SUMMARY
Migraine is a complex neurological disorder with a significant impact on patients and society. Clinical and preclinical studies have established the neuropeptide calcitonin gene-related peptide (CGRP) as a key player in migraine and other neurovascular headaches. To study the role of CGRP in these disorders, we have characterized the photophobic phenotype of nestin/hRAMP1 mice, a transgenic model with genetically engineered increased sensitivity to CGRP. These mice have increased nervous system expression of a regulatory subunit of the CGRP receptor, human receptor activity-modifying receptor (hRAMP1). We have previously demonstrated that nestin/hRAMP1 mice display a light aversive behavior that is greatly enhanced by CGRP and blocked by a CGRP receptor antagonist used to treat migraine. Here we have compared their behavior in two different experimental setups with testing chambers of different sizes and light intensities as well as in complete darkness. We demonstrated similar degrees of light aversion in nestin/hRAMP1 mice with 1000 and 50 lux. To control for other possible factors driving nestin/hRAMP1 mice to the dark zone, we tested them in the absence of any light and they showed identical behavior as littermates. Furthermore, both nestin/hRAMP1 and control mice have decreased motility in response to CGRP in the dark, but not the light side of the chamber. Our findings confirm the robust CGRP-induced light aversive phenotype of nestin/hRAMP1 mice, which can be a surrogate of photophobia, and validates its usefulness as a model of migraine and other disorders associated with photophobia.
Keywords: calcitonin gene-related peptide (CGRP), CGRP receptor, receptor activity-modifying protein 1 (RAMP1), migraine, photophobia
INTRODUCTION
Migraine is a chronic neurovascular disorder characterized by recurrent episodes of severe unilateral throbbing head pain and associated symptoms, such as photophobia (Goadsby et al., 2002). Mechanistic studies in the field of migraine pathophysiology are hampered by the lack of appropriate animal models. We have developed a transgenic mouse that displays a migraine-like behavioral phenotype, the nestin/hRAMP1 mouse (Zhang et al., 2007; Marquez de Prado, 2009; Recober et al., 2009). This mouse is sensitized to the calcitonin gene-related peptide (CGRP), a key player in migraine pathophysiology (Arulmani et al., 2004), by overexpression of the human receptor activity modifying protein 1 (hRAMP1) subunit of the CGRP receptor in the nervous system.
Nestin/hRAMP1 mice exhibit light aversive behavior that is greatly enhanced by exogenous CGRP (Recober et al., 2009). This behavior cannot be attributed to differences in anxiety or motor activity (Recober et al., 2009). The specificity of CGRP actions was confirmed by the use of a CGRP receptor antagonist, olcegepant, which prevented the CGRP-induced light-aversive behavior (Recober et al., 2009). A potential mechanism of the light-aversion observed at baseline may be the higher levels of CGRP present in cerebrospinal fluid of nestin/hRAMP1 mice (Recober et al., 2009).
We studied light aversive behavior as a surrogate of photophobia, a common symptom reported by patients both during migraine attack and to a lesser degree between attacks (Mulleners et al., 2001). While the mechanisms underlying photophobia remain unknown, the trigeminal system appears to be involved (Drummond and Woodhouse, 1993; Kowacs et al., 2001; Okamoto et al., 2009). As a major neuropeptide in the trigeminal system, CGRP is likely to be implicated in the development of photophobia. Our previous studies suggest that nestin/hRAMP1 mice are a useful model for mechanistic and therapeutic studies of migraine and other trigeminal mediated disorders and conditions associated with photophobia (Zhang et al., 2007; Marquez de Prado, 2009; Recober et al., 2009).
The aim of the current study is to fully characterize the photophobic phenotype of nestin/hRAMP1 mice and confirm the robustness of this behavior by replicating the findings in a different experimental setup that allows measurement of additional parameters. The times spent in light in chamber # 1 (Table 1) are taken from a previous publication for comparison (Recober et al. 2009). Transitions and latencies in chamber # 1 and all the parameters measured in chamber # 2 are original data for this study.
Table 1.
Summary of Photophobic behaviors in chamber # 1.
| Interval |
|||||||
|---|---|---|---|---|---|---|---|
| 1st 300 sec | 2nd 300 sec | ||||||
|
Time in Light (sec) |
(n) | Mean ± SEM | p | Mean ± SEM | p | ||
| Untreated | Control | (55) | 105.46 ± 4. | - | |||
| hRAMP1 | (36) | 17 73.67 ± 6.70 | ** | - | |||
| Vehicle | Control | (12) | 141.51 ± 16.24 | 111.03 +/− 17.80 | |||
| hRAMP1 | (12) | 108.43 ± 12.75 | 90.08 +/− 15.23 | ||||
| CGRP | Control | (22) | 133.02 ± 18.21 | 112.81 +/− 20.27 | |||
| hRAMP1 | (21) | 62.37 ± 7.50 | ** ## | 14.26 +/− 4.69 | ** ## | ||
|
No. of Transitions |
(n) | Mean ± SEM | Mean ± SEM | ||||
| Vehicle | Control | (8) | 10.75 ± 2.21 | 9.25 +/− 2.34 8. | |||
| hRAMP1 | (8) | 11.13 ± 2.53 | 88 +/− 2.72 | ||||
| CGRP | Control | (11) | 6.91 ± 1.94 | 4.45 +/− 1.72 | |||
| hRAMP1 | (10) | 3.30 ± 0.90 | ## | 1.20 +/− 1.00 | ## | ||
|
Latencies (sec) |
(n) |
Direction |
|||||
| Enter Dark | Exit Dark | ||||||
| Mean ± SEM | p | Mean ± SEM | p | ||||
| Untreated | Control | (55) | 23.84 ± 3.27 | 45.2 +/− 3.51 | |||
| hRAMP1 | (36) | 15.08 ± 3.44 | 82.28 +/− 12.08 | ** | |||
| Vehicle | Control | (8) | 63.75 ± 31.39 | 49.88 +/− 10.59 | |||
| hRAMP1 | (8) | 23.38 ± 5.42 | 61.63 +/− 17.61 | ||||
| CGRP | Control | (11) | 134.09 ± 62.83 | 39.45 +/− 6.75 | |||
| hRAMP1 | (10) | 26.80 ± 5.99 | 234.32 +/− 77.58 | * | |||
MATERIALS AND METHODS
Animals
Adult male and female mice between 10 and 20 weeks of age were used. Each mouse was tested only once in either chamber to ensure the novelty of the test. Animals were housed in groups of 2 to 5 per cage in standard conditions, on a 12-hour light cycle (lights on at 6 am), with access to water and food at libitum.
