Abstract
CD44 is a transmembrane glycoprotein expressed in various tissues including the skin. Previous studies indicated that CD44 is required for epidermal permeability barrier homeostasis and keratinocyte differentiation. Yet, while some studies have demonstrated that CD44 is critical for the development of inflammation, others have shown that CD44 is not essential for the development of cutaneous inflammation. In this study, we evaluated the changes in epidermal CD44 expression in a variety of skin inflammatory models and determined whether CD44 is required for the development of cutaneous inflammation. Inflammatory responses were compared in CD44 KO versus wild-type mice in acute models of irritant and allergic contact dermatitis, as well as in a subacute allergic contact dermatitis induced by repeated hapten treatment. Inflammatory responses were assessed by measuring ear thickness and epidermal hyperplasia in haematoxylin & eosin-stained sections. Our results demonstrate that: (i) epidermal CD44 expression increases in both acute and subacute cutaneous inflammatory models; and (ii) acute disruption of the epidermal permeability barrier function increases epidermal CD44 expression. Whereas inflammatory responses did not differ between CD44 KO and wild-type mice in acute models of irritant and allergic contact dermatitis, both inflammatory responses and epidermal hyperplasia increased in CD44 KO mice following repeated hapten challenges. These results show first, that permeability barrier disruption and inflammation stimulate epidermal CD44 expression, and second, that CD44 modulates epidermal proliferation and inflammatory responses in a subacute murine allergic contact dermatitis model.
Keywords: animal models, barrier function, CD44, CD44 KO mice, inflammation
Introduction
CD44 is a ubiquitous transmembrane glycoprotein that also is expressed on the plasma membrane of both epidermal keratinocytes and leucocytes (1–6). Previous studies demonstrated that CD44 plays an important role in cutaneous function. For example, CD44 knockout mice show (4): (i) a defect in epidermal permeability barrier homeostasis; (ii) epidermal thinning; and (iii) decreased keratinocyte differentiation. Likewise, in cultured keratinocytes, hyaluronan–CD44 interaction stimulates keratinocyte differentiation and lamellar body formation (4). Accordingly, hyaluronan induces keratinocyte proliferation both in primary cell cultures and epidermal hyperplasia in wild-type, but not in CD44-deficient mice (4,7). In addition, topical application of intermediate-size hyaluronan fragment (50–400 kDa) reverses skin atrophy in both aged and long-term corticosteroid-treated patients (8). Finally, keratinocyte proliferation declines in response to phorbol ester stimulation in CD44-deficient as compared with wild-type mice (7).
Whether CD44 is pro-inflammatory or anti-inflammatory in the skin, however, is controversial. CD44 regulates lymphocyte proliferation, maturation, activation, homing, extravasation and infiltration (9–18). Moreover, administration of anti-CD44 antibody reduced cell infiltration and cutaneous oedema in a delayed type hypersensitive dermatitis model (19–21), and allergic dermatitis induced by ovalbumin injection is reduced in CD44 KO mice in comparison with wild-type mice (22). In addition to cutaneous inflammation, CD44 also plays a positive role in other inflammatory disorders such as arthritis and colitis. Both the severity and the incidence of arthritis induced by proteoglycans are reduced in either CD44 knockout or anti-CD44 antibody-treated mice (23–28). Moreover, administration of anti-CD44v7 antibody improves colitis induced by dextran sulphate sodium or trinitrobenzene sulphonic acid (29,30). Finally, CD44v7-deficient mice developed only a minor colitis after trinitrobenezene sulphonic acid treatment (31).
Other studies indicate that CD44 suppresses inflammation (32). Inflammatory cell migration and cytokine production, including TNFα, IL-1β and IL-6, following lipopolysaccharide treatment, are enhanced in CD44 knockout mice (32). To delineate further the role of CD44 in cutaneous inflammation, in this study, we first determined the effects of barrier disruption and inflammation on epidermal CD44 expression, and then compared cutaneous inflammatory responses to various stimuli in CD44 knockout versus wild-type mice. Our results show that epidermal CD44 expression is up-regulated in response to both barrier disruption and various types of inflammation. We show further that CD44 negatively regulates cutaneous inflammation in a subacute allergic contact dermatitis model.
Materials and methods
All animal procedures were approved by the Animal Studies Subcommittee of the San Francisco Veterans Administration Medical Center and were performed in accordance with their guidelines.
Materials
Female hairless mice (hr/hr), 6–8 weeks old, were purchased from Charles River laboratories (Wilmington, MA, USA) and fed standard mouse diet (Ralston-Purina Co, St Louis, MO, USA) and water ad libitum. Six- to eight-week-old female CD44 knockout and wild-type mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Phorbol 12-myristate 13-acetate (TPA) and 4-ethoxymethylene-2-phenyl-2-oxazolin-5-one (oxazolone) were purchased from Sigma Chemical Co (St Louis, MO, USA). Biotinylated hyaluronic acid-binding protein and monoclonal rat anti-human CD44 antibody (clone 020; isotype IgG2b), which recognizes the ∼85–95 kDa CD44 protein (gp90) in humans and GP85 or Pgp-1 in mice, were from CMB-TECH Inc (San Francisco, CA, USA). ABC-peroxidase kit was purchased from Vector Laboratories (Burlingame, CA, USA). FITC-conjugated monoclonal anti-T-cell receptor (TCR) Vβ 8.1–3 (F23.1) antibody and polyclonal goat anti-IL-1α antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Models of inflammatory dermatoses
To minimize the skin damage caused by shaving, all experiments were carried out on hairless mice except, for experiments in which CD44 KO mice and wild-type control mice were used.
Acute irritant dermatitis model was induced by topical application of 10 µl of 0.03% TPA to both the inner and outer surfaces of the ears (33). Ear thickness was measured 20 h after TPA application, followed by biopsies.
Acute allergic contact dermatitis model was induced by first sensitizing animals with a topical application of 3% oxazolone to the back once daily for 2 days, followed by topical challenge with 0.5% oxazolone on both inner and outer surfaces of both ears 7 days later (33). Ears treated with acetone alone served as a vehicle control. Ear thickness was measured with a digital caliper (Mitutoyo, Tokyo, Japan) both before and 20 h after last oxazolone or TPA application, and samples were taken at that time-point for haematoxylin & eosin (H&E) staining and immunohisto-chemistry.
Subacute allergic contact dermatitis model was induced by first topical sensitizing mice with 3% oxazolone once daily for 2 days, followed by topical challenges with 0.1% oxazolone beginning on the seventh day, once every other day for 10 days (total of five challenge doses) (34).
Acute permeability barrier abrogation model was achieved by repeated tape-stripping (3 times) of mouse flank until transepidermal water loss exceeded 2 mg/cm2/h (35). Skin biopsies were taken at 3, 6 and 24 h following tape-stripping.
Immunohistochemistry
Determinations of cutaneous hyaluronic acid, epidermal CD44 and dermal IL-1α expression were performed as previously described (4). Briefly, 5-µm paraffin sections were incubated with an antibody against CD44 or hyaluronic acid-binding protein overnight at 4°C. For TCR Vβ 8.1–3 staining, 5-µm paraffin sections were incubated with FITC-conjugated monoclonal anti-Vβ 8.1–3 antibody overnight at 4°C. Immunostaining was detected by ABC peroxidase method and, in some cases, sections were counter-stained with haematoxylin. Sections were visualized with a Zeiss Microscope (Jena, Germany), and digital images were taken with Axio Vision software 3.1 (Carl Zeiss Vision, Munich, Germany) (33,34).
