SUMMARY
The interaction of longevity-conferring genes with longevity-conferring diets is poorly understood. The growth hormone receptor gene-disrupted (GHR-KO) mouse is long-lived; and this longevity is not responsive to 30% caloric restriction (CR), in contrast to wild-type animals from the same strain. To determine whether this may have been limited to a particular level of dietary restriction (DR), we subjected GHR-KO mice to a different dietary restriction regimen, an intermittent fasting (IF) diet.
The IF diet increased the survivorship and improved insulin sensitivity of normal males, but failed to affect either parameter in GHR-KO mice.
From the results of two paradigms of dietary restriction we postulate that GHR-KO mice would be resistant to any manner of DR; potentially due to their inability to further enhance insulin sensitivity. Insulin sensitivity may be a mechanism and/or a marker of the lifespan-extending potential of an intervention.
Keywords: aging, longevity, caloric restriction, intermittent fasting, growth hormone, insulin sensitivity
INTRODUCTION
In different animal species, aging can be postponed and longevity increased by dietary restriction (DR) or mutations of genes involved in somatotrophic/insulin signaling. The mechanisms by which these mutations and DR affect aging are apparently overlapping, although not identical, and dietary intake can interact with longevity genes in various ways (Piper & Bartke, [2008]). In mice, we have previously reported that an identical regimen of DR produces further longevity extension in long-lived Ames dwarf mice (Bartke et al., [2001]), but is ineffective in another long-lived mutant, the growth hormone (GH) resistant Ghr−/− (GHR-KO) mouse (Bonkowski et al., [2006]; Zhou et al., [1997]; Coschigano et al., [2000]; Coschigano et al., [2003]).
