Abstract
Objective
To determine the impact of environmental exposures (diesel exhaust particle (DEP), environmental tobacco smoke (ETS), and mold) that may contribute to oxidative stress on persistent wheezing in the Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS) birth cohort and to determine how the impact of these exposures is modified by the GST-P1 Ile105Val polymorphism.
Study design
A land-use regression model was used to derive an estimate of each child’s DEP exposure. ETS exposure was determined by questionnaire data. Each child’s home was evaluated for visible mold by a trained professional. Children in the CCAAPS cohort were genotyped for the GST-P1 polymorphism (N=570). Persistent wheezing was defined as wheezing at both 12 and 24 months.
Results
High DEP exposure conferred increased risk for wheezing phenotypes but only among the Val105 allele carriers. Infants with multiple exposures were significantly more likely to persistently wheeze despite their genotype.
Conclusion
There is evidence for an environmental effect of DEP among carriers of the GST-P1 Val105 allele in the development of persistent wheezing in children. The protective effect of the GST-P1 Ile105 genotype may be overwhelmed by multiple environmental exposures that converge on oxidative stress pathways.
Keywords: oxidative stress, gene:environment, diesel, smoking, children, Mold, ROS
The increasingly common occurrence of childhood wheeze and asthma, particularly in affluent westernized society, is well-documented.(1) Environmental factors associated with wheezing in early life include traffic exhaust exposure through diesel exhaust particles (DEP),(2, 3) environmental tobacco smoke exposure (ETS),(4, 5) and mold exposure.(3, 6, 7) The relationship between the glutathione S-transferase P1 (GST-P1) Ile105Val polymorphism and asthma has been reported in several populations, but these studies have not examined the interplay of the combined genetic and environmental factors on longitudinal wheezing status during early childhood.(8, 9)
In humans, the glutathione S-transferase (GST) class of multifunctional enzymes are divided into eight families: Alpha, Kappa, Mu, Omega, Pi, Sigma, Theta, and Zeta.(10, 11) A single gene in the Pi subfamily, GST-P1, is the predominant cytosolic GST expressed in lung epithelium.(12) GST-P1 is a 2.8 kb gene located on chromosome 11q13, a known “hot spot” for asthma-related genes.(13, 14) A single nucleotide polymorphism at position 313 in GST-P1 converts an adenine to a guanine (A→G).(15) The resulting isoleucine to valine substitution in codon 105 of exon 5 (Ile105→Val105) significantly lowers GST enzyme activity.(16)
Delineating the factors that are contributory or protective to persistent wheezing in early childhood is critical to advance our understanding of asthma. There is limited information about how genetic and environmental factors interact to influence longitudinal asthmatic/wheezing status over time. DEP, ETS, and mold exposures are common and each has been shown to aggravate respiratory symptoms. The gene-environment effect related to these individual and/or combined exposures has not been evaluated with regard to longitudinal wheezing status. The purpose of this study was to investigate whether exposure to DEP, ETS, and/or mold uniquely modifies wheezing and persistent wheezing in young children, especially among those with the GST-P1 I105V polymorphism. Our study evaluates the modified effect of this polymorphism upon exposure to not only ETS and mold, but distinctively DEP exposure associated with traffic and their combined exposures utilizing the well-characterized Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS) birth cohort.
METHODS
Study Participants
The CCAAPS study is a longitudinal birth cohort of high-risk children having at least one atopic parent. A complete description of the study’s recruitment, methods, and objectives has been published.(17) Briefly, infants with at least one atopic parent (based on allergy skin prick testing) were enrolled between 2001 and 2003 in a seven county area of Cincinnati, Ohio. Families were recruited based on the proximity of their home residence to truck and bus traffic by geocoding residential addresses located on birth records (Figure 1; available at www.jpeds.com). All infants recruited for the CCAAPS study were carried to term (>35 weeks), and no premature infants were eligible. Parental asthma diagnosis history and shortness of breath symptoms were collected at the time of the parent SPT. Infant subjects were evaluated by skin prick testing with a panel of 15 aeroallergens and two foods (egg white and milk) at both 12 and 24 months of age. Annual questionnaires administered to parents with regards to infant respiratory symptoms were adapted from the International Study of Asthma and Allergies in Children (ISAAC).(18) At the time of recruitment, administered questionnaires also collected information on household smoking habits and demographics. This study was approved by the Institutional Review Board.
Figure 1.
Geographical location of CCAAPS infant’s homes. Infants lived within a seven county area of Cincinnati, Ohio. Families were recruited based on the proximity of their home residence to truck and bus traffic by geocoding residential addresses located on infant birth records.
DNA Collection and GST-P1 Gene Polymorphism Genotyping
Buccal cells were collected using a nylon bristle cytology brush. Genomic DNA was isolated using the Zymo Research Genomic DNA II Kit™ (Orange, CA). Genotyping was accomplished using the LightTyper platform (Roche Diagnostics, GmbH, Mannheim, Germany). The PCR primers (GST-P1 Forward: 5’-TGGACATGGTGAATGACGGCG-3’ and GST-P1 Reverse: 5’-GGTCAGCCCAAGCCACCT-3’) and hybridization probes (5’-LCR640-AGGGAGACGTATTTGCAGCGGAGG-3’ and 5’-ACCCTGGTGCAGATGCTCACATAGTTGGTGTAGA-FL-3’) were designed using the LightCycler Probe Design Software 2.0 (Roche Diagnostics, GmbH, Mannheim, Germany). Genotypes were confirmed by randomly re-genotyping 10% of the population. Genotypes were dichotomized to carriers and non-carriers of the Val105 allele.
