Abstract
To investigate the potential efficacy of calcium and vitamin D in reducing risk for colorectal neoplasms and to develop ‘treatable’ phenotypic biomarkers of risk for colorectal neoplasms, we conducted a pilot, randomized, double-blind, placebo-controlled, 2×2 factorial clinical trial to test the effects of these agents on cell cycle markers in the normal colorectal mucosa.
Ninety-two men and women with at least one pathology-confirmed colorectal adenoma were treated with calcium 2 g/day and/or vitamin D3 800 IU/day vs. placebo over six months. Overall expression and distributions of p21waf1/cip1 (marker of differentiation), MIB-1 (marker of short-term proliferation), and hTERT (marker of long-term proliferation) in colorectal crypts in the normal-appearing rectal mucosa were detected by automated immunohistochemistry and quantified by image analysis.
In the calcium, vitamin D, and calcium plus vitamin D groups relative to the placebo, p21 expression increased by 201% (P=0.03), 242% (P=0.005), and 25% (P=0.47), respectively, along the full lengths of colorectal crypts after six months of treatment. There were no statistically significant changes in the expression of either MIB-1 or hTERT in the crypts overall; however, the proportion of hTERT, but not MIB-1, expression that extended into the upper 40% of the crypts was reduced by 15% (P=0.02) in the vitamin D plus calcium group relative to the placebo.
These results indicate that calcium and vitamin D promote colorectal epithelial cell differentiation and may “normalize” the colorectal crypt proliferative zone in sporadic adenoma patients, and support further investigation of calcium and vitamin D as chemopreventive agents against colorectal neoplasms.
Keywords: vitamin D, calcium, colonic neoplasms, cell differentiation, cell proliferation, clinical trial, normal colorectal mucosa, biomarkers
INTRODUCTION
Colorectal cancer is the second leading cause of cancer death in the United States (1). Despite advances in treatment, screening, and prevention, colorectal cancer mortality has declined only modestly in recent years, indicating the need for treatable preneoplastic biomarkers of risk for colorectal neoplasms in humans that could be used for monitoring the efficacy of preventive interventions and for developing chemopreventive agents against colorectal cancer.
Calcium and vitamin D are two plausible and evidentially well supported dietary potential anti-neoplastic agents. Proposed, likely complementary, mechanisms of calcium against colorectal cancer include protection of colonocytes against bile acids and fatty acids (2), direct effects on cell proliferation, and modulation of the APC colon carcinogenesis pathway (3). Proposed, likely complementary, mechanisms for vitamin D include effects on regulating cell cycle events; promoting bile acid degradation; influencing growth factor signaling, cell adhesion, inflammation, and immune function; and modulating more than 200 responsive genes (3, 4). Also, epidemiologic studies found that higher total calcium intakes have been consistently associated with reduced risk for colorectal neoplasms (5, 6), calcium supplementation reduced adenoma recurrence (7), and higher blood 25-OH-vitamin D levels have been associated with reduced risk for colorectal adenoma (5, 8).
Almost all carcinomas of the colon and rectum develop from adenomatous polyps, which are thought to arise from the “susceptible” colorectal epithelium characterized by hyperproliferation, impaired apoptosis, and reduced differentiation. Removal of the polyps does not eliminate risk for adenoma recurrence, suggesting that the normal-appearing colorectal epithelium possesses molecular phenotypic changes that put a person at risk for developing a neoplasm. Reduced differentiation and altered cell cycle control occur during the early stages of colon tumorigenesis, therefore markers of cell proliferation and differentiation in the colorectal epithelium may serve as phenotypic biomarkers of risk for colorectal neoplasia and may be modifiable by chemopreventive agents.
As described herein, we tested the effects of calcium and vitamin D on a marker of cell differentiation (p21waf1/cip1), and two markers of cell proliferation (MIB-1/Ki-67 and hTERT). P21 is a cyclin-dependent kinase inhibitor that plays an important role in cell differentiation, cell cycle control, apoptosis modulation, and tumorigenesis (9). In colorectal crypts, p21 is expressed only in fully differentiated cells (10), whereas telomerase (as indicated by detection of hTERT, the catalytic subunit of the telomerase) is expressed only in proliferative cells of colon crypts (11); and the proliferation-associated marker MIB-1/Ki-67 is expressed in all cells not in G0 phase of the cell cycle (12). We used telomerase expression as indicated by hTERT in colon crypt cells as a marker of long term proliferative activity, and the S-phase marker MIB-1 as a “snapshot” or short-term proliferative indicator.
Few published human studies tested the effect of calcium and vitamin D supplementation on colorectal epithelial cell proliferation and differentiation (13–16), which may serve as pre-neoplastic modifiable biomarkers of risk for colorectal neoplasms. To address this, we conducted a pilot, randomized, double-blind, placebo-controlled, 2×2 factorial chemoprevention clinical trial of supplemental calcium and vitamin D3, alone and in combination vs. placebo over six months, to estimate the efficacy of these agents on markers of differentiation and proliferation in the normal colorectal mucosa. We hypothesized that calcium and vitamin D3, alone and in combination, increase colonocyte differentiation, decrease the overall rate of proliferation, and normalize the distribution of proliferating cells in crypts within the normal-appearing colorectal epithelium.
PATIENTS AND METHODS
This study was approved by the Emory University IRB; written informed consent was obtained from each study participant.
Participant Population
The detailed protocol of study recruitment and procedures with detailed specific exclusions was published previously (17). Briefly, eligible patients, 30–75 years of age, in general good health, with a history of at least one pathology-confirmed colorectal adenoma within the past 36 months, and no contraindications to calcium or vitamin D supplementation or rectal biopsy procedures and no medical conditions, habits, or medication usage that would otherwise interfere with the study treatment or procedures, were recruited from the patient population attending the Digestive Diseases Clinic at the Emory Clinic, Emory University.
Clinical Trial Protocol
Between April 2005 and January 2006, 522 eligible patients were identified after initial screening of electronic medical records, and 224 (43%) patients were sent an introductory letter and contacted by telephone to see if they would be interested and eligible to participate in the study. Potential participants (n=105; 47%) attended the eligibility visit, during which they were interviewed, signed a consent form, completed questionnaires, and provided a blood sample (17). Diet was assessed with a semi-quantitative food frequency questionnaire (18). Medical and pathology records were reviewed. After a 30-day placebo run-in trial, 92 (88%) participants without significant perceived side effects and who had taken at least 80% of their tablets underwent a baseline rectal biopsy and, if still willing to participate, were randomly assigned to the following four treatment groups (n=23/treatment group) for six months (duration to ensure 25-OH-vitamin D steady state): a placebo control group, a 2.0 g elemental calcium (as calcium carbonate in equal doses twice daily) supplementation group, an 800 IU vitamin D3 supplementation group (400 IU twice daily), and a calcium plus vitamin D supplementation group taking 2.0 g elemental calcium plus 800 IU of vitamin D3 daily. Additional details on the rationale for the doses and forms of calcium and vitamin D supplements were previously published (17).