Because, in Drosophila melanogaster, longevity genes can alter energy intake requirements for optimal survival (Clancy et al., [2002]; Wang et al., [2009]) it was of interest to determine if GHR-KO mice will respond to a different DR regimen, intermittent fasting (IF) (Anson et al., [2005]).
RESULTS AND DISCUSSION
We determined the gender-specific caloric intake for ad libitum (AL) vs. IF mice at approximately 7–8, 14–15, and 32–33 months-of-age. All animals were fed AL for the first 8–10 weeks of age. Subsequently, the mice were either fed AL every day (AL group) or every other day (IF group). Average food intake was numerically reduced in almost all IF groups, but the difference between AL and IF animals was statistically significant only in normal males at 14–15 months of age (23% reduction, p < 0.02); thus confirming the reported compensatory feeding of IF animals during their ad lib. phase (Anson et al., [2003]; Mattson et al., [2003]).
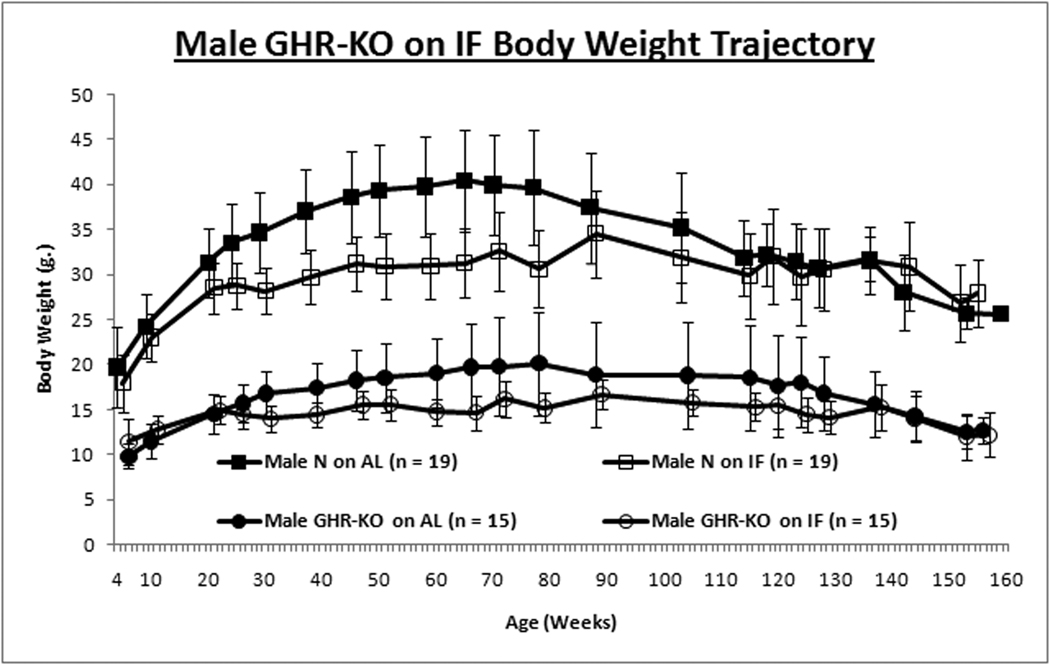
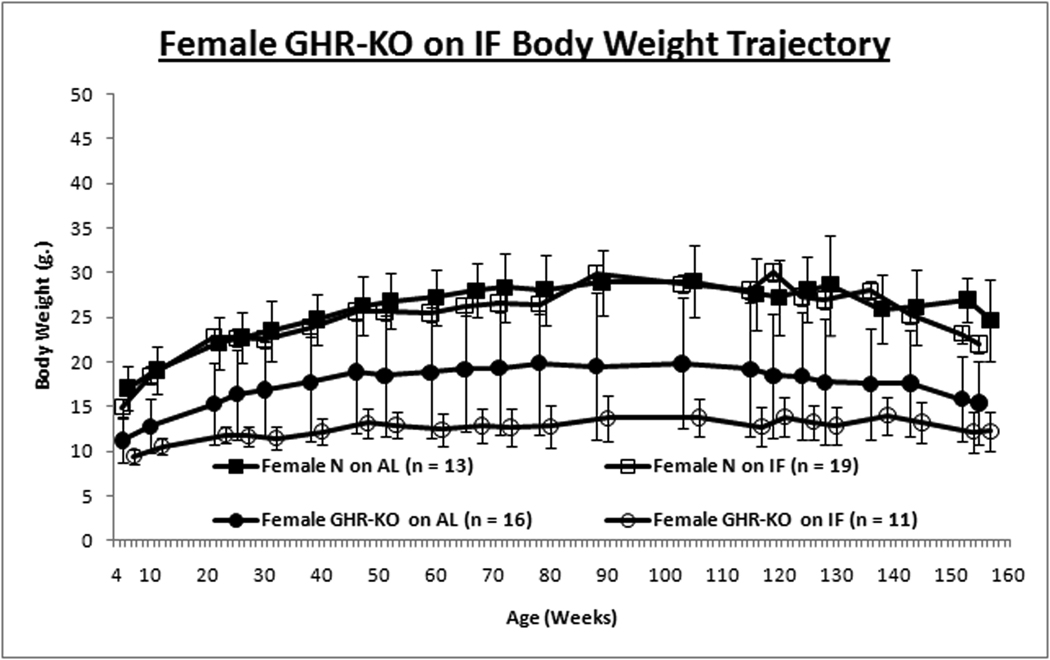
Intermittent fasting had no effect on the growth trajectory assessed by body weight of normal females or GHR-KO males, but reduced growth of normal males and female GHR-KO mice (Figs. 1A & 1B). This contrasts with no significant attenuation of growth trajectory in C57Bl/6 mice on IF (Goodrick et al., [1990]; (Anson et al., [2003]).
Figure 1. Gender-specific Effects of Intermittent Fasting (IF) on Body Weight (BW) and Insulin Sensitivity.
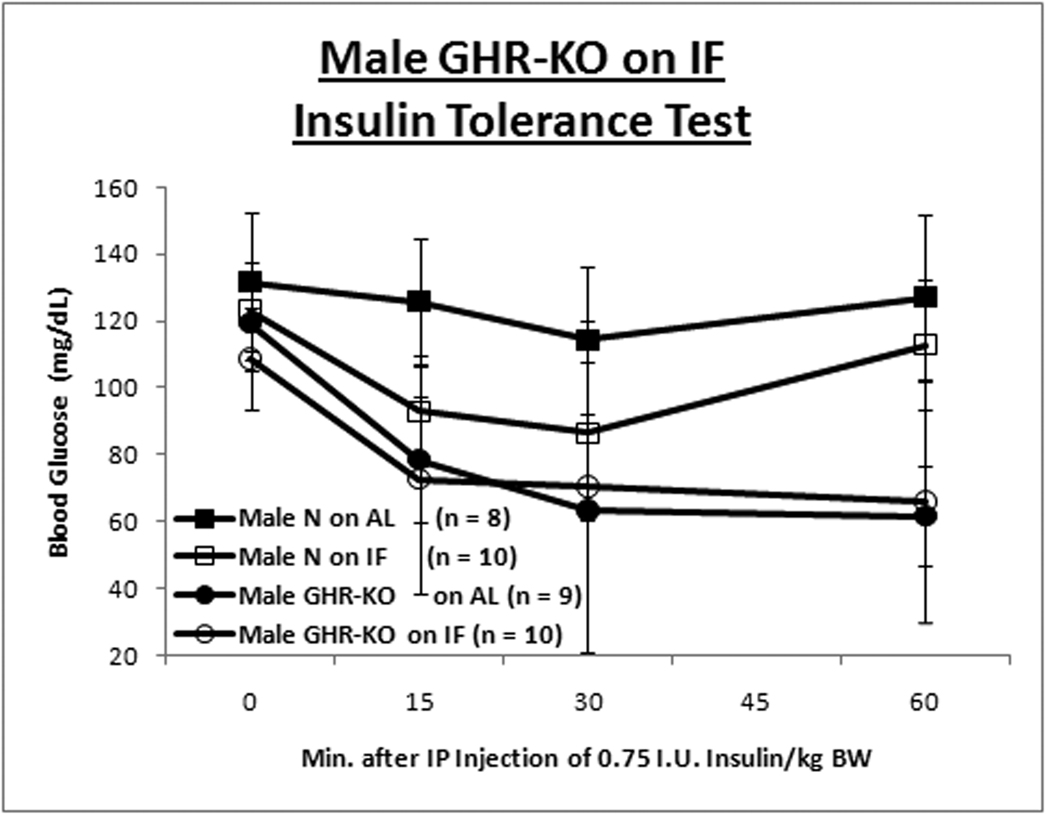
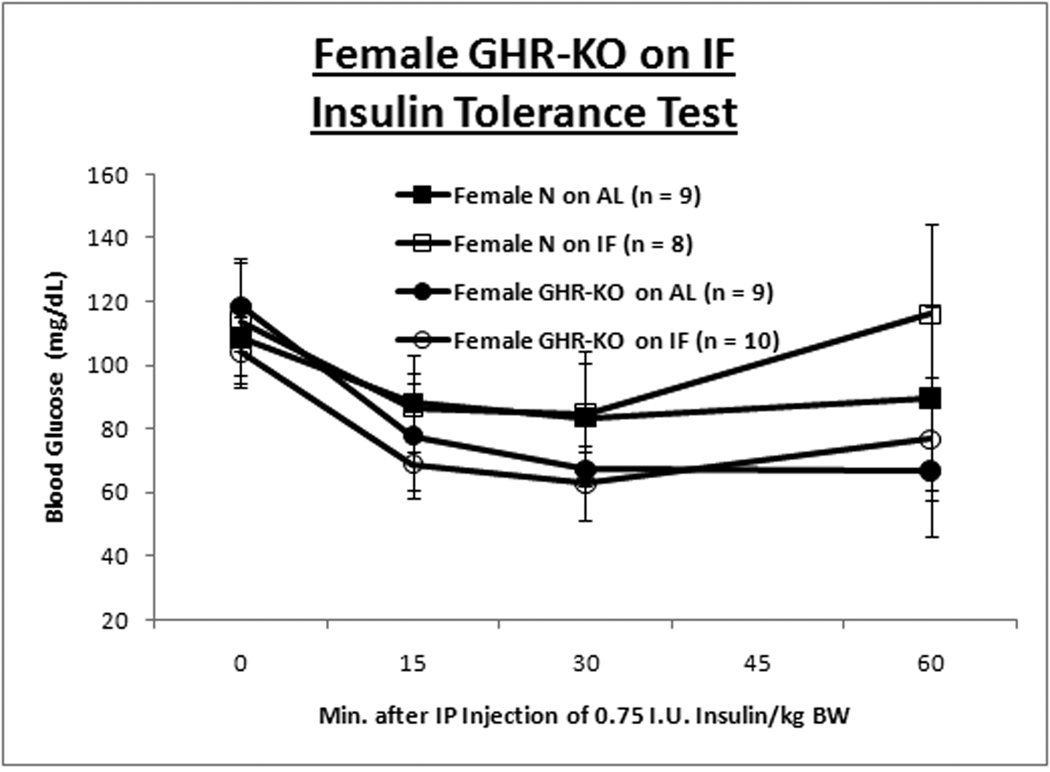
