 41.1) | 0.78 |
| P-selectin, ng/mL | 37.7 (36.3, 39.2) | 40.0 (38.4, 41.7) | 40.8 (39.1, 42.4) | 0.0003 |
| TNFR2, pg/mL | 1921 (1864, 1979) | 1978 (1916, 2041) | 1975 (1914, 2038) | 0.07 |
Estimated regression coefficients (standard error) for log marker concentrations; the reference group is <20 pack-years, marker concentrations standardized to mean 0, SD 1.
Effect modification
We tested for interactions by BMI <30 vs. ≥30kg/m2, age ≤60 vs. >60 years, sex (men vs. women), and cohort (Offspring vs. Omni). We observed 2 instances of significant (p<0.0045) effect modification (Table 4). First, there was an interaction between obesity and smoking for ICAM-1 concentrations: although obese participants had higher ICAM-1 concentrations than non-obese in 3 smoking categories, among acute smokers, the group with obesity had lower ICAM-1 concentrations than the non-obese group. Second, there was an interaction between age and smoking for CRP: whereas never smokers had similar CRP concentrations for younger and older participants, among former and current smokers, there were higher CRP concentrations in older as compared with younger participants.
Table 4.
Significant interactions: estimated geometric means (95% CI) of inflammatory markers by smoking category and clinical variable
| Smoking Category
|
Interaction p value* | ||||
|---|---|---|---|---|---|
| Never | Former | >6 hours | Acute | ||
| CRP (mg/L) Age, ≤60 vs. >60 years | |||||
| Age≤60 years | 1.89(1.74,2.05) | 1.98(1.82,2.16) | 2.00(1.63,2.44) | 2.61(2.24,3.05) | 0.002 |
| Age >60 years | 1.96(1.78,2.15) | 2.44(2.23,2.67) | 3.63(2.73,4.84) | 3.93(3.13,4.92) | |
|
| |||||
| ICAM-1 (ng/mL) BMI, kg/m2 | |||||
| BMI<30kg/m2 | 220(215,226) | 225(220,231) | 249(235,265) | 306(292,320) | 0.0003 |
| BMI≥30 | 230(222,239) | 233(225,241) | 264(240,290) | 262(242,282) | |
We display only statistically significant interactions (p<0.0045, to account for multiple testing). Analyses were conducted on log markers.
p-value from ANCOVA test of interaction between smoking categories and specified clinical variable adjusted for covariates listed in methods.
Discussion
Our panel of inflammatory markers was significantly elevated in current and former smokers as compared with never smokers. Examining individual markers across smoking groups, CRP concentrations were significantly elevated and plasma CD40L concentrations were significantly lower in former smokers. Current smokers had elevations of several systemic markers reflecting activation of many inflammatory pathways. Additionally, we observed a trend between lower plasma CD40L, and higher CRP, ICAM-1, IL-6, isoprostanes, P-selectin, and fibrinogen concentrations and cumulative tobacco exposure (pack-years) in ever smokers. Taken together, our results are consistent with the hypothesis that several systemic inflammatory pathways are activated by cigarette smoking, an association that is less notable in past smokers.
Individual Markers
It is unclear why we did not find significant elevations in serum CD40L, TNFR2, or MPO; although for the latter two markers the lack of significance was partly due to accounting for multiple testing. We also found a statistically significant inverse relation between smoking status and plasma CD40L, unlike others.21 Potential explanations for the contrast between our findings with respect to serum and plasma CD40L compared with others may be due to differences in biomarker collection techniques, study sample, covariate adjustment, and smoking status definitions. We are uncertain why plasma CD40L concentrations were higher in never smokers, and smokers with the fewest pack-years exposure, in contrast to the lack of a trend with serum CD40L. Our study is consistent with prior studies demonstrating that soluble CD40L concentrations vary by whether they are measured in serum or in plasma.28
Prior literature on serum TNFR2 concentrations in smokers is conflicting. Soluble TNF receptors compete with membrane-bound receptors for binding tumor necrosis factor, thereby acting as inhibitors of TNF activity on tissue, though in some circumstances they can also bind circulating TNF and prolong its activity in the circulation.29 Mizia-Stec and colleagues demonstrated that in 122 consecutive patients with stable coronary artery disease admitted to the hospital, TNFR2 levels were higher in those who had never smoked and lower in former smokers, which is consistent with our observations.30 However, this is in contrast to Fernandez-Real and colleagues, who found increased concentrations of soluble TNFR2 in obese smokers, and identified both fat mass and smoking as independent contributors to 4% of the variance of soluble TNFR2.31 It is also possible that the prolonged storage (eight years at −80°) for TNFR2 may have played a role. We hypothesize that there is a delicate balance between pro- and anti-inflammatory cytokines and receptors which we were unable to resolve in our sample.
