Abstract
Calcium/calmodulin-dependent kinase II (CaMKII) is a multifunctional serine/threonine kinase expressed abundantly in the heart. CaMKII targets numerous proteins involved in excitation contraction coupling and excitability and its activation may simultaneously contribute to heart failure and cardiac arrhythmias. In this review, we summarize the modulatory effects of CaMKII on cardiac ion channels function and expression and illustrate potential implications in the onset of arrhythmias via a computer model.
Keywords: CaM kinase, ion channels, arrhythmia, heart failure, computer modeling
Introduction
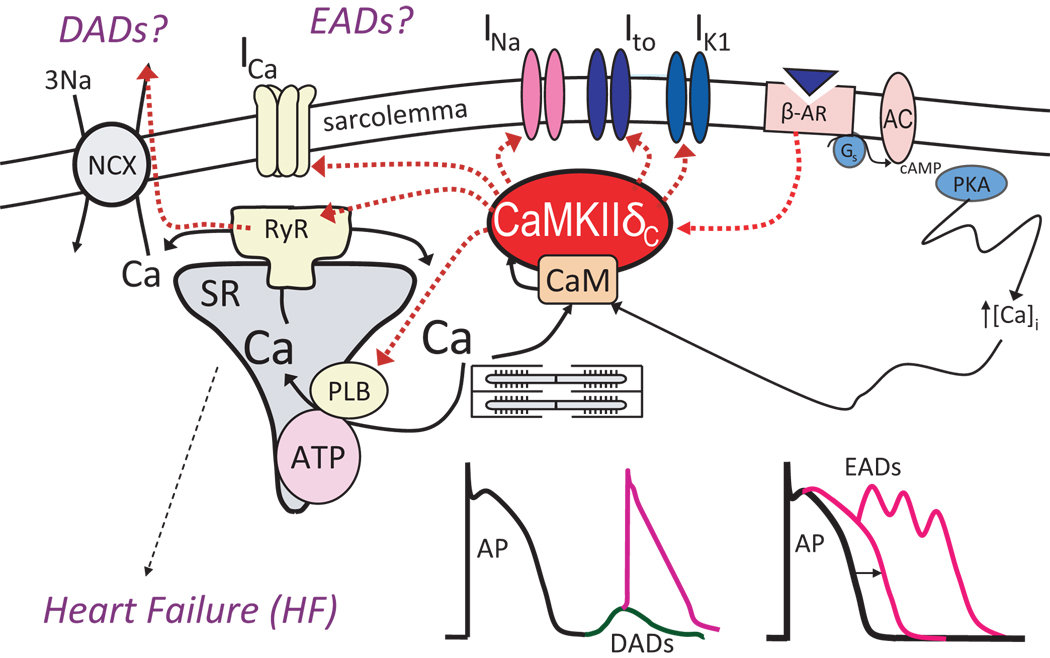
Excitation contraction coupling (ECC) is a mechanism by which membrane depolarization controls the amount of Ca entering the myoplasm and activating contraction, but it also includes feedbacks, whereby Ca regulates the gating of ion channels that are responsible for cardiac excitation. The Ca binding protein calmodulin (CaM) is a major mediator of this Ca-dependent modulation of ion channels through direct Ca-CaM binding or activation of Ca/CaM dependent kinases (e.g. CaMKII) and phosphatases (e.g. calcineurin). CaMKII is a multifunctional protein kinase expressed abundantly in the heart. CaMKII is known to phoshorylate numerous target proteins involved in Ca mobilization (Fig. 1), e.g. Ca influx, release from and uptake into the sarcoplasmic reticulum (SR). More recent findings are that CaMKII modulates sarcolemmal Na and K channels. This can influence myocyte Ca regulation and also confers further Ca-dependence to electrophysiological effects (and makes a complex feedback system).
Figure 1.
CaMKII influences EC coupling by affecting several electrical and Ca handling proteins, including phospholamban (PLB), SR Ca release channels (RyR), and L-type Ca channels responsible for transarcolemmal Ca influx (ICaL). In addition, Na channels (responsible for INa) and K channels (e.g., Ito and IK1) have shown to be regulated by CaMKII. By exerting multiple effects on these numerous targets CaMKII can simultaneously favor heart failure and arrhythmias.
CaMKII has also emerged recently as potentially a major mechanism of pathological signaling in heart failure (HF), where its expression and activity are greatly enhanced, and where CaMKII overexpression causes HF (1–3). Upregulation of CaMKII activity and expression seems to be a general feature of cardiomyopathy from diverse causes in patients and animal models, suggesting the hypothesis that CaMKII is a signaling molecule in cardiomyopathy. In this setting CaMKII may also be arrhythmogenic, due to altered cardiac repolarization and Ca handling. Indeed, CaMKII may be a key pharmacological target that acts upstream of ion channels and Ca regulatory pathways. Moreover, CaMKII inhibition may be a viable therapeutic target in the treatment of common forms of cardiac disease.
Mathematical models of cardiomyocytes provide a useful framework to advance our understanding of molecular mechanisms underlying cardiac arrhythmias and to consider therapeutic targets. Recent models have incorporated elements of the CaMKII signaling cascade to study the role of CaMKII in regulating cardiomyocyte contractility and excitability (4–8). In this review, we will discuss the functional consequences of CaMKII activity on cardiac ion channels (Fig. 1) and will illustrate using a mathematical model how these effects may underlie arrhythmogenic mechanisms for both triggered activity and reentry.
Ca Channels
The L-type Ca channel opens upon cell depolarization, serving as the primary entry point for Ca into cardiomyocytes. Gating of this channel is directly mediated by Ca/CaM through Ca dependent inactivation (CDI). Ca which either enters the myocyte via Ca current (ICa) or is released from the sarcoplasmic reticulum (SR) binds to CaM tethered to the channel C-terminus, altering the interaction of CaM with the Ca channel C-terminus, thereby accelerating inactivation (9). CDI is an important negative feedback mechanism. When the myocyte has relatively high Ca load and large Ca transients, enhanced ICa inactivation limits further Ca influx. Conversely, when myocyte Ca is low and SR Ca release is small, there is less CDI and enhanced Ca entry which increases SR Ca content. The presence of CDI also alters action potential duration (APD) which also controls Ca levels in the myocyte. With large Ca releases there is strong CDI, which limits integrated ICa influx thereby shortening the APD and further limiting Ca entry, and the earlier repolarization would also favor additional Ca extrusion via Na/Ca exchange (10).
Ca/CaM also indirectly regulates the Ca channel to enhance L-type ICa (ICaL) via activation of CaMKII. This process is functionally seen as a positive staircase of ICaL, in which amplitude increases and inactivation slows over a series of several pulses after a resting interval (11). CaMKII-mediated enhancement of ICaL may contribute to the increased contraction strength obtained with faster heart rates which improves cardiac performance during exercise (12). In fact, since CaMKII is sensitive to the frequency of Ca transients, CaMKII is ideally suited to respond to changes in cardiac rhythm. Thus, the positive regulation of the channel by CaMKII may partly offset reduced L-type Ca channel availability at high heart rates caused by direct Ca/CaM-dependent inactivation. This gating phenomenon requires Ca influx (it is not seen when Ba is the charge carrier and is more strongly apparent when local Ca influx is amplified by SR Ca release), and it is therefore termed Ca-dependent facilitation (CDF). CDF of ICaL coexist with CDI, where CDI responds rapidly (during the same beat), while CDF occurs more slowly (from beat to beat). Some studies even claimed that progressive decrease in SR Ca release (negative staircase in rat) and CDI is responsible for observed CDF (13, 14). However, CDF is quite similar in species which exhibit positive staircases, and even when SR Ca release is blocked (11).