GHR-KO mice and their littermate controls (derived from 129/Ola founders, provided by J. J. Kopchick [Ohio University, Athens, OH], and outbred to Balb/c, C57Bl/6J and C3HJ strains) were bred in a closed colony, housed under standard conditions (12-hr. light/12-hr. dark cycling and 20–23°C), and fed Lab Diet Formula 5001 (23% protein, 4.5% fat, 6% fiber) (Nestle Purina, St. Louis, MO). All animals were fed AL for the first 8–10 weeks of age. Thereafter, the mice were either fed AL every day (AL group) or every other day (IF group). Mice were weighed in the morning after a feeding day, approximately 16–20 hrs. after the IF group had been fed. Animal protocols were approved by the Animal Care and Use Committee of Southern Illinois University. A. Body weight trajectory for male mice shows IF reduces BW for only littermate control mice. B. Body weight trajectory for female mice shows IF reduces BW solely for GHR-KO mice. Unpaired Student’s t-test (Microsoft Excel, Redmond, WA) was employed to compare food consumption data. Body weight-gain data was contrasted with Repeated-Measures Analysis of Variance (ANOVA) (SPSS, Chicago, IL). Cohorts of mice not allocated for the lifespan assay, but similarly subjected to IF for ten months, were tested for insulin tolerance (ITT). After AL access to food overnight, food was removed, 0.75 international units (I.U.)/kg body weight of porcine insulin (Sigma-Aldrich, St. Louis, MO) was injected intra-peritoneal (IP) and tail-blood glucose was determined with a glucometer (Lifescan; Johnson & Johnson, New Brunswick, NJ) at 0, 15, 30, and 60 min. C. Insulin Tolerance Test for male mice depicts amelioration of insulin resistance of the littermate control mouse by IF. D. ITT for females shows IF has no effect on insulin sensitivity for either phenotype. Repeated-Measures ANOVA was used for comparisons of data from the ITT (SPSS, Chicago, IL). Graphs were generated with Excel (Microsoft, Redmond, WA). All measures of central tendency are arithmetic means, and all depictions of variation (error bars) represent standard deviations.