Participants attended follow-up visits 2 and 6 months after randomization and were contacted by telephone monthly between the second and final follow-up visits. Pill-taking adherence was assessed by questionnaire, interview, and pill count. Participants were instructed to remain on their usual diet and not take any nutritional supplements not being taken on entry into the study. At each of the follow-up visits participants were interviewed and completed questionnaires. At the last visit all participants underwent venipuncture and a rectal biopsy procedure. All visits for a given participant were scheduled at the same time of day to control for possible circadian variability in the outcome measures. Factors hypothesized to be related to the expression of biomarkers in normal colon mucosa (e.g., diet, age) were assessed at baseline and at the final follow-up visit. Participants did not have to be fasting for their visits and did not take a bowel cleansing preparation or enema.
All laboratory assays for serum 25-OH- and 1,25-(OH)2-vitamin D were performed by Dr. Bruce Hollis at the Medical University of South Carolina in a blinded manner using a radioimmunoassay method as previously described (19). The average intra-assay coefficient of variation for 25-OH-vitamin D was 2.3%, and for 1,25- (OH)2-vitamin D, 6.2%.
Six sextant 1-mm-thick biopsy specimens were taken from the rectal mucosa 10 cm proximal to the external anal aperture through a rigid sigmoidocsope with a jumbo cup flexible endoscopic forceps mounted on a semi-flexible rod. The biopsies were then immediately placed in phosphate buffered saline, reoriented under a dissecting microscope and transferred to 10% normal buffered formalin followed by transfer to 70% ethanol 24 hours after initial placement in formalin. Then, within a week the biopsies were processed and embedded in paraffin blocks (three biopsies per block).
Immunohistochemistry Protocol
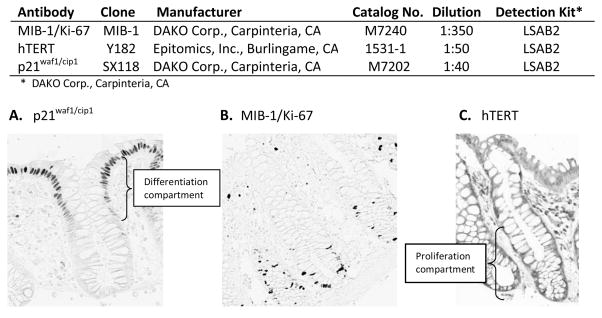
Five slides with four 3.0 μm-thick section levels each taken 40 microns apart were prepared for each antigen. Heat-mediated antigen retrieval was accomplished by placing the slides in a preheated Pretreatment Module (Lab Vision Corp., CA) with 100× Citrate Buffer pH 6.0 (DAKO S1699, DAKO Corp., Carpinteria, CA) and steaming them for 40 minutes. After antigen retrieval, slides were placed in a DAKO Automated Immunostainer and immunohistochemically processed using a labeled streptavidin-biotin method for p21, hTERT, and MIB-1 as summarized in Figure 1. The slides were not counterstained. After staining, the slides were coverslipped with a Leica CV5000 Coverslipper (Leica Microsystems, Inc., IL). In each staining batch of slides, positive and negative control slides were included. Tonsil was used as a control tissue for all biomarkers. The negative and the positive control slides were treated identically to the patients’ slides except that antibody diluent was used rather than primary antibody on the negative control slide.
Figure 1.
Summary of biomarker immunohistochemical protocols and images (at 200× magnification) of colon crypts immunohistochemically processed for: A. p21waf1/cip1, differentiation marker; B. MIB-1/Ki-67, marker of short term proliferative activity; C. hTERT, marker of long term proliferative activity.
Protocol for Quantifying Staining Density of Immunohistochemically Detected Biomarkers in Normal Colon Crypts (“Scoring”)
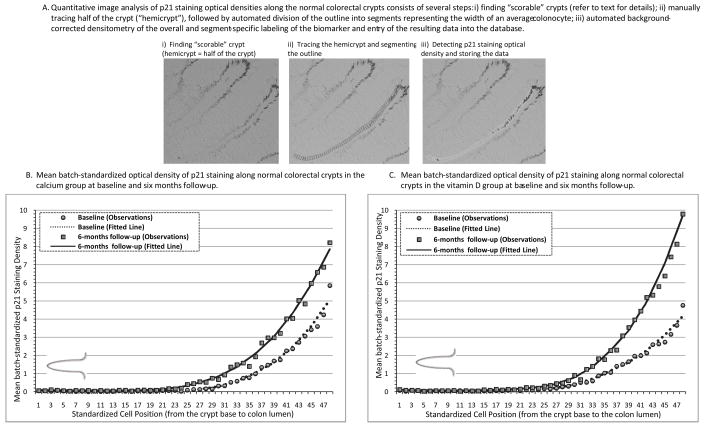
A quantitative image analysis method (“scoring”) was used to evaluate the expression of the biomarkers in the colon crypts, as described previously (17) and demonstrated in Figure 2, A. Briefly, a “scorable” crypt was defined as an intact crypt extending from the muscularis mucosa to the colon lumen (20). Before analysis, negative and positive control slides were checked for staining adequacy. Standardized settings were used on all equipment and software for the image analysis procedures: Olympus BX40 light microscope (Olympus America, Inc., PA), Polaroid DMC digital light microscope camera (Polaroid Corporation, MN), computer, digital drawing board, ImagePro Plus image analysis software (Media Cybernetics, Inc., MD), our custom plug-in software for colorectal crypt analysis, and Microsoft Access (Microsoft Corporation, WA). The technician reviewed slides under the light microscope and selected two of three biopsies with 8 to 10 “scorable” crypts per biopsy, then, created a background correction image for each slide, captured the 16-bit grayscale 1,600 × 1,200 pixel image of the crypt at 200× magnification, and traced the borders of the “hemicrypt” (one half of the crypt). The program then divided the tracing into equally spaced intervals to yield segments with the average widths of normal colonocytes, measured the optical density of the labeling across the entire hemicrypt as well as within each segment, and saved the resulting data into the database. Then, the technician moved to the next hemicrypt and repeated all the previously described analysis steps.
Figure 2.
A quantitative image analysis (A) with an example of resulting distributions of p21waf1/cip1 marker expression (staining optical densities) along the normal-appearing colorectal crypts in the calcium (B) and vitamin D (C) groups at baseline and follow-up visits.
One slide reader analyzed all of the stained slides throughout the study with high intra-reader reliability − 0.95 for MIB-1, 0.98 for hTERT, and 0.96 for p21.
Statistical Analysis
Treatment groups were assessed for comparability of characteristics at baseline and at final follow-up by the Fisher’s exact test for categorical variables and analysis of variance (ANOVA) for continuous variables. Slide “scoring” reliability was analyzed using intraclass correlation coefficients.
