Abstract
Pancreatic islet encapsulation within semi-permeable materials has been proposed for transplantation therapy of Type I diabetes mellitus. Polymer hydrogel networks used for this purpose have been shown to provide protection from islet destruction by immunoreactive cells and antibodies. However, one of the fundamental deficiencies with current encapsulation methods is that the permselective barriers cannot protect islets from cytotoxic molecules of low molecular weight that are diffusible into the capsule material, which subsequently results in β-cell destruction. Use of materials that can locally inhibit the interaction between the permeable small cytotoxic factors and islet cells may prolong the viability and function of encapsulated islet grafts. Here we report the design of anti-inflammatory hydrogels supporting islet cell survival in the presence of diffusible pro-inflammatory cytokines. We demonstrated that a poly(ethylene glycol)-containing hydrogel network, formed by native chemical ligation and presenting an inhibitory peptide for islet cell surface IL-1 receptor, was able to maintain the viability of encapsulated islet cells in the presence of a combination of cytokines including IL-1β, TNF-α, and INF-γ. In stark contrast, cells encapsulated in unmodified hydrogels were mostly destroyed by cytokines which diffused into the capsules. At the same time, these peptide-modified hydrogels were able to efficiently protect encapsulated cells against β-cell specific T-lymphocytes and maintain glucose-stimulated insulin release by islet cells. With further development, the approach of encapsulating cells and tissues within hydrogels presenting anti-inflammatory agents may represent a new strategy to improve cell and tissue graft function in transplantation and tissue engineering applications.
Introduction
Encapsulation of pancreatic islets in semi-permeable devices has been an attractive approach for islet transplantation to restore glycemic control in type I diabetic patients.[1, 2] Surrounding islet tissue with a barrier of immunoisolating materials can offer protection from host immunorejection, which permits allo- or xeno-transplantation in the absence of immunosuppressive medication. This approach also allows islet tissue to be modulated prior to implantation to improve graft acceptance and thereby improve the efficiency of islet transplantation for diabetic patients as well as help to resolve the shortage of organ sources[3, 4].
A variety of natural and synthetic polymers have been applied to islet encapsulation[5–8] and achievement of normal glycemia has been reported in rodent and canine models[9] and occasionally in humans [10, 11]. However, poor graft survival has been a major limitation of islet encapsulation for use in clinical implantation. Graft failure is usually attributed to several factors, including inadequate biocompatibility of the encapsulating materials, hypoxia within transplanted islets and incomplete immunoprotection [12–14]. For example, many studies have shown that the purity and composition of alginate, a widely-used natural polymer for islet encapsulation, substantially affect the survival of trapped islets [15–17]. Hypoxia is a problem due to the lack of vasculaturization within/around the islet transplant which limits the supply of oxygen to encapsulated cells [18, 19]. Revascularization is inhibited by the inability of vessels to penetrate the encapsulating materials. Furthermore, materials with poor biocompatibility tend to initiate nonspecific adsorption of protein and cells (fibroblast overgrowth) on the capsules, which further decreases oxygen diffusion into the encapsulated islets [20, 21]. Therefore, compared to natural materials that do not resist protein/cell adsorption, non-immunogenic and fouling-resistant synthetic biomaterials may be better candidates for cell encapsulation because of the easy control over their chemical purity and properties [8, 22]. Another limitation of currently used islet encapsulation approaches is incomplete immunoprotection from small molecules like cytokines and radicals [23–25]. Capsule permeability desired for islet encapsulation should block the entry of large cells and antibodies (MW ≥ 75 KD) of the immune system but still allow free transit of nutrients and metabolic wastes for maintaining cellular function [26]. More importantly, insulin secreted from cells must be able to freely diffuse out of the capsules in order to play its role in glycemic control [27, 28]. However, permeabilities that accommodate insulin diffusion out of the capsule will permit pro-inflammatory cytokines and other effector molecules of low molecular weight, such as IL-1β (17.5 KD) and TNF-α (51 KD), to enter the capsules and exert deleterious effects on β-cell function and islet vitality [12, 29, 30].
Encapsulation of islets within hydrogels bearing cytokine-suppressive molecules can provide protection to islets by both excluding immunocytes and mitigating the effects of permeable low molecular weight inflammatory factors. In this work, we demonstrate encapsulation of islet cells in an anti-inflammatory hydrogel network. The hydrogel networks are crosslinked in situ through native chemical ligation (NCL) upon mixing cysteine-terminated and thioester-terminated 4-armed poly(ethylene glycol) (PEG) under physiological conditions [31, 32]. This hydrogel efficiently isolated encapsulated insulin secreting cells from immunocytes and allowed straightforward attachment of bioactive molecules to modify the microenvironment so as to improve cell survival and function. In particular, presence of a cytokine-inhibitory peptide on the hydrogel exerted a protective effect on cells from damage induced by pro-inflammatory cytokines that were able to permeate the capsules, providing a new strategy to combat the incomplete immunoprotection of conventional islet encapsulation materials. This approach of using hydrogels with anti-inflammatory properties should be widely applicable in many other tissue engineering/ transplantation practices.
Experimental
Materials
4-Armed PEG amine (MW 10 KD) was purchased from Sunbio (South Korea). Rink amide resin, PyBop and Fmoc protected amino acids were purchased from Novabiochem (Gibbstown, NJ) and Anaspec (San Diego, CA). 2-Ethylpropriate thiol was purchased from TCI (Canada) and maleimide-OSU active ester from TRC (Canada). Dichloromethane (DCM), N′, N-dimethylformamide (DMF), acetonitrile (ACN), diethyl ether, N-methylmorphiline (NMM), methanol, piperidine, 2-mercaptoethanol (MCE), trifluoroacetic acid (TFA), triisopropylsilane (TIS) and α-cyano-4-hydroxycinnamic acid (CHCA) matrix were purchased from Sigma-Aldrich (Milwaukee, WI). Calcein-AM, and ethidium homodimer-1 were purchased from Molecular Probes (Eugene, OR). Dulbecco’s modified Eagle’s medium, fetal bovine serum, penicillin/streptomycin, trypsin-EDTA were obtained from American Type Culture Collection (Manassas, VA). Recombinant mouse IL-1β, TNF-α and INF-γ were purchased from R&D Systems (Minneapolis, MN). Transgenic T-lymphocyte cell line was provided as a gift from Dr. Xunrong Luo’s lab in the Division of Nephrology / Hypertension at Northwestern University.