Data on MPO is similarly conflicting. Whereas some investigators have shown that there is increased MPO activity in polymorphonuclear leukocytes in smokers (measured by flow cytometry), others have demonstrated no increased peroxidase activity (quantified by the MPXI, a parameter produced by automated blood counters) in neutrophils of smokers as compared with non-smokers.32,33 We conclude that current evidence is controversial, and our finding that there was no significant elevation in serum in smokers may reflect the distinction between circulating versus tissue concentrations or may reflect differences in MPO assays.
Effect modification
We noted effect modification for CRP by age ≥60 years, and for ICAM-1 by BMI <30kg/m2. Whereas we adjusted for prevalent clinically diagnosed CVD in our cohort, in our cross-sectional study we were unable to determine the degree to which variation in atherosclerotic burden might have contributed to elevated CRP concentrations in older participants.
Pathophysiology
Animal models have demonstrated that cigarette smoke increases alveolar macrophages and neutrophils in the lung tissue and increases epithelial permeability.34,35 A recent review examining the effect of acute cigarette exposure on inflammation and oxidative stress in humans concluded that acute smoke exposure was associated with increased neutrophil and macrophage chemotaxis and activation.36 Interestingly, it demonstrated that there is a suppressive effect of acute smoke exposure on eosinophils and several inflammatory cytokines, including IL-6, TNF-α, interleukin-2, and interferon-γ. It is hypothesized that a balance of pro-inflammatory and anti-inflammatory factors (such as carbon monoxide) exists in cigarette smoke that functions in concert to disturb pulmonary epithelial architecture.36 Animal studies examining the effects of smoke exposure on blood inflammatory markers have demonstrated a significant increase in MPO,37 but not in leukotriene B4 concentrations.38 Churg et al. exposed mice with knocked-out p55/p75 TNF-α receptors to cigarette smoke and compared them with control mice. Whereas the controls demonstrated increases in gene expression of TNF-α, neutrophil chemoattractant, macrophage inflammatory protein-2, and macrophage chemoattractant protein-1, the knockout mice did not demonstrate any increases in gene expression for those mediators. The authors concluded that TNF-α is central to acute smoke-induced inflammation and resulting connective tissue breakdown.35
In humans, autopsy studies have shown that there is a dose-dependent relation between smoking and degree of aortic and coronary atherosclerosis.39 However, the precise mechanisms by which cigarette smoking promotes atherosclerosis are not known. A recent review of pathobiological mechanisms of smoking and cardiovascular disease appropriately concluded that whereas epidemiological studies have clearly established an important relation between smoking and cardiovascular morbidity and mortality, the precise balance of thrombosis, endothelial dysfunction, and atherogenesis remains to be elucidated.40
Strengths and limitations
We recognize that our study has limitations that warrant consideration. First, the cross-sectional design does not allow us to infer causation between cigarette smoking and inflammatory markers; as an observational study we could neither randomize smoking exposure, nor observe pre- versus post smoking cessation. It is possible that elevations may have been caused by other chronic inflammatory states, particularly in smokers with possible co-existing chronic inflammatory conditions. Second, smoke exposure via other tobacco products (cigars, pipes, smokeless chewing tobacco) or second-hand smoke exposure, were not accounted for and may have led to misclassification of overall tobacco exposure. A recent cross-sectional evaluation of 7599 never-smoking adults from the Third National Health and Nutrition Examination Survey found that those with detectable, low-level (range, 0.05 to 0.215 ng/mL) cotinine were more likely to have elevated fibrinogen and homocysteine, but not WBC or CRP, as compared with subjects without any detectable cotinine.41 This very interesting finding warrants further investigation with a broader panel of markers to determine what degree of second-hand smoke exposure results in clinically significant elevations of inflammatory markers. Third, the mean cigarette pack-years and mean cigarette number per day is positively related to smoking status (Table 1) such that there was greater exposure in acute smokers vs. >6 hour smokers vs. former smokers. We estimated the mean marker concentrations by pack-year adjusting for smoking status; however, we acknowledge that with an observational study design we cannot completely eliminate confounding of exposure acuity by magnitude of smoking exposure. Fourth, we did not have serum cotinine concentrations available to corroborate self-reported smoking behaviors; we acknowledge that some misclassification of smoking exposure is inevitable. Fifth, there is limited on no data on the stability of several of our markers (ICAM-1, IL-6, MCP-1, MPO, P-selectin, fibrinogen) with long-term storage. It is possible that concentrations may be altered with storage. Sixth, the Framingham cohort is a predominantly white and middle-aged to elderly sample. We attempted to broaden the applicability of our study to other ethnicities and races by including subjects enrolled in the minority Omni study, but we lacked power to examine the relation between smoking exposure and specific ethnic/racial minority subgroup status. Finally, although the trend test between pack-years and marker concentrations was significant for CRP, fibrinogen and ICAM-1, we lacked power to examine threshold effects.