Several groups have demonstrated that CDF is mediated by CaMKII dependent phosphorylation. Early studies showed that pharmacological inhibition of CaMKII abolishes CDF in the heart (15–17). Moreover, addition of activated CaMKII to the cytoplasmic side of the sarcolemma results in phosphorylation of the LTCC complex, inducing high activity (mode 2) gating that is characterized by long, frequent openings and is consistent with CDF (18).
As for the targets of CaMKII phosphorylation, Hudmon et al. showed that CaMKII phosphorylates the α1C subunit and that tethering of CaMKII to the α1C carboxy-terminus is an essential molecular feature of CDF, because mutations to a putative C-terminus binding site prevent CDF. More recently, Grueter et al. (19) showed that CaMKII binds to the β2a subunit of the L-type Ca channel and preferentially phosphorylates Thr498 in β2a. In addition, mutation of Thr498 to Ala (T498A) in β2a results in ablation of CaMKII-mediated ICaL facilitation in cardiomyocytes.
By overexpressing CaMKIIδC in transgenic mouse myocytes as well as acutely in rabbit myocytes ICaL amplitude was increased and inactivation was slowed (20, 21), consistently with tonic CDF. ICaL amplitude could be reduced back to control levels by blocking CaMKII with KN-93 (20) or AIP (21).
It is now well accepted that CaMKII modulates ICaL, and that this modulation may be important under pathophysiological conditions (e.g. arrhythmias). Interestingly, a transgenic mouse model of cardiac hypertrophy that overexpresses CaMKIV and also shows increased CaMKII activity, showed an increased propensity for ventricular arrhythmias (22). This was caused by triggered activity due to early afterdepolarizations resulting from an inward current during the late plateau phase of the action potential. The increased propensity for arrhythmias has been attributed to enhanced open probability of L-type Ca channels due to increased activity of CaMKII (22). Additionally, in the presence of the CaMKII-inhibitor KN-93, both the increased Ca channel open probability as well as the increased occurrence of arrhythmias were reversed to control values (22). Enhanced CaMKII activation seems to play a major role in ICaL remodeling in a murine model of pressure overload HF (3). Indeed, in cardiomyocytes from these animals ICaL amplitude was increased and the decay slowed, suggesting a role for CaMKII. In HF myocytes CDF could not be induced, suggesting that ICaL was already maximally activated. In addition, the HF-related alterations were recapitulated by application of exogenous CaMKII on control myocytes, whereas exogenous CaMKII did not produce any effect on failing myocytes. These findings implicate increases in CaMKII activity as a direct mechanism governing HF-related changes in ICaL and foster the emerging importance of CaMKII as an anti-arrhythmic target.
T-type Ca (ICaT) current is present to a variable extent in pacemaking, atrial and conduction cells. In the ventricle T-type channels are expressed during cardiac development until the end of the fetal/neonatal period (23), then their expression decreases and there is little or no ICa,T in normal adult ventricular myocytes. Significant ICaT may reappear in ventricular myocytes during the development of cardiac hypertrophy and failure (24–26). T-type Ca channels are phosphorylated by CaMKII, which increases the probability of single channel openings (27, 28) and is also upregulated in hypertrophied ventricles (29). Thus, the enhanced CaMKII activity in hypertrophic and failing hearts could further enhance T-type Ca channel openings. However, the potential role of CaMKII signaling to regulate cardiac ICaT has not yet been fully explored. At this point one might only speculate that the enhanced ICa,T activation in myocytes may enhance their automaticity, and thus contribute to arrhythmogenesis.
Na Channels
The sodium current (INa) is responsible for the rapid depolarization of the membrane potential, which initiates the electrical cascade responsible for cardiac rhythm. Numerous life-threatening arrhythmias arise from Na channel dysfunction, thus illustrating its central role in controlling cardiac excitability. Gating of the Na channel appears to be modulated by Ca. However, the mechanism of this modulation (direct Ca binding and/or indirect regulation via CaM or CaMKII) is not yet fully resolved.
Wingo et al. (30) proposed a novel mechanism for modulation of Na channel function where Ca ions bind directly to hH1 via an EF-hand motif in the C-terminal domain. However, more recent and direct experiments have provided evidence against the possibility that Ca binds to this region (31).
Tan et al. (32) have shown that CaM enhances INa slow inactivation by binding to the carboxy-terminal IQ domain of the human cardiac Na channel (hH1) in a Ca-dependent manner. Direct interaction of CaM with the IQ motif of Nav1.5 has been shown in other studies, however, the effects on the Na channels have been discordant: Kim et al. (31) showed that CaM modulates the C-terminal interaction with the III–IV linker (suggested to be necessary to stabilize the inactivation gate), to minimize sustained channel activity during depolarization, and to prevent cardiac arrhythmias that lead to sudden death; Young and Cadwell (33) showed a hyperpolarizing shift in the voltage dependence of activation, whereas no effect was reported by Deschênes et al. (34).
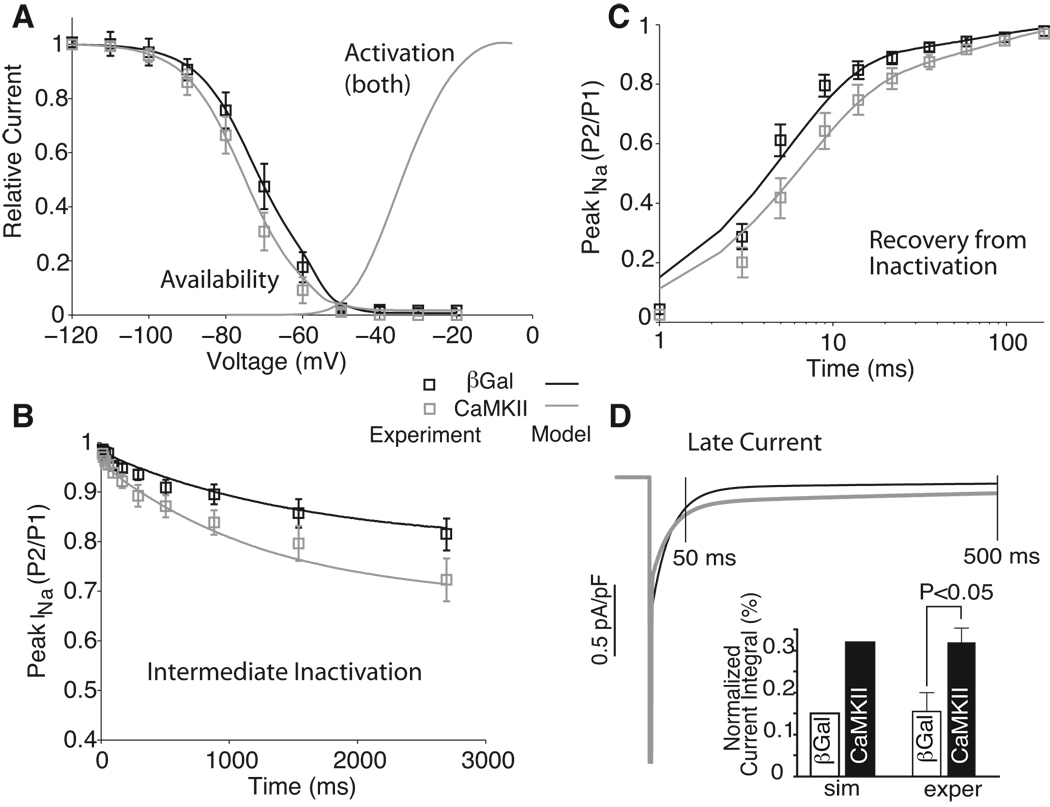
Recent reports have shown that also Na channels may be a target of CaMKII (35, 36). Wagner et al. (35) have shown that CaMKII coimmunoprecipitates with and phosphorylates Na channels. CaMKIIδC overexpression in rabbit myocytes (acutely) and in transgenic mice (chronically) slows fast INa inactivation, enhances late INa (Fig. 2D), but also enhances accumulation of intermediate INa inactivation (Fig. 2B), slows recovery from inactivation (Fig. 2C), and shifts steady-state inactivation of Na channels to more negative voltages (Fig. 2A) in a Ca-dependent manner (35). Moreover, all of these effects could be reversed with CaMKII inhibition (except impaired inactivation in TG mice). Deschênes et al. (34) found that inhibition of a CaM kinase (possibly CaMKIV) modifies cardiac Na channel inactivation, shifting the steady-state inactivation curve in the depolarizing direction, slowing entry into inactivated states, and hastening recovery from inactivation (KN-93 vs. KN-92 effects). They excluded CaMKII because the specific CaMKII inhibitor AIP did not seem to alter the Na channel gating. CaMKIV seems unlikely because it is poorly expressed in the heart and localizes to the nucleus. Maltsev et al. (36) tested the inhibition of CaMKII and CaM in failing vs. normal canine myocytes. They found that three of the above molecular mechanisms can be potentially involved in the effects of complex Ca/CaM/CaMKII modulation of the late Na current (INaL) characteristics: CaMKII slows the decay of INaL, Ca shifted steady-state inactivation towards more positive potentials (opposite the effect of CaMKII assessed by Wagner (35)). Thus, CaM and CaMKII effects on Na current may coexist.