Outcome Definitions
Parents were asked the following ISAAC adapted question: “In the past 12 months, have you ever noticed your child wheezing?” Infant wheezing at ages 12 and 24 months was defined as parental report of the child wheezing at the respective study visit one or more times in the past 12 months. Persistent wheezing was defined as parental report of the child wheezing at both the 12- and 24-month visits.
Environmental Exposure Definitions
The environmental exposures evaluated were DEP, ETS, and visible mold. Average daily levels of DEP at each infant’s home were calculated using a land-use regression (LUR) model of exposure as previously described.(2) Briefly, ambient levels of fine particulate matter with aerodynamic diameter <2.5 µm (PM2.5) were measured at 24 sampling sites located throughout the greater Cincinnati, Ohio metropolitan area. The PM2.5 chemical composition has been previously described.(19) Elemental carbon was measured at these different monitoring sites and estimated source signatures in the airshed were determined to determine how much elemental carbon was attributable to traffic alone. This estimate was used to estimate truck and bus DEP exposure as previously reported.(20) Geographic, traffic, and land-use data within 400 meters (m) of each sampling site was collected in a geographic information system (GIS). From these data a LUR model with a coefficient of determination (R2) of 0.75 was developed that included elevation, number of trucks within 400 m of the sampling site, and the length of bus routes within 100 m of the sampling site. The estimated model parameters were subsequently applied to the same geographic variables determined for each infant’s home residence at the time of study enrollment when they were approximately seven months of age. This estimate was used to obtain unique estimates of their early life exposure to DEP. The median exposure to DEP was estimated to be 0.34 µg/m3 (range=0.23–0.88). The level of 0.5µg/m3 was chosen to determine high vs. low exposure based upon the distribution of estimated DEP and prior results indicating an approximate 2-fold increased risk for wheezing at 12 months at this exposure level, and this level represented the top quintile.(2) The LUR model was further evaluated deriving a LUR model with 6 sampling sites removed. The estimated DEP was subsequently compared with the sampled DEP and was generally found to slightly underpredict the sampled values (manuscript currently in review).
Infants were defined as exposed to household ETS if the parent reported at least one smoker (person that smoked one or more cigarettes per day) living in the infant’s home. Infants were defined as exposed to mold if an in-home trained professional observed any visible mold, water damage, or moldy odor at the time of the home evaluation, generally before the infant’s first birthday.(21) Infants in homes that did not meet any of these criteria were considered unexposed to visible mold. Multivariate models were adjusted for race (Caucasian vs non-Caucasian) and sex. In analyses evaluating wheezing at 12 and 24 months, daycare attendance was also defined at 12 and 24 months.
Statistical Analysis
Racial differences for demographics, environmental exposures, health outcomes, and genotype and allele frequencies were compared using the chi-squared statistic. Unadjusted odds ratios (OR) and 95% Confidence Intervals (95% CI) were calculated using logistic regression to evaluate the univariate associations between outcomes, environmental exposures, and genotype. Three-way contingency tables were used to assess associations of environmental exposures, genotype, and health outcomes stratified by both genotype and exposure. Due to the significant difference in allele frequency between racial groups, a race-stratified analysis was also performed for Caucasians and non-Caucasians. The associations of DEP, ETS, and visible mold exposures and genotype with each outcome were evaluated using conditional logistic regression adjusting for daycare attendance, race, and sex. Since parent education, income, and health insurance were all highly correlated with race, we chose to adjust only for race. Race, however, was not found to be a significant covariate in adjusted models evaluating the independent associations of DEP (p=0.46), ETS (p=0.46), and mold (p=0.44) with wheezing at 12 months of age. Figure 2, therefore, present associations for racial groups combined between environmental exposures and GST-P1 genotype. All possible gene-environment interactions to evaluate the effect modification of DEP, ETS, and mold exposure by genotype were evaluated in each model. An interaction was removed from the model if the p-value was greater than 0.20. All analysis was performed using SAS software (version 8.2 for Windows, SAS Institute, Cary, NC, USA).
Figure 2.