Several outcome variables were defined to estimate the expression of the markers in the crypts overall as well as how they were distributed within the crypts. The mean optical density of staining for MIB-1, hTERT, and p21 in normal colon crypts was calculated for each patient at baseline and 6-months follow-up by summing all the densities from all analyzed crypts from the biopsy specimens and dividing by the number of crypts analyzed (this measure indicates the overall rate of proliferation or differentiation of rectal crypt epithelial cells and is further referred to as LI, labeling index (20)). The crypt differentiation compartment was defined a priori as the upper 40% of the crypts, and the crypt proliferation compartment as the bottom 60% of the crypts (Figure 1) (15, 20, 21). Measures of the within-crypt distributions of the proliferation markers (i.e., the ratio of expression in the upper 40% to that in the entire crypts, φh) were calculated for each patient by taking the mean of the biomarker densities in the upper 40% of crypts and dividing it by the biomarker densities in the entire crypt. For the proliferation markers, we decided a priori to use the φh because it is an indicator of an upward extension of the canonical proliferative zone of the colon crypt and was found previously to be modified by calcium and/or calcium plus vitamin D supplementation (15, 22, 23).
Primary analyses were based on assigned treatment at the time of randomization, regardless of adherence status (intent-to-treat analysis). The three biomarkers were analyzed separately. We transformed biomarker expression density data by dividing each individual measurement by the staining batch’s average density to adjust for possible batch effects (batch standardization). At baseline batch-specific mean staining densities were calculated using the measurements from all treatment groups, whereas for the follow-up visit, only measurements from the placebo group were used. Absolute treatment effects were calculated as the differences in the batch-standardized densities from baseline to the 6-months follow-up visit between patients in each active treatment group and the placebo group using a MIXED effects model. Interaction between calcium and vitamin D treatments was assessed in the MIXED model by including calcium and vitamin D as factors and interaction term between them. Since optical density is measured in arbitrary units, to provide perspective on the magnitude of the treatment effects we also calculated relative effects (17, 20), defined as: [treatment group follow-up mean/treatment group baseline mean]/[placebo follow-up mean/placebo baseline mean]. The relative effect provides an estimate of the proportional change in the treatment group relative to that in the placebo group, and its interpretation is somewhat analogous to that of an odds ratio (e.g., a relative effect of 2.0 would mean that the relative proportional change in the treatment group was two times as great as that in the placebo group). The Delta method was used to derive the 95% confidence intervals for the relative effects (24). Since the treatment groups were balanced on risk factors at baseline, no adjustment was made for other covariates in the primary intent-to-treat analyses.
Spearman’s rank and partial Spearman’s rank correlation coefficients were used to compare cell proliferation marker values at baseline and follow-up, respectively.
The distributions of the biomarkers’ staining densities were graphically evaluated using the LOESS procedure with smoothing parameter 0.5 and local quadratic fitting. First, the number of sections within a hemicrypt was standardized to 50. Then, the average for each section across all crypts was predicted by the LOESS model separately for each patient, and then for each treatment group by follow-up visit. The results were plotted in the graphs along with smoothing lines.
In sensitivity analyses, we also analyzed data without standardization for batch, as well as by including batch as a covariate and using different transformations. The results from these analyses did not differ materially from those reported. Statistical analyses were done using SAS System software (version 9.1.3; SAS Institute, Inc., NC). A cutoff level of P ≤ 0.05 (2-sided) was used for assessing statistical significance.
RESULTS
Characteristics of Study Participants
The treatment groups did not differ significantly on participant characteristics measured at baseline (Table 1) or at the end of the study (data not shown). The mean age of the participants was 61 years, 64% were men, 71% were white, and 20% had a family history of colorectal cancer in a first degree relative. Most participants were non-smokers, college graduates, and overweight. Biopsy specimens that were “scorable” were obtained for 87, 90, and 90 participants at baseline, and for 83, 85, and 84 participants at 6-month follow-up for the hTERT, MIB-1, and p21 markers, respectively.
Table 1.
Selected Baseline Characteristics of the Study Participants* (N=92).
| Treatment Group |
|||||
|---|---|---|---|---|---|
| Characteristics | Placebo (N=23) | Calcium (N=23) | Vitamin D (N=23) | Calcium + Vit. D (N=23) | P-value** |
| Demographics, medical history, habits, anthropometrics | |||||
| Age, years | 58.5 (8.2) | 61.9 (8.2) | 60.2 (8.1) | 62.1 (7.5) | 0.39 |
| Men (%) | 70 | 70 | 70 | 70 | 1.00 |
| White (%) | 74 | 83 | 65 | 61 | 0.39 |
| College graduate (%) | 65 | 61 | 57 | 44 | 0.53 |
| History of colorectal cancer in 1° relative (%) | 17 | 30 | 17 | 13 | 0.60 |
| Take NSAID*** regularly§ (%) | 22 | 13 | 9 | 22 | 0.60 |
| Take aspirin regularly§ (%) | 22 | 52 | 30 | 56 | 0.05 |
| If woman (n = 28), taking estrogens (%) | 4 | 9 | 4 | 4 | 1.00 |
| Current smoker (%) | 9 | 4 | 0 | 0 | 0.61 |
| Take multivitamin (%) | 30 | 30 | 26 | 39 | 0.86 |
| Body mass index (BMI), kg/m2 | 30.6 (7.2) | 29.4 (5.5) | 28.9 (5.6) | 31.6 (6.0) | 0.44 |
| Mean dietary intakes | |||||
| Total energy intake, kcal/d | 1,596 (528) | 1,788 (691) | 1,848 (821) | 1,845 (752) | 0.59 |
| Total§§ calcium, mg/d | 618 (308) | 746 (335) | 843 (526) | 824 (714) | 0.41 |
| Total§§ vitamin D, IU/d | 277 (230) | 336 (202) | 360 (317) | 415 (316) | 0.40 |
| Total fat, gm/d | 67 (32) | 72 (35) | 70 (32) | 74 (28) | 0.59 |
| Dietary fiber, gm/d | 15 (7) | 17 (9) | 18 (9) | 17 (11) | 0.97 |
| Alcohol, gm/d | 9 (14) | 11 (15) | 14 (18) | 10 (20) | 0.84 |
| Adenoma characteristic | |||||
| Multiple adenomas (%) | 17 | 22 | 39 | 26 | 0.45 |
| Large adenoma ≥ 1 cm£ (%) | 19 | 32 | 17 | 9 | 0.32 |
| Villous/tubulovillous adenoma££ (%) | 4 | 9 | 9 | 4 | 1.00 |
| Mild dysplasia (%) | 100 | 96 | 100 | 100 | 1.00 |
Data are given as means (SD) unless otherwise specified.
By Fisher’s exact χ2 test for categorical variables, and ANOVA for continuous variables.
Nonsteroidal anti-inflammatory drug.
At least once a week.
Diet plus supplements. At least two adenomas.
At least one large adenoma.
At least one villous or tubulovillous adenoma.
Adherence to visit attendance averaged 92% and did not differ significantly among the four treatment groups. On average, at least 80% of pills were taken by 93% of participants at the first follow-up visit and 84% at the final follow-up visit. There were no treatment or biopsy complications. Seven people (8%) were lost to follow-up due to perceived drug intolerance (n=2), unwillingness to continue participation (n=3), physician’s advice (n=1), and death (n=1). Dropouts included one person from the vitamin D supplementation group, and two persons from each of the other three groups.