Conclusion
Our data support previous findings that certain biomarkers are increased with cigarette smoke exposure, specifically, CRP, fibrinogen,42 ICAM-1,11 IL-6,9 urinary isoprostanes,43 and P-selectin.17 The pattern of marker elevation did not localize to a particular inflammatory pathway thereby confirming the complexity of biochemical events that occur in smokers. The influence of acute cigarette smoke on inflammation may not be completely reversible, as evidenced by the elevated CRP concentrations in the former smokers.
In an era when almost 25% of adolescents and up to 45% of adults in both the European Union and North America continue to smoke, it is essential to identify potential mechanistic pathways that can ultimately be intervened upon to reduce risk of cardiovascular complications in smokers.4,7,44
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute’s Framingham Heart Study N01-HC 25195; RO1 HL076784; RO1 HL064753, R01 AG028321; and the Flight Attendants Medical Research Institute’s Young Clinical Scientist Award (Dr. Walter).
Abbreviations
- BMI
body mass index
- CD40L
CD40 ligand
- CRP
C-reactive protein
- ICAM-1
intercellular adhesion molecule-1
- isoprostanes
urinary 8-epi-prostaglandin F2∞ indexed to urinary creatinine
- IL-6
interleukin-6
- MCP-1
monocyte chemoattractant protein-1
- MPO
myeloperoxidase
- TNFR2
tumor necrosis factor receptor 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ockene IS, Miller NH. Cigarette smoking, cardiovascular disease, and stroke: a statement for healthcare professionals from the American Heart Association. American Heart Association Task Force on Risk Reduction. Circulation. 1997;96:3243–3247. doi: 10.1161/01.cir.96.9.3243. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. The Health Consequences of Smoking: A Report of the Surgeon General. National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Rockville, Md: 2004. [Google Scholar]
- 4.Action on Smoking and Health: Tobacco Policy in the European Union Factsheet No. 20. [accessed 11/23/2007];2006 http://oldash.org.uk/html/factsheets/html/fact20.html.
- 5.Jha P, Peto R, Zatonski W, Boreham J, Jarvis MJ, Lopez AD. Social inequalities in male mortality, and in male mortality from smoking: indirect estimation from national death rates in England and Wales, Poland, and North America. Lancet. 2006;368:367–370. doi: 10.1016/S0140-6736(06)68975-7. [DOI] [PubMed] [Google Scholar]
- 6.Marmot M. Smoking and inequalities. Lancet. 2006;368:341–342. doi: 10.1016/S0140-6736(06)68976-9. [DOI] [PubMed] [Google Scholar]
- 7.Hublet A, De Bacquer D, Valimaa R, Godeau E, Schmid H, Rahav G, Maes L. Smoking trends among adolescents from 1990 to 2002 in ten European countries and Canada. BMC Public Health. 2006;6:280. doi: 10.1186/1471-2458-6-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collishaw NE, Lopez AD. Prevalence of cigarette smoking in developing countries. Tobacco Control. 1995;4:327. [Google Scholar]
- 9.Antoniades C, Tousoulis D, Vasiliadou C, Marinou K, Tentolouris C, Ntarladimas I, Stefanadis C. Combined effects of smoking and hypercholesterolemia on inflammatory process, thrombosis/fibrinolysis system, and forearm hyperemic response. Am J Cardiol. 2004;94:1181–1184. doi: 10.1016/j.amjcard.2004.07.090. [DOI] [PubMed] [Google Scholar]
- 10.Bazzano LA, He J, Muntner P, Vupputuri S, Whelton PK. Relationship between cigarette smoking and novel risk factors for cardiovascular disease in the United States. Ann Intern Med. 2003;138:891–897. doi: 10.7326/0003-4819-138-11-200306030-00010. [DOI] [PubMed] [Google Scholar]
- 11.Bermudez EA, Rifai N, Buring JE, Manson JE, Ridker PM. Relation between markers of systemic vascular inflammation and smoking in women. Am J Cardiol. 2002;89:1117–1119. doi: 10.1016/s0002-9149(02)02284-1. [DOI] [PubMed] [Google Scholar]