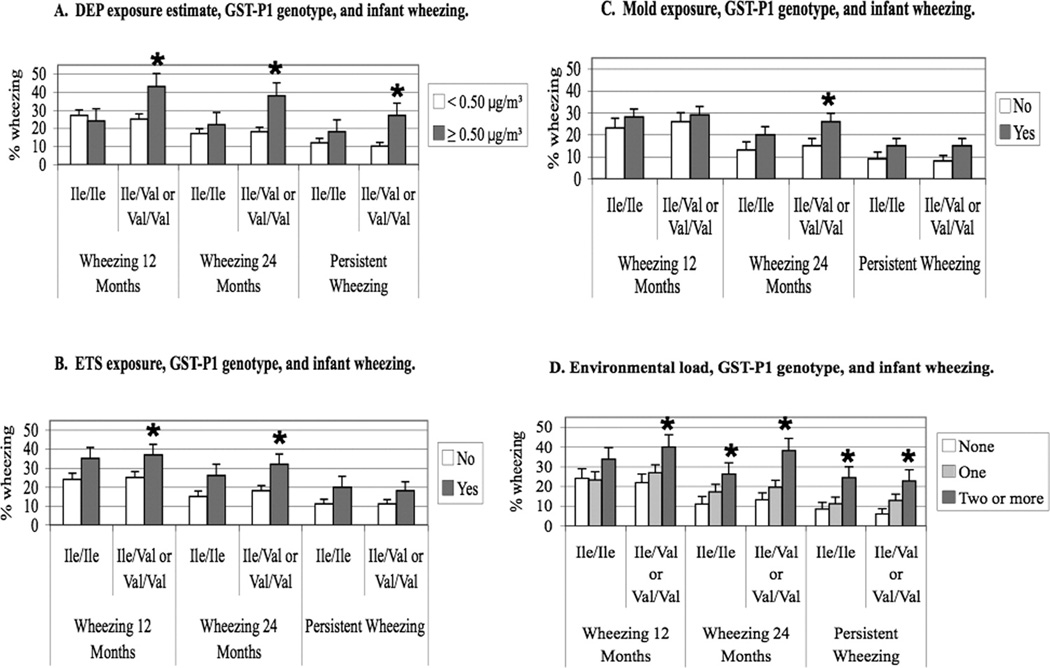
Individual environmental exposures, GST-P1 genotype, and infant wheezing. A. High DEP estimate exposure levels were significantly associated with wheezing at 12 months, 24 months, and with persistent wheezing only in infants carrying the Val105 allele. B. ETS exposure was significantly associated with wheezing at 12 and 24 months only in infants carrying the Val105 allele. C. Mold exposure was significantly associated with wheezing at 24 months in infants carrying the Val105 allele. D. Environmental load, GST-P1 genotype, and infant wheezing. At 12 months, only infants carrying the Val105 allele and who were exposed to two or more exposures were significantly likely to wheeze. At 24 months and with persistent wheezing, all infants despite their genotype were significantly likely to wheeze when exposed to two or more exposures. * = p-value<0.05
RESULTS
Subjects, exposures, and health outcomes
Of the 570 study participants, 464 (81.4%) infants were Caucasian and 106 (18.6%) were non-Caucasian (Table I). Of the non-Caucasian infants, 86.8% were African Americans defined as both parents being African American. Non-Caucasian infants were significantly more likely than Caucasians to have a household income less than $40,000 (69.5% versus 26.7%; p<0.001), have higher exposure to DEP ≥0.50 µg/m3 (p<0.001), and have visible mold in their homes (p=0.01). ETS exposure did not significantly differ between the racial groups. Wheezing at 24 months of age (34.6% versus 17.2%) and persistent wheezing (24.6% versus 10.9%) were significantly increased in non-Caucasian versus Caucasian infants.
Table I.
Demographics, Exposures, and Health Outcomes of Infants.
| Total | Caucasian | Non-Caucasian | p-value | |
|---|---|---|---|---|
| Gender | ||||
| Male | 298 (52.3) | 244 (52.6) | 54 (50.1) | 0.76 |
| Female | 272 (47.7) | 220 (47.4) | 52 (49.1) | |
| Family Income | ||||
| < $40,000 | 196 (34.7) | 123 (26.7) | 73 (69.5) | <0.001 |
| ≥ $40,000 | 369 (65.3) | 337 (73.3) | 32 (30.5) | |
| Daycare Attendance at 12 months | ||||
| Yes | 43 (7.6) | 35 (7.6) | 8 (7.7) | 0.96 |
| No | 524 (92.4) | 428 (92.4) | 96 (92.3) | |
| Daycare Attendance at 24 months | ||||
| Yes | 70 (12.4) | 54 (11.7) | 16 (15.4) | 0.30 |
| No | 497 (87.6) | 409 (88.3) | 88 (84.6) | |
| DEP Estimate (binary) | ||||
| < 0.50 µg/m3 | 469 (82.3) | 396 (85.3) | 73 (68.9) | <0.001 |
| ≥ 0.50 µg/m3 | 101 (17.7) | 68 (14.7) | 33 (31.1) | |
| ETS Exposure | ||||
| Yes | 158 (28.5) | 121 (27.0) | 37 (34.9) | 0.11 |
| No | 396 (71.5) | 327 (73.0) | 69 (65.1) | |
| Mold Exposure | ||||
| Yes | 297 (56.5) | 258 (59.2) | 39 (43.3) | 0.01 |
| No | 229 (43.5) | 178 (40.8) | 51 (56.7) | |
| Environmental Load* | ||||
| None | 178 (32.2) | 144 (31.0) | 34 (32.1) | 0.09 |
| One | 246 (43.2) | 209 (45.1) | 37 (34.9) | |
| Two or more | 146 (25.6) | 111 (23.9) | 35 (33.0) | |
| Wheezing at 12 months | ||||
| Yes | 145 (27.5) | 116 (26.6) | 29 (31.5) | 0.34 |
| No | 383 (72.5) | 320 (73.4) | 63 (68.5) | |
| Wheezing at 24 months | ||||
| Yes | 99 (19.9) | 72 (17.2) | 27 (34.6) | <0.001 |
| No | 399 (80.2) | 348 (82.8) | 51 (65.4) | |
| Persistent Wheezing | ||||
| Yes | 60 (12.9) | 43 (10.9) | 17 (24.6) | <0.001 |
| No | 404 (87.1) | 352 (89.1) | 52 (75.4) | |
| Total n† | 570 | 464 | 106 | |
DEP estimate ≥ 0.50 µg/m3, ETS exposure, and/or mold exposure
The total n is different from the n for each individual demographic because not all data was available for each subject.