At baseline, there were no significant differences between the four study groups in serum 25-OH - or 1,25-(OH)2-vitamin D levels. At the study end, the vitamin D and calcium plus vitamin D groups had significantly higher levels of serum 25-OH-vitamin D (P<0.001), whereas the placebo and calcium groups had slight non-significant decreases in 25-OH-vitamin D levels (17). As expected, serum levels of 1,25- (OH)2-vitamin D at the end of follow-up period did not differ significantly between study groups (17).
Effects of Calcium and/or Vitamin D on p21 Expression in Normal Colorectal Crypts
After six months treatment, p21 expression along the full lengths of crypts increased statistically significantly by 201% (P=0.03), 242% (P=0.005), and 25% (P=0.47) in the calcium, vitamin D, and calcium plus vitamin D groups, respectively, relative to the placebo group (Table 2, A). The graphical assessment of changes over six months in the distribution of p21 expression along crypts demonstrated that the largest post-supplemental increases in p21 were in the upper 40% of the crypts (Figure 2, B and C), and the numerical findings limited to the upper 40% of the crypts were essentially the same as for the entire crypt (Table 2, A). There was a statistically significant antagonistic interaction between calcium and vitamin D3 treatments on p21 expression (Table 2, A).
Table 2.
Colorectal expression of p21, MIB-1, and hTERT during the clinical trial.
| Baseline |
6-Months Follow-up |
Absolute Rx Effect** |
Relative Effect§ |
95% CI$ | Pinteraction¤ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SE | P* | N | Mean | SE | P* | N | Mean | SE | P* | |||||
| A. p21¥expression in colorectal crypts | ||||||||||||||||
| Entirecrypts (LI) | ||||||||||||||||
| Placebo | 22 | 1.23 | 0.17 | 21 | 1.00 | 0.18 | 20 | 0.00 | 1.00 | |||||||
| Calcium | 23 | 0.85 | 0.17 | 0.11 | 21 | 1.37 | 0.18 | 0.14 | 21 | 0.78 | 0.33 | 0.03 | 2.01 | 1.08 | 3.72 | |
| Vitamin D | 22 | 0.81 | 0.17 | 0.08 | 21 | 1.58 | 0.18 | 0.02 | 20 | 0.98 | 0.34 | 0.01 | 2.42 | 1.30 | 4.51 | |
| Ca + Vit. D | 23 | 1.12 | 0.17 | 0.62 | 21 | 1.13 | 0.18 | 0.60 | 21 | 0.23 | 0.33 | 0.47 | 1.25 | 0.69 | 2.26 | 0.01 |
| Upper 40% of crypts (LI40) | ||||||||||||||||
| Placebo | 22 | 1.10 | 0.15 | 21 | 0.91 | 0.16 | 20 | 0.00 | 1.00 | |||||||
| Calcium | 23 | 0.86 | 0.15 | 0.26 | 21 | 1.43 | 0.16 | 0.02 | 21 | 0.77 | 0.31 | 0.02 | 2.02 | 1.11 | 3.66 | |
| Vitamin D | 22 | 0.77 | 0.15 | 0.13 | 21 | 1.54 | 0.16 | 0.01 | 20 | 0.96 | 0.31 | 0.00 | 2.44 | 1.31 | 4.53 | |
| Ca + Vit. D | 23 | 1.02 | 0.15 | 0.70 | 21 | 1.09 | 0.16 | 0.43 | 21 | 0.26 | 0.31 | 0.41 | 1.29 | 0.71 | 2.34 | 0.004 |
| B. MIB-1¥expression in colorectal crypts | ||||||||||||||||
| Entire crypts (LI) | ||||||||||||||||
| Placebo | 22 | 1.01 | 0.10 | 21 | 1.00 | 0.10 | 20 | 0.00 | 1.00 | |||||||
| Calcium | 23 | 0.90 | 0.09 | 0.42 | 21 | 1.09 | 0.10 | 0.50 | 21 | 0.18 | 0.19 | 0.30 | 1.23 | 0.84 | 1.80 | |
| Vitamin D | 22 | 0.83 | 0.10 | 0.18 | 22 | 1.08 | 0.10 | 0.58 | 22 | 0.25 | 0.19 | 0.18 | 1.32 | 0.89 | 1.96 | |
| Ca + Vit. D | 23 | 1.25 | 0.09 | 0.09 | 21 | 1.10 | 0.10 | 0.49 | 21 | −0.13 | 0.19 | 0.50 | 0.89 | 0.62 | 1.27 | 0.04 |
| Ratio of upper 40% to entire crypts (φh) | ||||||||||||||||
| Placebo | 22 | 0.07 | 0.01 | 21 | 0.06 | 0.01 | 20 | 0.00 | 1.00 | |||||||
| Calcium | 23 | 0.09 | 0.01 | 0.40 | 21 | 0.07 | 0.01 | 0.64 | 21 | −0.01 | 0.03 | 0.80 | 0.94 | 0.47 | 1.87 | |
| Vitamin D | 22 | 0.08 | 0.01 | 0.56 | 22 | 0.07 | 0.01 | 0.71 | 22 | −0.003 | 0.03 | 0.89 | 0.97 | 0.48 | 1.94 | |
| Ca + Vit. D | 23 | 0.08 | 0.01 | 0.72 | 21 | 0.07 | 0.01 | 0.84 | 21 | −0.003 | 0.03 | 0.92 | 0.97 | 0.48 | 1.97 | 0.68 |
| C. hTERT¥expression in colorectal crypts | ||||||||||||||||
| Entire crypts (LI) | ||||||||||||||||
| Placebo | 21 | 1.08 | 0.10 | 20 | 1.00 | 0.10 | 19 | 0.00 | 1.00 | |||||||
| Calcium | 22 | 1.01 | 0.10 | 0.63 | 20 | 1.00 | 0.10 | 0.99 | 19 | 0.07 | 0.21 | 0.73 | 1.07 | 0.72 | 1.59 | |
| Vitamin D | 22 | 0.83 | 0.10 | 0.08 | 22 | 0.97 | 0.10 | 0.85 | 21 | 0.25 | 0.21 | 0.27 | 1.27 | 0.84 | 1.93 | |
| Ca + Vit. D | 22 | 1.08 | 0.10 | 0.98 | 21 | 1.06 | 0.10 | 0.70 | 20 | 0.14 | 0.21 | 0.80 | 1.05 | 0.72 | 1.54 | 0.33 |
| Ratio of upper 40% to entire crypts (φh) | ||||||||||||||||
| Placebo | 21 | 0.37 | 0.01 | 20 | 0.42 | 0.01 | 19 | 0.00 | 1.00 | |||||||
| Calcium | 22 | 0.39 | 0.01 | 0.33 | 20 | 0.39 | 0.01 | 0.24 | 19 | −0.04 | 0.03 | 0.13 | 0.90 | 0.78 | 1.03 | |
| Vitamin D | 22 | 0.37 | 0.01 | 0.81 | 22 | 0.41 | 0.01 | 0.63 | 21 | −0.01 | 0.03 | 0.61 | 0.97 | 0.85 | 1.11 | |
| Ca + Vit. D | 22 | 0.39 | 0.01 | 0.25 | 21 | 0.37 | 0.01 | 0.03 | 20 | −0.07 | 0.03 | 0.02 | 0.85 | 0.74 | 0.98 | 0.93 |
P-value for difference between each active treatment group and placebo group from Mixed model. Covariates included random intercept, follow-up visit, treatment group, and treatment group by follow-up visit interaction.