Epidermal thickness measurements and inflammatory cell quantification
Thickness of the epidermal nucleated cell layers was measured on 100× micrographs taken every 2 cm along the epidermis in biopsies from vehicle and oxazolone-treated skin of both wild-type and CD44 KO mice. The data presented represent the mean of all measured points ±SEM. The number of eosinophils and the number of inflammatory cells infiltrating the dermis in subacute allergic dermatitis model was identified and was counted on every 25 mm2 area at regions between basement membrane and 5 mm below basement membrane on H&E-stained section with a Zeiss microscope, equipped with AxioVision software 3.1 (34). Data are presented as the mean of all areas counted ± SEM.
All statistical analyses were performed using the two-tailed Student’s t-test. Data were expressed as mean ± SEM.
Results
Epidermal CD44 expression increases in inflamed skin
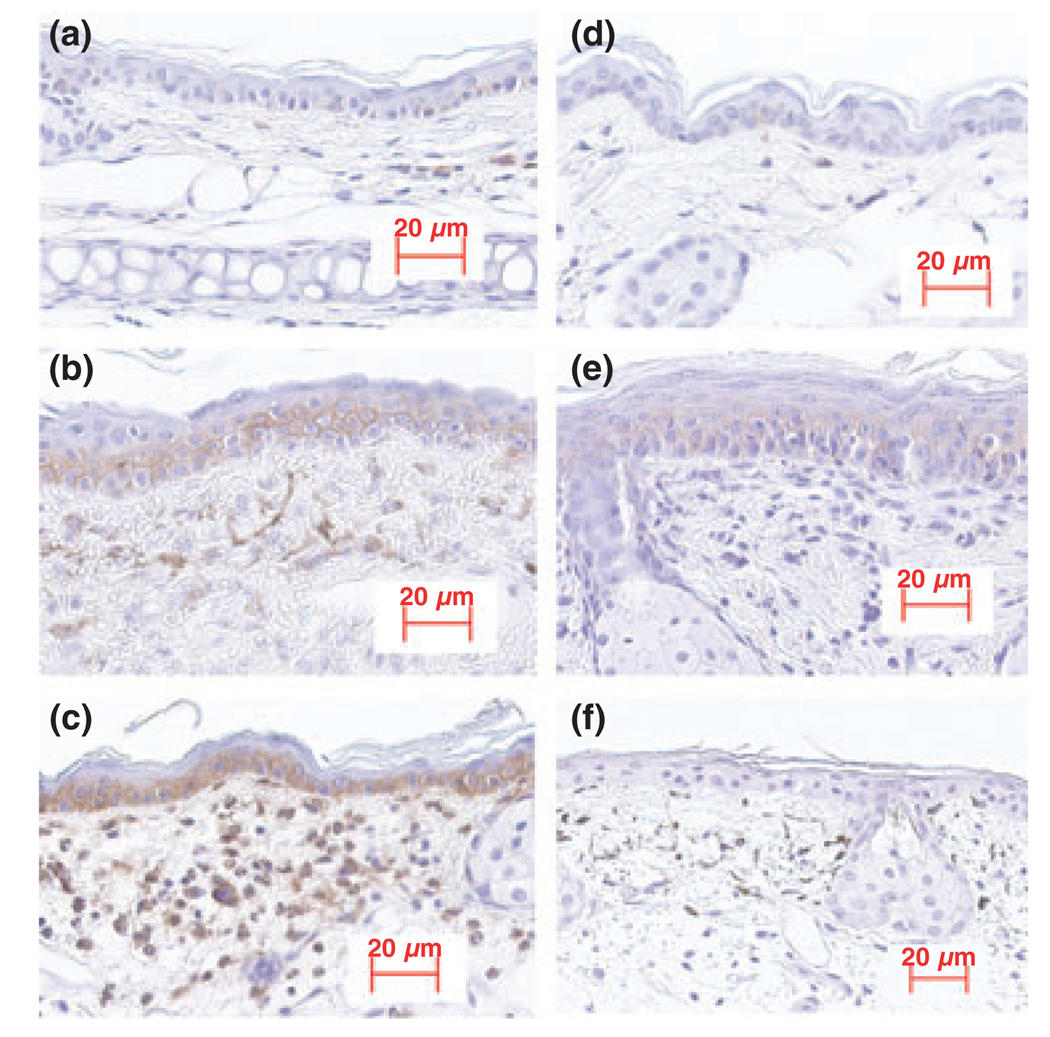
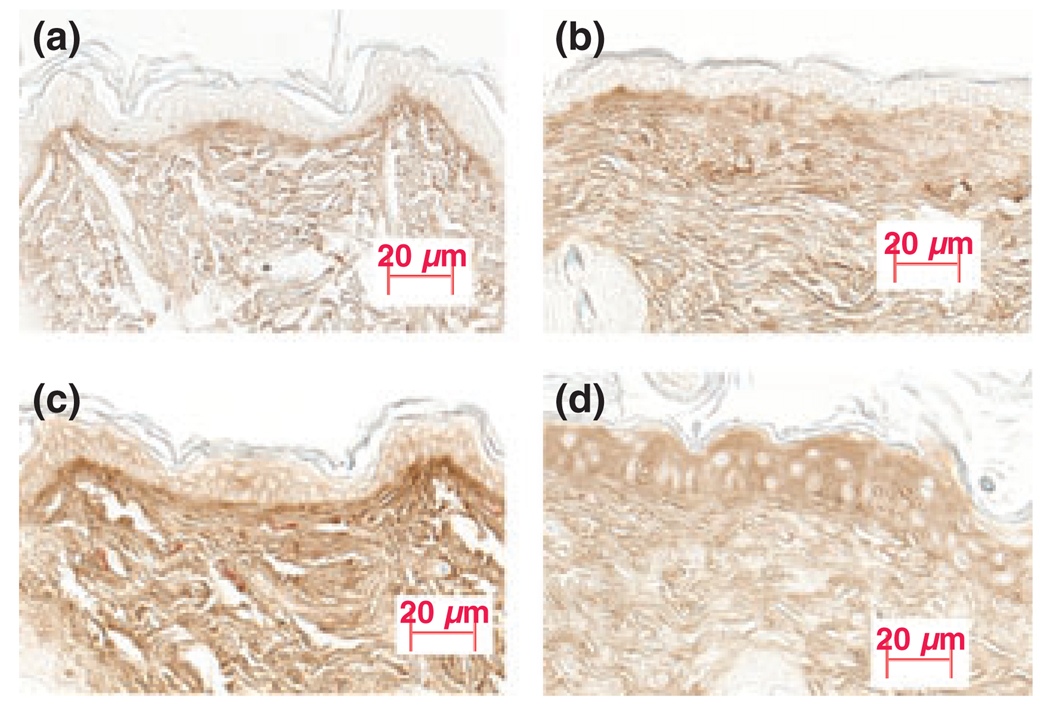
To determine the role of CD44 in cutaneous inflammation, we first assessed the effects of different types of cutaneous inflammation on epidermal CD44 expression in hairless mice. As shown in Fig. 1, a dramatic increase in epidermal CD44 expression occurred in animal models of both acute allergic (Fig. 1b) and irritant (Fig. 1c) contact dermatitis. In addition, epidermal CD44 expression increased in the subacute allergic contact dermatitis model (Fig. 1e). No epidermal CD44 expression was detected in skin samples from CD44 KO mice (Fig. 1f), further validating the specificity of the immunohistochemical results presented here. Together, these results demonstrate that epidermal CD44 expression increases in inflamed skin.