Figure 2.
Modulation of Na current gating upon adenoviral CaMKIIδC overexpression (vs. control β-galactosidase) in adult rabbit ventricular myocytes. Experimental data by Wagner et al. (35) and simulated results with our model (4) are reported. With CaMKII INa availability is shifted towards more negative potentials, whereas activation is unchanged (A), more INa accumulates in intermediate inactivation (B), INa recovers more slowly from inactivation (C) and there is more sustained current upon prolonged depolarization (D). Modified from ref. (4).
The CaMKII-induced changes in INa gating reported by Wagner (35) strikingly resemble those in a human genetic mutation in Nav1.5 (1795InsD) that is linked with simultaneous arrhythmogenic LQT3 and Brugada syndrome (37). In addition, CaMKII expression is upregulated and more active in HF (1, 38, 39) and arrhythmias are a major cause of death in HF. There are also reports of enhanced late INa in HF (40). Thus, increased CaMKII-dependent INa modulation in HF may be an acquired arrhythmogenic Na channel defect that could affect millions of people with HF.
K Channels
The transient outward K current (Ito) is known to be critical for initiating cardiac repolarization in the early phase of action potentials. Despite its transient nature Ito influences the overall duration of action potential as it profoundly influences phase 1 and the level of plateau, thereby affecting the currents that are active later in the action potential.
Tessier and colleagues (41) provided evidence that CaMKII regulates (slows) inactivation of Ito in human atrial myocytes thus contributing to atrial fibrillation. They showed that CaMKII inhibition accelerated Ito,fast inactivation, whereas okadaic acid (phosphatase inhibitor) had opposite effects (41). Similar results were obtained from rat ventricular myocytes (42) and in heterologeous expression of KV4.2/KV4.3 (42, 43). It was suggested that CaMKII acts on KV4.3 by a direct effect at Ser550, thereby prolonging open-state inactivation and accelerating the rate of recovery from inactivation (43). Recent evidence suggests that chronic inhibition of CaMKII in murine hearts results in APD shortening and prevents remodeling after myocardial infarction and excessive β-adrenergic stimulation (44). That study showed an enhancement of Ito during chronic CaMKII inhibition, suggesting that CaMKII may be involved in the regulation of repolarization in HF. However, acute dialysis of a CaMKII-inhibitory peptide into myocytes did not increase Ito (44), suggesting that Ito modulation is not caused by the acute effect of CaMKII dependent phosphorylation. Another recent report from Xiao et al. (45) has shown that Ito is downregulated in canine ventricular myocytes paced at 3 Hz, and suggested that CaMKII is involved in the Ito remodeling during sustained tachycardia via crosstalk with calcineurin/NFAT signaling.
Wagner et al. (46) studied how CaMKII alters Ito, both acutely by adenoviral CaMKII overexpression in rabbit myocytes and chronically in CaMKII overexpressing transgenic mice with heart failure. CaMKII activation accelerates Ito recovery from inactivation for both Ito,fast and Ito,slow, and this appears to be an acute regulatory effect of phosphorylation by CaMKII, as it is acutely reversed by CaMKII inhibitors. There are also longer term alterations in Ito,fast and Ito,slow which may result from CaMKII-dependent changes in the expression and function of the KV1.4 underlying Ito,slow, which was enhanced by both acute and transgenic CaMKIIδC-overexpression in rabbit and mouse myocytes. Conversely, KV4.2/4.3 (the molecular correlates of cardiac Ito,fast) was decreased during chronic CaMKIIδC-overexpression in mice.
An interesting finding by Wang and colleagues (47) suggests that the Ito inhibitor 4-AP binds to the Ito channel and facilitates ICaL via activation of CaMKII. Further studies are needed to test their hypothesis that a significant amount of inactive CaMKII forms molecular complex with KV4.3, and dissociation from KV4.3 facilitates CaMKII activation (48).
Differences in the expression pattern of Ito,fast and Ito,slow in different regions of the heart contribute to regional variations in AP duration. In addition, downregulation of Ito is a central and consistent electrophysiological change in cardiac diseases (e.g., HF). Thus, it is very important to understand how CaMKII modulation of Ito influences the electrical heterogeneity of the myocardium in diseased and undiseased states.
IK1 not only stabilizes the myocyte resting potential, but also plays an important role in modulating cellular excitability and regulating membrane repolarization, and it is therefore an important determinant of action potential initiation and termination in cardiac cells. In mice with chronic CaMKII inhibition, where the AP is shortened and Ito enhanced, IK1 density was significantly upregulated (44); however, exposure of ventricular myocytes to acute CaMKII inhibition failed to increase IK1, suggesting that IK1 upregulation in these mice is an adaptive response to chronic CaMKII inhibition (increased functional expression of IK1), rather than the acute effect of reduced CaMKII dependent phosphorylation.
Wagner et al. (46) have shown that either short term (24 hr) or chronic transgenic CaMKIIδC overexpression reduces Kir2.1 gene expression and reduces IK1 (when acute modulation during measurement is prevented by CaMKII inhibition with AIP). Thus CaMKIIδC alters expression level of the channel responsible for IK1. This is consistent with several HF studies (49, 50) where IK1 is downregulated ~40–50% and it raises the novel possibility that CaMKII (which is upregulated and more active in HF) might cause the depression of IK1 in HF. Additionally, CaMKII may acutely modulate IK1 amplitude because acute CaMKII inhibition with AIP in the patch pipette reduced IK1. This was true in both control and CaMKII overxpressing myocytes suggesting some basal level of CaMKII-dependent IK1 activation.