Absolute treatment effect = ([treatment group follow-up - treatment group baseline] - [placebo group follow-up - placebo group baseline]); actual calculations from the Mixed model, in which the interactions between treatment group and follow-up visits terms estimate absolute treatment effect in each active treatment group relative to the placebo.
Relative effect = [(treatment group follow-up/treatment group baseline)/(placebo follow-up/placebo baseline)]; interpretation similar to that for an odds ratio (e.g., a relative effect of 1.8 would indicate a proportional increase of 80% in the treatment group relative to that in the placebo group).
95% confidence interval for relative effect calculated by the Delta method (24).
P-value for interaction between calcium and vitamin D3 treatments from Mixed model; covariates included random intercept, two factors (calcium and vitamin D3 coded as 0/1 variable), follow-up visit, and all appropriate interaction terms between factors and follow-up visit.
Biomarkers detected immunohistochemically and then their labeling densities were quantified by image analysis; all biomarkers values shown as batch-standardized optical densities. Batch standardization for each biomarker was performed by dividing each individual measurement by the staining batch’s average optical density.
Effects of Calcium and/or Vitamin D on MIB-1 and hTERT Expression in Normal Colorectal Crypts
There were no statistically significant treatment effects on the expression of MIB-1 in the crypts overall or in the proportion of its overall expression that extended into the upper 40% of the crypts (φh) in any active treatment group relative to placebo (Table 2, B). Also, there were no statistically significant changes in the expression of hTERT in the entire crypt at the end of follow-up; however, the hTERT labeling index φh decreased by 10% (P=0.13), 3% (P=0.61), and 15% (P=0.02) in the calcium, vitamin D and calcium plus vitamin D groups relative to the placebo, respectively (Table 2, C).
Graphical assessments of changes in the distributions of MIB-1 and hTERT, and separate analyses of changes in the expression of these biomarkers in the upper 40% and lower 60% of the crypts over six months treatment indicated that the decrease in the φh observed in each active treatment group relative to the placebo at the end of the follow-up, while related to decreases in biomarker expression in the upper 40% of the crypts, was also related, in part, to slight increases in expression in the bottoms of the crypts (data not shown).
A statistically significant positive correlation was found between the baseline expression of MIB-1 and hTERT with Spearman’s rank correlation coefficients being 0.35 (P=0.001) and 0.28 (P=0.009) for the LI and φh, respectively. At the end of follow-up, the MIB-1 and hTERT labeling indices were positively correlated (ρpartial=0.35, P=0.001), but not the MIB-1 φh with the hTERT φh (ρpartial=0.13, P=0.24). A weak statistically non-significant correlation was noted between the LI and φh for each of the cell proliferation biomarkers at both study visits (ρ<|0.15|, P>0.31).
We also investigated whether VDR genotype, change in 25-OH-vitamin D levels, adherence to treatment, sex, family history of colorectal cancer, and NSAID use modified the observed associations; however, the sample size was too small for these results to be reliable (data not shown).
DISCUSSION
These data provide evidence for a substantial increase in cell differentiation, as indicated by increased expression of p21, in the normal colorectal epithelium of sporadic adenoma patients in response to vitamin D3 or calcium supplementation and, thus, are consistent with the hypothesis that increased levels of circulating vitamin D or a higher intake of calcium may reduce risk for colorectal neoplasms. Our data also suggest that vitamin D3 may have a slightly greater effect than calcium on p21 expression, and vitamin D combined with calcium may have a lesser treatment effect than either calcium or vitamin D alone on p21. Furthermore, the data provide no evidence that the overall colorectal epithelial cell proliferation rate, as indicated by the expression of short- and long-term markers of proliferation in the entire colorectal crypt, can be reduced by calcium and vitamin D, alone or in combination. However, our data suggested that calcium combined with vitamin D may shift downwards (“normalize”) the distribution of proliferating cells in the colorectal crypts as indicated by the expression of a long-, but not short-term marker of cell proliferation.
p21waf1/cip1, a cyclin-dependent kinase inhibitor used in this study as a marker of differentiation, is a potent inducer of differentiation in intestinal colonocytes (10), and its expression is known to be downregulated during the early stages of colon tumorigenesis (10, 25). p21 was also reported to participate in cell cycle regulation (9) and control of DNA methylation (26), and to interact with regulatory proteins, among which is calmodulin (27). As was found in colon cancer cells in vitro (28–32), we hypothesized that vitamin D and calcium would increase p21 expression in the normal human colorectal epithelium in vivo. The plausibility of this hypothesis is supported by the fact that the p21 gene is a primary 1,25-(OH)2-vitamin D3-responsive gene with at least three vitamin D response element (VDRE)-containing regions within its promoter (33); and that calcium, through the calcium-sensing receptor (CaSR), promotes differentiation in colorectal epithelial cells (31, 32). However, there is little literature regarding direct regulation of p21 by calcium, but there is some evidence that extracellular calcium activates protein kinase C, which is associated with the differential induction of p21 in the intestinal epithelium (3). Also, an intracellular calcium gradient along the colon crypt that coincides with the differentiation compartment may modulate differentiation of the colonocytes, thus, regulating p21 expression (34). As hypothesized, we observed the largest increase in p21 expression in the vitamin D group, and to a lesser extent in the calcium group; however, we found only a relatively small increase in the calcium plus vitamin D group, and a statistically significant antagonistic interaction between the two treatments. There are several possible explanations for the latter finding, including the possibility that the observed treatment effect in the calcium plus vitamin D group may have been due to chance, or that the two agents may have attenuated the effects of either alone. One animal study (35) found that calcium and vitamin D separately are more potent inhibitors of colon tumorigenesis than when combined. However, several other animal studies that investigated the combined effect of calcium and vitamin D reported stronger effects with vitamin D and calcium combined (36, 37); and the results of a large adenoma recurrence trial suggested that vitamin D enhanced the chemopreventive effect of calcium (38). Thus, the combined effect of calcium and vitamin D on colon crypt epithelial cell differentiation as indicated by p21 expression is not clear and a larger more definitive study is needed to clarify it.
No previous human studies tested the effect of calcium and/or vitamin D supplementation on p21 expression in the normal colorectal mucosa, but three small studies (15, 16, 21) investigated the effects of these agents or low fat dairy foods on other markers of differentiation (acidic mucins and/or cytokeratin AE1) in the normal colorectal mucosa with inconsistent results. Two small studies found no changes in the normal rectal crypt differentiation markers after supplementation with calcium and vitamin D3 (15), or with calcium or low fat dairy foods (16); but a third, larger (N=70), randomized, placebo-controlled trial reported significant changes in differentiation markers after supplementation with low fat dairy foods, which are rich in calcium and vitamin D, but contain other components that may also exert prodifferentiative effects (21). Taken altogether, the results of the present and past studies combined with the biological evidence suggest that calcium and vitamin D induce differentiation in the normal human colorectal mucosa, and that expression of p21 may be a more suitable biomarker of differentiation than other currently investigated markers.
