Abstract
In mice and in rats, reduced levels of the growth hormone secretagogue receptor (GHS-R1a) results in reduced body weight and lower levels of serum insulin-like growth factor I (IGF-I). However, the mechanism leading to these impairments has not been elucidated. Studies in primary cultures of pituitary cells from very young mice have shown that GHS-R1a agonists, including ghrelin, increase expression of the pituitary-specific transcription factor (Pit-1) that is critical for differentiation of pituitary cells into somatotrophs, lactotrophs and thyrotrophs. Hence, we hypothesized that ablation of Ghsr would reduce Pit-1 expression and as a consequence reduce growth hormone (GH) production explaining the lower body weight of Ghsr-/- mice. Here, we now show that Pit-1 mRNA levels are significantly lower in the pituitary gland of Ghsr-/- mice compared to wild type littermates and also with advancing age. This Pit-1 loss is associated with reduced GH mRNA and fewer GH producing cells. To determine whether reduced GH is caused by reduced expression of Pit-1 in Ghsr-/- mice, we also measured prolactin (PRL) expression in the pituitary gland and in the circulation. PRL mRNA was significantly reduced in Ghsr-/- mice compared to wild-type littermates and fewer cells expressed PRL. The reduction in expression of both GH and PRL is consistent with a Pit-1 regulated pathway and demonstrates that the GHS-R has an important role in the pituitary gland as a modulator of Pit-1 expression and provides a possible mechanism to explain the lower plasma IGF-1 and modestly reduced body weight exhibited by Ghsr-/- mice. We also believe that lower systemic and lymphoid hormone expression may also account, in part, for the enhanced thymic involution and reduced thymic output in Ghsr-/- mice.
Keywords: Ghrelin, GHS-R, Aging, Pituitary, GH, IGF-1, Prolactin, Pit-1, thymus
Introduction
The GHS-R was first characterized and expression cloned using a small molecule, MK-0677 that was designed to restore the amplitude of episodic GH release in elderly subjects to that of young adults (Howard et al., 1996; Patchett et al., 1995; Pong et al., 1996; Smith et al., 1997). GHS-R produces two transcripts that encode GHS-R1a and GHS-R1b (Howard et al., 1996); the former is a seven transmembrane domain (TM) G-protein coupled receptor, whereas the latter is a truncated form that lacks TM-VI and TM-VII and is localized in the nucleus rather than on the plasma membrane (Smith et al., 2005). Besides being expressed in cells that regulate GH pulse amplitude, the GHS-R is expressed in regions of the brain involved in memory and cognitive function (Guan et al., 1997), in the pancreas (Howard et al., 1996)and in immune cell subsets (Dixit et al., 2004; Dixit et al., 2007).
By fractionating tissue extracts and assaying for GHS-R1a activation in cells, Kojima and associates identified a 28-aminoacid octanoylated peptide agonist produced by enteroendocrine cell in the stomach and named this endogenous GHS-R1a agonist ghrelin. Amongst peptide agonists ghrelin is unique because octanoylation on Ser3 is essential for binding and activation of GHS-R1a (Dixit et al., 2007). Sun and colleagues (Sun et al., 2004) produced Ghsr-/- mice and showed they were refractory to the GH-releasing and orexigenic properties of ghrelin, which confirmed unambiguously that GHS-R1a was a biologically relevant receptor for ghrelin.
In addition to GH release, GHS-R1a agonists stimulate PRL release from primary cultures of female rat pituitary cells (Cheng et al., 1993). Reverse hemolytic plaque assays indicated that PRL is released from cells containing both GH and PRL (somatomammotrophs) (Smith et al., 1997). Acute oral administration of GHS-R1a agonists to obese humans also causes PRL release (Svensson et al., 1998). Subsequently, this observation was confirmed when the endogenous agonist ghrelin was injected into obese subjects (Tassone et al., 2003).
In the developing anterior pituitary gland of mice (e 12.5 - e 13.5), Pit-1 activation is required for differentiation and proliferation of somatotrophs, lactotrophs and thyrotrophs (Ingraham et al., 1988; Sornson et al., 1996). Furthermore, Pit-1 is a positive regulator of GH and PRL production. Ghrelin, as well as the synthetic GHS-R1a agonist GHRP-6, elicits time- and dose-dependent activation of Pit-1 expression in monolayer cultures of infant rat anterior pituitary cells, which is consistent with a physiological role for ghrelin signaling on somatotroph cell differentiation and function (Garcia et al., 2001).
In support for a role for ghrelin on somatotroph differentiation, serum insulin-like growth factor 1 (IGF-1) levels and body weights of GHS-R null mice are modestly reduced compared to wild-type littermates (Sun et al., 2004); however, the precise mechanism for these differences has not been established. Here, we have hypothesized that Ghsr ablation in mice may alter the pituitary expression of Pit-1, GH and PRL, especially with advancing age, thus influencing circulating hormone levels and body weight. Our findings may establish an important regulatory role for GHS-R and ghrelin as important modulators of optimal development and maintenance of the anterior pituitary gland over a lifespan.
Materials and Methods
GHS-R null mouse
GHS-R null mice were generated as previously described (10). GHS-R null and wild type male and female littermates were aged for 4 and 24 months at the Baylor College of Medicine and subsequent experiments were performed on these mice after providing at least 3-4 days of rest upon arrival at NIA animal facility. Female mice were utilized predominantly for this study (typically 4-5 mice per study), although similar results were used in male mice (data not shown). After sacrificing mice, the brain was removed and the pituitary was separated and weighted. These tissues were frozen and kept in -70°C for RT-PCR analysis. Some were fixed in 4% paraformaldehyde PBS for immunohistochemistry.