- 12.Gan WQ, Man SF, Sin DD. The interactions between cigarette smoking and reduced lung function on systemic inflammation. Chest. 2005;127:558–564. doi: 10.1378/chest.127.2.558. [DOI] [PubMed] [Google Scholar]
- 13.Kathiresan S, Larson MG, Vasan RS, Guo CY, Gona P, Keaney JF, Jr, Wilson PW, Newton-Cheh C, Musone SL, Camargo AL, Drake JA, Levy D, O’Donnell CJ, Hirschhorn JN, Benjamin EJ. Contribution of clinical correlates and 13 C-reactive protein gene polymorphisms to interindividual variability in serum C-reactive protein level. Circulation. 2006;113:1415–1423. doi: 10.1161/CIRCULATIONAHA.105.591271. [DOI] [PubMed] [Google Scholar]
- 14.Wannamethee SG, Lowe GD, Shaper AG, Rumley A, Lennon L, Whincup PH. Associations between cigarette smoking, pipe/cigar smoking, and smoking cessation, and haemostatic and inflammatory markers for cardiovascular disease. Eur Heart J. 2005;26:1765–1773. doi: 10.1093/eurheartj/ehi183. [DOI] [PubMed] [Google Scholar]
- 15.Keaney JF, Jr, Massaro JM, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Sutherland P, Vita JA, Benjamin EJ. Heritability and correlates of intercellular adhesion molecule-1 in the Framingham Offspring Study. J Am Coll Cardiol. 2004;44:168–173. doi: 10.1016/j.jacc.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 16.McDermott DH, Yang Q, Kathiresan S, Cupples LA, Massaro JM, Keaney JF, Jr, Larson MG, Vasan RS, Hirschhorn JN, O’Donnell CJ, Murphy PM, Benjamin EJ. CCL2 polymorphisms are associated with serum monocyte chemoattractant protein-1 levels and myocardial infarction in the Framingham Heart Study. Circulation. 2005;112:1113–1120. doi: 10.1161/CIRCULATIONAHA.105.543579. [DOI] [PubMed] [Google Scholar]
- 17.Mizia-Stec K, Zahorska-Markiewicz B, Gasior Z. Cigarette smoking and inflammatory indices in coronary artery disease. Int J Cardiol. 2004;93:169–174. doi: 10.1016/S0167-5273(03)00198-0. [DOI] [PubMed] [Google Scholar]
- 18.Tracy RP, Psaty BM, Macy E, Bovill EG, Cushman M, Cornell ES, Kuller LH. Lifetime smoking exposure affects the association of C-reactive protein with cardiovascular disease risk factors and subclinical disease in healthy elderly subjects. Arterioscler Thromb Vasc Biol. 1997;17:2167–2176. doi: 10.1161/01.atv.17.10.2167. [DOI] [PubMed] [Google Scholar]
- 19.Dogne JM, Hanson J, Pratico D. Thromboxane, prostacyclin and isoprostanes: therapeutic targets in atherogenesis. Trends Pharmacol Sci. 2005;26:639–644. doi: 10.1016/j.tips.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Moreno PR, Fuster V. The year in atherothrombosis. J Am Coll Cardiol. 2004;44:2099–2110. doi: 10.1016/j.jacc.2004.06.077. [DOI] [PubMed] [Google Scholar]
- 21.de Lemos JA, Zirlik A, Schonbeck U, Varo N, Murphy SA, Khera A, McGuire DK, Stanek G, Lo HS, Nuzzo R, Morrow DA, Peshock R, Libby P. Associations between soluble CD40 ligand, atherosclerosis risk factors, and subclinical atherosclerosis: results from the Dallas Heart Study. Arterioscler Thromb Vasc Biol. 2005;25:2192–2196. doi: 10.1161/01.ATV.0000182904.08513.60. [DOI] [PubMed] [Google Scholar]
- 22.Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Jr, Luster AD, Luscinskas FW, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 23.Keaney JF, Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 24.Dawber T, Kannel W, Lyell LP. An approach to longitudinal studies in a community: the Framingham Study. Ann N Y Acad Sci. 1963;107:539–556. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 25.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–525. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 26.Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O’Connor GT, Rapoport DM, Redline S, Robbins J, Samet JM, Wahl PW. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–1085. [PubMed] [Google Scholar]