Two strains of nestin/hRAMP1 mice with differing genetic background were utilized (Zhang et al., 2007). Both strains have identical Tg(Nes-cre)1Kln/J and Tg(RAMP1) alleles (Tronche et al., 1999). For Tg(Nes-cre)1Kln Tg(RAMP1), Nes-cre was introduced by an intercross involving a B6;129 mixed genetic background, yielding mice with contributions from the B6, 129, and SJL genetic backgrounds. For B6;SJL-Tg(Nes-cre)1Kln Tg(RAMP1), Nes-cre was introduced by an intercross involving mice obtained from The Jackson Laboratory (stock 003771) on a B6 genetic background, yielding mice without contributions from the 129 genetic background.
Nestin/hRAMP1 and control littermates from both strains have been shown to display almost identical behavior in the light-aversion test after treatment with CGRP or vehicle (Recober et al., 2009). In the current studies, we have used mice from both strains depending on animal availability. Control mice were littermates nontransgenic, single transgenic (not expressing hRAMP1) nestin-cre or Cx1-GFP-hRAMP1.
Animal care and procedures were approved by the University of Iowa Animal Care and Use Committee and performed in accordance with the standards set by the National Institutes of Health.
Drugs
Rat α-CGRP (Sigma) was diluted in phosphate buffered saline (PBS) (0.5 nmol). Inhaled 3% isoflurane was used for brief anesthesia during intracerebroventricular (icv) injections. Drug administration was by direct injection in the right lateral ventricle through the intact scalp aiming at 1 mm posterior to bregma and 1 mm right from the midline. All the injections were performed by the same person (AR) after a period of training yielding a success rate of 89% demonstrated by injections of dye in the ventricles. The avoidance of deep anesthesia and stereotaxic guidance was important to allow quick recovery of the mice and appropriate performance in the behavioral test. We used an injector made with a 30-gauge ½ inch needle connected by a polyethylene tube to a microsyringe. A 1 cm polyethylene tube was fitted to the needle to control the depth of the injection (2.5 mm). Mice were allowed to recover in individual cages for 30 minutes prior to behavioral testing.
Light Aversion Assay
Behavioral testing was performed always between 9 am and 2 pm, in a quiet room with constant temperature (23 ºC) and light intensity (200 lux inside the housing cage). Each mouse was tested only once to avoid decreased exploration due to habituation to the apparatus. Light aversion was tested in two different experimental setups.
Experimental setup using chamber # 1
Mice were individually tested in a custom made light aversion chamber (60 L × 60 W × 45 H cm) with two equally sized compartments; one brightly lit (1000 lux, thermal-neutral fiber optic source (Fiber-lite)), painted white and lacking a top; the other not lit, painted black and fully enclosed. A small opening (7 × 7 cm) connects the two compartments. Prior to testing mice were acclimated at least 1 hour in their cages in the testing room with the lights on. Each mouse was gently held on the investigator’s palm with minimal tail restrain and placed in the center of the open side of the box, facing away from the dark side. A video tracking system (View Point Life Sciences Inc.) was used to monitor and quantify behavior. Each test was conducted for five or ten minutes and the data were analyzed in 300 sec intervals. The light aversion chamber was thoroughly cleaned with 95% EtOH between animals. All behavioral studies were performed in untreated mice and 30 minutes after intracerebroventricular injection of 2 µl of CGRP (0.5 nmol) or vehicle.
The following parameters were assessed:
Total time in light. The total time spent in the light compartment was automatically calculated by software in seconds (sec).
Transitions. The number of times that the animal crossed from one compartment to another was determined by reviewing the videos. The four paws had to be in the compartment to be considered a transition.
Latency to enter the dark. The time taken by each animal to completely enter the dark (four paws inside) for the first time was quantified by reviewing the videos (sec).
Latency to exit the dark. The time taken by each animal to completely exit (four paws outside) the dark compartment after the first entry was determined by watching the videos (sec).
Experimental setup using chamber # 2
Mice were tested in a plexiglass chamber (27 × 27 × 20.3cm) equipped with three 16 beam infrared arrays (Med Associates Inc., St. Albans, VT). The chamber is divided in two equally sized compartments by a dark insert that is opaque to visible light. There is a small opening in the dark insert that allows the mouse to freely move between the two zones. This chamber is placed inside a sound-attenuating cubicle (56 × 38 × 36cm) (Med Associates Inc.). The light source is located in the back left and right corners of the cubicle and produces light intensity of 55 lux inside the lit area of the test chamber. As with the chamber # 1, mice were placed in the center of lit area facing away from the dark side and tested for 10 minutes. Six test chambers were used simultaneously, and the behavior was analyzed by the Activity Monitor software (Version 5.0; Med Associates Inc.). The behavioral data were analyzed in two 300 sec intervals. All the parameters were measured in untreated mice and 30 minutes after intracerebroventricular injection of 2 µl of CGRP (0.5 nmol) or vehicle, as done in setup # 1.
The following parameters were automatically obtained from the software and analyzed:
Total time in light. The total time spent in the light compartment was measured in seconds (sec).
Transitions. The number of times that the animal transitioned from one compartment to another was defined as the center of the mouse crossing the threshold between the two zones.
Latency to enter the dark. The time taken by each animal to enter the dark for the first time was quantified (sec).
Latency to exit the dark. The time taken by each animal to exit the dark compartment after the first entry was calculated (sec).
Rearings. The number of vertical movements anywhere in the chamber was assessed.
Ambulatory distance. The distance traveled by each animal, defined as the center of the mouse moving across a minimum of 3 beams (3.4 cm) was recorded (cm).
Ambulatory Time. The time that the mice spent moving was monitored (sec). The total time spent in the chamber is divided in ambulatory, resting and stereotypic, defined as small movements where the center of the mouse moves within the same 3 beams (3.4 cm)
Resting Time. The time that the mice spent inactive was monitored (sec).
Ambulatory Velocity. The average velocity of movement of each mouse was calculated from dividing distance traveled by the ambulatory time (cm/sec).
All the parameters to assess motility were normalized to the time that the animal spent in each zone and in each interval.
Statistical Analysis
Data are reported as mean ± S.E.M. Unpaired two tail Student’s t-test was used to assess two-sample comparisons. Levene’s test for equality of variances was performed prior to each comparison and the appropriate P value was reported when equal variances couldn’t be assumed. Outliers were defined as those with values between 1.5 and 3 times the size of the interquartile range from the upper or lower limit of the interquartile range. For the time spent in the light, the parameter of main interest, there were no outliers in any of the groups. Therefore all the animals were included in the analyses. Moreover, we did not find any mouse that was consistently an outlier in all or most of the parameters studied. P < 0.05 was considered significant.
RESULTS
Light aversion chambers
The features of chamber # 1 were selected to maximize the potential for light aversive behavior. This setup consists in a relatively large chamber size with very intense light and lacks a cover over the light compartment. The mice were monitored by a videotracking system.
Chamber # 2 allowed measurement of more parameters using infrared beams instead of a videotracking system. This chamber differs from the chamber #1 in its smaller size, use of a lower light intensity, and enclosure in a sound attenuating cabinet. A major advantage of this chamber is the ability to analyze motility in both the light and dark compartments.