Unlike other studies, we used two different markers of proliferation, hTERT and MIB-1, detected by immunohistochemical methods. MIB-1/Ki-67 is expressed in all cells not in G0 phase of the cell cycle (12); and hTERT protein, a catalytic subunit of telomerase, which functions to regenerate telomeres on the ends of chromosomes, is expressed in almost all human cancers and some normal proliferative epithelial cells such as in the colorectal crypt base (11, 39, 40). We hypothesized that hTERT expression in colorectal crypts better reflects average, long term proliferative activity than do “snapshot” proliferative indicators, such as the S-phase marker MIB-1, which demonstrate rapid, large responses to short term physiologic stimuli. Biological evidence supports the growth-restraining actions of calcium and vitamin D on colorectal epithelial cells (3), however few human studies tested the effect of vitamin D and calcium on cell proliferation in the colon.
There have been two large clinical trials of calcium and colorectal epithelial cell proliferation (13, 14) as well as several smaller trials (reviewed in (22), also (16, 21, 41–43)). One of these trials (N=193) found no evidence for a reduction in the labeling index (LI), but a marked, statistically significant proportional decrease in the φh (13), but the second trial (N=333) (14), with more methodological problems (22), found no effect on either measure of cell proliferation. The findings from several smaller controlled studies were inconsistent, with some suggesting decreases in the LI and/or φh, and other studies indicating no change or statistically non-significant increases in the LI and/or φh. The results of the present study for the LI are consistent with those from the previously conducted large clinical trials (13, 14); and for the φh with one large clinical trial (13) and several smaller clinical trials (reviewed in (22), also (42, 43)). However, it must be emphasized that the present study was a pilot study with limited statistical power; thus, our findings may have been due to chance. Other possible explanations for our findings may have been the use of an antibody that may have low specificity detecting hTERT (11); that the MIB-1 and/or hTERT markers may not be good biomarkers of cell proliferation in normal colorectal crypts; or that calcium may indeed have no substantial effect on colorectal cell proliferation in sporadic adenoma patients.
No published human studies tested the effect of vitamin D alone or combined with calcium on the hTERT or MIB-1 markers of proliferation, but one small randomized clinical trial (N=21) found a significantly decreased MIB-1 labeling index, but not the φh, in flat mucosa and resected polypoid tissue after 6-months supplementation with calcium (1,500 mg/day) plus vitamin D3 (400 IU/day) (15). Contrary to the results of one study (15), we did not find evidence for an effect of vitamin D alone or in combination with calcium on overall MIB-1 or hTERT labeling, but we did find a significant downward shift in hTERT expression in the calcium plus vitamin D group. However, as pointed out above, these findings may be due to chance, non-specific detection methods, or an insufficient vitamin D3 dose or duration.
Previous studies (44) and our study found that the LI and φh are statistically independent variables, and other controlled trials testing calcium or other agents on cell proliferation rates found statistically significant reductions in the φh, but not the LI (13, 45–47). Therefore, the LI and φh may represent different biological aspects of colon tumorigenesis, and serve as independent markers of risk for colorectal neoplasia.
The present study was conducted to test the joint and separate effects of calcium and vitamin D on the individual components and aggregate profile of a molecular phenotype panel of biomarkers of risk for colorectal cancer, which includes biomarkers of APC and mismatch repair pathways, cell cycle events, and others. We previously reported a statistically significant effect of vitamin D on the pro-apoptotic marker Bax (17), and analyses for other biomarkers in the panel are currently underway. Taken all together, the present and previously published data (13, 17) suggest that calcium and vitamin D may have stronger effects on cell differentiation and apoptosis than on proliferation; and that even relatively low dose vitamin D may have greater effects on colorectal epithelial cell differentiation and apoptosis than does high dose calcium alone or in combination with low dose vitamin D. However, larger, more definitive clinical studies are needed to confirm these results.
This study has several limitations. The most obvious limitation is the small sample size resulting in an increased role for chance in detecting or not detecting a treatment effect. The small size also did not allow us to conduct additional subgroup analyses. Another limitation is that, although human studies have found that cell proliferation rates observed in the rectal mucosa are correlated with those found throughout the colon (48, 49), animal studies found that calcium affects cell proliferation throughout the colon (50, 51), and one intervention trial found that calcium decreases the LI and φh in the rectum and sigmoid colon, but not in the descending colon (45), there are insufficient data to assume that the effect of calcium is the same in the distal and proximal parts of the colon in humans. Furthermore, the effects of vitamin D alone or in combination with calcium on proliferation and differentiation in different parts of the colon (other than the rectum) are not clear, as there were no such studies in humans. Also, it is unknown whether vitamin D and/or calcium may affect human normal colon, adenoma, and cancer tissue differently. Another potential limitation of this study is that proliferation and differentiation markers are evidentially well-supported, but not proven biomarkers of risk for colorectal neoplasms. Therefore, this study cannot prove that because calcium and vitamin D substantially increase p21 expression and may shrink the proliferative zone in the colorectal crypts, they can reduce risk for colorectal neoplasms. The findings of this study may not be generalizable to other populations. Finally, there may be more specific methods and antibodies to detect telomerase expression in colorectal crypts (11), and MIB-1 and hTERT may not adequately reflect cell proliferation rates in normal-appearing colorectal crypts.
The strengths of this study are that it is, to our knowledge, the first clinical trial of the effects of calcium and vitamin D3, alone and in combination on colorectal epithelial proliferation and differentiation in sporadic adenoma patients; the randomized, double-blind, placebo-controlled trial design; evaluation of both long- and short-term proliferation markers; high protocol adherence by study participants; automated biopsy processing and immunostaining procedures; the use of quantitative image analysis; and the strict quality control and consequent high scoring reliability of rectal biopsies.
In summary, these preliminary results from this pilot clinical trial indicate that calcium and vitamin D increase colorectal epithelial cell differentiation and may have relatively little, if any, effect on overall proliferation rates in the colorectal mucosa, but do not rule out a potential normalization of the proliferative zone in the colorectal crypts. This study suggests that p21 expression may be a treatable biomarker of risk for colorectal neoplasms and supports further investigation of calcium and vitamin D3 as chemopreventive agents against colorectal neoplasms.
Acknowledgments
Grant support: National Cancer Institute, National Institutes of Health (R01 CA104637 to R.M.B.); Georgia Cancer Coalition Distinguished Scholar award (to R.M.B.); the Franklin Foundation. The National Cancer Institute, the Georgia Cancer Coalition, and the Franklin Foundation had no influence on the design of the study; the collection, analysis, and interpretation of the data; the decision to submit the manuscript for publication; or the writing of the manuscript.