Figure 1.
Epidermal CD44 expression is increased in inflamed skin: cutaneous inflammatory models were established as described in the Materials and methods section. Tissue sections were stained with anti-CD44 antibody and counter-stained with haematoxylin. (a) Normal ear; (b) acute inflamed ear induced by topical oxazolone; (c) acute inflamed ear induced by topical TPA; (d) normal skin; (e) the subacute dermatitis model induced by repeated topical applications of oxazolone; (f) acute inflamed ear induced by topical TPA in CD44 KO mice, which served as a negative control. The magnifications are the same for all pictures. Scale bar = 20 µm.
Epidermal CD44 expression increases after acute permeability barrier disruption
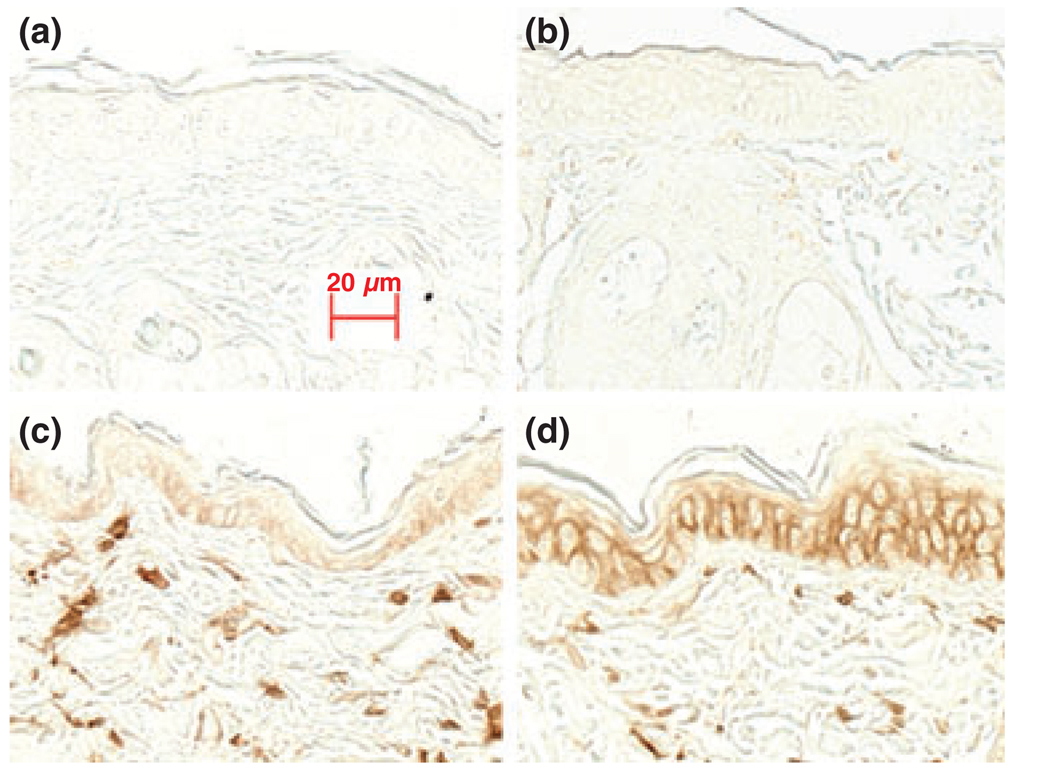
Previous studies from our laboratory and others have shown that acute barrier disruption increases epidermal cytokine expression, which if sustained can lead to inflammation (36,37). To assess whether epidermal CD44 expression changes in response to this pro-inflammatory stimulus, epidermal CD44 expression in hairless mice was evaluated 3, 6 and 24 h following acute barrier disruption. As shown in Fig. 2, epidermal CD44 expression did not appear to change 3 h following barrier disruption (Fig. 2b vs 2a), but by 6 h, there was a detectable increase in epidermal CD44 expression (Fig. 2c), which increased further at 24 h (Fig 2d). These results suggest that epidermal CD44 expression increases following acute permeability barrier abrogation.
Figure 2.
Permeability barrier disruption increases epidermal CD44 expression: Acute permeability barrier disruption was induced by repeated tape-stripping as described in the Materials and methods section. Skin samples were taken at the indicated times following barrier disruption. Sections were stained with anti-CD44 antibody without counter-staining. (a) Normal skin; (b) 3 h after barrier disruption; (c) 6 h after barrier disruption; and (d) 24 h after barrier disruption. The magnifications are the same for all pictures. Scale bar = 20 µm.
CD44 deficiency alters the severity of neither acute irritant nor allergic contact dermatitis
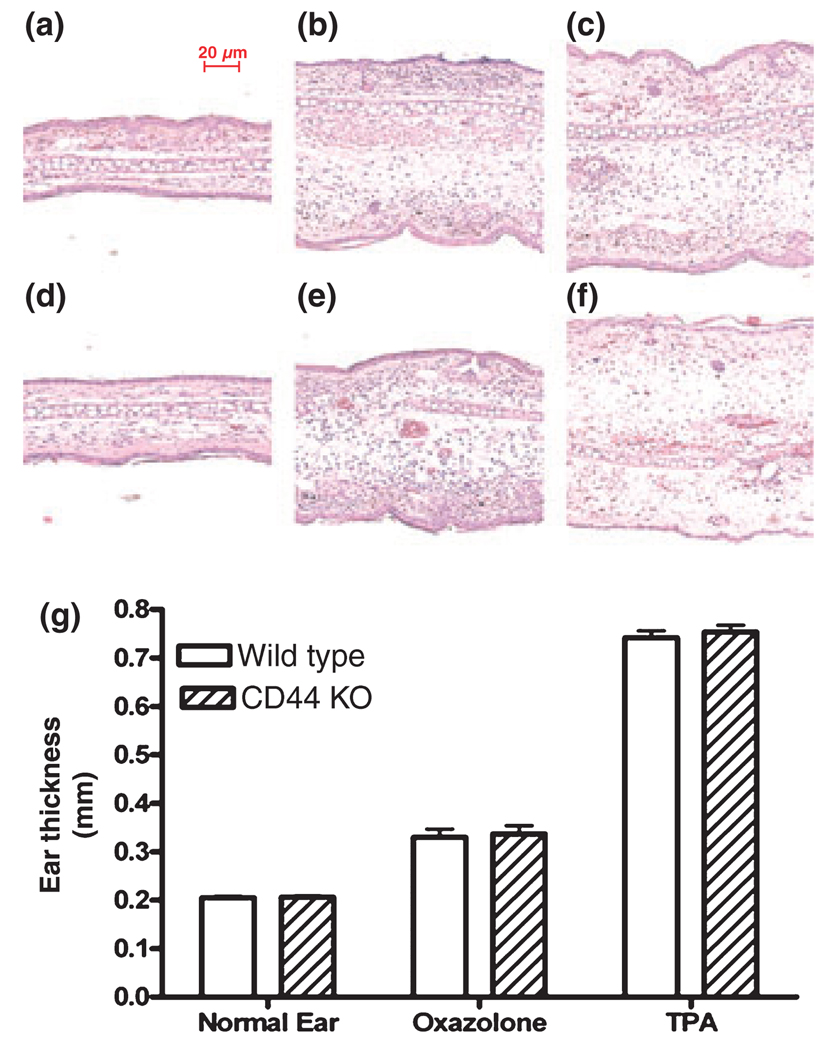
As the above results indicate that cutaneous inflammation increases epidermal CD44 expression, we next determined whether CD44 is required for the development of cutaneous inflammation. In both the acute irritant and allergic contact dermatitis models, the increase in ear thickness in CD44 knockout mice was comparable with that observed in wild-type mice (Fig. 3g). In addition, the density of cutaneous inflammatory cell infiltration did not differ substantially between CD44 KO and wild-type mice, as shown by H&E staining (Fig. 3b vs 3e; Fig. 3c vs 3f). Furthermore, there was no dramatic difference in cutaneous IL-1α immunostaining between CD44 KO and wild-type mice in both the acute dermatitis models (Fig. S1). These results suggest that CD44 may not play a crucial role in regulating cutaneous inflammation in either acute irritant or allergic contact dermatitis models.