Light aversion parameters in chamber # 1
Results of the light aversion test were collected as total time in the light compartment, number of transitions between the light and dark compartments, and the latencies to first enter and then exit the dark compartment. The results using chamber # 1 are summarized in Table 1. We had previously shown that untreated nestin/hRAMP1 mice spent less time in the light than control littermates, which was greatly enhanced by exogenous CGRP (Recober et al., 2009). Here we show that untreated nestin/hRAMP1 mice took longer than controls to exit the dark compartment after their first entry (P < 0.005). Similar behavior is seen after CGRP treatment (P < 0.05), but there is significant variability in this parameter after intracerebroventricular treatment. Nestin/hRAMP1 mice transitioned less between the light and dark compartments after CGRP injection compared with vehicle (P < 0.005). CGRP also appeared to decrease the number of transitions observed in control mice, although the difference was not statistically significant.
Time spent in light in chamber # 2
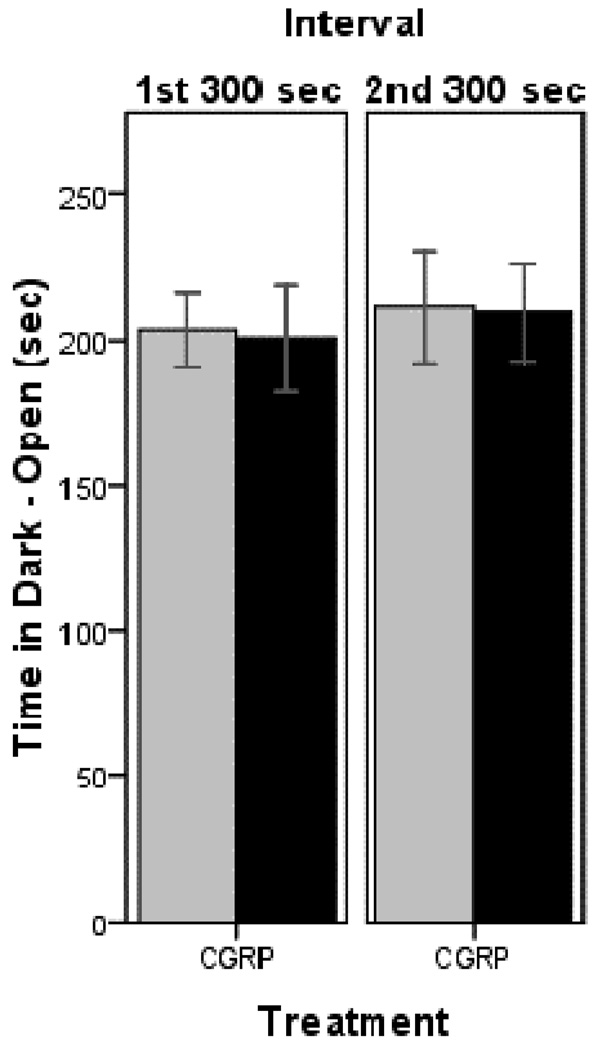
Having established the light aversive behavior in chamber # 1, we then tested a new cohort of mice in chamber # 2. To confirm that the CGRP-induced behavior is indeed caused by the light, we investigated the animals’ behavior in chamber # 2 in the absence of light in both zones. In this setting, CGRP-treated nestin/hRAMP1 and control mice spent two thirds of the time in the dark and open compartment (which is normally lit) where they were initially placed and only one third of the time in the dark and enclosed area (Fig 1). Similar behavior was seen in both testing intervals. Analyses of all the other parameters in this dark-dark paradigm did not reveal any difference between nestin/hRAMP1 and control mice (data not shown).
Figure 1. Time spent in dark and open zone of chamber # 2.
Both nestin/hRAMP1 and control mice spent similar amounts of time in the dark and open side of the chamber (normally light zone) in both testing intervals. For both groups: n = 8. Mean ± s.e.m.
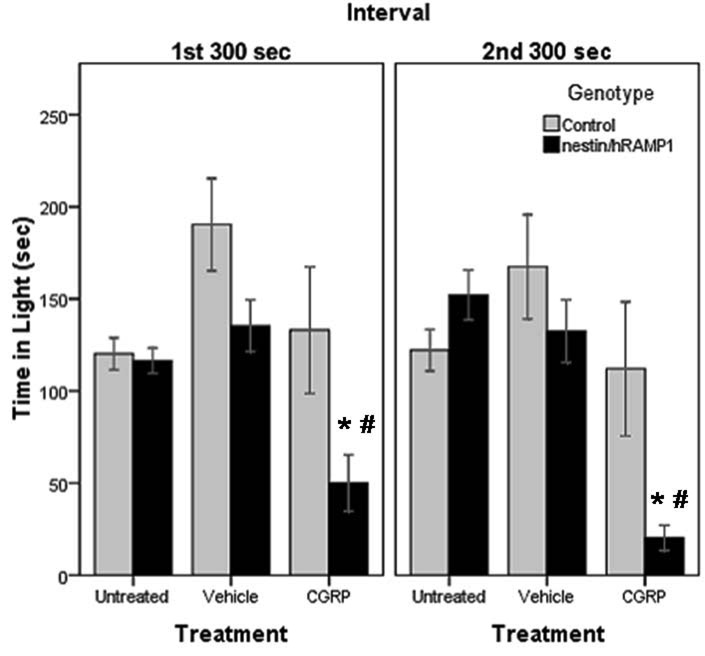
When a low intensity light (55 lux) was used, untreated nestin/hRAMP1 mice spent about the same amount of time in the light (116.5 ± 6.8 sec) as control littermates (120.2 ± 8.7 sec) (P = 0.74) during the first 300 sec interval (Fig 2). During the second interval similar behavior was observed (152.2 ± 13.5 and 122.1 ± 11.3 sec, respectively) (P = 0.09) (Fig. 2).
Figure 2. Time spent in light in chamber # 2.
CGRP-treated nestin/hRAMP1 spend less time in the light compared with control mice in the first interval (*P = 0.053) and in the second interval (*P = 0.04) and compared with vehicle treatment in the first interval (#P = 0.001) and in the second interval (#P < 0.001). Control, untreated: n = 13; nestin/hRAMP1, untreated : n = 12; all other groups: n = 8. Mean ± s.e.m.
After CGRP treatment, nestin/hRAMP1 mice spent less time in the light (50 ± 15.3 sec) than control mice (133 ± 34.4 sec) in the first 300 sec interval, although the difference did not quite reach statistical significance (P = 0.05) (Fig. 2). In the second testing interval, the difference became more noticeable (20.2 ± 6.9 and 112 ± 36.4 sec, respectively) (P = 0.04) (Fig. 2).
Nestin/hRAMP1 mice spent less time in the light after icv CGRP (50 ± 15.3 sec) compared with vehicle (135.4 ± 14.1 sec) (P < 0.001) in the first interval (Fig. 2). Again, the difference was even more pronounced (20.2 ± 6.9 and 132.5 ± 17.1 sec, respectively) during the second interval (P < 0.001) (Fig. 2). The effect of CGRP in control mice was not significant in either interval (Fig 2).