RT-PCR of GHS-R mRNA to confirm GHS-R null mice
Total RNA was isolated from the pituitary gland by using the Trizol reagent (Invitrogen, Basel, Switzerland) according to manufacturer’s instruction. The final pellet was air dried and dissolved into DEPC solution (Sigma Chemical Company, St. Louis, MO). The RNA concentration was determined using a spectrophotometer. cDNA was produced using 1 μg total RNA using the cDNA Synthesis Kit (Bio-Rad, Hercules, CA). The PCR for GH, PRL and Pit-1 was performed in a total volume of 25 μl containing 0.25 mM dNTPs, 1.5 mM MgCl2, 1U Taq DNA polymerase, 10 pmol of each sense and antisense primer, and distilled water. The following cycling condition was used for 35 cycles in a thermocycler (Bio-Rad): initial denaturation, 5 min at 94°C; denaturation 30 sec at 94°C; annealing 30 sec at 60°C; and elongation 30 sec at 72°C. The program was followed by a final elongation step for 5 min at 72°C. The primers utilized for the GHS-R RT-PCR are shown in Table I.
Table I.
Conventional and Real-time RT-PCR primer sequences utilized for the analysis of GHS-R, GH, PRL and Pit-1 gene expression in the pituitary glands of mice
| Primer | Forward | Reverse |
|---|---|---|
| GHS-R (conventional) | 5′-CTCCACCTTTCCTCAGCGTA-3′ | 5′-TGAAGAGTGGCATGTGGGTA-3′ |
| GHS-R (real time) | 5′-CTATCCAGCATGGCCTTCTC-3′ | 5′-GGAAGCAGATGGCGAAGTAG-3′ |
| GH | 5′-TAATGCTGTGCTCCGAGCC-3′ | 5′-GAATGGAATAGCGCTGTCCC-3′ |
| PRL | 5′-GGGTCAGCCCAGAAAGCAG-3′ | 5′-CAGTCACCAGCGGAACAGATT-3′ |
| Pit-1 | 5′-GTGCCTTCCTGTCATTATGGA-3′ | 5′-CAGGGTGTGGTCTGGAAACT-3′ |
| 18S | 5′-GTAACCCGTTGAACCCCATT-3′ | 5′-CCATCCAATCGGTAGTAGCG-3′ |
RNA isolation and quantitation of GH, PRL, and Pit-1 mRNAs
Total RNA was isolated from the pituitary gland using the Trizol reagent (Invitrogen) according to the manufacturer’s instructions. Total RNA (1 μg) was reverse transcribed into cDNA using the Superscript First-Strand Synthesis System (Invitrogen). GH, PRL, and Pit-1 mRNA were measured using real-time quantitative PCR using an ABL Prizm 7700 (Applied Biosystems, Foster City, CA). For real-time PCR, specific amplification primers and oligonucleotide hybridization probes were designed by Idaho Biochem Inc. The sequences of the amplification for GH, PRL, and Pit-1 mRNAs are shown in Table 1. Real time RT-PCR analysis was performed using standard protocols. A post amplification melting curve analysis was conducted to ensure specificity of the amplified product. For rRNA 18S evaluation, the Taqman ribosomal RNA control reagents were used according to the manufacturer’s instructions (Applied Biosystems).
Immunohistochemistry for GH and PRL
Sections of pituitary glands were washed with PBS and incubated in PBS containing 2% bovine serum albumin at 37°C for 30 min. Sections were reacted with 1:100 diluted goat anti-mouse GH and PRL antibody (GH [sc-10364] and PRL [sc-7805], Santa Cruz Biotechnology, Santa Cruz, CA) at 37°C for 1 h. After washing with PBS, samples were incubated with 1:200 diluted donkey anti-goat IgG (H+L) rhodamine-conjugated antibody (Jackson ImmunoResearch, Westbaltimore, CA, USA) at room temperature for 45 min. After washing, the sections were mounted using mounting media with DAPI for fluorescence (Dako Cytomation, Glostrup, Denmark). Subcellular localization was determined using a Zeiss fluorescence microscope.
PRL and IGF1 determinations
Murine PRL and IGF1 were estimated in the sera of wild type control and Ghsr-/- mice (n≥6 mice at the ages 4-6 months) using commercial R& D Systems (Minneapolis, MN) ELISA kits (PRL, DuoSet ELISA Development Kit; IGF1, Quantikine Immunoassay Kit) according to manufacturer’s instructions.
Statistical Analysis
All results are presented as means +/- SD of 4-5 mice. Significant differences between treatment groups were determined by the Student Newman-Keuls. A two factor ANOVA was used to analyze the effect of age and genotype or any interaction between age and genotype for each hormone mRNA assessment. If no significant interaction between age and genotype was present in the two-way ANOVA, a Tukey-Kramer post-hoc analysis was performed to determine statistically significant differences between each factor level of age and genotype. A P <0.05 was considered statistically significant for all analysis. All analysis was performed using SigmaStat version 3.11 (Systat software, San Jose, CA).