Synthesis of 4-armed PEG conjugated with cysteine and thioester groups
Cysteine-terminated and thioester-terminated 4-armed PEG macromers were synthesized using previously reported procedures [32]. Briefly, Boc-Cys(Trt)-OH (150 µmol), PyBop (150 µmol) and 20 µL NMM were dissolved in 5 mL DCM. The mixture was stirred for 10 minutes at room temperature and 4-armed PEG amine (30 µmol) was added. The final reaction mixture was stirred for 10 hours at room temperature. Solvents were removed on a rotavap and minimal methanol was added to dissolve the oily residue. The methanol solution was kept at −20 °C for 8 hrs and white precipitates were collected by centrifugation at −10 °C. The crude intermediate was dissolved in 5 mL TFA-ITS-H2O (95:2.5:2.5) and stirred for 3 hours at RT to carry out the deprotection of thiol and amine groups of cysteine conjugated to PEG macromers. The solvent was removed and crude cysteine-terminated 4-armed PEG (4A-PEG-Cys) was purified by preparative reversed phase high performance liquid chromatography (RP-HPLC). Similarly, to make thioester terminated 4-armed PEG (4A-PEG-ThE), equal equivalents of ethyl 3-mercaptopropionate-succinic acid [32], PyBop and NMM were dissolved in DCM prior to addition of 0.2 equivalents of 4-armed PEG amine. The reaction mixture was stirred for 12 hours, and the solvent was removed on a rotavap to give a pale-yellow oily residue. A minimal amount of methanol was added to dissolve the residue. The solution was kept at −20 °C for 2 hours and white precipitates were collected by centrifugation at −10 °C. The crude 4A-PEG-ThE was purified by preparative RP-HPLC.
Synthesis of peptides for functionalizing hydrogels
Maleimide-terminated peptides MA-GRGDSPG-NH2 and MA-OEG2-FEWTPGWYQPY-NH2 were synthesized using standard solid phase peptide synthesis protocols on Rink amide resin (0.69 meq/g) at 0.1 mmol scale. Each coupling step was carried out by mixing 3 equivalents of Fmoc protected amino acids, PyBop and NMM with the resin beads in the synthesis vessels with a rocking motion for 4 hours. Upon completion of coupling indicated by a ninhydrin test, the resin beads were washed thoroughly with DMF and then 20% piperidine in DMF was used to deprotect the Fmoc group to expose the amine groups on the beads for the next coupling. After the last amino acid was conjugated and its amine group was exposed, maleimide-OSU ester (2 eqv.) in DMF was used to attach the maleimide moieties to the N-terminal of resin-bound peptides. Cleavage of the maleimide-terminated peptides from the resin and deprotection of the amino acid side chains were accomplished by treating the resin with 95% (v/v) TFA, 2.5% H2O and 2.5% TIS for 2 hours at room temperature, after which the cleaved peptide solution was collected by filtration. Solvent was removed using a rotary evaporator, and the product residues were dissolved in a minimal amount of TFA. Upon addition of cold ether to the solution, solid precipitates were obtained and isolated by centrifugation at 4°C. The product pellets were dissolved in deionized water, frozen and lyophilized. Crude products were purified by preparative RP-HPLC and the molecular weight of each peptide was confirmed by MALDI-TOF MS.
MIN6 cell encapsulation in hydrogels formed by native chemical ligation
Mouse insulinoma (MIN6) cells were cultured in the DMEM containing 15% FBS, 100 IU/ ml penicillin, 100 µg/ml strepmycin and 50 µM MCE at 37°C under 5% CO2. Cells between passage number 25 and 28 were used for all studies. Prior to cell encapsulation, cells were detached from tissue culture plates with a 0. 25% trypsin-EDTA solution and resuspended in DMEM at a cell density of 4 × 105 / mL. 20 µL of a solution of 4A-PEG-Cys in DMEM (pH 7.4, w/v 10%,) was added to a mini-dialysis tube (Pierce, Rockford, IL) and immersed in 1mL DMEM. 40 µl of the cell suspension was then added to the tube followed by addition of 20 µL 4A-PEG-ThE in DMEM (pH 7, w/v 10%). To form hydrogels presenting functional peptides, maleimide-terminated peptides were added to the solution of 4A-PEG-Cys at a molar ratio of 1:25 (MA-peptide: PEG-Cys) prior to mixing with cells and 4A-PEG-ThE. The mixture was incubated at 37°C for 30 minutes to yield cell-embedded hydrogel disks (8mm O.D. × 1mm thick). The hydrogel disks were rinsed 3 times by incubating the gels in fresh DMEM for 5 minutes each time and then cultured in cell growth medium at 37°C under 5% CO2. Culture media were changed every 2 days.
Immunoisolation of encapsulated MIN6 cells from T-lymphocytes
The immunoisolating property of the hydrogel formed by native chemical ligation was tested by measuring cell viability upon co-culture with cytotoxic T-lymphocytes specific for islet β-cells. Transgenic T-lymphocytes were cultured and activated following previously reported protocols [33]. The T-cells were harvested by centrifugation to remove the culture media and resuspended in DMEM containing 15% FBS at a density of 106 cells/ mL. Hydrogels containing encapsulated MIN6 cells were immersed in the T-cell suspension under gentle shaking for 24 hours. The hydrogels were removed from the T-cell suspension and washed with fresh MIN6 cell culture medium followed by a cell viability assay. For comparison, unencapsulated MIN6 cells grown in monolayer on tissue culture plates (2 × 105 cell / cm2) were incubated with T-cell suspension for 24 hours. The media containing T-cells in suspension were removed and fresh culture media were used to wash the MIN6 cells followed by the cell viability assay.