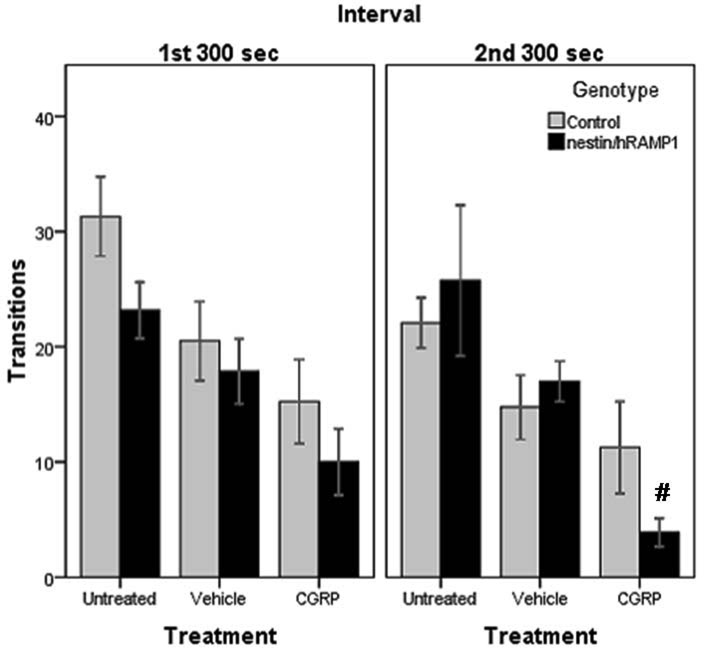
Transitions between light and dark zones in chamber # 2
While the number of transitions between the two zones is typically used as an index of exploratory behavior (Crawley and Goodwin, 1980) and has been reported to be unaffected by light intensity (Costall et al., 1989), others have used transitions as a measure of light aversion (Thiels et al., 2008). The numbers of transitions between the two sides of the chamber were similar in the first 300 sec interval for untreated nestin/hRAMP1 (23.2 ± 2.4) and control mice (31.2 ± 4) (P = 0.1) (Fig 3). Similar results were obtained in the second testing period (25.8 ± 6.5 and 22.2 ± 2.6, respectively) (P = 0.6) (Fig. 3). After CGRP treatment, the numbers of transitions between the two sides of the chamber were still similar for nestin/hRAMP1 (10 ± 2.9) and control mice (15.3 ± 3.7) (P = 0.3) during the first interval (Fig 3) and second interval (3.9 ± 1.2 and 11.3 ± 4, respectively) (P = 0.1) (Fig. 3).
Figure 3. Number of transitions between compartments in chamber # 2.
CGRP-treated nestin/hRAMP1 transition less than vehicle treated ones in the first interval (P = 0.071) and in the second interval (#P < 0.001). Both nestin/hRAMP1 and control mice display similar number of transitions after treatment with CGRP in both intervals. Control, untreated: n = 13; nestin/hRAMP1, untreated : n = 12; all other groups: n = 8. Mean ± s.e.m.
However, when compared to vehicle treated mice, nestin/hRAMP1 mice transitioned less between the two sides of the chamber after icv CGRP. While during the first interval the difference did not reach statistical significance (CGRP 10 ± 2.9, vehicle 17.9 ± 2.8) (P = 0.07) (Fig 3), it was more pronounced in the second interval (3.9 ± 1.2 and 17 ± 1.8, respectively) (P < 0.001) (Fig. 3). The effect of CGRP in control mice was not significant in either interval (Fig 3).
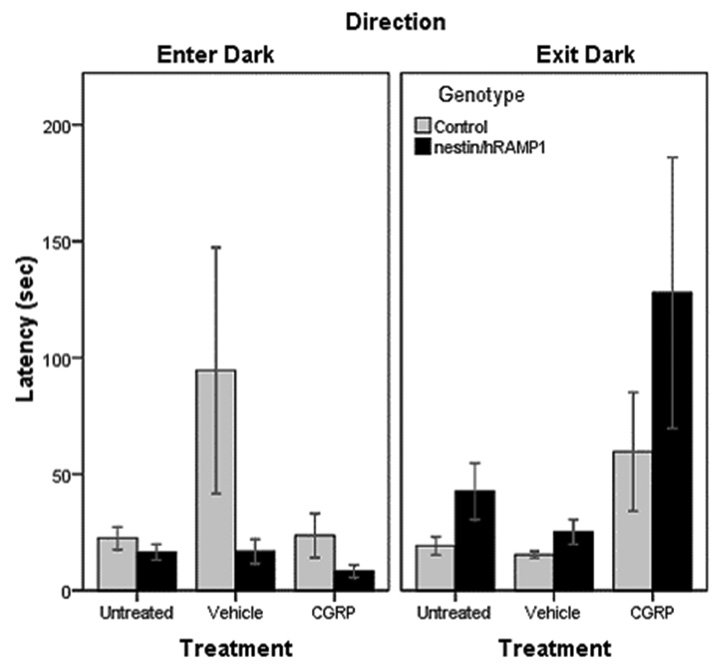
Latency to enter and exit the dark zone in chamber # 2
Untreated nestin/hRAMP1 displayed a slight tendency to enter the dark zone faster (16.5 ± 3.4 sec) than control mice (22.5 ± 4.9 sec) but this was not statistically significant (P = 0.33) (Fig. 4). Likewise, they showed a tendency to take longer to exit the dark zone (42.5 ± 12.1 sec) than control mice (19.2 ± 3.8 sec), although the difference did not quite reach significance (P = 0.07) (Fig. 4). Similarly, after CGRP treatment, there was a trend for nestin/hRAMP1 mice to enter the dark compartment faster (8.2 ± 2.7 sec) than control mice (23.6 ± 9.5 sec) or compared with vehicle (16.8 ± 5.3 sec), though they did not reach significance (P = 0.14 and 0.18, respectively) (Fig 4). There was also a trend for CGRP-treated nestin/hRAMP1 to take longer to exit the dark compartment for the first time (127.9 ± 58.2 sec) compared with littermates (59.7 ± 25.5 sec) or with vehicle (25.1 ± 5.3 sec) (P = 0.3 and 0.12, respectively) (Fig. 4). The effect of CGRP in control mice in these parameters was not significant (Fig 4).
Figure 4. Latency to enter and exit the dark zone in chamber # 2.
No differences between nestin/hRAMP1 and control mice are observed in the time taken to enter the dark zone. Untreated nestin/hRAMP1 mice showed a tendency to take longer to exit the dark zone than control mice (P = 0.07). Control, untreated: n = 13; nestin/hRAMP1, untreated : n = 12; all other groups: n = 8. Mean ± s.e.m.