Results
GHS-R mRNA expression in the pituitary of GHS-R null mice
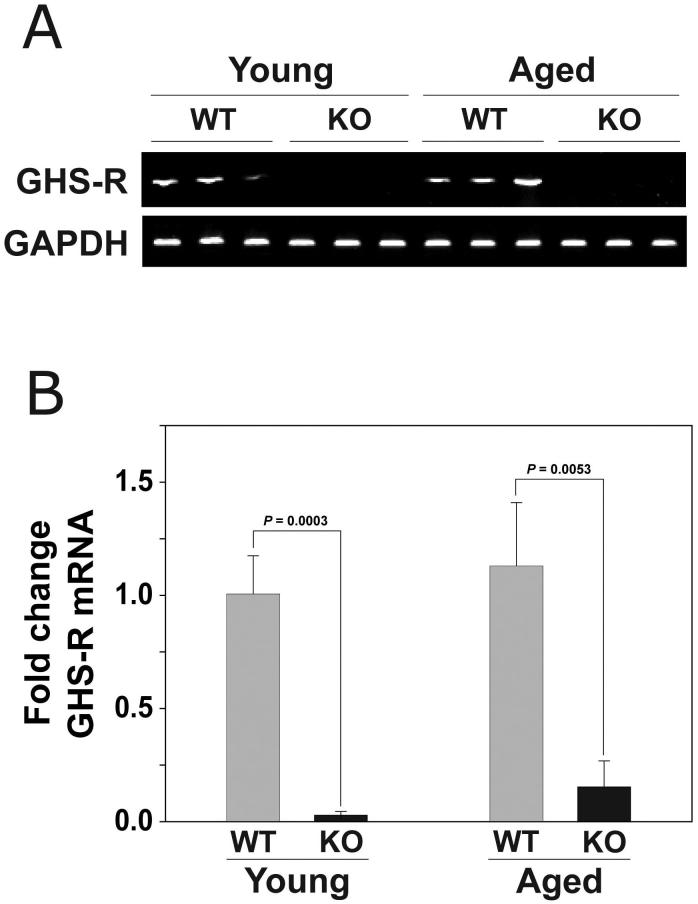
To determine the specificity of our quantitative PCR methodology we isolated RNA from the pituitary glands of Ghsr-/- mice and wild type littermates and performed RT-PCR using the GHSR primers described in Table I. These primers span the deleted sequence in the Ghsr-/- mice. RNA isolated from wild-type mice produced a PCR product of 400 bp, which did not appear when RNA from Ghsr-/- mice was used as template (Figure 1A). Similar to RT-PCR results, in real-time PCR analysis GHS-R mRNA levels were quite high in the wild-type mice in comparison to the non-specific amplification noted in the Ghsr-/- mice (Figure 1B).
Figure 1. The expression of GHS-R mRNA in the pituitary of wild-type and Ghrs-/- mice.
(A) Conventional and (B) Real-time RT-PCR of GHS-R mRNA expression in the pituitary glands isolated from young (4m) and old (24m) female wild-type and Ghsr-/- mice using the primers described in Table I. For Panel B, the data are expressed as fold mRNA change (+/- SD) compared to the young wild-type control mice. P values indicate significant differences. (n = 4-5 mice per group)
Pit-1 mRNA expression is lower in the pituitary gland of GHS-R null mice and is reduced according to age
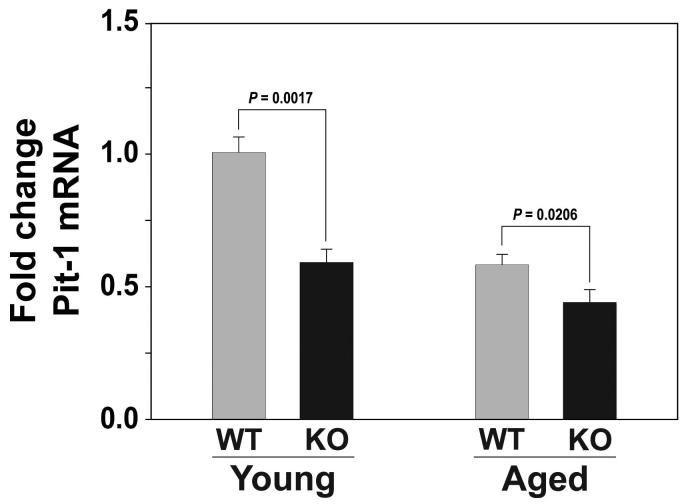
To determine whether Ghsr ablation would affect expression of Pit-1, RNA was isolated from the pituitary glands of young and old wild-type and Ghsr-/- mice. Pit-1 mRNA levels were measured by quantitative PCR. Pit-1 expression was found to be markedly lower in both young (P= 0.0017) and old (P=0.0206) Ghsr-/- mice compared to their wild-type littermates (Figure 2). Expression of Pit-1 in the pituitary gland was even lower in old wild-type (P=0.0008) and mutant (P=0.0082) genotypes compared to their young counterparts (Figure 2).
Figure 2. Pit-1 mRNA expression in the pituitary gland is reduced with age and in Ghsr-/-mice.
Real-time RT-PCR of Pit-1 mRNA expression in the pituitary glands isolated from young (4m) and old (24m) female wild-type and Ghsr-/- mice using the primers described in Table I. The data are expressed as fold change of GHS-R mRNA (+/- SD) compared to the young wild-type control mice. P values indicate significant differences. (n = 4-5 mice per group) There are significant age-associated changes in Pit-1 mRNA expression in both wild-type (P=0.0008) and GHSR-/- (P=0.0082) mice. In addition, there are significant differences in Pit-1 mRNA expression between the wild-type and Ghsr-/- mice at the younger (P= 0.0017) and older (P=0.0206) ages.