MIN6 cell death induced by cytokines
MIN6 cells were encapsulated in unmodified or peptide-functionalized hydrogel disks as described above and cultured for 48 hours. Cell culture media was changed to serum-free DMEM (containing 1% FBS), and cells were equilibrated at 37°C and 5% CO2 for 30 minutes. Cytokine-containing media were prepared by mixing IL-1β (5 ng / ml), TNF-α (10 ng / ml) and INF-γ (25 ng / ml) in serum-free DMEM. The encapsulated cells were treated with these media at 37°C for 2 hours followed by cell viability assay.
Viability assays on free and encapsulated MIN6 cells
MIN6 cells cultured on tissue culture plates or encapsulated in hydrogel disks were incubated with a PBS solution containing calcein-AM (2 µM) and ethidium homodimer-1 (2 µM) for 30 minutes at 37 °C. The cells were rinsed with PBS and imaged on an inverted fluorescence microscope. Cell viability was determined as the ratio of the number of live cells divided by the total number of live and dead cells.
Glucose-stimulated insulin release by free and encapsulated cells
MIN6 cells were seeded at a density of 2 × 105 cells / well in a 24-well plate and were allowed to grow for 48 hours. Encapsulated cells were used for this assay 48 hours after encapsulation in hydrogel. Culture media were removed and cells were rinsed twice with KRB (Krebs–Ringer bicarbonate) buffer supplemented with 0.2 % BSA containing 129 mM NaCl, 4.8 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 2.5 mM CaCl2, 5 mM NaHCO3, and 10 mM HEPES/NaOH (pH 7.4). Glucose concentrations of 3.3 and 16.7 mmol / L were prepared in the conditioned KRB buffer and subsequently conditioned at 37 °C and 5 % CO2 for 30 minutes. Cells were equilibrated at 3.3 mM glucose for 30 minutes and then incubated with fresh stimulation media with 3.3 mM glucose at 1mL/ well at 37 °C and 5 % CO2 for 60 minutes. 500 µL of the media were removed from each well, placed in an ice-cold enppendorf tube, centrifuged at 2,500 rpm for 5 minutes and 200 µL of the supernatant was used to measure the concentration of insulin. The remaining media was removed from each well and discarded. To measure glucose-stimulated insulin secretion, cells were incubated in 1 ml/well of medium containing 16.7 mM glucose at 37°C and 5% CO2 for 60 minutes. The medium was then collected, and the concentration of insulin was determined as described above. A mouse (pro)Insulin ELISA kit from Mercodia (Uppsala, Sweden) was used to determine the amount insulin concentration in the collected media following the manufacturer’s instructions.
Results and Discussion
Hydrogel formation and functionalization
In this work, in situ cross-linking of PEG-containing hydrogels was used to encapsulate mouse pancreatic islet-derived MIN6 cells. PEG is a well-studied polymer that has been widely used in drug development and tissue engineering [34]. 4-armed PEG cysteine (4A-PEG-Cys) and 4-armed PEG thioester (4A-PEG-ThE) molecules were utilized for cross-linking through native chemical ligation (NCL) to form hydrogels (Figure 1) [32]. One advantage of this method compared to other synthetic hydrogel formation techniques, is the high chemoselectivity of the NCL reaction, which is limited to reactions between the N-terminal cysteine and thioester groups on the PEG molecules due to the rarity of N-terminal cysteine residues in naturally occurring proteins and other biomolecules. In contrast, reactions employed in other covalent hydrogel cross-linking methods are often less selective and can occur between the macromers and biological components such as cell surface proteins and agents in the culture media [6]. Furthermore, the hydrogel formation by NCL occurs under mild alkaline conditions (pH 7-9) without the need for initiators or catalysts, resulting in minimal toxicity to the cells during encapsulation. In addition, the resulting hydrogel network presents thiol groups whose mild reductive properties can protect encapsulated cells from oxidative stress [35–37].
Figure 1.
Peptide-functionalized hydrogels formed by native chemical ligation. Prior to mixing 4-armed PEG-Cys (A) and 4-armed PEG-ThE (B) to form hydrogel, maleimide-terminated peptides are used to modify 1~5% cystine groups of PEG-Cys. The hydrogel resulting from NCL reaction between PEG-ThE and peptide modified PEG-Cys contains peptides covalently bound to the polymer network.
Due to chemoselectivity of the NCL reaction, hydrogel formation by this method also allows straightforward modification of gels with bioactive peptides and other biomolecules for improving the function of encapsulated cells including cell growth, development and secretion of cellular products in response to a biological stimulus [6, 32, 38, 39]. Weber and Anseth have reported the attachment of cell adhesive peptides on photocrosslinked hydrogels affected MIN6 cell survival and insulin release [40]. The cross-linking by NCL allows multiple strategies to be employed for hydrogel functionalization. In the present work, the hydrogels were functionalized with peptides through the Michael-Addition reaction between 4A-PEG-Cys and maleimide-terminated peptides prior to hydrogel formation. This reaction is quantitative and fast, providing good control over the density of peptides attached to the hydrogel. This strategy works especially well when physiological effects of peptides on cells can be achieved at low immobilization densities. For example, the presence of the cell adhesion peptide GRGDSPG at 1% of the total cysteine groups used for hydrogel formation was sufficient to provide good cell viability in our studies (Figure 2). If more peptides need to be presented (above 10% of total cysteine groups on 4A-PEG-Cys), the Michael-Addition approach may not be the best choice because it consumes a significant amount of cysteine groups that are used subsequently for hydrogel crosslinking, resulting in a slow gelation rate and gels with low stiffness. Alternatively, direct incorporation of peptides into the macromonomer design, for example through branched PEGs conjugated with N-terminal Cys containing peptides, may offer a better way to form a NCL-crosslinked hydrogel with bioactive peptides presented at high densities.
Figure 2.