Figure 3.
No difference in the inflammatory response between CD44 KO and wild-type mice in acute models of contact dermatitis: ear inflammation was induced by topical TPA or oxazolone treatment as described in the Materials and methods section. Ear samples were obtained 20 h after TPA or the last oxazolone application. No significant difference in inflammatory cell infiltration was seen between CD44 KO and wild-type mice (3b vs 3e; 3c vs 3f). (a) Normal skin from wild-type mice and d is normal skin from CD44 KO mice. (b, e) Acute dermatitis induced by topical oxazolone in wild-type and . (c, f) Acute dermatitis induced by topical TPA in wild-type and CD44 KO mice, respectively. (g) The ear thickness measured 20 h after last oxazolone or TPA application as described in Materials and methods section. The magnifications are the same for all pictures. Scale bar = 20 µm. N = 10 for normal and n = 4 for all other groups. Data are expressed as mean ± SEM.
CD44 deficiency enhances cutaneous inflammatory response in subacute allergic contact dermatitis
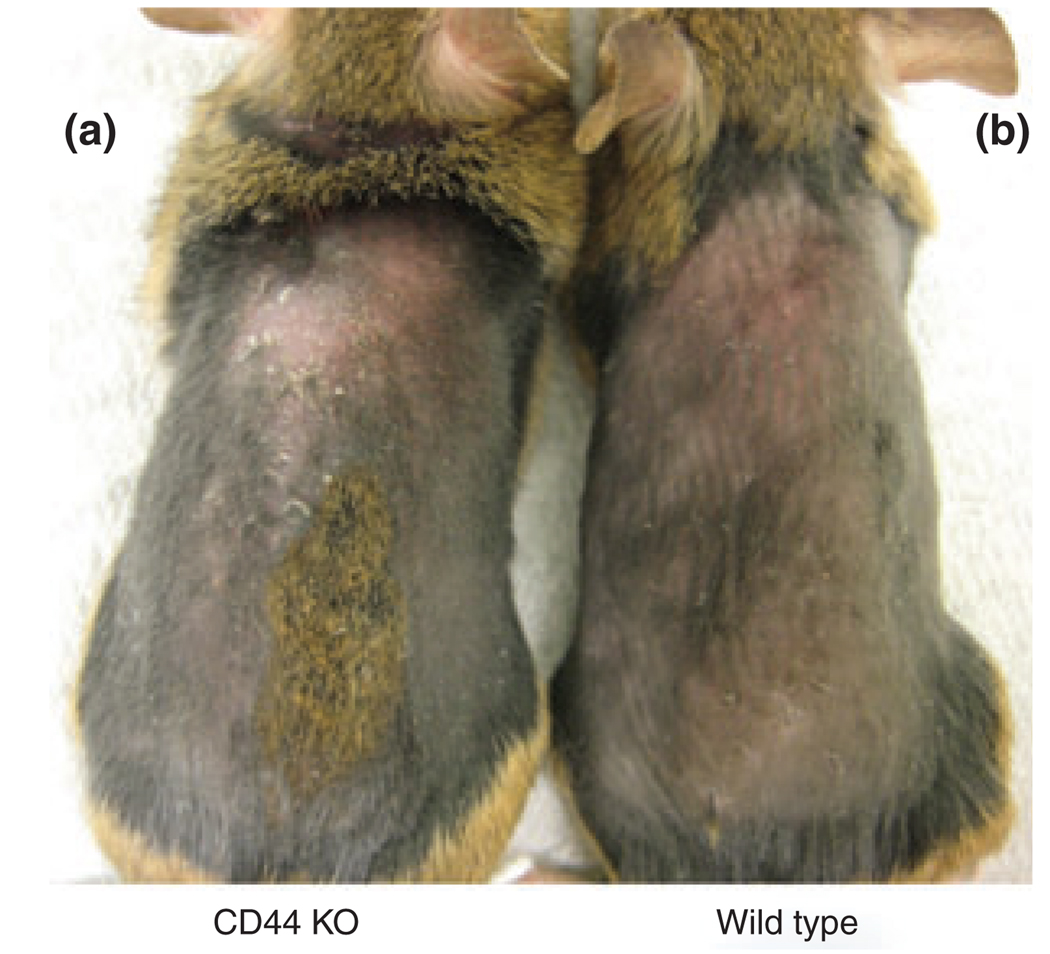
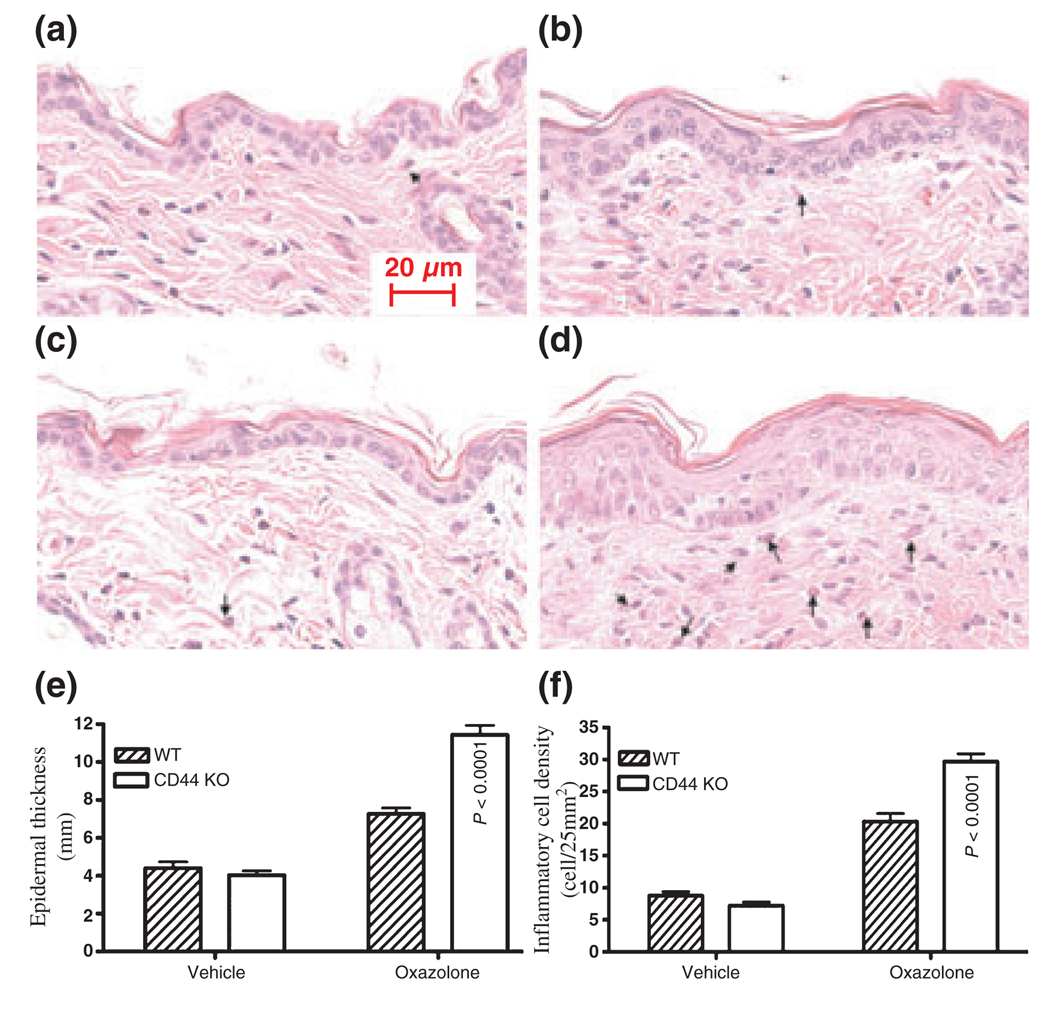
As no difference was found in the inflammatory response in the acute dermatitis models, we next tested whether CD44 deficiency alters the cutaneous inflammatory response in a model of subacute allergic contact dermatitis (chronic delayed-type hypersensitivity) induced by repeated hapten (oxazolone) challenges. As shown in Fig. 4, CD44 KO mice showed extensive skin abnormalities, drier skin and increased scaling, while the skin of wild-type mouse appeared near normal. Moreover, the epidermis was thicker in CD44 KO mice than in wild-type mouse (Fig. 5b vs 5d; Fig. 5e).