Effect of exposure on wheezing phenotypes
Exposure to DEP ≥0.50 µg/m3 increased the risk of wheezing at 24 months (OR=2.15, 95% CI=1.24–3.55) and persistent wheezing (OR=2.41, 95% CI=2.29–4.51) (data not shown). Similarly, ETS exposure was associated with wheezing at 12 months (OR=1.73, 95% CI=1.15–2.62), 24 months (OR=2.15, 95% CI=1.34–3.44), and persistent wheezing (OR=1.80, 95% CI=1.02–3.20). Mold exposure was significantly associated with infant wheezing at 24 months (OR=1.76, 95%CI=1.09–2.85) and persistent wheezing (OR=2.00, 95% CI=1.07–3.71). A comparison of the wheezing percentage among all infants versus those stratified by race revealed similar trends.
GST-P1 alleles in study subjects
The GST-P1 allele frequencies were significantly different (p=0.002) among Caucasians (Ile=67.2%, Val=32.8%) and non-Caucasians (Ile=56.1%, Val=43.9%). Similar allelic differences were noted in other studies.(16) Overall, 81.4% of the children were homozygous for the GST-P1 Ile105 allele, and 18.6% were carriers of the Val105 allele. When children carrying at least one Val105 allele (Ile/Val or Val/Val) were combined and compared with those homozygous for the Ile105 allele, the non-Caucasian children were significantly more likely than Caucasians to be carriers of the Val105 allele (21.9% versus 14.7%; p=0.02). GST-P1 genotype data were in Hardy-Weinberg equilibrium when stratified by race and sex.
GST-P1 and environmental exposures
After stratifying by genotype, high DEP exposure (≥0.50 µg/m3) was associated with an increased risk for wheezing among carriers of the Val105 allele (Figure 2A). Again, racial groups were combined because race was not found to be a significant covariate in evaluating the independent associations of each environmental exposure with wheezing at 12 months of age. The Ile/Ile genotype conferred a protective effect against wheezing when infants were exposed to high amounts of DEP. This finding was consistent at 12 months (p=0.01), 24 months (p=0.002), and with persistent wheezing (p=0.003) (Figure 2A). Similarly, carriers of the Val105 allele were significantly more likely to wheeze at 12 and 24 months if they were exposed to ETS (p=0.05 and p=0.01, respectively) compared with those infants with the protective Ile/Ile genotype (Figure 2B). Carriers of the Val105 allele exposed to visible mold were also significantly more likely to wheeze at 24 months of age (p=0.04) (Figure 2C).
The effect of each exposure (DEP, ETS, and visible mold) was independently evaluated with respect to wheezing at 12 months, 24 months, and persistent wheezing after adjusting for genotype, daycare attendance, race, and sex (Table II). High DEP exposure (≥0.50 µg/m3) conferred a significant risk for persistent wheezing (OR=2.13, 95% CI 1.11–4.07). There was a trend noted between high DEP exposure (≥0.50 µg/m3) and GST-P1 genotype on wheezing (DEP-GST-P1 interaction, p=0.08; Table IIA). Similar interactions between ETS and visible mold exposures with the GST-P1 Ile/Val or Val/Val genotypes were not observed and were subsequently removed from the models (Table IIB and C). ETS exposure was significantly associated with wheezing at both 12 (OR=1.78, 95% CI=1.17–2.71) and 24 months of age (OR=2.06, 95% CI=1.27–3.35), and an elevated risk was observed for persistent wheezing (Table IIB). Visible mold exposure significantly increased the risk of wheeze at 24 months (OR=2.18, 95% CI=1.30–3.63) and persistent wheezing (OR=2.57, 95% CI=1.33–4.96) (Table IIC). Thus, all three exposures were significant in a univariate model.
Table II.
Adjusted Odds Ratios (OR) for Infant Wheezing, Environmental Exposures, and GST-P1 Genotype.
| A. DEP Estimate Exposure | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Wheezing at 12 Months | Wheezing at 24 Months | Persistent Wheezing | |||||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| DEP Estimate ≥ 0.50 µg/m3 | 0.89 | 0.41–1.96 | 0.78 | 1.16 | 0.47–2.82 | 0.75 | 2.13 | 1.11–4.07 | 0.02 |