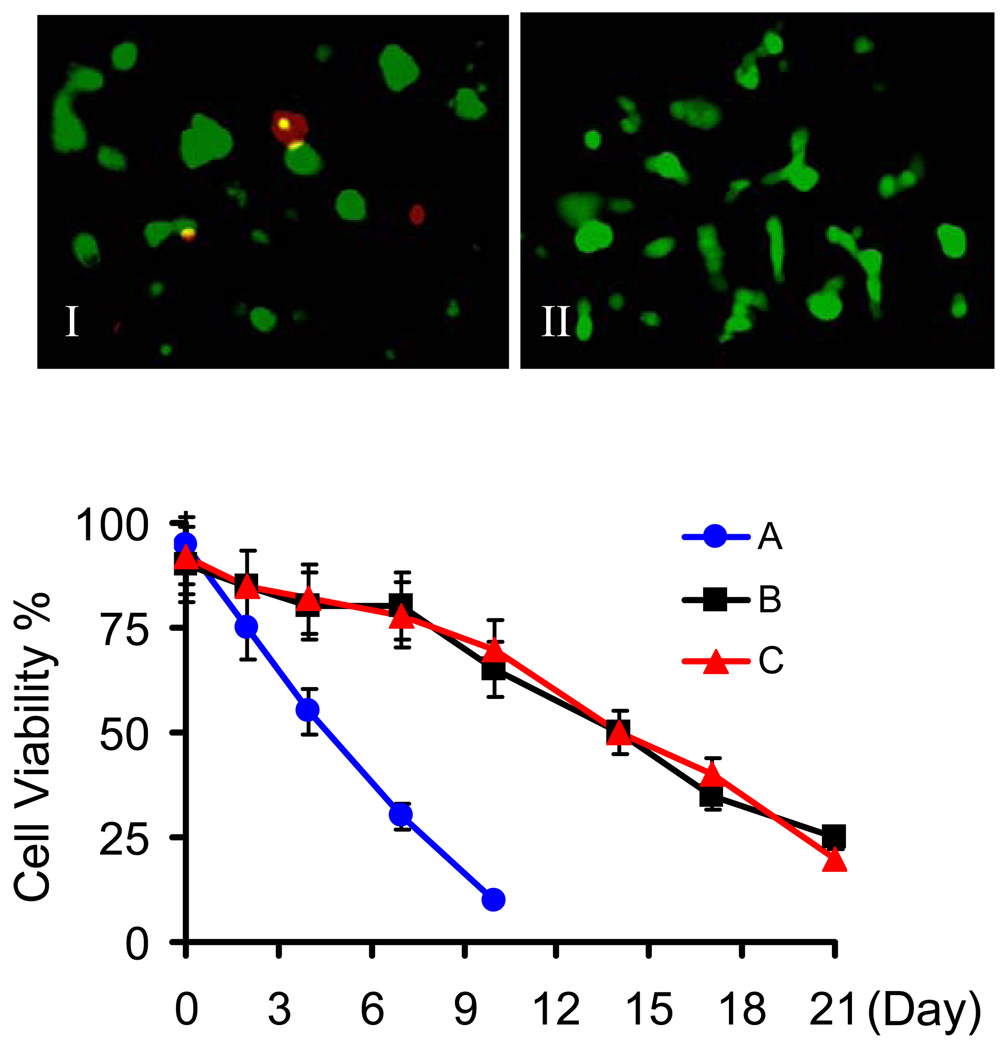
Viability of MIN6 cells encapsulated in hydrogels crosslinked by native chemical ligation. Representative photos of MIN6 cells encapsulated in unmodified hydrogel (I) and hydrogels modified with GRGDSPG and IL-1RIP (II) (Cells were stained with calcein-AM and imaged at 24 hours after encapsulation and shown as green at 100× magnification). Cell viability was monitored during a 21-day period for (A) unmodified hydrogel, (B) 1% GRGDSPG modified hydrogel, and (C) hydrogel modified with 1% GRGDSPG and 1% IL-1RIP. Assays were performed in triplicate with a standard deviation ≤ 10%.
MIN6 cell encapsulation in peptide-modified hydrogels
Cell encapsulation in hydrogels formed through NCL can be carried out in regular cell culture media whereas cell encapsulation through other chemical approaches are usually performed in simplified salt buffers (for example, photocross-linking is carried out in Hank’s buffer) due to the incompatibility of the cross-linking reaction with organic components in cell growth media [6, 29]. The chemoselective NCL cross-linking method is compatible with most organic nutrients in culture media including serum- and growth factor-containing media, allowing cells to stay in an optimal environment with minimal shock and stress during encapsulation.
In unmodified (peptide-free) hydrogels formed by NCL, MIN6 cells were 95% viable at 24 hours after encapsulation. However, cell viability dramatically decreased 96 hours post-encapsulation and no viable cells could be found after one week (Figure 2). We believe this result is due to a lack of cell-extracellular matrix (ECM) interaction that is critical to cell function because unmodified PEG hydrogel surfaces resist ECM deposition [40–42]. To improve the viability of encapsulated cells, the ECM-mimic peptide sequence GRGDSPG was conjugated onto 4A-PEG-Cys using the Michael-Addition reaction and crosslinked into hydrogels by NCL. Cells trapped in hydrogel presenting this cell adhesion peptide at a low molar ratio of 1% to total cysteine groups on 4A-PEG-Cys exhibited significantly improved cell survival through 21 days (Figure 2). Co-presence of a second peptide (the IL-1 receptor inhibitor peptide IL-1RIP) did not reduce the effect of the RGD-presenting peptide on cell adhesion and survival.
Immunoprotection of β-cells from pro-inflammatory cytokines and T-lymphocytes
An important goal of islet encapsulation in hydrogels is to provide islet transplants with a protective barrier from the host immunorejection. A number of hydrogels have been tested for isolation of encapsulated islet cells from immunoreactive T-cells and high MW antibodies (MW ≥ 75 KD) [43]. Recently a few examples have demonstrated that, other than physical barriers, encapsulation materials can also provide immunosuppression by targeting specific immunoreactive components. Teramura and Iwata investigated the immobilization of urokinase and heparin on an ultrathin synthetic membrane around islets in order to reduce inflammation triggered by blood coagulating on the surface of islets post-transplantation in the portal vein [44]. Cheung and Anseth reported the conjugation of anti-Fas MAbs to the exterior surface of PEG-containing-hydrogels for cell encapsulation [45]. Such hydrogels could induce apoptosis of T-cells in the vicinity of the hydrogel and therefore have the potential to down-regulate the immune response mediated by T-cells.