Motor activity during the light aversion test in chamber # 2
To ascertain the potential role of motor activity in the light aversion test, we assessed locomotion simultaneously. We have previously reported that movement was the same in vehicle and CGRP treated nestin/hRAMP1 and control mice in the light zone using experimental setup with chamber # 1 (Recober et al., 2009). However, a limitation of chamber # 1 was that measurements could be made only in the light zone due to the reliance on a camera system. We have now extended the motor activity evaluation using the experimental setup with chamber # 2. Rearings, distance traveled, ambulatory time, and resting time were calculated relative to the time spent in each compartment during each testing interval. Mean ambulatory velocity was also calculated.
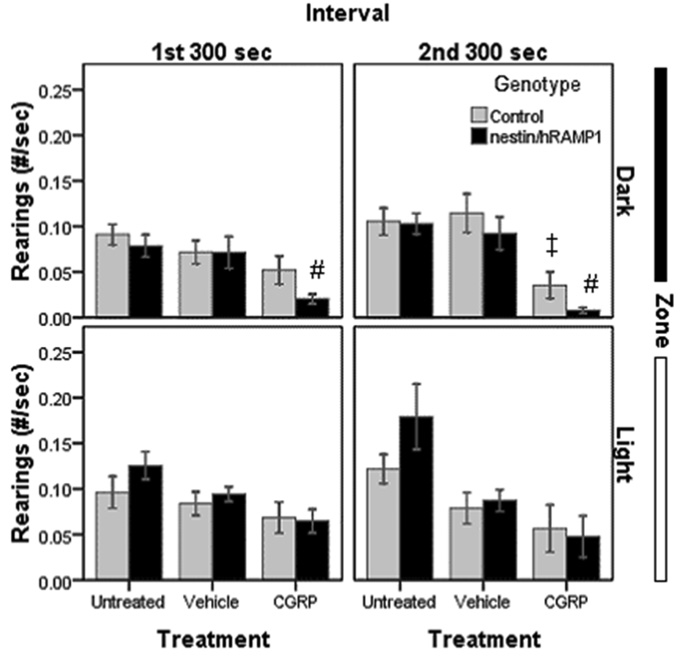
Rearings are an indicator of exploratory and motor activity. Untreated nestin/hRAMP1 and control mice had similar numbers of rearings (Fig. 5). CGRP-treated nestin/hRAMP1 displayed a tendency to rear less than control mice only when they were in the dark side during the first (P = 0.085) and the second intervals (P = 0.08), but no differences were seen in the light zone (Fig. 5). Nestin/hRAMP1 treated with CGRP showed a significant decrease in the number of rearings compared with vehicle only in the dark compartment during the first (P = 0.014) and second intervals (P = 0.002) (Fig. 5). Control mice also showed a decrease in rearings in response to CGRP that only reached significance in the dark in the second interval (P = 0.01) (Fig. 5).
Figure 5. Rearings in chamber # 2.
CGRP-treated nestin/hRAMP1 display less rearings than vehicle treated ones in the dark only (#P = 0.014 and 0.002, first and second interval respectively). There was a tendency to lower rearings in CGRP-treated nestin/hRAMP1 compared with control mice only in the dark in both intervals (P = 0.08). CGRP-treated control mice also reared less than vehicle treated control mice in the dark in the second interval (‡P =0.01). Rearings were normalized by dividing number of rearings (#) by total time spent in each zone (sec). Control, untreated: n = 13; nestin/hRAMP1, untreated : n = 12; all other groups: n = 8. Mean ± s.e.m.
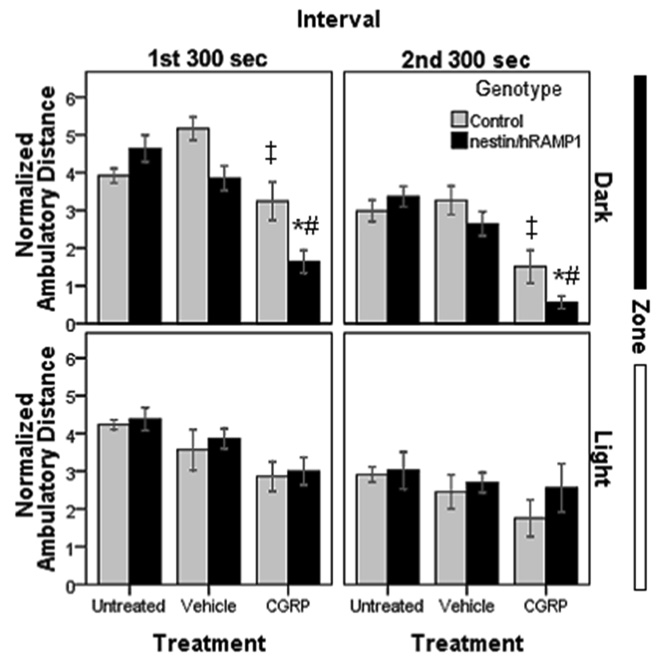
Another measure of motor activity is the distance traveled in the chamber. Untreated nestin/hRAMP1 and control mice traveled similar distances (Fig. 6). However, after CGRP treatment there was a striking difference between the light and dark zones. CGRP-treated nestin/hRAMP1 and control mice had a similar behavior in the light zone (Fig 6). In the dark zone nestin/hRAMP1 moved less than control mice, but it only reached significance in the firs interval (P = 0.019 and 0.054, respectively) (Fig. 6). CGRP-treated nestin/hRAMP1 mice also had decreased ambulatory distance in the dark compartment when compared with vehicle treatment during both testing intervals (P < 0.001) (Fig. 6). CGRP had a similar effect in control mice, decreasing the distance traveled only in the dark compartment in both intervals (P < 0.01) (Fig. 6).
Figure 6. Ambulatory distance in chamber # 2.
CGRP-treated nestin/hRAMP1 mice travelled less than control mice only in the dark zone during the first (*P < 0.02) and second (*P = 0.054) intervals. CGRP-treated nestin/hRAMP1 mice moved less in the dark zone when compared with vehicle treatment during both testing intervals (#P < 0.001). CGRP-treated control mice moved less than vehicle treated mice in the dark in both intervals (‡P < 0.01). Ambulatory distance was normalized by dividing the ambulatory distance (cm) by time spent in each zone (sec). Control, untreated: n = 13; nestin/hRAMP1, untreated : n = 12; all other groups: n = 8. Mean ± s.e.m.