| Ile/Val or Val/Val Gentoype | 0.94 | 0.61–1.44 | 0.78 | 1.03 | 0.61–1.72 | 0.93 | 0.92 | 0.52–1.61 | 0.76 |
| Daycare Attendance* | 1.48 | 0.76–2.88 | 0.25 | 1.86 | 1.03–3.38 | 0.04 | 1.13 | 0.52–2.48 | 0.76 |
| Non-Caucasian Race | 1.21 | 0.73–2.00 | 0.46 | 2.29 | 1.31–3.99 | <0.01 | 2.57 | 1.33–4.98 | 0.01 |
| Male Gender | 1.41 | 0.96–2.09 | 0.08 | 1.61 | 1.02–2.55 | 0.04 | 1.90 | 1.07–3.38 | 0.03 |
| DEP*Ile/Val or Val/Val Genotype | 2.52 | 0.91–7.01 | 0.08 | 2.23 | 0.72–6.92 | 0.16 | ns | ||
| B. ETS Exposure | |||||||||
| Wheezing at 12 Months | Wheezing at 24 Months | Persistent Wheezing | |||||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| ETS Exposure | 1.78 | 1.17–2.71 | 0.01 | 2.06 | 1.27–3.35 | <0.01 | 1.70 | 0.94–3.07 | 0.08 |
| Ile/Val or Val/Val Gentoype | 1.09 | 0.73–1.61 | 0.68 | 1.21 | 0.75–1.93 | 0.43 | 0.91 | 0.52–1.59 | 0.73 |
| Daycare Attendance* | 1.50 | 0.77–2.93 | 0.23 | 1.72 | 0.95–3.12 | 0.08 | 1.06 | 0.48–2.31 | 0.89 |
| Non-Caucasian Race | 1.21 | 0.73–1.99 | 0.46 | 2.36 | 1.36–4.10 | <0.01 | 2.60 | 1.35–5.02 | <0.01 |
| Male Gender | 1.50 | 1.01–2.23 | 0.05 | 1.73 | 1.08–2.77 | 0.02 | 1.98 | 1.11–3.52 | 0.02 |
| ETS*Ile/Val or Val/Val Genotype | ns | ns | ns | ||||||
| C. Mold Exposure | |||||||||
| Wheezing at 12 Months | Wheezing at 24 Months | Persistent Wheezing | |||||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Mold Exposure | 1.31 | 0.87–1.97 | 0.20 | 2.18 | 1.30–3.63 | <0.01 | 2.57 | 1.33–4.96 | 0.01 |
| Ile/Val or Val/Val Gentoype | 1.09 | 0.73–1.64 | 0.67 | 1.25 | 0.77–2.03 | 0.38 | 0.95 | 0.52–1.73 | 0.87 |
| Daycare Attendance* | 1.68 | 0.82–3.46 | 0.16 | 1.75 | 0.93–3.32 | 0.08 | 1.27 | 0.55–2.93 | 0.57 |
| Non-Caucasian Race | 1.37 | 0.81–2.34 | 0.24 | 3.06 | 1.68–5.60 | <0.01 | 3.64 | 1.75–7.59 | <0.01 |
| Male Gender | 1.34 | 0.89–2.01 | 0.16 | 1.74 | 1.07–2.82 | 0.02 | 2.00 | 1.08–3.69 | 0.03 |
| Mold*Ile/Val or Val/Val Genotype | ns | ns | ns | ||||||
| D. Environmental Load | |||||||||
| Wheezing at 12 Months | Wheezing at 24 Months | Persistent Wheezing | |||||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Environmental Load† | |||||||||
| None | ref | ref | ref | ||||||
| One | 1.21 | 0.75–1.95 | 0.43 | 1.82 | 1.00–3.32 | 0.05 | 2.05 | 0.96–4.41 | 0.06 |
| Two or more | 2.07 | 1.24–3.46 | 0.01 | 3.57 | 1.92–6.64 | <.01 | 3.86 | 1.78–8.37 | <0.01 |
| Ile/Val or Val/Val Gentoype | 1.15 | 0.78–1.69 | 0.50 | 1.30 | 0.81–2.08 | 0.28 | 0.96 | 0.55–1.70 | 0.89 |
| Daycare Attendance* | 1.52 | 0.78–2.97 | 0.22 | 1.78 | 0.98–3.24 | 0.06 | 1.13 | 0.51–2.50 | 0.75 |
| Non-Caucasian Race | 1.22 | 0.74–2.00 | 0.44 | 2.46 | 1.40–4.30 | <0.01 | 2.83 | 1.45–5.52 | <0.01 |
| Male Gender | 1.43 | 0.96–2.12 | 0.08 | 1.73 | 1.08–2.76 | 0.02 | 2.05 | 1.14–3.68 | 0.02 |
| Total Load*Ile/Val or Val/Val Geno | ns | ns | ns | ||||||
Daycare attendance at 12 months for 12 month wheezing; attendance at 24 months for 24 month and persistent wheezing.
ns: not significant at the 0.20 level and therefore removed from model (interaction only).
ETS exposure, DEP estimate ≥ 0.50 µg/m3 and/or mold exposure.
ref: referent category.
Multiple exposures associated with wheezing
We next adjusted for all three exposures simultaneously as well as genotype, daycare attendance, race, and sex (Table III). In this model, high DEP exposure (≥0.50 µg/m3) was associated with wheezing at 24 months (OR=1.93, 95% CI=1.06–3.53) and with persistent wheezing (OR=2.13, 95% CI=1.03–4.41). Exposure to ETS was significantly associated with wheezing at 12 (OR=1.73, 95% CI=1.11–2.70) and 24 months (OR=1.90, 95% CI=1.13–3.18). Similarly, visible mold exposure was associated with wheezing at 24 months (OR=2.12, 95% CI=1.25–3.60) and persistent wheezing (OR=2.47, 95% CI=1.27–4.80) (Table III).
Table III.
Adjusted Associations Estimated as Odds Ratios (OR) of Infant Wheezing with Demographic and Environmental Factors.