Expression of GH and PRL are reduced in pituitary glands from Ghsr-/- mice
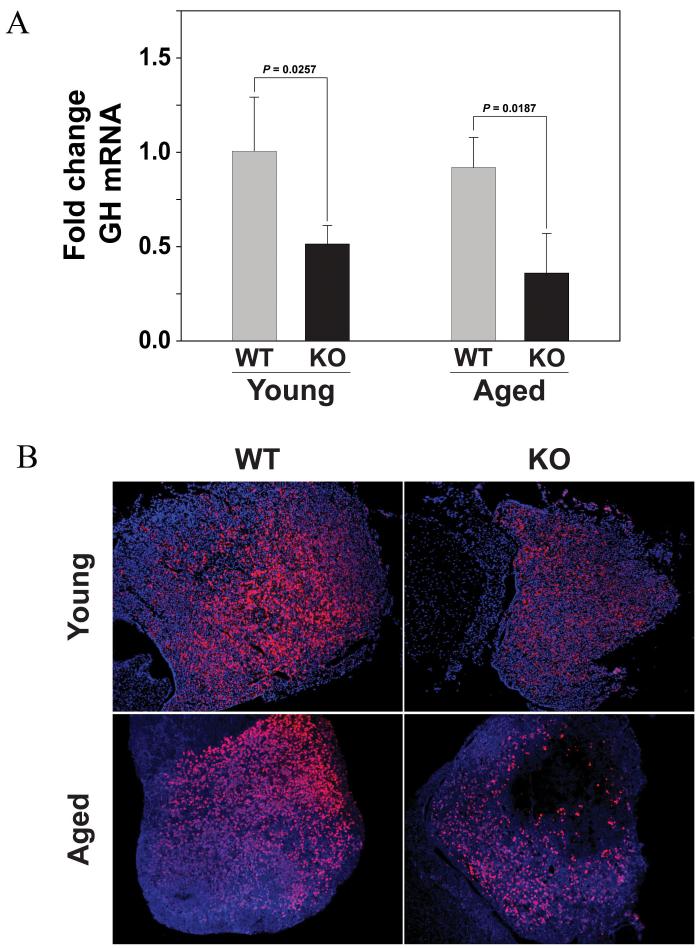
In addition to regulating differentiation of precursor cells into somatotrophs and lactotrophs, Pit-1 regulates GH and PRL gene expression. Hence, reduced Pit-1 expression might be associated with reduced production of GH and PRL. To determine whether pituitary GH and PRL mRNA levels were affected by Ghsr ablation, expression levels were measured in RNA isolated from pituitary glands of young (P=0.0257) and old (P=0.0187) Ghsr-/- mice and their wild-type littermates using quantitative RT-PCR. Figure 3A shows that GH mRNA is significantly lower in pituitary glands from young and old Ghsr-/- mice compared to wild type controls littermates. However, in comparing GH mRNA expression between young and old Ghsr-/- mice, there are no significant differences between these mice with age (P=0.121). Similarly, there is no significant difference in pituitary GH mRNA expression with age in the wild-type mice (P=0.251). To determine whether genotypic differences in GH mRNA correlated with altered GH protein levels, we performed immunohistochemistry on mouse pituitary gland sections (Figure 3B). The intensity of GH immunostaining is clearly weaker in sections from Ghsr-/- than in wild-type controls and, unlike the mRNA expression, there appears to be a difference in protein expression with age. However, with the lack of protein quantification in the pituitary glands of these mice and the lack of GH-producing somatotrophs and lactotrophs counts in these young and aged mice, our conclusions with regard to protein changes in the pituitary need to be viewed as qualitative assessments. Moreover, this reduction in pituitary GH levels is reflected in the significantly (P=0.04) reduced levels of serum IGF-1 levels in the Ghsr-/- mice (138.5 +/- 61 ng/ml) compared to the wild-type counterparts (399.7 +/- 90 ng/ml).
Figure 3. GH mRNA and protein expression in the pituitary gland of Ghsr-/- is reduced compared to wild-type control mice.
(A) GH mRNA levels are assessed using real-time RT-PCR using RNA isolated from the in pituitary glands of young (2m) and old (24m) female Ghsr-/- and Ghsr+/+ wild-type control mice. The data are expressed as fold change GH mRNA (+/- SD) compared to the young wild-type control mice. P values indicate significant differences. (n = 4-5 mice per group). In comparing GH mRNA expression between young and old Ghsr-/- mice, there is no significant difference between these mice with age (P=0.121). Similarly, there is no significant difference with age in the wild-type mice (P=0.251). However, there are significant differences in GH mRNA expression between the wild-type and GHSR-/- mice at the younger (P=0.0257) and older (P=0.0187) ages. [2-way ANOVA, Age: p = 0.207; Genotype p < 0.001; Tukey Kramer method: Genotype p < 0.001 Control vs Ghsr-/-] (B) Immunohistochemical analysis of GH protein expression in sections of pituitary glands of young (2m) and aged (24m) female Ghsr-/- compared to wild-type Ghsr+/+ mice. (n = 3 mice per group were examined)
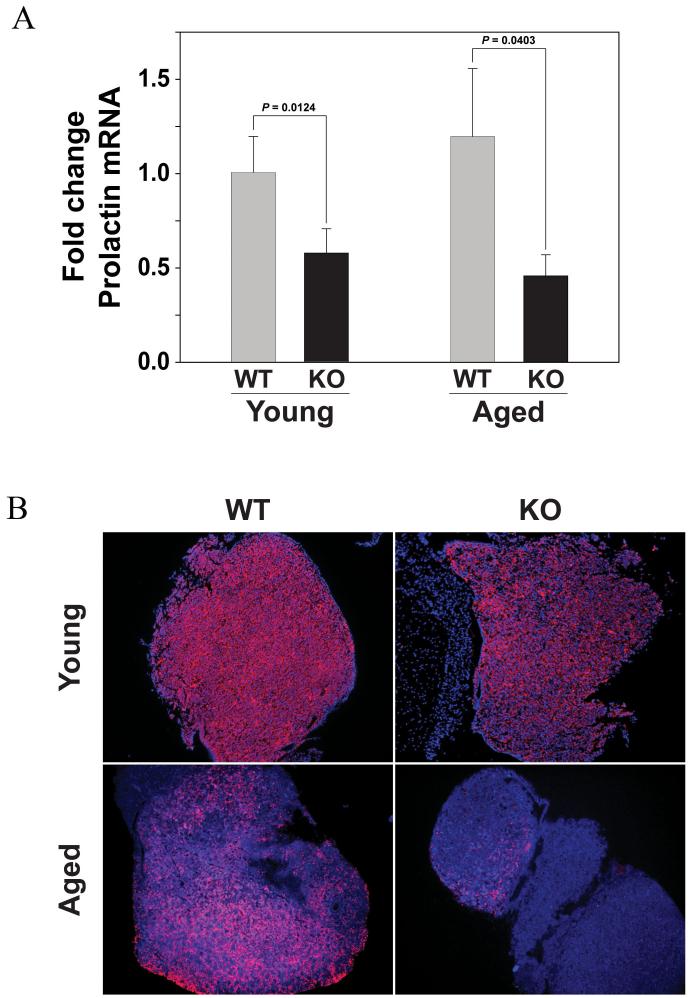