Insulin sensitivity, and the glucose homeostasis that it connotes, are a common correlate with longevity (Russell and Kahn, [2007]; Bartke, [2008]; Masternak et al., [2009]). Results of insulin tolerance tests (ITT) revealed that IF ameliorated the innate insulin resistance of male littermate control mice (Fig. 1C), but had no effect on insulin sensitivity of normal females (Fig. 1D) or GHR-KO mice of either gender. (Repeated Measures Analysis of Variance [ANOVA] p-value for male normal mice on AL (N-AL) vs. Male GHR-KO on AL (KO-AL) < 0.001 [Bonferroni’s Post-Hoc Test-corrected]; p-value for Male N-AL vs. Male normal mice on IF (N-IF) < 0.05 [Student-Newman-Keuls’ Post-Hoc Test-corrected]; p-value for Female N-AL vs. Female KO-AL < 0.05 [Student-Newman-Keuls’ Post-Hoc Test-corrected; data Log10-transformed]). Female mice for whom blood glucose levels increased after insulin injection, presumably indicative of an endocrine response to the stress of handling and injection, were excluded from the analysis; with their inclusion, the differences in insulin sensitivity between the female KO-AL and the female N-AL was not statistically significant.
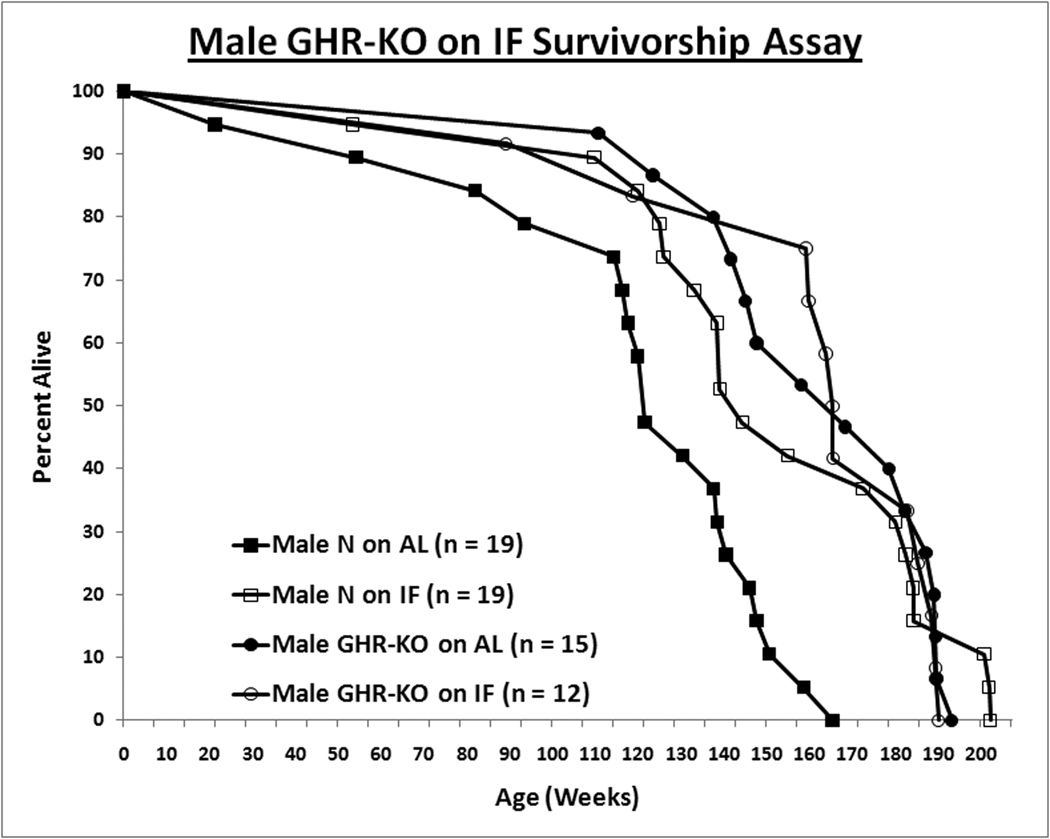
Intermittent fasting increased the lifespan of normal male mice but did not affect the longevity of GHR-KO males (Median Survival: Male N-AL = 851 days [d.], Male N-IF = 1010 d., Male KO-AL = 1178 d., and Male GHR-KO on IF (KO-IF) = 1157 d.; Log-rank [Mantel-Cox] Test p-value for Male N-AL vs. Male N-IF = 0.0002) (Fig. 2A). The results from the median lifespan were previously published by Bonkowski et al., [2009], and here we include the completed lifespan curves.