However, immunoprotection by these encapsulation methods may be incomplete because low MW inflammatory factors are still able to freely diffuse into the hydrogel and damage the encapsulated cells [12]. Entry of pro-inflammatory cytokines such as IL-1β, TNF-α and IFN-γ can not be blocked because these molecules have lower molecular weights than the MW cutoff value usually desired for encapsulation. These pro-inflammatory cytokines have been shown to be critical in causing early islet graft injury and lack of protection from them has significantly limited the success of islet encapsulation in clinical applications [46–49].
In this work, we developed an approach to provide insulin-secreting cells highly localized protection from the damage caused by cytokines infiltrating into the hydrogel capsules. A peptide inhibitor for cell surface IL-1 receptor (IL-1R) was attached covalently to the hydrogel scaffold in which cells were trapped, blocking the interaction between encapsulated cells and cytokines diffusing into the gel. This anti-inflammatory approach is highly localized to the encapsulated cells and thus avoids the harmful side-effects encountered during the use of conventional immunosuppressive drugs following transplantation [49].
The IL-1R inhibitory peptide sequence FEWTPGWYQPY-NH2 (IL-1RIP) was designed based on a previously reported inhibitory peptide for IL-1R [50]. Maleimide-terminated IL-1RIP was attached to hydrogels using Michael-Addition reaction as described previously, with the peptide density in the hydrogel varying from 1% to 5% of total thiol groups (calculated values). Cell-embedded hydrogels were treated with serum-free DMEM containing a combination of cytokines IL-1β, TNF-α and IFN-γ as used in previous studies [51]. Compared to unmodified hydrogel capsules, the IL-1RIP modified hydrogel was able to reduce the death of encapsulated MIN6 cells by up to 60% as shown in Figure 3. The pro-survival effect could be achieved at an IL-1RIP density as low as 1% of total cysteine / thiol groups in the hydrogel. Interestingly, our data also indicated that co-presence of IL-1RIP and the adhesion peptide sequence GRGDSPG further enhances the anti-cytokine effects compared to hydrogels containing either of the two peptides alone.
Figure 3.
Peptide-functionalized hydrogels protect MIN6 cells from cytokine-induced cell death. 24 hours after encapsulation, cells were treated with a combination of IL-1β (5 ng/ml), TNF-α (10 ng/ml) and INF-γ (25 ng/ml) in serum-free DMEM for 2 hours. A fluorescent cell viability assay (calcein-AM for live and ethidium homodimer-1 for dead cells) was used to determine the amount of cell death. Cells encapsulated in hydrogels modified with both GRGDSPG and IL-1RIP showed significantly increased viability compared to those in nonmodified and single-peptide modified hydrogels. Assays were performed in triplicates with a standard deviation ≤ 10%.
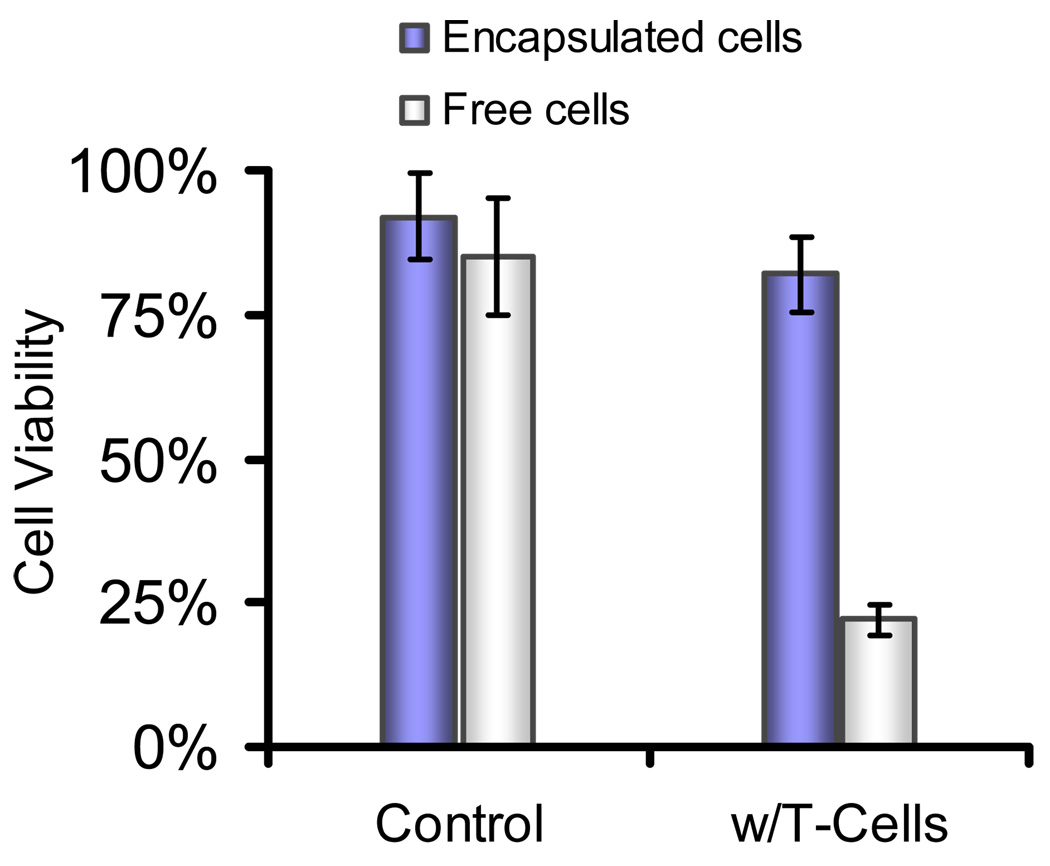
The immunoisolating property of the hydrogel network formed through NCL was also tested by co-culture of encapsulated MIN6 cells with cytotoxic T-lymphocytes. Islet-specific CD4+ T-lymphocyte cells were collected from transgenic mice and activated using BDC peptide following previously reported procedures [33]. Co-culture of these activated CD4+ T-cells with free MIN6 cells resulted in cell death, whereas cells encapsulated in hydrogels showed dramatically increased survival under the same conditions (Figure 4). This demonstrated that the NCL-crosslinked hydrogel barrier could efficiently protect MIN6 cells from cell infiltration and fatal attack by immunoreactive T-lymphocytes.