The levels of PRL mRNA in the pituitary glands from both young (P=0.0124) and old (P=0.0403) mice are also significantly decreased in Ghsr-/- mice compared to wild type littermates (Figure 4A). In comparing PRL mRNA expression between young and old GHSR-/- mice, there is no significant difference between these mice with age (P=0.124). Similar to the GH mRNA studies described above, there are also no significant differences with age in PRL mRNA expression in the wild-type mice (P=0.209). PRL immunohistochemical staining of sections from the pituitary gland of each genotype suggests that the reduced PRL mRNA expression correlates with lower levels of PRL protein in Ghsr-/- mice compared to wild type mice. However, similar to the GH immunostaining, the concentration of PRL positive cells is also dramatically reduced with age in both the wild type and mutant tissues (Figure 4B). However, similar to our GH protein assessments in the pituitary glands of these mice, the lack of direct protein quantification and counts of PRL-producing somatotrophs and lactotrophs limits the conclusions that can be drawn on the pituitary protein changes in these animals with progressive aging. In addition, while the PRL mRNA data did not reach statistical significance (P=0.09), we did observe a trend (albeit not significant) for reduced circulating serum levels of PRL in Ghsr-/- mice (26401 +/- 18134 pg/ml) compared to their wild-type counterparts (49082 +/- 44806 pg/ml).
Figure 4. PRL mRNA and protein expression in the pituitary gland of Ghsr-/- is reduced compared to wild-type control mice.
(A) PRL mRNA levels are assessed using real-time RT-PCR using RNA isolated from the in pituitary glands of young (2m) and old (24m) female Ghsr-/- and Ghsr+/+ wild-type control mice. The data are expressed as fold change PRL mRNA (+/- SD) compared to the young wild-type control mice. P values indicate significant differences. (n = 4-5 mice per group). In comparing PRL mRNA expression between young and old Ghsr-/- mice, there is no significant difference between these mice with age (P=0.124). Similarly, there is also no significant difference with age in the wild-type mice (P=0.209). There are only significant differences in PRL mRNA expression between the wild-type and Ghsr-/- mice at the younger (P=0.0124) and older (P=0.0403) ages. [2-way ANOVA, Age: p = 0.626; Genotype p < 0.001; Tukey Kramer method: Genotype p < 0.001 Control vs Ghsr-/-]. (B) Immunohistochemical analysis of PRL protein expression in sections of pituitary glands of young (2m) and aged (24m) female Ghsr-/- compared to wild-type mice. (n = 3 mice per group were examined).
Pituitary gland weight declines during aging of Ghsr-/- mice
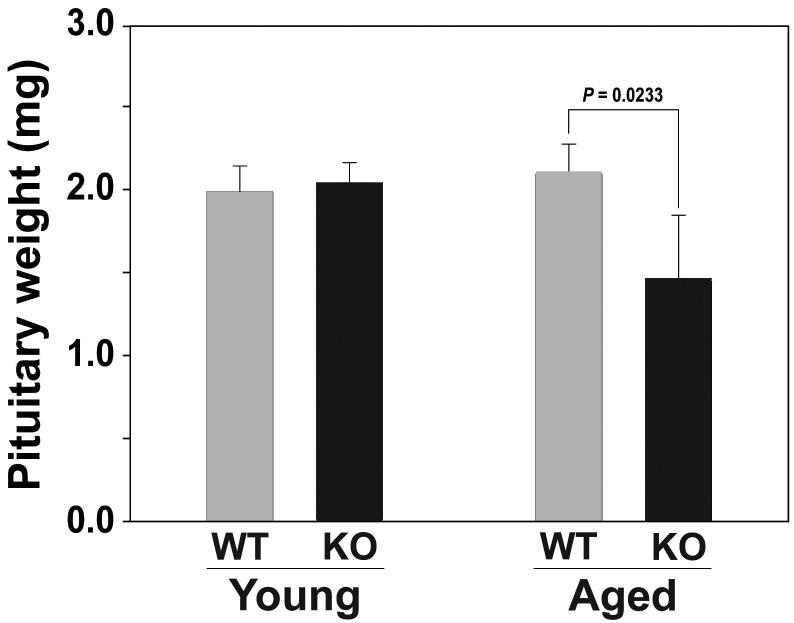
Since Pit-1 is critical for the differentiation and maintenance of somatotrophs, lactotrophs and thyrotrophs in the anterior pituitary gland, reduced levels of these cell types might be anticipated as a consequence of reduced Pit-1 expression in Ghsr-/- mice. Therefore, we compared the pituitary gland weights of young and old Ghsr-/- and littermate control mice. In 4 month old mice, the pituitary weights are identical irrespective of the Ghsr genotype. However, in contrast to wild-type mice, 24 month old Ghsr-/- mice exhibited significantly reduced pituitary weights (P=0.0233; Figure 5).
Figure 5. Pituitary gland weight is lower in old Ghsr-/- mice compared to age-matched wild-type littermates.
Isolated pituitary glands of young (4m) and old (24m) female Ghsr-/- and wild type mice (n = 4-5 mice per group) were isolated and weighed to assess the pituitary weight. The data are expressed in mg (+/- SD). P values indicate significant differences. Old Ghsr-/- demonstrate a significant reduction in pituitary weight compared to age-matched wild-type mice (P=0.0233). Moreover, Ghsr-/- mice also demonstrate a significantly lower pituitary weight with advancing age (young vs. old Ghsr-/-, P=0.0330; young vs. old wild-type, NS).