Figure 4.
Gross appearance of wild-type and CD44 KO mice treated with oxazolone: mice were topically sensitized with 3% oxazolone once daily for 2 days, followed by topical challenge with 0.1% oxazolone 7 days later, once every other day for 10 days. (a) (left) CD44 KO mice treated with oxazolone and (b) (right) wild-type mice treated with oxazolone.
Figure 5.
The inflammatory cell infiltrate and epidermal thickness were increased in CD44 KO mice in the subacute dermatitis model: subacute dermatitis was induced as described in the Materials and methods section. Skin samples were taken 24 h after the last oxazolone treatment. (a) Wild-type mice treated with vehicle; (b) wild-type mice treated with oxazolone; (c) CD44 KO mice treated with vehicle; (d) CD44 KO mice treated with oxazolone. The arrows indicate eosinophils. The epidermal thickness was measured on 100× micrographs as described in the Materials and methods section. The number of inflammatory cells in dermis was randomly counted in H&E stained sections with a Zeiss microscope, equipped with AxioVision software 3.1. The counted areas were 5 mm below the basement membrane. The density of inflammatory cells was expressed as the number of cell per 25 mm2. There were four mice for each treatment group. (e) The segments counted were 32–37 on three sections; (f) the segments counted were 13–19 on three sections. The differences were significant between wild-type and CD44 KO mice. Scale bar = 20 µm.
Previous studies demonstrated increased eosinophil infiltration in subacute allergic dermatitis (34), and TCR Vβ 8.1–3 positive T cells are a major cell type involved in delayed-type hypersensitivity (38–40). Indeed, there was a more extensive inflammatory cell infiltrate, with a higher density of eosinophils (Fig. 5b vs 5d; arrows indicate eosinophils), and an increase in TCR Vβ 8.1–3 positive T cells (Fig. S2), in CD44 KO mice versus wild-type mice. Finally, dermal IL-1α expression was also increased in CD44 KO mice as compared with wild-type mice in this subacute allergic contact dermatitis model (Fig. S3). These results suggest that the absence of CD44 predisposes to increase cutaneous inflammation in a subacute allergic contact dermatitis model.
Previous studies have demonstrated that lung inflammation is increased in CD44-deficient mice, and this increase has been attributed to the accumulation of hyaluronic acid (41). Therefore, we next assessed whether hyaluronic acid expression also increases in the subacute dermatitis model in CD44 KO mice. As shown in Fig. 6, after 10 days of vehicle treatment, there was a modest increase in dermal hyaluronic acid expression in CD44 KO mice compared with wild-type mice (Fig. 6a,b). Moreover, there was also a significant increase in both dermal and epidermal hyaluronic acid expression in wild-type mice with the induction of subacute dermatitis (Fig. 6c). Dermal hyaluronic acid expression did not appear to change (Fig. 6d), while epidermal hyaluronic acid expression dramatically increased in CD44 KO mice as compared with wild-type mice with the induction of subacute dermatitis (Fig. 6c vs 6d). These results suggest that the enhanced cutaneous inflammatory response in CD44 KO mice is not likely because of dermal hyaluronic acid accumulation. The increased epidermal hyaluronic acid expression could contribute to enhanced epidermal hyperproliferation as demonstrated previously (42).
Figure 6.
Epidermal hyaluronic acid expression was increased in CD44 KO mice in the subacute allergic dermatitis model: subacute allergic dermatitis was induced as described in the Materials and methods section. Skin samples were taken 24 h after the last oxazolone treatment. (a) Wild-type mice treated with vehicle; (b) CD44 KO mice treated with vehicle; (c) wild-type mice treated with oxazolone; (d) CD44 KO mice treated with oxazolone. Cutaneous hyaluronic acid staining was performed as described in the Materials and methods section and pictures were taken with a digital camera, equipped with AxioVision software 3.1. There were four mice for each treatment group. Figures shown are representative. Scale bar = 20 µm.
Discussion
Previous studies have shown that CD44 regulates epidermal proliferation, keratinocyte differentiation and permeability barrier function (4). In this study, we demonstrate that cutaneous inflammation stimulates epidermal CD44 expression regardless of the method used to induce inflammation. The mechanisms by which inflammation enhances epidermal CD44 expression remain unclear. However, previous studies demonstrated that cytokines such as TNFα, IL-1α, IL-1β and IL-10 stimulate CD44 expression in other systems (43–46) and we showed here that IL-1 level increased in CD44 KO mice with the induction of subacute dermatitis. Thus, the enhanced epidermal CD44 expression during cutaneous inflammation could be because of the increased cytokine levels.
This study further demonstrates that CD44 may not be crucial for acute cutaneous inflammation, although other studies have demonstrated the importance of CD44 in acute inflammation (16–31,47). There were no significant differences in inflammatory responses induced by either acute irritant or allergic contact dermatitis in CD44 knock-out and wild-type mice. These results are consistent with previous studies (48–50), which showed that CD44 deficiency did not alter the inflammatory response in an allergic contact dermatitis model and anti-CD44 antibody did not reduce inflammation in either irritant or allergic contact dermatitis. Moreover, it has been reported that T cells from CD44-deficient mice showed normal immune responses to type II collagen or other antigen (51). There are at least two mechanisms by which CD44-deficient mice could develop inflammation. First, other adhesion molecules, such as L-selectin, E-selectin, as well as ICAM-1, also play roles in inflammation (51–54). Adhesion molecules, other than CD44, may play a key role in inflammatory cell extravasation during inflammation (51). Second, there are other proteins, which could bind hyaluronan and initiate inflammation, thereby compensating for the absence of CD44 (reviewed in (55). Thus, it is not entirely unexpected that CD44 deficiency does not decrease acute cutaneous inflammation.