We thank Vaunita Cohen and Eileen Veronica Smith for excellent technical support; Dr. Bruce W. Hollis for conducting the blood vitamin D assays; Christopher Farino and Stuart Myerberg for the development of the study database; the physicians of the Emory Clinic for work on biopsy procurement; and all study participants for their time and dedication to the study.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Newmark HL, Lipkin M. Calcium, vitamin D, and colon cancer. Cancer Res. 1992;52:2067s–2070s. [PubMed] [Google Scholar]
- 3.Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer. 2003;3:601–14. doi: 10.1038/nrc1144. [DOI] [PubMed] [Google Scholar]
- 4.Ebert R, Schutze N, Adamski J, Jakob F. Vitamin D signaling is modulated on multiple levels in health and disease. Mol Cell Endocrinol. 2006;248:149–159. doi: 10.1016/j.mce.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 5.Bostick RM, Goodman M, Sidelnikov E. Calcium and vitamin D. In: Potter JD, Lindor NM, editors. Genetics of Colorectal Cancer. New York, NY: Springer Science + Business Media, LLC; 2009. pp. 277–296. [Google Scholar]
- 6.Cho E, Smith-Warner SA, Spiegelman D, et al. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. J Natl Cancer Inst. 2004;96:1015–1022. doi: 10.1093/jnci/djh185. [DOI] [PubMed] [Google Scholar]
- 7.Weingarten MA, Zalmanovici A, Yaphe J. Dietary calcium supplementation for preventing colorectal cancer and adenomatous polyps. Cochrane Database Syst Rev. 2008;1:CD003548. doi: 10.1002/14651858.CD003548.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei MY, Garland CF, Gorham ED, Mohr SB, Giovannucci E. Vitamin D and prevention of colorectal adenoma: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17:2958–69. doi: 10.1158/1055-9965.EPI-08-0402. [DOI] [PubMed] [Google Scholar]
- 9.Dotto GP. p21(WAF1/Cip1): more than a break to the cell cycle? Biochim Biophys Acta. 2000;1471:M43–56. doi: 10.1016/s0304-419x(00)00019-6. [DOI] [PubMed] [Google Scholar]
- 10.el-Deiry WS, Tokino T, Waldman T, et al. Topological control of p21WAF1/CIP1 expression in normal and neoplastic tissues. Cancer Res. 1995;55:2910–9. [PubMed] [Google Scholar]
- 11.Yan P, Benhattar J, Seelentag W, Stehle JC, Bosman FT. Immunohistochemical localization of hTERT protein in human tissues. Histochem Cell Biol. 2004;121:391–7. doi: 10.1007/s00418-004-0645-5. [DOI] [PubMed] [Google Scholar]
- 12.Pinder SE, Wencyk P, Sibbering DM, et al. Assessment of the new proliferation marker MIB1 in breast carcinoma using image analysis: associations with other prognostic factors and survival. Br J Cancer. 1995;71:146–9. doi: 10.1038/bjc.1995.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bostick RM, Fosdick L, Wood JR, et al. Calcium and colorectal epithelial cell proliferation in sporadic adenoma patients: a randomized, double-blinded, placebo-controlled clinical trial. J Natl Cancer Inst. 1995;87:1307–1315. doi: 10.1093/jnci/87.17.1307. [DOI] [PubMed] [Google Scholar]
- 14.Baron JA, Tosteson TD, Wargovich MJ, et al. Calcium supplementation and rectal mucosal proliferation: a randomized controlled trial. J Natl Cancer Inst. 1995;87:1303–1307. doi: 10.1093/jnci/87.17.1303. [DOI] [PubMed] [Google Scholar]
- 15.Holt PR, Bresalier RS, Ma CK, et al. Calcium plus vitamin D alters preneoplastic features of colorectal adenomas and rectal mucosa. Cancer. 2006;106:287–96. doi: 10.1002/cncr.21618. [DOI] [PubMed] [Google Scholar]
- 16.Holt PR, Wolper C, Moss SF, Yang K, Lipkin M. Comparison of calcium supplementation or low-fat dairy foods on epithelial cell proliferation and differentiation. Nutr Cancer. 2001;41:150–5. doi: 10.1080/01635581.2001.9680626. [DOI] [PubMed] [Google Scholar]
- 17.Fedirko V, Bostick RM, Flanders WD, et al. Effects of vitamin D and calcium supplementation on markers of apoptosis in normal colon mucosa: a randomized, double-blind, placebo-controlled clinical trial. Cancer Prev Res. 2009;2:213–23. doi: 10.1158/1940-6207.CAPR-08-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988;127:188–99. doi: 10.1093/oxfordjournals.aje.a114780. [DOI] [PubMed] [Google Scholar]
- 19.Hollis BW. Quantitation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D by radioimmunoassay using radioiodinated tracers. Methods Enzymol. 1997;282:174–86. doi: 10.1016/s0076-6879(97)82106-4. [DOI] [PubMed] [Google Scholar]
- 20.Bostick RM, Fosdick L, Lillemoe TJ, et al. Methodological findings and considerations in measuring colorectal epithelial cell proliferation in humans. Cancer Epidemiol Biomarkers Prev. 1997;6:931–42. [PubMed] [Google Scholar]
- 21.Holt PR, Atillasoy EO, Gilman J, et al. Modulation of abnormal colonic epithelial cell proliferation and differentiation by low-fat dairy foods: a randomized controlled trial. J Am Med Assoc. 1998;280:1074–1079. doi: 10.1001/jama.280.12.1074. [DOI] [PubMed] [Google Scholar]
- 22.Bostick RM. Human studies of calcium supplementation and colorectal epithelial cell proliferation. Cancer Epidemiol Biomarkers Prev. 1997;6:971–980. [PubMed] [Google Scholar]
- 23.Holt PR, Arber N, Halmos B, et al. Colonic epithelial cell proliferation decreases with increasing levels of serum 25-hydroxy vitamin D. Cancer Epidemiol Biomarkers Prev. 2002;11:113–119. [PubMed] [Google Scholar]
- 24.Rao CR. Wiley series in probability and mathematical statistics. New York: Wiley; 1973. Linear statistical inference and its applications. [Google Scholar]
- 25.Polyak K, Hamilton SR, Vogelstein B, Kinzler KW. Early alteration of cell-cycle-regulated gene expression in colorectal neoplasia. Am J Pathol. 1996;149:381–7. [PMC free article] [PubMed] [Google Scholar]
- 26.Chuang LS, Ian HI, Koh TW, Ng HH, Xu G, Li BF. Human DNA-(cytosine-5) methyltransferase-PCNA complex as a target for p21WAF1. Science. 1997;277:1996–2000. doi: 10.1126/science.277.5334.1996. [DOI] [PubMed] [Google Scholar]
- 27.Taules M, Rodriguez-Vilarrupla A, Rius E, et al. Calmodulin binds to p21(Cip1) and is involved in the regulation of its nuclear localization. J Biol Chem. 1999;274:24445–8. doi: 10.1074/jbc.274.35.24445. [DOI] [PubMed] [Google Scholar]