Discussion
In the anterior pituitary gland, the transcription factor Pit-1 regulates GH and PRL gene expression (Ingraham et al., 1988; Sornson et al., 1996). The GHS-R1a agonists GHRP-6 and ghrelin increase Pit-1 expression in primary cultures of anterior pituitary cells from infant rats (Garcia et al., 2001). Hence, in the present study, we measured Pit-1 mRNA in the pituitary gland of Ghsr-/- mice and wild-type littermates to determine whether GHS-R1a modulated Pit-1 expression. Pit-1 mRNA levels were markedly lower in the Ghsr-/- mice compared to wild type littermates and in both genotypes expression declined markedly during aging (Figure 2). Since Pit-1 is a positive regulator of GH and PRL production, it was anticipated that GH and PRL expression would be reduced in Ghsr-/- mice (Ingraham et al., 1988; Sornson et al., 1996).
As expected, our studies revealed that GH mRNA in the pituitary of Ghsr-/- mice was significantly lower than in wild-type littermates but surprisingly GH mRNA levels did not change with age in the pituitary gland (Figure 3A). Immunohistochemical analyses also suggested, although not directly quantified, that less GH protein was produced by the pituitary of Ghsr-/- mice (Figure 3B), which is consistent with reduced body weight and lower IGF-1 levels exhibited by Ghsr-/- mice (Sun et al., 2007; Sun et al., 2004). Interestingly, in contrast to the mRNA data (Figure 3A), there appeared to be an age-associated decrease in GH protein expression in the pituitary glands of both wild-type and Ghsr-/- mice. Similarly, we found that PRL mRNA and protein in the pituitary glands of Ghsr-/- mice were also markedly reduced compared to wild type littermates (Figure 4A), along with a trend (albeit not significant) towards reduced PRL levels in the circulation. Moreover, similar to GH protein expression, there appears to be an age-associated decrease with PRL protein producing cells within the pituitary; however, no significant age-related differences in PRL mRNA levels were observed in null or wild-type mice. However, our conclusions on GH and PRL protein changes in the pituitary need to be qualified as no direct quantitation of protein levels or hormone expressing cells was performed in these mice at any age. Moreover, it is indeed unclear why no age-related changes in hormone mRNA expression were observed in Ghsr-/- and wild-type mice. Perhaps the dramatic age-associated changes in Pit-1 mRNA expression in both wild-type and Ghsr-/- mice account for these differences (Figure 2). This is in line with our hypothesis that ablation of Ghsr would reduce pituitary Pit-1 expression and as a consequence reduce pituitary and systemic GH and PRL production, thus possibly explaining the lower body weights and lower IGF-1 levels in Ghsr-/- mice.
Besides regulating GH and PRL gene transcription, Pit-1 is also involved in the differentiation, proliferation and maintenance of somatotrophs, lactotrophs and thyrotrophs. Immunohistochemistry studies show fewer GH and PRL containing cells in the pituitary gland of Ghsr-/- mice and with advancing age (Figure 4B). While differences in pituitary gland weights between young mutant and wild-type mice were not observed, the weights of pituitary glands from old Ghsr-/- mice were markedly lower than those of their wild-type counterparts. Moreover, we have also observed a significant difference in the pituitary weight of young and old GHSR-/- mice (P=0.0330) but not young and old wild-type mice. These results suggest that reduced numbers of somatotrophs and lactotrophs did not affect pituitary weight in young Ghsr-/- mice, but that reduced Pit-1, GH and PRL expression combined may result in an impaired maintenance of pituitary gland weight during the aging process (Figure 5). Again, without direct quantitation of the hormone producing somatotrophs and lactotrophs in the pituitary of these animals, the immunohistological assessments are merely qualitative in nature and conclusions regarding protein levels should bear this in mind.
Overall, the secretion of GH and PRL from the anterior pituitary somatotrophs is regulated by the hypothalamic factors, growth hormone releasing hormone (GHRH) and somatostatin (Plotsky and Vale, 1985; Rivier et al., 1982; Vance et al., 1985). It was subsequently established that GHS-R1a agonists act directly on somatotrophs to increase GH release, as well as amplifying GHRH-induced GH release and antagonizing somatostatin action (Smith et al., 1997). Furthermore, GHS-R1a agonists act directly on GHRH neurons to stimulate the release of GHRH and inhibit release of somatostatin (Smith et al., 1997). Besides their stimulatory effects on GH release, early studies with GHS-R1a agonists in humans showed modest increases in PRL when administered acutely, but this effect on PRL was not sustained during chronic treatment (Chapman et al., 1996). More marked PRL responses were observed in obese subjects (Svensson et al., 1998). The results of studies on primary cultures of female rat pituitary cells suggests PRL release is mediated by activation of GHS-R1a agonists on pituitary cells that contain both GH and PRL (Cheng et al., 1993; Smith et al., 1997). These observations along with our current findings support a critical regulatory role for ghrelin and its receptor in the maintenance and function of the pituitary gland throughout the lifetime of a host. Alterations in these pathways, such as the age-associated loss of Pit-1 mRNA expression and hormone production, may result in significant metabolic and physiologic alterations including weight loss, changes in adiposity, loss of muscle mass, sarcopenia, fatigue and changes in food intake. Many of these changes are already associated with advancing age in humans suggesting that the ghrelin-GHS-R system may also undergo some dramatic changes in expression and activity with age. Such possible changes in the ghrelin-GHS-R signaling pathway need to be further examined.