IKs is enhanced by intracellular Ca. This may be a consequence of allosteric interaction of CaM rather than phosphorylation by CaMKII, because a CaM inhibitor (W7), but not a CaMKII inhibitor (KN62) inhibited the Ca-induced IKs enhancement (51). Interestingly, the α subunit of the IKs channel, KCNQ1, has two potential binding sites for Ca-free CaM (apo-CaM), an IQ motif and a Φ motif, in its intracellular C-terminal region (52). Yeast two-hybrid assay also suggests that KCNQ1 associates with apo-CaM. Functional and biochemical analysis of LQTS mutations in the KCNQ1 C-terminus showed that disruption of CaM interaction with KCNQ1 interfered with subunit assembly and that the resultant channels generated very small currents. CaM also affects IKs channel gating, since channels with defective Ca/CaM interaction show prominent inactivation not typical of this current (53). Thus, IKs is enhanced by increases in [Ca]i at a physiological range via a calmodulin-dependent pathway, which does not involve phosphorylation.
Ca has been shown to play a role in the regulation of If in guinea pig (54) and rabbit (55) sinoatrial node cells. Alterations in intracellular calcium during the cardiac cycle are involved in fine tuning the voltage-dependent properties of If and may thus determine its relative contribution to pacemaking. It seems likely that CaM may be involved in this process, since three structurally unrelated CaM antagonists decreased If and shifted its voltage-dependence of activation to more negative potentials. However, If was not affected by inhibition of CaMKII. CaMKII has been found to regulate the pacemaker activity of the sinus node mainly via modulating ICaL inactivation and reactivation (56), and the local subsarcolemmal SR Ca releases which drive sinoatrial node cell automaticity, seem to involve basal phosphorylation of Ca cycling proteins by both PKA and CaMKII (57).
RyRs
RyR channels directly control SR Ca release in cardiac muscles, activating contraction during EC coupling. CaMKII associates with RyRs, but the molecular targeting is not yet completely resolved. CaMKII has been shown to phosphorylate RyR at Ser2809 (58) (non exclusive site, (59)) and Ser2815 (59). However, additional phosphorylation sites might also exist (60).
The functional consequences of RyR phosphorylation by CaMKII remain somewhat controversial. In early studies in planar bilayer reconstitution experiments CaMKII has been shown to either increase (58, 59, 61) or decrease (62) RyR open probabilities. In the natural ventricular myocyte environment, Li et al. (63) showed that, for a given SR Ca load and ICaL trigger, inhibition of endogenous CaMKII (with KN-93) decreased Ca release. These results were confirmed by Currie et al. (39) showing that the CaMKII peptide inhibitor AIP depresses Ca spark frequency in rabbit myocytes due to decreased endogenous CaMKII binding to RyR. Guo et al. (64) used saponin-permeabilized myocytes and found that both endogenous and exogenous CaMKII dramatically enhanced spontaneous SR Ca release events (Ca sparks) in a controlled setting where ICa, phospholamban effects and SR Ca load effects were prevented. A contrasting study (65) showed that adenoviral expression of a constitutively active CaMKII depresses SR Ca release, whereas a dominant negative CaMKII enhances it. Another group found that acute adenoviral overexpression of wild type CaMKIIδC only enhanced Ca sparks (21), as in the permeabilized myocyte studies noted above. Transgenic overexpression of CaMKII (3-fold increase in CaMKII activity) increased the fractional SR Ca release during ECC, diastolic and spontaneous SR Ca release. Indeed, the frequency of Ca sparks was greatly enhanced, demonstrating increased diastolic SR Ca leak, which could be reversed by CaMKII inhibition (20).
In a rabbit heart failure model of left ventricular pressure and volume overload, Ai et al. (1) showed that CaMKII expression is increased and more CaMKII is autophosphorylated and associated with the RyR2. In addition the HF myocyte had enhanced SR Ca leak, which was inhibited by CaMKII inhibition (thereby enhancing SR Ca content). This suggests a CaMKII-dependent phosphorylation of RyR in HF that is involved in the enhancement of diastolic SR Ca leak and decreased SR Ca load. Thus, CaMKII effects on RyR seem to be implicated in the phenotype of HF, both in the diastolic dysfunction (depletion of SR Ca stores), and in arrhythmogenesis (because the diastolic Ca release may activate NCX mediated Iti causing DADs and triggered arrhythmias). On the other hand, the CaMKII effect on RyR may not contribute appreciably to the depressed systolic function in HF. That is because the decreased SR Ca load is offset by a greater fractional release also attributable to CaMKII-dependent RyR modulation (63). The enhanced diastolic leak and fractional release during ECC may be manifestations of the same RyR2 activating effect of CaMKII.
CaMKII regulation of RyR seems important and most work points to an activating effect of CaMKII on RyR2. However, the fact that some observations differ, may indicate that there are multiple CaMKII effects on RyR2, possibly mediated by different target sites on RyR2 or auxiliary proteins. More work is needed to clarify this issue.
InsP3 Receptors
Inositol 1,4,5-trisphosphate receptors (InsP3Rs) are intracellular Ca release channels that are present in cardiac myocytes, at much lower levels than RyRs. The type 2 InsP3R (InsP3R2) is the predominant subtype in cardiac myocytes (66), but its role in heart is poorly understood. In atrial myocytes, InsP3Rs have been shown to exert positive inotropic and arrhythmogenic effects on EC coupling (67). Ventricular myocytes express InsP3R, but at lower levels than atrial myocytes (68), and InsP3Rs are mainly in the nuclear envelope where they colocalize with CaMKII (69). CaMKII phosphorylates the InsP3R2, which may feedback on channel gating. In fact, incorporation of CaMKII-treated InsP3R2 into planar lipid bilayers revealed that InsP3-mediated channel open probability is significantly reduced by phosphorylation via CaMKII (69). The phosphorylation of InsP3R by CaMKII may represent a mechanism through which activated CaMKII feeds back to terminate InsP3R2-dependent Ca release before exhaustion of the Ca store. Indeed, reports have shown that CaMKII activity is inhibitory with InsP3-mediated Ca release in Xenopus oocytes and HeLa cells (70, 71).
Wu et al. (72) recently demonstrated an InsP3R-CaMKII signaling in the nuclear envelope of adult ventricular myocytes which can participate in transcriptional regulation. The physiological hypertrophic agonist endothelin-1, which activates plasmalemmal G protein–coupled receptors and InsP3 production, elicits local nuclear envelope Ca release via InsP3R. This local Ca release activates nuclear CaMKII, which triggers phosphorylation of a class II histone deacetylase (HDAC5) and HDAC5 nuclear export (derepressing transcription) (72). Remarkably, this Ca-dependent pathway cannot be activated by the global Ca transients that cause contraction at each heartbeat, suggesting a Ca-dependent reactive signaling circuit that is controlled separately from ECC-based Ca cycling. This is analogous to the very local control involved in Ca-induced Ca release during ECC and to Ca-dependent inactivation of Ca current. These results are relevant since CaMKII activation and its ability to regulate HDAC5 nuclear shuttling represent a critical Ca-dependent signaling circuit for controlling cardiac hypertrophy and heart failure. In fact, InsP3R2 expression is enhanced in HF (1, 73).