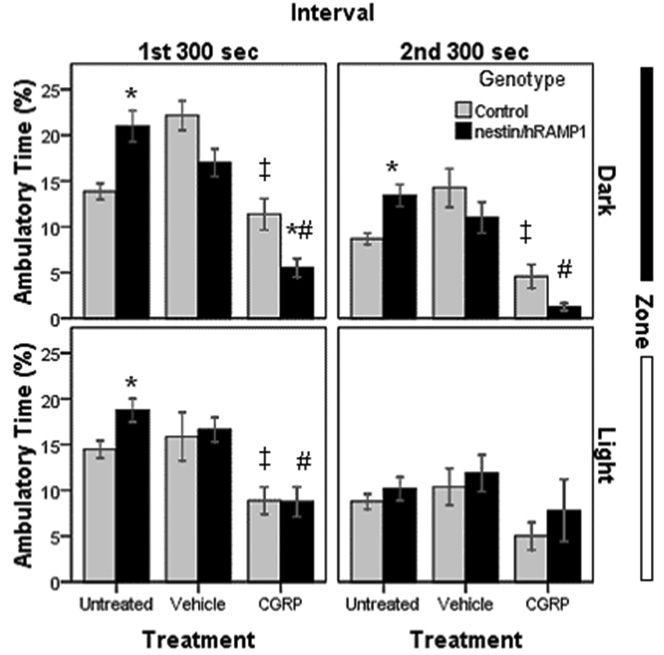
In general, the amount of time the mice spent moving followed a similar trend as the distances traveled although there were some differences. Untreated nestin/hRAMP1 mice exhibited greater ambulatory time than littermates (P < 0.01) except for when they were in the light during the second interval (Fig. 7). CGRP caused nestin/hRAMP1 mice to spend less time moving than littermates only in the dark compartment during the first interval (P = 0.017) although the same trend was observed in the second interval (P = 0.059) (Fig. 7). Nestin/hRAMP1 mice treated with CGRP spent less time moving compared with vehicle (P < 0.002), except in the light compartment during the second interval (Fig. 7). CGRP also decreased the time that control mice spent moving, more noticeably in the dark zone (P = 0.001 and 0.002, in the first and second interval, respectively) and to a lesser degree in the light compartment (P = 0.04 and 0.052, in the first and second interval, respectively) (Fig. 7).
Figure 7. Ambulatory time in chamber # 2.
Untreated nestin/hRAMP1 mice spent more time moving than littermates (*P < 0.01) except for when they were in the light during the second interval. CGRP-treated nestin/hRAMP1 mice spent less time moving than littermates only in the dark compartment during the first interval (*P = 0.017) although the same trend was observed in the second interval (P = 0.059). Nestin/hRAMP1 mice treated with CGRP spent less time moving compared with vehicle (#P < 0.002), except in the light compartment during the second interval. CGRP-treated control mice spent less time moving than vehicle-treated mice in the dark in both intervals (‡P < 0.002) and in the light in the first interval (‡P = 0.04). Ambulatory time was normalized to a percentage by dividing ambulatory time (sec) by total time spent in each zone (sec). Control, untreated: n = 13; nestin/hRAMP1, untreated : n = 12; all other groups: n = 8. Mean ± s.e.m.
Likewise, in general, the amount of time the mice spent resting followed the inverse trend of the amount of time spent moving. Percentage of time resting time was similar in untreated nestin/hRAMP1 (51 ± 1.8 %) and control littermates (50 ± 4 %) (P = 0.84) in the light zone in the first interval and also in the second interval (62 ± 2.2 % and 58 ± 1.6 %, nestin/hRAMP1 and control mice, respectively) (P = 0.115). Similar results were observed in the dark zone in both intervals. CGRP-treated nestin/hRAMP1 mice spend more time inactive (76 ± 3.8 %) than control mice (59 ± 3.8 %) (P = 0.009) only in the dark zone in the first and in the second intervals (88 ± 3 % and 75 ± 4.8 %, nestin/hRAMP1 and control mice, respectively) (P = 0.032). CGRP- treated nestin/hRAMP1 mice spend more time resting compared with vehicle treated mice only in the dark in the first interval (76 ± 3.8 % and 54 ± 2.1, CGRP and vehicle, respectively) (P < 0.001) and in the second interval (88 ± 3 % and 61 ± 2 %, CGRP and vehicle, respectively) (P < 0.001). Similarly, CGRP only had an effect in control mice in the dark compartment where it increased the proportion of time spent resting in the first interval (59 ± 3.8 % and 47 ± 2.2 %, CGRP and vehicle, respectively) (P = 0.016) and in the second interval (75 ± 4.9 % and 58 ± 2.6 %, CGRP and vehicle, respectively) (P = 0.007).
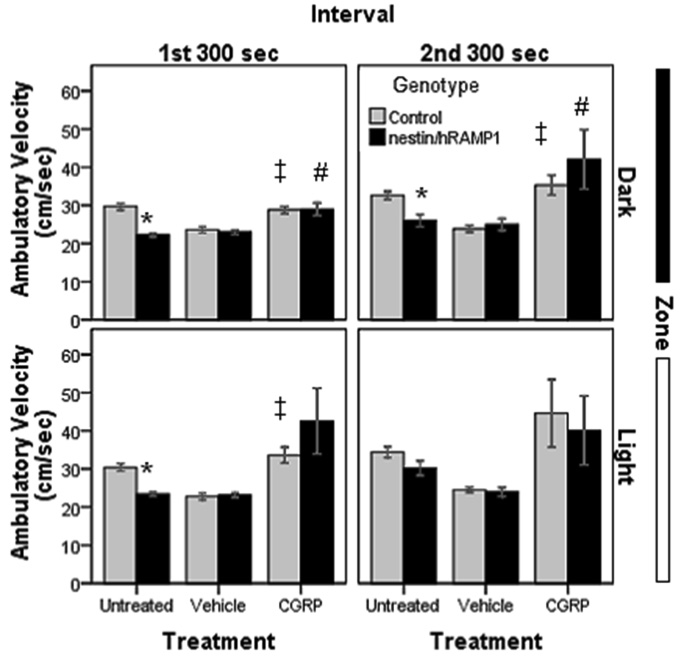
The mean ambulatory velocity was calculated by dividing the total distance traveled by the total amount of ambulatory time in each zone and in each interval. Untreated nestin/hRAMP1 moved slower than control mice (P < 0.005) except in the second interval in the light (Fig. 8). There were not differences between CGRP-treated nestin/hRAMP1 and control mice (Fig. 8). Nestin/hRAMP1 mice treated with CGRP moved faster compared with vehicle, but this only reached significance in the dark in the first (P = 0.007) and second (P = 0.049) intervals (Fig.8). Similarly, CGRP-treated control mice moved faster than vehicle-treated mice in the dark in the first (P = 0.001) and second (P = 0.003) intervals. In the light compartment, the increased ambulatory velocity only reached significance in the first (P < 0.001) but not in the second (P =0.07) interval (Fig.8).
Figure 8. Ambulatory velocity in chamber # 2.
Untreated nestin/hRAMP1 move slower than control mice (*P < 0.01) except in the second interval in the light. There were not differences between CGRP-treated nestin/hRAMP1 and control mice. Nestin/hRAMP1 mice treated with CGRP moved faster compared with vehicle, but this only reached significance in the dark in the first (#P = 0.007) and second (#P = 0.049) intervals. CGRP-treated control mice moved faster than vehicle-treated mice in the dark in the first (‡P = 0.001) and second (‡P = 0.003) intervals and in the light in the first interval (‡P < 0.001) and second interval (P = 0.07). Control, untreated: n = 13; nestin/hRAMP1, untreated : n = 12; all other groups: n = 8. Mean ± s.e.m.