Figure 4.
Immunoisolation of MIN6 cells from cytotoxic T-lymphocytes. Free MIN6 cells and cells encapsulated in hydrogels presenting 1% GRGDSPG and 1% IL-1RIP were co-cultured with β-cell specific CD4+ T-cells. Encapsulated MIN6 cells had significantly increased viability compared to cells exposed freely to T-cells. Assays were performed in triplicates with a standard deviation ≤ 5%.
Insulin-releasing property of encapsulated β-cells in peptide-modified hydrogels
The failure of islet grafts following transplantation has been attributed to not only β-cell death over time, but also the inability of surviving cells to secrete insulin in response to changes in blood glucose [28, 40, 52]. An ideal material for islet cell encapsulation should help encapsulated β-cells maintain glucose-stimulated insulin secretion.
We examined the effect of the NCL hydrogel network on glucose-stimulated insulin secretion from encapsulated MIN6 cells. As Figure 5 shows, MIN6 cells trapped in hydrogels maintained their ability to secrete insulin in response to increasing the ambient glucose concentration from 3.3 mM to 16.7 mM. Interestingly, even in the absence of cytokines the presence of IL-1RIP on hydrogels enhanced glucose-stimulated insulin secretion by cells encapsulated in this hydrogel compared to unmodified and GRGDSPG–modified gels. We also observed this sensitizing effect of soluble IL-RIP on insulin release from untrapped MIN6 cells (data not shown). This property of IL-1RIP has not been previously reported, suggesting that the underlying mechanism merits further investigation.
Figure 5.
Glucose-stimulated insulin release from encapsulated MIN6 cells. Peptide-functionalized hydrogels did not affect the capability of cells to produce insulin in response to an increase in glucose concentration from 3.3 mM to 16.7 mM; in particular, the IL-1RIP peptide-modified hydrogel augmented the response of the encapsulated cells to the glucose stimulus. Assays were performed in triplicates with a standard deviation ≤ 8%.
Conclusions
We have reported the use of peptide-functionalized hydrogels to improve cell survival and function following cell encapsulation and during early immunorejection of islet grafts. The hydrogel capsule used in this work was generated in situ by the native chemical ligation reaction between PEG-containing macromers. This cross-linking method was highly chemoselective and able to maintain the function of encapsulated cells. The NCL hydrogels modified with an anti-inflammatory peptide and an adhesion peptide substantially reduced cell death induced by pro-inflammatory cytokines while the hydrogel was also able to isolate encapsulated β-cells from the toxic effects of immunocytes. This hydrogel provides a new approach to address the incomplete protection from permeable immunoreactive factors which has been a long-standing obstacle with conventional islet encapsulation devices. Our work demonstrated that such peptide-functionalized hydrogels could maintain and even enhance glucose-stimulated insulin secretion by encapsulated cells. Encapsulating cells and tissue transplants within hydrogel biomaterials presenting anti-inflammatory constituents may prove to be a more general approach to improving cell and tissue graft function in transplantation, tissue engineering and regenerative medicine applications.
Acknowledgement
This work is supported by NIH grant R01 EB003806. Jing Su is supported by Baxter Early Career Development Award at Northwestern University. We gratefully acknowledge the research group of Dr. Xunrong Luo (Department of Medicine, Northwestern University) for providing CD4+ T-lymphocytes and for sharing their knowledge of islet transplantation and islet immunotolerance, and Mr. Warren R. Sands for providing experimental advice on MIN6 cell culture.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Orive G, Hernandez RM, Gascon AR, Calafiore R, Chang TMS, de Vos P, et al. History, challenges and perspectives of cell microencapsulation. Trends Biotechnol. 2004;22:87–92. doi: 10.1016/j.tibtech.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Efrat S. Cell replacement therapy for type 1 diabetes. Trends Mol Med. 2002;8:334–339. doi: 10.1016/s1471-4914(02)02365-1. [DOI] [PubMed] [Google Scholar]
- 3.Eftat S. Beta-cell replacement for insulin-dependent diabetes mellitus. Adv Drug Deliver Rev. 2008;60:114–123. doi: 10.1016/j.addr.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 4.Gazda LS, Vinerean HV, Laramore MA, Diehl CH, Hall RD, Rubin AL, et al. Encapsulation of porcine islets permits extended culture time and insulin independence in spontaneously diabetic BB rats. Cell Transplant. 2007;16:609–620. doi: 10.3727/000000007783465028. [DOI] [PubMed] [Google Scholar]
- 5.Wang T, Lacik I, Brissova M, Anilkumar AV, Prokop A, Hunkeler D, et al. An encapsulation system for the immunoisolation of pancreatic islets. Nat Biotechnol. 1997;15:358–362. doi: 10.1038/nbt0497-358. [DOI] [PubMed] [Google Scholar]
- 6.Weber LM, He J, Bradley B, Haskins K, Anseth KS. PEG-based hydrogels as an in vitro encapsulation platform for testing controlled beta-cell microenvironments. Acta Biomater. 2006;2:1–8. doi: 10.1016/j.actbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Teramura Y, Kaneda Y, Iwata H. Islet-encapsulation in ultra-thin layer-by-layer membranes of poly(vinyl alcohol) anchored to poly(ethylene glycol)-lipids in the cell membrane. Biomaterials. 2007;28:4818–4825. doi: 10.1016/j.biomaterials.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 8.Zimmermann H, Ehrhart F, Zimmermann D, Muller K, Katsen-Globa A, Behringer M, et al. Hydrogel-based encapsulation of biological, functional tissue: fundamentals, technologies and applications. Appl Phys A-Mater. 2007;89:909–922. [Google Scholar]