In contrast to the acute dermatitis models, this study did demonstrate that CD44 deficiency enhances chronic cutaneous inflammation, as demonstrated by an increase in dermal inflammatory cell infiltration and cytokine expression in a subacute allergic contact dermatitis model. This result is in agreement with others, who showed that CD44 is a negative regulator in pulmonary inflammation induced by lipopolysaccharide or bleomycin (32,41). Studies indicated that there are several mechanisms that could cause exaggerated inflammation in CD44 deficiency. For example, increased inflammatory gene expression occurs in CD44 KO mice (32). In addition, studies also showed that interaction of hyaluronic acid with CD44 exhibits anti-allergic effect, and topical application of hyaluronic acid improves inflammation in DNFB-induced atopic dermatitis model (56). Therefore, the enhanced cutaneous inflammation in CD44 mice may be because of both elevated cytokine production and decreased interaction of hyaluronic acid with CD44. Thus, while the absence of CD44 does not affect acute inflammation, CD44 deficiency enhances chronic inflammation.
Previous studies have shown a decrease in epidermal thickness and keratinocyte proliferation in CD44-deficient mice (4). However, in the studies reported here, we observed that chronic inflammation provoked an increase in epidermal thickness in CD44-deficient mice. The mechanism accounting for these discordant results is not clear. It is possible that the increased inflammation and cytokine production in CD44-deficient mice with hapten challenges results in a secondary increase in epidermal thickness. It is well-recognized that inflammation and a concurrent increase in cytokine levels are associated with keratinocyte proliferation (35,56,57). In addition, increased accumulation of hyaluronic acid in the epidermis of CD44-deficient mice could result in epidermal thickening. Accordingly, prior studies have shown that the accumulation of epidermal hyaluronic acid increases epidermal thickness following epidermal permeability barrier disruption (42), and topical application of low molecular weight hyaluronic acid fragment accelerates wound healing (58,59). Thus, a number of pathways could account for the increase in epidermal thickness in CD44-deficient mice with chronic inflammation.
In conclusion, this study demonstrates that epidermal CD44 expression is stimulated by cutaneous inflammations regardless of the methods by which inflammation is induced. Conversely, CD44 may not be crucial for acute cutaneous inflammation, but it appears to play a role in suppressing chronic inflammation.
Acknowledgements
The authors greatly appreciate the invaluable help of Sandra Chang. This work was supported by National Institutes of Health grants AR 050629, AR 19098 and the Medical Research Service, Department of Veterans Affairs Medical Center.
Footnotes
Conflict of interest
All authors have no conflicts of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. No difference in cutaneous IL-1α expression between CD44 KO and wild-type mice in acute contact dermatitis models: ear inflammation was induced by topical TPA or oxazolone treatment as described in the Materials and methods section. Ear samples were obtained 20 h after TPA or the last oxazolone application. No significant difference in IL-1α expression was seen between CD44 KO and wild-type mice (Fig. S1c vs S1d; Fig. S1e vs S1f). Figure S1a is normal skin from wild-type mice and Fig. S1b is normal skin from CD44 KO mice. Figure S1c and d are acute dermatitis induced by topical oxazolone in wild-type and CD44 KO mice, respectively. Figure S1e and f are acute dermatitis induced by topical TPA in wild-type and CD44 KO mice, respectively. Figure S1g is negative control. The magnifications are the same for all pictures. Scale bar = 20 µm.
Figure S2. Increased TCR Vβ8.1-3 cell infiltration in CD44 KO mice in subacute allergic dermatitis models: subacute allergic dermatitis was induced as described in the Materials and methods section. Skin samples were taken 24 h after the last oxazolone treatment. TCR Vβ8.1-3 staining was performed as described in the Materials and methods section and pictures were taken with a digital camera, equipped with AxioVision software 3.1. There were four mice for each treatment group. Figures shown are representative. Scale bar = 20 µm.
Figure S3. Increased dermal IL-1α expression in CD44 KO mice in subacute allergic dermatitis models: subacute allergic dermatitis was induced as described in the Materials and methods section. Skin samples were taken 24 h after the last oxazolone treatment. IL-1α expression was determined as described in the Materials and methods section and pictures were taken with a digital camera, equipped with AxioVision software 3.1. There were four mice for each treatment group. Figures shown are representative. Scale bar = 20 εm.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Seiter S, Schmidt DS, Zoller M. The CD44 variant isoforms CD44v6 and CD44v7 are expressed by distinct leukocyte subpopulations and extent non-overlapping functional activities. Int Immunol. 2000;12:37–49. doi: 10.1093/intimm/12.1.37. [DOI] [PubMed] [Google Scholar]
- 2.Witting B, Seiter S, Schmidt DS, Zuber M, Zoller M. CD44 variant isoforms on blood leukocytes in chronic inflammatory bowel disease and other systemic autoimmune diseases. Lab Invest. 1999;79:747–759. [PubMed] [Google Scholar]
- 3.Tuhkanen AL, Agren UM, Tammi MI, Tammi RH. CD44 Expression marks the onset of keratinocyte stratification and mesenchymal maturation into fibrous dermis in fetal human skin. J Histochem Cytochem. 1999;47:1617–1624. doi: 10.1177/002215549904701213. [DOI] [PubMed] [Google Scholar]
- 4.Bourguignon LY, Ramez M, Gilad E, et al. Hyaluronan-CD44 interaction stimulates keratinocyte differentiation, lamellar body formation/secretion, and permeability barrier homeostasis. J Invest Dermatol. 2006;126:1356–1365. doi: 10.1038/sj.jid.5700260. [DOI] [PubMed] [Google Scholar]
- 5.Wang C, Tammi M, Tammi R. Distribution of hyaluronan and its CD44 receptor in the epithelia of human skin appendages. Histochemistry. 1992;98:105–112. doi: 10.1007/BF00717001. [DOI] [PubMed] [Google Scholar]
- 6.Seiter S, Schadendorf D, Tilgen W, Zoller M. CD44 variant isoform expression in a variety of skin-associate autoimmune diseases. Clin Immunol Immunopathol. 1998;89:79–93. doi: 10.1006/clin.1998.4565. [DOI] [PubMed] [Google Scholar]
- 7.Kaya G, Rodriguez I, Jorcano JL, Vassalli P, Stamenkovic I. Selective suppression of CD44 in keratinocytes of mice bearing an antisense CE44 transgene driven by a tissue-specific promoter disrupts hyaluronate metabolism in the skin and impairs keratinocyte proliferation. Genes Dev. 1997;11:996–1007. doi: 10.1101/gad.11.8.996. [DOI] [PubMed] [Google Scholar]