Whether InsP3R in HF contributes to arrhythmogenesis besides transcriptional control in HF is not yet known. Recent work from Domeier et al. (68) showed that activation of IP3R facilitates SR Ca release through RyR release clusters and enhances the Ca transient during ECC, thus the IP3 signaling pathway may modulate ventricular ECC under conditions in which IP3/IP3R signaling is altered, such as HF.
Modeling of CaMKII signaling
CaMKII has numerous effects on transmembrane electrical processes and intracellular Ca fluxes, which can be both acute regulatory effects (phosphorylation), but can also alter the expression levels of ion channels (to alter protein transcription, translation, or trafficking). Transgenic mice overexpressing CaMKII exhibit arrhythmias (22, 35), which could have multiple causes via diverse mechanisms. Pharmacologic CaMKII inhibition has also been shown to be protective against arrhythmias (22). We have investigated in silico the effects of CaMKII overexpression on cardiac myocyte ionic currents and APs in a comprehensive computer model of ECC (4). By incorporating the modulatory effects on INa, ICaL and Ito we showed that APs from rabbit myocytes overexpressing CaMKII are shorter compared to control (Figure 3A left) as has been shown experimentally (46). However, when Ito expression is low (e.g., in HF, or endocardial myocytes), the effects of INa and ICaL would be predominant, thus potentially leading to a LQT3-like phenotype (Figure 3A middle and right) and possibly EAD-induced triggered arrhythmias. In fact, the prolongation of AP plateau may allow for the recovery and reactivation of ICaL that depolarizes the membrane and generates EADs (74). In addition, experimental and modeling data indicate that phoshorylation events promoting mode 2 gating of ICaL contribute to EADs and ventricular arrhythmias (22, 75). We have also shown that CaMKII-induced Na channel loss of function effect may reduce AP rate of rise and conduction velocity, especially at fast heart rates (BrS) (4). Heterogeneity of transmural ventricular repolarization has been linked to a variety of arrhythmic manifestations. A certain degree of transmural dispersion of repolarization is normal (Fig 3B left), and may be attributed in part to differential expression of Ito (where there is higher Ito in myocytes at the epicardial side). If CaMKII prolongs APDs in the endocardium (Figure 3A middle and right) and shortens APDs in the epicardium (Figure 3A left), this could amplify transmural dispersion of repolarization (Fig. 3B, right), thus predisposing to the development of reentrant arrhythmias.
Figure 3.
Simulated APs at 1 Hz in βGal and CaMKIIδC-overexpressing cardiac myocytes in the presence of 100% (A, left) 25% (A, middle), and 10% (A, right) Ito. When Ito is fully expressed (A, epi or control), CaMKII shortens the AP, whereas the AP is prolonged due to Ito downregulation (e.g., endo or HF). This could amplify transmural dispersion of repolarization (B, endo-epi: 78 ms for βGal vs. 183 ms for CaMKII), which may predispose cells to reentry phenomena. Modified from ref. (4).
When incorporating in the ECC model the full spectrum of HF-induced changes, such as CaMKII mediated RyR-Ca sensitization, enhanced SR Ca leak, decreased IK1 and Ito (besides the changes in INa, ICaL and Ito gating), coupled with other cellular changes, such as increased INCX, we observed DAD-induced triggered arrhythmias upon β-AR stimulation. DADs are a consequence of increased [Ca]SR activating SR Ca release and inward INCX, which depolarizes the membrane toward threshold for a triggered AP. HF myocytes were stimulated to steady state (1 Hz), then stimulation was stopped and DADs and spontaneous APs were monitored. DADs were not seen at baseline in control (not shown) or HF (Fig. 4, left). However, when simulating isoproterenol administration in HF myocytes only (i.e., doubling ICaL, IKs, SR Ca pump Ca affinity, RyR Ca affinity) DADs and triggered APs were seen upon cessation of 1-Hz stimulation (Fig. 4, right).
Figure 4.
(A) Stimulation of steady-state AP and Ca transients followed by a period of rest in digital HF cell (left) and with isoproterenol (right) at 1 Hz. In isoproterenol cessation of 1-Hz stimulation, leads to spontaneous SR Ca release (right, lower panel) activating inward INCX, which depolarizes the membrane generating DADs (right, upper panel). DADs were not seen in HF (left).
Thus, the computer model shows that there are multiple targets and potential pathways through which CaMKII may be arrhythmogenic. Given the emerging role for CaMKII in cardiac pathogenesis it is important that computer models evolve to capture the dynamic range of CaMKII activity and the functional consequences on cardiomyocyte electrical activity (5–8).
Conclusions
It is increasingly clear that CaMKII has numerous ion channel and Ca regulatory targets in cardiac myocytes, and alters their function. Several of these channels are critically involved in altered electrophysiological and Ca handling properties in pathophysiological settings (e.g. HF), where CaMKII may also have enhanced expression and function. Further studies are needed to clarify details of how CaMKII modulates these and other targets, and especially in the dynamic interactive environment of the cardiac myocyte. Because of the complexity of these interacting systems, computer modeling can be helpful in discerning the relative roles of different pathways and also in terms of testing how well we understand the individual and collective systems. The involvement of CaMKII in the electrical remodeling responsible for pro-arrhythmic signaling and contractile disfunction (e.g. in HF) suggests that this kinase may be a promising therapeutic target in the treatment of common forms of cardiac disease.
Acknowledgments
Sources of support: NIH grants (R37-HL30077, R01-HL64274, P01-HL80101) and from the Fondation Leducq.
Footnotes
Conflict of interest: Nothing to disclose.