- 9.Oshea GM, Sun AM. Encapsulation of Rat Islets of Langerhans Prolongs Xenograft Survival in Diabetic Mice. Diabetes. 1986;35:943–946. doi: 10.2337/diab.35.8.943. [DOI] [PubMed] [Google Scholar]
- 10.Soonshiong P, Heintz RE, Merideth N, Yao QX, Yao ZW, Zheng TL, et al. Insulin Independence in a Type-1 Diabetic Patient after Encapsulated Islet Transplantation. Lancet. 1994;343:950–951. doi: 10.1016/s0140-6736(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 11.Soon-Shiong P. Treatment of type I diabetes using encapsulated islets. Adv Drug Deliver Rev. 1999;35:259–270. doi: 10.1016/s0169-409x(98)00076-3. [DOI] [PubMed] [Google Scholar]
- 12.de Groot M, Schuurs TA, van Schilfgaarde R. Causes of limited survival of microencapsulated pancreatic islet grafts. J Surg Res. 2004;121:141–150. doi: 10.1016/j.jss.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Wilson JT, Chaikof EL. Challenges and emerging technologies in the immunoisolation of cells and tissues. Adv Drug Deliver Rev. 2008;60:124–145. doi: 10.1016/j.addr.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Vos P, van Hoogmoed CG, van Zanten J, Netter S, Strubbe JH, Busscher HJ. Long-term biocompatibility, chemistry, and function of microencapsulated pancreatic islets. Biomaterials. 2003;24:305–312. doi: 10.1016/s0142-9612(02)00319-8. [DOI] [PubMed] [Google Scholar]
- 15.Figliuzzi M, Plati T, Cornolti R, Adobati F, Fagiani A, Rossi L, et al. Biocompatibility and function of microencapsulated pancreatic islets. Acta Biomater. 2006;2:221–227. doi: 10.1016/j.actbio.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Tam SK, Dusseault J, Polizu S, Menard M, Halle JP, Yahia L. Impact of residual contamination on the biofunctional properties of purified alginates used for cell encapsulation. Biomaterials. 2006;27:1296–1305. doi: 10.1016/j.biomaterials.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 17.DeVos P, DeHaan B, VanSchilfgaarde R. Effect of the alginate composition on the biocompatibility of alginate-polylysine microcapsules. Biomaterials. 1997;18:273–278. doi: 10.1016/s0142-9612(96)00135-4. [DOI] [PubMed] [Google Scholar]
- 18.Wright JR, Yang H, Dooley KC. Tilapia - A source of hypoxia-resistant islet cells for encapsulation. Cell Transplant. 1998;7:299–307. doi: 10.1177/096368979800700308. [DOI] [PubMed] [Google Scholar]
- 19.Schrezenmeir J, Kirchgessner J, Gero L, Kunz LA, Beyer J, Muellerklieser W. Effect of Microencapsulation on Oxygen Distribution in Islets Organs. Transplantation. 1994;57:1308–1314. doi: 10.1097/00007890-199405150-00003. [DOI] [PubMed] [Google Scholar]
- 20.Bakeine GJ, Bertolotti A, Latina M, Congiu T, Prati U, Roveda L, et al. Surface properties and implantation site affect the capsular fibrotic overgrowth. J Biomed Mater Res A. 2007;83:965–969. doi: 10.1002/jbm.a.31342. [DOI] [PubMed] [Google Scholar]
- 21.Chae SY, Kim YY, Kim SW, Bae YH. Prolonged glucose normalization of streptozotocin-induced diabetic mice by transplantation of rat islets coencapsulated with crosslinked hemoglobin. Transplantation. 2004;78:392–397. doi: 10.1097/01.tp.0000128617.14309.26. [DOI] [PubMed] [Google Scholar]
- 22.Miura S, Teramura Y, Iwata H. Encapsulation of islets with ultra-thin polyion complex membrane through poly(ethylene glycol)-phospholipids anchored to cell membrane. Biomaterials. 2006;27:5828–5835. doi: 10.1016/j.biomaterials.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 23.Siebers U, Endl U, Horcher A, Bretzel RG, Federlin K, Zekorn T. Evaluation of the effect of microencapsulation and immuno-modulation on islet immunogenicity in vitro. Exp Clin Endocr Diab. 1995;103:140–142. doi: 10.1055/s-0029-1211412. [DOI] [PubMed] [Google Scholar]
- 24.de Vos P, de Haan BJ, de Haan A, van Zanten J, Faas MM. Factors influencing functional survival of microencapsulated islet grafts. Cell Transplant. 2004;13:515–524. doi: 10.3727/000000004783983738. [DOI] [PubMed] [Google Scholar]
- 25.Wiegand F, Kroncke KD, Kolbbachofen V. Macrophage-Generated Nitric-Oxide as Cytotoxic Factor in Destruction of Alginate-Encapsulated Islets - Protection of Arginine Analogs and/or Coencapsulated Erythrocytes. Transplantation. 1993;56:1206–1212. doi: 10.1097/00007890-199311000-00030. [DOI] [PubMed] [Google Scholar]
- 26.Brissova M, Lacik I, Powers AC, Anilkumar AV, Wang T. Control and measurement of permeability for design of microcapsule cell delivery system. J Biomed Mater Res. 1998;39:61–70. doi: 10.1002/(sici)1097-4636(199801)39:1<61::aid-jbm8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 27.Risbud MV, Bhargava S, Bhonde RR. In vivo biocompatibility evaluation of cellulose macrocapsules for islet immunoisolation: Implications of low molecular weight cut-off. J Biomed Mater Res A. 2003;66:86–92. doi: 10.1002/jbm.a.10522. [DOI] [PubMed] [Google Scholar]
- 28.de Haan BJ, Faas MM, de Vos P. Factors influencing insulin secretion from encapsulated islets. Cell Transplant. 2003;12:617–625. doi: 10.3727/000000003108747226. [DOI] [PubMed] [Google Scholar]