- 8.Kaya G, Tran C, Sorg O, et al. Hyaluronate fragments reverse skin atrophy by a CD44-dependent mechanism. PLoS Med. 2006;3:2291–2303. doi: 10.1371/journal.pmed.0030493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kincade PW, He Q, Ishihara K, Miyaka K, Lesley J, Hyman R. CD44 and other cell interaction molecules contributing to B lymphopoiesis. Curr Top Microbiol Immunol. 1993;184:215–222. doi: 10.1007/978-3-642-78253-4_17. [DOI] [PubMed] [Google Scholar]
- 10.Sugimoto K, Tsurumaki Y, Hoshi H, et al. Effects of anti-CD44 monoclonal antibody on adhesion of erythroid leukemic cells (ELM-I-1) to hematopoietic supportive cells (MS-5): CD44, but not hyaluronate-mediated, cell–cell adhesion. Exp Hematol. 1994;22:488–494. [PubMed] [Google Scholar]
- 11.Funaro A, Spagnoli GC, Momo M, Knapp W, Malavasi F. Stimulation of T cells via CD44 requires leukocyte-function-associated antigen interactions and interleukin-2 production. Hum Immunol. 1994;40:267–278. doi: 10.1016/0198-8859(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 12.Huet S, Groux H, Caillou B, Valentin H, Prieur AM, Bernard A. CD44 contributes to T cell activation. J Immunol. 1989;143:798–801. [PubMed] [Google Scholar]
- 13.Foger N, Marhaba R, Zoller M. CD44 supports T cell proliferation and apoptosis by apposition of protein kinases. Eur J Immunol. 2000;30:2888–2899. doi: 10.1002/1521-4141(200010)30:10<2888::AID-IMMU2888>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Zoller M, Gupta P, Marhaba R, Vitacolonna M, Freyschmidt-Paul P. Anti-CD44-mediated blockade of leukocyte migration in skin-associated immune diseases. J Leukoc Biol. 2007;82:57–71. doi: 10.1189/jlb.0107063. [DOI] [PubMed] [Google Scholar]
- 15.DeGrengele HC, Estess P, Siegelman MH. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science. 1997;278:672–675. doi: 10.1126/science.278.5338.672. [DOI] [PubMed] [Google Scholar]
- 16.Rothman BL, Blue ML, Kelley KA, Wunderlich D, Mierz DV, Aune TM. Human T cell activation by OKT3 is inhibited by a monoclonal antibody to CD44. J Immunol. 1991;147:2493–2499. [PubMed] [Google Scholar]
- 17.Pierres A, Lipcey C, Mawas C, Olive D. A unique CD44 monoclonal antibody identifies a new T cell activation pathway. Eur J Immunol. 1992;22:413–417. doi: 10.1002/eji.1830220219. [DOI] [PubMed] [Google Scholar]
- 18.Witting B, Seiter S, Foger N, Schwarzler C, Gunthert U, Zoller M. Functional activity of murine CD44 variant isoforms in allergic and delayed type hypersensitivity. Immunol Lett. 1997;57:217–223. doi: 10.1016/s0165-2478(97)00060-6. [DOI] [PubMed] [Google Scholar]
- 19.Seiter S, Engel P, Gohr N, Zoller M. Mitigation of delayed-type hypersensitivity reaction by a CD44 variant isoform v3-specific antibody: blockade of leukocyte egress. J Invest Dermatol. 1999;113:11–21. doi: 10.1046/j.1523-1747.1999.00635.x. [DOI] [PubMed] [Google Scholar]
- 20.Rosel M, Seiter S, Zoller M. CD44v10 expression in the mouse and functional activity in delayed type hypersensitivity. J Cell Physiol. 1997;171:305–317. doi: 10.1002/(SICI)1097-4652(199706)171:3<305::AID-JCP9>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 21.Camp RL, Scheynius A, Johansson C, Pure E. CD44 is necessary for optimal contact allergic responses but is not required for normal leukocyte extravasation. J Exp Med. 1993;178:497–507. doi: 10.1084/jem.178.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonda A, Gal I, Szanto S, et al. CD44, but not I selectin, is critically involved in leukocyte migration into the skin in a murine model of allergic dermatitis. Exp Dermatol. 2005;14:700–708. doi: 10.1111/j.0906-6705.2005.00348.x. [DOI] [PubMed] [Google Scholar]
- 23.Sarraj B, Ludanyi K, Glant TT, Finnegan A, Mikecz K. Expression of CD44 and l-selectin in the innate immune system in required for severe joint inflammation in the proteoglycan-induced murine model of rheumatoid arthritis. J Immunol. 2006;177:1932–1940. doi: 10.4049/jimmunol.177.3.1932. [DOI] [PubMed] [Google Scholar]
- 24.Stoop R, Kotani H, McNeish JD, Otterness IG, Mikecz K. Increased resistance to collagen-induced arthritis in CD44-deficient DBA/1 mice. Arthritis Rheum. 2001;44:2922–2931. doi: 10.1002/1529-0131(200112)44:12<2922::aid-art480>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 25.Verdrengh M, Holmdahl R, Tarkowski A. Administration of antibodies to hyaluronan receptor (CD44) delays the start and ameliorates the severity of collagen II arthritis. Scand J Immunol. 1995;42:353–358. doi: 10.1111/j.1365-3083.1995.tb03667.x. [DOI] [PubMed] [Google Scholar]
- 26.Mikecz K, Brennan FR, Kim JH, Glant TT. Anti-CD44 treatment abrogates tissue oedema and leukocyte infiltration in murine arthritis. Nat Med. 1995;1:558–563. doi: 10.1038/nm0695-558. [DOI] [PubMed] [Google Scholar]
- 27.Nedvetzki S, Walmsley M, Alpert E, Williams RO, Feldmann M, Naor D. CD44 involvement in experimental collagen-induced arthritis (CIA) J Autoimmun. 1999;13:39–47. doi: 10.1006/jaut.1999.0294. [DOI] [PubMed] [Google Scholar]
- 28.Mikecz K, Dennis K, Shi M, Kim JH. Modulation of hyaluronan receptor (CD44) function in vivo in a murine model of rheumatoid arthritis. Arthrithis Rheum. 1999;42:659–668. doi: 10.1002/1529-0131(199904)42:4<659::AID-ANR8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 29.Farkas S, Hornung M, Sattler C, et al. Short-term treatment with anti-CD44v7 antibody, but not VD44v4, restores the gut mucosa in established chronic dextran sulfate sodium (DSS)-induced colitis in mice. Clin Exp Immunol. 2005;142:260–267. doi: 10.1111/j.1365-2249.2005.02911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witting B, Schwarzler C, Fohr N, Gunthert U, Zoller M. Curative treatment of an experimentally induced colitis by a CD44 variant V7-specific antibody. J Immunol. 1998;161:1069–1073. [PubMed] [Google Scholar]
- 31.Witting BM, Johansson B, Zoller M, Schwarzler C, Gunthert U. Abrogation of experimental colitis correlates with increased apoptosis in mice deficient for CD44 variant exon 7 (CD44v7) J Exp Med. 2000;191:2053–2064. doi: 10.1084/jem.191.12.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang J, Jiang D, Griffith J, et al. CD44 is a negative regulator of acute pulmonary inflammatory and lipopolysaccharide-TLR signaling in mouse macrophages. J Immunol. 2007;178:2469–2475. doi: 10.4049/jimmunol.178.4.2469. [DOI] [PubMed] [Google Scholar]