- 28.Evans SR, Soldatenkov V, Shchepotin EB, Bogrash E, Shchepotin IB. Novel 19-nor-hexafluoride vitamin D3 analog (Ro 25–6760) inhibits human colon cancer in vitro via apoptosis. Int J Oncol. 1999;14:979–85. doi: 10.3892/ijo.14.5.979. [DOI] [PubMed] [Google Scholar]
- 29.Gaschott T, Wachtershauser A, Steinhilber D, Stein J. 1,25-Dihydroxycholecalciferol enhances butyrate-induced p21(Waf1/Cip1) expression. Biochem Biophys Res Commun. 2001;283:80–5. doi: 10.1006/bbrc.2001.4756. [DOI] [PubMed] [Google Scholar]
- 30.Scaglione-Sewell BA, Bissonnette M, Skarosi S, Abraham C, Brasitus TA. A vitamin D3 analog induces a G1-phase arrest in CaCo-2 cells by inhibiting cdk2 and cdk6: roles of cyclin E, p21Waf1, and p27Kip1. Endocrinology. 2000;141:3931–9. doi: 10.1210/endo.141.11.7782. [DOI] [PubMed] [Google Scholar]
- 31.Chakrabarty S, Radjendirane V, Appelman H, Varani J. Extracellular calcium and calcium sensing receptor function in human colon carcinomas: promotion of E-cadherin expression and suppression of beta-catenin/TCF activation. Cancer Res. 2003;63:67–71. [PubMed] [Google Scholar]
- 32.Kirchhoff P, Geibel JP. Role of calcium and other trace elements in the gastrointestinal physiology. World J Gastroenterol. 2006;12:3229–36. doi: 10.3748/wjg.v12.i20.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carlberg C, Dunlop TW, Saramaki A, Sinkkonen L, Matilainen M, Vaisanen S. Controlling the chromatin organization of vitamin D target genes by multiple vitamin D receptor binding sites. J Steroid Biochem Mol Biol. 2007;103:338–43. doi: 10.1016/j.jsbmb.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 34.Brenner BM, Russell N, Albrecht S, Davies RJ. The effect of dietary vitamin D3 on the intracellular calcium gradient in mammalian colonic crypts. Cancer Lett. 1998;127:43–53. doi: 10.1016/s0304-3835(98)00005-6. [DOI] [PubMed] [Google Scholar]
- 35.Pence BC, Buddingh F. Inhibition of dietary fat-promoted colon carcinogenesis in rats by supplemental calcium or vitamin D3. Carcinogenesis. 1988;9:187–90. doi: 10.1093/carcin/9.1.187. [DOI] [PubMed] [Google Scholar]
- 36.Sitrin MD, Halline AG, Abrahams C, Brasitus TA. Dietary calcium and vitamin D modulate 1,2-dimethylhydrazine-induced colonic carcinogenesis in the rat. Cancer Res. 1991;51:5608–13. [PubMed] [Google Scholar]
- 37.Beaty MM, Lee EY, Glauert HP. Influence of dietary calcium and vitamin D on colon epithelial cell proliferation and 1,2-dimethylhydrazine-induced colon carcinogenesis in rats fed high fat diets. J Nutr. 1993;123:144–52. doi: 10.1093/jn/123.1.144. [DOI] [PubMed] [Google Scholar]
- 38.Grau MV, Baron JA, Sandler RS, et al. Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J Natl Cancer Inst. 2003;95:1765–1771. doi: 10.1093/jnci/djg110. [DOI] [PubMed] [Google Scholar]
- 39.Cech TR. Beginning to understand the end of the chromosome. Cell. 2004;116:273–9. doi: 10.1016/s0092-8674(04)00038-8. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura Y, Tahara E, Tahara H, Yasui W, Ide T. Quantitative reevaluation of telomerase activity in cancerous and noncancerous gastrointestinal tissues. Mol Carcinog. 1999;26:312–20. [PubMed] [Google Scholar]
- 41.van Gorkom BA, Karrenbeld A, van der Sluis T, et al. Calcium or resistant starch does not affect colonic epithelial cell proliferation throughout the colon in adenoma patients: a randomized controlled trial. Nutr Cancer. 2002;43:31–8. doi: 10.1207/S15327914NC431_3. [DOI] [PubMed] [Google Scholar]
- 42.Rozen P, Lubin F, Papo N, et al. Calcium supplements interact significantly with long-term diet while suppressing rectal epithelial proliferation of adenoma patients. Cancer. 2001;91:833–40. [PubMed] [Google Scholar]
- 43.van Gorkom BA, van der Meer R, Karrenbeld A, et al. Calcium affects biomarkers of colon carcinogenesis after right hemicolectomy. Eur J Clin Invest. 2002;32:693–9. doi: 10.1046/j.1365-2362.2002.01048.x. [DOI] [PubMed] [Google Scholar]
- 44.Risio M, Lipkin M, Candelaresi G, Bertone A, Coverlizza S, Rossini FP. Correlations between rectal mucosa cell proliferation and the clinical and pathological features of nonfamilial neoplasia of the large intestine. Cancer Res. 1991;51:1917–21. [PubMed] [Google Scholar]
- 45.Cats A, Kleibeuker JH, van der Meer R, et al. Randomized, double-blinded, placebo-controlled intervention study with supplemental calcium in families with hereditary nonpolyposis colorectal cancer. J Natl Cancer Inst. 1995;87:598–603. doi: 10.1093/jnci/87.8.598. [DOI] [PubMed] [Google Scholar]
- 46.Paganelli GM, Biasco G, Brandi G, et al. Effect of vitamin A, C, and E supplementation on rectal cell proliferation in patients with colorectal adenomas. J Natl Cancer Inst. 1992;84:47–51. doi: 10.1093/jnci/84.1.47. [DOI] [PubMed] [Google Scholar]
- 47.Anti M, Marra G, Armelao F, et al. Effect of omega-3 fatty acids on rectal mucosal cell proliferation in subjects at risk for colon cancer. Gastroenterology. 1992;103:883–91. doi: 10.1016/0016-5085(92)90021-p. [DOI] [PubMed] [Google Scholar]
- 48.Terpstra OT, van Blankenstein M, Dees J, Eilers GA. Abnormal pattern of cell proliferation in the entire colonic mucosa of patients with colon adenoma or cancer. Gastroenterology. 1987;92:704–8. doi: 10.1016/0016-5085(87)90021-7. [DOI] [PubMed] [Google Scholar]
- 49.Mills SJ, Mathers JC, Chapman PD, Burn J, Gunn A. Colonic crypt cell proliferation state assessed by whole crypt microdissection in sporadic neoplasia and familial adenomatous polyposis. Gut. 2001;48:41–6. doi: 10.1136/gut.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bird RP, Schneider R, Stamp D, Bruce WR. Effect of dietary calcium and cholic acid on the proliferative indices of murine colonic epithelium. Carcinogenesis. 1986;7:1657–61. doi: 10.1093/carcin/7.10.1657. [DOI] [PubMed] [Google Scholar]
- 51.Wargovich MJ, Eng VW, Newmark HL. Calcium inhibits the damaging and compensatory proliferative effects of fatty acids on mouse colon epithelium. Cancer Lett. 1984;23:253–8. doi: 10.1016/0304-3835(84)90091-0. [DOI] [PubMed] [Google Scholar]