References
- 1.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/Calmodulin-Dependent Protein Kinase Modulates Cardiac Ryanodine Receptor Phosphorylation and Sarcoplasmic Reticulum Ca2+ Leak in Heart Failure. Circ Res. 2005;97(12):1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 2.Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Jr., Bers DM, Brown JH. The {delta}C Isoform of CaMKII Is Activated in Cardiac Hypertrophy and Induces Dilated Cardiomyopathy and Heart Failure. Circ Res. 2003;92(8):912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Tandan S, Cheng J, Yang C, Nguyen L, Sugianto J, Johnstone JL, Sun Y, Hill JA. Ca2+/Calmodulin-dependent Protein Kinase II-dependent Remodeling of Ca2+ Current in Pressure Overload Heart Failure. J. Biol. Chem. 2008;283(37):25524–25532. doi: 10.1074/jbc.M803043200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grandi E, Puglisi JL, Wagner S, Maier LS, Severi S, Bers DM. Simulation of Ca-Calmodulin-Dependent Protein Kinase II on Rabbit Ventricular Myocyte Ion Currents and Action Potentials. Biophys. J. 2007;93(11):3835–3847. doi: 10.1529/biophysj.107.114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hund TJ, Rudy Y. Rate Dependence and Regulation of Action Potential and Calcium Transient in a Canine Cardiac Ventricular Cell Model. Circulation. 2004;110(20):3168–3174. doi: 10.1161/01.CIR.0000147231.69595.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Livshitz LM, Rudy Y. Regulation of Ca2+ and electrical alternans in cardiac myocytes: Role of CaMKII and repolarizing currents. Am J Physiol Heart Circ Physiol. 2007 doi: 10.1152/ajpheart.01347.2006. 01347.02006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hund TJ, Decker KF, Kanter E, Mohler PJ, Boyden PA, Schuessler RB, Yamada KA, Rudy Y. Role of activated CaMKII in abnormal calcium homeostasis and INa remodeling after myocardial infarction: Insights from mathematical modeling. 2008;45(3):420. doi: 10.1016/j.yjmcc.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saucerman JJ, Bers DM. Calmodulin Mediates Differential Sensitivity of CaMKII and Calcineurin to Local Ca2+ in Cardiac Myocytes. Biophys. J. 2008;95(10):4597–4612. doi: 10.1529/biophysj.108.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitt GS, Zuhlke RD, Hudmon A, Schulman H, Reuter H, Tsien RW. Molecular Basis of Calmodulin Tethering and Ca2+-dependent Inactivation of L-type Ca2+ Channels. J. Biol. Chem. 2001;276(33):30794–30802. doi: 10.1074/jbc.M104959200. [DOI] [PubMed] [Google Scholar]
- 10.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415(6868):198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 11.Hryshko LV, Bers DM. Ca current facilitation during postrest recovery depends on Ca entry. Am J Physiol Heart Circ Physiol. 1990;259(3):H951–H961. doi: 10.1152/ajpheart.1990.259.3.H951. [DOI] [PubMed] [Google Scholar]
- 12.Ross J, Jr., Miura T, Kambayashi M, Eising GP, Ryu K-H. Adrenergic Control of the Force-Frequency Relation. Circulation. 1995;92(8):2327–2332. doi: 10.1161/01.cir.92.8.2327. [DOI] [PubMed] [Google Scholar]
- 13.Guo J, Duff HJ. Inactivation of ICa-L is the major determinant of use-dependent facilitation in rat cardiomyocytes. J Physiol. 2003;547(3):797–805. doi: 10.1113/jphysiol.2002.033340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo J, Duff HJ. Calmodulin kinase II accelerates L-type Ca2+ current recovery from inactivation and compensates for the direct inhibitory effect of [Ca2+]i in rat ventricular myocytes. J Physiol. 2006;574(2):509–518. doi: 10.1113/jphysiol.2006.109199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson ME, Braun AP, Schulman H, Premack BA. Multifunctional Ca2+/calmodulin-dependent protein kinase mediates Ca2+-induced enhancement of the L-type Ca2+ current in rabbit ventricular myocytes. Circ Res. 1994;75(5):854–861. doi: 10.1161/01.res.75.5.854. [DOI] [PubMed] [Google Scholar]
- 16.Yuan W, Bers DM. Ca-dependent facilitation of cardiac Ca current is due to Ca-calmodulin-dependent protein kinase. Am J Physiol Heart Circ Physiol. 1994;267(3):H982–H993. doi: 10.1152/ajpheart.1994.267.3.H982. [DOI] [PubMed] [Google Scholar]
- 17.Xiao RP, Cheng H, Lederer WJ, Suzuki T, Lakatta EG. Dual regulation of Ca2+/calmodulin-dependent kinase II activity by membrane voltage and by calcium influx. Proc Natl Acad Sci U S A. 1994;91(20):9659–9663. doi: 10.1073/pnas.91.20.9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dzhura I, Wu Y, Colbran RJ, Balser JR, Anderson ME. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nat Cell Biol. 2000;2(3):173–177. doi: 10.1038/35004052. [DOI] [PubMed] [Google Scholar]
- 19.Grueter CE, Abiria SA, Dzhura I, Wu Y, Ham A-JL, Mohler PJ, Anderson ME, Colbran RJ. L-Type Ca2+ Channel Facilitation Mediated by Phosphorylation of the [beta] Subunit by CaMKII. 2006;23(5):641. doi: 10.1016/j.molcel.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic CaMKII{delta}C Overexpression Uniquely Alters Cardiac Myocyte Ca2+ Handling: Reduced SR Ca2+ Load and Activated SR Ca2+ Release. Circ Res. 2003;92(8):904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 21.Kohlhaas M, Zhang T, Seidler T, Zibrova D, Dybkova N, Steen A, Wagner S, Chen L, Heller Brown J, Bers DM, Maier LS. Increased Sarcoplasmic Reticulum Calcium Leak but Unaltered Contractility by Acute CaMKII Overexpression in Isolated Rabbit Cardiac Myocytes. Circ Res. 2006;98(2):235–244. doi: 10.1161/01.RES.0000200739.90811.9f. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y, Temple J, Zhang R, Dzhura I, Zhang W, Trimble R, Roden DM, Passier R, Olson EN, Colbran RJ, Anderson ME. Calmodulin Kinase II and Arrhythmias in a Mouse Model of Cardiac Hypertrophy. Circulation. 2002;106(10):1288–1293. doi: 10.1161/01.cir.0000027583.73268.e7. [DOI] [PubMed] [Google Scholar]
- 23.Cribbs LL, Martin BL, Schroder EA, Keller BB, Delisle BP, Satin J. Identification of the T-Type Calcium Channel (CaV3.1d) in Developing Mouse Heart. Circ Res. 2001;88(4):403–407. doi: 10.1161/01.res.88.4.403. [DOI] [PubMed] [Google Scholar]
- 24.Ferron L, Capuano V, Ruchon Y, Deroubaix E, Coulombe A, Renaud J-F. Angiotensin II Signaling Pathways Mediate Expression of Cardiac T-Type Calcium Channels. Circ Res. 2003;93(12):1241–1248. doi: 10.1161/01.RES.0000106134.69300.B7. [DOI] [PubMed] [Google Scholar]
- 25.Nuss HB, Houser SR. T-type Ca2+ current is expressed in hypertrophied adult feline left ventricular myocytes. Circ Res. 1993;73(4):777–782. doi: 10.1161/01.res.73.4.777. [DOI] [PubMed] [Google Scholar]