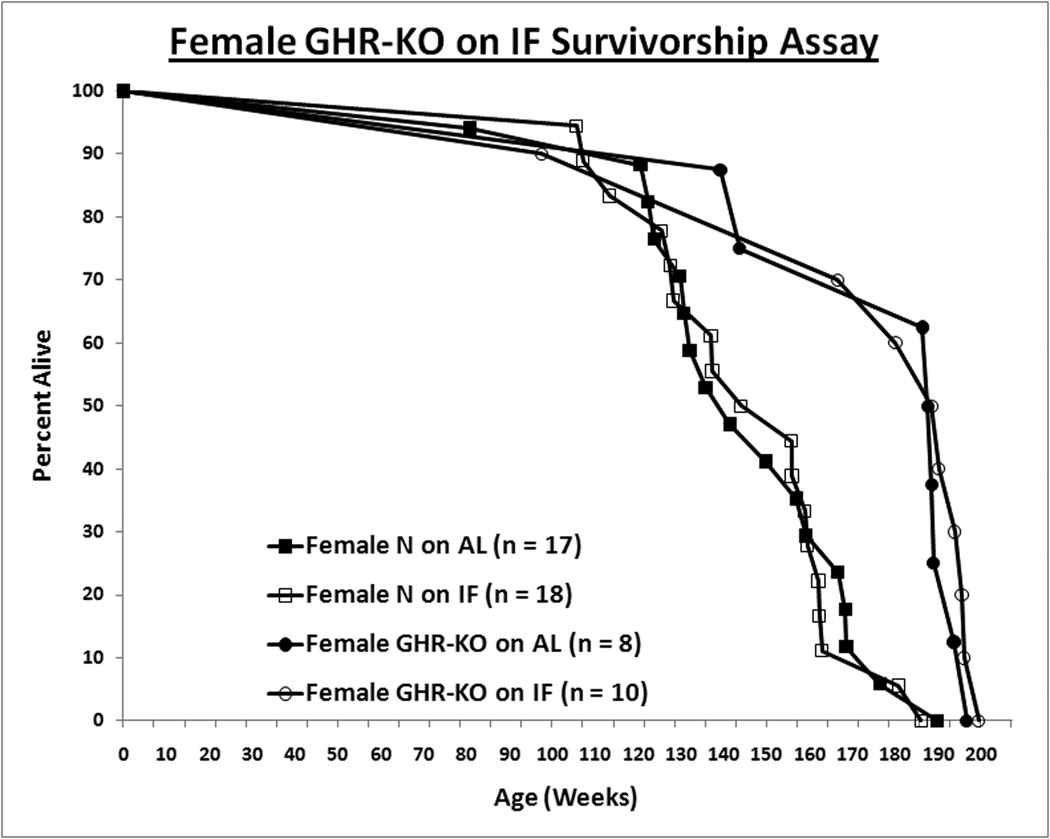
Figure 2. Sexual Dimorphism of Effect of Intermittent Fasting (IF) on Survivorship.