In summary, CGRP induced motility changes in both nestin/hRAMP1 and control mice that were consistently more noticeable in the dark compartment. CGRP-treated nestin/hRAMP1 mice exhibited less rearing, traveled less and were more inactive than control mice when they were in the dark zone, but they had similar behavior in the light compartment. Furthermore, CGRP caused both nestin/hRAMP1 and control mice to move faster, especially in the light zone.
DISCUSSION
Induction of light aversion is a novel function for the neuropeptide CGRP that opens a new field of study. We have previously demonstrated that CGRP induces light aversive behavior in nestin/hRAMP1 mice (Recober et al., 2009). In the current study, we report additional parameters indicative of light aversion that strengthen our initial findings. In particular, the identical behavior of nestin/hRAMP1 and control mice when tested in the in complete darkness confirms that the nestin/hRAMP1 preference for the dark compartment is indeed due exclusively to the aversive light stimulus. While we cannot be certain that the animals are experiencing photophobia, we presume that their aversion to light must be a very unpleasant sensation to overcome their strong, innate exploratory drive.
The light-dark exploratory test is known to yield inconsistent results associated to methodological modifications (Bourin and Hascoet, 2003). For this reason, we have analyzed the nestin/hRAMP1 behavior in a different testing apparatus with a considerably lower light level and a completely different analytical system. Consequently, this study, replicating the CGRP-induced light aversive behavior, corroborates the robust photophobic phenotype of the nestin/hRAMP1 model.
In contrast to CGRP-induced light aversion, the baseline behavior observed with the more intense light, where untreated nestin/hRAMP1 mice spent 30% less time in the light than their littermates, was not observed in the new experimental setup. We speculate that this may be due to the lower light intensity, but cannot rule out a contribution of the smaller size of the chamber or the smaller sample size.
Transitions are frequently used in the light-dark test to assess exploratory behavior in response to anxiolytic dugs (Crawley et al., 1997). It is important to note that we have previously excluded enhanced anxiety-like behavior in untreated and CGRP-treated nestin/hRAMP1 compared with control mice in two different paradigms (Recober et al., 2009). Transitions have also been proposed as a measure of photophobia in mice (Thiels et al., 2008). We observed a higher number of transitions overall in the chamber # 2, which could be attributed to the smaller size of the light and dark zones or the lower light intensity. Another possibility is that the slightly different definition of transition, one being automatically obtained from the software and the other obtained after reviewing the video (see Methods), may account for the differences. Nonetheless, a similar decrease in the number of transitions in response to treatment was seen in control and nestin/hRAMP1 mice in both setups.
The shorter latency to exit the dark compartment after the first entry and the trend to enter the dark zone faster for the first time, are consistent with the light aversive phenotype displayed by nestin/hRAMP1 mice. In untreated mice, the time to exit the dark compartment for the first time may represent a very useful parameter indicative of light aversion, especially when testing small number of animals (n = 8–10). While the total time spent in light requires large number of animals of 20 or more per group to observe a significant difference in untreated mice, the latency to exit the dark is a parameter that we have consistently found to be higher in untreated nestin/hRAMP1 mice. Unfortunately, the variability of this parameter after treatment with either CGRP or vehicle seen in both chambers makes these measures less informative. We propose that the latency to exit the dark compartment for the first time may be an especially useful parameter when CGRP treatment needs to be avoided and only a small number of animals can be tested. This may be the case for therapeutic studies of preventive drugs were the baseline behavior is of interest, and the potential benefit from the drug may be lost after intracerebroventricular treatment with CGRP.
Whether the trait of light aversion observed in untreated nestin/hRAMP1 mice represents a model of chronic, as opposed to episodic migraine, is unknown. We believe that this baseline behavior is equivalent to the constant mild photophobia that patients with episodic migraine report between attacks (Mulleners et al., 2001), but future studies are needed to solve this question.
In addition to measuring light aversive parameters, we have performed an extensive analysis of motor activity in both the light and dark zones of the testing chamber. Our findings revealed that CGRP-induced effects on motor activity (rearing, distance traveled, ambulatory time, resting time, and velocity) were similar in nestin/hRAMP1 and control mice. Therefore the distinct light aversive behavior displayed by nestin/hRAMP1 mice after CGRP treatment appears to be independent of the CGRP-induced decreased motility or exploration.
The role of CGRP in motor activity remains unclear. CGRP has been reported to increase rearings and decrease ambulatory activity in rats in the open field test (Kovacs et al., 1999). However, recent work by Schorscher-Petcu et al in C57BL/6 mice did not find a significant effect of centrally administered CGRP in motor activity in two different paradigms or anxiety-like behavior in the elevated plus maze test (Schorscher-Petcu et al., 2009).
We recognize that CGRP-induced decreased ambulatory activity and simultaneously increased velocity seems paradoxical. This has to be interpreted with caution given the relatively low amount of time that the mice spend moving after CGRP treatment. Nevertheless, the main reason to analyze motor activity in the light aversion test is to rule out a prominent abnormality in ambulation that could keep the mice from transitioning between the light and dark compartments. Given the striking preference that nestin/hRAMP1 showed for the dark in response to CGRP and the similar effects of CGRP in motility in both genotypes, we conclude that CGRP effects in motility are not responsible for the photophobic phenotype.
Whereas the subjective perception of headache can not be assessed in mice, we noticed during the course of our studies that CGRP had other general effects, some of them suggestive of discomfort or pain (decreased escape response, decreased exploration, piloerection and diarrhea). These were observed in both nestin/hRAMP1 and control littermates, but we could not objectively quantify them. Future studies are needed to formally examine these effects of CGRP.
An interesting finding of our study is the differences in motility in the light and dark compartments. We speculate that the CGRP-induced reduction of motor activity or exploration, observed only in the dark, represents a deliberate avoidance of physical activity as oppose to a motor deficit per se. Furthermore, one of the diagnostic criteria of migraine is aggravation of the headache by routine physical activity (walking or climbing stairs) (Headache Classification Subcommittee of the International Headache Society, 2004).
The greater effect of CGRP on motor activity in nestin/hRAMP1 compared with control mice in the dark is most likely due to their higher sensitivity to CGRP. This is also consistent with clinical evidence showing that migraine patients develop a migraine-like headache in response to intravenous CGRP (Lassen et al., 2002) while non-migraine individuals only experience a sensation of head fullness (Petersen et al., 2005)
Whereas our studies establish an essential role for CGRP in the light aversive behavior, further studies are required to elucidate the exact mechanisms of CGRP action and possible contributions from other neuropeptides. We have previously investigated the specificity of CGRP actions by co-administering CGRP and the CGRP receptor antagonist, olcegepant (Recober et al, 2009). We found that olcegepant prevented the CGRP-induced light-aversive behavior. These studies established the involvement of CGRP receptors because CGRP can interact with other receptors at lower affinities. In addition, RAMP1 interacts with the calcitonin receptor to generate the amylin receptor, AMY1(a). AMY1(a) has a relatively high affinity for CGRP (Hay et al., 2006a), but olcegepant has approximately 150-fold higher affinity for the CGRP receptor than for the AMY1(a) receptor (Hay et al., 2006b). A potential caveat is that the drug concentrations at the relevant sites are not known; thus, we cannot exclude a combination of CGRP and AMY1(a) receptor actions contributing to the light-aversive phenotype.