| Wheezing at 12 Months | Wheezing at 24 Months | Persistent Wheezing | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Male Gender | 1.47 | 0.97–2.23 | 0.07 | 1.92 | 1.16–3.17 | 0.01 | 2.13 | 1.15–3.97 | 0.02 |
| Non-Caucasian Race | 1.20 | 0.69–2.07 | 0.52 | 2.54 | 1.36–4.75 | 0.00 | 2.91 | 1.36–6.21 | 0.01 |
| Daycare Attendance* | 1.88 | 0.91–3.91 | 0.09 | 1.81 | 0.95–3.47 | 0.07 | 1.36 | 0.66–2.79 | 0.41 |
| DEP Estimate Exposure ≥ 0.50 µg/m3 | 0.92 | 0.40–2.14 | 0.85 | 1.93 | 1.06–3.53 | 0.03 | 2.13 | 1.03–4.41 | 0.04 |
| ETS Exposure | 1.73 | 1.11–2.70 | 0.02 | 1.90 | 1.13–3.18 | 0.02 | 1.49 | 0.76–2.81 | 0.22 |
| Mold Exposure | 1.22 | 0.79–1.86 | 0.37 | 2.12 | 1.25–3.60 | 0.01 | 2.47 | 1.27–4.80 | 0.01 |
| Ile/Val or Val/Val GST-P1 Genotype | 0.94 | 0.59–1.47 | 0.77 | 1.25 | 0.76–2.07 | 0.38 | 0.94 | 0.52–1.73 | 0.85 |
| Ile/Val or Val/Val GST-P1*DEP Estimate ≥ 0.50µg/m3 | 2.09 | 0.69–6.30 | 0.19 | ns | ns | ||||
Defined as daycare attendance during the first year of life for wheezing at age 12 months, attendance during the second year of life for wheezing at 24 months of age and lifetime attendance for persistent wheezing.
ns: not significant at the 0.20 level and therefore removed from model (interaction only).
ETS, mold and/or high DEP
ref: referent category
Environmental exposure load overwhelms genotype effect
In order to evaluate the relationship of total environmental “load” with wheezing, the additive or synergistic effect of having none, one, or two or more environmental exposures (DEP, ETS, or mold) was investigated. One-third (32.2%) of CCAAPS infants were not exposed to any of the three environmental exposures whereas 43.2% were exposed to one pollutant and 25.6% were exposed to two or more. Carriers of the Val105 allele were found to be at risk for wheezing if they were exposed to two or more pollutants compared with unexposed infants at 12 months (p=0.02), 24 months (p<0.01), and with persistent wheezing (p<0.01). In addition, infants homozygous for the Ile105 allele exposed to two or more air pollutants were at significantly increased risk for wheezing compared with those who were not exposed at 24 months (p=0.03) and with persistent wheezing (p=0.01) (Figure 2D). The protective effect of the Ile/Ile genotype against wheezing previously observed with each of the individual exposures alone disappears when infants have multiple exposures. This trend is most evident with DEP exposure alone, but this same trend can be seen when infants are exposed to any one exposure (Figure 2). Environmental load was not significantly different between races (Table I). Clearly, long-term exposure to more pollutants places the infant at greater risk (almost 4 fold) of persistent wheezing irrespective GST-P1 genotype (Table IID).
DISCUSSION
To our knowledge, this study is the first to investigate the impact of complex environmental exposures (DEP, ETS, and mold) along with genetics, specifically GST-P1 on persistent wheezing in children. Our data supports that DEP, ETS, and/or mold exposure are risk factors for wheezing by 24 months of age. Furthermore, the presence of the Val105 allele, which has been shown to significantly lower GST enzyme activity,(16) confers susceptibility to these environmental exposures compared with the Ile105 allele. The Ile/Ile GST-P1 genotype conferred protection against wheezing among the DEP exposed group, however, infants exposed to multiple environmental exposures were significantly more likely to persistently wheeze irrespective of genotype. Thus, the Ile105 GST-P1 genotype may confer protection from persistent wheezing, but strong environmental exposure converging on a similar pathway may overwhelm the genetic effect.
Other investigations have reported associations between asthma and the GST-P1 polymorphism.(8, 22, 23) The Val105 allele was shown to have a protective effect in children age 8 to 11 years against respiratory illness (23), and in adults age 20 to 34 years exposed to DEP and secondhand smoke against increased nasal allergic responses.(22) Contrary to these studies, our study examined infancy and early childhood, a period of time in which the lung undergoes critical development and asthma symptoms are just beginning to develop. Early childhood wheezing and early persistent wheezing may be a precursor to asthma in these young children. One limitation of this study is that it is difficult to definitively determine the cause of wheezing in this young age group. The use of the well-studied ISAAC-adapted questions was used to characterize wheezing as a precursor to asthma.
One mechanism by which environmental exposures may lead to lung injury is by inducing inflammatory cells to generate reactive oxygen species (ROS) leading to oxidative injury.(6, 24, 25) DEP, ETS, and mold are three common environmental exposures that lead to increased generation of ROS and have been shown to cause respiratory symptoms.(2, 4, 21) DEP are respirable, with over 90% in the fine (0.1–2.5 µm) or ultrafine (<0.1 µm) size range. The DEP are composed of elemental carbon cores with large surface areas capable of binding organic polycyclic aromatic hydrocarbons and transition metals, which have the potential to induce ROS.(6) Cigarette smoke has also been shown to contain a high concentration of ROS.(5) Studies have demonstrated an association between ETS exposure during early childhood with the subsequent development of asthma.(26) Mold exposure has also been associated with increased intracellular levels of ROS(27) and respiratory illness in children.(28) Though the mechanism by which DEP, ETS, and mold may contribute to the development of asthma and asthma symptoms is unknown, there is mounting evidence implicating oxidative stress as a contributor to the airway inflammatory response.(29, 30) GSTs can neutralize the electrophilic sites of reactive oxygen species (ROS) by conjugation to the tripeptide thiol, glutathione (GSH), which has an electron-donating capacity. The resulting product is more water-soluble promoting ROS detoxification and thereby protecting the lung from oxidative damage.(31) This may be one mechanism for the observed genetic effect of GST-P1 in this study. We cannot rule out that the observed association between GST-P1 and wheezing is due to a linked polymorphism in the same gene and/or another gene. Another limitation of the study is the relatively small sample size of non-Caucasian children in the cohort (N=106). Race was not found to be a significant covariate in multivariate models evaluating independent associations of the three environmental exposures with wheezing at 12 months resulting in the combining of the racial groups. Although ideally it would be beneficial to stratify by race, the power of the study would have been severely compromised, particularly for African-Americans.