For the survivorship assay, all animals were fed AL for the first 8–10 weeks of age. Henceforth, the mice were either fed AL every day (AL group) or every other day (IF group). Mice found either moribund or with a neoplasm approximately 10% of their total body volume were euthanized, and the date of euthanasia was recorded as the date of death. A. IF confers longevity to male littermates. B. For females, IF has no effect on either phenotype. Log-rank (Mantel-Cox) test analysis was utilized to compare the overall survivorship data with GraphPad Prism 5.01 (GraphPad Software Inc., La Jolla, CA); and maximal lifespan, of the 90th percentile of the appropriate control population, was analyzed with quantile regression exploiting an exact unconditional (Score Statistic) test (http://www.stat.ncsu.edu/exact/); for the latter, a binomial model was used, the hypothesis was two-sided, 99.9000% confidence interval assigned, and the test statistic employed was Fisher's Exact-Boschloo Test Statistic. Graphs were generated with Excel (Microsoft, Redmond, WA). All measures of central tendency are arithmetic means, and all depictions of variation (error bars) represent standard deviations. Mice physically lost from the study, euthanized due to senescence-independent matters, or included in a supplemental cohort in the survivorship assay account for the discrepancies in the sample sizes (within subgroup) between the body weight data and the survivorship data.
When maximal lifespans (the survivorships of the longest-lived deciles of the respective populations) were contrasted, they concurred with the mean lifespan results, with only male littermate control mice on IF exhibiting increased longevity relative to their AL counterparts (Exact Unconditional Homogeneity/Independence Test p-value for Male N-AL vs. Male N-IF = 0.0151; p-value for Male N-AL vs. Male KO-AL = 0.0028; Female N-AL vs. Female KO-AL = 0.0030) (Berger, [1994]; Berger, [1996]; Wang et al., [2004]; Miller et al., [2007]; Arum and Johnson, [2007]).
Intermittent fasting did not increase mean or maximal lifespan of female normal or GHR-KO mice (Median Survival: Female N-AL = 990 d., Female N-IF = 1049 d., Female KO-AL = 1316 d., and Female KO-IF = 1325 d.; Log-rank [Mantel-Cox] Test p-value for Female N-AL vs. Female KO-AL = 0.0056) (Fig. 2B).
The key novel finding of the present study is that IF fails to affect insulin sensitivity and average as well as maximal longevity of male GHR-KO mice, even though it significantly increases all of these parameters in normal males from the same strain. These observations indicate that the previously reported inability of 30% reduction of caloric intake to cause further increases in longevity or insulin sensitivity in these long-lived mutants (Bonkowski et al., [2006]) was not limited to that particular regimen of DR, and that this presumably applies to DR in general. As it would address whether it was simply the lengthened fasting schedule or that and a minor reduction in caloric intake that was responsible for the IF effects observed in this report, a further IF study involving mice fed daily with a diet isocaloric to that of IF mice is warranted.
We have also found striking sexual dimorphism in the response of normal animals from this strain to IF, with significant improvements in insulin sensitivity and longevity in males only. Gender-based differential responses to caloric restriction have been reported (Willott et al., [1995]; Turturro et al., [1999]; Forster et al., [2003]; Valle et al., [2007]; Porter et al., [2004]; Bonkowski et al., [2006]; Selman et al., [2008]). Although C57Bl/6J male mice on IF exhibit hyperphagia, and thus gain weight at a rate very similar to that of AL counterparts (Anson et al., [2003]), IF has been reported to decrease body weight gain substantially in female C57Bl/6J mice (Barrows and Kokkonen, [1978]); moreover, the incidence of mammary tumors in female rats is also decreased by IF (Carlson and Hoelzel, [1946]). Yet, to the best of our knowledge, this is the first documentation of a sexually dimorphic effect of IF on insulin sensitivity or survivorship.
Association of the effects of IF on insulin sensitivity and longevity across the genotype/gender groups support the suggested causative link between the two: male littermates have improvements in both upon IF treatment, but the other three subgroups do not have any effect on either. Of further note in this regard are the concordant results on female littermates in both insulin tolerance tests and survivorship assays in the two related studies: in Bonkowski et al., 2006, 30% CR-induced insulin sensitivity in normal females concurred with enhanced survivorship; in this report, IF failed to affect both insulin sensitivity and survivorship in females. Together with the results, from this report, of IF allaying insulin resistance and increasing survival in male littermates, these results further buttress the hypothesis that insulin sensitivity may be a mechanism and/or a marker of the lifespan-extending potential of an intervention.
The manner in which increased insulin sensitivity might increase lifespan may involve multiple mechanisms including reduction of post-prandial blood glucose content, decreased potential for hyperglycemic cytotoxicity, whether via Maillard reactions (Thorpe and Baynes, [1996], Cerami and Ulrich, [2001]) or other means (Cerami, [1985], Ceriello, [2001]), increased rate of ATP production that would provide the cellular currency to resist the post-prandial oxidative surge (Jenkins et al., [2006], Charpentier et al., [2006]) and to increase the levels of damage-preventing and/or repairing constituents, and/or altered stress-responsive kinase Erk signaling. Another potential mechanism is enhanced protein turnover, particularly through autophagy, induced by conditions of hypoinsulinemia, such as CR or decreased GH signaling (Cuervo, [2008]). Reduced basal insulin concentrations themselves, independent of insulin sensitivity, blunt the insulin-mediated shift in metabolism (towards anabolism), increase autophagy, and lead to 1) the preservation of cellular energetics (for efficient production of ATP to be used for maintenance and repair processes), 2) the degradation of perniciously effete proteins and subcellular organelles (most notably mitochondria), 3) the enhancement of innate and acquired immunity, and 4) protection from malignant neoplasia (Mizushima et al., [2008]); these actions may engender longevity by counterbalancing the age-associated decline in autophagy (Cuervo et al., [2005]).