This study provides an appreciation of the significance of the neuropeptide CGRP in migraine. The results demonstrate that CGRP-induced light aversion behavior in nestin/hRAMP1 mice can be objectively quantified using the light aversion test and may represent a good surrogate to study photophobia. The development of nestin/hRAMP1 mice that exhibit some behaviors consistent with migraine, including photophobia and mechanical allodynia (Marquez de Prado et al., 2009), suggest that a genetic and/or epigenetic-based sensitivity to CGRP may contribute to migraine susceptibility. In this scenario, CGRP is proposed to act as a neuromodulator to increase synaptic transmission from multiple sensory inputs. With increased RAMP1 in the nervous system, a threshold might be reached so that the increased input results in migraine. While caution is necessary when translating rodent to human behavior, nestin/hRAMP1 mice may represent a model for mechanistic studies of migraine and other disorders associated with photophobia. Future studies with nestin/hRAMP1 mice should prove useful for unraveling the sites of action, target genes, and mechanisms of CGRP action that contribute to photophobia and other migraine-like symptoms.
Acknowledgements
We thank Pedro Gonzalez-Alegre for invaluable suggestions and use of his video tracking system in early studies, Beverly Davidson for sharing her light-dark boxes, Eric van Otterloo for initial development of the light aversion test, Jacqueline N. Crawley for her suggestions on the light aversion test, and Donna Hammond and members of the Russo lab for stimulating discussions.
Studies were supported by NIH grants DE016511, DE018149, UL1 RR024979 and T32 NS045449, and the National Headache Foundation. We thank the University of Iowa Clinical and Translational Science Program for their support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- Arulmani U, Maassenvandenbrink A, Villalon CM, Saxena PR. Calcitonin gene-related peptide and its role in migraine pathophysiology. Eur J Pharmacol. 2004;500:315–330. doi: 10.1016/j.ejphar.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Bourin M, Hascoet M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- Costall B, Jones BJ, Kelly ME, Naylor RJ, Tomkins DM. Exploration of mice in a black and white test box: validation as a model of anxiety. Pharmacol Biochem Behav. 1989;32:777–785. doi: 10.1016/0091-3057(89)90033-6. [DOI] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Drummond PD, Woodhouse A. Painful stimulation of the forehead increases photophobia in migraine sufferers. Cephalalgia. 1993;13:321–324. doi: 10.1046/j.1468-2982.1993.1305321.x. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N Engl J Med. 2002;346:257–270. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- Hay DL, Poyner DR, Sexton PM. GPCR modulation by RAMPs. Pharmacol Ther. 2006a;109:173–197. doi: 10.1016/j.pharmthera.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Hay DL, Christopoulos G, Christopoulos A, Sexton PM. Determinants of 1-piperidinecarboxamide, N-[2-[[5-amino-l-[[4-(4-pyridinyl)-l-piperazinyl]carbonyl]pentyl]amino]-1- [(3,5-dibromo-4-hydroxyphenyl)methyl]-2-oxoethyl]-4-(1,4-dihydro-2-oxo-3(2 H)-quinazolinyl) (BIBN4096BS) affinity for calcitonin gene-related peptide and amylin receptors--the role of receptor activity modifying protein 1. Mol Pharmacol. 2006b;70:1984–1991. doi: 10.1124/mol.106.027953. [DOI] [PubMed] [Google Scholar]
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders. Cephalalgia. (2nd edition) 2004;24 Suppl 1:9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- Kovacs A, Telegdy G, Toth G, Penke B. Neurotransmitter-mediated open-field behavioral action of CGRP. Life Sci. 1999;64:733–740. doi: 10.1016/s0024-3205(99)00002-8. [DOI] [PubMed] [Google Scholar]
- Kowacs PA, Piovesan EJ, Werneck LC, Tatsui CE, Lange MC, Ribas LC, da Silva HP. Influence of intense light stimulation on trigeminal and cervical pain perception thresholds. Cephalalgia. 2001;21:184–188. doi: 10.1046/j.1468-2982.2001.00178.x. [DOI] [PubMed] [Google Scholar]
- Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- Marquez de Prado B, Hammond DL, Russo AF. Genetic enhancement of calcitonin gene-related peptide-induced central sensitization to mechanical stimuli in mice. The Journal of Pain. 2009 doi: 10.1016/j.jpain.2009.03.018. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulleners WM, Aurora SK, Chronicle EP, Stewart R, Gopal S, Koehler PJ. Self-reported photophobic symptoms in migraineurs and controls are reliable and predict diagnostic category accurately. Headache. 2001;41:31–39. doi: 10.1046/j.1526-4610.2001.111006031.x. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Thompson R, Tashiro A, Chang Z, Bereiter DA. Bright light produces Fos-positive neurons in caudal trigeminal brainstem. Neuroscience. 2009;160:858–864. doi: 10.1016/j.neuroscience.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Petersen KA, Lassen LH, Birk S, Lesko L, Olesen J. BIBN4096BS antagonizes human alpha-calcitonin gene related peptide-induced headache and extracerebral artery dilatation. Clin Pharmacol Ther. 2005;77:202–213. doi: 10.1016/j.clpt.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Austin JS, Mogil JS, Quirion R. Role of Central Calcitonin Gene-Related Peptide (CGRP) in Locomotor and Anxiety- and Depression-Like Behaviors in Two Mouse Strains Exhibiting a CGRP-Dependent Difference in Thermal Pain Sensitivity. J Mol Neurosci. 2009 doi: 10.1007/s12031-009-9201-z. [DOI] [PubMed] [Google Scholar]
- Recober A, Kuburas A, Zhang Z, Wemmie JA, Anderson MG, Russo AF. Role of calcitonin gene-related peptide in light-aversive behavior: Implications for migraine. J Neurosci. 2009 doi: 10.1523/JNEUROSCI.1727-09.2009. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiels E, Hoffman EK, Gorin MB. A reliable behavioral assay for the assessment of sustained photophobia in mice. Curr Eye Res. 2008;33:483–491. doi: 10.1080/02713680802130347. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Dickerson IM, Russo AF. Calcitonin gene-related peptide receptor activation by receptor activity-modifying protein-1 gene transfer to vascular smooth muscle cells. Endocrinology. 2006;147:1932–1940. doi: 10.1210/en.2005-0918. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Winborn CS, Marquez de Prado B, Russo AF. Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J Neurosci. 2007;27:2693–2703. doi: 10.1523/JNEUROSCI.4542-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]