- 27.SAS Institute Inc. SAS/STAT User’s Guide, Version 8. Cary, N.C.: SAS Institute, Inc.; 1999. [Google Scholar]
- 28.Ahn ER, Lander G, Jy W, Bidot CJ, Jimenez JJ, Horstman LL, Ahn YS. Differences of soluble CD40L in sera and plasma: implications on CD40L assay as a marker of thrombotic risk. Thromb Res. 2004;114:143–148. doi: 10.1016/j.thromres.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 30.Mizia-Stec K, Zahorska-Markiewicz B, Gasior Z. Cigarette smoking and inflammatory indices in coronary artery disease. Int J Cardiol. 2004;93:169–174. doi: 10.1016/S0167-5273(03)00198-0. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez-Real JM, Broch M, Vendrell J, Ricart W. Smoking, fat mass and activation of the tumor necrosis factor-alpha pathway. Int J Obes Relat Metab Disord. 2003;27:1552–1556. doi: 10.1038/sj.ijo.0802472. [DOI] [PubMed] [Google Scholar]
- 32.Bain BJ, Gresham N, Addison G. High neutrophil myeloperoxidase content in smokers. Blood. 1992;80:845–846. [PubMed] [Google Scholar]
- 33.van Eeden SF, Hogg JC. The response of human bone marrow to chronic cigarette smoking. Eur Respir J. 2000;15:915–921. doi: 10.1034/j.1399-3003.2000.15e18.x. [DOI] [PubMed] [Google Scholar]
- 34.Burns AR, Hosford SP, Dunn LA, Walker DC, Hogg JC. Respiratory epithelial permeability after cigarette smoke exposure in guinea pigs. J Appl Physiol. 1989;66:2109–2116. doi: 10.1152/jappl.1989.66.5.2109. [DOI] [PubMed] [Google Scholar]
- 35.Churg A, Dai J, Tai H, Xie C, Wright JL. Tumor necrosis factor-alpha is central to acute cigarette smoke-induced inflammation and connective tissue breakdown. Am J Respir Crit Care Med. 2002;166:849–854. doi: 10.1164/rccm.200202-097OC. [DOI] [PubMed] [Google Scholar]
- 36.van der Vaart H, Postma DS, Timens W, ten Hacken NH. Acute effects of cigarette smoke on inflammation and oxidative stress: a review. Thorax. 2004;59:713–721. doi: 10.1136/thx.2003.012468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bosken CH, Doerschuk CM, English D, Hogg JC. Neutrophil kinetics during active cigarette smoking in rabbits. J Appl Physiol. 1991;71:630–637. doi: 10.1152/jappl.1991.71.2.630. [DOI] [PubMed] [Google Scholar]
- 38.Witten ML, Quan SF, Sobonya RE, Bruck D, Devine L, Lemen RJ. Acute cigarette smoke exposure alters lung eicosanoid and inflammatory cell concentrations in rabbits. Exp Lung Res. 1988;14:727–742. doi: 10.3109/01902148809087840. [DOI] [PubMed] [Google Scholar]
- 39.Strong JP, Richards ML. Cigarette smoking and atherosclerosis in autopsied men. Atherosclerosis. 1976;23:451–476. doi: 10.1016/0021-9150(76)90007-1. [DOI] [PubMed] [Google Scholar]
- 40.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43:1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 41.Venn A, Britton J. Exposure to secondhand smoke and biomarkers of cardiovascular disease risk in never-smoking adults. Circulation. 2007;115:990–995. doi: 10.1161/CIRCULATIONAHA.106.648469. [DOI] [PubMed] [Google Scholar]
- 42.Yasue H, Hirai N, Mizuno Y, Harada E, Itoh T, Yoshimura M, Kugiyama K, Ogawa H. Low-grade inflammation, thrombogenicity, and atherogenic lipid profile in cigarette smokers. Circ J. 2006;70:8–13. doi: 10.1253/circj.70.8. [DOI] [PubMed] [Google Scholar]
- 43.Helmersson J, Larsson A, Vessby B, Basu S. Active smoking and a history of smoking are associated with enhanced prostaglandin F(2alpha), interleukin-6 and F2-isoprostane formation in elderly men. Atherosclerosis. 2005;181:201–207. doi: 10.1016/j.atherosclerosis.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 44.Trosclair A, Caraballo R, Malarcher A, Husten C, Pechacek T. Cigarette Smoking Among Adults --- United States, 2003. Morbidity and Mortality Weekly Report. 2005;54(MM20):509–513. [PubMed] [Google Scholar]