ACKNOWLEDGEMENTS
We thank Dr. Rafael de Cabo, Dr. Michal M. Masternak, Jacob A. Panici, Adam Spong, and Reyhan Westbrook for scientific assistance; and Steve Sandstrom and Pam Barnett for copyediting. This work was supported by National Institute on Aging Grants AG19899, U19 AG023122, and 3R01AG019899-07S1, as well as a Senior Scholar Award in Aging from The Ellison Medical Foundation and The Glenn Foundation for Medical Research.
Contributor Information
Michael S. Bonkowski, Email: mbonkowski@siumed.edu.
Andrzej Bartke, Email: abartke@siumed.edu.
REFERENCES
- Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A. 2003 May 13;100(10):6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anson RM, Jones B, de Cabo R. The diet restriction paradigm: a brief review of the effects of every-other-day feeding. Age. 2005;27:17–25. doi: 10.1007/s11357-005-3286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arum O, Johnson TE. Reduced expression of the Caenorhabditis elegans p53 ortholog cep-1-results in increased longevity. J Gerontol A Biol Sci Med Sci. 2007;62(9):951–959. doi: 10.1093/gerona/62.9.951. [DOI] [PubMed] [Google Scholar]
- Barrows CH, Jr, Kokkonen G. The effect of various dietary restricted regimes on biochemical variables in the mouse. Growth. 1978;42(1):71–85. [PubMed] [Google Scholar]
- Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS. Extending the lifespan of long-lived mice. Nature. 2001 Nov 22;414(6862):412. doi: 10.1038/35106646. [DOI] [PubMed] [Google Scholar]
- Bartke A. Insulin and aging. Cell Cycle. 2008 Nov;7(21):3338–3343. doi: 10.4161/cc.7.21.7012. [DOI] [PubMed] [Google Scholar]
- Berger RL. Power comparison of exact unconditional tests for comparing two binomial proportions. Institute of Statistics Mimeo Series No. 2266. 1994. [Google Scholar]
- Berger RL. More powerful tests from confidence interval p values. American Statistician. 1996;50:314–318. [Google Scholar]
- Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci U S A. 2006 May 16;103(20):7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowski MS, Dominici FP, Arum O, Rocha JS, Al Regaiey KA, Westbrook R, Spong A, Panici J, Masternak MM, Kopchick JJ, Bartke A. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS ONE. 2009;4(2):e4567. doi: 10.1371/journal.pone.0004567. Epub 2009 Feb 23. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Carlson AJ, Hoelzel F. Apparent prolongation of the lifespan of rats by intermittent fasting. J Nutr. 1946;31:363–375. doi: 10.1093/jn/31.3.363. [DOI] [PubMed] [Google Scholar]
- Cerami A. Hypothesis. Glucose as a mediator of aging. J Am Geriatr Soc. 1985 Sep;33(9):626–634. doi: 10.1111/j.1532-5415.1985.tb06319.x. [DOI] [PubMed] [Google Scholar]
- Cerami A, Ulrich P. Pharmaceutical intervention of advanced glycation end products. Novartis Found Symp. 2001;235:202–212. doi: 10.1002/0470868694.ch16. discussion 212–6, 217–20. [DOI] [PubMed] [Google Scholar]
- Ceriello A. Mechanisms of tissue damage in the postprandial state. Int J Clin Pract Suppl. 2001 Sep;(123):7–12. [PubMed] [Google Scholar]
- Charpentier G, Dardari D, Riveline JP. How should postprandial glycemia be treated? Diabetes Metab. 2006 Sep;32(Spec No2:2):S21–S27. doi: 10.1016/s1262-3636(06)70481-5. [DOI] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Hafen E, Leevers SJ, Partridge L. Dietary restriction in long-lived dwarf flies. Science. 2002 Apr 12;296(5566):319. doi: 10.1126/science.1069366. [DOI] [PubMed] [Google Scholar]
- Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000 Jul;141(7):2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003 Sep;144(9):3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Bergamini E, Brunk UT, Droge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining "clean" cells. Autophagy. 2005 Oct;1:131–140. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- Cuervo AM. Calorie restriction and aging: the ultimate "cleansing diet". J Gerontol A Biol Sci Med Sci. 2008 Jun;63(6):547–549. doi: 10.1093/gerona/63.6.547. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Morris P, Sohal RS. Genotype and age influence the effect of caloric intake on mortality in mice. FASEB J. 2003;17:690–692. doi: 10.1096/fj.02-0533fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider N. Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mech Ageing Dev. 1990 Jul;55(1):69–87. doi: 10.1016/0047-6374(90)90107-q. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Kendall CW, Josse AR, Salvatore S, Brighenti F, Augustin LS, Ellis PR, Vidgen E, Rao AV. Almonds decrease postprandial glycemia, insulinemia, and oxidative damage in healthy individuals. J Nutr. 2006 Dec;136(12):2987–2992. doi: 10.1093/jn/136.12.2987. [DOI] [PubMed] [Google Scholar]