An important strength of this study is the longitudinal birth cohort design. In particular, DEP estimate exposure using multiple monitoring sites and a LUR model is unique to the CCAAPS cohort. Given the importance of early-life exposures, DEP, ETS, and mold exposure were determined through age two. Although we did collect data regarding treatment in the wheezing infants, we did not have complete data on all the children. In-utero smoke exposure was an independent risk factor for wheezing at 12 and 24 months but not persistent wheezing in only the Caucasian infants. There was no association among the non-Caucasian infants. We recognize that one limitation of the exposure assessment is that infants are not only exposed in the home. In order to address this, we adjusted for daycare attendance (also used as a proxy for exposure to respiratory infections). Future analyses will consider cumulative exposures and effect on asthma development as children age.
Overall, the relationship between DEP and wheezing was found to be stronger at 24 months of age than 12 months suggesting that longer exposure results in an elevated risk of wheezing. Interestingly, ETS exposure at 12 months of age was associated with wheezing at both 12 and 24 months of age suggesting that a shorter exposure time is needed to see an effect in infants. Since infants’ lungs are still developing, household ETS exposure is generally much closer to the child’s personal living space than is exposure to traffic exhaust and therefore highly likely to have a stronger impact during this sensitive stage of development. This reflects the findings of a recent study that reported exposure to parental smoking during the first year of life is associated with persistent wheezing.(32)
In conclusion, these data suggest that the GST-P1 genotype should be considered when evaluating asthma/wheezing in young children exposed to high DEP levels, ETS, and/or visible mold. These data provide evidence that carriers of the Val105 allele may be more susceptible to high DEP exposure with regard to the development of persistent wheezing. High environmental load converging on an oxidative stress pathway may overwhelm the genetic effect.
Acknowledgments
This work was supported by NIEHS R01 ES11170 and ES10957.
Abbreviations
- CCAAPS
Cincinnati Childhood Allergy and Air Pollution Study
- DEP
Diesel exhaust particle
- ETS
Environmental tobacco smoke
- ROS
Reactive oxygen species
- SPT
Skin prick test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors[H1] declare no conflicts of interest.
REFERENCES
- 1.Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet. 1998;351:1225–1232. [PubMed] [Google Scholar]
- 2.Ryan PH, LeMasters GK, Biswas P, Levin L, Hu S, Lindsey M, et al. A comparison of proximity and land use regression traffic exposure models and wheezing in infants. Environ Health Perspect. 2007;115:278–284. doi: 10.1289/ehp.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Systematic review of health aspects of air pollution in Europe. 2004
- 4.Biagini JM, LeMasters GK, Ryan PH, Levin L, Reponen T, Bernstein DI, et al. Environmental risk factors of rhinitis in early infancy. Pediatr Allergy Immunol. 2006;17:278–284. doi: 10.1111/j.1399-3038.2006.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang MF, Lin WL, Ma YC. A study of reactive oxygen species in mainstream of cigarette. Indoor Air. 2005;15:135–140. doi: 10.1111/j.1600-0668.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 6.Riedl M, Diaz-Sanchez D. Biology of diesel exhaust effects on respiratory function. J Allergy Clin Immunol. 2005;115:221–228. doi: 10.1016/j.jaci.2004.11.047. quiz 9. [DOI] [PubMed] [Google Scholar]
- 7.Iossifova YY, Reponen T, Bernstein DI, Levin L, Kalra H, Campo P, et al. House dust (1–3)-beta-D-glucan and wheezing in infants. Allergy. 2007;62:504–513. doi: 10.1111/j.1398-9995.2007.01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilliland FD, Gauderman WJ, Vora H, Rappaport E, Dubeau L. Effects of glutathione-S-transferase M1, T1, and P1 on childhood lung function growth. Am J Respir Crit Care Med. 2002;166:710–716. doi: 10.1164/rccm.2112065. [DOI] [PubMed] [Google Scholar]
- 9.Fryer AA, Bianco A, Hepple M, Jones PW, Strange RC, Spiteri MA. Polymorphism at the glutathione S-transferase GSTP1 locus. A new marker for bronchial hyperresponsiveness and asthma. Am J Respir Crit Care Med. 2000;161:1437–1442. doi: 10.1164/ajrccm.161.5.9903006. [DOI] [PubMed] [Google Scholar]
- 10.Mannervik B, Awasthi YC, Board PG, Hayes JD, Di Ilio C, Ketterer B, et al. Nomenclature for human glutathione transferases. Biochem J. 1992;282(Pt 1):305–306. doi: 10.1042/bj2820305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pemble SE, Taylor JB. An evolutionary perspective on glutathione transferases inferred from class-theta glutathione transferase cDNA sequences. Biochem J. 1992;287(Pt 3):957–963. doi: 10.1042/bj2870957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fryer AA, Hume R, Strange RC. The development of glutathione S-transferase and glutathione peroxidase activities in human lung. Biochim Biophys Acta. 1986;883:448–453. doi: 10.1016/0304-4165(86)90283-7. [DOI] [PubMed] [Google Scholar]