- 33.Man MQ, Shi Y, Man M, et al. Chinese herbal medicine (Tuhuai extract) exhibits topical anti-proliferative and anti-inflammatory activity in murine disease models. Exp Dermatol. 2008;17:681–687. doi: 10.1111/j.1600-0625.2007.00690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Man MQ, Hatano Y, Lee SH, et al. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J Invest Dermatol. 2008;128:79–86. doi: 10.1038/sj.jid.5701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Man MQ, Wood L, Elias PM, Feingold KR. Cutaneous barrier repair and pathophysiology following barrier disruption in IL-1 and TNF type I receptor deficient mice. Exp Dermatol. 1999;8:261–266. [PubMed] [Google Scholar]
- 36.Wood LC, Elias PM, Calhoun C, Tsai JC, Grunfeld C, Feingold KR. Barrier disruption stimulates interleukin-1 alpha expression and release from a preformed pool in murine epidermis. J Invest Dermatol. 1996;106:397–403. doi: 10.1111/1523-1747.ep12343392. [DOI] [PubMed] [Google Scholar]
- 37.Wood LC, Jackson SM, Elias PM, Grundfeld C, Feingold KR. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest. 1992;90:482–487. doi: 10.1172/JCI115884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang R, Yang PZ, Wu CY, et al. Role of T-cell receptor V beta 8.3 peptide vaccine in the prevention of experimental autoimmune uveoretinitis. Chin Med J (Engl) 2006;119:740–748. [PubMed] [Google Scholar]
- 39.Egwuagu CE, Mahdi RM, Nussenblatt RB, Gery I, Caspi RR. Evidence for selective accumulation of V beta 8+ T lymphocytes in experimental autoimmune uveoretinitis induced with two different retinal antigens. J Immunol. 1993;151:1627–1636. [PubMed] [Google Scholar]
- 40.Egwuagu CE, Bahmanyar S, Mahdi RM, Nussenblatt RB, Gery I, Caspi RR. Predominant usage of V beta 8.3 T cell receptor in a T cell line that induces experimental autoimmune uveoretinitis (EAU) Clin Immunol Immunopathol. 1992;65:152–160. doi: 10.1016/0090-1229(92)90218-d. [DOI] [PubMed] [Google Scholar]
- 41.Teder P, Vandivier RW, Jiang D, et al. Resolution of lung inflammation by CD44. Science. 2002;296:155–158. doi: 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]
- 42.Maytin EV, Chung HH, Seetharaman VM. Hyaluronan participates in the epidermal response to disruption of the permeability barrier in vivo. Am J Pathol. 2004;165:1331–1341. doi: 10.1016/S0002-9440(10)63391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kulseth MA, Kolset SO, Ranheim T. Stimulation of serglycin and CD44 mRNA expression in endothelial cells exposed to TNF-alpha and IL-1alpha. Biochim Biophys Acta. 1999;1428:225–232. doi: 10.1016/s0304-4165(99)00096-3. [DOI] [PubMed] [Google Scholar]
- 44.van Grevenstein WM, Hofland LJ, Jeekel J, van Eijck CH. The expression of adhesion molecules and the influence of inflammatory cytokines on the adhesion of human pancreatic carcinoma cells to mesothelial monolayers. Pancreas. 2006;32:396–402. doi: 10.1097/01.mpa.0000220865.80034.2a. [DOI] [PubMed] [Google Scholar]
- 45.Mishra JP, Mishra S, Gee K, Kumar A. Differential involvement of calmodulin-dependent protein kinase II-activated AP-1 and c-Jun N-terminal kinase-activated EGR-1 signaling pathways in tumor necrosis factor-alpha and lipopoly-saccharide-induced CD44 expression in human monocytic cells. J Biol Chem. 2005;280:26825–26837. doi: 10.1074/jbc.M500244200. [DOI] [PubMed] [Google Scholar]
- 46.Gee K, Lim W, Ma W, et al. Differential regulation of CD44 expression by lipopolysaccharide (LPS) and TNF-alpha in human monocytic cells: distinct involvement of c-Jun N-terminal kinase in LPS-induced CD44 expression. J Immunol. 2002;169:5660–5672. doi: 10.4049/jimmunol.169.10.5660. [DOI] [PubMed] [Google Scholar]
- 47.Zoller M, Gupta P, Marhaba R, Vitacolonna M, Freyschmidt-Paul P. Anti-CD44-mediated blockade of leukocyte migration in skin-associated immune diseases. J Leukoc Biol. 2007;82:57–71. doi: 10.1189/jlb.0107063. [DOI] [PubMed] [Google Scholar]
- 48.Catalina MD, Estess P, Siegelman MH. Selective requirements for leukocyte adhesion molecules in models of acute and chronic cutaneous inflammation: participation of E- and P- but not L-selectin. Blood. 1999;93:580–589. [PubMed] [Google Scholar]
- 49.Schmits R, Filmus J, Gerwin N, et al. CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood. 1997;90:2217–2233. [PubMed] [Google Scholar]
- 50.Stoop R, Gal I, Glant TT, McNeish JD, Mikecz K. Trafficking of CD44-defienct murine lymphocytes under normal and inflammatory conditions. Eur J Immunol. 2002;32:2532–2542. doi: 10.1002/1521-4141(200209)32:9<2532::AID-IMMU2532>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 51.Szanto S, Gal I, Glant TT, Mikecz K. Expression of L-selectin, but not CD44, is required for early extravasation in antigen-induced arthritis. J Immunol. 2004;172:6723–6734. doi: 10.4049/jimmunol.172.11.6723. [DOI] [PubMed] [Google Scholar]
- 52.Fujita T, Fujimoto M, Matsushita T, et al. Phase-dependent roles of E-selectin during chronic contact hypersensitivity responses. Am J Pathol. 2007;170:1649–1658. doi: 10.2353/ajpath.2007.060747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Staite ND, Justen JM, Sly LM, Beaudet AL, Bullard DC. Inhibition of delayed-type contact hypersensitivity in mice deficient in both E-selectin and P-selectin. Blood. 1996;88:2973–2979. [PubMed] [Google Scholar]
- 54.Shimada Y, Hasegawa M, Kaburagi Y, et al. L-selectin or ICAM-1deficiency reduces an immediate-type hypersensitivity response by preventing mast cell recruitment in repeated elicitation of contact hypersensitivity. J Immunol. 2003;170:4325–4334. doi: 10.4049/jimmunol.170.8.4325. [DOI] [PubMed] [Google Scholar]
- 55.Naor D, Nedvetzki S, Walmsley M, et al. CD44 involvement in autoimmune inflammations: the lesson to be learned from CD44-targeting by antibody or from knockout mice. Ann N Y Acad Sci. 2007;1110:233–247. doi: 10.1196/annals.1423.025. [DOI] [PubMed] [Google Scholar]
- 56.Kim Y, Lee YS, Hahn JH, et al. Hyaluronic acid targets CD44 and inhibits FcepsilonRI signaling involving PKCdelta, Rac1, ROS, and MAPK to exert antiallergic effect. Mol Immunol. 2008;45:2537–2547. doi: 10.1016/j.molimm.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 57.Hagemann I, Proksch E. Topical treatment by urea reduces epidermal hyperproliferation and induces differentiation in psoriasis. Acta Derm Venereol. 1996;76:353–356. doi: 10.2340/0001555576353356. [DOI] [PubMed] [Google Scholar]
- 58.Vazquez JR, Short B, Findlow AH, et al. Outcomes of hyaluronan therapy in diabetic foot wounds. Diabetes Res Clin Pract. 2003;59:123–127. doi: 10.1016/s0168-8227(02)00197-3. [DOI] [PubMed] [Google Scholar]
- 59.Sobotka L, Smahelova A, Pastorova J, Kusalova M. A case report of the treatment of diabetic foot ulcers using a sodium hyaluronate and iodine complex. Int J Low Extrem Wounds. 2007;6:143–147. doi: 10.1177/1534734607304684. [DOI] [PubMed] [Google Scholar]