In addition, these data also suggest a possible role for GHS-R and ghrelin in the expression of hormones within other physiological systems in the body, such as the immune system. GH and IGF1 have been shown to be produced and mediate function on immune cells (Taub, 2007; Welniak et al., 2002). Moreover, thymic atrophy has been associated with the ablation of the pituitary gland and in mice with Pit-1 defects (Taub, 2007; Welniak et al., 2002). Hypophysectomized rats or Snell dwarf mice (dw/dw), which are both defective in the production of GH and PRL, display deficiencies in thymic and lymphocyte development, which were corrected upon administration of exogenous GH to these animals. Stress also seems to be a major factor influencing the altered immune systems in these animals (Dorshkind et al., 2003). Interestingly, the pituitary defects associated with immune alterations in these mouse models are actually quite similar to the defects we have recently reported for aged Ghsr-/- mice (Dixit et al., 2007). We have observed significant alterations in hematopoiesis, thymic function, thymopoiesis and thymic output in aged but not young Ghsr-/- and Ghrl -/- mice (Dixit et al., 2007). Interestingly, infusion of acylated ghrelin into aged but not young mice results in a restoration in many of the defects associated with age-associated thymic involution. These results support a role for ghrelin and the GHS-R in thymic physiology and development. While it is unclear what is the precise mechanisms behind these thymic defects, it seems plausible that the diminished capacity of aged Ghsr -/- mice to express both pituitary and immune-derived GH, PRL and Pit-1 may contribute, in part, to these immunological alterations observed in these animals with advancing age (Figure 6). We have recently found that the thymi of Ghsr-/- mice express significantly diminished levels of GH, PRL and IGF1 with age compared to wild-type mice (data not shown). While the role of immune-derived hormones remain controversial [e.g., the lack of immunological defects in GH receptor knockout mice (Zhou et al., 1997)], there is ample evidence for a role for pituitary hormones in immune modulation including data supporting an immunostimulatory role for GH under conditions of stress, aging and post transplantation in mice and humans ((Dorshkind et al., 2003; Taub, 2007). How GHSR, ghrelin and circulating and lymphoid-derived hormones crosstalk within the immune system to influence cellular function and thymic integrity remains to be elucidated. These Ghsr-/- mice should prove valuable in furthering our understanding of lymphoid-produced hormones in immune homeostasis.
Figure 6. Possible role for GH and ghrelin in the thymic defects associated with aging and in Ghsr-/- mice.
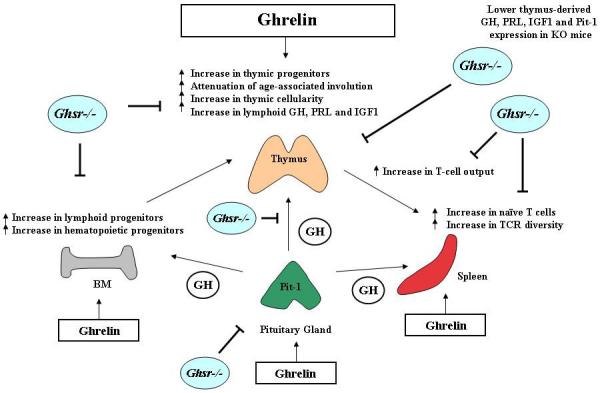
The thymus is critical for the development, selection and maintenance of the peripheral T cell pool possessing a broad spectrum of TCR specificities. Post puberty and with advancing age, the thymic space becomes progressively filled with adipocytes coupled with a dramatic loss of thymocytes leading to a reduction in output of naive T cells (a process called “thymic involution”). Involution of the thymus with age and the paucity of newly formed naïve CD4+ T cells are therefore believed to be responsible for much of the deterioration in adaptive immunity and the resultant immune dysfunction in the elderly and immunosuppressed patients. GH and ghrelin levels decrease with age in the thymus and circulation, notably coinciding with thymic involution. This impaired thymic function with aging or in mice possessing a pituitary defect could be restored by the administration of recombinant GH or ghrelin. Given ghrelin’s ability to influence peripheral T cell responses and its ability to stimulate the GH-IGF-1 axis, we recently demonstrated the ability of ghrelin and its receptor to reverse age-related thymic involution by increasing hematopoietic, lymphoid and thymic progenitors, redistribution of thymic structure, increasing T cell output and promoting peripheral TCR diversity (which is significantly diminished with age). In Ghsr-/- mice, many of the alterations associated with normal immunological aging (e.g., bone marrow, thymus and spleen) were found to be further enhanced in these mice compared to wild-type controls. This enhancement may be due to even further diminished pituitary and immune-associated GH, PRL and IGF1 levels in the Ghrs-/- mice. Based on the ability of exogenous GH and ghrelin to restore age-associated thymic function, these ligands may serve as promising therapeutics for intervention in aged and immunocompromised hosts.
Acknowledgements
We would like to thank the staff of the Comparative Medicine Section of the NIA animal facility for their support in maintaining and monitoring our animals. We would also like to thank Drs. Dan L. Longo, Michel Mark Mattson and Arya Biragyn for reviewing this manuscript and Mrs. Dawn Tripp for her administrative assistance with the manuscript. This research was supported in part by the Intramural Research Program of the National Institute on Aging, National Institutes of Health and R01 AG19230-01 and R01 AG18895-02 NIH grants to RGS.