- 26.Izumi T, Kihara Y, Sarai N, Yoneda T, Iwanaga Y, Inagaki K, Onozawa Y, Takenaka H, Kita T, Noma A. Reinduction of T-Type Calcium Channels by Endothelin-1 in Failing Hearts In Vivo and in Adult Rat Ventricular Myocytes In Vitro. Circulation. 2003;108(20):2530–2535. doi: 10.1161/01.CIR.0000096484.03318.AB. [DOI] [PubMed] [Google Scholar]
- 27.Barrett PQ, Lu H-K, Colbran R, Czernik A, Pancrazio JJ. Stimulation of unitary T-type Ca2+ channel currents by calmodulin-dependent protein kinase II. Am J Physiol Cell Physiol. 2000;279(6):C1694–C1703. doi: 10.1152/ajpcell.2000.279.6.C1694. [DOI] [PubMed] [Google Scholar]
- 28.Welsby PJ, Wang H, Wolfe JT, Colbran RJ, Johnson ML, Barrett PQ. A Mechanism for the Direct Regulation of T-Type Calcium Channels by Ca2+/Calmodulin-Dependent Kinase II. J. Neurosci. 2003;23(31):10116–10121. doi: 10.1523/JNEUROSCI.23-31-10116.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colomer JM, Mao L, Rockman HA, Means AR. Pressure Overload Selectively Up-Regulates Ca2+/Calmodulin-Dependent Protein Kinase II in Vivo. Mol Endocrinol. 2003;17(2):183–192. doi: 10.1210/me.2002-0350. [DOI] [PubMed] [Google Scholar]
- 30.Wingo TL, Shah VN, Anderson ME, Lybrand TP, Chazin WJ, Balser JR. An EF-hand in the sodium channel couples intracellular calcium to cardiac excitability. 2004;11(3):219. doi: 10.1038/nsmb737. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Ghosh S, Liu H, Tateyama M, Kass RS, Pitt GS. Calmodulin Mediates Ca2+ Sensitivity of Sodium Channels. J. Biol. Chem. 2004;279(43):45004–45012. doi: 10.1074/jbc.M407286200. [DOI] [PubMed] [Google Scholar]
- 32.Tan HL, Kupershmidt S, Zhang R, Stepanovic S, Roden DM, Wilde AAM, Anderson ME, Balser JR. A calcium sensor in the sodium channel modulates cardiac excitability. 2002;415(6870):442. doi: 10.1038/415442a. [DOI] [PubMed] [Google Scholar]
- 33.Young KA, Caldwell JH. Modulation of skeletal and cardiac voltage-gated sodium channels by calmodulin. J Physiol. 2005;565(2):349–370. doi: 10.1113/jphysiol.2004.081422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deschenes I, Neyroud N, DiSilvestre D, Marban E, Yue DT, Tomaselli GF. Isoform-Specific Modulation of Voltage-Gated Na+ Channels by Calmodulin. Circ Res. 2002;90(4):e49–e57. doi: 10.1161/01.res.0000012502.92751.e6. [DOI] [PubMed] [Google Scholar]
- 35.Wagner S, Dybkova N, Rasenack ECL, Jacobshagen C, Fabritz L, Kirchhof P, Maier SKG, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J. Clin. Invest. 2006;116(12):3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maltsev VA, Reznikov V, Undrovinas NA, Sabbah HN, Undrovinas A. Modulation of late sodium current by Ca2+, calmodulin, and CaMKII in normal and failing dog cardiomyocytes: similarities and differences. Am J Physiol Heart Circ Physiol. 2008;294(4):H1597–H1608. doi: 10.1152/ajpheart.00484.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veldkamp MW, Viswanathan PC, Bezzina C, Baartscheer A, Wilde AAM, Balser JR. Two Distinct Congenital Arrhythmias Evoked by a Multidysfunctional Na+ Channel. Circ Res. 2000;86(9):91e–97e. doi: 10.1161/01.res.86.9.e91. [DOI] [PubMed] [Google Scholar]
- 38.Kirchhefer U, Schmitz W, Scholz H, Neumann J. Activity of cAMP-dependent protein kinase and Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human hearts. Cardiovascular Research. 1999;42(1):254. doi: 10.1016/s0008-6363(98)00296-x. [DOI] [PubMed] [Google Scholar]
- 39.Currie S, Loughrey CM, Craig MA, Smith GL. Calcium/calmodulin-dependent protein kinase IIdelta associates with the ryanodine receptor complex and regulates channel function in rabbit heart. Biochem J. 2004;377(Pt 2):357–366. doi: 10.1042/BJ20031043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, Kamp TJ, Makielski JC. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. Journal of Molecular and Cellular Cardiology. 2005;38(3):475. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Tessier S, Karczewski P, Krause E-G, Pansard Y, Acar C, Lang-Lazdunski M, Mercadier J-J, Hatem SN. Regulation of the Transient Outward K+ Current by Ca2+/Calmodulin-Dependent Protein Kinases II in Human Atrial Myocytes. Circ Res. 1999;85(9):810–819. doi: 10.1161/01.res.85.9.810. [DOI] [PubMed] [Google Scholar]
- 42.Colinas O, Gallego M, Setien R, Lopez-Lopez JR, Perez-Garcia MT, Casis O. Differential modulation of Kv4.2 and Kv4.3 channels by calmodulin-dependent protein kinase II in rat cardiac myocytes. Am J Physiol Heart Circ Physiol. 2006;291(4):H1978–H1987. doi: 10.1152/ajpheart.01373.2005. [DOI] [PubMed] [Google Scholar]
- 43.Sergeant GP, Ohya S, Reihill JA, Perrino BA, Amberg GC, Imaizumi Y, Horowitz B, Sanders KM, Koh SD. Regulation of Kv4.3 currents by Ca2+/calmodulin-dependent protein kinase II. Am J Physiol Cell Physiol. 2005;288(2):C304–C313. doi: 10.1152/ajpcell.00293.2004. [DOI] [PubMed] [Google Scholar]
- 44.Li J, Marionneau C, Zhang R, Shah V, Hell JW, Nerbonne JM, Anderson ME. Calmodulin Kinase II Inhibition Shortens Action Potential Duration by Upregulation of K+ Currents. Circ Res. 2006;99(10):1092–1099. doi: 10.1161/01.RES.0000249369.71709.5c. [DOI] [PubMed] [Google Scholar]
- 45.Xiao L, Coutu P, Villeneuve LR, Tadevosyan A, Maguy A, Le Bouter S, Allen BG, Nattel S. Mechanisms Underlying Rate-Dependent Remodeling of Transient Outward Potassium Current in Canine Ventricular Myocytes. Circ Res. 2008;103(7):733–742. doi: 10.1161/CIRCRESAHA.108.171157. [DOI] [PubMed] [Google Scholar]
- 46.Wagner S, Hacker E, Grandi E, Weber S, Dybkova N, Sossalla S, Sowa T, Bers D, Maier L. Ca/Calmodulin Kinase II Differentially Modulates Potassium Currents. Circ Arrhythmia Electrophysiol. doi: 10.1161/CIRCEP.108.842799. (under revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Cheng J, Tandan S, Jiang M, McCloskey DT, Hill JA. Transient-Outward K+ Channel Inhibition Facilitates L-Type Ca2+ Current in Heart. Journal of Cardiovascular Electrophysiology. 2006;17(3):298–304. doi: 10.1111/j.1540-8167.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- 48.Keskanokwong T, Cheng J, Wang Y. Abstract 1536: The CaMKII-Ito Channel Functional Unit in Cardiomyocytes. Circulation. 2008;118 (18_MeetingAbstracts):S_346-b- [Google Scholar]
- 49.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and Contractile Dysfunction in Heart Failure: Roles of Sodium-Calcium Exchange, Inward Rectifier Potassium Current, and Residual {beta}-Adrenergic Responsiveness. Circ Res. 2001;88(11):1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- 50.Beuckelmann D, Näbauer M, Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993;73:379–385. doi: 10.1161/01.res.73.2.379. [DOI] [PubMed] [Google Scholar]
- 51.Nitta J, Furukawa T, Marumo F, Sawanobori T, Hiraoka M. Subcellular mechanism for Ca2+-dependent enhancement of delayed rectifier K+ current in isolated membrane patches of guinea pig ventricular myocytes. Circ Res. 1994;74(1):96–104. doi: 10.1161/01.res.74.1.96. [DOI] [PubMed] [Google Scholar]