- 29.Jang JY, Lee DY, Park SJ, Byun Y. Immune reactions of lymphocytes and macrophages against PEG-grafted pancreatic islets. Biomaterials. 2004;25:3663–3669. doi: 10.1016/j.biomaterials.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 30.Rabinovitch A, Suarez-Pinzon WL. Cytokines and their roles in pancreatic islet beta-cell destruction and insulin-dependent diabetes mellitus. Biochem Pharmacol. 1998;55:1139–1149. doi: 10.1016/s0006-2952(97)00492-9. [DOI] [PubMed] [Google Scholar]
- 31.Dawson PE, Muir TW, Clarklewis I, Kent SBH. Synthesis of Proteins by Native Chemical Ligation. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 32.Hu B-H, Su J, Messersmith PB. Polymer Hydrogels Cross-Linked by Native Chemical Ligation. Biomacromolecules. 2009 doi: 10.1021/bm900366e. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo XR, Tarbell KV, Yang H, Pothoven K, Bailey SL, Ding RC, et al. Dendritic cells with TGF-beta 1 differentiate naive CD4+CD25(−) T cells into isletprotective Foxp3(+) regulatory T cells. Proc Natl Acad Sci USA. 2007;104:2821–2826. doi: 10.1073/pnas.0611646104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veronese FM, Harris JM. Peptide and protein PEGylation III: advances in chemistry and clinical applications. Adv Drug Deliver Rev. 2008;60:1–2. [Google Scholar]
- 35.Ammon HPT, Hagele R, Youssif N, Eujen R, Elamri N. A Possible Role of Intracellular and Membrane Thiols of Rat Pancreatic-Islets in Calcium-Uptake and Insulin Release. Endocrinology. 1983;112:720–726. doi: 10.1210/endo-112-2-720. [DOI] [PubMed] [Google Scholar]
- 36.Robertson RP, Harmon JS. Pancreatic islet beta-cell and oxidative stress: The importance of glutathione peroxidase. FEBS Lett. 2007;581:3743–3748. doi: 10.1016/j.febslet.2007.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pi JB, Bai YS, Zhang Q, Wong V, Floering LM, Daniel K, et al. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007;56:1783–1791. doi: 10.2337/db06-1601. [DOI] [PubMed] [Google Scholar]
- 38.Rydholm AE, Held NL, Benoit DSW, Bowman CN, Anseth KS. Modifying network chemistry in thiol-acrylate photopolymers through postpolymerization functionalization to control cell-material interactions. J Biomed Mater Res A. 2008;86:23–30. doi: 10.1002/jbm.a.31526. [DOI] [PubMed] [Google Scholar]
- 39.Lee HJ, Lee JS, Chansakul T, Yu C, Elisseeff JH, Yu SM. Collagen mimetic peptide-conjugated photopolymerizable PEG hydrogel. Biomaterials. 2006;27:5268–5276. doi: 10.1016/j.biomaterials.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Weber LM, Hayda KN, Haskins K, Anseth KS. The effects of cell-matrix interactions on encapsulated beta-cell function within hydrogels functionalized with matrix-derived adhesive peptides. Biomaterials. 2007;28:3004–3011. doi: 10.1016/j.biomaterials.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Velten F, Laue C, Schrezenmeir J. The effect of alginate and hyaluronate on the viability and function of immunoisolated neonatal rat islets. Biomaterials. 1999;20:2161–2167. doi: 10.1016/s0142-9612(99)00119-2. [DOI] [PubMed] [Google Scholar]
- 42.Lee JI, Nishimura R, Sakai H, Sasaki N, Kenmochi T. A newly developed immunoisolated bioartiricial pancreas with cell sheet engineering. Cell Transplant. 2008;17:51–59. doi: 10.3727/000000008783907035. [DOI] [PubMed] [Google Scholar]
- 43.Prakash S, Bhathena JR, Chen H, editors. Artificial cells, cell engineering and therapy. Abington : Woodhead ; Roca Raton, Fla: CRC; 2007. Introduction to artificial cells : concept, history, design, current status and future; pp. 5–8. [Google Scholar]
- 44.Teramura Y, Iwata H. Islets surface modification prevents blood-mediated inflammatory responses. Bioconjug Chem. 2008;19:1389–1395. doi: 10.1021/bc800064t. [DOI] [PubMed] [Google Scholar]
- 45.Cheung CY, Anseth KS. Synthesis of immunoisolation barriers that provide localized immunosuppression for encapsulated pancreatic islets. Bioconjug Chem. 2006;17:1036–1042. doi: 10.1021/bc060023o. [DOI] [PubMed] [Google Scholar]
- 46.Amoli MM, Larijani B. Would blockage of cytokines improve the outcome of pancreatic islet transplantation? Med Hypotheses. 2006;66:816–819. doi: 10.1016/j.mehy.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 47.Zumsteg U, Frigerio S, Hollander GA. Nitric oxide production and Fas surface expression mediate two independent pathways of cytokine-induced murine betacell damage. Diabetes. 2000;49:39–47. doi: 10.2337/diabetes.49.1.39. [DOI] [PubMed] [Google Scholar]
- 48.Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol. 2005;77:587–597. doi: 10.1189/jlb.1104649. [DOI] [PubMed] [Google Scholar]
- 49.Shapiro AMJ, Lakey JRT, Paty BW, Senior PA, Bigam DL, Ryan EA. Strategic opportunities in clinical islet transplantation. Transplantation. 2005;79:1304–1307. doi: 10.1097/01.tp.0000157300.53976.2a. [DOI] [PubMed] [Google Scholar]
- 50.Akeson AL, Woods CW, Hsieh LC, Bohnke RA, Ackermann BL, Chan KY, et al. AF12198, a novel low molecular weight antagonist, selectively binds the human type I interleukin (IL)-1 receptor and blocks in vivo responses to IL-1. J Biol Chem. 1996;271:30517–30523. doi: 10.1074/jbc.271.48.30517. [DOI] [PubMed] [Google Scholar]
- 51.Baker MS, Chen XJ, Rotramel A, Nelson J, Kaufman DB. Proinflammatory cytokines induce NF-kappa B-dependent/NO-independent chemokine gene expression in MIN6 beta cells. J Surg Res. 2003;110:295–303. doi: 10.1016/s0022-4804(03)00027-1. [DOI] [PubMed] [Google Scholar]
- 52.Lau J, Mattsson G, Carlsson C, Nyqvist D, Kohler M, Berggren PO, et al. Implantation site-dependent dysfunction of transplanted pancreatic islets. Diabetes. 2007;56:1544–1550. doi: 10.2337/db06-1258. [DOI] [PubMed] [Google Scholar]