- 13.Kano T, Sakai M, Muramatsu M. Structure and expression of a human class pi glutathione S-transferase messenger RNA. Cancer Res. 1987;47:5626–5630. [PubMed] [Google Scholar]
- 14.Doull IJ, Lawrence S, Watson M, Begishvili T, Beasley RW, Lampe F, et al. Allelic association of gene markers on chromosomes 5q and 11q with atopy and bronchial hyperresponsiveness. Am J Respir Crit Care Med. 1996;153:1280–1284. doi: 10.1164/ajrccm.153.4.8616554. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad H, Wilson DE, Fritz RR, Singh SV, Medh RD, Nagle GT, et al. Primary and secondary structural analyses of glutathione S-transferase pi from human placenta. Arch Biochem Biophys. 1990;278:398–408. doi: 10.1016/0003-9861(90)90277-6. [DOI] [PubMed] [Google Scholar]
- 16.Watson MA, Stewart RK, Smith GB, Massey TE, Bell DA. Human glutathione S-transferase P1 polymorphisms: relationship to lung tissue enzyme activity and population frequency distribution. Carcinogenesis. 1998;19:275–280. doi: 10.1093/carcin/19.2.275. [DOI] [PubMed] [Google Scholar]
- 17.LeMasters GK, Wilson K, Levin L, Biagini J, Ryan P, Lockey JE, et al. High prevalence of aeroallergen sensitization among infants of atopic parents. J Pediatr. 2006;149:505–511. doi: 10.1016/j.jpeds.2006.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–491. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 19.Martuzevicius D, Grinshpun SA, Reponen T, Gorny RL, Shukla R, Lockey J, et al. Spatial and temporal variations of PM2.5 concentration and composition throughout an urban area with high freeway density-the Greater Cincinnati study. Atmospheric Environment. 2004;38:1091–1105. [Google Scholar]
- 20.Hu S, McDonald R, Martuzevicius D, Biswas P, Turner J, Kelly A, et al. UNMIX calculations of ambient PM2.5 near an interstate highway in the greater Cincinnati area. Atmospheric Environment. 2005;40:378–395. doi: 10.1016/j.atmosenv.2006.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho SH, Reponen T, LeMasters G, Levin L, Huang J, Meklin T, et al. Mold damage in homes and wheezing in infants. Ann Allergy Asthma Immunol. 2006;97:539–545. doi: 10.1016/S1081-1206(10)60947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilliland FD, Li YF, Gong H, Jr, Diaz-Sanchez D. Glutathione-S-Transferase M1 and P1 Prevent Aggravation of Allergic Responses by Secondhand Smoke. Am J Respir Crit Care Med. 2006 doi: 10.1164/rccm.200509-1424OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilliland FD, Rappaport EB, Berhane K, Islam T, Dubeau L, Gauderman WJ, et al. Effects of glutathione S-transferase P1, M1, and T1 on acute respiratory illness in school children. Am J Respir Crit Care Med. 2002;166:346–351. doi: 10.1164/rccm.2111048. [DOI] [PubMed] [Google Scholar]
- 24.Vachier I, Damon M, Le Doucen C, de Paulet AC, Chanez P, Michel FB, et al. Increased oxygen species generation in blood monocytes of asthmatic patients. Am Rev Respir Dis. 1992;146:1161–1166. doi: 10.1164/ajrccm/146.5_Pt_1.1161. [DOI] [PubMed] [Google Scholar]
- 25.Owen S, Pearson D, Suarez-Mendez V, O'Driscoll R, Woodcock A. Evidence of free-radical activity in asthma. N Engl J Med. 1991;325:586–587. doi: 10.1056/NEJM199108223250816. [DOI] [PubMed] [Google Scholar]
- 26.von Mutius E. Environmental factors influencing the development and progression of pediatric asthma. J Allergy Clin Immunol. 2002;109:S525–S532. doi: 10.1067/mai.2002.124565. [DOI] [PubMed] [Google Scholar]
- 27.Jussila J, Komulainen H, Huttunen K, Roponen M, Halinen A, Hyvarinen A, et al. Inflammatory responses in mice after intratracheal instillation of spores of Streptomyces californicus isolated from indoor air of a moldy building. Toxicol Appl Pharmacol. 2001;171:61–69. doi: 10.1006/taap.2000.9116. [DOI] [PubMed] [Google Scholar]
- 28.Jaakkola JJ, Jaakkola N, Ruotsalainen R. Home dampness and molds as determinants of respiratory symptoms and asthma in pre-school children. J Expo Anal Environ Epidemiol. 1993;3 Suppl 1:129–142. [PubMed] [Google Scholar]
- 29.Nel AE, Diaz-Sanchez D, Li N. The role of particulate pollutants in pulmonary inflammation and asthma: evidence for the involvement of organic chemicals and oxidative stress. Curr Opin Pulm Med. 2001;7:20–26. doi: 10.1097/00063198-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Leem JH, Kim JH, Lee KH, Hong Y, Lee KH, Kang D, et al. Asthma attack associated with oxidative stress by exposure to ETS and PAH. J Asthma. 2005;42:463–467. doi: 10.1080/02770900500200733. [DOI] [PubMed] [Google Scholar]
- 31.Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 32.De Sario M, Di Domenicantonio R, Corbo G, Forastiere F, Pistelli R, Rusconi F, et al. Characteristics of early transient, persistent, and late onset wheezers at 9 to 11 years of age. J Asthma. 2006;43:633–638. doi: 10.1080/02770900600878974. [DOI] [PubMed] [Google Scholar]