- Masternak MM, Panici JA, Bonkowski MS, Hughes LF, Bartke A. Insulin Sensitivity as a Key Mediator of Growth Hormone Actions on Longevity. J Gerontol A Biol Sci Med Sci. 2009 May;64(5):516–521. doi: 10.1093/gerona/glp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Duan W, Guo Z. Meal size and frequency affect neuronal plasticity and vulnerability to disease: cellular and molecular mechanisms. J Neurochem. 2003 Feb;84(3):417–431. doi: 10.1046/j.1471-4159.2003.01586.x. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, Nadon NL, Warner HR, Strong R. An Aging Interventions Testing Program: study design and interim report. Aging Cell. 2007 Aug;6(4):565–575. doi: 10.1111/j.1474-9726.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo A, Klionsky D. Autophagy fights disease through cellular self-digestion. Nature. 2008 Feb;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper MD, Bartke A. Diet and aging. Cell Metab. 2008 Aug;8(2):99–104. doi: 10.1016/j.cmet.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Porter MH, Fine JB, Cutchins AG, Bai Y, DiGirolamo M. Sexual dimorphism in the response of adipose mass and cellularity to graded caloric restriction. Obes Res. 2004 Jan;12(1):131–140. doi: 10.1038/oby.2004.18. [DOI] [PubMed] [Google Scholar]
- Russell SJ, Kahn CR. Endocrine regulation of ageing. Nat Rev Mol Cell Biol. 2007 Sep;8(9):681–691. doi: 10.1038/nrm2234. [DOI] [PubMed] [Google Scholar]
- Selman C, Lingard S, Choudhury AI, Batterham RL, Claret M, Clements M, Ramadani F, Okkenhaug K, Schuster E, Blanc E, Piper MD, Al-Qassab H, Speakman JR, Carmignac D, Robinson IC, Thornton JM, Gems D, Partridge L, Withers DJ. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 2008 Mar;22(3):807–818. doi: 10.1096/fj.07-9261com. [DOI] [PubMed] [Google Scholar]
- Thorpe SR, Baynes JW. Role of the Maillard reaction in diabetes mellitus and diseases of aging. Drugs Aging. 1996 Aug;9(2):69–77. doi: 10.2165/00002512-199609020-00001. [DOI] [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the biomarkers of aging program. J. Gerontol. 1999;54A:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- Valle A, Guevara R, García-Palmer FJ, Roca P, Oliver J. Sexual dimorphism in liver mitochondrial oxidative capacity is conserved under caloric restriction conditions. Am J Physiol Cell Physiol. 2007 Oct;293(4):C1302–C1308. doi: 10.1152/ajpcell.00203.2007. [DOI] [PubMed] [Google Scholar]
- Wang C, Li Q, Redden DT, Weindruch R, Allison DB. Statistical methods for testing effects on "maximum lifespan". Mech Ageing Dev. 2004 Sep;125(9):629–632. doi: 10.1016/j.mad.2004.07.003. Erratum in: Mech. Ageing Dev. 2006 Jul;127(7):652. [DOI] [PubMed] [Google Scholar]
- Wang PY, Neretti N, Whitaker R, Hosier S, Chang C, Lu D, Rogina B, Helfand SL. Long-lived Indy and calorie restriction interact to extend life span. Proc Natl Acad Sci U S A. 2009 Jun 9;106(23):9262–9267. doi: 10.1073/pnas.0904115106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott JF, Erway LC, Archer JR, Harrison DE. Genetics of age-related hearing loss in mice. II. Strain differences and effects of caloric restriction on cochlear pathology and evoked response thresholds. Hear. Res. 1995;88:143–155. doi: 10.1016/0378-5955(95)00107-f. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Cataldo L, Coschigamo K, Wagner TE, Baumann G, Kopchick JJ. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc Natl Acad Sci U S A. 1997 Nov 25;94(24):13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]