- 52.Yus-Najera E, Santana-Castro I, Villarroel A. The Identification and Characterization of a Noncontinuous Calmodulin-binding Site in Noninactivating Voltage-dependent KCNQ Potassium Channels. J. Biol. Chem. 2002;277(32):28545–28553. doi: 10.1074/jbc.M204130200. [DOI] [PubMed] [Google Scholar]
- 53.Ghosh S, Nunziato DA, Pitt GS. KCNQ1 Assembly and Function Is Blocked by Long-QT Syndrome Mutations That Disrupt Interaction With Calmodulin. Circ Res. 2006;98(8):1048–1054. doi: 10.1161/01.RES.0000218863.44140.f2. [DOI] [PubMed] [Google Scholar]
- 54.Rigg L, Mattick PAD, Heath BM, Terrar DA. Modulation of the hyperpolarization-activated current (If) by calcium and calmodulin in the guinea-pig sino-atrial node. Cardiovasc Res. 2003;57(2):497–504. doi: 10.1016/s0008-6363(02)00668-5. [DOI] [PubMed] [Google Scholar]
- 55.Hagiwara N, Irisawa H. Modulation by intracellular Ca2+ of the hyperpolarization-activated inward current in rabbit single sino-atrial node cells. J Physiol. 1989;409(1):121–141. doi: 10.1113/jphysiol.1989.sp017488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vinogradova TM, Zhou Y-Y, Bogdanov KY, Yang D, Kuschel M, Cheng H, Xiao R-P. Sinoatrial Node Pacemaker Activity Requires Ca2+/Calmodulin-Dependent Protein Kinase II Activation. Circ Res. 2000;87(9):760–767. doi: 10.1161/01.res.87.9.760. [DOI] [PubMed] [Google Scholar]
- 57.Sirenko S, Li Y, Yang D, Lukyanenko Y, Lakatta EG, Vinogradova TM. Abstract 1537: Basal Phosphorylation Of Ca2+ Cycling Proteins By Both PKA And CAMKII is Required For Robust Generation Of Local Subsarcolemmal Ca2+ Releases To Drive Sinoatrial Node Cell Automaticity. Circulation. 2008 118; (18_MeetingAbstracts):S_346-c- [Google Scholar]
- 58.Witcher DR, Kovacs RJ, Schulman H, Cefali DC, Jones LR. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J. Biol. Chem. 1991;266(17):11144–11152. [PubMed] [Google Scholar]
- 59.Wehrens XHT, Lehnart SE, Reiken SR, Marks AR. Ca2+/Calmodulin-Dependent Protein Kinase II Phosphorylation Regulates the Cardiac Ryanodine Receptor. Circ Res. 2004;94(6):e61–e70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 60.Rodriguez P, Bhogal MS, Colyer J. Stoichiometric Phosphorylation of Cardiac Ryanodine Receptor on Serine 2809 by Calmodulin-dependent Kinase II and Protein Kinase A. J. Biol. Chem. 2003;278(40):38593–38600. doi: 10.1074/jbc.C301180200. [DOI] [PubMed] [Google Scholar]
- 61.Hain J, Onoue H, Mayrleitner M, Fleischer S, Schindler H. Phosphorylation Modulates the Function of the Calcium Release Channel of Sarcoplasmic Reticulum from Cardiac Muscle. J. Biol. Chem. 1995;270(5):2074–2081. doi: 10.1074/jbc.270.5.2074. [DOI] [PubMed] [Google Scholar]
- 62.Lokuta AJ, Rogers TB, Lederer WJ, Valdivia HH. Modulation of cardiac ryanodine receptors of swine and rabbit by a phosphorylation-dephosphorylation mechanism. J Physiol. 1995;487(Pt3):609–622. doi: 10.1113/jphysiol.1995.sp020904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li L, Satoh H, Ginsburg KS, Bers DM. The effect of Ca2+-calmodulin-dependent protein kinase II on cardiac excitation-contraction coupling in ferret ventricular myocytes. J Physiol. 1997;501(Pt1):17–31. doi: 10.1111/j.1469-7793.1997.017bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo T, Zhang T, Mestril R, Bers DM. Ca2+/Calmodulin-Dependent Protein Kinase II Phosphorylation of Ryanodine Receptor Does Affect Calcium Sparks in Mouse Ventricular Myocytes. Circ Res. 2006;99(4):398–406. doi: 10.1161/01.RES.0000236756.06252.13. [DOI] [PubMed] [Google Scholar]
- 65.Yang D, Zhu W-Z, Xiao B, Brochet DXP, Chen SRW, Lakatta EG, Xiao R-P, Cheng H. Ca2+/Calmodulin Kinase II-Dependent Phosphorylation of Ryanodine Receptors Suppresses Ca2+ Sparks and Ca2+ Waves in Cardiac Myocytes. Circ Res. 2007;100(3):399–407. doi: 10.1161/01.RES.0000258022.13090.55. [DOI] [PubMed] [Google Scholar]
- 66.Perez PJ, Ramos-Franco J, Fill M, Mignery GA. Identification and Functional Reconstitution of the Type 2 Inositol 1,4,5-Trisphosphate Receptor from Ventricular Cardiac Myocytes. J.Biol. Chem. 1997;272(38):23961–23969. doi: 10.1074/jbc.272.38.23961. [DOI] [PubMed] [Google Scholar]
- 67.Zima AV, Blatter LA. Inositol-1,4,5-trisphosphate-dependent Ca2+ signalling in cat atrial excitation-contraction coupling and arrhythmias. J Physiol. 2004;555(3):607–615. doi: 10.1113/jphysiol.2003.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Domeier TL, Zima AV, Maxwell JT, Huke S, Mignery GA, Blatter LA. IP3 receptor-dependent Ca2+ release modulates excitation-contraction coupling in rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol. 2008;294(2):H596–H604. doi: 10.1152/ajpheart.01155.2007. [DOI] [PubMed] [Google Scholar]
- 69.Bare DJ, Kettlun CS, Liang M, Bers DM, Mignery GA. Cardiac Type 2 Inositol 1,4,5-Trisphosphate Receptor: Interaction and modulation by calcium/calmodulin-dependent protein kinase II. J. Biol. Chem. 2005;280(16):15912–15920. doi: 10.1074/jbc.M414212200. [DOI] [PubMed] [Google Scholar]
- 70.Matifat F, Hague F, Brûlé G, Collin T. Regulation of InsP3-mediated Ca2+ release by CaMKII in Xenopus oocytes. Pflügers Archiv European Journal of Physiology. 2001;441(6):796–801. doi: 10.1007/s004240000479. [DOI] [PubMed] [Google Scholar]
- 71.Zhu DM, Tekle E, Chock PB, Huang CY. Reversible Phosphorylation as a Controlling Factor for Sustaining Calcium Oscillations in HeLa Cells: Involvement of Calmodulin-Dependent Kinase II and a Calyculin A-Inhibitable Phosphatase. Biochemistry. 1996;35(22):7214–7223. doi: 10.1021/bi952471h. [DOI] [PubMed] [Google Scholar]
- 72.Wu X, Zhang T, Bossuyt J, Li X, McKinsey T, Dedman J, Olson E, Chen J, Brown J, Bers D. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J Clin Invest. 2006;116(3):675–682. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Go L, Moschella M, Watras J, Handa K, Fyfe B, Marks A. Differential regulation of two types of intracellular calcium release channels during end-stage heart failure. J Clin Invest. 1995;95(2):888–894. doi: 10.1172/JCI117739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clancy CE, Rudy Y. Na+ Channel Mutation That Causes Both Brugada and Long-QT Syndrome Phenotypes: A Simulation Study of Mechanism. Circulation. 2002;105(10):1208–1213. doi: 10.1161/hc1002.105183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tanskanen AJ, Greenstein JL, O'Rourke B, Winslow RL. The Role of Stochastic and Modal Gating of Cardiac L-Type Ca2+ Channels on Early After-Depolarizations. 2005;88(1):85. doi: 10.1529/biophysj.104.051508. [DOI] [PMC free article] [PubMed] [Google Scholar]