References
- Chapman IM, Bach MA, Van Cauter E, Farmer M, Krupa D, Taylor AM, Schilling LM, Cole KY, Skiles EH, Pezzoli SS, Hartman ML, Veldhuis JD, Gormley GJ, Thorner MO. Stimulation of the growth hormone (GH)-insulin-like growth factor I axis by daily oral administration of a GH secretogogue (MK-677) in healthy elderly subjects. J Clin Endocrinol Metab. 1996;81:4249–4257. doi: 10.1210/jcem.81.12.8954023. [DOI] [PubMed] [Google Scholar]
- Cheng K, Chan WW, Butler B, Wei L, Smith RG. A novel non-peptidyl growth hormone secretagogue. Horm Res. 1993;40:109–115. doi: 10.1159/000183777. [DOI] [PubMed] [Google Scholar]
- Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, Lillard JW, Jr., Taub DD. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit VD, Yang H, Sun Y, Weeraratna AT, Youm YH, Smith RG, Taub DD. Ghrelin promotes thymopoiesis during aging. J Clin Invest. 2007;117:2778–2790. doi: 10.1172/JCI30248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorshkind K, Welniak L, Gault RA, Hixon J, Montecino-Rodriguez E, Horseman ND, Gertner JM, Murphy WJ. Effects of housing on the thymic deficiency in dwarf mice and its reversal by growth hormone administration. Clin Immunol. 2003;109(2):197–202. doi: 10.1016/s1521-6616(03)00181-5. [DOI] [PubMed] [Google Scholar]
- Garcia A, Alvarez CV, Smith RG, Dieguez C. Regulation of Pit-1 expression by ghrelin and GHRP-6 through the GH secretagogue receptor. Mol Endocrinol. 2001;15:1484–1495. doi: 10.1210/mend.15.9.0694. [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, Patchett AA, Nargund R, Griffin PR, DeMartino JA, Gupta SK, Schaeffer JM, Smith RG, Van der Ploeg LH. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- Ingraham HA, Chen RP, Mangalam HJ, Elsholtz HP, Flynn SE, Lin CR, Simmons DM, Swanson L, Rosenfeld MG. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell. 1988;55:519–529. doi: 10.1016/0092-8674(88)90038-4. [DOI] [PubMed] [Google Scholar]
- Patchett AA, Nargund RP, Tata JR, Chen MH, Barakat KJ, Johnston DB, Cheng K, Chan WW, Butler B, Hickey G, et al. Design and biological activities of L-163,191 (MK-0677): a potent, orally active growth hormone secretagogue. Proc Natl Acad Sci U S A. 1995;92:7001–7005. doi: 10.1073/pnas.92.15.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky PM, Vale W. Patterns of growth hormone-releasing factor and somatostatin secretion into the hypophysial-portal circulation of the rat. Science. 1985;230:461–463. doi: 10.1126/science.2864742. [DOI] [PubMed] [Google Scholar]
- Pong SS, Chaung LY, Dean DC, Nargund RP, Patchett AA, Smith RG. Identification of a new G-protein-linked receptor for growth hormone secretagogues. Mol Endocrinol. 1996;10:57–61. doi: 10.1210/mend.10.1.8838145. [DOI] [PubMed] [Google Scholar]
- Rivier J, Spiess J, Thorner M, Vale W. Characterization of a growth hormone-releasing factor from a human pancreatic islet tumour. Nature. 1982;300:276–278. doi: 10.1038/300276a0. [DOI] [PubMed] [Google Scholar]
- Smith RG, Jiang H, Sun Y. Developments in ghrelin biology and potential clinical relevance. Trends Endocrinol Metab. 2005;16:436–442. doi: 10.1016/j.tem.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Smith RG, Van der Ploeg LH, Howard AD, Feighner SD, Cheng K, Hickey GJ, Wyvratt MJ, Jr., Fisher MH, Nargund RP, Patchett AA. Peptidomimetic regulation of growth hormone secretion. Endocr Rev. 1997;18:621–645. doi: 10.1210/edrv.18.5.0316. [DOI] [PubMed] [Google Scholar]
- Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O’Connell SM, Gukovsky I, Carriere C, Ryan AK, Miller AP, Zuo L, Gleiberman AS, Andersen B, Beamer WG, Rosenfeld MG. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384:327–333. doi: 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- Sun Y, Garcia JM, Smith RG. Ghrelin and growth hormone secretagogue receptor expression in mice during aging. Endocrinology. 2007;148:1323–1329. doi: 10.1210/en.2006-0782. [DOI] [PubMed] [Google Scholar]
- Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci U S A. 2004;101:4679–4684. doi: 10.1073/pnas.0305930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson J, Lonn L, Jansson JO, Murphy G, Wyss D, Krupa D, Cerchio K, Polvino W, Gertz B, Boseaus I, Sjostrom L, Bengtsson BA. Two-month treatment of obese subjects with the oral growth hormone (GH) secretagogue MK-677 increases GH secretion, fat-free mass, and energy expenditure. J Clin Endocrinol Metab. 1998;83:362–369. doi: 10.1210/jcem.83.2.4539. [DOI] [PubMed] [Google Scholar]
- Tassone F, Broglio F, Destefanis S, Rovere S, Benso A, Gottero C, Prodam F, Rossetto R, Gauna C, van der Lely AJ, Ghigo E, Maccario M. Neuroendocrine and metabolic effects of acute ghrelin administration in human obesity. J Clin Endocrinol Metab. 2003;88:5478–5483. doi: 10.1210/jc.2003-030564. [DOI] [PubMed] [Google Scholar]
- Taub DD. Novel connections between the neuroendocrine and immune systems: the ghrelin immunoregulatory network. Vitam Horm. 2007;77:325–346. doi: 10.1016/S0083-6729(06)77014-5. [DOI] [PubMed] [Google Scholar]
- Vance ML, Kaiser DL, Evans WS, Furlanetto R, Vale W, Rivier J, Thorner MO. Pulsatile growth hormone secretion in normal man during a continuous 24-hour infusion of human growth hormone releasing factor (1-40). Evidence for intermittent somatostatin secretion. J Clin Invest. 1985;75:1584–1590. doi: 10.1172/JCI111864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welniak LA, Sun R, Murphy WJ. The role of growth hormone in T-cell development and reconstitution. J Leukoc Biol. 2002;71:381–387. [PubMed] [Google Scholar]
- Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Cataldo L, Coschigamo K, Wagner TE, Baumann G, Kopchick JJ. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc Natl Acad Sci U S A. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]